ABSTRACT
Introduction
Vitamin D levels have been reported to be associated with COVID-19 susceptibility, severity, and mortality events. We performed a meta-analysis of randomized controlled trials (RCTs) to evaluate the use of vitamin D intervention on COVID-19 outcomes.
Areas covered
Literature search was conducted using PubMed, Cochrane library, and ClinicalTrials.gov databases. We included RCTs reporting the use of vitamin D intervention to control/placebo group in COVID-19. The study was registered at PROSPERO: CRD42021271461.
Expert opinion
A total of 6 RCTs with 551 COVID-19 patients were included. The overall collective evidence pooling all the outcomes across all RCTs indicated the beneficial use of vitamin D intervention in COVID-19 (relative risk, RR = 0.60, 95% CI 0.40 to 0.92, Z = 2.33, p = 0.02, I2 = 48%). The rates of RT-CR positivity were significantly decreased in the intervention group as compared to the non-vitamin D groups (RR = 0.46, 95% CI 0.24 to 0.89, Z = 2.31, p = 0.02, I2 = 0%). Conclusively, COVID-19 patients supplemented with vitamin D are more likely to demonstrate fewer rates of ICU admission, mortality events, and RT-PCR positivity.
KEYWORDS: Cholecalciferol, COVID-19, randomized controlled trial, SARS-cov-2, vitamin D
1. Introduction
Since December 2019, millions have been infected with severe acute respiratory syndrome associated with coronavirus-2 (SARS-CoV-2) causing Coronavirus disease 2019 (COVID-19), a global pandemic the World Health Organization (WHO). The COVID-19 symptoms range from mildly symptomatic to moderate, severe to critical with patients needing hospitalization and intensive care unit (ICU) admissions. As of 23 November 2021 and WHO, there have been 257,469,528 confirmed COVID-19 cases, including 5,158,211 deaths [1]. Multiple risk factors in the form of age, comorbidities, exaggerated immune response in the form of cytokine storm, oxidative stress, activation of pro-coagulation factors, and severe inflammation contribute to the disease progression [2].
It has been documented that vitamin D deficiency is associated with severity of viral infections such as influenza [3]. Recent evidence shows the potential of vitamin D to affect SARS-CoV-2 gene expression and alleviate infection upon binding to the vitamin D response element [4,5]. Vitamin D regulates the renin-angiotensin system and expression of angiotensin converting enzyme 2 (ACE2), and its receptor that mediates SARS-CoV-2 infection. Furthermore, vitamin D is known to exert immuno-modulatory effects in innate and adaptive immune responses, induces the production of antimicrobial proteins, and could act as anti-inflammatory agent [6–9].
Despite vaccination rollouts, much focus has been documented on additional preventive measures such as using vitamin D supplementation to be promising in COVID-19 [7,10]. While strong observational evidence [11–16] indicate the association of low vitamin D levels to the COVID-19 susceptibility, severity, and mortality outcomes, the beneficial use of vitamin D supplements in COVID-19 has been reported in some non-randomized observational cohorts [17,18]. Vitamin D supplementation has also been suggested as a putative useful tool to enhance immune responses to COVID-19 vaccines [19,20]. However, there is still a scarcity of information through randomized controlled trials (RCTs) on the use of vitamin D supplementation in COVID-19 patients. With many of the trials in the ongoing stage, there is a greater need for supportive evidence through meta-analysis of available RCTs [21–26]. Therefore, our objective of this study was to evaluate the effect of vitamin D intervention in relationship to several COVID-19 outcomes reported in all available RCTs.
2. Material and methods
This meta-analysis was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [27]. The protocol was registered at PROSPERO: CRD42021271461.
