Abstract
Background:
Long-term loss of arm function after ischaemic stroke is common and may be improved by Vagus Nerve Stimulation (VNS) paired with rehabilitation.
Methods:
In this pivotal, randomised, triple-blind, sham-controlled trial, we assigned participants with moderate to severe arm weakness, at least nine months after ischaemic stroke, to receive rehabilitation paired with active VNS or rehabilitation paired with sham stimulation (Control). All participants were implanted with a VNS device and received six weeks of in-clinic therapy followed by a home exercise program. The primary outcome was the change in impairment measured by the Fugl-Meyer Assessment Upper Extremity (FMA-UE) score on the first day after completion of in-clinic therapy. All analyses were by intention to treat. The trial was registered on ClinicalTrials.gov (NCT03131960).
Findings:
We randomised 108 participants between Oct 2, 2017 and Sept 12, 2019 (53 to VNS and 55 to Control). A total of 106 completed the study. On the first day after completion of in-clinic therapy, the mean (±SD) FMA-UE score increased by 5.0 points (SD 4.4) in the VNS group and by 2.4 points (SD 3.8) in the Control group (p=0.001, between group difference 2.6, 95% CI 1.03 to 4.2). Ninety days later, a clinically meaningful response on the FMA-UE score was achieved in 47% with VNS versus 24% in controls (p=0.01; between group difference 24%, 95% CI 6 to 41%). The Wolf Motor Function Test (WMFT) functional score increased by 0.46 (±0.40) points in the VNS group compared to 0.16 (±0.30) points in the Control group (p<0.0001, between group difference 0.30, 95% CI 0.16 to 0.43). The FMA-UE score increased by 5.8 points (±6.0) from baseline with VNS and by 2.8 points (±5.2) in controls (p=0.008, between group difference 2.96, 95% CI 0.83 to 5.08). There was one serious adverse event related to surgery (vocal cord paresis).
Interpretation:
Participants with moderate to severe arm impairment after ischaemic stroke showed clinically meaningful improvements in motor impairment and function with paired VNS compared to rehabilitation with sham VNS.
Funding:
The trial was funded by MicroTransponder Inc.
Keywords: vagus nerve, stroke, rehabilitation, neuromodulation, physical therapy, occupational therapy, plasticity, upper extremity
Introduction
Approximately 80% of people with acute stroke have upper limb motor impairment and as many as 50%−60% of these survivors still have persistent problems six months later.1,2 Persistent arm impairment is linked with poorer quality of life and reduced well-being.3 Identifying new treatments to improve upper limb function after stroke is a research priority for both stroke survivors and caregivers.4
There are few effective treatments to enhance upper limb recovery after stroke. Trials of increased therapy dose and of adjuvant drug or brain stimulation therapies have not been effective5–8. Constraint induced movement therapy has been shown to improve measures of upper limb impairment and function in selected people with stroke, possibly through helping them re-learn how to use intact motor pathways9.
One potential method to enhance the reorganisation potential of the brain following stroke is via cholinergic and monoaminergic modulation of motor cortex neurons10,11. This may be achieved by Vagus Nerve Stimulation (VNS). VNS paired with sensory input or motor training has been shown to result in input-specific reorganization of rat cortical neurons12,13. In rodent models of ischemic stroke, VNS combined with movement training significantly improved forelimb motor recovery and tripled the synaptic connectivity of motor cortex neurons compared to movement training alone14. Two pilot studies of VNS paired with intensive upper limb rehabilitation have been conducted in people with long-term moderate to severe arm weakness after stroke. VNS-treated participants had greater improvement in Fugl-Meyer Assessment-Upper Extremity (FMA-UE) score compared to participants that received intense rehabilitation alone15,16.
We performed a pivotal, randomised, blinded, controlled trial comparing active VNS paired with rehabilitation versus sham stimulation paired with rehabilitation in people with moderate to severe arm impairment after ischaemic stroke. The purpose of this trial was to determine whether VNS paired with rehabilitation is a safe and effective treatment for improving arm function after stroke.
Methods
Further details regarding the design of the trial have been published previously.17 The study was approved by the review boards at each institution and subject to appropriate regulatory approvals (FDA Investigational Device Exemption (IDE, #G170031) and UK MHRA No #CI/2015/0011). The study was registered on clinicaltrials.gov (NCT03131960). Written informed consent was obtained from all participants. The study was conducted according to the Declaration of Helsinki and was undertaken in 19 sites in the UK and USA.
Participants
Study participants were male and female adults aged ≥ 22 years and ≤ 80 years old with a history of unilateral supratentorial ischaemic stroke that occurred between nine months to ten years prior to enrollment. People with moderate to severe arm impairment defined as a FMA-UE score between 20–50 were eligible for inclusion. Full inclusion and exclusion criteria are provided in the supplement.
Randomisation and Masking
We randomised participants at the time of VNS implant surgery to either rehabilitation paired with active VNS (VNS group) or rehabilitation paired with sham stimulation (Control group) on a 1:1 basis. Randomisation was done by ResearchPoint Global (USA) using SAS PROC PLAN, with stratification by region (US/UK), age (≤30, >30), and baseline FMA-UE score (20–35, 36–50). The randomisation allocation was sent via email to an unblinded clinical engineer at each site who tested and programmed the device with the appropriate stimulation settings for group assignment during implantation. Participants, outcomes assessors, and treating therapists were blinded to group assignment. In an effort to maximize masking of treatment allocation, all participants were implanted with the VNS device. In addition, both treatment groups participants received 5 stimulations in reducing strengths (0.8 mA and then lower) at the beginning of each therapy session followed by stimulation according to randomised allocation. This was designed to minimize risk of participants being able to guess treatment allocation by exposing all participants to the same stimulation parameters at the start of each session. After the primary endpoint assessment, participants were asked to rate their certainty regarding group allocation by picking one of five options; knew they received VNS; thought they received VNS; knew they were in the sham stimulation group; thought they were in the sham stimulation group; or had ‘no idea.’
