Abstract
Aryl hydrocarbon receptor (AHR) was initially discovered as a cellular protein involved in mediating the detoxification of xenobiotic compounds. Extensive research in the past two decades has identified several families of physiological ligands and uncovered important functions of AHR in normal development and homeostasis. Deficiency in AHR expression disrupts major signaling systems and transcriptional programs, which appear to be responsible for the development of numerous developmental abnormalities including cardiac hypertrophy and epidermal hyperplasia. This mini review primarily summarizes recent advances in our understanding of AHR functions in normal physiology with an emphasis on the cardiovascular, gastrointestinal, integumentary, nervous, and immunomodulatory systems.
Keywords: Aryl hydrocarbon receptor, physiology, endogenous ligands, review
1. Introduction
Aryl hydrocarbon receptor (AHR) was originally named for its function in the metabolism of xenobiotics, in particular, compounds with aromatic hydrocarbons [1]. In 1976, a study by Poland et al., demonstrated that 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) bound to a cellular molecule with high affinity in mouse liver cells, which was a key step leading to the determination of hepatic absorption of the compound [2]. Subsequent studies revealed that AHR is evolutionarily conserved in both its domain structures and functions, expressed in various tissues, and plays a variety of roles in homeostasis [3]. After ligand binding, AHR is translocated from the cytoplasm into the nucleus where it associates with AHR nuclear translocator (ARNT), the complex of which subsequently binds to xenobiotic responsive element (XRE) [4]. The AHR/ARNT complex functions as a transcription factor responsible for expression of genes belonging to the cytochrome P450 family and in particular of CYP1A1, CYP1A2 and CYP1B1 [5].
Early studies on AHR were primarily focused on the toxicological aspect due to its activation by dioxins, which are environmental toxicants [6, 7]. However, extensive studies in the past two decades have identified many endogenous AHR ligands and uncovered numerous physiological functions of the receptor [8]. Significantly, mouse genetic studies have revealed that AHR-deficiency causes cardiac hypertrophy and epidermal hyperplasia among other abnormalities, underscoring that AHR is important for normal development in vivo [9, 10]. This concise review attempts to summarize major advances in the regulation of physiological functions by AHR with an emphasis on its roles in cardiovascular, gastrointestinal, nervous, immune, and circadian systems.
2. Basic Biology of AHR
2.1.1. AHR structures
AHR belongs to the family of basic helix-loop-helix (bHLH)/PAS proteins. Its primary structure can be divided into three distinct domains: namely, an N-terminal bHLH domain, Per-ARNT-Sim (PAS) domains (A and B), and a C-terminal transactivation domain (TAD) [11, 12] (Figure 1). Although three-dimensional structure of AHR remains unavailable, the crystal structure of AHR-ARNT-XRE complex is determined [13]. Based on the analysis of the complex structure, as well as on study of homologous bHLH-PAS family proteins, it is proposed that the stability of heterodimerization between AHR and ARNT and the interaction of AHR interdomains are mainly controlled by bHLH and PAS domains [14].
Figure 1.

Domain structure of Aryl hydrocarbon receptor. AHR consists of three major domains including bHLH, PAS (A&B) and TAD.
The AHR activation axis involves ligand association, nuclear translocation, and binding to the canonical xenobiotic response element (XRE) of target genes [15]. PAS-B domain, a conserved ligand binding pocket, functions to sense xenobiotic signals [16]. Mutational studies demonstrate that mouse residues of Ala375, His285, and Gln377 play a key role in ligand binding [17]. Different from PAS-B, PAS-A domain primarily controls the specificity and stability of heterodimerization with ARNT. Biochemical and deletional studies indicate that an α-helical structure at the N-terminus forms an indispensable dimer interface, as well as hydrophobic contacts with residues in opposing PAS-A domain, to maintain the stability between AHR and ARNT [18]. The bHLH domain specifically recognizes and interacts with the XRE consensus sequence (TTGCGTG) with two α-helices at the N-terminus and a flexible connector [13] [19]. Human AHR residues His39, Ser36, and Arg40 in the helices play a major role in forming phosphate and/or hydrogen bonds with corresponding nucleotide bases on the target DNA strand [20].
2.1.2. AHR ligands
TCDD, a byproduct of Agent Orange, was identified as an AHR agonist, largely because of chloracne, a skin disease it caused [21]. Subsequent analyses revealed that other xenobiotic compounds with structures similar to TCDD, especially those with halogenated aromatic (HAs) and polynuclear aromatic hydrocarbons, are also efficient AHR agonists although their binding affinity to AHR pocket and transactivating capacity may substantially differ [22].
Physiological functions of AHR were largely ignored until the characterization of AHR-deficient mice, as well as the identification of many endogenous/physiological ligands [8, 9]. Among endogenous ligands of AHR are a series of metabolic products of tryptophan [23]. Metabolism of commensal microorganisms and the host and photo-oxidation are the primary processes for the generation of tryptophan derivatives including indirubin, kynurenine and 6-formylindolo[3,2-b] carbazole (FICZ) [24]. Three permeases (Mtr, AroP, and TnaB) transport tryptophan into bacteria where tryptophanase converts tryptophan to indole and pyruvate [25]. Indole subsequently enters liver by passive diffusion or with the help of AcrEF-TolC and Mtr transporters where cytochrome CYP2E1 catalyzes indole hydroxylation to generate indirubin [26]. In hepatocyte and dendritic cells, tryptophan is primarily metabolized to N′-formylkynurenine by rate-limiting enzymes indoleamine 2,3-dioxygenase (IDO) and tryptophan 2,3-dioxygenase (TDO) [27]. N′-formylkynurenine is later hydrolyzed to kynurenine by NFK formamidase, which regulates the AHR-mediated transcription [27]. Furthermore, UV irradiation or visible light catabolizes tryptophan into indole-3-acetaldehyde, which is subsequently rearranged into FICZ [28]. Of note, the FICZ-mediated AHR activation is transient due to efficient removal catalyzed by CYP isozymes and is a part of the negative feedback loop [29].
Besides tryptophan metabolites, the endogenous ligands of AHR are produced with various metabolic pathways. Lumichrome, a riboflavin metabolite, is identified as an endogenous AHR ligands in rat as early as 1980s [30]. Additionally, heme degradation products bilirubin and its metabolic precursor biliverdin are both recognized as AHR endogenous ligands to directly active AHR transformation and CYP isozymes transcription [31]. In hyperhomocysteinemia (HHcy) mouse model, lipoxin A4 (a metabolite of arachidonic acid) is found to markedly increase AHR activity and up-regulate CD36 expression [32]. These metabolites along with tryptophan metabolites, have an important impact on the homeostasis through regulating AHR activity.
2.1.3. AHR signaling
AHR activation and its downstream signaling transduction consist of both canonical and non-canonical pathways [33]. In the canonical pathway, AHR forms complexes with molecular chaperons and is kept inactive in the cytosol [34] (Figure 2). Among molecular chaperones are heat shock protein 90 (Hsp90), AHR-interacting protein (AIP /XAP2/ARA9), p23 [35] [36]. Upon ligand binding, AHR undergoes a conformational change, and the AHR/ligand complex is released from chaperone proteins; AHR is subsequently translocated into the nucleus where AHR heterodimerizes with ARNT; AHR/ARNT binds to XREs of target genes, regulating their expression. AHR target genes include those encoding cytochrome P450 superfamily enzymes (Cyp1a1, Cyp1a2 and Cyp1b1) and AHR repressor (AHRR), which play important roles in detoxication of environmental carcinogens and negative regulation of AHR-dependent genes, respectively [37–39].
Figure 2.