2.1. Literature search and study selection
The literature search was conducted with no language restrictions using PubMed/MEDLINE, Cochrane library, EMBASE, SCOPUS, Science Direct, and ClinicalTrials.gov from inception to 5 August 2021. The search strategy included both the MeSH and broad text-word search terms: (“vitamin D” (MeSH Terms) OR “vitamin D” (All Fields) OR “ergocalciferols” (MeSH Terms) OR “ergocalciferols” (All Fields)) AND (“SARS-CoV-2 OR COVID-19” (MeSH Terms) OR “SARS-CoV-2 OR COVID-19” (All Fields)). The other terms used for vitamin D were 25-Hydroxyvitamin D, 25-Hydroxycholecalciferol, calcidiol, 1,25-dihydroxyvitamin D3, Calcifediol, Cholecalciferol, Vitamin D3, and Calcitriol. The other terms used for SARS-CoV-2/COVID-19 are Coronavirus and 2019-nCoV Disease. The bibliographies of published articles were manually hand-searched for additional studies.
The inclusion criteria were: (1) RCTs comparing supplementation of vitamin D to placebo/control; (2) RCTs reporting the use of vitamin D supplementation on one or more of the following; COVID-19 severity, ICU care, mortality events, seropositivity, and RT-PCR positivity or any other adverse events. No prespecified limitations applied for dose or type of vitamin D and follow-up durations. The exclusion criteria were: (1) studies with no control/comparator group; (2) study types other than RCTs such as observational studies and trial-protocols. In case of duplicate articles, only a recent report with all relevant information was included. All the relevant RCTs were screened at the title, abstract, and full-text levels for their suitability in this systematic review and meta-analysis.
2.2. Data extraction and risk of bias assessment
The information extracted from eligible RCTs include: first author names, study country and setting, sample sizes, randomization, blinding, vitamin D form and dose, follow-up details, number of events for study outcomes (severity, ICU care, mortality, seropositivity and RT-PCR positivity) in treatment and comparator groups, and other study characteristics. Two investigators (S.R.V. and B.T.) independently assessed the potential risks of bias of the RCTs using the Cochrane Risk of Bias Tool [28]. Two authors have independently performed the literature search, study selection, and assessment. Any discrepancies were resolved upon discussion with a third investigator. When required, the corresponding authors of respective articles were contacted through e-mail to obtain data/clarification.
2.3. Data analysis
For this meta-analysis of RCTs, we reported the effect sizes as risk ratio (RR) for the number of events on the outcomes such as severity, ICU admissions, mortality, seropositivity, and RT-PCR positivity in treated and control groups. We reported RR values with their 95% confidence intervals (CI) using Mantel–Haenszel analysis method and random-effects model. The overall effect size for RR was presented Z-score. A Z-score with a p value of <0.05 was considered statistically significant. The between-study heterogeneity was examined by the I2 statistics, and the values >50% were considered to indicate a high degree of heterogeneity [29]. We examined the funnel plot asymmetry for publication bias followed by Begg and Egger’s tests.
2.4. Subgroup and sensitivity analysis
We conducted subgroup analysis based on vitamin D form, vitamin D-deficient studies, single- or multi-centric trials, and double-blinded status. We also performed a one-study leave-out sensitivity analysis for individual outcomes by excluding one trial at a time and by repeating the analysis. The meta-regression analysis was not possible due to the small number of available trials.
3. Results
We reviewed 755 articles for eligibility, 6 RCTs [21–26] comprising 551 COVID-19 patients were selected for final analysis (Figure 1).
Figure 1.
