ABSTRACT
The novel coronavirus outbreak was declared a pandemic in March 2020. We are reviewing the COVID-19 vaccines authorized for use in the United States by discussing the mechanisms of action, administration, side effects, and efficacy of vaccines developed by Pfizer, Moderna, and Johnson & Johnson. Pfizer and Moderna developed mRNA vaccines, encoding the spike protein of SARS-CoV-2, whereas Johnson & Johnson developed an adenovirus vector-based vaccine. Safety has been shown in a large cohort of participants in clinical trials as well as the general population since emergency approval of vaccine administration in the US. Clinical trial results showed the Pfizer and Moderna vaccines to be 95.0%, and the Johnson & Johnson vaccine to be 66.0% effective in protecting against moderate and symptomatic SARS-CoV-2 infection. It is important to keep medical literature updated with the ongoing trials of these vaccinations, especially as they are tested among different age groups and upon the emergence of novel variants of the SARS-CoV-2 coronavirus.
KEYWORDS: Vaccine, COVID-19, coronavirus, SARS-CoV-2, Pfizer, Moderna, Johnson & Johnson
Introduction
The SARS-CoV-2 virus is responsible for the COVID-19 pandemic that has infected hundreds of millions and resulted in millions of deaths worldwide.1 In the majority of the patients, SARS-CoV-2 presents as a respiratory infection causing fever, chills, cough, difficulty breathing, and other associated symptoms.2,3 Serious COVID-19 infection resulted in the use of ventilator support and organ damage.4 As a result of the rapid spread and burden of disease of the novel coronavirus, researchers and pharmaceutical companies had to develop vaccines using preexisting or novel technologies rapidly. In the U.S., there are currently three vaccines being administered broadly. These vaccines were developed by Pfizer-BioNTech, Moderna, and Johnson & Johnson. Pfizer-BioNTech and Moderna developed mRNA vaccines targeting the surface protein of SARS-CoV-2. Johnson & Johnson used preexisting technology with an adenovirus vector to trigger an immune response and offer protection for subsequent infection.5 Currently, data from clinical phase III trials have been released and show that these vaccines are effective in preventing infection and disease.6 The Pfizer-BioNTech BNT162b2 vaccine was approved by the FDA on August 23, 2021, after meeting the FDA requirements for efficacy, manufacturing, and safety.7 This review will evaluate current data showing the efficacy of these three vaccines in preventing coronavirus infection. Additionally, new variants of SARS-CoV-2 have emerged recently. Each vaccine has had differing responses to the variants that will be discussed as well as other differences between the vaccines, such as administration and adverse effects.8
Pfizer–BioNTech mRNA COVID-19 vaccine BNT162b2
Development
Pfizer’s COVID-19 vaccine was the quickest vaccine to be developed, taking just about 7 months after its phase I/II trial took place in May 2020 for the FDA to allow for its emergency use in December 2020.8 The previous record set by pharmaceutical company Merck took 4 years to develop the world’s first effective vaccine against mumps in 1967. With cases nearing 5 million and deaths just shy of 150,000 globally in May 2020, Pfizer began Phase I/II testing. The study included 45 participants divided into three groups, with each group containing 15 participants. Group 1 was supplied two 10 μg doses, separated by 21 days. Group 2 was given 2 30 μg doses, separated by 21 days. Group 3 was administered a single 100 μg dose. To compare the immunogenicity of the vaccine to individuals who recovered from infection, human convalescent sera (HCS) were extracted from 38 patients 14 days after the PCR-confirmed diagnosis. The receptor-binding domain (RBD) is part of the virus that binds to a receptor within the host and facilitates entry of the virus into host cells. The RBD IgG is sufficient for serological testing because neutralizing antibodies can act here and prevent SARS-CoV-2 entry into host cells. Remarkably, HCS revealed an RBD-binding IgG concentration of 602 U ml−1 compared to group 2, which showed an RBD-binding IgG concentration of 16,166 U ml-1. Group 1 demonstrated an RBD IgG concentration of 5,880 U ml−1 d. Group 3 displayed an RBD-binding IgG concentration of 1,260. All RBD-binding IgG concentrations were measured 35 days after administration of the first dose. These findings demonstrated that the Pfizer vaccine was effective in producing antibodies against SARS-CoV-2 signifying a pivotal moment in the fight against the pandemic.8
With the success of the Phase I/II trial, Pfizer and BioNTech were approved to proceed to phase III testing. As the ability of the BNT162b2 vaccine to produce neutralizing antibodies to the RBD of SARS-CoV-2 was now well established, the phase III trial would determine the safety and efficacy of the vaccine. A total of 43,448 participants 16 years or older were gathered and received the vaccine. Of which, 21,720 received 2 30 μg doses of the BNT162b2 vaccine separated by 21 days and 21,728 received placebos.9
Figure 1 explains the developmental milestone of Pfizer-BioNTech mRNA vaccine. On January 12, 2020, the genetic sequence of SARS-CoV-2 was made public.5 In late April, Pfizer and BioNTech began phase I/II testing in Germany with 12 participants dosed with BNT162. Phase I/II testing in the US begun less than two weeks later with 360 subjects receiving doses. In late July 2020, Pfizer announced that they will start phase III testing with 2, 30 µg doses administered to over 30,000 subjects. In November 2020, Pfizer concluded phase III testing and announced an efficacy of 95.0%. On December 11, 2020, the FDA authorized emergency use of BNT162b28 and on August 23, 2021, the US FDA approved the Pfizer vaccine making it the first approved COVID-19 vaccine.10
Figure 1.