Study Procedures
A pre-surgery assessment was performed. Device implantation was done under general anaesthesia. A horizontal neck crease incision was created left of the midline at the level of the cricoid cartilage. After the vagus nerve was identified, the stimulation lead was wrapped around the vagus nerve. The lead was then tunnelled subcutaneously to the pulse generator device which was contained in a subcutaneous pocket in the pectoral region.18
Baseline assessments were performed one week after device implantation. Stimulation was tested in increments of 0.1 mA to assess if participants felt and tolerated stimulation. If stimulation at 0.8 mA was uncomfortable, stimulation settings were lowered to a comfortable level, and this level was used in the study. This process was performed in both groups regardless of treatment allocation. In two participants, stimulation settings were lowered to 0.7 mA and 0.6 mA.
In-clinic rehabilitation therapy began the next day and was provided three times per week for six weeks (total of 18 sessions). Details about the upper limb rehabilitation delivered in the trial have been reported previously.16 Briefly, in-clinic rehabilitation consisted of high repetition, task-based, functional, individualised, and progressive upper limb exercises. All participants received the same goal-oriented and intense upper limb rehabilitation following specific guidelines16. Therapy tasks were divided into six categories: reach and grasp, gross movement, object flipping, simulated eating tasks, inserting objects, and opening/closing containers. For a given task, the object, movement direction and/or environment factors were adjusted to maintain difficulty level and subject motivation. Since participants had varying degrees of impairment and functional deficit, the exact number of repetitions and tasks per session varied. However, it was expected that six tasks would be performed in the same order at each session and that approximately 30–50 repetitions would be performed on each task giving >300 repetitions per session. The therapist timed the VNS pulse with each repetition of movement (Appendix Figure S1). The VNS group received 0.8 mA (or 0.7 and 0.6 mA in two participants as described above), 100 μs, 30 Hz stimulation pulses, lasting 0.5 seconds, during each movement repetition. The Control group received 0 mA pulses.
Following the six weeks of in-clinic therapy, all participants began daily, therapist-prescribed home exercises. The home therapy session lasted 30 minutes and included tasks following the same principles as the in-clinic therapy. During home exercises, participants activated the VNS device via a single magnet swipe over the device and 30 minutes of either active or sham VNS was then delivered according to their randomised allocation. The stimulation output current was kept the same as during in-clinic therapy. Bi-monthly phone calls between the therapist and participant were conducted to ensure compliance and adequate exercise intensity.
Study Outcome Measures
Outcome assessments were performed on days one and 90 after the completion of the six weeks of in-clinic therapy. These included the FMA-UE, Wolf-Motor Function Test (WMFT function and time score), Motor Activity Log (MAL), Stroke Impact Scale (SIS) score, Stroke Specific Quality of Life (SS-QOL), EuroQol-5D (EQ-5D), and the Beck Depression Inventory (BDI). The WMFT and FMA-UE were also assessed at day 30 following completion of in-clinic therapy. A description of each of the measures is provided in the supplement. Assessments were performed by the same assessor at baseline and at follow-up.
The primary outcome was change in FMA-UE score from baseline to the first day following completion of in-clinic therapy19,20. The secondary outcomes measures were 1) clinically meaningful response on FMA-UE score at day 90, 2) change in day 90 WMFT-Functional score, and 3) change in day 90 FMA-UE score. We defined a clinically meaningful response as a six 6 point or greater improvement in FMA-UE score based on previous research demonstrating that a 5.25-point change was associated with an excellent improvement (greater than 50% improvement) in arm function21.
Tertiary outcome measures were the MAL, SIS score, SS-QOL score, EQ-5D score and the BDI score. We added WMFT response rate as a post-hoc outcome measure to assess response on a functional outcome measure. A clinically meaningful response was defined as a ≥ 0.4-point change in WMFT-Functional score at day 90.22
Safety reporting
Data on all adverse events and serious adverse events were recorded prospectively. Events were coded with the use of the Medical Dictionary for Regulatory Activities (MedDRA, version 22). Severity and causality/relationship to study treatment (rehabilitation and VNS) or implant surgery was assigned by the site Principal Investigator.
Sample Size
The a priori sample size calculation was based on data from our pilot studies.15,16 A sample size of 100 participants (50 per group) was determined to provide 80% power (alpha = 0.05) to detect a FMA-UE difference of 2.3 (SD 4) points between the two treatment groups. We enrolled 108 participants to allow for drop-outs.
Statistical Analysis
Statistical analyses were independently performed by ResearchPoint Global using SAS Version 9.4 or higher.
A pre-defined futility analysis was performed by the Data Safety Monitoring Board (DSMB) based on data from the first 40 participants. The criteria for futility were not met and DSMB determined that the trial could continue.
All efficacy and safety summaries were performed on the intent-to-treat (ITT) population, defined as all participants who have any surgical portion of the implant procedure attempted, regardless of the treatment to which they are assigned, and regardless of the amount of intervention completed. A Per Protocol (PP) population was a priori defined to include participants who completed at least 12 sessions without major protocol violations that could impact and/or compromise the safety or efficacy of the treatment.
For the primary outcome measure, an analysis of covariance (ANCOVA) model was used, with the change from baseline to day one following completion of in-clinic therapy as the dependent variable, and treatment arm, region (UK or USA), treatment by region interaction as factors, and with age and baseline FMA-UE score as covariates. A significance level of 0.05 was used. The region by treatment interaction was to be removed from the final model if it was not significant (p>0.1). For the responder analysis at day 90 post completion of therapy, we used a logistic regression model with treatment arm, region, age and baseline FMA-UE score as factors. An ANCOVA model, with the change from baseline as the dependent variable, and treatment / randomisation strata as factors was used for the analysis of the WMFT-functional change and the FMA-UE change at day 90 following completion of in-clinic therapy. The three secondary outcomes measures were tested for significance in a hierarchical manner in the order listed. Significance was declared for the first secondary outcome at 0.05, and each subsequent outcome only if all higher ranked endpoints were significant at 0.05. For the responder analyses, a number needed to treat to achieve an additional clinically meaningful response was calculated. For the post-hoc outcome measure of WMFT response rate at day 90 we used a Fisher Exact test to assess the between-group difference. Summary statistics for tertiary measures were tabulated but formal statistical analysis was not performed. In additional post hoc analyses we compared response rates on the FMA-UE score at 3 additional levels (≥4 points, ≥5 points and ≥7 points). We also compared the proportion who guessed they received VNS and who correctly guessed their treatment allocation.
A ‘last observation carried forward’ approach was used if an assessment was missing after baseline. We assessed the effect of missing data by first performing a Mixed Model Repeated Measures test (SAS PROC MIXED) on the full data set. We then performed multiple imputation with missing at random assumptions (SAS PROC MI).
