Abstract
A striking increase in the numbers of cultures positive for Mycobacterium tuberculosis was noticed in a mycobacterial reference laboratory in Campinas, Sao Paulo State, Brazil, in May 1995. A contaminated bronchoscope was the suspected cause of the increase. All 91 M. tuberculosis isolates grown from samples from patients between 8 May and 18 July 1995 were characterized by spoligotyping and IS6110 fingerprinting. Sixty-one of the 91 isolates had identical spoligotype patterns, and the pattern was arbitrarily designated S36. The 61 specimens containing these isolates had been processed and cultured in a 21-day period ending on 1 June 1995, but only 1 sample was smear positive for acid-fast bacilli. The patient from whom this sample was obtained was considered to be the index case patient and had a 4+ smear-positive lymph node aspirate that had been sent to the laboratory on 10 May. Virtually all organisms with spoligotype S36 had the same IS6110 fingerprint pattern. Extensive review of the patients’ charts and investigation of laboratory procedures revealed that cross-contamination of specimens had occurred. Because the same strain was grown from all types of specimens, the bronchoscope was ruled out as the outbreak source. The most likely source of contamination was a multiple-use reagent used for specimen processing. The organism was cultured from two of the solutions 3 weeks after mock contamination. This investigation strongly supports the idea that M. tuberculosis grown from smear-negative specimens should be analyzed by rapid and reliable strain differentiation techniques, such as spoligotyping, to help rule out laboratory contamination.
The inexorable global increase in tuberculosis cases (1) has resulted in a substantial rise in the numbers of specimens cultured in diagnostic mycobacteriology laboratories. This increase and the advent and use of molecular tools to subtype or fingerprint Mycobacterium tuberculosis have resulted in increased recognition of specimen contamination events linked either to laboratory procedures or, in rare cases, to contaminated bronchoscopes (2–5, 7, 9–13, 15). Generally, contamination of only a relatively few specimens from patients has been documented, and this suggests that the number of individuals detrimentally affected has been small and that the associated treatment and contact investigation costs have been low. In the work described here, we used molecular strain typing and epidemiologic techniques to document an extensive pseudo-outbreak of tuberculosis involving 60 patients, all of whom were treated for the disease.
MATERIALS AND METHODS
The mycobacterial laboratory located at the Hospital das Clinicas da Universidade Estadual de Campinas in Campinas, Sao Paulo State, Brazil, serves an area with an estimated population of 5 million people. The laboratory receives about 5,000 to 8,000 specimens each year, and mycobacteria are grown from 5.4% of the specimens. M. tuberculosis complex isolates are identified in approximately 60% of the positive cultures.
One M. tuberculosis isolate was examined from each culture-positive patient identified during the study period (91 specimens from 91 patients). The specimens positive by culture were bronchoalveolar lavage (n = 23); sputum (n = 23); pleural fluid (n = 12); urine (n = 10); stool (n = 6); lymph node (n = 4); ascites fluid (n = 4); gastric lavage (n = 3); and synovial fluid, middle ear aspirate, lung biopsy, pleural biopsy, hepatic biopsy, and bone biopsy (n = 1 each) specimens. The patients’ charts were reviewed to identify signs and symptoms associated with tuberculosis.
Specimen processing was performed in a laminar-flow hood in a biosafety level 3 laboratory. All reagents used in the decontamination protocol were made in 1,000-ml volumes. Sterile pipettes were used to transfer the solutions from the main stock bottle into individual specimen tubes. All specimens, including normally sterile material (except cerebrospinal fluid specimens) were decontaminated by a standard NaOH procedure. An equal volume of 4% NaOH was added to the specimen, and the contents were mixed by inversion for 15 min and diluted with phosphate-buffered saline to a final volume of 50 ml. After centrifugation at 3,000 rpm for 15 min, the supernatant was discarded. The pH of the pellet was checked by adding a few drops of phenol red and was adjusted to pH 7 with 3 M HCl. The pellet was then resuspended in a few drops of 0.9% NaCl, and the sediment was used to prepare Ziehl-Neelsen smears and to inoculate one Löwenstein-Jensen slant. The cultures were incubated at 35°C and were checked for growth weekly for 8 weeks. The BACTEC radiometric culture method was not used in this period. The AccuProbe M. tuberculosis test (Gen-Probe Inc., San Diego, Calif.) was used for species confirmation. The bacteria analyzed were recovered from frozen Löwenstein-Jensen cultures.