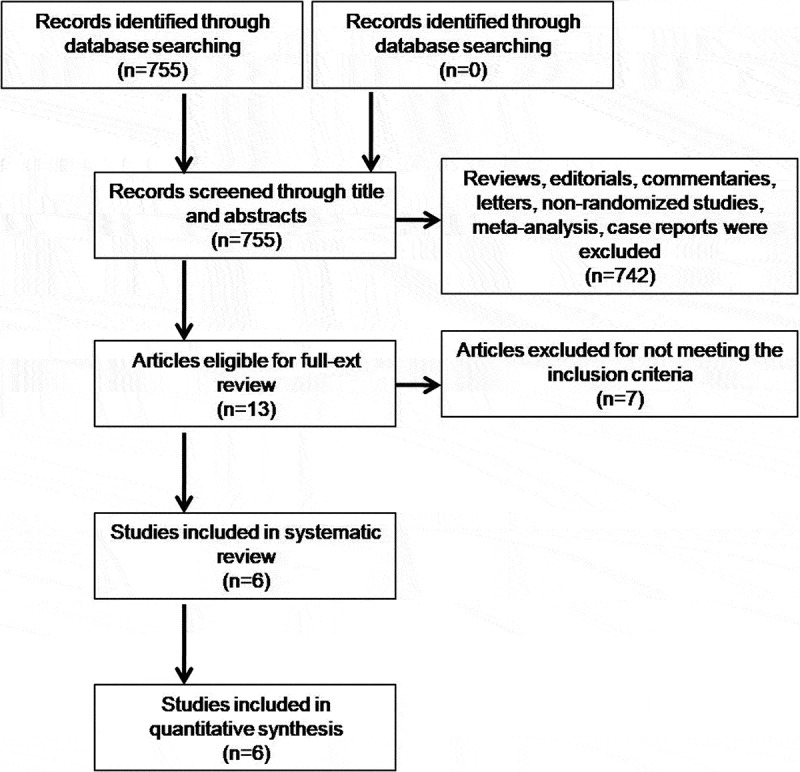
Literature search results.
While all the studies enrolled participants aged >18 years with mean age in individual studies range from 36 to 56 years, the proportion of men varied from 44% to 59%. The symptoms of COVID-19 patients diagnosed by RT-PCR (viral RNA) or ELISA and/or radiographic testing varied across the individual studies (mild-moderate-severe). The criteria for inclusion and exclusion, varied study settings, participant characteristics, number of participants with preexisting comorbidities and treatment strategies, vitamin D form, dosage, reported outcomes, and other study characteristics are presented in Supplementary Table 1.
There were two multicenter [23,25] and four single center RCTs [21,22,24,26], one double-blinded [23], and four registered clinical trials 21–23,25]. Vitamin D treatment was compared to placebo in two studies [23,24], non-vitamin control in three studies [21,22,26], and standard treatment comparator group in one study [25]. While Castillo et al. [21] used calcifediol with an allocation ratio of 2:1; all other studies used cholecalciferol with an allocation ratio of 1:1. The baseline vitamin D statuses in three studies [22,24,25] were reported to be sub-optimal, and one study reported a separate outcome analysis in vitamin D deficient participants [23]. The vitamin D sufficiency status, treatment doses, follow-up durations, adverse events, and study limitations are detailed in Supplementary Table 1. The risk of bias assessment based on five domains, and the overall bias of included RCTs is presented the supplementary appendix.
The collective evidence in Figure 2 shows that vitamin D treatment was significantly associated with reduced risk of COVID-19 severity when six observations on the number events for symptom severity, ICU care, and mechanical ventilation were pooled (RR = 0.46, 95% CI 0.23 to 0.93, Z = 2.16, p = 0.03, I2 = 52%). But the pooled estimate from four studies showed that the use of vitamin D was not significantly associated with ICU outcome alone (RR = 0.11, 95% CI 0.15 to 1.30, Z = 1.48, p = 0.14, I2 = 66%).
Figure 2.
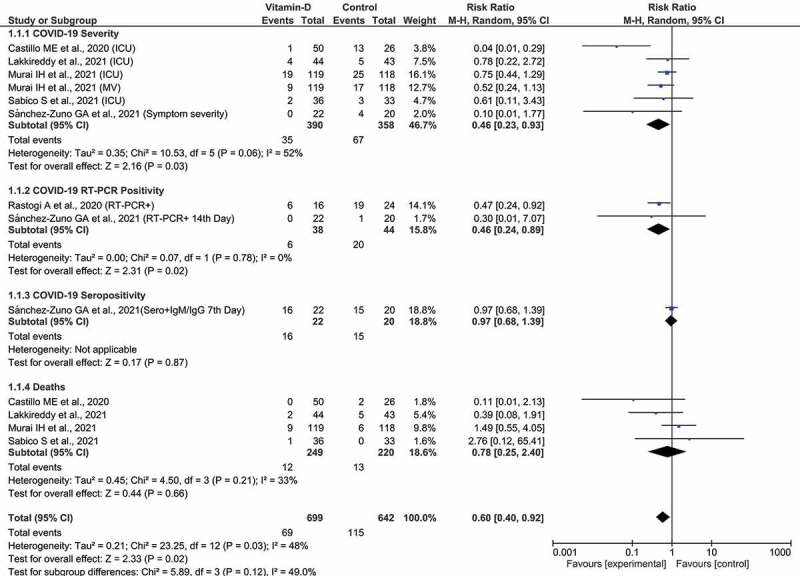
The Forest plot for association of vitamin D intervention in COVID-19.