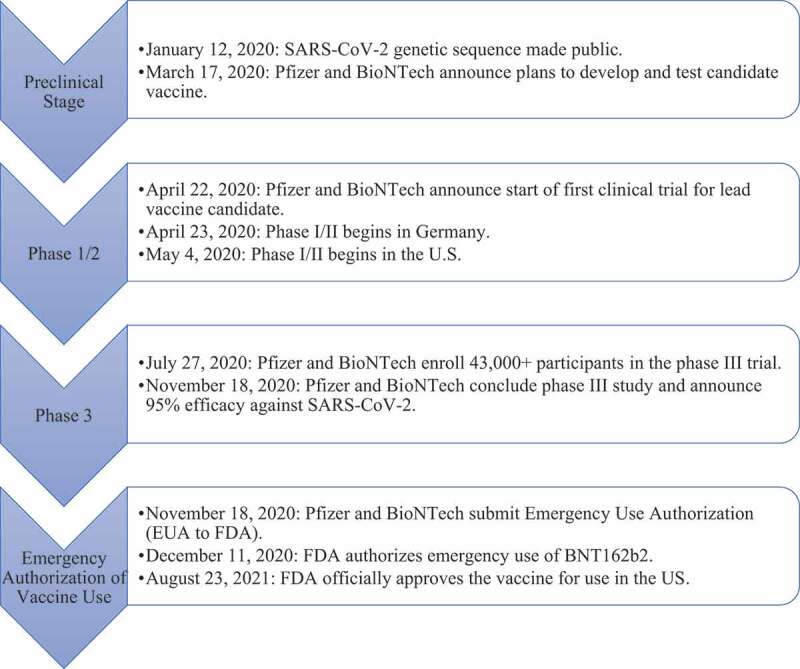
Mechanism of action
The Pfizer and Moderna vaccines express similar mechanisms of action. The vaccine contains a nucleoside-modified mRNA that encodes the SARS-CoV-2 spike glycoprotein and is delivered in lipid nanoparticles for more efficient delivery into host cells.11 The mRNA specifically encodes for the S2-P antigen, consisting of the SARS-CoV-2 glycoprotein with a transmembrane anchor. Binding antibody responses against S2-P were used as a method of evaluating efficacy. The vaccine’s goal is to elicit both B- and T-cell responses against the spike protein. As per published data, the vaccine was successful in inducing antibody responses to both full-length S2-P and receptor-binding domain. The potent lipid-nanoparticle delivery system used by the vaccine in combination with the use of modified nucleotides that avoid early activation of interferon-associated genes are unique features that contribute to its efficacy.12 The mRNA vaccine intends to produce prolonged protein expression, induction of antigen-specific T-follicular helper cells, and activation of germinal center B cells.13
Efficacy
In a study conducted by Polack et al., among 36,523 participants with evidence or no evidence of existing or previous SARS-CoV-2 infections, 8 cases were observed in participants who received the vaccine (at least 7 days after administration of the second dose) and 162 cases were observed in placebo recipients, equating to a 94.6% efficacy against infection.14 While not the primary focus of the study, a vaccine efficacy of 52.0% was observed which demonstrates the early protection against infection the BNT162b2 vaccine provides 12 days after the first dose. This 52.0% efficacy is based on the occurrence of 39 cases in the BNT162b2 vaccine group and 82 cases in the placebo group between the time of administration of the first dose and second dose.14 The Pfizer Phase III study includes over 46,000 participants globally, and on November 18, 2020, efficacy data were sufficient for the FDA to grant an EUA for vaccine administration in the US. The study assesses infections occurring 7 and 14 days after the second dose in participants who have either not had or had a previous infection. The results found a 95.0% efficacy in preventing infection 7 days after second dose administration in participations, with these results being pivotal in the FDA granting an EUA for the vaccine in the US.14 On March 31, 2021, Pfizer and BioNTech announced a remarkable 100% vaccine efficacy in young adolescents after a phase 1/2/3 trial that involved the administration of the BNT162b2 vaccine in over 2000 adolescents aged 12–15.15
Effectiveness against multiple genetic variants of SARS-CoV-2
The first case of a mutant variant reported in the United States was December 29, 2020, in Denver, Colorado. Cases caused by two common mutant variants of SARS-CoV-2 were initially arose in the United Kingdom and South Africa. Since then, there have been many reports of several other mutant variants globally. These variants have caught public attention for two reasons: A.) uncertainty of the vaccine coverage against these mutants and B.) the ability for these mutants to spread more rapidly. The latter has been explored by Tian et al., who demonstrated that the B.1.1.7 variant, which contains the mutation N501Y, a mutation in the spike protein that allows for binding to the host cell receptor angiotensin-converting enzyme (ACE2) showed 4 times higher binding affinity to ACE2 than the wild-type virus.16 This significant finding, among other factors, contributes to the ability of these variants to spread faster. As the cases of variants increase globally, a question that has dominated headlines was how effective currently available vaccines will be against these variants. Common among several variants is the N501Y mutation, a mutation in a gene that codes for the receptor-binding domain (RBD) that is selectively targeted by the BNT162b2 vaccine. In January 2021, Xuping et al. extracted serum from patients vaccinated with the BNT162b2 vaccine and measured the ability of their antibodies to neutralize variants expressing the N501Y mutation.17 Despite the N501Y mutation found within the spike protein, which is targeted by the BNT162b2 vaccine, this study demonstrates that there is no reduction in the efficacy of the BNT162b2 vaccine against mutant strain N501Y relative to the original strain.17 Given these findings, there is no current indication for the need to develop a new vaccine against these mutant strains. It is important to mention that as more mutant variants continue to be discovered, more research needs to be conducted to identify potential changes in vaccine coverage and spreading rates.
In Israel, the Pfizer vaccine efficacy was determined in individuals ages 16 and up, a population of approximately 6.5 million people. The goal was to approximate the efficacy of the two-dose regimen. The authors interpreted data from January 24 to April 23, 2021. Within this time, there were 232,268 infections, 7694 hospitalizations, 4481 critical hospitalizations, and 1113 deaths. By April 3, 2021, 72.1% of individuals in this age group had received both Pfizer vaccine doses. Additionally, infected individuals were tested for the prevalence of variant strains. Of these samples, 94.5% were the B.1.1.7 variant. The authors concluded that the B.1.1.7 variant was the predominant strain in Israel. After both doses were administered, the data showed that the Pfizer vaccine was effective in all ages for asymptomatic and symptomatic infection, hospitalization, and death, including infections with the B.1.1.7 variant. In individuals above the age of 16, the vaccine was 95.3% effective in preventing infection, 91.5% for asymptomatic infections, 97.0% for symptomatic infections, 97.2% for hospitalizations, 97.5% for critical hospitalizations, and 96.7% effective in preventing death. Critical hospitalizations were defined as situations requiring the use of a mechanical ventilator or the occurrence of organ failure. There were zero deaths in individuals 16–44, and the mortality incidence rate was 0.1 out of 100,000 persons per day. This showed that the two-dose Pfizer vaccine was effective in preventing asymptomatic and symptomatic infection, hospitalization, and death in regard to SARS-CoV-2 as well as the B1.1.7 variant.18,19
A booster, or third dose, of the vaccine was approved for use in patients over 60 in Israel on July 30, 2021. The immunogenicity of the booster was analyzed by Bar-On et al. and showed a lower rate of infection in participants receiving the booster. Additionally, incidence of severe infection was decreased by a factor of 19.5 in the booster group.20 The safety profile was also similar to the already reported systemic reactions in previous clinical trials, with preliminary results showing no increased risk of adverse events or systemic reactions caused by the third booster dose.21
Efficacy of Pfizer vaccine on B.1.617.2 strain
The first case of infection caused by the Delta variant, B.1.617.2, was reported in India in October 2020.22 This mutant variant has been dubbed the “Double Mutant” because of two mutations found in the RBD. Research has suggested that these mutations resulted in increased binding capacity to the ACE2 receptor resulting in increased transmissibility. The impact these mutations have on current vaccines is still being researched; however, research in several countries has shown a decline in efficacy against the Delta variant, which has been categorized as a variant of concern by the World Health Organization. In Qatar, vaccine efficacy against B.1.617.2 infection was found to be 64.2% 14 days after the first dose and 53.5% 14 days after the second dose in preventing hospitalization and death. The vaccine was less effective in preventing infection but was able to prevent the worst outcomes of hospitalization and death.23 In the United Kingdom, there was a 33.5% efficacy after the first dose and 59.8% efficacy after the second dose against the B.1.617.2 variant in preventing symptomatic infection.24 In the United States, there has been further analysis of the B.1.617.2 variant. After two doses, there was an initial 88.0% protection against symptomatic infection in the first month and a progressive decline to 47.0% efficacy after five months. In the first month after vaccination, there was a 75.0% efficacy in preventing moderate-to-severe infection. In all ages, the vaccine was 93.0% in preventing hospitalization. These results raise the question whether the gradual decline in efficacy is due to the decreasing antibody response over time or the mutations in the B.1.617.2 variant allowing for immune evasion.25 In Israel, sampling of infected patients showed a high prevalence of the Delta strain, and efficacy showed a 39.0% efficacy against asymptomatic infection, 40.5% against symptomatic infection, 88.0% against hospitalization, and 91.4% against severe or critical cases.18 Table 1 presents efficacy values for symptomatic B.1.617.2 infection and severe or fatal infection requiring hospitalization.
