The use of ChAdOx1 and Ad26.COV2.S anti-COVID-19 adenovirus vector vaccines has been associated with unusual cases of cerebral venous sinus thrombosis (CVST) with thrombocytopenia and anti-platelet factor 4 (anti-PF4) antibodies. In this syndrome, which has been named vaccine-induced immune thrombotic thrombocytopenia (VITT) [1], the binding between anti-PF4 antibodies and platelet FcγRIIA receptor causes platelets activation with the release of procoagulant microparticles leading to CVST [2,3]. A similar mechanism has been observed in cases of heparin-induced thrombocitopenia [4–7].
For both vaccines, on April 7 and 20, 2021, the European Medicines Agency (EMA) stated ‘[…] COVID-19 is associated with a risk of hospitalization and death. The reported combination of blood clots and low blood platelets is very rare, and the overall benefits of the vaccine in preventing COVID-19 outweigh the risks of side effects.’ This statement stemmed from the analysis of individual case reports (169 for ChAdOx1 and 12 for Ad26.COV2.S) [8,9] of adverse events reported for these adenovirus vaccines and the demonstrated efficacy of vaccination against COVID-19 transmission and clinical severity [10–12]. Indeed, the burden of thromboembolic events reported after vaccination (469 reports, 191 of them from the European Economic Area [EEA]) was lower than that expected in the general population [13]. Although the EMA did not impose age-related limits for the use these vaccines, several EU countries opted for preferential use among subjects older than 60 years, given that CVST case reports mainly concerned younger women (median age: 42 years) (female/male ratio: 49/12). Nevertheless, these decisions did not consider the individual risk of venous thromboembolism (VTE) among younger individuals. In this respect, the EMA also provided a visual risk contextualization in which ChAdOx1 showed a favorable benefit/risk profile for almost all age categories [14], and a recent statement of CHMP (EMA) further confirmed this position [15].
The discussion regarding VITT was also featured by misinformation and miscommunication, which clearly contributed to vaccine resistance and hesitancy [16,17], although the pandemic in the UK was initially controlled using this type of vaccine [18].
Thus, to fuel the discussion on the individual benefit/risk evaluation for anti-COVID-19 vaccines and to support the public health and ethical role of vaccination against COVID-19, we used the Health Search Database (HSD) [19], a general practice research database that covers the electronic healthcare records of about 1 million patients, to quantify the individual risk of VTE among those subjects who developed CVST before vaccine availability, according to age and a validated score employed to predict such a risk [20]. Including 21 clinical risk factors, our VTE score showed good accuracy with an AUC of 0.82 (95% CI: 0.82–0.83), explaining 27.9% of the variation for VTE occurrence, and a margin of error between the predicted and observed risk of less than 10% (under or overestimation) in 70% of the validation cohort [20].
We identified all subjects who were at least 18 years old between 1 January 2000and 31 December 2019. They were followed from the start date (1 January 2000) or their 18th birthday until the occurrence of whichever of these events came first: diagnosis of CVST (ICD-9-CM: 437.6x, 325x), death, transfer out of general practice or end of the study period (31 December 2019). The individual VTE risk among CVST cases was assessed using the aforementioned score, whose determinants were operationally defined on 31 December 2019. We identified 92 cases of CVST (mean age: F: 57.4 ± 18.2 vs. M: 58.5 ± 17.9) with an incidence rate equal to 3.44 (95% CI 2.77–4.22) per 1,000,000 person-years. Figure 1 displays those subjects diagnosed with CVST (squares and triangles according to age) within the background risk of VTE for the general population. Of the 92 cases, the mean score of VTE was equal to 1.67 ± 0.41, thus predicting 30-day risk of VTE equal to 0.7 per 1000 (vs. 0.5 per 1000 in the overall population). Sixteen were younger than 60 years and were staged as very high (n = 10) or high risk (n = 6), and most were female (n = 11) (Figure 2). Also, the total number of patients younger than 60 years who were at high or very high risk in the overall population was not negligible.
Figure 1.
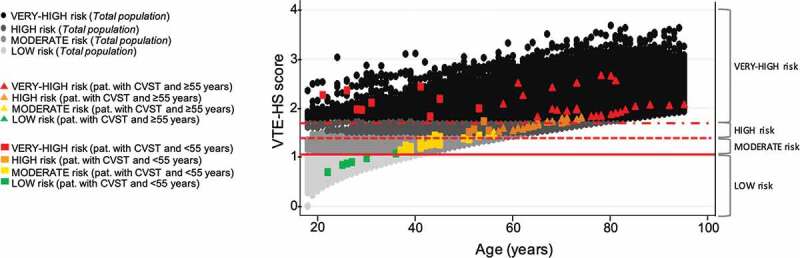
Distribution of venous thromboembolism (VTE) risk among subjects with a history of cerebral venous sinus thrombosis (CVST) and general population included according to age and risk categories. Solid line: cutoff point 1.01 (risk of VTE equal to 0.03 per 1000); dashed line: cutoff point 1.43 (risk of VTE equal to 0.1 per 1000); dash-dotted line: cutoff point 1.80 (risk of VTE equal to 0.3 per 1000). This risk categories have been obtained using Cox’s method.