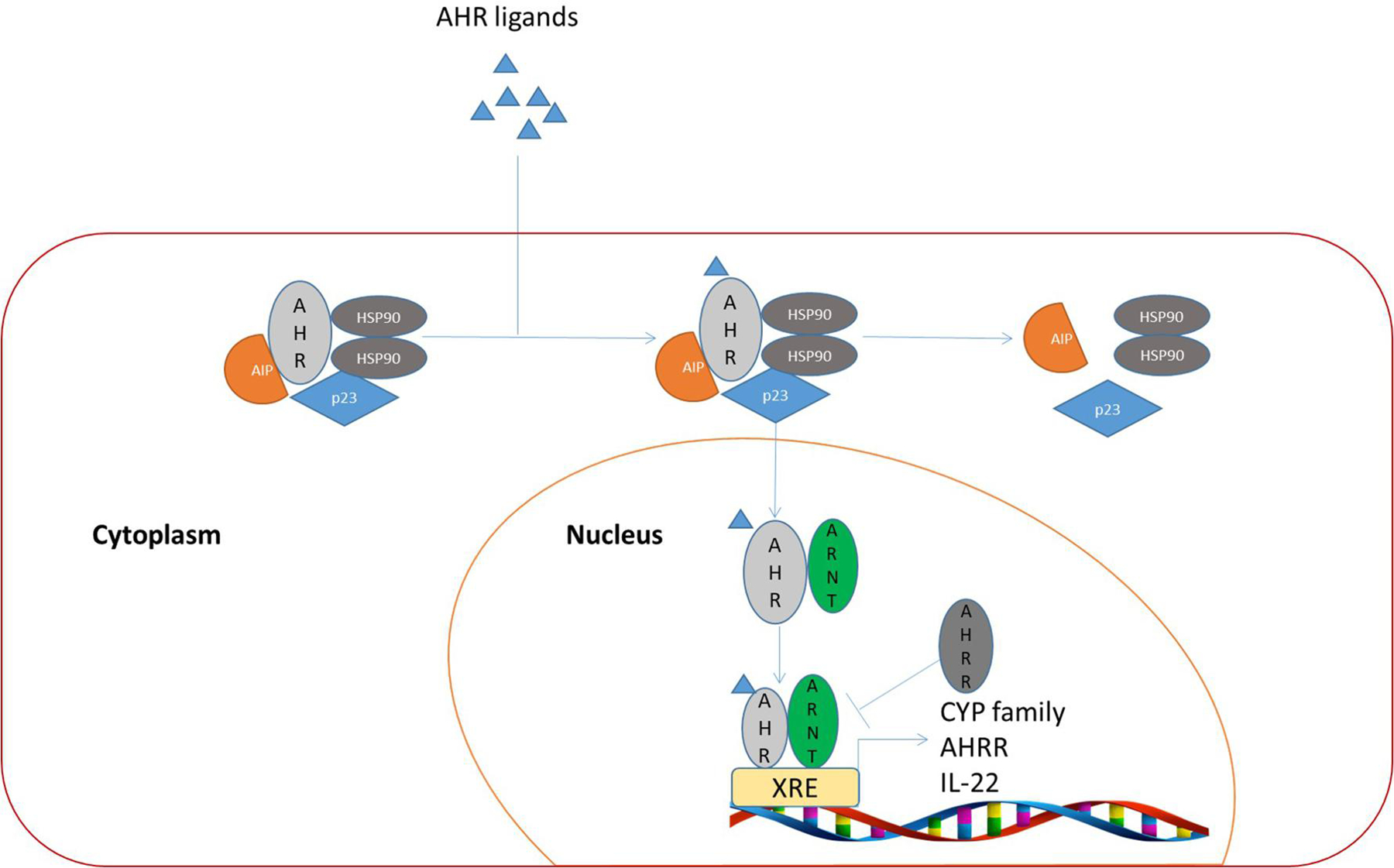
AHR canonical pathway. After ligand binding, chaperon proteins dissociate from the AHR/chaperon complex in the cytoplasm, leading the translocation of ligand-bound AHR into the nucleus. AHR/ligand heterodimerizes with ARNT in the nucleus, where they bind to XRE cis-elements of target genes, leading to their transactivation.
In the non-canonical signaling pathway, activated AHR forms complexes with different transcription factors in the nucleus, leading to its binding to non-XRE DNA elements to regulate expression of target genes [40]. For example, estrogen receptor (ER) has been shown to interact with the AHR/ARNT complex, which subsequently binds to DNA elements in the absence of an ER ligand [41]. Several lines of evidence indicated that Src (a tyrosine kinase) dissociates from the AHR complex after AHR activation to positively regulate MAPK and FAK, leading to enhanced cell migration and inflammation [37, 42]. It has been also shown that KLF6, a tumor suppressor, interacts with AHR, resulting in its recruitment to a novel DNA sequence [43]. Moreover, increasing evidence suggests that AHR regulates NF-κB activity directly via the association with RELA or RELB, or indirectly via suppressing SOX2 [44–46]. Given that promoters of a majority of AHR-regulated genes bear no identifiable XRE [47], it is considered that AHR may team up with many other nuclear factors, regulating gene expression following exposure to various exogenous and endogenous ligands.
3. AHR in Cardiovascular System
3.1.1. AHR in cardiac function and development
It has been shown that dioxins induce AHR activation in cardiomyocyte progenitors, exert adverse effects on cardiogenesis and cardiomyocyte differentiation, and cause cardiac fibrosis [48]. At the molecular level, cardiac fibrosis induced by dioxins is mediated at least in part by perturbation of the WNT signaling pathway and downregulation of various homeobox transcription factors [48]. AHR knockout mice display abnormalities including cardiac hypertrophy, reduced cardiac ejection fraction, and AHR-independent high blood pressure after treatment with TCDD [49], suggesting that dioxins may cause cardiomyopathy and cardiac dysfunction due to toxicity. Although cardiac AHR expression is low in both fetal and adult tissues its activation remains important in cardiac development and function [50]. It has been shown that during the embryonic development, cardiogenesis is sensitive to AHR expression [51]. Supporting this notion, AHR knockdown significantly inhibits differentiation toward the cardiomyocyte lineage [52]. Moreover, in AHR-silenced P19 mouse embryonic carcinoma cells, expression of GATA4 and Nkx2.5, two early biomarkers of precardiac cells, is increased that facilitates cardiac development in committed precursors. This observation strongly suggests that AHR plays an important role in cardiac stem cell differentiation [53, 54].
In adult mice, systolic and diastolic volumes are higher than those of wild-type mice after ablating both AHR alleles, and male mice appear to be more sensitive to Cre recombinase-mediated AHR ablation [55]. In addition, cardiac output, ejection fraction, and stroke volume in AHR-deficient mice are all different from those of wild-type mice [49, 51]. It has also been shown that cardiac hypertrophy is regarded as a severe symptom caused by the disorder of vasoactive peptide endothelin-1 (ET-1) signaling in AHR-null mice [56]. ET-1, one of the strongest vasoconstrictors, increases about 10 folds in plasma in the absence of AHR, leading to hyper-activation of ETA receptor and elevation of reactive oxygen species (ROS) [57]. In the meantime, elevated angiotensin II due to the absence of AHR acts synergistically with ET-1 to keep the mean arterial pressure high [58]. In addition to increased ROS and high blood pressure, hypertrophic growth of the heart in AHR-null mice is associated with the deficiency of Vav3, a Rho/Rac GTPases activator [59]. It has been reported that low expression of Vav3 can lead to excess catecholamine levels and overactive renin-angiotensin system, thus disrupting cardiovascular homeostasis. [60] In summary, AHR is important to maintain normal cardiac functions including the development and differentiation of cardiomyocyte progenitors, as well as the prevention of cardiac hypertrophy.
3.1.2. AHR in angiogenesis
Recent studies indicate that AHR displays differential effects between species in angiogenesis and vascular cell development [61]. Aberrations in kidney vascular structures, immature hepatic sinusoids, and hyaloid arteries in the eyes are observed in AHR-null mice, while adult AHR-KO rats do not exhibit ductus venosus and hyaloid arteries comparing to wild-type rats. [62, 63]. Treatment with an AHR agonist (3-methylcholanthrene) induces a dose-dependent reduction of cell adhesion, which is associated with decrease in phosphorylated FAK in human umbilical vascular endothelial cells [64]. Consistent with this observation, alpha-naphthoflavone, an AHR antagonist, reverses the effect of 3-methylcholanthrene, strongly implicating that angiogenesis is mediated by AHR [65]. In AHR-null mice, ischemia-induced angiogenesis is enhanced, which is coupled with high levels of HIF-1α and ARNT [66]. Combined, these observations suggest that AHR may compete with HIF-1α for ARNT, thereby reducing active HIF-1α/ARNT heterodimer at the promoters of target genes, leading to downregulation of genes encoding proteins including VEGF with a significant role in angiogenesis [66, 67].
