ABSTRACT
Introduction
From July through October of 2021, several countries issued recommendations for increased COVID-19 vaccine protection for individuals with one or more immunocompromised (IC) conditions. It is critically important to understand the vaccine effectiveness (VE) of COVID-19 vaccines among IC populations as recommendations are updated over time in response to the evolving COVID-19 pandemic.
Areas covered
A targeted literature review was conducted to identify real-world studies that assessed COVID-19 VE in IC populations between December 2020 and September 2021. A total of 10 studies from four countries were identified and summarized in this review.
Expert opinion
VE of the widely available COVID-19 vaccines, including BNT162b2 (Pfizer/BioNTech), mRNA-1273 (Moderna), Ad26.COV2.S (Janssen), and ChAdOx1 nCoV-19 (Oxford/AstraZeneca), ranged from 64% to 90% against SARS-CoV-2 infection, 73% to 84% against symptomatic illness, 70% to 100% against severe illness, and 63% to 100% against COVID-19-related hospitalization among the fully vaccinated IC populations included in the studies. COVID-19 VE for most outcomes in the IC populations included in these studies were lower than in the general populations. These findings provide preliminary evidence that the IC population requires greater protective measures to prevent COVID-19 infection and associated illness, hence should be prioritized while implementing recommendations of additional COVID-19 vaccine doses.
KEYWORDS: COVID-19-related hospitalization, COVID-19 vaccines, immunocompromised, SARS-CoV-2 infection, symptomatic COVID-19 illness, vaccine effectiveness
1. Introduction
As of 30 September 2021 approximately 45% of the worldwide population had received at least one dose of a coronavirus disease 2019 (COVID-19) vaccine [1]. Scientific evidence gained from real-world studies conducted in multiple countries is increasingly showing that widely available COVID-19 vaccines, including BNT162b2 (Pfizer/BioNTech), mRNA-1273 (Moderna), Ad26.COV2.S (Janssen), and ChAdOx1 nCoV-19 (Oxford/AstraZeneca), are effective against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, symptomatic COVID-19 illness, and COVID-19-related hospitalization and death [2,3]. Such findings from real-world studies are generally consistent with the efficacy results of the randomized clinical trials (RCTs) of these vaccines [4–7]. Vaccine efficacy in clinical trials and vaccine effectiveness (VE) measured in real-world studies both calculate the risk of disease among vaccinated and unvaccinated individuals and the percentage reduction in risk of disease among vaccinated individuals relative to unvaccinated individuals; VE equates to the reduction in disease occurrence for those who are vaccinated (i.e. a VE of 85% = an 85% reduction in disease occurrence among the vaccinated) [8].
From July through October of 2021, several countries across the world issued recommendations for increased COVID-19 vaccine protection for individuals with one or more immunocompromised (IC) conditions; many of these recommendations also included other subpopulations (e.g. elderly) [9]. IC individuals are generally defined as those with suppressed immunity resulting from health conditions, including active cancers, organ/stem cell transplants, primary immunodeficiencies, advanced/untreated human immunodeficiency virus [HIV] infections, and/or active usage of immunosuppressive medications [10]. These recommendations were informed by real-world studies of IC populations, who were largely excluded from the RCTs of the COVID-19 vaccines [4–7], that observed a reduced immune response to COVID-19 vaccines in IC individuals compared to the general population [11–16]. Whether a reduced immune response to COVID-19 vaccines correlates with diminished VE is not well understood. However, results from several recent real-world studies conducted in the United States (US), Israel, England, and Qatar that assessed VE in IC populations [17–27] indicate that IC individuals are at increased risk for severe COVID-19 outcomes. These studies found that although the COVID-19 vaccines provided a high level of protection against SARS-CoV-2 infection, symptomatic COVID-19 illness, and/or COVID-19-related hospitalization, VE for the IC populations tended to be lower than that observed in the general population [17–27].
Multiple case reports suggest that IC individuals may serve as a reservoir for the development of novel escape variants given their risk for prolonged viral replication enabled by altered immune function [28–31]. Variants of concern (VOC) and variants of interest (VOI) have similarities with those variants identified in IC individuals including a relatively large number of mutations, particularly in the receptor-binding domain of the spike protein, as well as convergent mutations [32]. Garnering a clearer understanding of VE in this population is therefore imperative to inform immunization recommendations to protect this vulnerable population and mitigate the evolution of VOCs and VOIs. Furthermore, as new COVID-19 vaccine recommendations are implemented and updated over time in response to the evolving COVID-19 pandemic, it is necessary to rapidly and more comprehensively understand the effectiveness of COVID-19 vaccines in IC populations. From a policy perspective, such information could provide decision makers with the data to help to fill vaccine coverage gaps and instill greater protective measures toward the IC population, measures such as additional dose/booster prioritization. This objective has become even more critical given the continuing risk of emergence of more transmissible variants (i.e. Omicron). Toward, this objective, in this review, we have summarized the findings of real-world studies that have assessed COVID-19 VE in IC populations.
2. Methods
2.1. Research question and study inclusion criteria
The research question and study eligibility criteria were developed based on the Population, Intervention, Comparator, Outcomes (PICO) framework [33]. The research question was, what is the reported COVID-19 VE in IC populations? IC populations were defined according to the definitions used in the individual studies. The interventions assessed were any of the widely available COVID-19 vaccines in the world versus no COVID-19 vaccination (control/comparator). The outcomes explored included COVID-19 VE against SARS-CoV-2 infection, symptomatic COVID-19 illness, severe COVID-19 illness, and COVID-19-related hospitalization/death. We targeted real-world observational studies, either cross-sectional or longitudinal in design, conducted in any country that assessed these outcomes and reported calculated VE estimates. Studies that evaluated vaccine efficacy in the context of a clinical trial or immunogenicity were not included in this review.
2.2. Search strategy and screening
The best practice in systematic literature reviews is to prioritize searches and to include studies that are peer reviewed and published [34]. Given that the interventions (i.e., COVID-19 vaccines), in the scope of this review were recently introduced, and that there has been a high influx of COVID-19 research being posted on pre-print servers, we included both peer-reviewed and non-peer-reviewed preprint studies. While this approach strengthens the comprehensiveness of this review, the author team recognizes the potential limitations in the reproducibility of the review and the quality of the collected evidence base.
Based on the above, a targeted search was performed using PubMed and the preprint servers, medRxiv and Khub, to identify real-world studies that assessed COVID-19 VE in IC populations between December 2020 and 30 September 2021 (inclusive). The following list of terms was generated and searched across all study fields: ‘COVID-19,’ ‘SARS-CoV-2,’ ‘vaccine effectiveness,’ and ‘immunocompromised.’ To maximize the scope of the search, no search terms were included for interventions or outcomes. All studies found written in the English language, without restrictions of countries, but with reported COVID-19 VE against SARS-CoV-2 infection, symptomatic COVID-19 illness, severe COVID-19 illness, and/or COVID-19-related hospitalization/death were examined for inclusion. Titles, abstracts, and full study contents publicly available were screened by one independent reviewer (MLS). Since there was only one reviewer, random selection, and inter-rater reliability scores (e.g. kappa) were not determined.
2.3. Data extraction
Study characteristics (i.e. countries, vaccines included in analyses, study periods, study designs, and data sources), general characteristics of the overall study population (i.e. sample size, follow-up duration, proportion of fully vaccinated, median age, and sex distribution), IC definitions, and IC population characteristics, and details of the study outcome measures related to VE (i.e. controls, VE follow-up durations, VE calculations, and analysis methods) were extracted and incorporated into an Excel spreadsheet. When possible, COVID-19 VE in the general and non-IC population was also extracted. Since all data presented in this review were extracted from already published and/or publicly available preprint studies, this review is not subject to ethical approval.
2.4. Narrative synthesis
Given the diversity of the studies included, the quality of the selected studies was not compared, and meta-analyses were not performed. The assembled body of evidence was drawn together and interpreted in a narrative synthesis. After tabulating the individual studies, we assessed if the observed outcomes of interest were consistent across studies, which were interpreted in the context of their similarities (e.g. definition of symptomatic COVID-19 illness) and differences (e.g. VE follow-up durations). We qualitatively grouped the studies by outcome measures and investigated any reasons for inconsistencies among the results. This approach is supported by guidance for undertaking reviews [35].
3. Results
3.1. Search results
With an end search date of 30 September 2021 a total of 10 studies were identified in which COVID-19 VE was assessed in IC populations [17–27]; six, with one study accounting for two publications, were peer-reviewed [17–20,22–24] and four were preprints [21,25–27] at the time this review was written. The study outcomes included COVID-19 VE against SARS-CoV-2 infection, symptomatic COVID-19 illness, severe COVID-19 illness, and COVID-19-related hospitalization, which were summarized for the study IC populations, as well as the general populations, when such data were available. Although one study assessed COVID-19 VE against all-cause death [20], VE specifically against COVID-19-related death was not reported in the included studies for IC populations and therefore was not summarized in this review.
3.2. Key study characteristics
Tables 1, 2, and 3 show study characteristics, general characteristics of the overall study populations, and a comparison of IC definitions and IC populations across studies, respectively.
Table 1.