Spoligotyping was performed with a commercially available kit (Isogen Bioscience BV, Maarssen, The Netherlands) according to the instructions supplied by the manufacturer. Briefly, the M. tuberculosis direct repeat region (6) was amplified with primers DRa (biotinylated) and DRb (8). The amplified DNA was hybridized to a membrane containing 43 oligonucleotide probes derived from the spacer sequences in the direct repeat region of M. tuberculosis with a 45-lane blotter (Miniblotter 45; Immunetics, Cambridge, Mass.). The membrane was incubated with horseradish peroxidase-labeled streptavidin, and hybridization was detected by enhanced chemiluminescence (ECL Direct Labeling and Detection System; Amersham, Arlington Heights, Ill.). IS6110 fingerprinting was performed by an internationally standardized protocol (14).
RESULTS
The investigation was initiated when an attending physician noted that all bronchoalveolar lavage specimens cultured in the laboratory in May 1995 were reported to be positive for M. tuberculosis. Concern was raised that a bronchoscope had not been sterilized properly. However, because molecular typing techniques were not available to the laboratory at that time and because there was clinical suspicion of tuberculosis, treatment of these patients was continued.
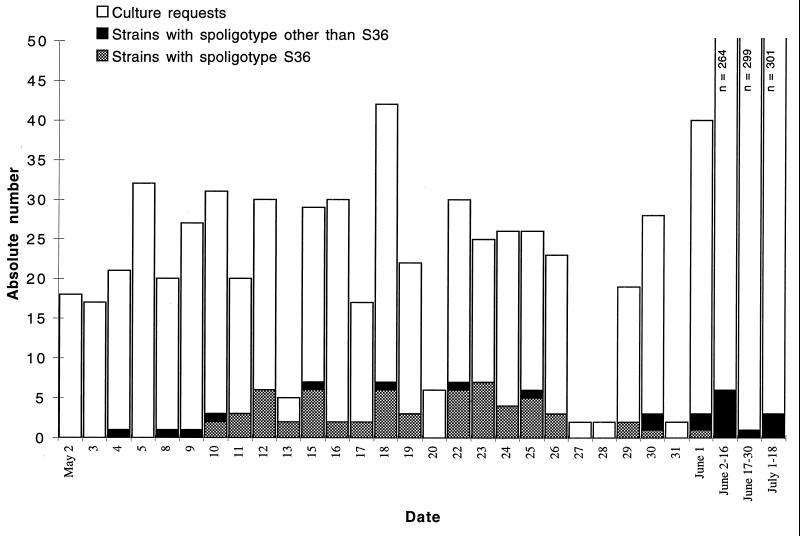
To conduct a retrospective investigation examining the possibility of specimen contamination, all M. tuberculosis isolates obtained between 8 May and 18 July 1995 were characterized by spoligotyping and IS6110 fingerprinting. Normally the laboratory identifies one to three culture-positive specimens per week, but for a 3-week period in May this number increased dramatically (Fig. 1). Ninety-one patients had culture-positive specimens in the study period. Eleven of the 91 specimens were smear positive for acid-fast bacilli (AFB).
FIG. 1.
Temporal distribution of culture-positive specimens in the study period. The number of culture requests from 2 May through 18 July 1995 is represented by bars. Solid and cross-hatched areas, total number of positive cultures; cross-hatched area, strains with spoligotype pattern S36.
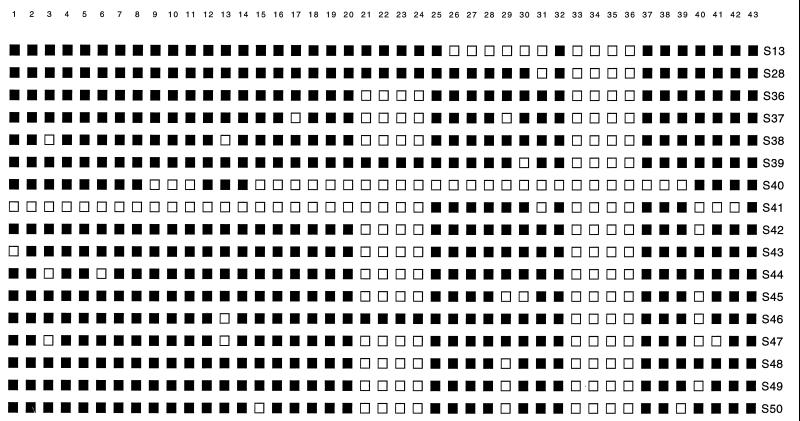
Seventeen different spoligotypes were identified among the 91 M. tuberculosis isolates (Fig. 2). Sixty-one isolates had a spoligotype arbitrarily designated S36, and 60 of these isolates were cultured from specimens processed between 10 May and 1 June 1995. A review of the laboratory records revealed that only one patient was smear positive. On 10 May a 4+ AFB-positive purulent specimen was obtained from a lymph node aspirate from this patient, who was considered to be the index case patient. All other isolates grown from smear-positive specimens had different spoligotypes.