Table 1.
| Variant |
Country |
Efficacy against symptomatic Infection |
Efficacy against severe or fatal infection |
| B.1.617.2 | Qatar | 53.5% | 89.7% |
| United Kingdom | 88.0% | 93.1% | |
| United States | 75.0% | 93.0% | |
| Israel | 40.5% | 91.4% |
Safety profile: side effects and contraindications
Safety results from the phase III trial were obtained up to 14 weeks after the second dose was administered to capture potential delayed adverse reactions. Tolerability to the vaccine, defined as local and immediate reactions, and systemic adverse effects in individuals after receiving the second dose are shown in Table 2. The most common local reaction reported among participants was mild-moderate pain at the site of injection, which lasted up to one week. Younger patients reported pain at the site of injection (83.0% after the first dose, 78.0% after the second) more frequently than older patients (71.0% after the first dose, 66.0% after the second dose).4 Systemic reactions were far more common in the second dose and again, more common in younger participants than in older patients with the most common adverse effects including fatigue, headaches, and less frequently reported fever (≥38°C). Of the older adults, 39.0% reported headaches, 51.0% reported fatigue and 11.0% reported fever. Of the younger patients, 52.0% reported headaches, 59.0% reported fatigue and 16.0% reported fever.4 It should be noted that the reports of fatigue and headache were commonly reported among placebo participants as well (23.0% and 24.0% respectively).10,14 In a study published in the British Journal of Anesthesia, Lene H. Garvey and Shuaib Nasser discuss the occurrence of two anaphylactic responses seen in two National Health Service workers who received the BNT162b2 vaccine on the second day of the vaccine trial.26 The anaphylactic response was potentially linked to the role polyethylene glycols (PEG) might have played in these reactions. PEG is a component of the BNT162b2 vaccine and is used as an adjuvant, helping the body produce a stronger immune response. Anaphylactic responses seen in patients with PEG allergies are typically major systemic reactions with a rapid onset. Due to the rapid nature as well as the severity of the anaphylactic response observed in the two NHS workers, Garvey and Nasser emphasize that the role of PEG cannot be overlooked.
Table 2.
Tolerability and systemic adverse effects experienced by Pfizer vaccine recipients after second dose10.
| |
Individuals 18 to 55 |
|
Individuals 55 and older |
|
|---|---|---|---|---|
| Mild to moderate | Severe (symptoms prevent daily activity) | Mild to moderate | Severe (symptoms prevent daily activity) | |
| Tolerability (Local and immediate reactions) | ||||
| Injection Site Pain | 76.6% | 1.2% | 65.7% | 0.5% |
| Erythema | 5.4% | 0.5% | 6.8% | 0.5% |
| Swelling | 5.9% | 0.3% | 7.3% | 0.2% |
| Systemic Reactions | ||||
| Headache | 48.5% | 3.2% | 38.4% | 0.5% |
| Fatigue | 54.8% | 4.6% | 47.7% | 2.8% |
| Myalgia | 37.3% | 2.2% | 27.8% | 1.0% |
| Vomiting | 1.7% | 0.2% | 0.6% | 0.1% |
| Fever | 30.2% | 1.2% | 21.5% | 0.3% |
Myocarditis has been reported after administration, especially after the second dose. In adults, the prevalence of myocarditis was 3.5 cases per 1 million doses. When discussing vaccines, a risk and benefit analysis is always necessary and the FDA and CDC determined the efficacy of the vaccine in preventing hospitalization and mortality offset the increased risk of myocarditis.27 Another rare AE reported was Bell’s Palsy. Bell’s Palsy has previously been associated with administration of other viral vaccines, in addition to SARS-CoV-2. Renoud et al. compared this to cases of Bell’s Palsy associated with other viral vaccines and did not find an increased or disproportionate risk of contracting Bell’s Palsy with the Pfizer-BioNTech vaccine.28 As the vaccine distribution increases globally, the adverse effects should continue to be carefully monitored. Contraindications to the BNT162b2 vaccine, as highlighted in FDA guidelines include those with known severe allergic reactions to any components of the Pfizer-BioNTech vaccine.27
Administration
According to FDA guidelines, the BNT162b2 vaccines are administered as a series of two intramuscular (deltoid) injections of 0.3 mL separated by 3 weeks.27 The BNT162b2 vaccines are distributed in thermal containers in dry ice. Once received, according to FDA storage guidelines, vials may be stored in ultra-low temperature freezers −80°C to −60°C for up to six months or in common hospital freezers between −25°C and −15°C for up to two weeks. If needed, vials may be kept in special transport thermals for up to 30 days (refilled every 5 days with dry ice). Vials may be thawed at room temperature (25°C) for no more than 2 hours or thawed in refrigerators (2°C-8°C) for no more than 5 days.27
According to the FDA, the full list of ingredients of the BNT162b2 vaccine includes: mRNA, lipids, potassium chloride, monobasic potassium phosphate, sodium chloride, dibasic sodium phosphate dihydrate, and sucrose.27
Moderna COVID-19 vaccine (mRNA-1273)
Development
Moderna was one of the first pharmaceutical companies to pledge to develop a vaccine against COVID-19. On February 7, the first batch of the Moderna vaccine was developed and ready to use for analytical testing. The NIH was an early proponent of the mRNA-1273 vaccine, given that it worked in collaboration with Moderna in investigating the mRNA sequence for COVID-19 virus.29 On March 16th, the first patient in Phase 1 clinical trials was dosed with the mRNA-1273 vaccine. One month later, BARDA announced a grant of up to $483 million to accelerate the development of the vaccine. On May 1, Moderna in collaboration with Lonza announced a strategic partnership to develop around 1 billion mRNA-1273 vaccine doses per year. On May 12, Moderna received the FDA Fast Track designation for mRNA-1273, as the global need for a vaccine increased. Vaccine trial results were published in the New England Journal of Medicine and were well received internationally. After positive results from the Phase 1 and Phase 2 trials, BARDA continued their support, and Phase 3 trials of the mRNA-1273 vaccine began on July 26, 2020. Moderna signed an initial supply agreement with the U.S. government to provide the first 100 million doses of mRNA-1273 vaccine. After the first analysis of the Phase 3 trial was released on November 16, 2020, it was determined that the Moderna vaccine has a longer shelf life at refrigerated temperatures. This finding was significant, as it positively influenced the logistics of distributing the vaccine. On November 30th, 2020, Moderna filed for Emergency Use Authorization with the U.S. FDA, and received approval shortly after.29
The U.S. CDC Advisory Committee on Immunization Practices has recommended the use of the Moderna vaccine in individuals 18 years and older. Adolescents were included in the Phase 2/3 trials, and results are yet to be published. In the meanwhile, the Moderna mRNA-1273 vaccine is currently undergoing Phase 1/2 trials in Japan, led by Takeda Pharmaceutical Company. In recent months, clinical data suggesting that there are new variants of COVID-19 has emerged. On January 25, 2021, Moderna published results from in vitro neutralization studies from sera from infected individuals indicating that the vaccine has activity against variants identified in the U.K. and the Republic of South Africa.29 On May 10, 2021, the FDA granted approval for the emergency use of Moderna vaccine in adolescents between 12 and 18 years of age.10
Figure 2 explains the developmental milestone of the Moderna mRNA vaccine. The duration of time from the start of Phase 1 trials to FDA approval for the Moderna vaccine was approximately 9 months. Prior to the initiation of clinical trials, the NIH and Moderna worked in tandem to finalize the mRNA sequence for COVID-19. Phase 1 and Phase 2 study subjects included healthy adult volunteers from ages 18–55. After Moderna published primary efficacy results of Phase 3 studies in late November, the FDA announced emergency use authorization shortly thereafter.11,29
Figure 2.