The pooled estimate from two studies showed a statistically significant RR for COVID-19 RT-PCR positivity (RR = 0.46, 95% CI 0.24 to 0.89, Z = 2.31, p = 0.02, I2 = 0%). Whereas the pooled evidence from four studies showed that the association of vitamin D with mortality outcome was not statistically significant (RR = 0.78, 95% CI 0.25 to 2.40, Z = 0.66, p = 0.02, I2 = 33%). However, when all the observations on all reported outcomes were pooled, there was statistically significant evidence on the use of vitamin D treatment in reducing overall COVID-19-related outcomes (RR = 0.60, 95% CI 0.40 to 0.92, Z = 2.33, p = 0.02, I2 = 48%). The test for subgroup differences was not statistically significant (I2 = 49%, p = 0.12).
The results of subgroup analysis are presented in Supplementary Table 2. None of the outcomes in different categories of subgroups showed statistically significant RR values. No statistically significant difference was observed for the pooled estimate of outcomes from studies with vitamin D suboptimal status. The sensitivity analysis performed leaving-out any one of the included trials at a time and repeating the analysis showed statistically non-significant RR values for individual outcomes. Whereas, for all studied outcomes together, the pooled RR remained statistically significant after leaving our any particular study/observation. The I2 value significantly changed from 48% to 5% after leaving-out a study by Castillo et al. (ICU and mortality observations) and repeating the analysis suggestive of major source of heterogeneity. The funnel plot analysis (Figure 3) with Begg’s (p = 0.17) and Egger tests (p = 0.14) on all the outcomes across all the RCTs indicated no significant publication bias.
Figure 3.
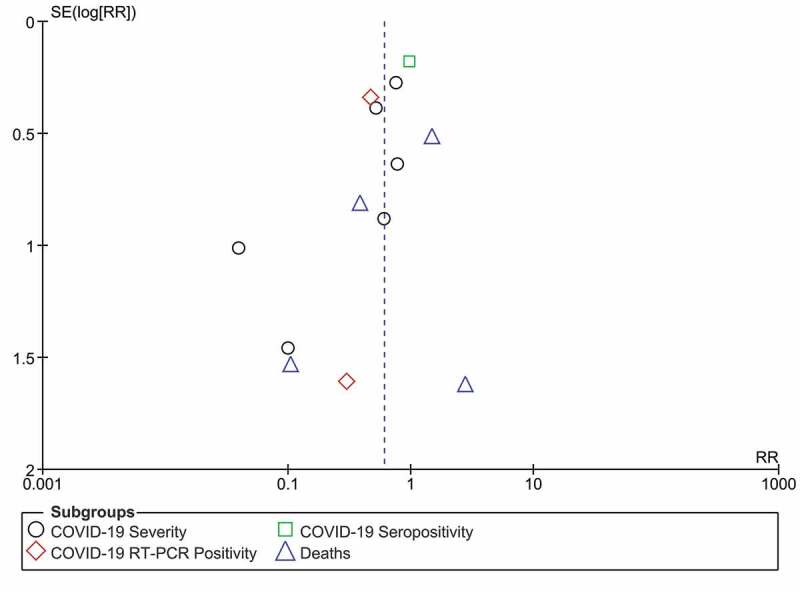
The Funnel plot for publication bias.