FIG. 2.
Spoligotype patterns identified among M. tuberculosis isolates. Solid squares, hybridization with the designated spacer probe; open squares, lack of hybridization.
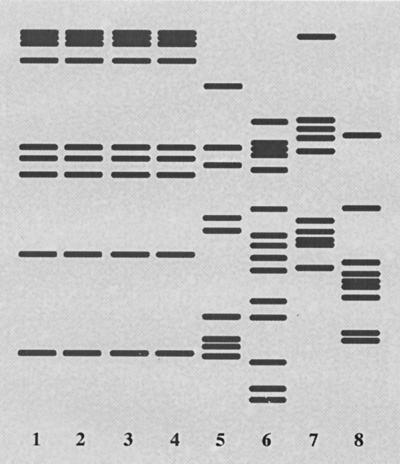
The results of the spoligotype analysis suggested that the 61 strains with pattern S36 were related by recent clonal descent. However, M. tuberculosis isolates grouped together on the basis of spoligotype sometimes can be differentiated by IS6110 fingerprinting (8). In this light, analysis of a random sample of 53 of the 61 isolates with the S36 spoligotype by IS6110 typing found that 49 of these 53 organisms also had the same IS6110 fingerprint, arbitrarily designated AK (Fig. 3). Four isolates with the S36 spoligotype had different IS6110 fingerprints, including two with unique fingerprints. Two specimens had a unique fingerprint mixed with the AK pattern, suggesting that two M. tuberculosis clones were present in these samples. IS6110 fingerprint analysis of 17 M. tuberculosis isolates with a spoligotype other than S36 found that none had the AK fingerprint characteristic of the contaminating organism (Fig. 3).
FIG. 3.
IS6110 fingerprint patterns obtained for selected M. tuberculosis isolates. Isolates 1 to 4 have fingerprint pattern AK characteristic of the organism causing the pseudo-outbreak; isolates 5 to 8 have unique fingerprint patterns that differentiate them from the organism responsible for the pseudo-outbreak.
A detailed retrospective chart review found that only 19 of the 91 patients, including the index case patient, had typical manifestations of tuberculosis (fever, hemoptysis, weight loss, and good response to treatment). The great majority of patients (58 of 61) infected with the S36 spoligotype strain did not have the full spectrum of manifestations.
DISCUSSION
Taken together, the data presented here indicate that laboratory cross-contamination of specimens resulted in a pseudo-outbreak of tuberculosis that affected 60 patients. Review of the literature indicated that this is the largest documented episode of laboratory cross-contamination (2–5, 7, 9–13, 15).
Although the investigation was initiated because of a concern about bronchoscope contamination, molecular characterization of cultures revealed that the same strain was grown from all types of specimens. Hence, the bronchoscope was ruled out as the outbreak source. Instead, the most likely cause of contamination was found to be the procedure used to decontaminate specimens. All false-positive cultures were obtained after a highly AFB-positive sample that grew a strain with the same fingerprint had been processed in the laboratory. The solutions used in the decontamination procedure were generally used for approximately 1 month. It is likely that this organism was inadvertently introduced into one of these reagents. Use of the same solution during all of May then resulted in contamination of many specimens processed in that period. Importantly, we found that M. tuberculosis could be grown from the phosphate-buffered saline solution and the 0.9% NaCl solution 3 weeks after mock contamination. The fact that all 244 cerebrospinal fluid specimens were culture negative is also consistent with the idea that the contaminant organism was introduced during the specimen decontamination procedure. (These specimens were cultured directly and were not decontaminated.) Recognition of the pseudo-outbreak and elucidation of its probable cause have resulted in the revision of laboratory procedures. For example, all specimens obtained from sterile body sites are now cultivated without decontamination. Most importantly, each solution used in the decontamination protocol is now aliquoted daily from the main stock container into a smaller vessel.
To avoid an erroneous diagnosis of tuberculosis and the resulting unnecessary treatment and contact investigation, mycobacteriology laboratories should institute strict procedures to prevent and identify cross-contamination episodes. Excellent guidelines have been provided by Small et al. (12). Although IS6110 analysis is the current standard method for the differentiation of M. tuberculosis strains, this technique requires a mature culture, is time-consuming, and is available only in specialized laboratories. Our results suggest that spoligotyping can be used as an initial screening method to rapidly identify potential episodes of laboratory cross-contamination. In addition, laboratories should adopt surveillance mechanisms designed to promptly detect and investigate culture-positive specimens from patients lacking clinical manifestations of tuberculosis (4).