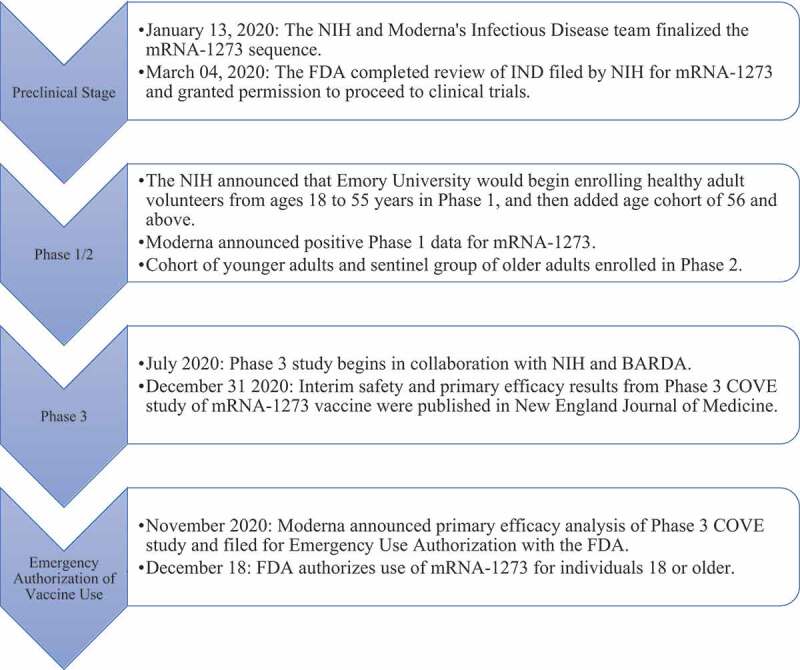
Timeline of Moderna’s mRNA-1273 vaccine development for COVID-19.11,27
Mechanism of action
The Pfizer and Moderna vaccine both use mRNA technology and are considered to have similar mechanisms of action.30 This mechanism is discussed on page 5.
Efficacy
The Moderna mRNA-1273 vaccine is 94.1% effective at protecting against symptomatic COVID-19 after administration of the second dose. The vaccine’s efficacy in the age cohort of >65 appears to be slightly lower, at 86.4%. There were no significant differences in efficacy across ethnic and racial groups. The Phase 3 COVE trial was selected for cohorts that have risk factors to severe COVID-19 infection such as chronic lung disease, cardiac disease, severe obesity, liver disease, or infection with human immunodeficiency virus. The trial enrolled 30,240 volunteers. Participants were randomly assigned to either the control group or to receive two doses of mRNA-1273. Symptomatic illness was confirmed in 185 participants in the control group, whereas it was confirmed in 11 participants in the vaccinated group. Vaccine efficacy was 94.1% in this study. Serious adverse effects were rare, with incidence being similar in both the control and vaccinated group. Overall, the COVE trial provides evidence of efficacy among adult trial participants against COVID-19.31
Effectiveness against multiple genetic variants of SARS-CoV-2
Recent reports about variants in the United Kingdom (B.1.1.7 lineage) and in South Africa (B.1.351 lineage) raised concerns about these new variants evading the Moderna vaccine.32 This led Moderna researchers to investigate the efficacy of the vaccine against new variants. Serum neutralizing activity was evaluated against the B.1.1.7 and B.1.351 variants. Results indicate that the vaccine maintains its neutralizing activity against the B.1.1.7 variant. However, a decrease in titers of neutralizing antibodies was reported against the B.1.351 variant. Protection against the B.1.351 variant by the Moderna mRNA vaccine remains to be seen.32
Efficacy of Moderna vaccine on B.1.617.2 (Delta) strain
There are currently limited studies on the effectiveness of the Moderna vaccine against the Delta lineage of SARS-CoV-2. One published study found that the Delta strain is 6.8-fold more resistant to neutralization by sera from individuals who have received both doses of the vaccine. Overall, all vaccinated individuals were able to neutralize the Delta strain by the time of the conclusion of the study. Further studies investigating the effectiveness of the Moderna vaccine on the Delta and other emerging strains will be beneficial for the vaccine manufacturer.33 A study performed by the Mayo Clinic Health System investigated the efficacy of the Moderna vaccine against the Delta variant. This study was conducted from January to July 2021, in which individuals infected with the Delta variant in geographic areas that Mayo Clinic locations covered increased sevenfold. The study, which has not been peer-reviewed at this time, suggests that the Moderna vaccine efficacy against the Delta variant is 76.0%. Despite reduced efficacy against acquiring infection, the vaccine is maintaining 90.0–95.0% efficacy against hospitalization and death across all publicly available studies.34
Safety profile: side effects and contraindications
The safety profile of the Moderna vaccine was and is continuously being assessed by monitoring local and systemic adverse events for 7 days after each injection, adverse reactions 28 days after each reaction, and adverse reactions leading to discontinuation from a dose. Medically related adverse effects will continue to be monitored through day 759. Local adverse effects were identified as pain, erythema, swelling, and lymphadenopathy. Systemic adverse effects were identified as fever, headache, fatigue, myalgia, arthralgia, nausea/vomiting, and chills.31 As per study results, local adverse events at the injection site occurred significantly more frequently in the mRNA group compared to the placebo group. Similarly, systemic adverse events occurred more frequently in the mRNA group after dose 1 and dose 2 when compared to the placebo group. Moderate-to-severe systemic side effects such as fever, myalgia, and arthralgia were noted in about 50.0% of study participants in the mRNA vaccine group after the second dose. However, these systemic side effects were resolved within day 2 after receiving the vaccine.
A severe or immediate allergic reaction such as urticaria, lymphedema, respiratory distress, or anaphylaxis after a previous dose or to an mRNA component of the Moderna vaccine serves as a contraindication.31
Adverse effects of the second dose of the Moderna vaccine are contrasted between the two age groups shown in Table 3. These adverse effects include local and immediate reactions, including injection site pain, erythema, swelling, and systemic adverse effects including headache, fatigue, myalgia, nausea, and fever.20
Table 3.
Tolerability and systemic adverse effects experienced by Moderna vaccine recipients after second dose35.