Figure 2.
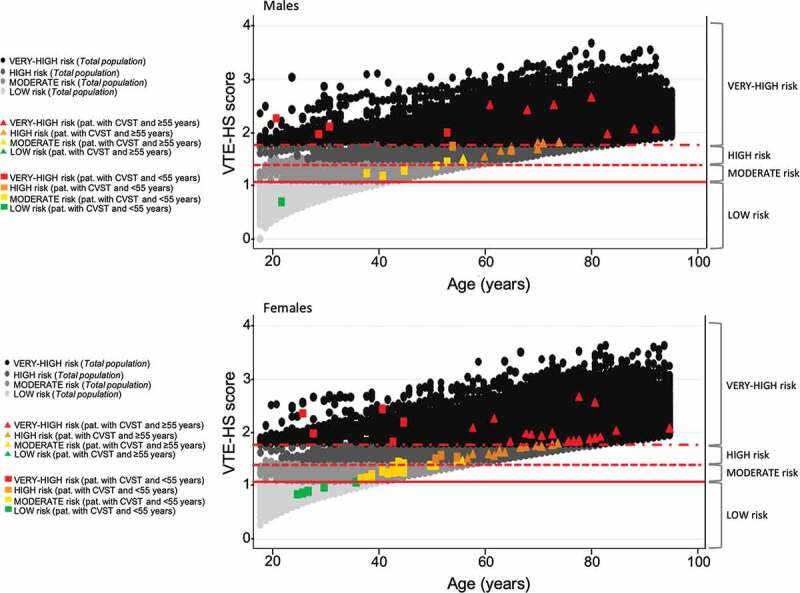
Distribution of venous thromboembolism (VTE) risk among subjects with a history of cerebral venous sinus thrombosis (CVST) and general population included according to age and risk categories and stratified by gender. Solid line: cutoff point 1.01 (risk of VTE equal to 0.03 per 1000); dashed line: cutoff point 1.43 (risk of VTE equal to 0.1 per 1000); dash-dotted line: cutoff point 1.80 (risk of VTE equal to 0.3 per 1000). This risk categories have been obtained using Cox’s method.
Our results show that the risk of CVST in the Italian general population is in line with the most recent estimates.
According to the VTE risk estimated using our score, CVST cases are subjects who, irrespective of age, are at a high risk of thrombosis, even excluding the additional risk due to Sars-CoV2 infection. In this context, data from nine tertiary stroke centers showed that the rate of thrombotic events in patients who develop COVID-19 ranges 15–30% [21]. Along this line, a recent study showed an incidence of CVT in the two weeks after a Sars-CoV-2 infection equal to 42.8 per million people [22], compared to an incidence of 1.52 per 100,000 person-months after ChAdOx1 vaccination [23], thus clearly emphasizing the favorable benefit/risk profile for anti-COVID vaccination [15].
These results are consistent with the fact that the potential risk of COVID-related thrombosis needs more attention than the potential risk of vaccine-related CVST, especially among women. This evidence is not limited to older individuals, who certainly cover most of the vulnerable population, but concerns a number of younger individuals, including those with a history of CVST and a high risk of VTE, as quantified by our score. Unfortunately, there are still several young women who are unvaccinated and/or reluctant to be vaccinated, whose risk of infection-related thrombosis might be sensibly reduced by using the indicated vaccine for this category of subjects.
Declaration of interests
F Lapi and E Marconi provided consultancies in protocol preparation for epidemiological studies and data analyses for AstraZeneca and Pfizer; C Cricelli provided clinical consultancies for AstraZeneca and Pfizer. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclsed.
Reviewer disclosures
A reviewer on this manuscript is a member of the UK Dept. Health and Social Care’s (DHSC) Joint Committee on Vaccination & Immunisation (JCVI) but does not participate in policy decisions on COVID19 vaccine. He/she is also a member of the WHO’s SAGE, and is an investigator on clinical trials of Oxford University’s COVID19 vaccine funded by NIHR. Oxford University has entered a joint COVID19 vaccine development partnership with Astra Zeneca. Peer reviewers on this manuscript have no other relevant financial or other relationships to disclose.