Trial management and role of the funding source
An independent data safety monitoring board (DSMB) reviewed adverse events, safety information and the planned futility analysis. The funder, MicroTransponder Inc, supported the writing committee in the writing of the manuscript. MicroTransponder played no role in data collection, data interpretation or the decision to submit the manuscript. The decision to submit the manuscript was the responsibility of JD, TJK, and CL. The corresponding author had full access to all the data in the study.
Results
108 participants were randomised between Oct 2, 2017 and Sept 12, 2019. A total of 195 participants consented and were screened for eligibility. 140 people met eligibility criteria and 32 withdrew prior to device implantation and randomisation. Of the 108 randomised participants, 53 were assigned to the active VNS group and 55 to the Control sham stimulation group. A total of 107 completed the study intervention and were included in the per-protocol population, and 106 attended for primary endpoint assessment (see trial profile, Figure 1). There were no significant protocol deviations that affected the rights, safety, or well-being of participants or the scientific integrity of the study (Appendix text and Appendix Table S1). Baseline demographics are shown in Table 1. Groups were well matched at baseline. Enrollment by site is shown in the supplement (Appendix Table S2).
Figure 1:
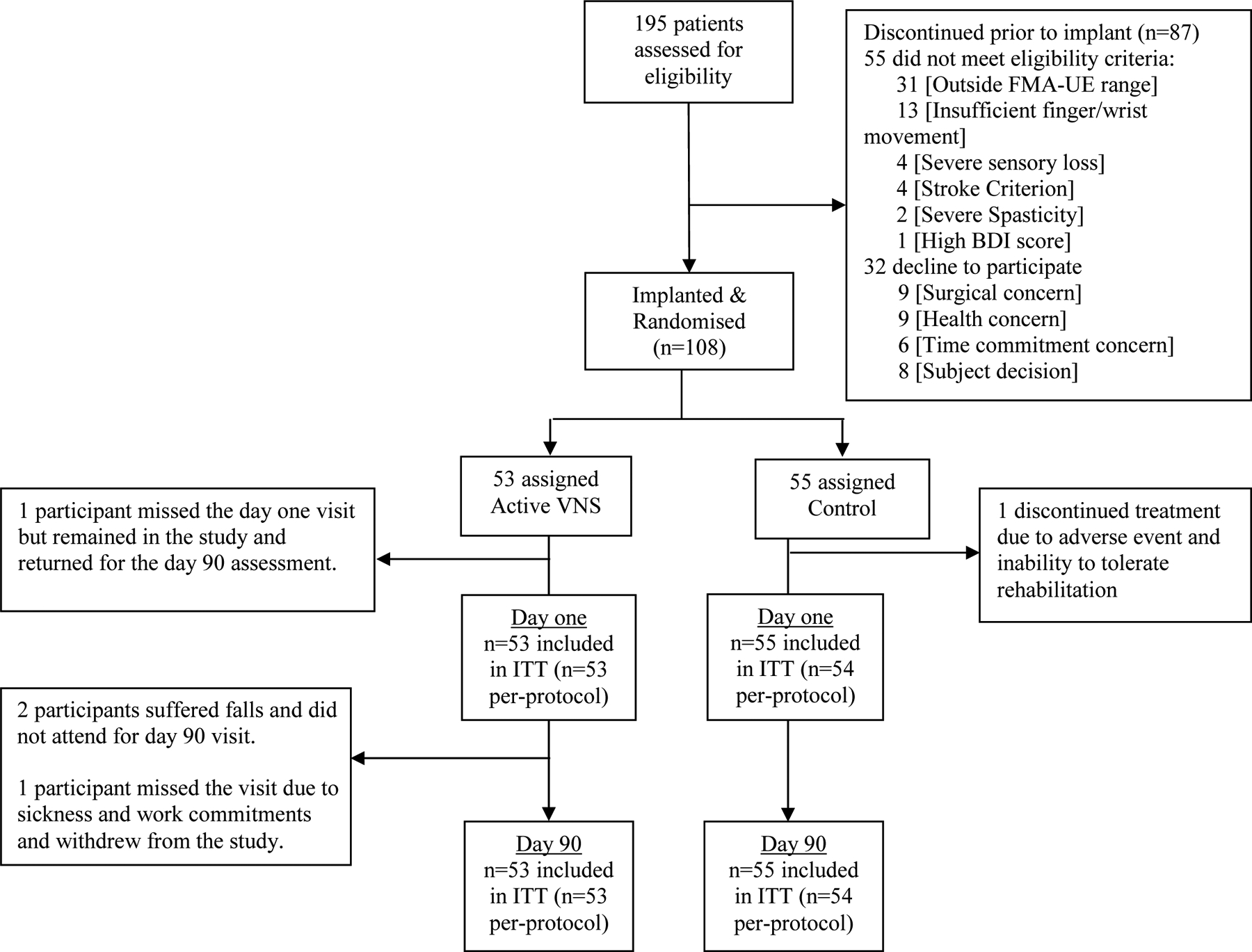
Trial profile
Table 1.
Baseline Characteristics
| VNS (n=53) |
Control (n=55) |
|
|---|---|---|
| Gender (N, %) | ||
| Male | 34 (64%) | 36 (65.5%) |
| Female | 19 (37%) | 19 (35%) |
| Ethnicity (N, %) | ||
| Caucasian | 42 (79%) | 43 (78%) |
| African-American | 9 (17%) | 9 (16%) |
| Asian, Indian, Other | 1 (2%) | 4 (7%) |
| Not Reported | 1 (2%) | 1 (2%) |
| Age (years, Mean ± SD) | 59.1 ± 10.2 | 61.1 ± 9.2 |
| Time since stroke (years.) | 3.1 ± 2.3 | 3.3 ± 2.6 |
| Handedness (Right/Left/Ambidextrous) | 48 (91%) / 4 (8%) / 1 (2%) | 50 (91%) / 5 (9) / 0 |
| Side of Paresis (Right/Left) | 25 (47%) / 28 (53%) | 26 (47%) / 29 (53%) |
| FMA-UE Baseline Score (Mean ± SD) | 34.4 ± 8.2 | 35.7 ± 7.8 |
| WMFT Functional Score | 2.71 ± 0.70 | 2.83 ± 0.65 |
Baseline demographic and clinical characteristics by randomisation group in the intention to treat population. FMA-UE is Fugl-Meyer Assessment Upper Extremity. WMFT is Wolf Motor Function Test. Participants could select more than one option for ethnicity.