| Individuals 18 to 64 |
Individuals 65 and older |
|||
| |
Mild |
Severe (resulting in debilitating symptoms) |
Mild |
Severe (resulting in debilitating symptoms) |
| Tolerability (Local and immediate reactions) | ||||
| Injection Site Pain | 90.1% | 4.6% | 83.4% | 2.7% |
| Erythema | 9.0% | 2.0% | 7.4% | 2.1% |
| Swelling | 12.6% | 1.7% | 10.8% | 1.9% |
| Systemic Reactions | ||||
| Headache | 62.8% | 5.0% | 46.4% | 3.0% |
| Fatigue | 67.6% | 10.6% | 58.4% | 6.9% |
| Myalgia | 61.3% | 10.0% | 46.9% | 5.6% |
| Nausea | 21.3% | <0.1% | 11.8% | 0.3% |
| Fever | 17.4% | 1.6% | 10.2% | 0.5% |
Administration
The Moderna vaccine is administered through an intramuscular (IM) injection in the deltoid muscle. The dosage per injection is 0.5 mL and is scheduled as a 2-dose series separated by 28 days. Each vial contains 10 doses, and it is imperative to not mix with a diluent or combine residual vaccine from different vials. The vaccine must be initially delivered in a freezer or refrigerator at a temperature between −13 and 5°F. Vaccine vials then may be stored in a refrigerator at a temperature between 36 and 46°F for up to 30 days before the vials are punctured. Unpunctured vials may be held at room temperature between 46 and 77°F for up to 12 hours.35 Ingredients include mRNA, lipids, tromethamine, acetic acid, sodium acetate trihydrate, and sucrose.35
Johnson & Johnson SARS-CoV-2 Vaccine
Development
The emergence of the novel coronavirus disease prompted a rapid response from major pharmaceutical companies. Johnson & Johnson pledged over $1 billion toward the development of a vaccine in conjunction with the Biomedical Advanced Research and Development and the U.S. Department of Health and Human Science. On March 30, 2020, a lead vaccine candidate was announced. The vaccine was designed with AdVac technology previously used by Johnson & Johnson for the development of an Ebola vaccine during the West Africa Ebola epidemic.36 In July 2020, Phase I and II trials were combined as part of an accelerated vaccine development timeline to assess vaccine safety and determine the appropriate dosage with participants from the United States and Belgium. After Phase I/IIa trials were completed, Phase III officially started September 27, 2020, with 44,325 participants from several countries, including the U.S., Brazil, and South Africa. Preliminary outcomes to determine the efficacy of the vaccine have been published as of February 9, 2021, with researchers evaluating the prevalence of moderate and severe infection after 14 and 28 days of vaccine administration.37
After preliminary Phase III results were released, the U.S. Food and Drug Administration unanimously approved an Emergency Use Authorization for the Johnson & Johnson vaccine to be used in the United States. This would be the third vaccine approved for use in the U.S., following the Pfizer and Moderna two-dose regimen vaccines. As of May 2021, the Johnson & Johnson vaccine is being manufactured on a wide scale and doses are being administered to eligible individuals.38
Globally, the Johnson & Johnson vaccine has also been approved for use. South Africa began the administration of the vaccine on February 17, 2021. South Africa approved the Johnson & Johnson vaccine earlier due to the emergence of a more contagious variant of COVID-19, the South African variant 501.V2. The South African government initially had purchased AstroZeneca’s vaccine, but early trials showed that the AstraZeneca vaccine did not provide the same level of protection against mild, moderate, and severe coronavirus infection that the Johnson & Johnson vaccine did. Key aspects of the Johnson & Johnson vaccine that allowed for its rapid approval were that it is only required as a single-dose and could be stored at higher temperatures than its competitors, allowing for easier implementation in rural and underdeveloped communities that do not have access to the freezers and technology required to store mRNA vaccines.39 Bahrain was the next country to authorize its use on February 25, 2021, approving vaccination for individuals 65 or older and those with preexisting conditions.40
Johnson & Johnson officially announced a lead candidate for their vaccine using an adenovirus vector. Phase 1 and 2 started in July 20202 and were combined to determine safety and dosage in the United States and Belgium. Phase 3 started September 7, 2020, with the goal of determining vaccine efficacy. Phase 3 was the Ensemble trial and approximately 44,000 individuals participated. In February 2021, the U.S. FDA issued an emergency use authorization of the Johnson & Johnson and vaccine administration began, with this timeline being shown graphically in Figure 3.22
Figure 3.
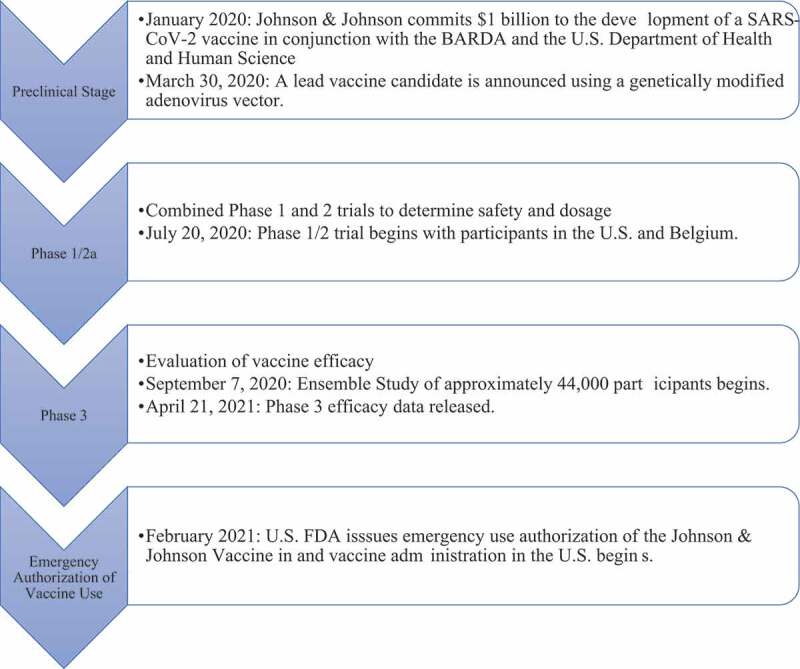
Timeline of Johnson & Johnson vaccine development for COVID-19.37,38
Table 4 summarizes the key clinical trials for the Pfizer, Moderna, and Johnson & Johnson vaccines and includes the timeline for Phase 3 clinical trials and the approval of an Emergency Use Authorization for each vaccine by the US Food and Drug Administration.
Table 4.
Summary of key clinical trials for Pfizer, Moderna, and Johnson & Johnson SARS-CoV-2 Vaccines9,29,38.
| Key clinical trials for Pfizer, Moderna, and Johnson & Johnson vaccine candidates | |
| Pfizer–BioNTech mRNA COVID-19 Vaccine BNT162b2 |
|
| Moderna mRNA-1273 COVID-19 Vaccine |
|
| Johnson & Johnson Adenovirus Type 26 COVID-19 Vaccine |
|
Mechanism of action
The Johnson & Johnson (Janssen) Vaccine is a product of a recombination and replication-incompetent human adenovirus type 26 vector that expresses the SARS-CoV-2 spike antigen. The adenovirus is normally responsible for symptoms similar to the common cold and serves as the viral vector. Adenovirus type 26 is a naturally occurring virus and occurs in low prevalence in humans. Without replication, the virus is unable to propagate within its host. A replication gene is deleted in the vaccine, so it is incapable of replicating within humans and causing infection. As a result, it is an appropriate method to deliver genetic material encoding the spike antigen into human cells. The spike (S) antigen is what is responsible for the immune protection offered by the vaccination. Protective antibodies are produced and protected from future infection.28 An immune response is mounted against the S antigen and results in a vaccine-induced antibody response against the spike protein in SARS-CoV-2.41
Efficacy
Currently, Johnson & Johnson vaccine is in a Phase 3 trial, entitled “Ensemble,” determining the efficacy of the single-dose regimen. “Ensemble II” is also undergoing to evaluate the efficacy of the vaccine in a dual-dose regimen, but results are not expected until late 2021.42 Ensemble is a randomized, double-blind, and placebo-controlled study including participants from the United States, South Africa, Mexico, Brazil, Peru, Argentina, Colombia, and Peru. Of 44,325 participants, all individuals above the age of 18 were included, and outcomes will be measured spanning 2 years. Researchers are evaluating the efficacy of the vaccine in preventing moderate and severe infections. Moderate infection is defined as a respiratory rate less than or equal to 20 breaths per minute, pneumonia, shortness of breath, difficulty breathing, fever, sore throat, and chills. Severe infection is defined as respiratory failure, presence of shock, intensive care unit admission, organ failure, and death.37
For protection against moderate infection, efficacy was 66.9% 14 days after vaccination and 66.1% 28 days after vaccination. Against severe infection, efficacy was 76.7% 14 days after vaccination and 85.4% 28 days after vaccination. Primary outcomes were released on January 5, 2021, and there were no COVID-19 associated hospitalizations or deaths in participants who received the Johnson & Johnson vaccine. Researchers also found no differences in safety and efficacy in different age groups.36
Primary vaccine trial data from Ensemble showed that vaccine was approximately 66.0% effective in the prevention of moderate infection 14 and 28 days after vaccine administration. The vaccine was approximately 80.0% effective in preventing severe infection 14 and 28 days after vaccine administration.43 In the phase III study, 39,321 participants were given the Johnson and Johnson vaccine or a placebo in an international, randomized, and double-blind trial. The study assessed prevention of moderate-to-severe infection within 14 and 28 days of vaccine administration. Within 14 days, there was 66.9% efficacy in preventing moderate infection and 66.1% efficacy within 28 days. Protection against critical infection was 76.7% within 14 days and 85.4% within 28 days.44 These results show that the vaccine is effective in preventing the most serious outcomes, which are admission into the ICU and death.45 This is important because the onset of coronavirus pandemic had overwhelmed hospitals globally. Preventing moderate and severe infections would reduce the number of hospitalizations and reduce the burden of COVID-19 on the healthcare infrastructure.