ACKNOWLEDGMENTS
This research was supported by Public Health Service grant DA-09238 (to J.M.M.).
We thank S. Siddiqui for excellent assistance with the graphics.
REFERENCES
- 1.Anonymous. Tuberculosis notification update. Weekly Epidemiol Rec. 1996;71:65–72. [PubMed] [Google Scholar]
- 2.Bauer J, Thomsen V O, Poulsen S, Andersen A B. False-positive results from cultures of Mycobacterium tuberculosis due to laboratory cross-contamination confirmed by restriction fragment length polymorphism. J Clin Microbiol. 1997;35:988–991. doi: 10.1128/jcm.35.4.988-991.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhattacharya M, Dietrich S, Mosher L, Siddiqui F, Reisberg B E, Paul W S, Warren J R. Cross contamination of specimens with Mycobacterium tuberculosis: clinical significance, causes, and prevention. Microbiol Infect Dis. 1997;109:324–330. doi: 10.1093/ajcp/109.3.324. [DOI] [PubMed] [Google Scholar]
- 4.Braden C R, Templeton G L, Stead W W, Bates J H, Cave M D, Valway S E. Retrospective detection of laboratory cross-contamination of Mycobacterium tuberculosis cultures with use of DNA fingerprint analysis. Clin Infect Dis. 1997;24:35–40. doi: 10.1093/clinids/24.1.35. [DOI] [PubMed] [Google Scholar]
- 5.Dunlap N E, Harris R H, Benjamin W H, Jr, Harden J W, Hafner D. Laboratory contamination of Mycobacterium tuberculosis cultures. Am J Respir Crit Care Med. 1995;152:1702–1704. doi: 10.1164/ajrccm.152.5.7582316. [DOI] [PubMed] [Google Scholar]
- 6.Groenen P M A, Bunschoten A E, van Soolingen D, van Embden J D A. Nature of DNA polymorphism in the direct repeat cluster of Mycobacterium tuberculosis: application for strain differentiation by a novel typing method. Mol Microbiol. 1993;10:1057–1065. doi: 10.1111/j.1365-2958.1993.tb00976.x. [DOI] [PubMed] [Google Scholar]
- 7.Jones W D., Jr Bacteriophage typing of Mycobacterium tuberculosis cultures from incidents of suspected laboratory cross-contamination. Tubercle. 1988;69:43–49. doi: 10.1016/0041-3879(88)90039-6. [DOI] [PubMed] [Google Scholar]
- 8.Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Embden J. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35:907–914. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacGregor, R. R., L. W. Clark, and F. Bass. The significance of isolating low numbers of Mycobacterium tuberculosis in culture of sputum specimens. Chest 68:518–523. [DOI] [PubMed]
- 10.Maurer J R, Desmond E P, Lesser M D, Jones W D. False-positive cultures of Mycobacterium tuberculosis. Chest. 1984;86:439–442. doi: 10.1378/chest.86.3.439. [DOI] [PubMed] [Google Scholar]
- 11.Murray P R. Mycobacterial cross-contamination with the modified BACTEC 460 TB system. Diagn Microbiol Infect Dis. 1991;14:33–35. doi: 10.1016/0732-8893(91)90085-t. [DOI] [PubMed] [Google Scholar]
- 12.Small P M, McClenny N B, Singh S P, Schoolnik G K, Tompkins L S, Mickelsen P A. Molecular strain typing of Mycobacterium tuberculosis to confirm cross-contamination in the mycobacteriology laboratory and modification of procedures to minimize occurrence of false-positive cultures. J Clin Microbiol. 1993;31:1677–1682. doi: 10.1128/jcm.31.7.1677-1682.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith W B, Vance D W., Jr Specimen cross-contamination by a strain of Mycobacterium tuberculosis lacking nitrate reductase activity. Diagn Microbiol Infect Dis. 1991;14:523–526. doi: 10.1016/0732-8893(91)90012-5. [DOI] [PubMed] [Google Scholar]
- 14.van Embden J D A, Cave M D, Crawford J T, Dale J W, Eisenach K D, Gicquel B, Hermans P, Martin C, McAdam R, Shinnick T M, Small P M. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wurtz R, Demarais P, Trainor W, McAuley J, Kocka F, Mosher L, Dietrich S. Specimen contamination in mycobacteriology laboratory detected by pseudo-outbreak of multidrug-resistant tuberculosis: analysis by routine epidemiology and confirmation by molecular technique. J Clin Microbiol. 1996;34:1017–1019. doi: 10.1128/jcm.34.4.1017-1019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]