4. Discussion
This meta-analysis of RCTs showed that COVID-19 patients supplemented with vitamin D had reduced overall risk for all outcomes. The collective overall evidence on severity, ICU care, mortality, sero, and RT-PCR positivity events reported in all trials indicated that COVID-19 patients treated with vitamin D showed lower rates of these outcomes relative to patients receiving no-vitamin D/standard/placebo. Although there were no statistically significant differences in the individual outcomes of ICU admission and mortality, the respective RRs indicated a decrease in the rates of these outcomes in vitamin D-treated groups. However, there was a statistically significant decrease in the rates of RT-PCR positivity in COVID-19 patients supplemented with vitamin D.
The first multicenter double-blind RCT study by Murai et al. [23] enrolled 237 moderate–severe COVID-19 patients. It had 119 patients in the experimental group treated with a single high dose of vitamin D3 (200,000 IU orally) and 118 patients in the placebo group receiving peanut oil. The results do not support the use of a high dose of vitamin D, as it did not significantly reduce the length of hospital stay, hospital discharge, ICU admission and rates of mechanical ventilation, and mortality. Similar findings were reported in subgroups of patients (57 in intervention and 58 in placebo arms) with vitamin D deficiency at baseline (<20 ng/mL), despite of achieving sufficient status (≥30 ng/mL) in 86.7% of the vitamin D3 group post-intervention. This study reports more mortality events in the intervention arm (9/119) than the placebo (6/118) group.
In another multicenter RCT [25] randomizing 73 mild–moderate COVID-19 patients with suboptimal vitamin D status into experimental (n = 36) and standard-comparator (n = 33) groups receiving 5000 IU and 1000 IU of oral cholecalciferol daily for 2 weeks. This study though reports a significantly shorter recovery time to symptoms (even after adjusting for age, sex, BMI, and D-dimer) in the intervention arm, no significant differences in ICU, mortality events and days to discharge were reported between groups. This study differs from that of Murai et al. [23] as it excludes severe COVID-19 cases, vitamin D dosage and duration, using standard comparator group in place of placebo, and in defining the suboptimal vitamin D status (<50 nmol/L). Furthermore, this study also differs from all other trials as 47% of randomized participants had also received vitamin C supplements. The significant increase in vitamin D levels reported in treatment arm (5000 IU) post-intervention along with other study findings are to be interpreted with caution to the baseline vitamin D levels in the comparator arm. The post-treatment vitamin D levels (62.5 nmol/L) of the intervention arm are similar to that of the pre-treatment levels (63 nmol/L) in comparator arm (p = 0.67).
In an RCT by Sánchez-Zuno et al. [26], 42 mild COVID-19 patients were randomized to intervention arm (22 cases receiving 10,000 IU of vitamin D3 orally for 14 days) and comparator arm that receives no vitamin D3 (n = 20). A stratified analysis based on the sufficient (≥30 ng/mL) and insufficient (<30 ng/mL) baseline vitamin status indicated a significantly increased number of COVID-19 symptoms in the later group (p = 0.03). It was found that the intervention arm had significantly increased vitamin D levels post-treatment and presented fewer symptom severities on the 7th and 14th day of follow-up. The intervention arm also had lesser rates of seropositivity and RT-PCR positivity on the 7th and 14th day, respectively. In a study by Rastogi et al. [24], a similar observation was reported with a significant decrease in the proportion of SARS-CoV-2 RNA negativity in the intervention arm before day-21 (p < 0.01). This study randomized 40 mildly symptomatic or symptomatic COVID-19 patients into intervention and placebo arms of 20 cases each. The intervention arm received 60,000 IU of cholecalciferol daily for 7 days and continued for another 7 days in six cases (who did not achieve a therapeutic target of >50 ng/mL on day 7) and distilled water was supplied to placebo group.