Study characteristics
| Study/Peer-reviewed | Country | Vaccines included in analyses | Study period | Study design | Data source |
|---|---|---|---|---|---|
| Young-Xu Y, et al./Yes [17] | United States |
|
Pre-Delta: Dec 14 2020– Mar 14, 2021 |
|
EMR data from VHA Corporate Data Warehouse |
| Tenforde MW, et al./Yes [18] | United States |
|
Intermediate: Mar 11 – May 5, 2021 |
Case-control analysis | Hospital admission logs and EMRs (18 academic hospitals/16 states) |
| Tenforde MW, et al./Yes [19] | United States |
|
Including Delta: Mar 11 – Jul 14, 2021 |
Case-control analysis | Hospital admission logs and EMRs (21 academic hospitals/18 states) |
| Khan N & Mahmud N/Yes [20] | United States |
|
Pre-Delta: Dec 18 2020 – Apr 20, 2021 |
Retrospective cohort analysis | VHA data sources, including VHA Corporate Data Warehouse |
| Polinski JM, et al./No, medRxiv preprint [21] | United States |
|
Including Delta: Mar 1 – Jul 31, 2021 |
Matched control analysis | Administrative insurance claims in Health Verity database |
| Dagan N, et al.; Barda N, et al./Yes [22,23] | Israel |
|
Pre-Delta: Dec 20 2020 – Feb 1, 2021 |
Matched control analysis | EMR data from Clalit Health Services data repositories |
| Chodick G, et al./Yes [24] | Israel |
|
Pre-Delta: Dec 19 2020 – Feb 20, 2021 |
Retrospective cohort analysis | EMR data from Maccabi Healthcare Services databases |
| Yelin I, et al./No, medRxiv preprint [25] | Israel |
|
Pre-Delta: Dec 1 2020– Feb 25, 2021 |
Prospective Patient-level analysis | EMR data from Maccabi Healthcare Services databases |
| Whitaker HJ, et al./No, Khub preprint [26] | England |
|
Including Delta: Dec 7 2020– Jun 13, 2021 |
Nested test-negative case-control analysis | EMR data of 718 general practices |
| Chemaitelly H, et al./No, medRxiv preprint [27] | Qatar |
|
Including Delta a: Feb 1 2021– Jul 21, 2021 |
Retrospective cohort analysis with cross-over design | Hamad Medical Corporation: integrated nationwide digital-health information platform |
EMR: Electronic medical record; mRNA: messenger ribonucleic acid; VHA: Veterans Health Administration.
Covariant predominance period was defined as Pre-Delta, Intermediate, and Including Delta (when the study period extended to months when the Delta variant was predominant) for countries referring to [36; https://covariants.org/per-country] as below.
US: Dec 2020-April 2021 as Pre-delta; May 2021-June 2021: Intermediate; July 2021-Sept 2021: Delta.
Israel: Dec 2020 – Feb 2021 was Alpha, which was defined as Pre-delta.
England: Dec 2020 – April 2021 was Alpha (Pre-delta), May 2021: Intermediate, June 2021 was during Delta variant, hence defined as including Delta.
Qatar: Feb–Apr 2021 was Beta (Pre-delta), May 2021: Intermediate, and June–July 2021 was Delta, hence as defined as including Delta.
aIn Qatar, the Delta variant was preceded by the Beta variant, as opposed to the Alpha variant in the US, Israel, and England. Authors reported that as of 28 July 2021 Delta was at low incidence in Qatar.
Table 2.
General characteristics of the overall study populations
| Study | Count | Follow-up duration | Proportion fully vaccinated | Age | Sex distribution |
|---|---|---|---|---|---|
| Young-Xu Y, et al. [17] | Overall: N = 6,647,733 in VHA cohort
|
≥14 days after 2nd dose up to 3 months |
|
Median age not reported.
|
|
| Tenforde MW, et al. [18] | Overall: N = 1,212 in general hospitalized population
|
Median: 43 days; maximum: 113 days |
|
|
|
| Tenforde MW, et al. [19] | Overall: N = 3,089 in general hospitalized population
|
Up to 24 weeks |
|
|
|
| Khan N & Mahmud N. [20] | Overall: N = 14,697 in VHA cohort with IBD
|
|
91% of Pfizer (N = 3,017) and 89% of Moderna (N = 3,561) patients received both vaccine doses during full study period |
|
|
| Polinski JM, et al. [21] | General population cohort
|
≥14 days after single dose and up to 5 months | 100% of vaccinated cohort |
|
|
| Dagan N, et al.; Barda N, et al. [22,23] | General population cohort
|
|
Among those with ≥21 days of follow-up, 96% received a 2nd dose |
|
|
| Chodick G, et al. [24] | Overall: N = 1,178,597 in general population
|
|
N = 872,454 (74%) |
|
|
| Yelin I, et al. [25] | Overall in general population: 1.8 million/ 1.3 million were vaccinated | 67 days, >93 million observations after exclusion | >98% of patients administered with the 1st dose administered with 2nd dose; 1,721,377 vaccinated | Not included in preprint | Not included in preprint |
| Whitaker HJ, et al. [26] | Overall: N = 5,642,687 in general population Clinical risk groups: N = 1,054,510 |
≥14 days after 2nd dose up to 6.5 months | Data not available; to be published in Supplementary material S2. | Data not available; to be published in Supplementary material S2. | Data not available; to be published in Supplementary material S2. |
| Chemaitelly H, et al. [27] | N = 782 kidney transplant recipients
|
≥14 days after 2nd dose up to ~6 months | N = 601 (77% of 782 with ≥1st dose) |
|
|
COVID-19: Coronavirus disease 2019; IBD: Inflammatory bowel disease; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; Yrs: Years; VHA: Veterans Health Administration.
Table 3.
Comparison of IC definitions and populations across studies
| Study | IC definition | Study population with IC condition (% of overall population or case/control cohort) |
|---|---|---|
| Young-Xu Y, et al. [17] | HIV, asymptomatic HIV, pneumocystosis, retrovirus disease, post-transplant lymphoproliferative disorder, neutropenia, functional disorders of polymorphonuclear neutrophils, white blood cell disorders, spleen diseases, other diseases with lymphoreticular and reticulohistiocytic tissue, immunodeficiencies, other disorders involving immune mechanisms, rheumatoid arthritis, enteropathic arthropathies, juvenile arthritis, polyarteritis nodosa and related conditions, other necrotizing vasculopathies, systemic lupus erythematosus, dermatopolymyositis, systemic sclerosis, Sjögren syndrome, systemic connective tissue disorders, absence/malformation of spleen, antineoplastic and immunosuppressive drug unintentional poisoning, organ transplant, antineoplastic radiation therapy, antineoplastic chemotherapy and immunotherapy | N = 16,315 (22%)
|
| Tenforde MW, et al. [18] | Active solid organ cancer with or without metastases (active cancer defined as treatment for the cancer or newly diagnosed cancer in the past 6 months), active hematologic cancer (such as leukemia/lymphoma/myeloma) or active cancer defined as treatment for the cancer or newly diagnosed cancer in the past 6 months, HIV infection without AIDS, AIDS, congenital immunodeficiency syndrome, prior splenectomy, prior solid organ transplant, immunosuppressive medication usage, systemic lupus erythematosus, rheumatoid arthritis, psoriasis, scleroderma, IBD including Crohn’s disease or ulcerative colitis | N = 254 (21%)
|
| Tenforde MW, et al. [19] | Active solid organ cancer (active cancer defined as treatment for the cancer or newly diagnosed cancer in the past 6 months), active hematologic cancer (such as leukemia, lymphoma, or myeloma), HIV infection without AIDS, AIDS, congenital immunodeficiency syndrome, previous splenectomy, previous solid organ transplant, immunosuppressive medication usage, systemic lupus erythematosus, rheumatoid arthritis, psoriasis, scleroderma, or inflammatory bowel disease, including Crohn’s disease or ulcerative colitis | N = 652 (21%)
|
| Khan N & Mahmud N. [20] | IBD diagnosis w/ IBD medication exposure (mesalamine, thiopurines, anti-tumor necrosis factor biologic agents, vedolizumab, ustekinumab, tofacitinib, methotrexate, and corticosteroid use) | N = 14,697 (100%) |
| Polinski JM, et al. [21] | Any diagnosis for active cancer, history of organ/stem cell transplant, primary immunodeficiency (e.g. DiGeorge syndrome, Wiskott-Aldrich syndrome), or HIV infection, AND/OR recent use (within 60 days of index) of immunosuppressive medications including high-dose corticosteroids (i.e. ≥20 mg prednisone or equivalent per day), transplant-related immunosuppressives, antimetabolites, alkylating agents, and other severely immunosuppressive cancer chemotherapeutics, tumor necrosis factor blockers, and other immunosuppressive biologics. IC subgroup definitions were based on CDC vaccine guidance for moderately to severely immunocompromised status [10]. | N = 131,820 (7%)
|
| Dagan N, et al.; Barda N, et al. [22,23] | HIV, asymptomatic HIV, organ transplant, bone marrow or hematopoietic stem cell transplant, immunosuppressive medication usage | N = 1,674 (0.5% of full study population with 7–28 days after 2nd dose: N = 310,696) |
| Chodick G, et al. [24] | Hematopoietic cell or solid organs transplant, immunosuppressive medication usage, asplenia, and chronic renal failure (advanced kidney disease, dialysis, or nephrotic syndrome) | N = 27,822 (2%) |
| Yelin I, et al. [25] | Not included in preprint | Not included in preprint |
| Whitaker HJ, et al. [26] | Data not available; to be published in Supplementary material S2. | Data not available; to be published in Supplementary material S2. |
| Chemaitelly H, et al. [27] | Kidney transplant recipients with maintenance immunosuppressive medication usage | N = 782 (100%) |
Bolded text shows the only 2 common conditions across six of the studies included in this review.
AIDS: Acquired immunodeficiency syndrome; CDC: Centers for Disease Control and Prevention; HIV: Human immunodeficiency virus; IBD: Inflammatory bowel disease; IC: Immunocompromised.
3.2.1. Study geographical location and design
The 10 studies summarized in this review were conducted in four countries, the US, Israel, England, and Qatar. Four of the five studies conducted in the US assessed VE of the mRNA vaccines, BNT162b2 and mRNA-1273, using electronic medical records (EMRs) and/or hospital admission logs. Young-Xu et al. [17] conducted a matched analysis of COVID-19 cases and controls among US veterans with an IC subgroup; the CDC COVID-19 Response Team conducted two unmatched hospitalized COVID-19 case–control analyses among the general hospitalized population with IC subgroups [18,19], and Khan & Mahmud [20] conducted a retrospective cohort analysis of US veterans with inflammatory bowel disease (IBD). The fifth US study, by Polinski et al. [21], performed a matched control analysis of Ad26.COV2.S VE among the general population and an IC subgroup using the administrative insurance claims database of Health Verity.
