Abstract
A male with C7 complete tetraplegia participated in 14 weeks of body weight supported treadmill training (BWSTT) combined with spinal cord epidural stimulation (SCES), 4 weeks of no intervention, and two more weeks of BWSTT + SCES. The participant presented with unstable resting seated blood pressure (BP; 131/66 mmHg). After retrospective analysis, resting systolic BP decreased and diastolic BP increased, yielding a safe mean arterial BP. There was a fivefold increase in BWSTT bouts per session, and percentage of body weight support decreased to 69%. BWSTT + SCES safely and effectively regulated resting BP and mitigated symptoms of orthostatic intolerance. These effects were not maintained after 4 weeks without training.
Unstable blood pressure (BP) is a common problem in tetraplegic spinal cord injury (SCI), and induces a spectrum of hemodynamic consequences owing to a loss of homeostatic regulation of the autonomic nervous system. 1 This manifests as aberrant resting BP and/or BP fluctuations in response to sudden positional changes, such as orthostatic hypotension or autonomic dysreflexia. 1 , 2 These hemodynamic challenges may interfere with participation in active rehabilitation programs, and lead to social isolation and poor quality of life. Thus, regulation of BP is a key rehabilitation goal for persons with tetraplegia. 3
Recent reports have demonstrated the efficacy and the mechanisms of spinal cord epidural stimulation (SCES) in enhancing motor recovery, 4 and restoration of standing and volitional overground stepping. 5 , 6 , 7 Furthermore, multiple reports show that SCES is effective in modulating lumbosacral neural circuits to regulate BP in persons with complete SCI. 8 , 9 , 10 Potential mechanisms of SCES‐induced regulation of orthostatic hypotension and cardiovascular function have been proposed. 11 , 12 This may include increasing the resting membrane potential of the sympathetic circuitry via the stimulation of dorsal afferent relays, modulating local efferent outflow of spinal sympathetic neurons, changes in hormonal and inflammatory profiles, or vasodilation induced by antidromic stimulation of the afferent terminals. 12 Others showed that SCES ameliorated orthostatic hypotension, increasing BP from 81/48 mmHg to 120/72 mmHg. 9 While these trials have focused on orthostatic intolerance after SCI, we are unaware of any research investigating the effects of SCES on unstable resting BP and orthostatic intolerance. It is likely that SCES may either normalize unstable BP in individuals with tetraplegia or offer carryover effects after cessation of stimulation. 9 SCES may normalize BP regulation by simultaneously mitigating unstable resting BP and orthostatic intolerance.
To examine the role of body weight supported treadmill training (BWSTT) + SCES in modulating unstable BP, clinical data were retrospectively analyzed to investigate the training effects of BWSTT + SCES on resting BP as well as symptoms of orthostatic intolerance in a person with complete tetraplegia. The participant presented with resting borderline resting hypertension (131/66 mmHg) in a seated position and symptoms of orthostatic intolerance during standing challenges. The unstable BP was not related to any other illness or chronic comorbidities. Two different configurations of SCES were used independent from one another to either facilitate walking during manually assisted BWSTT or to activate core muscles to relieve symptoms of orthostatic indolence during standing (Fig. 1A–C). We also explored the carryover effects of the treatment at 4 weeks post‐intervention. We hypothesized that BWSTT + SCES would favorably modulate unstable BP and symptoms of orthostatic intolerance in a person with complete tetraplegia.
Figure 1.

(A) Radiographic scan of the SCES paddle placed at the T9‐T10 interspace and extended to the mid T11 vertebral body. (B) Schematic and settings of the SCES program used for stepping during BWSTT. Cathodes (red); anodes (gray); inactive (white). (C) Schematic and settings of the SCES program used for activation of core muscles after developing symptoms of orthostatic intolerance. Cathodes (red); anodes (gray); inactive (white). [Colour figure can be viewed at wileyonlinelibrary.com]
Case Report
A 34‐year‐old male with a clinically complete traumatic SCI (C7, American Spinal Injury Association Impairment Scale [AIS] A, zone of partial preservation at T1, sustained in 2014) participated in the current trial. After 3.75 years, the participant was implanted with a SCES array (5–6‐5 Restore ADVANCED, Medtronic, Fridley, MN; Fig. 1A–C) that covered the T9‐T10 interspace and extended to the mid T11 vertebral body (Fig. 1A). This was an elective surgery followed by 45 days of inpatient rehabilitation which the participant received at an outside medical facility. 7 The participant underwent SCES implantation with the goal of restoring his ability to stand and perform overground stepping. The team at the outside medical facility created multiple SCES configurations for the participant to use, including SCES configurations intended to facilitate walking (Fig. 1B) or core muscle activity (Fig. 1C).