There were two open label RCTs [21,22]. Lakkireddy et al. [22] randomized 130 mild–moderate COVID-19 cases, of which 87 cases who completed the study were analyzed in the intervention (n = 44) and comparator (n = 43) groups. The intervention arm received 60,000 IU of oral vitamin D3 daily for 8–10 days, and the outcomes were recorded till 21 days. Supplementation resulted in a significant increase in vitamin D levels with a lower rate of ICU and mortalities in the intervention arm as compared to the comparator group. In the only trial using calcifediol, Castillo et al. [21] randomized 76 patients into intervention (n = 50) and comparator (n = 26) groups depending on whether or not supplemented with calcifediol. The oral calcifediol supplemented varied at admission (0.532 mg), on days 3 and 7 (0.266 mg), and weekly until ICU/discharge (0.266 mg). This study concludes that vitamin D treatment resulted in significantly less probability of ICU admission, and the statistical significance retained even after adjusting for comorbidities like diabetes and hypertension. However, there is no information available on the baseline and post-treatment vitamin D levels.
In general, it has been demonstrated that vitamin D induce antimicrobial peptides and mediates anti-inflammatory, antiviral, apoptotic, and autophagic activities [6–9,17,30]. The protective immuno-modulatory effects of this fat-soluble steroid vitamin have been reported in respiratory diseases [31,32] including its role in enhancing immune responses to COVID-19 vaccines [19,20]. Studies have proposed vitamin D deficiency as leading candidate in association with COVID-19 susceptibility, severity, and progression [33,34]. However, there is no strong evidence through RCTs on the therapeutic benefits of vitamin D supplementation in COVID-19 outcomes. Our study results based on the available RCTs are suggestive of the overall beneficial effect of vitamin D treatment when all the observations across all RCTs were pooled as an overall effect size. Although no statistically significant differences were observed for ICU care and mortality outcomes individually, the observed RR values are suggestive of decrease in the rate of these outcomes in vitamin D-treated COVID-19 patients. This meta-analysis based on RCTs is first of its kind on the subject, and the results are supportive of vitamin D use in COVID-19. Furthermore, as there is compatible evidence in the form of a meta-analysis of observational studies on the use of vitamin D in COVID-19 [18], the results of this study strongly suggest the need for future/ongoing RCTs to consider better designs, large sample sizes adequate enough to assess the effect of vitamin D supplementation on the individual COVID-19-related outcomes.
However, this study has some limitations. First, the heterogeneity observed in the meta-analysis could be due to methodological, participant and treatment variations of the included trials. While the single-center RCTs have mainly contributed to the heterogeneity, leaving-out a study by Castillo et al. [21] decreased the I2 values from 48% to 5%, 66% to 0%, and 33% to 13% for the overall outcome pooling the results of all RCTs, ICU, and mortality outcomes, respectively. This open label trial differs from all other RCTs as it uses calcifediol in varied concentrations at different time periods of the study. Second, there are only two placebo-controlled trials, one double-blinded study that uses a single high dose of vitamin D. Third, although no significant loss to the follow-up were reported in the RCTs, the proportion of participants and the criteria for sufficient and deficient vitamin D status varied across the trials. Fourth, the variations in the COVID-19 severity, comorbidities proportions and standard care treatment strategies could have influenced the heterogeneity and the overall result. Finally, the difference in the study settings, timings, randomization, blinding, and data collection strategies could have influenced the outcomes. None of the trials reported any adverse events due to vitamin supplementation. As there are only two and three trials respectively in the years 2020 [21,24] and 2021 [22,23,25,26] including small sample sizes, this meta-analysis strongly recommends for more RCTs for better evaluating the role of vitamin D in COVID-19 patients. Therefore, the evidence obtained upon completion of several ongoing trials [35] (CORONAVIT, COVITD-19, COVIDIOL, VIVID, and COVIT-TRIAL) will be crucial in better determination on vitamin D in association with COVID-19.
5. Conclusion
In conclusion, vitamin D use was associated with significant decrease in rates of COVID-19-related events when all the outcomes were pooled across all RCTs. However, there was no significant difference observed for the relative risk of ICU admission and mortality outcomes upon vitamin D supplementation. The overall pooled results in addition to a significant decrease in the rates of RT-PCR positivity observed in this study are suggestive of the possible beneficial effects of vitamin D. These inconclusive results would indicate the need for more RCTs in support of the beneficial effect of vitamin D in COVID-19. Furthermore, it is reasonable to speculate that Vitamin D deficiency could be a proxy of other conditions, as advanced age, BMI, diabetes, liver disease, etc., all known to negatively impact on the outcome of COVID-19. Despite of randomization done in the included trials, these conditions may act as confounders, and hence, the potential benefits of Vitamin D in COVID-19 has to be interpreted with caution and needs to be investigated further in large-scale studies.