4. AHR in the Gastrointestinal System
4.1.1. AHR and intestinal barrier function
The contiguous layer of the intestinal epithelium not only supports the transport of water, electrolytes, and nutrients, but also acts as a barrier that otherwise is constantly exposed to external substances including numerous xenobiotic compounds and AHR dietary ligands [68]. It has been shown that AHR ligands that enter the intestinal track play an important role in dampening local gut inflammation and protecting intestinal barrier integrity in an AHR-dependent manner [69]. It is suggested that AHR affects the barrier function by controlling cell permeability and muscle contraction via regulating tight-junction proteins and myosin kinase [70]. In the colitis mouse model induced by dextran sodium sulphate, AHR ablation leads to susceptibility to bacterial infection due to disruption of tight junction [71] (Figure 3). Claudin-2, a biomarker of leaky gut barrier, is highly inducible in AHR-null cells by IL-6 compared with that of wild-type cells [72]. Consistent with this, it has been observed that ZO-1, a tight-junction protein, is absent in gut epithelium when AHR activity is low, leading to an epithelial barrier dysfunction [73]. Moreover, Par-6 in the Par complex (Par-6/Par-3/aPKC) is over-expressed in inflammatory bowel disease, causing leaky junctions [74]. Moreover, Par-6 overexpression is repressed by AHR activation through repression of activating protein-2γ [74, 75]. When AHR is activated by dietary ligands or FICZ, the epithelial barrier injury is significantly ameliorated, which is associated with suppression of Claudin-2 and its downstream signal component CDX-2 and HNF-1α [72]. Furthermore, in cells with FICZ-induced AHR activation, ZO-1 expression is restored to facilitate tight-junctions [73], whereas myosin light chain phosphorylation and NF-κB p65 signaling are suppressed [71]. In summary, AHR is essential for intestinal homeostasis and the absence of AHR disturbs the epithelial barrier integrity, leading to inflammation.
Figure 3.

AHR ablation impairs integrity of the intestinal barrier through multiple steps: (1) Inhibition of goblet cell differentiation by downregulation of Muc2 and Car4, 2; (2) Suppression of ISC differentiation by reduction of Rnf43 and Znf43; (3) Disruption of tight junction due to comprised expression of multiple tight junction proteins.
In addition to its role in mediating tight junction of epithelial cells, AHR is believed to regulate goblet cells in the protection of the intestinal barrier. Goblet cells are known to protect mucous membranes from disruption by a variety of enteric pathogens by producing and secreting mucins [76]. In the colitis animal model, not only the number of goblet cells is significantly decreased but also the amount of mucin-2 secreted from goblet cells is reduced in the absence of AHR; Consistent with its role in positively regulating goblet cells, AHR activation by FICZ promotes goblet cell differentiation and enhances mucin-2 expression, leading to restoration of the intestinal barrier and alleviation of infection [77].
It is suggested that the intestinal barrier homeostasis is regulated by AHR through inhibition of intestinal stem cell (ISC) proliferation and repression of Wnt signaling pathway [78, 79]. AHR ablation causes uncontrolled ISC proliferation and even transformation, which is coupled with significantly reduced differentiation [78] (Figure 3). Decreased expression of Muc2 and Car4, the latter being an enterocyte marker, indicates that impaired differentiation of ISCs reduces the number of goblet cells and enterocytes that are essential for maintaining proper intestinal barrier functions [78, 80]. Consistently, AHR activation curtails ISC proliferation and restores ISC differentiation through regulation of ubiquitin E3 ligases, Rnf43 and Znrf3 [78, 81]. Both Rnf43 and Znrf3 suppress Wnt-β-catenin pathway overactivation by targeting Wnt receptor for a R-spondin-sensitive degradation, thus restoring epithelial cell differentiation and preventing potential tumorigenesis [78].
4.1.2. Effect of AHR in regulating gut microbiota
Commensal microbiota is known to be essential for the maintenance of host’s metabolism, immunity, and homeostasis [82]. Extensive research in the past has shown that AHR plays an important role in the communication with microbiota and regulation of host metabolic homeostasis in the intestine [83] albeit the specific mechanism underlying the AHR effect in this process remains unclear. It is well known that certain strains of commensal microbiota generate metabolites that are AHR agonists [84]. For example, Enterobacteriaceae and Lactobacillus produce phenazines and indole-3-aldehyde, respectively [85]. Germ-free mice display low levels of expression of IDO1, AHRs, and cytochrome P450 genes in liver compared with mice carrying normal microbiota [86]. In contrast, administration of short-chain fatty acids derived from microbiota-driven anaerobic fermentation restores the expression of AHR target genes to normal levels and the process appears to be mediated via G protein-coupled receptor transduction [86, 87]. Moreover, it has been reported that AHR activation affects the microbiome composition and functions but not the diversity, which in turn affects the amount of microbiota-derived metabolic compounds (ligands) that are capable of activating AHR [88].
Both preclinical and clinical studies reveal that the role of AHR is important in the maintenance of energy homeostasis. The composition of intestinal microbiota is very different between wild-type mice following a normal diet and those with disrupted AHR functions which is achieved either by feeding wild-type mice an AHR ligand-free diet or by knocking out the AHR gene [86, 88, 89]. It has been shown that there is a higher prevalence of Bacteroidetes, Proteobacteria, Actinobacteria, Tenericutes, Erysipelotrichaceae, and Clostridia in mice on AHR ligand-free diet than in those on the conventional diet [86, 88, 89]. The change in the microbiota composition due to the absence of dietary AHR ligands can further decrease metabolic components catalyzed by certain microbial populations and these microbial-derived compounds otherwise bind to AHR and function as its activators [88, 90]. Such a vicious cycle can lead to impairments of microbial homeostasis and severe metabolic disorders including liver steatosis [91]. Moreover, through studying AHR knockout mice, it has been shown that deficiency in AHR activation impairs fatty acid oxidation as the synthesis of 3-hydroxybutyrate significantly decreases with a concomitant increase in gluconeogenesis and elevated lactate and alanine in blood [86]. Consistent with the role of AHR in metabolic homeostasis, it has been shown that supplementation of FICZ or AHR ligand-producing microbiota alleviates the metabolic stress via upregulation GLP-1 expression [89]. Clinical data are also available to support the notion that AHR is important for metabolic homeostasis. It has been reported that patients with metabolic syndrome associated with obesity, diabetes, and hypertension have fewer AHR ligands in fecal samples than those of healthy individuals [89, 92].
AHR activities can also positively influence commensal microbiota [93]. Down-regulation of AHR and its downstream components including IL-22 and p-Stat3 is associated with decreased host antimicrobials, which is proposed to alleviate the antibiotic-associated intestinal mucosa injury. Supporting this notion, exogenous activation of the AHR/IL-22/Stat3 signaling pathway by FICZ is capable to restore the level of host antimicrobial molecules following reconstitution of intestinal microbiota by fecal microbiota transplantation [94]. In addition, it has been reported that AHR stimulated by indole-3-aldehyde upregulates IL-22, which in turn supports commensal microbial balance and protects mucosa from inflammation [84]. Combined, these studies underscore the role of AHR and its signaling axis in modulating the production of intestinal antimicrobial molecules and maintaining proper mucosal functions.
5. AHR in the Integumentary System
Skin functions as the first line of defense against injuries and microbial infections and it is composed of dermis and epidermis [95]. AHR plays an important role in the skin barrier formation and wound healing [96]. In AHR knockout mice, the dorsal skin displays morphological defects with a ragged and delaminated appearance, and it is prone to bacterial infection due to impaired barrier functions [9]. Treatment with AHR antagonist(s) can lead to compromised epidermal differentiation of keratinocytes and reduction of formation of the cornified layer [97]. In a murine model, FICZ enhances skin barrier functions by increasing expression of filaggrin (FLG) and loricrin through the AHR-OVOL1 pathway and reduces the skin inflammation [98, 99]. On the other hand, prolonged AHR activation can be detrimental to the skin [100]. For example, although TCDD, a potent exogenous AHR ligand, enhances expression of filaggrin, keratin, and keratinocyte proline-rich protein to accelerate epidermal maturation it can also cause epidermal hyperplasia and the hyperkeratotic skin disorder with severe inflammation with high levels of IL-1β expression [101–103].