Effectiveness against multiple genetic variants of SARS-CoV-2
The Johnson & Johnson phase 3 trial occurred later than Moderna’s and Pfizer’s. As a result, results were able to evaluate the efficacy of the vaccine to new variants of SARS-CoV-2. In South Africa, a new and more contagious variant entitled 501Y.V2 has been discovered. 501Y.V2 is a variant of the B.1.351 lineage. In the Ensemble study by Johnson & Johnson, various strains were identified in COVID-19 positive participants. In the United States, 96.4% of COVID-19 infections were due to the original Wuhan-H1 variant D614G. In Brazil, 69.4% were from the P.2 lineage and 30.6% were due to Wuhan-H1 D614G. In South Africa, 94.5% of variants identified were 501Y.V2. These results show the evolution of the pandemic due to multiple genetic variants and the necessity of emerging vaccines to be effective against different variants. In the US and Brazil, efficacy was similar to the initial study at approximately 66.0% against moderate infection and 80.0% against severe infection. However, the vaccine efficacy on the South African variant was only 52.0% after 14 days and 73.1% after 14 days. For severe infection, efficacy was 64.0% after 14 days and 81.7% after 28 days. These results are important as South Africa has adjusted their vaccine implementation and focus toward the Johnson & Johnson vaccine to combat the 501Y.V2 variant.43 In terms of neutralizing antibodies and immunogenicity of the vaccine, preliminary studies by Johnson & Johnson show there is a 1.6-fold reduction in neutralizing antibodies to the Delta variant, but there is still strong evidence the vaccine can prevent hospitalization and death, which are the most important goals of vaccination.46,47 These preliminary studies currently show that the single-dose vaccine efficacy is high in preventing mortality due to the Delta variant despite lower neutralizing antibody levels.
Safety profile: side effects and contraindications
Contraindications to the Johnson & Johnson vaccine include individuals with a known history of a severe allergic reaction to the ingredients of the vaccine. There is not enough data to guide the administration of the vaccine to pregnant women.48
Local adverse reactions were measured in a cohort of vaccine recipients, with 2,036 individuals ages 18 to 59 and 1,320 individuals ages 60 and above. Table 5 shows the prevalence of local adverse reactions and whether mild symptoms were experienced or severe symptoms were expressed in each age group. In this group, only one serious adverse event was recorded, with one patient experiencing a hypersensitivity reaction to the vaccine, resulting in facial swelling with no other debilitating symptoms.49 The most common side effect was injection site pain. Thrombotic events were also studied in vaccine recipients. There were six thrombotic events recorded, occurring as a deep vein thrombosis. However, further analysis suggested that these events were not related to the Johnson & Johnson vaccine.36
Table 5.
Tolerability and systemic adverse effects experienced by Johnson & Johnson vaccine recipients43.
| Individuals 18 to 59 |
Individuals 60 and older |
|||
| |
Mild |
Severe (resulting in debilitating symptoms) |
Mild |
Severe (resulting in debilitating symptoms) |
| Tolerability (Local and immediate reactions) | ||||
| Injection Site Pain | 58.6% | 0.4% | 33.3% | 0.2% |
| Erythema | 9.0% | 0.3% | 4.6% | 0.1% |
| Swelling | 7.0% | 0.2% | 2.7% | 0.2% |
| Systemic Reactions | ||||
| Headache | 44.4% | 0.9% | 30.4% | 0.4% |
| Fatigue | 43.8% | 1.2% | 29.7% | 0.8% |
| Myalgia | 39.1% | 1.4% | 24.0% | 0.2% |
| Nausea | 15.5% | 0.1% | 12.3% | 0.2% |
| Fever | 12.8% | 0.3% | 3.1% | 0.1% |
Adverse effects experienced by recipients of the Johnson & Johnson vaccine are listed in Table 5, with the majority of individuals aged over 18 experiencing local and immediate reactions such as injection site pain, erythema, swelling, and systemic reactions including headache, fever, fatigue, and myalgia. These symptoms were reported to be mild in most participants, with less than 1% of the cohort experiencing debilitating symptoms that impeded the ability to carry out daily tasks or required hospitalization.23 Additional serious AEs have also occurred. The FDA has issued changes to the EUA fact sheet warning of the potential but rare risk of Guillain-Barré syndrome (GBS) and thrombosis with thrombocytopenia syndrome (TTS). In the US, as of September 15, 2021, over 15 million doses have been administered, and there have only been 47 confirmed cases of thrombosis with thrombocytopenia syndrome. This is a rare occurrence noted in women under the age of 50. Relative to the mRNA vaccines, significant risk of developing myocarditis has not been reported.50 Guillain-Barré syndrome (GBS) is a neurological disorder that has been reported as a post-vaccine complication of the Johnson & Johnson vaccine. According to the CDC Vaccine Adverse Events Reporting System, 7.8 GBS cases per million vaccine doses were found in males and females. Similar to the myocarditis risk seen in mRNA vaccines, the CDC determined the possible benefits of the vaccine in preventing hospitalization and mortality was more significant than the risk of developing TTS or GBS.51
Johnson & Johnson vaccine administration halt in the United States
On April 13, 2021, the administration of the Johnson & Johnson vaccine was halted due to the incidence of cerebral venous sinus thromboses (CVST). There were six reported cases, all women ages 18 to 48, and all had received the Johnson & Johnson vaccine 6 to 13 days before the onset of CVST symptoms. The FDA advised a halt in vaccine administration while also assuring that this was an extremely rare occurrence as over 7 million doses had already been administered in the United States. Johnson & Johnson vaccine administration was paused until April 23 after the FDA and CDC analyzed whether the vaccine did increase the risk for thromboses and other blood disorders and will continue to monitor adverse events. The FDA and CDC concluded that the vaccine is safe and effective, with the risk of CVST and thrombosis-thrombocytopenia syndrome being determined to be extremely low relative to the benefits of receiving the vaccine.52
Administration
The key difference between the Johnson & Johnson vaccine from others currently being used is that it is delivered as a single dose. Administration occurs through an intramuscular (IM) injection at a dose of 0.5 mL. Each vial contains approximately 5 doses.51
Vaccine vials can also be stored in refrigerators at higher temperatures, unlike the Pfizer and Moderna vaccines. Punctured multi-dose vials are stored at 36 to 46°F and unpunctured vials can be stored at 47 to 77°F for up to 12 hours. After the first puncture of the vial, it should be stored at 36 to 46°F for up to 6 hours or at room temperature, 77°F, for up to 2 hours. Vials should be thrown away if they are not used within this window.51 The vaccine ingredients are composed of citric acid monohydrate, trisodium citrate dihydrate, ethanol, 2-hydroxypropyl-β-cyclodextrin, polysorbate-80, and sodium chloride. No preservatives, eggs, or latex have been added to the formulation.51
Conclusion
Throughout this article, data including the efficacy, mechanism of action, administration, safety, and development of the three vaccines currently in use in the US were reviewed and summarized. Pfizer and Moderna shared many commonalities including efficacy, with both companies demonstrating the efficacy of approximately 95.0% in preventing infection during early clinical trials. Mechanisms of actions and administration were also a common point, with both companies delivering two separate doses of mRNA vaccines to develop an immune response. The Johnson and Johnson vaccine was shown to have significantly lower efficacy than both Pfizer and Moderna in preventing infection with approximately 66.0% efficacy. The Johnson and Johnson vaccine also differed from Pfizer and Moderna in that it did not utilize mRNA technology, rather involving the single-dose administration of a mutated adenovirus to replicate an immune response to the virus. The efficacy of all three vaccines against variants, especially the emerging variant must be a topic of further research, as it could elucidate a need for additional dose administration. All three vaccines have very high effectiveness in preventing hospitalization and death, which are the most important goals of vaccination. The swift response of pharmaceutical companies to the COVID-19 pandemic cannot be undermined and as vaccine distribution increases globally, the fight against a virus that has taken nearly millions of lives looks to finally be tilting in humanity’s favor.