Supplementary Material
Acknowledgement
Dr. S.R. Varikasuvu specially acknowledge Bhairavi Sisters (Sahasra and Aagneya) for the time I could not give them during this work, as it was performed before 8 am and after 6 pm from its initiation to completion.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
S.R.V., B.T., A.V., and M.M.: Conceptualization, literature search, data extraction, methodology, analysis, interpretation, writing, supervising, reviewing, and editing. P.D., and S.A.: literature search, assistance in data extraction, analysis, review, and writing. H.R: conduction and verification of literature search, data extraction results, and analysis.
Supplementary Material
Supplemental data for this article can be accessed here.
References
- 1.WHO Coronavirus (COVID-19) Dashboard . WHO Coronavirus (COVID-19) Dashboard With Vaccination Data. n.d.. (cited 2021 Aug 6. https://covid19.who.int
- 2.Farshbafnadi M, Kamali Zonouzi S, Sabahi M, et al. Aging & COVID-19 susceptibility, disease severity, and clinical outcomes: the role of entangled risk factors. Exp Gerontol. 2021;154:111507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watkins RR, Lemonovich TL, Salata RA.. An update on the association of vitamin D deficiency with common infectious diseases. Can J Physiol Pharmacol. 2015;93(5):363–368. [DOI] [PubMed] [Google Scholar]
- 4.Brito DTM, Ribeiro LHC, Daltro CHDC, et al. The possible benefits of vitamin D in COVID-19. Nutrition. 2021;91-92:111356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glinsky GV. Tripartite Combination of Candidate Pandemic Mitigation Agents: vitamin D, Quercetin, and Estradiol Manifest Properties of Medicinal Agents for Targeted Mitigation of the COVID-19 Pandemic Defined by Genomics-Guided Tracing of SARS-CoV-2 Targets in Human Cells. Biomedicines. 2020;8(5):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gois PHF, Ferreira D, Olenski S, et al. Vitamin D and Infectious Diseases: simple Bystander or Contributing Factor? Nutrients. 2017;9(7):651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.AlSafar H, Grant WB, Hijazi R, et al. COVID-19 Disease Severity and Death in Relation to Vitamin D Status among SARS-CoV-2-Positive UAE Residents. Nutrients. 2021;13(5):1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malek Mahdavi A. A brief review of interplay between vitamin D and angiotensin-converting enzyme 2: implications for a potential treatment for COVID-19. Rev Med Virol. 2020;30(5):e2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinheiro MM, Fabbri A, Infante M. Cytokine storm modulation in COVID-19: a proposed role for vitamin D and DPP-4 inhibitor combination therapy (VIDPP-4i). Immunotherapy. 2021;13(9):753–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham BS. Rapid COVID-19 vaccine development. Science. 2020;368(6494):945–946. [DOI] [PubMed] [Google Scholar]
- 11.Orchard L, Baldry M, Nasim-Mohi M, et al. Vitamin-D levels and intensive care unit outcomes of a cohort of critically ill COVID-19 patients. Clin Chem Lab Med. 2021;59(6):1155–1163. [DOI] [PubMed] [Google Scholar]
- 12.Angelidi AM, Belanger MJ, Lorinsky MK, et al. Vitamin D Status Is Associated With In-Hospital Mortality and Mechanical Ventilation: a Cohort of COVID-19 Hospitalized Patients. Mayo Clin Proc. 2021;96(4):875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bychinin MV, Klypa TV, Mandel IA, et al. Low Circulating Vitamin D in Intensive Care Unit-Admitted COVID-19 Patients as a Predictor of Negative Outcomes. J Nutr. 2021;151(8):2199–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Infante M, Buoso A, Pieri M, et al. Low Vitamin D Status at Admission as a Risk Factor for Poor Survival in Hospitalized Patients With COVID-19: an Italian Retrospective Study. J Am Coll Nutr. 2021;1–16. DOI: 10.1080/07315724.2021.1877580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Infante M, Pieri M, Lupisella S, et al. Low testosterone levels and high estradiol to testosterone ratio are associated with hyperinflammatory state and mortality in hospitalized men with COVID-19. Eur Rev Med Pharmacol Sci. 2021;25(19):5889–5903. [DOI] [PubMed] [Google Scholar]