Several studies demonstrate that AHR is involved in normal skin formation during fetal development and wound healing in the adulthood. In TCDD-treated mice at embryonic day 16 (E16), as well as in AHR knockout fetuses, premature expression of filaggrin was detected [104], suggesting that AHR modulates skin terminal differentiation in fetal mice. It remains unclear whether AHR activation affects wound healing in adults. Previous studies reported that a loss of cell-cell contact stimulates AHR signaling by dissociating Src, which in turn regulates the MAPK signaling pathway [105]. Topical treatment with tryptophan improves wound healing, an effect that potentially involves the AHR pathway. From a clinical point of view, this treatment benefits patients with leg ulcers; the metabolites of tryptophan including known AHR ligands appear to promote wound-healing via an AHR-independent pathway [106]. Likewise, topical application of an AHR agonist accelerates wound-healing although the mechanism of the wound-healing seems independent of the AHR pathway [105]. Moreover, indirubin is capable of promoting migration of keratinocytes and enhancing wound-healing in both in vitro and in vivo models [107]. Intriguingly, enhancement of wound-healing by indirubin is diminished by PXR inhibition but not by AHR inhibition, suggesting that the indirubin-pregnane X receptor/JNK axis, but not the indirubin/AHR signaling axis, plays a primary role in mediating wound-healing given the known function of indirubin on pregnane X receptor (PXR) [107]. In support of this hypothesis is the fact that, accelerated wound healing is also observed following FICZ treatment. Furthermore, AHR inhibition by its antagonist (CH-223191) did not reduce the FICZ-induced wound-healing [108]. In contrast to indirubin, FICZ accelerates keratinocytes migration via activation of ERK but not JNK [108]. Furthermore, compared with the controls AHR-null mice do not exhibit a significant difference on the total wound-healing time [109]. Taken together, it appears that AHR is involved in wound healing but does not play a major role in the process.
6. AHR in the Nervous System
AHR mRNA expression is widely distributed in various nervous tissues with its high level of expression in cerebellum, cortex, and hippocampus [110]. During embryonic development and early infancy, AHR expression is detectable in hippocampal stem cells, retinal ganglion cells and nerve myelin sheath [111]. The function of AHR is highly conserved in neurodevelopment in invertebrates [112]. In Caenorhabditis elegans, AHR-1, the AHR ortholog, controls the fate determination of GABAergic neurons, as well as the development, orientation, and axonal migration [113]. In D. melanogaster, Spineless, the AHR ortholog, controls movement and dendrite diversity in dendritic arborization (da) sensory neurons [112, 114]. Deficiency in spineless expression robustly improves the movement by 25% and distorts the original morphologies of different classes da neurons [112, 114]. AHR knockout mice exhibit a visual-information-processing defect due to downregulation of myelin-associated glycoprotein, as well as an alteration in optic nerve myelin sheath [115]. Although AHR does not appear to directly bind to myelin gene promoter, AHR inhibition stimulates Wnt/β-catenin signaling and promotes the binding of β-catenin to the myelin gene promoter [116].
Deficiency in AHR expression delays the maturation of hippocampal neuronal synapses in newborns, leading to impaired hippocampus-dependent memory and learning functions [117]. It remains controversial the exact mechanism of hippocampal functions regulated by AHR. One possibility is that AHR cross-talks with Wnt/β-catenin, which in turn regulates neurogenesis through modulating neuron-specific transcription factors such as neurogenin-2, doublecortin, and NeuN [118]. On the other hand, it has been shown that AHR deficiency does not impair neurogenesis whereas it compromises hippocampus-dependent memory due to aberrant dendritic arborization and an increased spine density [119]. Despite of the discrepancy, it is generally agreed that AHR is a possible drug target for the management and treatment of cognitive disorders [119].
The blood–brain barrier is composed of brain capillary endothelium that controls the exchange of xenobiotics between the brain parenchyma and the systemic circulation to maintain homeostasis in the central nervous system (CNS) [120]. Recently, it has been shown that AHR controls the absorption, accumulation, distribution and excretion of xenobiotics, especially therapeutic drugs in the brain by interacting with efflux transporter proteins that are expressed in the apical surface of capillary endothelial cells [121]. Toxicological studies shows that expression of P-glycoprotein, also called multidrug resistance protein 1 (MDR1), is increased by TCDD-mediated AHR activation, leading to an increased transport activity coupled with reduced accumulation of therapeutic drugs in CNS [121]. Besides, AHR is involved in the increase of MDR1 expression after treatment with histone deacetylases (HDAC) inhibitors [122]. AHR agonist synergizes withe HDAC inhibitors in upregulation of MDR1 whereas AHR inhibitor attenuates this induction; Subsequent studies reveal that HDAC inhibitors facilitate the binding of AHR to XRE of the MDR1 gene promoter in brain capillary endothelial cells [122].
7. Roles of AHR in the Immune System
7.1.1. AHR/IDO1/kynurenine loop
The AHR/IDO1/kynurenine regulatory loop starts from tryptophan, an essential amino acid, whose degradation accounts for 95% of kynurenine production in the body [123]. Indoleamine 2,3-dioxygenase-1 (IDO1) is a rate-limiting enzyme for tryptophan metabolism [124]. IDO1 first converts tryptophan into N-formylkynurenine and then to kynurenine with the participation of formamidase [125]. Kynurenine is capable of binding to and activating AHR, thus functioning as an endogenous ligand [126]. Moreover, active AHR induces pro-inflammatory IL-6 expression [127, 128]. It has been observed that inhibition of IL-6 or STAT3 expression/activity reduces IDO1 expression [127, 129]. Therefore, IDO1 activity sustains its own expression via an autocrine AHR-IL-6/STAT3 signaling loop [127, 130]. It has also been shown that both IDO1 enzymatic activity and IFN-γ-induced AHR expression are required for sustained IDO1 expression [131]. Although IFN-γ-induced IDO1 is transient IDO1 expression has a long-term maintenance effect on switching dendritic cells (DCs) from immunogenic to tolerogenic [132]. Moreover, addition of kynurenine to DC cultures in which IDO1 activity has been blocked can restore long-term IDO1 expression in wild-type but not in AHR-deficient DCs, indicating the central role of AhR/kynurenine pathway in the maintenance of IDO1 expression [132]. It has been proposed that the IDO1-AHR-IL-6-STAT3 signaling loop should be thoroughly investigated as a potential therapeutic target for human malignancies [133].
At the cellular level, the immuno-modulatory role of the AHR/IDO1/kynurenine loop appears to be mediated through regulation of DC functions and naive T cell differentiation [134]. In DCs, AHR activated by kynurenine regulates differentiation of naive T cells into regulatory T (Treg) cells but not IL-17–producing helper T (Th17) cells [135]. AHR-deficient DCs derived from bone marrow decrease the production of both kynurenine and IL-10, leading to promotion of naive T-cell proliferation, generation of Th17 cells, and blockage of naïve T cell differentiation into Treg cells [136]. In contrast, AHR activation increases production of Treg cells, which plays an important role in immunologic tolerance, that is essential for the maintenance of feto-maternal interface during pregnancy [137].
7.1.2. Cytokine expression mediated by AHR
T cells are required for adaptive immune response [138]. Upon stimulation by specific cytokines, naïve CD4+ cells differentiate into various subtypes, including Th (T helper)1, Th2, Th17 and Treg (regulatory T cells) [139]. Th1 is mainly for cell-mediated response while Th2 oversees B cells activation [140]. Th17 is responsible for host-defense combating bacterial infections [141] and Treg mediates immunosuppression for peripheral tolerance [142]. Recent studies show that AHR is also involved in regulating differentiation of various T cell subtypes and modulating cytokine homeostasis. It has been shown that cytokines (e.g., IL-4 and IL-5) secreted by Th2 cells are negatively regulated by AHR whereas cytokines secreted by Th1 (e.g., INF-γ) are positively regulated by AHR [143]. Intriguingly, it has also been shown that AHR can indirectly promote a wide range of cytokines production to modulate pathogen defense and autoimmunity, as well as immunosuppression and tolerance [144]. Secretion of IL-17A and IL-22 by Th17 cells is enhanced by AHR, which is mediated by promoting Th17 cell differentiation and Aiolos expression that in turn suppresses IL-2 [145–147]. Conversely, Th17 cells with AHR deficiency or treated with an AHR antagonist are unable to produce and secret normal levels of IL-17A and IL-22, suggesting that AHR may be recruited to promoters of these cytokines in Th17 cells and transactivate expression of these genes [148]. Moreover, Previous studies suggest that AHR-mediated IL-10 production is induced in type-1 regulatory T (Treg1) cells to restrict T cell responses [149]. In support of this hypothesis is the fact that, IL-27/STAT3/AHR axis stimulates Treg1 cell differentiation and promotes expression of IL-10 and IL-21 [150, 151]. Furthermore, Th17 cells are capable of trans-differentiation into Treg cells during immune responses and in the presence of TGF-β1, AHR activation is capable of promoting this conversion [152].