Three studies, all of which assessed VE of the BNT162b2 mRNA vaccine in the general population and IC subgroups, were conducted in Israel [22–25], including the largest matched population analysis of vaccinated and unvaccinated persons (N = 596,618 matched pairs) conducted to date by Dagan et al. [22], with follow-up subgroup analyses, which included an IC population, provided in Barda et al. [23]; the data source of this study was EMRs in Clalit Health Services data repositories. Chodick et al. [24] conducted a retrospective cohort analysis and Yelin et al. [25] conducted a prospective patient-level analysis; both studies extracted data from EMRs in Maccabi Healthcare Services databases.
The nested test-negative case–control study conducted in England by Whitaker et al. [26] was the only study that evaluated ChAdOx1 nCoV-19 VE in the general population with an IC subgroup. This study also assessed VE of the BNT162b2 mRNA vaccine; it utilized EMR data from 718 general practices [26]. Chemaitelly et al. [27] conducted a retrospective cohort analysis with a cross-over design of mRNA (BNT162b2 and mRNA-1273 vaccine) VE in a population of kidney transplant recipients in Qatar; data were extracted from an integrated nationwide digital-health information platform from the Hamad Medical Corporation.
The study periods (i.e. follow-up) were all shorter than 6.5 months. Five of the included studies in this review assessed COVID-19 VE primarily from December 2020 through February/March 2021 [17,20,22–25]; the three studies conducted in Israel fall within this group. Tenforde et al. [19], Polinski et al. [21], Whitaker et al. [26], and Chemaitelly et al. [27] had study periods that extended into the summer months (up until July) of 2021. Only Tenforde et al. [19] and Polinski et al. [21] separated their VE analyses by time periods to better understand if VE was affected by the emergence of the Delta variant in the US.
3.2.2. Characteristics of the study IC populations
The health conditions used to define IC populations varied across the studies. In two studies, Yelin et al. [25] and Whitaker et al. [26], the definitions of IC were not available in the preprint materials. Khan & Mahmud [20] and Chemaitelly et al. [27] assessed COVID-19 VE in specific IC populations, IBD patients among US veterans and kidney transplant recipients, respectively; in both studies, patients also had maintenance immunosuppressive medication usage. In the other six studies [17–19,21–24], IC populations were defined according to various IC conditions; only two IC conditions, organ transplant and immunosuppressive medication usage, were common across the six studies. Other IC conditions common across multiple studies included HIV infection in five studies, active cancer in four, immunodeficiencies in four, rheumatoid arthritis/other related inflammatory conditions in three; chronic kidney disease (CKD) was included in only one study. In these six studies, some patient groups with other IC diseases that were not specifically defined may have been captured among those grouped with immunosuppressive medication usage.
The sample sizes of the IC populations were reported in eight studies and are summarized in Table 3. Sample sizes included 16,315 (22% of overall study population) in Young-Xu et al. [17], 254 (21% of overall study population) in Tenforde et al. [18], 652 (21% of overall study population) in Tenforde et al. [19], 14,697 (100% IBD population) in Khan & Mahmud [20], 131,820 (7% of overall study population) in Polinski et al. [21], 1,674 (0.5% of overall study population) in Dagan et al.; Barda et al. [22,23], 27,822 (2% of overall study population) in Chodick et al. [24], and 782 (100% kidney transplant recipients) in Chemaitelly et al. [27]. No information on sample size was available for Yelin et al. [25] and Whitaker et al. [26] at the time of writing this review.
Only three studies provided characteristics of the IC populations in which COVID-19 VE was assessed [20,21,27]. Khan & Mahmud [20] conducted their study specifically among Veterans Health Administration (VHA) patients with IBD who took immunosuppressive medications; median age was 68 years, 92% were male, 80% were White, approximately 44% were from the South US region, and 62% had ulcerative colitis. The frequency of breakthrough infections was 0.11% (N = 7) in those who were fully vaccinated compared to 1.34% (N = 197) among those who were not vaccinated [20]. Polinski et al. [21] defined their IC population according to the guidance of the US Centers for Disease Control and Prevention (CDC) for moderate-to-severe IC status [10]. In this study, the IC represented 6.8% (N = 26,720) of the overall vaccinated population and 6.9% (N = 105,100) of the overall unvaccinated population; mean age of matched vaccinated and unvaccinated IC groups was 59 years, 60% were female, ethnicity/race was not reported, and approximately 41% resided in the South US region [21]. Chemaitelly et al. [27] conducted their study specifically in kidney transplant recipients who were on maintenance immunosuppressive medication; the study population (N = 782) was in Qatar; median age of the vaccinated cohort was 52 years and 70% were male; the median age of the unvaccinated cohort was 49 years and 63% were male [27]. The incidence of breakthrough infections was 2.58% in those who were vaccinated compared to 4.74% among those who were unvaccinated (follow-up: 120 days after 14 days after 2nd dose) [27].
3.3. COVID-19 VE
Table 4 reports the details of the study outcome measures related to COVID-19 VE (i.e. outcome measures, controls, VE follow-up duration, VE calculations, and analysis methods), while Table 5 presents the reported VE estimates, including 95% confidence intervals (95% CI), against SARS-CoV-2 infection, symptomatic COVID-19 illness, severe COVID-19 illness, and COVID-19-related hospitalization across the studies included in this review. Figure 1 graphically presents COVID-19 VE in IC populations relative to overall study populations from those studies with such available data.
Table 4.
Details of the study outcome measures related to COVID-19 VE
| Study | Outcome measures | Controls | VE follow-up duration | VE calculation | Analysis method |
|---|---|---|---|---|---|
| Young-Xu Y, et al. [17] |
|
|
≥14 days after 2nd dose up to 3 months | (1− OR) X 100 OR: SARS-CoV-2 infection in vaccinated vs. unvaccinated |
Logistic regression |
| Tenforde MW, et al. [18] |
|
|
≥14 days before reference date (date of hospitalization for cases) | (1‒OR) X 100 OR: Prior vaccination in cases vs. controls |
Logistic regression |
| Tenforde MW, et al. [19] |
|
|
|
(1‒OR) X 100 OR: Prior vaccination in cases vs. controls |
Logistic regression |
| Khan N & Mahmud N. [20] |
|
Unvaccinated controls |
|
(1 – incidence vaccinated/incidence unvaccinated) X 100 |
|
| Polinski JM, et al. [21] |
|
Unvaccinated controls |
|
(1-HR) X 100 HR: COVID-19 infection/hospitalization in vaccinated vs. unvaccinated |
|
| Dagan N, et al.; Barda N, et al. [22,23] |
|
Matched unvaccinated controls | 7 days after 2nd dose up to 28 days: mean follow-up: 18 days in subgroup analyses | 1 – RR RR: vaccinated vs. unvaccinated |
|
| Chodick G, et al. [24] |
|
Between 7 to 27 days after 2nd dose (protection-period) vs. days 1 to 7 after the 1st dose (reference period) | Days 7–27 after 2nd dose vs. days 1–7 after 1st dose | (1 – Relative Risk) X 100 Relative Risk: Protection-period vs. reference-period |
|
| Yelin I, et al. [25] |
|
Unvaccinated controls |
All post-vaccination |
(1-OR) X 100 OR: Positive test/symptomatic infection in vaccinated vs. unvaccinated |
Logistic regression |
| Whitaker HJ, et al. [26] |
|
Test-negative controls: Patients with symptoms within 10 days of SARS-CoV-2 negative test | ≥14 days after 2nd dose up to ~6.5 months | Adjusted VE OR OR: Cases in vaccinated vs. unvaccinated |
Logistic regression |
| Chemaitelly H, et al. [27] |
|
Unvaccinated controls |
|
1 – HR HR: vaccinated vs. unvaccinated |
A proportional hazards model was used to calculate adjusted hazard ratios |
COVID-19: Coronavirus disease 2019; EMR: Electronic medical records; HR: Hazard ratio; IC: Immunocompromised; NIH: National Institutes of Health; OR: Odds ratio; RR: Risk ratio; RT-PCR: Reverse transcription polymerase chain reaction; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; VE: Vaccine effectiveness; VHA: Veterans Health Administration; WHO: World Health Organization.
Table 5.