Author contributions
All authors substantially contributed to the conception and design of the review article and interpreting the relevant literature and been involved in writing the review article or revised it for intellectual content.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Klok FA, Pai M, Huisman MV, et al. Vaccine-induced immune thrombotic thrombocytopenia. Lancet Haematol. 2022;9(1):e73–e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marchandot B, Carmona A, Trimaille A, et al. Procoagulant microparticles: a possible link between vaccine-induced immune thrombocytopenia (VITT) and cerebral sinus venous thrombosis. J Thromb Thrombolysis. 2021;52(3):689–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greinacher A, Selleng K, Palankar R, et al. Insights in ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. Blood. 2021;138(22):2256–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(22):2124–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simpson CR, Shi T, Vasileiou E, et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med. 2021;27(7):1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greinacher A, Thiele T, and Warkentin TE, et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]; ••This article provides an overview about the mechanisms behind thrombotic and thrombocytopenic events after vaccination.
- 7.European Medicines Agency . AstraZeneca’s COVID-19 vaccine: EMA finds possible link to very rare cases of unusual blood clots with low blood platelets. Apr. 2021;7. [cited 2021 Apr 30]. Available from: https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood. [Google Scholar]
- 8.MacNeil JR, Su JR, Broder KR, et al. Updated recommendations from the advisory committee on immunization practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients — United States, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70(17):651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CHMP . Assessment report EMA/239822/2021. [cited 2021 Apr 26]. Available from: https://www.ema.europa.eu/en/documents/referral/use-vaxzevria-prevent-covid-19-article-53-procedure-assessment-report_en.pdf.
- 10.Voysey M, Costa Clemens SA, Madhi SA, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomized trials. Lancet. 2021;397(10277):881–891. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This article provides evidence of vaccine’s efficacy.
- 11.Johnson & Johnson COVID-19 Vaccine Authorized by U.S . FDA for emergency use | johnson & Johnson. [cited 2021 May 3]. Available from: https://www.jnj.com/johnson-johnson-covid-19-vaccine-authorized-by-u-s-fda-for-emergency-usefirst-single-shot-vaccine-in-fight-against-global-pandemic.
- 12.Janssen Investigational COVID-19 vaccine: interim analysis of phase 3 clinical data released | national Institutes of Health (NIH). [cited 2021 May 3]. Available from: https://www.nih.gov/news-events/news-releases/janssen-investigational-covid-19-vaccine-interim-analysis-phase-3-clinical-data-released.
- 13.Østergaard SD, Schmidt M, Horváth-Puhó E, et al. Thromboembolism and the Oxford–AstraZeneca COVID-19 vaccine: side-effect or coincidence? Lancet. 2021;397(10283):1441–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This article provides evidence about the natural risk of venous thromboembolism before the introduction of COVID-19 vaccines.
- 14.Annex to Vaxzevria Art.5.3 - Visual risk contextualization. [cited 2021 Apr 30]. Available from: https://www.ema.europa.eu/en/documents/chmp-annex/annex-vaxzevria-art53-visual-risk-contextualisation_en.pdf.
- 15.CHMP . Committee for Medicinal Products for Human Use (CHMP) Assessment report; 2021. [cited 2021 Oct 11]. Available from: www.ema.europa.eu/contact.
- 16.Fischer A. SARS-CoV-2 vaccines, where do we stand? C R Biol. 2021;344(1):43–55. [DOI] [PubMed] [Google Scholar]
- 17.Salerno L, Craxì L, Amodio E, et al. Factors affecting hesitancy to mRNA and viral vector COVID-19 vaccines among college students in Italy. Vaccines (Basel). 2021;9(8):927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phe PHE monitoring of the early impact and effectiveness of COVID-19 vaccination in England; 2021. [Accessed 11 October 2021]. Available from: https://www.gov.uk/government/publications/phe-monitoring-of-the-effectiveness-of-covid-19-vaccination.
- 19.HealthSearch . [cited 2021 Oct 11]. Available from: https://www.healthsearch.it/.
- 20.Dentali F, Fontanella A, Cohen AT, et al. Derivation and validation of a prediction model for venous thromboembolism in primary care. Thromb Haemost. 2020;120(4):692–701. [DOI] [PubMed] [Google Scholar]
- 21.Bilaloglu S, Aphinyanaphongs Y, and Jones S, et al. Thrombosis in hospitalized patients with COVID-19 in a New York City Health System. JAMA. 2020;324(8):799–801. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This article evaluates the risk for venous and arterial thrombosis in patients hospitalized with COVID-19
- 22.Taquet M, Husain M, Geddes JR, et al. Cerebral venous thrombosis and portal vein thrombosis: a retrospective cohort study of 537,913 COVID-19 cases. EClinicalMedicine. 2021;39:101061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulz JB, Berlit P, Diener HC, et al. COVID-19 vaccine-associated cerebral venous thrombosis in Germany. Ann Neurol. 2021;90(4):627–639. [DOI] [PMC free article] [PubMed] [Google Scholar]