Participants in the VNS and Control groups received a similar number of stimulations per therapy session (VNS: 422 (SD 99) stimulations, Control: 419 (SD 86) sham stimulations). The mean duration of each in-clinic rehabilitation session was 90 (SD 16) minutes.
103 participants (49 VNS and 54 Control) rated their certainty regarding treatment allocation (Appendix Table S3). Nine VNS (18%) and 9 Control participants (18%) in each group believed they received VNS (p>0.999). Nine VNS (18%) and 13 (24%) Controls participants guessed their treatment allocation correctly (p=0.631).
The primary outcome (change in FMA-UE score from baseline to the first day after in-clinic therapy) was significantly higher in the VNS group than the Control group (VNS: 5.0, SD 4.4, Control: 2.4, SD 3.8; p=0.001; between group difference 2.60, 95% CI 1.03 to 4.2) (Figure 2, Appendix Table S4). There was no significant interaction between treatment allocation and geographic region (p>0.1).
Figure 2. Change in Primary and Secondary Outcome Measures.
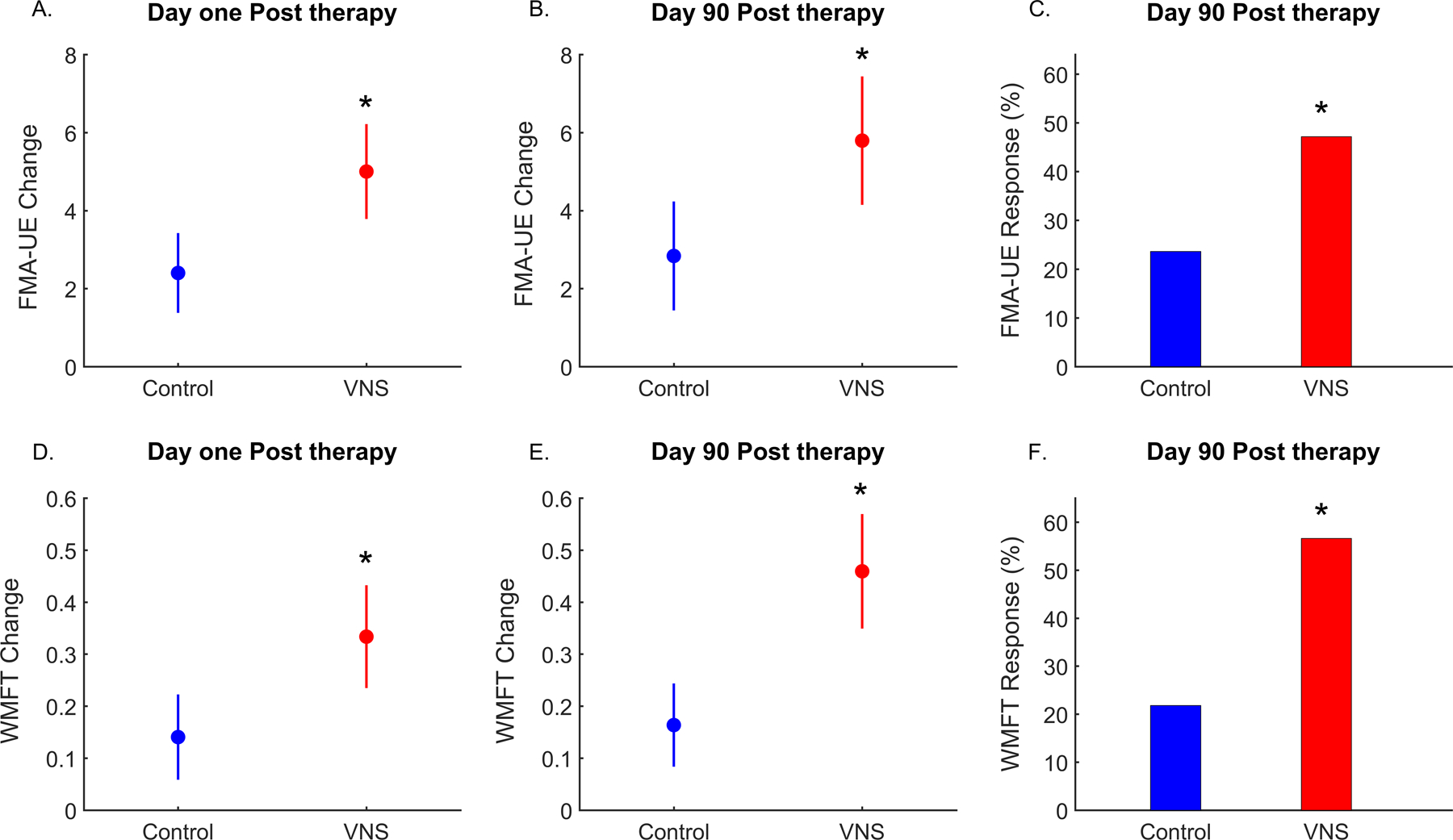
A. Change in Fugl-Meyer Assessment-Upper Extremity (FMA-UE) score between baseline and day one post completion of in-clinic therapy. (Primary End-point). B. Change in FMA-UE score between baseline and day 90 post completion of in-clinic therapy. C. FMA-UE response rate (≥6 point change from baseline) at day 90 post completion of in-clinic therapy. D. Change in Wolf Motor Function Test-Functional (WMFT) score between baseline and day one post completion of in-clinic therapy. E. Change in WMFT score between baseline and day 90 post completion of in-clinic therapy. F. WMFT response rate (≥ 0.4 point change from baseline) at day 90 post completion of in-clinic therapy. The circle is the mean group value and the vertical lines denote 95% confidence intervals. * denotes p<0.05 for the between group difference. Red: VNS group; Blue: Control group.
A clinically meaningful response on the FMA-UE score occurred in more participants in the VNS group compared to the control group at day 90 following completion of in-clinic therapy (47% versus 24%, p=0.01; between group difference 24%, 95% CI 6 to 41%), resulting in a number needed to treat of 4.3 for VNS. Response rates defined as a ≥4 point, ≥5 point and ≥7 point increase on the FMA-UE score were consistent higher with VNS and are shown in Appendix table S5.