At the time of admission into an exercise program at our hospital, the participant weighed 77.5 kg, with a height of 170 cm and body mass index of 26.2 kg/m2 with mild muscle spasms. The participant was on Midodrine HCL (5 mg) to manage orthostatic hypotension which remained unchanged over the course of his participation in the program. Electrocardiography examination was normal.
The participant previously completed 24 sessions (12 weeks) of exoskeleton training with SCES which resulted in greatly improved motor activity during exoskeleton‐assisted walking. 7 After completion of the exoskeleton training phase, the participant was invited to participate in BWSTT + SCES for an additional 14 weeks, twice weekly for 9 weeks (training phase) and then once weekly from weeks 10 to14 (maintenance phase). The decision to decrease the frequency was based on prior research demonstrating a frequency of once weekly was adequate to maintain physiological adaptations, ensure adherence, and reduce burden in persons with SCI. 13 The maintenance phase was followed by 4 weeks with no intervention due to family and travelling commitments. After this 18‐week period, the participant completed two more BWSTT sessions over the course of 2 weeks, enabling exploration of any carryover effects after 4 weeks without training (total of 20 weeks).
Seated resting BP, heart rate, and rate of perceived exertion (RPE) were measured 5–10 min before and after every session. Measurements were captured once at the level of the brachial artery with the arm maintained in a horizontal position at the level of the heart (COSMED 740). Mean arterial BP was calculated using the formula: mean arterial BP = Diastolic BP + 1/3 × (Systolic BP − Diastolic BP). The number of BWSTT bouts per session and the duration of each bout were recorded across the 20 weeks and used as a surrogate index of orthostatic intolerance. A bout of BWSTT was stopped when the participant reported symptoms of orthostatic intolerance, such as dizziness or shortness of breath. Once symptoms of orthostatic intolerance developed, the participant was asked to turn on the core muscle SCES program in a standing position to offset the drop in BP (Fig. 1C). The SCES amplitude of the core program remained unchanged throughout the trial and the stimulation lasted 3–5 min. Increasing the number of bouts per single training session reflected the ability to maintain the BP against orthostatic challenges.
Manual‐assisted BWSTT was provided via the Therastride System (Innoventor Engineering, Louisville, KY) according to standard procedures. 6 During BWSTT, the SCES walking configuration was only used to facilitate stepping on the treadmill (Fig. 1B), while the core muscle activity configuration was used to alleviate symptoms of orthostatic intolerance during standing breaks. Stepping speed was initially set at 1.5 mph and then increased by 0.2 mph to a maximum of 2.7 mph for the first 4 weeks, with the aim of cardiovascular conditioning. From weeks 5–14, the speed was set at 0.3 mph to facilitate volitional effort during assisted stepping with SCES. The decision to increase speed was made based on the participant's tolerance to maintain erect body posture and to successfully control rotation of his pelvis. For warm‐up purposes, the first training bout of each session (range: 1.16–5.30 min) was provided at 95% body weight support (%BWS) without SCES. The percentage of BWS was lowered by 5% during the BWSTT bouts of each session, provided the participant maintained adequate trunk control and knee extension in the stance phase. The decrease in percentage of BWS of the participant necessitated a subsequent gradual increase in the SCES amplitude to ensure stepping with erect posture.
Results
At the time of the admission, the participant experienced unstable BP and symptoms of orthostatic intolerance according to ranges previously described. 2 , 14 Blood pressure results are summarized in Figure 2. In weeks 1–4, the participant's seated resting BP averaged 125/65 mmHg; 75% of BP measurements were borderline hypertensive and 25% were normotensive according to ranges previously described. 2 In weeks 5–9, the participant's seated resting BP averaged 122/71 mmHg, with 20% of measurements hypertensive, 60% of measurements borderline hypertensive, and 20% of measurements hypotensive. The participant then switched to training once per week from weeks 10 to 14, when his seated resting BP averaged 118/71 mmHg, with 20% of measurements hypertensive, 60% normotensive, and 20% hypotensive. After a 4‐week break, the participant's seated resting BP across the two follow‐up sessions was reduced to 100/55 mmHg and 98/61 mmHg.
Figure 2.