8. Roles of AHR in Circadian Rhythm
In the animal kingdom, animals in a higher evolutionary hierarchy usually synchronize their circadian activity largely to the light and dark cycles of the nature. Extensive research in the past reveals that ocular photoreception relays signals to the suprachiasmatic nucleus (SCN) in the hypothalamus. Proteins with a Per-Arnt-Sim (PAS) domain are known to play an important role in controlling circadian rhythm, among which CLOCK and BMAL1 are the two best characterized proteins [153]. CLOCK and BMAL1 form a heterodimeric transcription complex, regulating expression of key circadian genes including CRY1 and CRY2 and PER1, PER2, and PER3 genes. CRY and PER gene products can dimerize as transcriptional repressors, negatively controlling CLOCK/BMAL1 activities in the nucleus and participating in a negative feedback loop controlling the circadian clock [154].
Because AHR shares a PAS domain with circadian regulatory proteins it is postulated that AHR may physically interact with circadian proteins such as CLOCK and BMAL1, thus playing a role in regulating normal circadian rhythm [155]. It is well established that the AHR expression pattern in SCN alters during the day following the circadian rhythm, peaking at the time of lights-off, and that the CLOCK/Bmal1/Ebox complex is involved in the regulation [156]. Moreover, it has been reported that FICZ, derived from tryptophan after exposure to light, disrupts the circadian rhythm via the formation of AHR/BMAL1 heterodimer, which down-regulates PER1 [157]. In case of lack of a feedback control by PER1, the normal circadian rhythm in the human body is dysfunctional, a condition that increases the risk of developing insomnia, metabolic syndrome, and cardiovascular diseases [158].
9. Summary
Although AHR was initially characterized as a cellular receptor mediating toxicological responses to xenobiotic compounds it plays essential roles in development, normal organ functions, and metabolic homeostasis in human. AHR deficiency or dysfunctional regulation underlies pathophysiology of various disease states including circadian rhythm disturbances, myocardial hypertrophy, and intestinal barrier disruption. Significantly, AHR exerts a major influence in modulating a wide spectrum of cytokine expression and immune cell differentiation and functions. On the other hand, it is important to bear in mind that many functional studies are based on rodent models. Given known physiological differences between species, many findings from the animal models cannot be directly extrapolated into human conditions. Therefore, additional studies are necessary to elucidate new roles of AHR in human physiology.
Acknowledgement
We thank Dr. Byeong Hyeok Choi and other lab members for various discussions during the course of this work. This work was supported in part by NIEHS Center grant (ES000260).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Stevens EA, Mezrich JD, and Bradfield CA, The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology, 2009. 127(3): p. 299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poland A, Glover E, and Kende AS, Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J Biol Chem, 1976. 251(16): p. 4936–46. [PubMed] [Google Scholar]
- 3.Tian J, et al. , The Aryl Hydrocarbon Receptor: A Key Bridging Molecule of External and Internal Chemical Signals. Environ Sci Technol, 2015. 49(16): p. 9518–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ikuta T, et al. , Nucleocytoplasmic shuttling of the aryl hydrocarbon receptor. J Biochem, 2000. 127(3): p. 503–9. [DOI] [PubMed] [Google Scholar]
- 5.Fujii-Kuriyama Y and Mimura J, Molecular mechanisms of AhR functions in the regulation of cytochrome P450 genes. Biochem Biophys Res Commun, 2005. 338(1): p. 311–7. [DOI] [PubMed] [Google Scholar]
- 6.Bock KW, Aryl hydrocarbon receptor (AHR): From selected human target genes and crosstalk with transcription factors to multiple AHR functions. Biochem Pharmacol, 2019. 168: p. 65–70. [DOI] [PubMed] [Google Scholar]
- 7.Murray IA, Patterson AD, and Perdew GH, Aryl hydrocarbon receptor ligands in cancer: friend and foe. Nat Rev Cancer, 2014. 14(12): p. 801–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujii-Kuriyama Y and Kawajiri K, Molecular mechanisms of the physiological functions of the aryl hydrocarbon (dioxin) receptor, a multifunctional regulator that senses and responds to environmental stimuli. Proc Jpn Acad Ser B Phys Biol Sci, 2010. 86(1): p. 40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-Salguero PM, et al. , Lesions of aryl-hydrocarbon receptor-deficient mice. Vet Pathol, 1997. 34(6): p. 605–14. [DOI] [PubMed] [Google Scholar]
- 10.Carreira VS, et al. , Disruption of Ah Receptor Signaling during Mouse Development Leads to Abnormal Cardiac Structure and Function in the Adult. PLoS One, 2015. 10(11): p. e0142440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nebert DW, Aryl hydrocarbon receptor (AHR): “pioneer member” of the basic-helix/loop/helix per-Arnt-sim (bHLH/PAS) family of “sensors” of foreign and endogenous signals. Prog Lipid Res, 2017. 67: p. 38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu D and Rastinejad F, Structural characterization of mammalian bHLH-PAS transcription factors. Curr Opin Struct Biol, 2017. 43: p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seok SH, et al. , Structural hierarchy controlling dimerization and target DNA recognition in the AHR transcriptional complex. Proc Natl Acad Sci U S A, 2017. 114(21): p. 5431–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corrada D, Denison MS, and Bonati L, Structural modeling of the AhR:ARNT complex in the bHLH-PASA-PASB region elucidates the key determinants of dimerization. Mol Biosyst, 2017. 13(5): p. 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swanson HI, DNA binding and protein interactions of the AHR/ARNT heterodimer that facilitate gene activation. Chem Biol Interact, 2002. 141(1–2): p. 63–76. [DOI] [PubMed] [Google Scholar]
- 16.Wu D, et al. , Structure and dimerization properties of the aryl hydrocarbon receptor PAS-A domain. Mol Cell Biol, 2013. 33(21): p. 4346–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bisson WH, et al. , Modeling of the aryl hydrocarbon receptor (AhR) ligand binding domain and its utility in virtual ligand screening to predict new AhR ligands. J Med Chem, 2009. 52(18): p. 5635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pandini A, et al. , Detection of the TCDD binding-fingerprint within the Ah receptor ligand binding domain by structurally driven mutagenesis and functional analysis. Biochemistry, 2009. 48(25): p. 5972–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones S, An overview of the basic helix-loop-helix proteins. Genome Biol, 2004. 5(6): p. 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulte KW, et al. , Structural Basis for Aryl Hydrocarbon Receptor-Mediated Gene Activation. Structure, 2017. 25(7): p. 1025–1033 e3. [DOI] [PubMed] [Google Scholar]
- 21.Beischlag TV, et al. , The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr, 2008. 18(3): p. 207–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denison MS and Nagy SR, Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol, 2003. 43: p. 309–34. [DOI] [PubMed] [Google Scholar]
- 23.Murray IA and Perdew GH, Ligand activation of the Ah receptor contributes to gastrointestinal homeostasis. Curr Opin Toxicol, 2017. 2: p. 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hubbard TD, Murray IA, and Perdew GH, Indole and Tryptophan Metabolism: Endogenous and Dietary Routes to Ah Receptor Activation. Drug Metab Dispos, 2015. 43(10): p. 1522–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yanofsky C, Horn V, and Gollnick P, Physiological studies of tryptophan transport and tryptophanase operon induction in Escherichia coli. J Bacteriol, 1991. 173(19): p. 6009–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roager HM and Licht TR, Microbial tryptophan catabolites in health and disease. Nat Commun, 2018. 9(1): p. 3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Badawy AA, Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int J Tryptophan Res, 2017. 10: p. 1178646917691938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rannug A and Rannug U, The tryptophan derivative 6-formylindolo[3,2-b]carbazole, FICZ, a dynamic mediator of endogenous aryl hydrocarbon receptor signaling, balances cell growth and differentiation. Crit Rev Toxicol, 2018. 48(7): p. 555–574. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell KA and Elferink CJ, Timing is everything: consequences of transient and sustained AhR activity. Biochem Pharmacol, 2009. 77(6): p. 947–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurl RN and Villee CA, A metabolite of riboflavin binds to the 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) receptor. Pharmacology, 1985. 30(5): p. 241–4. [DOI] [PubMed] [Google Scholar]