The WMFT-functional score was significantly increased in the VNS group compared to the Control group at 90 days after the end of in-clinic therapy (VNS: 0.46, SD 0.40, Control: 0.16, SD 0.30; p<0.0001; between group difference 0.30, 95% CI 0.16 to 0.43). The FMA-UE score was also significantly increased in the VNS group compared to the Control group at 90 days (VNS: 5.8, SD 6.0, Control: 2.8, SD 5.2; p=0.008; between group difference 2.96, 95% CI 0.83 to 5.08). A clinically meaningful response on the WMFT-Functional test occurred in significantly more participants in the VNS group than the Control group (57% vs 22%, p=0.01), resulting in a number needed to treat of 2.8 with VNS.
A total of 334 adverse events (163 VNS, 171 Control) were reported in 85 (78%) participants. The majority of these (n=242) were mild. A total of 21 (40%) participants in the active VNS group and 24 (55%) controls reported an adverse event rated as either possibly, probably, or definitely related to device implantation. These were mostly due to post-operative pain. A total of 13 participants in the active VNS group and 9 controls reported an adverse event rated as either possibly, probably or definitely related to device use. The number of events, the number of participants reporting at least one event, and the number of severe events were similar in both groups (Appendix Table S5 and Table S6). There were no unexpected adverse events or serious adverse device events reported. There was one case of vocal cord palsy in a control participant, which resolved after five weeks.
For tertiary outcomes, there was a numerically greater difference between baseline and follow-up in the VNS group than in the Control group for the MAL, SIS, SS-QoL, EQ-5D and BDI scores (Appendix Table S7).
Results for all outcomes were similar on the per-protocol analysis and sensitivity analyses revealed no significant effect of missing data (Appendix Table S8).
Discussion
In our trial involving participants with moderate to moderately-severe arm impairment after chronic ischaemic stroke, participants who were assigned to VNS paired with rehabilitation demonstrated clinically meaningful improvements in motor impairment and function compared to participants assigned to rehabilitation and sham stimulation. The number of participants achieving a clinically meaningful improvement in upper limb impairment in the active VNS group was approximately double that of the Control group, with nearly half of the participants in the active VNS group achieving a clinically meaningful response. Notably, the responder rate was also significantly higher in the VNS group for the WMFT, a measure of arm function and was consistent across different FMA-UE score thresholds. The greater improvement in the VNS group was consistent across the primary outcome measure and all secondary outcome measures.
All participants were at least nine months post stroke, with a mean time from stroke of over three years. Treatment options for people with arm impairment at this stage typically focus on treatment of complications, rather than concerted efforts to improve function. Our data show it is possible to achieve meaningful improvements many years after stroke. Any improvements are unlikely to be attributable to spontaneous or expected recovery; indeed, many stroke survivors suffer functional decline at this time point.23 Many recent large clinical trials have not found additional clinically important improvements in arm impairment or function with intensive rehabilitation treatment, despite the use of rehabilitation devices, when compared to usual care.5,24 We saw a small improvement in the Control group, consistent with other trials. However, the amount of improvement was 2–3 times higher across multiple measures of arm function in participants who received active VNS paired with therapy. These findings are consistent with improvements seen in numerous experimental studies of motor recovery after stroke and in our clinical pilot studies (Appendix Figure S2).10,15,16,25
Nearly half of participants receiving VNS had a clinically meaningful improvement assessed by the FMA-UE score.26 We found a similar rate of clinically meaningful response rate for the WMFT.22 In addition, tertiary outcome measures, including the MAL,27, SIS-ADL,28 and SS-QoL,29 suggested greater improvement in the VNS group. The consistency of findings across WHO outcome dimensions provides further evidence that the VNS-related improvements demonstrated are important to stroke survivors. Further, responses were maintained at 90 days after completion of in-clinic therapy.
In preclinical models of ischaemic and hemorrhagic stroke, VNS paired with task-specific rehabilitation significantly enhanced post-stroke recovery compared to rehabilitation alone.10 When VNS was dissociated from rehabilitation or when rehabilitation was delivered alone, rats showed relatively less motor improvement, suggesting that task-specific rehabilitation paired with VNS is key to driving plastic changes in the motor cortex.30 Pairing VNS with rehabilitation has been shown to triple the synaptic connectivity in the corticospinal tract networks controlling the impaired forelimb compared to rehabilitation alone.14 This task-specific neuroplasticity is believed to result from molecular and neuronal mechanisms induced by VNS that include activation of noradrenergic, cholinergic and serotonergic systems.31 It is possible that VNS-mediated heterosynaptic neuromodulation facilitates long-term synaptic changes in motor neurons during a temporal learning window for spike-timing dependent plasticity.32,33 This pre-clinical evidence would suggest that VNS as used in this clinical human trial may exploit similar neuroplastic mechanisms34, although this remains to be verified.
This intervention requires surgical device implantation. VNS devices are used for the treatment of epilepsy and depression, and over 100,000 devices have been implanted worldwide for such clinical indications. The risk of implantation and side effects of stimulation have been well described.35,36 We found a similar low rate of vocal cord palsy, as has previously been documented, suggesting that the risk of vocal cord palsy is not substantially increased in well-selected people with a history of chronic ischaemic stroke. We saw no serious adverse device events. The stimulation parameters of 0.8 mA, 100 μs, 30 Hz and 0.5 second duration were used in all our preclinical stroke studies and in our two pilot studies of VNS for post-stroke rehabilitation10. These settings have been shown to cause desynchronization of the rat cortical EEG12 suggesting activation of cholinergic and noradrenergic neurons37,38 and to be associated with cortical plasticity and motor recovery39,40. Non-invasive methods of stimulating the vagus nerve are now available41. However, it is unclear whether non-invasive VNS activates the nerve to the same degree as with cervical implantable VNS42. The optimum site to deliver non-invasive VNS and which, if any, stimulation parameters cause task specific plasticity is unclear.