Vaccine effectiveness against SARS-CoV-2 infection, symptomatic COVID-19 illness, severe COVID-19 illness, and COVID-19-related hospitalization across studies
| Study | Vaccine | Outcome | Patient group | VE | 95% CI Lower | 95% CI Upper |
|---|---|---|---|---|---|---|
| Young-Xu Y, et al. [17] | mRNA vaccines | Infection | IC | 87% | 79% | 92% |
| Young-Xu Y, et al. [17] | mRNA vaccines | Infection | Overall population | 95% | 93% | 96% |
| Khan N & Mahmud N. [20] | mRNA vaccines | Infection | IC: IBD | 80% | NR | NR |
| Polinski JM, et al. [21] | Ad26.COV2.S | Infection | IC | 64% | 57% | 70% |
| Polinski JM, et al. [21] | Ad26.COV2.S | Infection | Overall population | 79% | 77% | 80% |
| Polinski JM, et al. [21] | Ad26.COV2.S | Infection | Non-IC | 79% | 78% | 81% |
| Dagan N, et al.;Barda N, et al. [22,23] | BNT162b2 | Infection | IC | 90% | 49% | 100% |
| Dagan N, et al.;Barda N, et al. [22,23] | BNT162b2 | Infection | Overall population | 93% | 91% | 94% |
| Chodick G, et al. [24] | BNT162b2 | Infection | IC | 71% | 37% | 87% |
| Chodick G, et al. [24] | BNT162b2 | Infection | IC ≥65 yrs | 52% | 26% | 82% |
| Chodick G, et al. [24] | BNT162b2 | Infection | Overall population | 90% | 79% | 95% |
| Yelin I, et al. [25] | BNT162b2 | Infection | IC | OR:0.67 | 0.53 | 0.83 |
| Yelin I, et al. [25] | BNT162b2 | Infection | Overall population | 95% | 93% | 96% |
| Chemaitelly H, et al. [27] | mRNA vaccines | Infection | Kidney transplant recipients ≥14 days after 2nd dose | 47% | 0% | 74% |
| Chemaitelly H, et al. [27] | mRNA vaccines | Infection | Kidney transplant recipients ≥42 days after 2nd dose | 66% | 21% | 85% |
| Chemaitelly H, et al. [27] | mRNA vaccines | Infection | Kidney transplant recipients ≥56 days after 2nd dose | 74% | 33% | 90% |
| Dagan N, et al.;Barda N, et al. [22,23] | BNT162b2 | Symptomatic illness | IC | 84% | 19% | 100% |
| Dagan N, et al.;Barda N, et al. [22,23] | BNT162b2 | Symptomatic illness | Overall population | 96% | 94% | 97% |
| Chodick G, et al. [24] | BNT162b2 | Symptomatic illness | IC | 75% | 44% | 88% |
| Chodick G, et al. [24] | BNT162b2 | Symptomatic illness | Overall population | 94% | 88% | 97% |
| Whitaker HJ, et al. [26] | BNT162b2 | Symptomatic illness | IC; age range not provided | 73% | 34% | 89% |
| Whitaker HJ, et al. [26] | ChAdOx1 nCoV-19 | Symptomatic illness | IC; age range not provided | 75% | 19% | 92% |
| Whitaker HJ, et al. [26] | BNT162b2 | Symptomatic illness | Overall population 16–64 yrs |
93% | 86% | 97% |
| Whitaker HJ, et al. [26] | ChAdOx1 nCoV-19 | Symptomatic illness | Overall population 16–64 yrs |
78% | 70% | 84% |
| Whitaker HJ, et al. [26] | BNT162b2 | Symptomatic illness | Overall population ≥65 yrs |
87% | 80% | 91% |
| Whitaker HJ, et al. [26] | ChAdOx1 nCoV-19 | Symptomatic illness | Overall population ≥65 yrs |
76% | 59% | 86.5% |
| Khan N & Mahmud N. [20] | mRNA vaccines | Severe illnessa | IC: IBD | 70% | NR | NR |
| Dagan N, et al.;Barda N, et al. [22,23] | BNT162b2 | Severe illnessb | IC | 100% | 1 vs. 0c | |
| Dagan N, et al.;Barda N, et al. [22,23] | BNT162b2 | Severe illnessb | Overall population | 95% | 89% | 99% |
| Chemaitelly H, et al. [27] | mRNA vaccines | Severe illnessd | Kidney transplant recipients ≥14 days after 2nd dose | 72% | 0% | 91% |
| Chemaitelly H, et al. [27] | mRNA vaccines | Severe illnessd | Kidney transplant recipients ≥42 days after 2nd dose | 85% | 36% | 96.5% |
| Chemaitelly H, et al. [27] | mRNA vaccines | Severe illnessd | Kidney transplant recipients ≥56 days after 2nd dose | 84% | 31% | 96% |
| Tenforde MW, et al. [18] | mRNA vaccines | Hospitalization | IC | 63% | 21% | 83% |
| Tenforde MW, et al. [18] | mRNA vaccines | Hospitalization | IC subgroup: solid organ or hematologic malignancy or solid organ transplant | 51% | −31% | 82% |
| Tenforde MW, et al. [18] | mRNA vaccines | Hospitalization | Overall population | 87% | 81% | 91% |
| Tenforde MW, et al. [18] | mRNA vaccines | Hospitalization | Non-IC | 91% | 86% | 95% |
| Tenforde MW, et al. [19] | mRNA vaccines | Hospitalization | IC | 63% | 44% | 76% |
| Tenforde MW, et al. [19] | mRNA vaccines | Hospitalization | Overall population | 86% | 82% | 88% |
| Tenforde MW, et al. [19] | mRNA vaccines | Hospitalization | Non-IC | 90% | 87% | 92% |
| Polinski JM, et al. [21] | Ad26.COV2.S | Hospitalization | IC | 68% | 54% | 77% |
| Polinski JM, et al. [21] | Ad26.COV2.S | Hospitalization | Overall population | 81% | 79% | 84% |
| Polinski JM, et al. [21] | Ad26.COV2.S | Hospitalization | Non-IC | 83% | 80% | 85% |
| Dagan N, et al.;Barda N, et al. [22,23] | BNT162b2 | Hospitalization | IC | 100% | 2 vs. 0c | |
| Dagan N, et al.;Barda N, et al. [22,23] | BNT162b2 | Hospitalization | Overall population | 92% | 85% | 97% |
aHospitalization or death [20].
bAccording to National Institutes of Health criteria [37]: Individuals who have SpO2 < 94% on room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) <300 mmHg, respiratory frequency >30 breaths/min, or lung infiltrates >50%; critical illness: Individuals who have respiratory failure, septic shock, and/or multiple organ dysfunction.
cNumber of events in unvaccinated vs. vaccinated.
dSevere disease (acute-care hospitalization) and critical disease (intensive care unit hospitalization) were defined per World Health Organization guidelines [38]: Oxygen saturation of <90% on room air, and/or respiratory rate of >30 breaths/minute in adults and children >5 years old (or ≥60 breaths/minute in children <2 months old or ≥50 breaths/minute in children 2–11 months old or ≥40 breaths/minute in children 1–5 years old), and/or signs of severe respiratory distress (accessory muscle use and inability to complete full sentences, and, in children, very severe chest wall indrawing, grunting, central cyanosis, or presence of any other general danger signs).
aHR: Adjusted hazard ratio; CI: Confidence interval; COVID-19: Coronavirus disease 2019; IBD: Inflammatory bowel disease; IC: Immunocompromised; mRNA: messenger ribonucleic acid; OR: Odds ratio; NR: Not reported; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; yrs: Years; VE: Vaccine effectiveness
Figure 1.
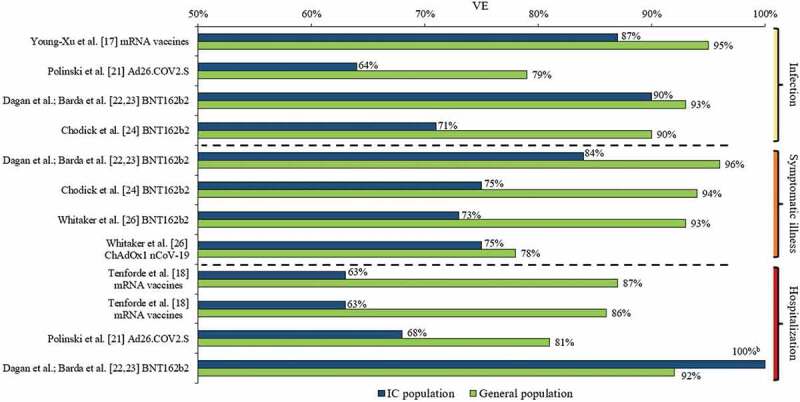
VE against SARS-CoV-2 infection, symptomatic COVID-19 illness, and COVID-19-related hospitalization in IC populations versus general populationsa.
a See Table 5 for VE including 95% CIs.
b Dagan et al.; Barda et al. [22,23] reported a BNT162b2 VE against COVID-19-related hospitalization of 100% in their IC population; however, only two such events occurred in the unvaccinated IC group and none in the vaccinated group.
CI: Confidence interval; COVID-19: Coronavirus disease 2019; IC: Immunocompromised; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; VE: Vaccine effectiveness
3.3.1. COVID-19 VE against SARS-CoV-2 infection
Seven studies (Young-Xu et al. [17]; Khan & Mahmud [20]; Polinski et al. [21]; Dagan et al.; Barda et al. [22,23]; Chodick et al. [24]; Yelin, et al. [25]; and Chemaitelly et al. [27]) assessed VE against SARS-CoV-2 infection in IC populations; the time periods of measured VE varied in these seven studies (e.g., ≥7 or ≥14 days after second vaccine dose to end of follow-up; 5 months maximum). Additionally, the definition of SARS-CoV-2 infection differed to some extent. All studies required a positive RT-PCR test; Young-Xu et al. [17] also included a positive antigen test, while Polinski et al. [21] defined SARS-CoV-2 infection as a medical claim with a COVID-19 diagnosis code (85% of cases) and/or a positive RT-PCR test (15% of cases). Across the five studies that assessed VE of mRNA vaccines against SARS-CoV-2 infection [17,20,22–25], VE ranged from 52% to 90% in the IC populations, versus a VE of 90% to 95% in the overall study populations. The lowest mRNA VE (52%; 95% CI: 26%–82%) was observed among individuals who were ≥65 years of age and IC (IC conditions included: hematopoietic cell or solid organs transplant, immunosuppressive medication usage, asplenia, and chronic renal failure [advanced kidney disease, dialysis, or nephrotic syndrome]) [24]. In this study by Chodick et al. [24], mRNA VE was 71% (95% CI: 37%–87%) among those who were IC (age range not provided). The highest VE against SARS-CoV-2 infection (90%; 95% CI: 49%–100%) among an IC population was observed in the Israeli study of Dagan et al. [22,23] (IC conditions included HIV, asymptomatic HIV, organ transplant, bone marrow or hematopoietic stem cell transplant, immunosuppressive medication usage). Young-Xu et al. [17], additionally conducted a post-hoc IC subgroup analysis of mRNA VE against SARS-CoV-2 infection in US veterans with hematological malignant neoplasms, which was 69% (95% CI: 17%–88%).