- 31.Phelan D, et al. , Activation of the Ah receptor signal transduction pathway by bilirubin and biliverdin. Arch Biochem Biophys, 1998. 357(1): p. 155–63. [DOI] [PubMed] [Google Scholar]
- 32.Yao L, et al. , Hyperhomocysteinemia activates the aryl hydrocarbon receptor/CD36 pathway to promote hepatic steatosis in mice. Hepatology, 2016. 64(1): p. 92–105. [DOI] [PubMed] [Google Scholar]
- 33.Wright EJ, et al. , Canonical and non-canonical aryl hydrocarbon receptor signaling pathways. Curr Opin Toxicol, 2017. 2: p. 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Avilla MN, et al. , The Ah Receptor: Adaptive Metabolism, Ligand Diversity, and the Xenokine Model. Chem Res Toxicol, 2020. 33(4): p. 860–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kazlauskas A, Poellinger L, and Pongratz I, Evidence that the co-chaperone p23 regulates ligand responsiveness of the dioxin (Aryl hydrocarbon) receptor. J Biol Chem, 1999. 274(19): p. 13519–24. [DOI] [PubMed] [Google Scholar]
- 36.Meyer BK, Petrulis JR, and Perdew GH, Aryl hydrocarbon (Ah) receptor levels are selectively modulated by hsp90-associated immunophilin homolog XAP2. Cell Stress Chaperones, 2000. 5(3): p. 243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larigot L, et al. , AhR signaling pathways and regulatory functions. Biochim Open, 2018. 7: p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsuji N, et al. , The activation mechanism of the aryl hydrocarbon receptor (AhR) by molecular chaperone HSP90. FEBS Open Bio, 2014. 4: p. 796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Androutsopoulos VP, Tsatsakis AM, and Spandidos DA, Cytochrome P450 CYP1A1: wider roles in cancer progression and prevention. BMC Cancer, 2009. 9: p. 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jackson DP, Joshi AD, and Elferink CJ, Ah Receptor Pathway Intricacies; Signaling Through Diverse Protein Partners and DNA-Motifs. Toxicol Res (Camb), 2015. 4(5): p. 1143–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jensen KA, Luu TC, and Chan WK, A truncated Ah receptor blocks the hypoxia and estrogen receptor signaling pathways: a viable approach for breast cancer treatment. Mol Pharm, 2006. 3(6): p. 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomkiewicz C, et al. , The aryl hydrocarbon receptor regulates focal adhesion sites through a non-genomic FAK/Src pathway. Oncogene, 2013. 32(14): p. 1811–20. [DOI] [PubMed] [Google Scholar]
- 43.Wilson SR, Joshi AD, and Elferink CJ, The tumor suppressor Kruppel-like factor 6 is a novel aryl hydrocarbon receptor DNA binding partner. J Pharmacol Exp Ther, 2013. 345(3): p. 419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Formosa R and Vassallo J, The Complex Biology of the Aryl Hydrocarbon Receptor and Its Role in the Pituitary Gland. Horm Cancer, 2017. 8(4): p. 197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vogel CF, et al. , Cross-talk between aryl hydrocarbon receptor and the inflammatory response: a role for nuclear factor-kappaB. J Biol Chem, 2014. 289(3): p. 1866–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vogel CF and Matsumura F, A new cross-talk between the aryl hydrocarbon receptor and RelB, a member of the NF-kappaB family. Biochem Pharmacol, 2009. 77(4): p. 734–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang G and Elferink CJ, A novel nonconsensus xenobiotic response element capable of mediating aryl hydrocarbon receptor-dependent gene expression. Mol Pharmacol, 2012. 81(3): p. 338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Q, et al. , Ah Receptor Activation by Dioxin Disrupts Activin, BMP, and WNT Signals During the Early Differentiation of Mouse Embryonic Stem Cells and Inhibits Cardiomyocyte Functions. Toxicol Sci, 2016. 149(2): p. 346–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurita H, et al. , Ah receptor expression in cardiomyocytes protects adult female mice from heart dysfunction induced by TCDD exposure. Toxicology, 2016. 355–356: p. 9–20. [DOI] [PubMed] [Google Scholar]
- 50.Jiang YZ, et al. , Expression of aryl hydrocarbon receptor in human placentas and fetal tissues. J Histochem Cytochem, 2010. 58(8): p. 679–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carreira VS, et al. , Ah Receptor Signaling Controls the Expression of Cardiac Development and Homeostasis Genes. Toxicol Sci, 2015. 147(2): p. 425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Q, et al. , Disruption of aryl hydrocarbon receptor homeostatic levels during embryonic stem cell differentiation alters expression of homeobox transcription factors that control cardiomyogenesis. Environ Health Perspect, 2013. 121(11–12): p. 1334–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu C, et al. , ShRNA-mediated gene silencing of AHR promotes the differentiation of P19 mouse embryonic carcinoma cells into cardiomyocytes. Mol Med Rep, 2012. 6(3): p. 513–8. [DOI] [PubMed] [Google Scholar]
- 54.Durocher D, et al. , The cardiac transcription factors Nkx2–5 and GATA-4 are mutual cofactors. EMBO J, 1997. 16(18): p. 5687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Q, et al. , Aryl Hydrocarbon Receptor Ablation in Cardiomyocytes Protects Male Mice From Heart Dysfunction Induced by NKX2.5 Haploinsufficiency. Toxicol Sci, 2017. 160(1): p. 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yi T, et al. , Aryl Hydrocarbon Receptor: A New Player of Pathogenesis and Therapy in Cardiovascular Diseases. Biomed Res Int, 2018. 2018: p. 6058784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lund AK, et al. , Endothelin-1-mediated increase in reactive oxygen species and NADPH Oxidase activity in hearts of aryl hydrocarbon receptor (AhR) null mice. Toxicol Sci, 2005. 88(1): p. 265–73. [DOI] [PubMed] [Google Scholar]
- 58.Lund AK, et al. , Cardiac hypertrophy in aryl hydrocarbon receptor null mice is correlated with elevated angiotensin II, endothelin-1, and mean arterial blood pressure. Toxicol Appl Pharmacol, 2003. 193(2): p. 177–87. [DOI] [PubMed] [Google Scholar]
- 59.Sauzeau V, et al. , Transcriptional factor aryl hydrocarbon receptor (Ahr) controls cardiovascular and respiratory functions by regulating the expression of the Vav3 proto-oncogene. J Biol Chem, 2011. 286(4): p. 2896–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sauzeau V, et al. , Vav3 proto-oncogene deficiency leads to sympathetic hyperactivity and cardiovascular dysfunction. Nat Med, 2006. 12(7): p. 841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang N, The role of endogenous aryl hydrocarbon receptor signaling in cardiovascular physiology. J Cardiovasc Dis Res, 2011. 2(2): p. 91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lahvis GP, et al. , Portosystemic shunting and persistent fetal vascular structures in aryl hydrocarbon receptor-deficient mice. Proc Natl Acad Sci U S A, 2000. 97(19): p. 10442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harrill JA, et al. , Knockout of the aryl hydrocarbon receptor results in distinct hepatic and renal phenotypes in rats and mice. Toxicol Appl Pharmacol, 2013. 272(2): p. 503–18. [DOI] [PubMed] [Google Scholar]
- 64.Chang CC, et al. , Mediating effects of aryl-hydrocarbon receptor and RhoA in altering brain vascular integrity: the therapeutic potential of statins. Am J Pathol, 2012. 181(1): p. 211–21. [DOI] [PubMed] [Google Scholar]
- 65.Wang ML, et al. , alpha-Naphthoflavone Increases Lipid Accumulation in Mature Adipocytes and Enhances Adipocyte-Stimulated Endothelial Tube Formation. Nutrients, 2015. 7(5): p. 3166–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ichihara S, et al. , A role for the aryl hydrocarbon receptor in regulation of ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol, 2007. 27(6): p. 1297–304. [DOI] [PubMed] [Google Scholar]
- 67.Hashimoto T and Shibasaki F, Hypoxia-inducible factor as an angiogenic master switch. Front Pediatr, 2015. 3: p. 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Groschwitz KR and Hogan SP, Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol, 2009. 124(1): p. 3–20; quiz 21–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Postal BG, et al. , AhR activation defends gut barrier integrity against damage occurring in obesity. Mol Metab, 2020. 39: p. 101007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He WQ, et al. , Contributions of Myosin Light Chain Kinase to Regulation of Epithelial Paracellular Permeability and Mucosal Homeostasis. Int J Mol Sci, 2020. 21(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu M, et al. , Aryl Hydrocarbon Receptor Activation Modulates Intestinal Epithelial Barrier Function by Maintaining Tight Junction Integrity. Int J Biol Sci, 2018. 14(1): p. 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma Y, et al. , 6-Formylindolo(3,2-b)carbazole induced aryl hydrocarbon receptor activation prevents intestinal barrier dysfunction through regulation of claudin-2 expression. Chem Biol Interact, 2018. 288: p. 83–90. [DOI] [PubMed] [Google Scholar]