Funding Statement
This is supported by no funding.
Authors’ contribution
R.P., M.K., V.P., P.K., D.K. assisted in drafting the manuscript, editing, interpretation of the referenced articles, and review. D.K. served as principal investigator, provided manuscript oversight, directed, and finalized the manuscript. All authors reviewed and approved this submission.
Availability of Data and Material (Data Transparency)
Not applicable since it is a review manuscript.
Code availability
Not applicable since it is a review manuscript.
Consent to participate
Not applicable since it is a review manuscript.
Consent for publication
All the authors have given permission for publication.
Ethics approval
Not applicable since it is a review manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.WHO . Global coronavirus statistics. United Nations. Published 2021. [Accessed 2021 Mar 30]. https://covid19.who.int/region/amro/country/us [Google Scholar]
- 2.Hapshy V, Aziz D, Kahar P, Khanna D, Johnson KE, Parmar MS.. COVID-19 and pregnancy: risk, symptoms, diagnosis, and treatment. SN Compr Clin Med. 2021;1–12. doi: 10.1007/s42399-021-00915-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suri JS, Agarwal S, Gupta SK, Puvvula A, Biswas M, Saba L, Bit A, Tandel GS, Agarwal M, Patrick A, et al. A narrative review on characterization of acute respiratory distress syndrome in COVID-19-infected lungs using artificial intelligence. Comput Biol Med. 2021;130:104210. doi: 10.1016/j.compbiomed.2021.104210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CDC . Symptoms of coronavirus. Centers for Disease Control. Published 2021. Updated 2021 [Accessed 2021 Mar 29]. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html [Google Scholar]
- 5.WHO . Timeline: WHO’s COVID-19 response. World Health Organization. Published 2021. [Accessed 2021 Mar 30]. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/interactive-timeline [Google Scholar]
- 6.Calina D . Towards effective COVID19 vaccines: updates, perspectives and challenges (Review). Int J Mol Med. 2020;46(1):3–16. doi: 10.3892/ijmm.2020.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.FDA . FDA approves first COVID-19 vaccine. FDA. Published 2021. Updated 2021 Aug 23. [Accessed 2021 Sept 20]. https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine [Google Scholar]
- 8.Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Raabe V, Bailey R, Swanson KA. Publisher correction: phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2021;590(7844):E26–E26. doi: 10.1038/s41586-020-03098-3. [DOI] [PubMed] [Google Scholar]
- 9.Biontech PA . Pfizer And Biontech Conclude Phase 3 Study Of Covid-19 Vaccine Candidate, Meeting All Primary Efficacy Endpoints; 2020. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccine
- 10.FDA . Pfizer-BioNTech COVID-19 vaccine EUA Amendment Review Memorandum. fda.gov. USA: Food and Drug Administration; 2021. April 9. [Google Scholar]
- 11.Moderna . Emergency use authorization (EUA): moderna COVID-19 Vaccine. Moderna. Published 2021. [Accessed 2021 Mar 16]. https://www.modernatx.com/covid19vaccine-eua/ [Google Scholar]
- 12.Jackson LA, Anderson EJ, Rouphael NG. An mRNA Vaccine against SARS-CoV-2 - preliminary report. New Eng J Med. 2020;383:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pardi NH, Hogan MJ, Naradikian MS. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J Exp Med. 2018;6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C. Safety and efficacy of the BNT162b2 mRNA Covid-19 Vaccine. New Eng J Med. 2020;383(27):2603–15. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biontech Pfizer 31 Mar. Pfizer-BioNTech Announce Positive Topline Results of Pivotal COVID-19 Vaccine Study in Adolescents; 2021. United States: Pfizer. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-biontech-announce-positive-topline-results-pivotal [Google Scholar]
- 16.Tian F. Mutation N501Y in RBD of Spike Protein Strengthens the Interaction between COVID-19 and its Receptor ACE2. Elife 2021;10. doi: 10.7554/eLife.69091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie X. Neutralization of N501Y mutant SARS-CoV-2 by BNT162b2 vaccine-elicited sera. Res Sq [Preprint]. 2021. doi: 10.21203/rs.3.rs-143532/v1. [DOI] [PubMed] [Google Scholar]
- 18.Haas E, Angulo F, McLaughlin J, Anis E, Singer S, Khan F, Brooks N, Smaja M, Mircus G, Pan K. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–29. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosen B, Waitzberg R, Israeli A. Israel’s rapid rollout of vaccinations for COVID-19. Isr J Health Policy Res. 2021;10(1):6. doi: 10.1186/s13584-021-00440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bar-On YM . Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N Engl J Med. 385(15): 1393–1400. doi: 10.1056/NEJMoa21142552021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mofaz M, Yechezkel M, and Guan G. [Preprint] 15 Sep 2021. . Self-reported and physiological reactions to the third BNT162b2 mRNA COVID-19 (booster) vaccine dose. medRxiv doi: 10.1101/2021.09.15.21263633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization . COVID-19 weekly epidemiological update. World Health Organization. Published 2021. Updated 2021 May 9. [Accessed 2021 May 14]. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—11-may-2021 [Google Scholar]
- 23.Tang P, Hasan MR, Chemaitelly H et al. 2021. . BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the Delta (B.1.617.2) variant in Qatar. Nat Med. 27(12): 2136–2143. doi: 10.1038/s41591-021-01583-4. [DOI] [PubMed] [Google Scholar]
- 24.Bernal JL, Andrews N, Gower C et al. 2021. . Effectiveness of COVID-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 385(7): 585–594. doi: 10.1038/s41591-021-01583-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tartof SY, Slezak JM, Fischer H, et al. [Preprint] . Six-Month effectiveness of BNT162B2 mRNA COVID-19 Vaccine in a large US integrated health system: a retrospective cohort study. SSRN; 2021. doi: 10.2139/ssrrn.3909743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garvey LH, Nasser S. Anaphylaxis to the first COVID-19 vaccine: is polyethylene glycol (PEG) the culprit? Br J Anaesth. 2021;126(3):e106–e108. doi: 10.1016/j.bja.2020.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.FDA . Pfizer-BioNTech COVID-19 vaccine. 2021.