In this trial, the risk of bias was low and groups were well matched at enrolment. All participants were implanted with a VNS device; and blinding of therapists, participants, and outcome assessors was achieved. There was no evidence of expectation bias or unmasking of participants. The majority of participants were uncertain or incorrect regarding their treatment allocation and there was no difference between groups in the number who guessed they received VNS or who guessed correctly. This suggests that the study was well-blinded. Randomisation was performed by an independent service with allocation concealment. The outcome measures used here are common in stroke rehabilitation trials and are valid, reliable, and sensitive to change. There were low levels of missing data and all but two participants completed the study to day 90. While the long-term data from this study are not yet available, our earlier pilot study suggests that benefits of paired VNS therapy are maintained over time43.
Our study has some limitations. We cannot generalise our findings to people who do not meet trial eligibility criteria or to people with other types of stroke or other neurological disorders. In particular it is unclear whether VNS paired with rehabilitation improves motor outcomes in people with a more severely affected upper limb, spasticity and severe sensory loss. Although improvements were maintained for at least 90 days, we cannot be certain that the benefits of VNS paired with rehabilitation will be maintained in the longer-term and this should be investigated in future research. The sample size of our study limits our ability to assess the effect of VNS treatment in different sub-groups and two-thirds of participants in our study were male.
Participants with arm impairment, an average of three years after ischaemic stroke, who received rehabilitation showed clinically meaningful improvements in impairment and function that were 2–3 times greater with VNS compared to sham VNS. Improvements with paired VNS therapy were also reflected in quality-of-life measures. VNS combined with rehabilitation is a novel strategy to help people achieve improvement in arm and hand function after stroke.
Supplementary Material
Research in Context Panel.
Evidence before this study
Intense task-specific rehabilitation has a limited effect on upper limb impairment in people with long-term problems after ischaemic stroke. Vagus nerve stimulation paired with rehabilitation has been shown to improve forelimb function after experimental stroke and showed promise in two clinical pilot studies. However, no large adequately powered clinical study has been performed.
Added value of this study
VNS-REHAB is the first multicenter trial with adequate statistical power to compare rehabilitation plus active VNS paired with rehabilitation and sham stimulation. Participants treated with VNS had clinically meaningful improvements in measures of upper limb function and impairment on the first day after completion of in-clinic therapy and similar improvements 90-days later after a period of home exercise. The clinical response rate with active VNS was double that of sham stimulation on both the FMA-UE and WMFT, and almost 50% of active VNS treated participants achieved a clinical response. Improvements were also reflected in quality of life measures. The rate of surgical complications due to VNS implantation was similar to that seen with use of VNS in epilepsy.
Implications of the available evidence
The results of this trial support the use of VNS paired with rehabilitation for the treatment of selected people with upper limb impairment at least 9 months after ischaemic stroke. Further research should explore how to implement this approach in clinical practice and whether VNS can be used to improve other impairments after stroke, including more severe degrees of arm impairment.
Acknowledgements
We would like to thank participants for their contribution to this research project. We thank all staff at VNS Rehab study centres including, Ozzy Dincarslan, Elizabeth Colquhoun, Omar Hilmi and David Alexander Dickie (University of Glasgow / NHSGGC); Jon T. Willie and Susan Murphy (Emory University); Allan Block, Nicole C Hank, Brandon McCravey, Laura Cristians (Perseverance Research Center); Katherine Gaylarde, Christopher Uff, Geetha Boyapati (Royal London Hospital), Muhib Khan, Konstantin Elisevich, Sanjay Patra, Laurel Packard (Spectrum Health); Tomoko Kitago, K. Zoe Tsagaris, Marissa Wuennemann, Jennifer Cole, Heather Pepper Lane (Burke Neurological Institute); Mary-Joan MacLeod (University of Aberdeen / NHS Grampian); Kathy Bell, Maria Pia Ka Kabamalan, Megan Sbrochi, Sara Milligan, Cindy Dolezal, Erika Molina, Nneka L. Ifejika, Candice Osborne (University of Texas Southwestern Medical Center); Michelle P. Lin, Veronica Munet Diaz, Luke Partridge (Mayo Clinic Jacksonville); Taisiya Matev, Brooke Hoisington, Mary Mohay, Mona Moraska, (Brooks Rehabilitation); Nuray Yozbatiran, Kathryn Nedley, Ruta Paranjape (University of Texas Health McGovern Medical School, Houston); Grace Cole, Alex Radford, Sarah Peck, Lindsay Maidment, Janine Blackshire, Louise Patterson, Daniel Ireland (University of Sheffield); S Shaw, E Peter, C Kim (Ranchos Los Amigos Research Institute, Los Angeles).
We would also like to thank staff at MicroTransponder Inc and their affiliates for their work on this trial including W. Brent Tarver, Sue Lesly, Reema Adham Hinds, Asya Lesly, Chester Burress, Ravi Jain, David Ng, Kathy Galvan, Diana Hansen and study monitors from Med-Q monitors.
AM and JR were supported by the NIHR Sheffield Biomedical Research Centre (BRC) and NIHR Sheffield Clinical Research Facility (CRF). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care (DHSC).
Declaration of interests
Jesse Dawson and Teresa J. Kimberley have received reimbursement for conference attendance where results of the study were presented from MicroTransponder Inc. Steven C. Cramer has served as a consultant for Constant Therapeutics, Neurolutions, MicroTransponder, SanBio, Fujifilm Toyama Chemical Co., Medtronic and TRCare. David Pierce, Navzer D. Engineer and Cecília N. Prudente are employees of MicroTransponder, Inc. Steven L. Wolf is a consultant to Enspire, Inc and serves on the Scientific Advisory Board of SAEBO, Inc. Gerard E. Francisco has received research grants and/or consulting honoraria from Allergan, Ipsen, Merz, MicroTransponder, Ottobock/Hangar Orthopedics, Parker Hannifin, Revance Therapeutics, ReWalk, Sword Health.
The MGH Translational Research Center has a clinical research support agreement with Neuralink, Paradromics, and Synchron, for which Leigh R. Hochberg provides consultative input.
Footnotes
Data Sharing Statement
Data collected for the study, including deidentified individual participant data, data dictionary defining each field in the set, study protocol, and statistical analysis plan will be available after the completion of the post market study requirements of regulatory approval. Data will only be shared upon the approval of the proposal with the principal investigators, the sponsor of the study, and requires a signed data access agreement with specific funding to access the database without any support from investigators. Requests should be sent to VNSdatarequest@gmail.com.
Contributor Information
Prof. Jesse Dawson, Institute of Cardiovascular and Medical Sciences, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, G12 9QQ, UK..