- 73.Han B, et al. , Aryl Hydrocarbon Receptor Activation in Intestinal Obstruction Ameliorates Intestinal Barrier Dysfunction Via Suppression of MLCK-MLC Phosphorylation Pathway. Shock, 2016. 46(3): p. 319–28. [DOI] [PubMed] [Google Scholar]
- 74.Yu K, et al. , AhR activation protects intestinal epithelial barrier function through regulation of Par-6. J Mol Histol, 2018. 49(5): p. 449–458. [DOI] [PubMed] [Google Scholar]
- 75.Ahmed SM and Macara IG, Mechanisms of polarity protein expression control. Curr Opin Cell Biol, 2016. 42: p. 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim JJ and Khan WI, Goblet cells and mucins: role in innate defense in enteric infections. Pathogens, 2013. 2(1): p. 55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yin J, et al. , Aryl hydrocarbon receptor activation alleviates dextran sodium sulfate-induced colitis through enhancing the differentiation of goblet cells. Biochem Biophys Res Commun, 2019. 514(1): p. 180–186. [DOI] [PubMed] [Google Scholar]
- 78.Metidji A, et al. , The Environmental Sensor AHR Protects from Inflammatory Damage by Maintaining Intestinal Stem Cell Homeostasis and Barrier Integrity. Immunity, 2018. 49(2): p. 353–362 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Santos AJM, et al. , The Intestinal Stem Cell Niche: Homeostasis and Adaptations. Trends Cell Biol, 2018. 28(12): p. 1062–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pelaseyed T, et al. , The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev, 2014. 260(1): p. 8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koo BK, et al. , Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature, 2012. 488(7413): p. 665–9. [DOI] [PubMed] [Google Scholar]
- 82.Wu HJ and Wu E, The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes, 2012. 3(1): p. 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ji J and Qu H, Cross-regulatory Circuit Between AHR and Microbiota. Curr Drug Metab, 2019. 20(1): p. 4–8. [DOI] [PubMed] [Google Scholar]
- 84.Zelante T, et al. , Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity, 2013. 39(2): p. 372–85. [DOI] [PubMed] [Google Scholar]
- 85.Ma N and Ma X, Dietary Amino Acids and the Gut-Microbiome-Immune Axis: Physiological Metabolism and Therapeutic Prospects. 2019. 18(1): p. 221–242. [DOI] [PubMed] [Google Scholar]
- 86.Korecka A, et al. , Bidirectional communication between the Aryl hydrocarbon Receptor (AhR) and the microbiome tunes host metabolism. NPJ Biofilms Microbiomes, 2016. 2: p. 16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Postler TS and Ghosh S, Understanding the Holobiont: How Microbial Metabolites Affect Human Health and Shape the Immune System. Cell Metab, 2017. 26(1): p. 110–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brawner KM, et al. , Depletion of dietary aryl hydrocarbon receptor ligands alters microbiota composition and function. Sci Rep, 2019. 9(1): p. 14724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Natividad JM, et al. , Impaired Aryl Hydrocarbon Receptor Ligand Production by the Gut Microbiota Is a Key Factor in Metabolic Syndrome. Cell Metab, 2018. 28(5): p. 737–749 e4. [DOI] [PubMed] [Google Scholar]
- 90.Gao J, et al. , Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front Cell Infect Microbiol, 2018. 8: p. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wada T, et al. , Aryl Hydrocarbon Receptor Plays Protective Roles against High Fat Diet (HFD)-induced Hepatic Steatosis and the Subsequent Lipotoxicity via Direct Transcriptional Regulation of Socs3 Gene Expression. J Biol Chem, 2016. 291(13): p. 7004–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Boulange CL, et al. , Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med, 2016. 8(1): p. 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pernomian L, Duarte-Silva M, and de Barros Cardoso CR, The Aryl Hydrocarbon Receptor (AHR) as a Potential Target for the Control of Intestinal Inflammation: Insights from an Immune and Bacteria Sensor Receptor. Clin Rev Allergy Immunol, 2020. [DOI] [PubMed] [Google Scholar]
- 94.Wang J, et al. , Aryl hydrocarbon receptor/IL-22/Stat3 signaling pathway is involved in the modulation of intestinal mucosa antimicrobial molecules by commensal microbiota in mice. Innate Immun, 2018. 24(5): p. 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim JY and Dao H, Physiology, Integument, in StatPearls. 2020: Treasure Island (FL). [PubMed] [Google Scholar]
- 96.Furue M, et al. , Role of AhR/ARNT system in skin homeostasis. Arch Dermatol Res, 2014. 306(9): p. 769–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van den Bogaard EH, et al. , Genetic and pharmacological analysis identifies a physiological role for the AHR in epidermal differentiation. J Invest Dermatol, 2015. 135(5): p. 1320–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Furue M, Hashimoto-Hachiya A, and Tsuji G, Aryl Hydrocarbon Receptor in Atopic Dermatitis and Psoriasis. Int J Mol Sci, 2019. 20(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jux B, et al. , The aryl hydrocarbon receptor mediates UVB radiation-induced skin tanning. J Invest Dermatol, 2011. 131(1): p. 203–10. [DOI] [PubMed] [Google Scholar]
- 100.Lozza L, et al. , The Henna pigment Lawsone activates the Aryl Hydrocarbon Receptor and impacts skin homeostasis. Sci Rep, 2019. 9(1): p. 10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rademacher F, et al. , Staphylococcus epidermidis Activates Aryl Hydrocarbon Receptor Signaling in Human Keratinocytes: Implications for Cutaneous Defense. J Innate Immun, 2019. 11(2): p. 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Haas K, et al. , Aryl Hydrocarbon Receptor in Keratinocytes Is Essential for Murine Skin Barrier Integrity. 2016. 136 11: p. 2260–2269. [DOI] [PubMed] [Google Scholar]
- 103.Furue M, et al. , Gene regulation of filaggrin and other skin barrier proteins via aryl hydrocarbon receptor. J Dermatol Sci, 2015. 80(2): p. 83–8. [DOI] [PubMed] [Google Scholar]
- 104.Loertscher JA, et al. , In utero exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin causes accelerated terminal differentiation in fetal mouse skin. Toxicol Sci, 2002. 68(2): p. 465–72. [DOI] [PubMed] [Google Scholar]
- 105.Owens DW, et al. , The catalytic activity of the Src family kinases is required to disrupt cadherin-dependent cell-cell contacts. Mol Biol Cell, 2000. 11(1): p. 51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barouti N, et al. , L-Tryptophan as a Novel Potential Pharmacological Treatment for Wound Healing via Aryl Hydrocarbon Receptor Activation. Dermatology, 2015. 230(4): p. 332–9. [DOI] [PubMed] [Google Scholar]
- 107.Tanaka Y, et al. , Indirubin-pregnane X receptor-JNK axis accelerates skin wound healing. Sci Rep, 2019. 9(1): p. 18174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Morino-Koga S, et al. , 6-Formylindolo[3,2-b]Carbazole Accelerates Skin Wound Healing via Activation of ERK, but Not Aryl Hydrocarbon Receptor. J Invest Dermatol, 2017. 137(10): p. 2217–2226. [DOI] [PubMed] [Google Scholar]
- 109.Ikuta T, et al. , AhR protein trafficking and function in the skin. Biochem Pharmacol, 2009. 77(4): p. 588–96. [DOI] [PubMed] [Google Scholar]
- 110.Kimura E and Tohyama C, Embryonic and Postnatal Expression of Aryl Hydrocarbon Receptor mRNA in Mouse Brain. Front Neuroanat, 2017. 11: p. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu PY, et al. , Novel Endogenous Ligands of Aryl Hydrocarbon Receptor Mediate Neural Development and Differentiation of Neuroblastoma. ACS Chem Neurosci, 2019. 10(9): p. 4031–4042. [DOI] [PubMed] [Google Scholar]