- 28.Wan E, Chui C, Lai F, et al . Bell’s palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis. 2022;22(1): 64–7. doi: 10.1016/S1473-3099(21)00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moderna . Moderna’s work on our Covid-19 vaccine. Moderna. Published 2021. Updated 2021 Mar 5. [Accessed 2021 Mar 16. https://www.modernatx.com/modernas-work [Google Scholar]
- 30.Fessenden J. Inside the new mRNA vaccines for COVID-19. UMass Medical School. 2020 Dec 18. https://www.umassmed.edu/news/news-archives/2020/12/inside-the-new-mrna-vaccines-for-covid-19/ [Google Scholar]
- 31.Baden LS, Essink HM. B. efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. New Eng J Med. 2021;384(5):13. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu KK, Werner AP, Koch M, Choi A, Narayanan E, Stewart-Jones GBE, Colpitts T, Bennett H, Boyoglu-Barnum S, Shi W. M. serum neutralizing activity elicited by mRNA-1273 vaccine - preliminary report. New Eng J Med. 2021;384(15):1468–70. doi: 10.1056/NEJMc2102179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edara VV, et al. [Preprint]. Infection and vaccine-induced neutralizing antibody responses to the SARS-CoV-2 B.1.617.1 variant. bioRxiv. 2021. doi: 10.1101/2021.05.09.443299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puranik A, Lenehan PJ, Silvert E, et al. Preprint . Comparison of two highly-effective mRNA vaccines for COVID-19 during periods of Alpha and Delta variant prevalence. medRxiv. 2021 Oct 18. doi: 10.1101/2021.08.06.21261707. [DOI] [Google Scholar]
- 35.CDC . Moderna COVID-19 vaccine information. CDC. Published 2021. Updated February 22, 2021. [Accessed 2021 Mar 16]. https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/index.html [Google Scholar]
- 36.Johnson J. The Janssen COVID-19 Vaccine. Johnson & Johnson. Published 2021. [Accessed 2021 Mar 8]. https://www.janssencovid19vaccine.com/hcp.html [Google Scholar]
- 37.Vaccines J, Randomized A. Double-blind, Placebo-controlled Phase 3 Study to Assess the Efficacy and Safety of Ad26.COV2.S for the Prevention of SARS-CoV-2-mediated COVID-19 in Adults Aged 18 Years and Older. Johnson & Johnson. Published 2020. Updated 2020 Dec 14. [Accessed 2021 Mar 8]. https://www.jnj.com/coronavirus/ensemble-1-study-protocol [Google Scholar]
- 38.Levine H. The 5 Stages of COVID-19 Vaccine Development: what You Need to Know About How a Clinical Trial Works. Johnson & Johnson. Published 2020. Sept 23. [Accessed 2021 Mar 10]. https://www.jnj.com/innovation/the-5-stages-of-covid-19-vaccine-development-what-you-need-to-know-about-how-a-clinical-trial-works [Google Scholar]
- 39.Steinhauser G. South Africa Rolls Out J&J Covid-19 Vaccine to Healthcare Workers. The Wall Street Journal. Published 2021. [Accessed 2021 Mar 8]. https://www.wsj.com/articles/south-africa-rolls-out-j-j-covid-19-vaccine-to-healthcare-workers-11613564630 [Google Scholar]
- 40.Staff R. Bahrain first to approve Johnson & Johnson COVID-19 vaccine for emergency use - regulator. Reuters. Published 2021. [Accessed 2021 Mar 9]. https://www.reuters.com/article/health-coronavirus-bahrain-idUSS8N2I4021 [Google Scholar]
- 41.Pharmaceuticals J. Vaccine Technology. Janssen Global Services. Published 2021. [Accessed 2021 Mar 12]. https://www.janssen.com/infectious-diseases-and-vaccines/vaccine-technology [Google Scholar]
- 42.Sadoff J, Le Gars M, Shukarev G, Heerwegh D, Truyers C, de Groot AM, Stoop J, Tete S, Van Damme W, Leroux-Roels I, et al. Interim results of a phase 1–2a Trial of Ad26.COV2.S Covid-19 Vaccine. New Eng J Med. 2021;384(19):1824–35. doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Control CfD . Johnson & Johnson’s Janssen. Center for Disease Control. Published Updated 2021. Mar 4 [Accessed 2021 Mar 9]. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/janssen.html [Google Scholar]
- 44.Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, Goepfert PA, Truyers C, Fennema H, Spiessens B, et al. Safety and efficacy of single-dose Ad26.COV2.S Vaccine against Covid-19. New Eng J Med. 2021;384(23):2187–201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker M. J&J COVID-19 vax effective against severe disease - but overall efficacy falls well short of competitors. MedPage. Published 2021. [Accessed 2021 Mar 11]. https://www.medpagetoday.com/infectiousdisease/covid19/90942 [Google Scholar]
- 46.Schwartz F. J&J Vaccine Highly Effective Against Delta Variant in South African Trial. The Wall Street Journal. Published 2021. Updated 2021 Aug 6 [Accessed 2021 Sept 20]. https://www.wsj.com/articles/j-j-vaccine-highly-effective-against-delta-variant-in-south-african-trial-11628292645 [Google Scholar]
- 47.Jongeneelen M, Kaszas K, Veldman D, et al. [Preprint] . Ad26.COV2.S elicited neutralizing activity against Delta and other SARS-CoV-2 variants of concern. bioRxiv. 2021. 10.1101/2021.07.01.450707. [DOI] [Google Scholar]
- 48.Oliver SE, Gargano JW, Scobie H, et al . The advisory committee on immunization practices’ interim recommendation for use of Janssen COVID-19 Vaccine - United States, February 2021. MMWR Morb Mortal Wkly Rep. 2021;70(9):329–332. doi: 10.15585/mmwr.mm7009e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mahase E. Covid-19: Johnson and Johnson vaccine trial is paused because of unexplained illness in participant. BMJ. 2020;371:m3967. doi: 10.1136/bmj.m3967. [DOI] [PubMed] [Google Scholar]
- 50.Rosenblum H, Hadler S, Moulia DE, Shimabukuro TT, Su JR, Tepper NK, Ess KC, Woo EJ, Mba-Jonas A, Alimchandani M. Use of COVID-19 vaccines after reports of adverse events among adult recipients of Janssen (Johnson & Johnson) and mRNA COVID-19 vaccines (Pfizer-BioNTech and Moderna): update from the advisory committee on immunization practices — United States, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70(32):1094–99. doi: 10.15585/mmwr.mm7032e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fact sheet for healthcare providers administering vaccine [press release]. Johnson & Johnson. 2021. Feb 27. [Google Scholar]
- 52.FDA . FDA and CDC lift recommended pause on Johnson & Johnson (Janssen) COVID-19 vaccine use following thorough safety review. U.S. Department of Health and Human Services. Published 2021. Updated April 23, 2021 [Accessed 2021 May 10]. https://www.fda.gov/news-events/press-announcements/fda-and-cdc-lift-recommended-pause-johnson-johnson-janssen-covid-19-vaccine-use-following-thorough [Google Scholar]