In the study of Chemaitelly et al. [27] of kidney transplant recipients, mRNA VE was measured at different time periods post-second dose, and as the duration increased, VE against SARS-CoV-2 infection increased from 47% (95% CI: 0%–74%) at ≥14 days to 66% (95% CI: 21%–85%) and 74% (95% CI: 33%–90%) at ≥42 days and ≥56 days, respectively, indicating vaccine protection in this IC patient group did not reach a high level until several weeks after the second dose. Yelin et al. [25] assessed VE of BNT162b2 and only reported an odds ratio (0.67; 95% CI: 0.53–0.83), and not VE for the IC population, relative to the overall population with a VE of 95% (95% CI: 93%–96%), indicating that the IC population had a 33% reduction in VE relative to the overall population. Polinski et al. [21] assessed Ad26.COV2.S VE against SARS-CoV-2 infection, which was 64% (95% CI: 57%–70%) in the IC population and 79% in both the overall (95% CI: 77%–80%), and non-IC (95% CI: 78%–81%) populations.
3.3.2. VE against symptomatic COVID-19 illness
Three studies (Dagan et al.; Barda et al. [22,23]; Chodick et al. [24]; and Whitaker et al. [26]) assessed VE against symptomatic COVID-19 illness in IC populations; Dagan et al.; Barda et al. [22,23] assessed VE 7–28 days after the second vaccine dose, Chodick et al. [24] assessed VE on days 7–27 after the second vaccine dose versus days 1–7 after the first vaccine dose, and Whitaker et al. [26] assessed VE ≥14 days after the second vaccine dose up to approximately 6.5 months. The definition of symptomatic COVID-19 illness was generally similar across the studies; Dagan et al. [22]; Barda et al. [23] and Chodick et al. [24] required documentation of symptomatic COVID-19 illness in EMRs, while Whitaker et al. [26] required a diagnosis of COVID-19 or clinical illness consistent with COVID-19 within 10 days before or after a positive RT-PCR test. In the two Israeli studies by Dagan et al.; Barda et al. [22,23] and Chodick et al. [24], BNT162b2 VE against symptomatic COVID-19 illness was 84% (95% CI: 19%–100%) and 75% (95% CI: 44%–88%) in the IC populations, respectively, while it ranged 94% (95% CI: 88%–97% [24]) to 96% (95% CI: 94%–97% [22,23]) in the overall study populations. From the study conducted in England, Whitaker et al. [26] reported a BNT162b2 VE of 73% (95% CI: 34%–89%) against symptomatic COVID-19 illness among the IC population (age range not provided); VE in the overall population 16–64 years of age was 93% (95% CI: 86%–97%), while it was 87% (95% CI: 80%–90%) for those ≥65 years. Whitaker et al. [26] also assessed ChAdOx1 nCoV-19 VE against symptomatic COVID-19 illness, which was 75% (95% CI: 19%–92%) among the IC population, 78% (95% CI:70%–84%) among the overall population 16–64 years of age, and 76% (95% CI: 59%–86.5%) among the overall population ≥65 years of age.
3.3.3. VE against severe COVID-19 illness
Three studies (Khan & Mahmud [20]; Dagan et al.; Barda et al. [22,23], and Chemaitelly et al. [27]) assessed mRNA VE in IC populations against severe COVID-19 illness, which was defined differently across these studies. Khan & Mahmud [20] defined severe COVID-19 illness as hospitalization or death, while Dagan et al.; Barda et al. [22,23] defined it according to National Institutes of Health (NIH) criteria [37], and Chemaitelly et al. defined it as per the World Health Organization (WHO) criteria [38]. Khan & Mahmud [20] reported a mRNA VE against severe illness of 70% (95% CI: NR) among their IBD patient population. Dagan et al.; Barda et al. [22,23] reported a BNT162b2 VE of 100% (95% CI: could not be determined) against severe illness in the IC population compared to 95% (95% CI: 89%–99%) in the overall population; however, the interpretation of such a finding should consider the low statistical power due to the small numbers of events (i.e. only one such event of severe illness occurred in the unvaccinated IC group and none in the vaccinated group). Similar to VE against SARS-CoV-2 infection, Chemaitelly et al. [27] observed an increase in mRNA VE against severe illness with increased time post-second dose in kidney transplant recipients, from 72% (95% CI: 0%–91%) at ≥14 days to 85% (95% CI: 36%–96.5%) and 84% (95% CI: 31%–96%) at ≥42 days and ≥56 days, respectively.
3.3.4. VE against COVID-19-related hospitalization
Four studies assessed VE against COVID-19-related hospitalization in IC populations; three (Tenforde et al. [18]; Tenforde et al. [19]; Dagan et al.; Barda et al. [22,23]) assessed VE of mRNA vaccines and Polinski et al. [21] assessed VE of Ad26.COV2.S. In the first CDC study by Tenforde et al. [18], mRNA VE against COVID-19-related hospitalization was 63% (95% CI: 21%–83%) in the IC population, 87% (95% CI: 81%–91%) in the overall study population, and 91% (95% CI: 86%–95%) in the non-IC population. The second CDC study by Tenforde et al. [19], which included nearly three times more hospitalized patients, had similar findings, with mRNA VE against COVID-19 related hospitalization reported at 63% (95% CI: 44%–76%) in the IC population, 86% (95% CI: 82%–88%) in the overall study population, and 90% (95% CI: 87%–92%) in the non-IC population over the full surveillance period (March–July 2021). Although overall VE in the IC population was lower than that in the non-IC population, it was sustained over the two study periods (March–May: 2–12 weeks and June–July: 13–24 weeks post full vaccination), which was consistent with the sustained VE observed in the overall population [19]. Dagan et al.; Barda et al. [22,23] reported a BNT162b2 VE against COVID-19-related hospitalization of 100% (95% CI: could not be determined) in their IC population; however, only two such events occurred in the unvaccinated IC group and none in the vaccinated group; VE was 92% (95% CI: 85%–97%) in the overall study population. In the study of Polinski et al. [21], VE of Ad26.COV2.S against COVID-19-related hospitalization was 68% (95% CI: 54%–77%) in the IC population compared to 81% (95% CI: 79%–84%) in the overall study population and 83% (95% CI: 80%–85%) in the non-IC population [21].
4. Discussion
This targeted literature review of 10 real-world studies conducted in four different countries gives an early view of COVID-19 VE in IC populations. Among the fully vaccinated IC populations included in the studies, VE of widely available COVID-19 vaccines ranged from 64% to 90% against SARS-CoV-2 infection, 73% to 84% against symptomatic COVID-19 illness, 70% to 100% against severe COVID-19 illness, and 63% to 100% against COVID-19-related hospitalization. COVID-19 VE for most outcomes in the IC populations included in these studies was lower than in the general populations, in which VE ranged from 79% to 95% against SARS-CoV-2 infection, from 76% to 96% against symptomatic COVID-19 illness, and from 81% to 92% against COVID-19-related hospitalization. Important to consider when interpreting the reported VE estimates for the IC populations are the accompanying confidence intervals, ranges of which were wider than those reported among the general populations across studies; such findings are related to the smaller sample sizes of the IC populations, but also stress the variability in COVID-19 VE across individuals with various IC conditions within overall IC populations. Moreover, the confidence intervals ranged substantially even among kidney transplant recipients only in the study of Chemaitelly et al. [27] suggesting that even when COVID-19 VE is assessed in one specific IC patient group, there is significant variability among individuals. These summarized findings provide a preliminary evidence base supporting greater protective measures to prevent COVID-19 infection and associated illness in those who are IC.
In the rapidly changing COVID research environment, new studies continue to be conducted, published or posted as preprints. Some of these noteworthy, late-breaking studies were not available during the study timeframe as defined for this current review. A recently medRxiv posted systematic review and meta-analysis of 54 observational and longitudinal studies of general populations [39], wherein COVID-19 VE was assessed among fully vaccinated (post-second vaccine dose) individuals, estimated a pooled VE for the BNT162b2, mRNA-1273, and ChAdOx1 nCoV-19 vaccines against SARS-CoV- 2 infection of 87% (pooled odds ratio [OR] = 0.13; 95% CI: 0.08–0.21). Against COVID-19 related hospitalization, a pooled VE of 89% (pooled OR = 0.11; 95% CI: 0.07–0.17) was estimated [39]. The findings of this meta-analysis of COVID-19 VE are relatively consistent and in the range of the COVID-19 VE estimates reported in the general populations of the summarized studies herein. Altogether, these study findings emphasize the effectiveness of widely used COVID-19 vaccines across populations from different countries.
Embi et al. [40] published a study on 5 November 2021 in which COVID-19 mRNA VE against COVID-19-related hospitalization was estimated in a fully vaccinated (i.e. after completing 2 doses of an mRNA vaccine with ≥14 days prior to index hospitalization date) US population. This test-negative designed study utilized data from the VISION network, a CDC collaboration with seven US healthcare systems and research centers, including 187 hospitals in nine US states; it included over 89,000 COVID-19-associated hospitalizations of IC and immunocompetent adults [40]. The IC population in this study was defined as individuals with a diagnosis of solid malignancy, hematologic malignancy, rheumatologic, or other inflammatory disorders, other intrinsic immune conditions or immunodeficiencies, or organ or stem cell transplants; immunosuppressive medication usage was not included in this study since the data were not available [40]. Embi et al. [40] reported a COVID-19 mRNA VE against COVID-19-associated hospitalization of 77% (95% CI: 74%–80%) among 10,564 fully vaccinated IC individuals during January 17 through 5 September 2021 and a VE of 90% (95% CI: 89%–91%) among those considered immunocompetent [40]. Additionally, Embi et al. [40] assessed COVID-19 mRNA VE before and during Delta variant predominance in the US; they consistently found a lower VE against COVID-19-associated hospitalization among the IC compared to the immunocompetent before (76%; 95% CI: 69%–81% versus 91%; 95% CI: 90%–93%) and during Delta variant predominance (79%; 95% CI: 74%–83% versus 90%; 95% CI: 89%–91%). COVID-19 mRNA VE in the IC population relative to the immunocompetent population did not significantly differ by age group (18–64 years of age and aged ≥65 years) or mRNA vaccine type, nor by time periods of assessment [40].