- 112.Williams EG, et al. , An evolutionarily conserved role for the aryl hydrocarbon receptor in the regulation of movement. PLoS Genet, 2014. 10(9): p. e1004673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee HU, et al. , Host-microbiome interactions: the aryl hydrocarbon receptor and the central nervous system. J Mol Med (Berl), 2017. 95(1): p. 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim MD, Jan LY, and Jan YN, The bHLH-PAS protein Spineless is necessary for the diversification of dendrite morphology of Drosophila dendritic arborization neurons. Genes Dev, 2006. 20(20): p. 2806–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Juricek L, et al. , AhR-deficiency as a cause of demyelinating disease and inflammation. Sci Rep, 2017. 7(1): p. 9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shackleford G, et al. , Involvement of Aryl hydrocarbon receptor in myelination and in human nerve sheath tumorigenesis. Proc Natl Acad Sci U S A, 2018. 115(6): p. E1319–E1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Latchney SE, et al. , Deletion or activation of the aryl hydrocarbon receptor alters adult hippocampal neurogenesis and contextual fear memory. J Neurochem, 2013. 125(3): p. 430–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Keshavarzi M, et al. , An Endogenous Ligand of Aryl Hydrocarbon Receptor 6-Formylindolo[3,2-b]Carbazole (FICZ) Is a Signaling Molecule in Neurogenesis of Adult Hippocampal Neurons. J Mol Neurosci, 2020. 70(5): p. 806–817. [DOI] [PubMed] [Google Scholar]
- 119.de la Parra J, et al. , AhR Deletion Promotes Aberrant Morphogenesis and Synaptic Activity of Adult-Generated Granule Neurons and Impairs Hippocampus-Dependent Memory. eNeuro, 2018. 5(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Abbott NJ, et al. , Structure and function of the blood-brain barrier. Neurobiol Dis, 2010. 37(1): p. 13–25. [DOI] [PubMed] [Google Scholar]
- 121.Wang X, Hawkins BT, and Miller DS, Aryl hydrocarbon receptor-mediated upregulation of ATP-driven xenobiotic efflux transporters at the blood-brain barrier. FASEB J, 2011. 25(2): p. 644–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.You D, et al. , Increased MDR1 Transporter Expression in Human Brain Endothelial Cells Through Enhanced Histone Acetylation and Activation of Aryl Hydrocarbon Receptor Signaling. Mol Neurobiol, 2019. 56(10): p. 6986–7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Borghi M, et al. , Tryptophan as a Central Hub for Host/Microbial Symbiosis. 2020. 13: p. 1178646920919755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Soliman H, Mediavilla-Varela M, and Antonia S, Indoleamine 2,3-dioxygenase: is it an immune suppressor? Cancer J, 2010. 16(4): p. 354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ball HJ, et al. , Indoleamine 2,3-dioxygenase-2; a new enzyme in the kynurenine pathway. Int J Biochem Cell Biol, 2009. 41(3): p. 467–71. [DOI] [PubMed] [Google Scholar]
- 126.Noakes R, The Aryl Hydrocarbon Receptor: A Review of Its Role in the Physiology and Pathology of the Integument and Its Relationship to the Tryptophan Metabolism. 2015. 8: p. IJTR.S19985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Litzenburger UM, et al. , Constitutive IDO expression in human cancer is sustained by an autocrine signaling loop involving IL-6, STAT3 and the AHR. Oncotarget, 2014. 5(4): p. 1038–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hollingshead BD, et al. , Inflammatory signaling and aryl hydrocarbon receptor mediate synergistic induction of interleukin 6 in MCF-7 cells. Cancer Res, 2008. 68(10): p. 3609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Orabona C, et al. , Cutting Edge: Silencing Suppressor of Cytokine Signaling 3 Expression in Dendritic Cells Turns CD28-Ig from Immune Adjuvant to Suppressant. 2005. 174(11): p. 6582–6586. [DOI] [PubMed] [Google Scholar]
- 130.Wirthgen E and Hoeflich A, Endotoxin-Induced Tryptophan Degradation along the Kynurenine Pathway: The Role of Indolamine 2,3-Dioxygenase and Aryl Hydrocarbon Receptor-Mediated Immunosuppressive Effects in Endotoxin Tolerance and Cancer and Its Implications for Immunoparalysis. J Amino Acids, 2015. 2015: p. 973548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee SM, et al. , Inhibition of acute lethal pulmonary inflammation by the IDO-AhR pathway. Proc Natl Acad Sci U S A, 2017. 114(29): p. E5881–E5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li Q, et al. , Tolerogenic Phenotype of IFN-gamma-Induced IDO+ Dendritic Cells Is Maintained via an Autocrine IDO-Kynurenine/AhR-IDO Loop. J Immunol, 2016. 197(3): p. 962–70. [DOI] [PubMed] [Google Scholar]
- 133.Gunther J, Dabritz J, and Wirthgen E, Limitations and Off-Target Effects of Tryptophan-Related IDO Inhibitors in Cancer Treatment. Front Immunol, 2019. 10: p. 1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Harden JL and Egilmez NK, Indoleamine 2,3-dioxygenase and dendritic cell tolerogenicity. Immunol Invest, 2012. 41(6–7): p. 738–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mezrich JD, et al. , An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol, 2010. 185(6): p. 3190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nguyen NT, et al. , Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci U S A, 2010. 107(46): p. 19961–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hao K, et al. , Possible role of the ‘IDO-AhR axis’ in maternal-foetal tolerance. Cell Biol Int, 2013. 37(2): p. 105–8. [DOI] [PubMed] [Google Scholar]
- 138.Alberts B, Molecular biology of the cell. 2002, New York: Garland Science. [Google Scholar]
- 139.Raphael I, et al. , T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine, 2015. 74(1): p. 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Golubovskaya V and Wu L, Different Subsets of T Cells, Memory, Effector Functions, and CAR-T Immunotherapy. Cancers (Basel), 2016. 8(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ouyang W, Kolls JK, and Zheng Y, The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity, 2008. 28(4): p. 454–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sharma A and Rudra D, Emerging Functions of Regulatory T Cells in Tissue Homeostasis. Front Immunol, 2018. 9: p. 883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Negishi T, et al. , Effects of aryl hydrocarbon receptor signaling on the modulation of TH1/TH2 balance. J Immunol, 2005. 175(11): p. 7348–56. [DOI] [PubMed] [Google Scholar]
- 144.Gutierrez-Vazquez C and Quintana FJ, Regulation of the Immune Response by the Aryl Hydrocarbon Receptor. Immunity, 2018. 48(1): p. 19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kimura A, et al. , Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci U S A, 2008. 105(28): p. 9721–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Quintana FJ, et al. , Aiolos promotes TH17 differentiation by directly silencing Il2 expression. Nat Immunol, 2012. 13(8): p. 770–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen Y, et al. , Foxp3(+) regulatory T cells promote T helper 17 cell development in vivo through regulation of interleukin-2. Immunity, 2011. 34(3): p. 409–21. [DOI] [PubMed] [Google Scholar]
- 148.Veldhoen M, et al. , The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature, 2008. 453(7191): p. 106–9. [DOI] [PubMed] [Google Scholar]
- 149.Hao N and Whitelaw ML, The emerging roles of AhR in physiology and immunity. Biochem Pharmacol, 2013. 86(5): p. 561–70. [DOI] [PubMed] [Google Scholar]
- 150.Gandhi R, et al. , Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat Immunol, 2010. 11(9): p. 846–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Apetoh L, et al. , The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol, 2010. 11(9): p. 854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gagliani N, et al. , Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature, 2015. 523(7559): p. 221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tischkau SA, Mechanisms of circadian clock interactions with aryl hydrocarbon receptor signalling. Eur J Neurosci, 2020. 51(1): p. 379–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Buhr ED and Takahashi JS, Molecular components of the Mammalian circadian clock. Handb Exp Pharmacol, 2013(217): p. 3–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Xu CX, et al. , Disruption of CLOCK-BMAL1 transcriptional activity is responsible for aryl hydrocarbon receptor-mediated regulation of Period1 gene. Toxicol Sci, 2010. 115(1): p. 98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jaeger C and Tischkau SA, Role of Aryl Hydrocarbon Receptor in Circadian Clock Disruption and Metabolic Dysfunction. Environ Health Insights, 2016. 10: p. 133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mukai M and Tischkau SA, Effects of tryptophan photoproducts in the circadian timing system: searching for a physiological role for aryl hydrocarbon receptor. Toxicol Sci, 2007. 95(1): p. 172–81. [DOI] [PubMed] [Google Scholar]
- 158.Xu CX, et al. , Aryl hydrocarbon receptor activation attenuates Per1 gene induction and influences circadian clock resetting. Toxicol Sci, 2013. 132(2): p. 368–78. [DOI] [PMC free article] [PubMed] [Google Scholar]