- 16.Campi I, Gennari L, Merlotti D, et al. Vitamin D and COVID-19 severity and related mortality: a prospective study in Italy. BMC Infect Dis. 2021;21(1):566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mercola J, Grant WB, Wagner CL. Evidence Regarding Vitamin D and Risk of COVID-19 and Its Severity. Nutrients. 2020;12(11):3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrelli F, Luciani A, Perego G, et al. Therapeutic and prognostic role of vitamin D for COVID-19 infection: a systematic review and meta-analysis of 43 observational studies. J Steroid Biochem Mol Biol. 2021;211:105883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Velikova T, Fabbri A, Infante M. The role of vitamin D as a potential adjuvant for COVID-19 vaccines. Eur Rev Med Pharmacol Sci. 2021;25(17):5323–5327. [DOI] [PubMed] [Google Scholar]
- 20.Chiu SK, Tsai KW, Wu CC, et al. Putative Role of Vitamin D for COVID-19 Vaccination. Int J Mol Sci. 2021;22(16):8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castillo EM, Entrenas Costa LM, Vaquero Barrios JM, et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203:105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lakkireddy M, Gadiga SG, Malathi RD, et al. Impact of daily high dose oral vitamin D therapy on the inflammatory markers in patients with COVID 19 disease. Sci Rep. 2021;11(1):10641. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Murai IH, Fernandes AL, Sales LP, et al. Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients With Moderate to Severe COVID-19: a Randomized Clinical Trial. JAMA. 2021;325(11):1053–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rastogi A, Bhansali A, and Khare N, et al. Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study). Postgrad Med J. 2020;98:87–90. [DOI] [PubMed] [Google Scholar]
- 25.Sabico S, Enani MA, Sheshah E, et al. Effects of a 2-Week 5000 IU versus 1000 IU Vitamin D3 Supplementation on Recovery of Symptoms in Patients with Mild to Moderate Covid-19: a Randomized Clinical Trial. Nutrients. 2021;13(7):2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sánchez-Zuno GA, González-Estevez G, Matuz-Flores MG, et al. Vitamin D Levels in COVID-19 Outpatients from Western Mexico: clinical Correlation and Effect of Its Supplementation. J Clin Med. 2021;10(11):2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, et al., PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 29.Turner RM, Davey J, Clarke MJ, et al. Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int J Epidemiol. 2012;41(3):818–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teymoori-Rad M, Shokri F, Salimi V, et al. The interplay between vitamin D and viral infections. Rev Med Virol. 2019;29(2):e2032. [DOI] [PubMed] [Google Scholar]
- 31.Jolliffe DA, Camargo CA Jr, Sluyter JD, et al. Vitamin D supplementation to prevent acute respiratory infections: a systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021;9(5):276–292. [DOI] [PubMed] [Google Scholar]
- 32.Lips P. Vitamin D to prevent acute respiratory infections. Lancet Diabetes Endocrinol. 2021;9(5):249–251. [DOI] [PubMed] [Google Scholar]
- 33.Menshawey E, Menshawey R, Nabeh OA. Shedding light on vitamin D: the shared mechanistic and pathophysiological role between hypovitaminosis D and COVID-19 risk factors and complications. Inflammopharmacology. 2021;29(4):1017–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar R, Rathi H, Haq A, et al. Putative roles of vitamin D in modulating immune response and immunopathology associated with COVID-19. Virus Res. 2021;292:198235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raisi-Estabragh Z, Martineau AR, Curtis EM, et al. Vitamin D and coronavirus disease 2019 (COVID-19): rapid evidence review. Aging Clin Exp Res. 2021;33(7):2031–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