In the four studies reviewed herein that estimated VE against COVID-19-related hospitalization, VE ranged from 63% to 100% in the IC populations and 81% to 92% in the general populations [18,19,21–23]. Only Tenforde et al. [19] included a time period in which the Delta variant emerged as predominant; similar to the above findings of Embi et al. [40], during emerging Delta variant predominance (June–July 2021), COVID-19 mRNA VE against COVID-19-associated hospitalization did not significantly change among the IC or the overall study population from the earlier study period of March–May 2021. Embi et al. [40] also performed subgroup analyses among the IC population, in which mRNA VE against COVID-19-related hospitalization was estimated between 17 January and 5 September 2021 in organ or stem cell transplant recipients at 59% (95% CI: 38%–73%), in those with solid malignancy at 79% (95% CI: 73%–84%), in those with hematologic malignancy at 74% (95% CI: 62%–83%), in those with intrinsic immune conditions or primary immunodeficiencies at 73% (95% CI: 66%–80%), and in those with rheumatic or inflammatory disorders at 81% (95% CI: 75%–86%); all IC subgroups exhibited lower VE than among the immunocompetent population of this study. Of the summarized studies in this review, only Tenforde et al. [18] reported mRNA VE against COVID-19 related hospitalization for a subgroup of the IC population; the estimated VE was 51% (95% CI: −31%–82%) against COVID-19 related hospitalization for IC patients with an active solid organ or hematologic malignancy or solid organ transplant. These study findings further highlight that certain IC patient groups exhibit significantly lower COVID-19 VE than the general population, as well as the variability in VE between groups with different IC conditions, further warranting greater research of VE in particular IC groups.
On 17 November 2021 Galmiche et al. [41] published a systematic review of studies, in which COVID-19 VE in IC populations in real-world settings was assessed in four of the included studies. The other studies included in this systematic review assessed COVID-19 vaccine immunogenicity in IC populations (N = 157 studies) and one study assessed vaccine efficacy in a clinical trial setting [41]. Three of the four studies that assessed COVID-19 VE in IC populations included in this systematic review, Tenforde et al. [18], Khan & Mahmud [20], and Chodick et al. [24], have already been included in our targeted literature review. The fourth study by Aslam et al. [42], reported incidence rates of symptomatic COVID-19 illness in solid organ transplant recipients (N = 2,151) and not a calculated VE; in those who were vaccinated the incidence rate was 0.065 per 1000/person days (95% CI: 0.024–0.17) and in those who were unvaccinated or partially vaccinated, the incidence rate was 0.34 per 1000/person days (95% CI: 0.26–0.44).
In a US real-world study of nearly 1.2 million people fully vaccinated with the BNT162b2 mRNA vaccine, over 212,000 (18%) individuals were designated as having an IC condition [43]. This study utilized the broadest IC case algorithm of real-world studies to date, wherein 12 mutually exclusive IC conditions were identified (e.g. symptomatic HIV, solid/hematologic malignancy, organ transplant, rheumatologic/inflammatory condition, primary immunodeficiency, chronic kidney disease, usage of immunosuppressive/antimetabolite medication) [43]. Although this study did not directly measure VE, it reported the number of COVID-19 vaccine breakthrough infections following a second BNT162b2 dose between 10 December 2020 and 8 July 2021 [43]. The total number of breakthrough infections was low (N = 978; 0.08%) but nearly 40% of cases occurred among the IC population, which only accounted for approximately 18% of the overall study population [43]. The calculated incidence rate of COVID-19 vaccine breakthrough infections was 2.6 times higher among the IC population than in the non-IC population (0.89 vs. 0.34 per 100 person-years) [43]. Moreover, approximately 60% (N = 74 of 124) of the breakthrough infections that resulted in hospitalization and 100% (N = 2 of 2) of those that resulted in inpatient death, occurred in the IC population [43]. In this study, subgroup analyses of the 12 IC condition groups were also conducted; organ transplant recipients excluding bone marrow transplant had the highest incidence rate of breakthrough infections (3.66 per 100 person-years) [43]. Additionally, compared to the incidence rate among the overall IC population in this study, incidence rates of breakthrough infections were higher in those who had >1 IC condition, those with usage of antimetabolites, those with a primary immunodeficiency, those with a hematologic malignancy, and those with kidney disease [43]. The findings of this study underscore the need to standardize the definition of IC across research studies evaluating COVID-19 VE and to also conduct studies of specific IC patient groups, so that a risk stratification can be established across the overall IC population.
At the time this review was written, only 10 real-world studies, four of which were preprints without peer-review, were available that assessed COVID-19 VE in IC populations. Although our approach of including preprints for this targeted literature review strengthens the comprehensiveness of this review, we acknowledge the potential limitations in the reproducibility of this review and the quality of the collected evidence base. Only one reviewer performed data extraction for this targeted review and no inter-rater reliability/agreement scores were determined. Of the 10 included studies, study designs, follow-up periods after full vaccination, IC definitions, and IC populations, methods of computing VE, and adjustment for confounders significantly varied across these real-world studies. Hence, a comparison of study findings or a meta-analysis estimating the pooled VE for outcomes of interest was considered unfeasible. As discussed earlier, the most notable inconsistency across the studies summarized in this review, was the substantial variability in the definitions of IC populations. In this context, the COVID-19 VE estimates across these studies should be interpreted cautiously.
Additionally, the reviewed studies had limited follow-up after vaccination ranging from 7 days to 6.5 months. Four studies by Tenforde et al. [19], Polinski et al. [21], Whitaker et al. [26], and Chemaitelly et al. [27] included VE analyses during time periods of Delta variant predominance; however, only Tenforde et al. [19] reported, albeit in a figure only, mRNA VE in the IC during March to May (Alpha variant predominance) and June to July (Delta variant emerging as predominant) 2021. The study period in Tenforde et al. [19] went through July 2021, which covered only the early period of Delta variant predominance in the US (approximately the first six weeks), and a Delta-specific VE was not reported [19]. As mentioned earlier, Embi et al. [40] did not observe a significant change in COVID-19 mRNA VE against COVID-19-associated hospitalization among IC or immunocompetent individuals during Delta variant predominance compared with an earlier time period. Altogether, the studies summarized in this review covered up to eight months after COVID-19 vaccines became available. Thus, waning COVID-19 vaccine protection remains relatively undescribed, particularly among the IC, and further follow-up studies are needed to better understand not only waning vaccine protection but also the impact of increased vaccine protection with an additional dose. Only a few studies performed subgroup analyses by IC condition groups or severity of IC conditions. The included studies were also from only four countries, including the US, Israel, England, and Qatar. Since only studies published/publicly available in the English language were included in this review, there is the potential for important missing data from studies conducted in other countries and reported in languages other than English. Additionally, due in part to such exclusion criteria, other COVID-19 vaccines (e.g. Sputnik V, Sinovac-CoronaVac, etc.) more widely used in some countries did not get evaluated in this review. Furthermore, our study findings may not be generalizable to populations with IC conditions endemic to specific regions. While this review provides an early view of COVID-19 VE in IC populations, mostly as an aggregate group, further study is warranted.
5. Expert opinion
As the COVID-19 pandemic continues across the world and if in the future, COVID-19 becomes endemic to societies, it may be of clinical utility to more consistently and precisely define IC populations across research studies evaluating COVID-19 VE. A consensus on defining IC condition groups will provide more useful evidence for policymakers and healthcare providers in the decision-making process when recommending and updating vaccination protocols and treating patients at high-risk for COVID-19. Across the studies included in this review, only two IC conditions, organ transplant and immunosuppressive medication usage, were common in the definitions of IC populations. Only a few studies included in this review focused on particular IC conditions and only one study included CKD as an IC condition. A consensus on the list of immunosuppressive medications to designate individuals as IC also needs to be developed. Moreover, it may be useful to stratify overall IC populations into low-, medium-, and high-risk patient groups for COVID-19 illness. This may also involve the identification of IC groups with comorbidities known to increase the risk for severe COVID-19 (e.g. older age, type 2 diabetes, obesity) [44,45] and their risk stratification. Furthermore, individuals with IC conditions that are endemic to certain countries and regions that heighten the risk for COVID-19 illness may also need to be identified so that the necessary preventive and protective measures can be put in place
This review highlights the most current findings of real-world studies that have assessed COVID-19 VE in IC populations. Our summarized findings provide preliminary evidence that individuals who are IC require greater protective measures to prevent COVID-19 infection and associated illness; hence, should be prioritized while implementing recommendations of additional/booster COVID-19 vaccine doses. Indeed, in the US, the CDC recommends an additional primary mRNA COVID-19 vaccine dose for moderately or severely IC people at least 5 years of age (BNT162b2 only) and at least 18 years of age (mRNA-1273) who received a two-dose mRNA vaccine primary series [46], and countries, including Israel, the United Kingdom (UK), and France, as well as the World Health Organization, have similar recommendations [47,48]. Initial data from studies of clinical trial participants have shown that additional/booster COVID-19 vaccine doses increase immune responses [49]. Further, emerging real-world evidence indicates that VE of two mRNA doses against severe COVID-19 illness wanes in the IC population (from 93% to 68% over 6 months) but not in the non-IC population, and that a third dose can restore initial high levels of protection against hospitalization (87%) as well as against infection [50]. The benefits of improved protection against infection and hospitalization with additional doses should be balanced against risks of reactogenicity and the safety profile. Safety monitoring of additional/booster COVID-19 vaccine doses has been implemented in the US by the CDC; a CDC study of 22,191 persons who had received an additional COVID-19 vaccine dose from 12 August 2021 to 19 September 2021 a time period in the US in which an additional COVID-19 vaccine dose was primarily recommended for those with moderate-to-severe IC conditions (98% received third dose of same vaccine as initial doses), found that among those with a completed health check-in survey (N = 12,591), the prevalence of local/systemic reactions after a third dose was similar to that observed after a second dose [51].