Prof. Charles Y. Liu, USC Neurorestoration Center and Department of Neurological Surgery, USC Keck School of Medicine, Los Angeles, CA USA, and Rancho Los Amigos National Rehabilitation Center, Downey, CA USA..
Prof. Gerard E. Francisco, Department of Physical Medicine and Rehabilitation, The University of Texas Health Science Center McGovern Medical School, and The Institute for Rehabilitation and Research (TIRR) Memorial Hermann Hospital; Houston, Texas, USA..
Prof. Steven C. Cramer, Dept. Neurology, David Geffen School of Medicine at UCLA, and California Rehabilitation Institute; Los Angeles, CA USA..
Prof. Steven L. Wolf, Emory University School of Medicine, Department of Rehabilitation Medicine, Division of Physical Therapy, Atlanta, Georgia, USA..
Anand Dixit, Stroke Service, The Newcastle Upon Tyne Hospitals NHS Foundation Trust, UK..
Jen Alexander, Institute of Cardiovascular and Medical Sciences, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, G12 9QQ, UK..
Rushna Ali, Department of Neurosciences, Spectrum Health, Grands Rapids, MI, USA..
Benjamin L. Brown, Ochsner Neuroscience Institute, Department of Neurosurgery, Louisiana, USA..
Prof. Wuwei Feng, Department of Neurology, Duke University School of Medicine, North Carolina, USA..
Louis DeMark, Brooks Rehabilitation, Jacksonville, Florida, USA.
Prof. Leigh Hochberg, Center for Neurotechnology and Neurorecovery, Dept. of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA; School of Engineering and Carney Institute for Brain Science, Brown University; VA RR&D Center for Neurorestoration and Neurotechnology, VA Medical Center, Providence, RI, USA..
Prof. Steven A. Kautz, Ralph H. Johnson VA Medical Center, Charleston, South Carolina, USA and Department of Health Sciences and Research, Medical University of South Carolina, Charleston, South Carolina, USA..
Prof. Arshad Majid, Sheffield Institute for Neurological Sciences (SITraN) and Sheffield Teaching Hospitals, 385 Glossop Road, Sheffield, S10 2HQ, UK..
Prof. Michael W. O’Dell, Clinical Rehabilitation Medicine, Weill Cornell Medicine, New York City, NY, USA.
David Pierce, MicroTransponder Inc, Austin, Texas, USA..
Cecília N. Prudente, MicroTransponder Inc, Austin, Texas, USA..
Jessica Redgrave, Sheffield Institute for Neurological Sciences (SITraN), 385 Glossop Road, Sheffield, S10 2HQ, UK..
Prof. Duncan L Turner, School of Health, Sport and Bioscience, University of East London, London, UK..
Navzer D. Engineer, MicroTransponder Inc., Austin, Texas, USA..
Prof. Teresa J Kimberley, Department of Physical Therapy, Massachusetts General Hospital, Institute of Health Professions. Boston, MA, USA..
References
- 1.Kelly-Hayes M, Beiser A, Kase CS, Scaramucci A, D’Agostino RB, Wolf PA. The influence of gender and age on disability following ischemic stroke: the Framingham study. J Stroke Cerebrovasc Dis 2003; 12: 119–26. [DOI] [PubMed] [Google Scholar]
- 2.Wade DT, Langton-Hewer R, Wood VA, Skilbeck CE, Ismail HM. The hemiplegic arm after stroke: measurement and recovery. J Neurol Neurosurg Psychiatry 1983; 46: 521–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wyller TB, Sveen U, Sedring KM, Pettersen AM, Bautz-Holter E. Subjective well-being one year after stroke. Clin Rehabil 1997; 11: 139–45. [DOI] [PubMed] [Google Scholar]
- 4.Pollock A, St George B, Fenton M, Firkins L. Top 10 research priorities relating to life after stroke - consensus from stroke survivors, caregivers, and health professionals. Int J Stroke 2014; 9: 313–20. [DOI] [PubMed] [Google Scholar]
- 5.Rodgers H, Bosomworth H, Krebs HI, et al. Robot assisted training for the upper limb after stroke (RATULS): a multicentre randomised controlled trial. Lancet 2019; 394: 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy RM, Harvey RL, Kissela BM, et al. Epidural Electrical Stimulation for Stroke Rehabilitation. Neurorehabil Neural Repair 2016; 30: 107–19. [DOI] [PubMed] [Google Scholar]
- 7.Hankey GJ, Hackett ML, Almeida OP, et al. Safety and efficacy of fluoxetine on functional outcome after acute stroke (AFFINITY): a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2020; 19: 651–60. [DOI] [PubMed] [Google Scholar]
- 8.Harvey RL, Edwards D, Dunning K, et al. Randomized sham-controlled trial of navigated repetitive transcranial magnetic stimulation for motor recovery in stroke the NICHE trial. Stroke 2018; 49: 2138–46. [DOI] [PubMed] [Google Scholar]
- 9.Kwakkel G, Veerbeek JM, van Wegen EEH, Wolf SL. Constraint-induced movement therapy after stroke. Lancet Neurol. 2015; 14: 224–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engineer ND, Kimberley TJ, Prudente CN, Dawson J, Tarver WB, Hays SA. Targeted Vagus Nerve Stimulation for Rehabilitation After Stroke. Front Neurosci 2019; 13: 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hays SA, Rennaker RL, Kilgard MP. Targeting plasticity with vagus nerve stimulation to treat neurological disease. Prog Brain Res 2013; 207: 275–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engineer ND, Riley JR, Seale JD, et al. Reversing pathological neural activity using targeted plasticity. Nature 2011; 470: 101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porter BA, Khodaparast N, Fayyaz T, et al. Repeatedly pairing vagus nerve stimulation with a movement reorganizes primary motor cortex. Cereb Cortex 2012; 22: 2365–74. [DOI] [PubMed] [Google Scholar]
- 14.Meyers EC, Solorzano BR, James J, et al. Vagus nerve stimulation enhances stable plasticity and generalization of stroke recovery. Stroke 2018; 49: 710–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dawson J, Pierce D, Dixit A, et al. Safety, feasibility, and efficacy of vagus nerve stimulation paired with upper-limb rehabilitation after ischemic stroke. Stroke 2016; 47: 143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimberley TJ, Pierce D, Prudente CN, et al. Vagus Nerve Stimulation Paired With Upper Limb Rehabilitation After Chronic Stroke. Stroke 2018; 49: 1–5. [DOI] [PubMed] [Google Scholar]