For moderately or severely IC individuals with a primary series of mRNA vaccination, the CDC in the US recommends an additional dose of homologous mRNA vaccine [10,46]; however, some countries such as Canada and the UK have also recommended an mRNA additional dose for such individuals who have completed a primary series with a homologous or heterologous schedule of mRNA or viral vector-based vaccines (e.g. ChAdOx1 nCoV-19) [52,53]. VE data comparing homologous and heterologous additional doses among IC individuals are limited, but a few studies have found VE after the various booster combinations in general populations [54]. In the UK, Andrews et al. [55] reported substantial protection against symptomatic COVID-19 illness in persons 50 years of age and older after a BNT162b2 booster regardless of the primary vaccine schedule (mRNA vaccine or ChAdOx1 nCoV-19). Similarly, the Chilean government reported preliminary results of VE of booster doses based on data from two million individuals of a total cohort of 11 million people, showing that heterologous or homologous booster doses provided a high level of protection against hospitalization with no major differences across boosters (BNT162b2, CoronaVac, or ChAdOx1 nCoV-19) after a primary series with the inactivated vaccine CoronaVac [56].
Safety monitoring of additional COVID-19 vaccine doses is ongoing worldwide and further studies in overall populations, as well as in IC populations, are awaited on the effectiveness and safety of additional/booster vaccine doses, optimal time intervals between primary series and additional/booster vaccine doses, duration of protection, prevention of emergence of highly mutated novel SARS-CoV-2 variants, receipt of vaccine doses from different manufacturers, etc.
In a recent study of approximately 22,000 US survey respondents with comorbid conditions all participating in an online health community, of whom 27% reported having cancer and 23% reported having an autoimmune disease, approximately 20% expressed COVID-19 vaccine hesitancy [57]. In light of this finding, in addition to the substantial number of people who have IC conditions and/or take immunosuppressive medications, and the potential for waning COVID-19 VE and emergence of new SARS-CoV-2 variants, it is critical to rapidly advance our understanding of COVID-19 VE and duration of response among IC populations, including specific IC condition groups and IC individuals who have other COVID-19 risk factors (e.g. elderly, comorbidities, etc.), as the COVID-19 pandemic continues worldwide. The importance of this undertaking is explicitly emphasized by the recent emergence of the highly transmissible Omicron variant, which again underscores the ongoing need to provide the most up-to-date scientific information to decision makers so that measures, such as immunization scheduling and additional/booster vaccine-dose prioritization, can be rapidly implemented.
Funding Statement
This work was supported by Pfizer, Inc., which had a role in study design, data collection and the review of this paper.
Article highlights
Scientific evidence gained from real-world studies conducted in multiple countries is increasingly showing that widely available COVID-19 vaccines, including BNT162b2 (Pfizer/BioNTech), mRNA-1273 (Moderna), Ad26.COV2.S (Janssen), and ChAdOx1 nCoV-19 (Oxford/AstraZeneca), are highly effective for protecting against SARS-CoV-2 infection, symptomatic COVID-19 illness, and COVID-19-related hospitalization and death.
From July through October of 2021, several countries across the world issued recommendations for increased COVID-19 vaccine protection for individuals with one or more immunocompromised (IC) conditions.
As new COVID-19 vaccine recommendations are implemented and updated over time in response to the evolving COVID-19 pandemic, it is necessary to rapidly and more comprehensively understand the effectiveness of COVID-19 vaccines in IC populations.
In this review, we have summarized the findings of real-world studies that have assessed COVID-19 VE in IC populations.
Among the fully vaccinated IC populations included in the reviewed studies, VE of widely available COVID-19 vaccines ranged from 64% to 90% against SARS-CoV-2 infection, 73% to 84% against symptomatic COVID-19 illness, 70% to 100% against severe COVID-19 illness, and 63% to 100% against COVID-19-related hospitalization.
VE for most outcomes in the IC populations included in these studies was lower than in the general populations, in which VE ranged from 79% to 95% against SARS-CoV-2 infection, from 76% to 96% against symptomatic COVID-19 illness, and from 81% to 92% against COVID-19-related hospitalization.
Our summarized findings provide preliminary evidence that individuals who are IC require greater protective measures to prevent COVID-19 infection and associated illness; hence, should be prioritized while implementing recommendations of additional/booster COVID-19 vaccine doses.
Declaration of interest
Manuela Di Fusco, Jennifer L Nguyen, Thomas Scassellati Sforzolini, Jennifer Judy, Alejandro Cane, and Mary M Moran are employees of Pfizer. Shailja Vaghela is an employee of HealthEcon Consulting, Inc. and external consultant for Pfizer. Melissa Lingohr-Smith and Jay Lin are employees of Novosys Health, which received funding support from Pfizer, Inc. for the preparation of this review article.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they receive a study drug from the BMS-Pfizer Alliance for Eliquis for a federally funded, investigator-initiated (NIH) clinical trial of apixaban vs aspirin for stroke prevention. Peer reviewers on this manuscript have no other relevant financial or other relationships to disclose.
Author contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article. All authors contributed to study conception and design, analysis, and interpretation, drafting and revising of the manuscript, and have given their approval for this manuscript version to be published.
Data availability statement
All data contained within this review were extracted from the published/preprint referenced articles and are available to the public.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.OurWorldinData.org [Internet] . Statistics and research: coronavirus (COVID-19) vaccinations. London (England): Oxford; [cited 2021 Sep 30]. Available from: https://ourworldindata.org/covid-vaccinations [Google Scholar]
- 2.Cdc.gov [Internet] . Atlanta (GA): centers for disease control and prevention. Science brief: COVID-19 vaccines and vaccination. Updated 2021 Sep 15. cited 2021 Oct 19]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/fully-vaccinated-people.html. [PubMed]
- 3.Henry DA, Jones MA, Stehlik P, et al. Effectiveness of COVID-19 vaccines: findings from real-world studies. Med J Aust. 2021;215(4):149–151e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polack FP, Thomas SJ, Kitchin N, et al., Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27): 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Of importance: a clinical trial of COVID-19 vaccine efficacy
- 5.Baden LR, El Sahly HM, Essink B, et al., Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5): 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Of importance: a clinical trial of COVID-19 vaccine efficacy
- 6.Sadoff J, Gray G, Vandebosch A, et al., Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N Engl J Med. 2021;384(23): 2187–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Of importance: a clinical trial of COVID-19 vaccine efficacy
- 7.Falsey AR, Sobieszczyk ME, Hirshch I, et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 vaccine. N Engl J Med. 2021. Sep 29;385(25):2348–2360.Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Of importance: a clinical trial of COVID-19 vaccine efficacy
- 8.Cdc.gov [Internet] . Atlanta (GA): centers for disease control and prevention. Principles of epidemiology in public health practice, third edition: an introduction to applied epidemiology and biostatistics. [cited 2021 Oct 19]. Available from: https://www.cdc.gov/csels/dsepd/ss1978/lesson3/section6.html
- 9.Reuters.com. [Internet] . London (England): Reuters staff; Factbox: countries weigh need for booster COVID-19 shots. 2021 Oct 5. cited 2021 Oct 19]. Available from: https://www.reuters.com/business/healthcare-pharmaceuticals/countries-weigh-need-booster-covid-19-shots-2021-09-24 [Google Scholar]
- 10.Cdc.gov [Internet] . COVID-19 vaccines for moderately to severely immunocompromised people. Atlanta (GA): Centers for Disease Control and Prevention;. Updated. 2021. Sep 2 [cited 2021 Sep 30]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html. ** Of considerable importance: provides definition of moderately to severely immunocompromised individuals. [Google Scholar]
- 11.Haidar G, Mellors JW.. Improving the outcomes of immunocompromised patients with COVID-19. Clin Infect Dis. 2021;73(6):e1397–e1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325(17):1784–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yi SG, Knight RJ, Graviss EA, et al. Kidney transplant recipients rarely show an early antibody response following the first COVID-19 vaccine administration. Transplantation. 2021;105(7):e72–e73. [DOI] [PubMed] [Google Scholar]
- 14.Agha ME, Blake M, Chilleo C, et al. Suboptimal response to coronavirus disease 2019 messenger RNA vaccines in patients with hematologic malignancies: a need for vigilance in the postmasking era. Open Forum Infect Dis. 2021;8(7):ofab353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyarsky BJ, Ruddy JA, and Connolly CM, et al. Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021. Mar 23;80(8):1098–1099 doi: 10.1136/annrheumdis-2021-220289. annrheumdis-2021-220289. annrheumdis-2021-220289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee ARYB, Wong SY, Chai LYAC, et al. Efficacy of COVID-19 vaccines in immunocompromised patients: a systematic review and meta-analysis. medRxiv. 2021. Oct 1. DOI: 10.1101/2021.09.28.21264126. Preprint. [DOI] [Google Scholar]
- 17.Young-Xu Y, Korves C, Powell EI, et al. Coverage and effectiveness of mRNA SARS-CoV-2 vaccines among United States veterans. JAMA Network Open. 2021;4(10):e2128391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tenforde MW, Patel MM, Ginde AA, et al. Effectiveness of SARS-CoV-2 mRNA vaccines for preventing Covid-19 hospitalizations in the United States. Clin Infect Dis. 2021. Aug 6;ciab687. doi: 10.1093/cid/ciab687. Online ahead of print. [DOI] [Google Scholar]
- 19.Tenforde MW, Self WH, Naioti EA, et al. Sustained effectiveness of Pfizer-BioNTech and moderna vaccines against COVID-19 associated hospitalizations among adults — United States, March–July 2021. MMWR Morb Mortal Wkly Rep. 2021;70(34):1156–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan N, Mahmud N. Effectiveness of SARS-CoV-2 vaccination in a veterans affairs cohort of patients with inflammatory bowel disease with diverse exposure to immunosuppressive medications. Gastroenterology. 2021;161(3):827–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polinski JM, Weckstein AR, Batech M, et al. Effectiveness of the single-dose Ad26.COV2.S COVID vaccine. medRxiv. 2021. Sep 10. DOI: 10.1101/2021.09.10.21263385. Preprint. [DOI] [Google Scholar]
- 22.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barda N, Dagan N, Balicer RD. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting: reply. N Engl J Med. 2021;384:1970. [DOI] [PubMed] [Google Scholar]
- 24.Chodick G, Tene L, Rotem RS, et al. The effectiveness of the two-dose BNT162b2 vaccine: analysis of real-world data. Clin Infect Dis. 2021. May 17;ciab438. doi: 10.1093/cid/ciab438. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yelin I, Katz R, Herzel E, et al. Associations of the BNT162b2 COVID-19 vaccine effectiveness with patient age and comorbidities. medRxiv. 2021. May 24. DOI: 10.1101/2021.03.16.21253686. Preprint. [DOI] [Google Scholar]
- 26.Whitaker HJ, Tsang RSM, Byford R, et al. Pfizer-BioNTech and Oxford AstraZeneca COVID-19 vaccine effectiveness and immune response among individuals in clinical risk groups. Khub.net. Posted July 2021. Preprint. [DOI] [PMC free article] [PubMed]
- 27.Chemaitelly H, AlMukdad S, Paravila J, et al. SARS-CoV-2 vaccine effectiveness in immunosuppressed kidney transplant recipients. medRxiv. 2021. Aug 9. DOI: 10.1101/2021.08.07.21261578. Preprint. [DOI] [Google Scholar]
- 28.Kemp SA, Collier DA, Datir RP, et al. SARS-CoV-2 evolution during treatment of chronic infection. Nature. 2021;592(7853):277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Truong TT, Ryutov A, Pandey U, et al. Persistent SARS-CoV-2 infection and increasing viral variants in children and young adults with impaired humoral immunity. medRxiv. 2021. Mar 2. DOI: 10.1101/2021.02.27.21252099. Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi B, Choudhary MC, Regan J, et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med. 2020;383(23):2291–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avanzato VA, Matson MJ, Seifert SN, et al. Case study: prolonged infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell. 2020;183(7):1901.e9–1912.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corey L, Beyrer C, Cohen MS, et al. SARS-CoV-2 variants in patients with immunosuppression. N Engl J Med. 2021;385(6):562–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. [DOI] [PubMed] [Google Scholar]
- 34.Cochrane.org [Internet] . 10.3.2 Including unpublished studies in systematic reviews. The Cochrane Collaboration. [cited 2021 Nov 18]. Available from: https://handbook-5-1.cochrane.org/chapter_10/10_3_2_including_unpublished_studies_in_systematic_reviews.htm. [Google Scholar]
- 35.Cochrane.org [Internet] . 6.2.2.5 other reviews, guidelines and reference lists as sources of studies. The Cochrane Collaboration. [cited 2021 Nov 18]. Available from: https://handbook-5-1.cochrane.org/chapter_6/6_2_2_5_other_reviews_guidelines_and_reference_lists_as.htm [Google Scholar]
- 36.Covariants.org [Internet] . Munich (Germany): GISAID; Overview of variants in countries. [cited 2021 Dec 30]. Available from: https://covariants.org/per-country [Google Scholar]
- 37.Nih.gov [Internet] . Coronavirus disease 2019 (COVID-19) treatment guidelines. Bethesda (MD): National Institutes of Health; 2021. [cited 2021 Dec 30]. Available from: https://www.covid19treatmentguidelines.nih.gov [PubMed] [Google Scholar]
- 38.Who.int [Internet] . COVID-19 clinical management: living guidance. Geneva (Switzerland): World Health Organization; [cited 2021 Dec 30]. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1 [Google Scholar]
- 39.Rahmani K, Shavaleh R, Forouhi M, et al. Effectiveness of COVID-19 vaccines and post-vaccination SARS-COV 2 infection, hospitalization, and mortality: a systematic review and meta-analysis of observational studies. medRxiv. 2021. Nov 3. DOI: 10.1101/2021.11.03.21265819. Preprint. [DOI] [Google Scholar]
- 40.Embi PJ, Levy ME, Naleway AL, et al. Effectiveness of 2-dose vaccination with mRNA COIVD-19 vaccines against COVID-19-associated hospitalizations among immunocompromised adults – nine states, January-September 2021. MMWR Morb Mortal Wkly Rep. 2021;70(44):1553–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galmiche S, Nguyen LBH, Tartour E, et al. Immunological and clinical efficacy of COVID-19 vaccines in immunocompromised populations: a systematic review. Clin Microbiol Infect. 2021. Nov 17. DOI: 10.1016/j.cmi.2021.09.036. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aslam S, Adler E, Mekeely K, et al. Clinical effectiveness of COVID-19 vaccination in solid organ transplants. Transpl Infect Dis. 2021;e13705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Fusco M, Moran MM, Cane A, et al. Evaluation of COVID-19 vaccine breakthrough infections among immunocompromised patients fully vaccinated with BNT162b2. J Med Econ. 2021;24(1):1248–1260. [DOI] [PubMed] [Google Scholar]
- 44.Cdc.gov [Internet] . Atlanta (GA): centers for disease control and prevention: advisory committee on immunization practices COVID-19: people with certain medical conditions. Updated. 2021. Aug 20 [cited 2021 Nov 22]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html
- 45.Sentinelinitiative.org [Internet] . White Oak (MD): US Food and Drug Administration. Sentinel initiative: master protocol development: COVID-19 natural history. 2020. Oct 13 [cited 2021 Nov 22]. Available from: https://www.sentinelinitiative.org/methods-data-tools/methods/master-protocol-development-covid-19-natural-history
- 46.Cdc.gov [Internet] . Atlanta (GA): centers for disease control and prevention: interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Updated. 2021. Dec 17 [cited 2021 Dec 22]. Available from: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html
- 47.Cdc.gov [Internet] . Atlanta (GA): centers for disease control and prevention: sara oliver-data and clinical considerations for additional doses in immunocompromised people. 2021. Jul 22 [cited 2021 Dec 22]. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-07/07-COVID-Oliver-508.pdf.
- 48.Who.int [Internet] . Geneva (Switzerland): World Health Organization; WHO advisory group recommends extra COVID-19 vaccine dose for immunocompromised. [cited 2021 Dec 30]. Available from: https://news.un.org/en/story/2021/10/1102732 [Google Scholar]
- 49.Cdc.org [Internet] . Atlanta (GA): centers for disease control and prevention: COVID-19 vaccine booster shots. Updated. 2022. Jan 22 . [cited 2022 Jan 11]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html
- 50.Tartof SY, Slezak JM, Puzniak L, et al. Effectiveness of a third dose of BNT162b2 mRNA COVID-19 vaccine in a large US health system: a retrospective cohort study. Lancet. 2021. Dec 21;398(10309):1407–1416. Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hause AM, Baggs J, Gee J, et al. Safety monitoring of an additional dose of COVID-19 vaccine – United States, August 12-September 19, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(39):1379–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Canada.ca [Internet]. An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI) . Interim guidance on booster COVID-19 vaccine doses in Canada. Public Health Agency of Canada. 29 Oct 2021. [cited 2022 Jan 20]. Available from: https://www.canada.ca/content/dam/phac-aspc/documents/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines/statement-guidance-booster-doses/statement-guidance-booster-doses.pdf
- 53.Gov.UK [Internet] . Independent report: Joint Committee on Vaccination and Immunisation (JCVI) advice on third primary dose vaccination. United Kingdom: Department of Health & Social Care. 1 Sep 2021. [cited 2022 Jan 20]. Available from: https://www.gov.uk/government/publications/third-primary-covid-19-vaccine-dose-for-people-who-are-immunosuppressed-jcvi-advice/joint-committee-on-vaccination-and-immunisation-jcvi-advice-on-third-primary-dose-vaccination#fn:2
- 54.Ema.europa.eu [Internet] . Heterologous primary and booster COVID-19 vaccination: evidence based regulatory considerations. Amsterdam (The Netherlands): European Medicines Agency; 13 Dec 2021. [cited 2022 Jan 20]. Available from: https://www.ema.europa.eu/en/documents/report/heterologous-primary-booster-covid-19-vaccination-evidence-based-regulatory-considerations_en.pdf [Google Scholar]
- 55.Andrews N, Stowe J, Kirsebom F, et al. Effectiveness of BNT162b2 (Comirnaty, PfizerBioNTech) COVID-19 booster vaccine against COVID-19 related symptoms in England: test negative case-control study. medRxiv. 2021. Nov 15.DOI: 10.1101/2021.11.15.21266341. Preprint. [DOI] [Google Scholar]
- 56.Immunization campaign against SARS-CoV-2: early estimates of the effectiveness of booster shots in Chile. Chile: Ministerio de Salud; Oct 2021. [cited 2022 Jan 20]. Available from: https://www.minsal.cl/wp-content/uploads/2021/10/2021-10-07-EFECTIVIDAD-DOSIS-DE-REFUERZO_ENG.pdf [Google Scholar]
- 57.Tsai R, Hervey J, Hoffman K, et al. COVID-19 vaccine hesitancy and acceptance among individuals with cancer, autoimmune diseases, and other serious comorbid conditions: across-sectional internet-based survey. JMIR Public Health Surveill. 2021. Oct 20. 10.2196/29872. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data contained within this review were extracted from the published/preprint referenced articles and are available to the public.