- 17.Kimberley TJ, Prudente CN, Engineer ND, et al. Study protocol for a pivotal randomised study assessing vagus nerve stimulation during rehabilitation for improved upper limb motor function after stroke. Eur Stroke J 2019; : 239698731985530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Townsley RB, Hilmi OJ. The use of nerve monitoring in the placement of vagal nerve stimulators. Clin Otolaryngol 2017; 42: 959–61. [DOI] [PubMed] [Google Scholar]
- 19.Bushnell C, Bettger JP, Cockroft KM, et al. Chronic stroke outcome measures for motor function intervention trials: Expert panel recommendations. Circ Cardiovasc Qual Outcomes 2015; 8: S163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.See J, Dodakian L, Chou C, et al. A Standardized Approach to the Fugl-Meyer Assessment and Its Implications for Clinical Trials. Neurorehabil Neural Repair 2013; 27: 732–41. [DOI] [PubMed] [Google Scholar]
- 21.Page SJ, Fulk GD, Boyne P. Clinically Important Differences for the Upper-Extremity Fugl-Meyer Scale in People With Minimal to Moderate Impairment Due to Chronic Stroke. Phys Ther 2012; 92: 791–8. [DOI] [PubMed] [Google Scholar]
- 22.Lin KC, Hsieh YW, Wu CY, Chen CL, Jang Y, Sen Liu J. Minimal detectable change and clinically important difference of the wolf motor function test in stroke patients. Neurorehabil Neural Repair 2009; 23: 429–34. [DOI] [PubMed] [Google Scholar]
- 23.Dhamoon MS, Moon YP, Paik MC, et al. Long-term functional recovery after first ischemic stroke: The Northern manhattan study. Stroke 2009; 40: 2805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stinear CM, Lang CE, Zeiler S, Byblow WD. Advances and challenges in stroke rehabilitation. Lancet Neurol. 2020; 19: 348–60. [DOI] [PubMed] [Google Scholar]
- 25.Cramer SC, Sur M, Dobkin BH, et al. Harnessing neuroplasticity for clinical applications. Brain. 2011; 134: 1591–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Page SJ, Fulk GD, Boyne P. Clinically Important Differences for the Upper-Extremity Fugl-Meyer Scale in People With Minimal to Moderate Impairment Due to Chronic Stroke. Phys Ther 2012; 92: 791–8. [DOI] [PubMed] [Google Scholar]
- 27.van der Lee JH, Wagenaar RC, Lankhorst GJ, Vogelaar TW, Devillé WL, Bouter LM. Forced use of the upper extremity in chronic stroke patients: results from a single-blind randomized clinical trial. Stroke 1999; 30: 2369–75. [DOI] [PubMed] [Google Scholar]
- 28.Lin KC, Fu T, Wu CY, et al. Minimal detectable change and clinically important difference of the stroke impact scale in stroke patients. Neurorehabil Neural Repair 2010; 24: 486–92. [DOI] [PubMed] [Google Scholar]
- 29.chung Lin K, Fu T, yi Wu C, ju Hsieh C. Assessing the Stroke-Specific Quality of Life for Outcome Measurement in Stroke Rehabilitation: Minimal Detectable Change and Clinically Important Difference. Health Qual Life Outcomes 2011; 9. DOI: 10.1186/1477-7525-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khodaparast N, Kilgard MP, Casavant R, et al. Vagus Nerve Stimulation during Rehabilitative Training Improves Forelimb Recovery after Chronic Ischemic Stroke in Rats. Neurorehabil Neural Repair 2016; 30: 676–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hulsey D Neuromodulatory pathways required for targeted plasticity therapy. Dissertation. 2018. [Google Scholar]
- 32.He K, Huertas M, Hong SZ, et al. Distinct Eligibility Traces for LTP and LTD in Cortical Synapses. Neuron 2015; 88: 528–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seol GH, Ziburkus J, Huang S, et al. Neuromodulators Control the Polarity of Spike-Timing-Dependent Synaptic Plasticity. Neuron 2007; 55: 919–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ni Z, Gunraj C, Kailey P, Cash RFH, Chen R. Heterosynaptic modulation of motor cortical plasticity in human. J Neurosci 2014; 34: 7314–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robinson LC, Winston KR. Relationship of vocal cord paralysis to the coil diameter of vagus nerve stimulator leads. J Neurosurg 2015; 122: 532–5. [DOI] [PubMed] [Google Scholar]
- 36.Kahlow H, Olivecrona M. Complications of vagal nerve stimulation for drug-resistant epilepsy: A single center longitudinal study of 143 patients. Seizure 2013; 22: 827–33. [DOI] [PubMed] [Google Scholar]
- 37.Metherate R, Cox CL, Ashe JH. Cellular bases of neocortical activation: Modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. J Neurosci 1992; 12: 4701–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayat H, Regev N, Matosevich N, et al. Locus coeruleus norepinephrine activity mediates sensory-evoked awakenings from sleep. Sci Adv 2020; 6. DOI: 10.1126/sciadv.aaz4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pruitt DT, Danaphongse TT, Lutchman M, et al. Optimizing Dosing of Vagus Nerve Stimulation for Stroke Recovery. Transl Stroke Res 2020. DOI: 10.1007/s12975-020-00829-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morrison RA, Danaphongse TT, Pruitt DT, et al. A limited range of vagus nerve stimulation intensities produce motor cortex reorganization when delivered during training. Behav Brain Res 2020; 391. DOI: 10.1016/j.bbr.2020.112705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yap JYY, Keatch C, Lambert E, Woods W, Stoddart PR, Kameneva T. Critical Review of Transcutaneous Vagus Nerve Stimulation: Challenges for Translation to Clinical Practice. Front. Neurosci 2020; 14: 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burger AM, Verkuil B. Transcutaneous nerve stimulation via the tragus: are we really stimulating the vagus nerve? Brain Stimul 2018; 11: 945–6. [DOI] [PubMed] [Google Scholar]
- 43.Dawson J, Engineer ND, Prudente CN, et al. Vagus Nerve Stimulation Paired With Upper-Limb Rehabilitation After Stroke: One-Year Follow-up. Neurorehabil Neural Repair 2020; 34: 609–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