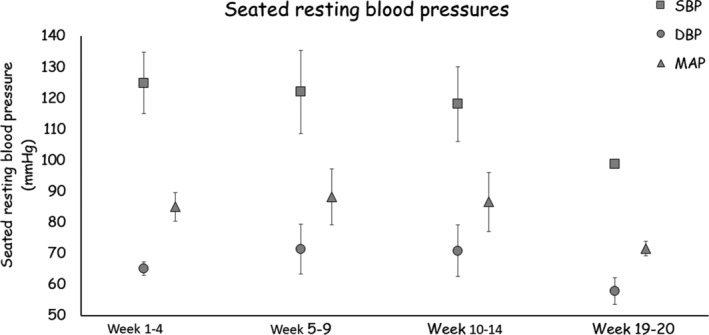
Average seated resting systolic, diastolic, and mean arterial BP over the course of 14 weeks and the 2 weeks of follow‐up visits. During the follow‐up visits, the effects on BP were attenuated but remained in a safe range.
During the 14 weeks of combined BWSTT + SCES, the percentage of BWS decreased to an average of 69% for up to 20 sec without buckling of knees or developing any symptoms of orthostatic intolerance (Fig. 3D). Standing RPE was six at the beginning of the session and reached 16–18 at the end of the session, even at speeds as low as 0.3 mph. The participant's RPE during the development of symptoms of orthostatic intolerance was 14–15 during the training phase (i.e., weeks 1–9). In the maintenance phase (i.e., weeks 10–14), his RPE ranged from 10 to 14.
Figure 3.
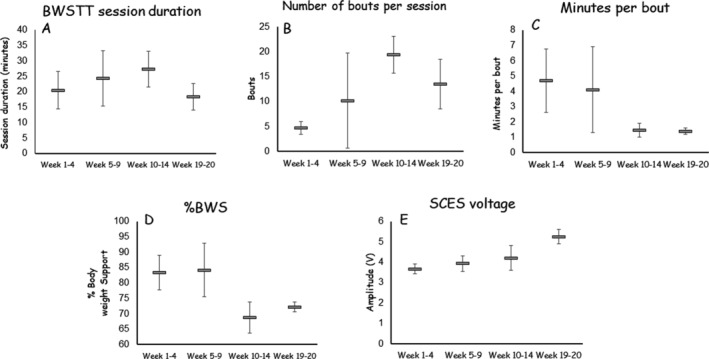
(A) Average time (minutes) of walking during BWSTT sessions over weeks 1–4 (2 visits per week), weeks 5–9 (2 visits per week), weeks 10–14 (once weekly), and during the two weekly follow‐ups (once weekly) at 4 weeks after cessation to determine carry over effects. (B) Average number of walking bouts during BWSTT sessions over weeks 1–4, 5–9, 10–14, and during the 2 weeks of follow‐up after cessation of training for 4 weeks. The total number of bouts per session was determined based on the ability to withstand orthostatic standing challenges. There was a threefold increase in the average number of bouts per session and a 33% increase in the duration of the session in weeks 10–14 compared to weeks 1–4. (C) Average minutes per bout over the course of training. The minutes per bout decreased by 69% over the course of 14 weeks and was maintained during the 2‐week follow‐up visits. This reflected the decrease in percentage of BWS as indicated in 3D. (D) The percentage of BWS provided by the suspension that ensured erect posture without buckling of the knees. The percentage of BWS decreased from 83% to 69% over the course of the initial 14 weeks. The percentage of BWS was maintained at 72% in the 2‐week follow‐up visits. (E) A 14.4% increase in SCES amplitude from 3.67 V (weeks 1–4) to 4.2 V (weeks 10–14). After 4 weeks of cessation of BWSTT, the SCES amplitude was increased to 5.5 V (43%) to maintain 72%BWS.
Over the course of training, the minutes per bout decreased, though number of bouts and the total BWSTT session duration increased (Fig. 3A–C). Considering this along with the fact that resting recovery periods between BWSTT bouts ranged from 5 to 10 min in weeks 1–9, while resting recovery periods were less than 5 min in weeks 10–14, suggests improved tolerance to orthostatic challenges. The SCES amplitude (V) increased by 14.4% at the end of week 14 and by 43% at the end of week 20 (Fig. 3E). Figure 4 presents the relationships between percentage of BWS and different outcome variables. A decrease in the percentage of BWS was associated with an increase in the duration of BWSTT sessions (Fig. 4A), decreased duration per bout (Fig. 4B), and increased SCES amplitude (Fig. 4C). Finally, lower seated resting systolic BP (Fig. 4D) and mean arterial BP values (Fig. 4E) appeared to be related to higher SCES amplitudes targeted toward stepping over the course of the trial.
Figure 4.
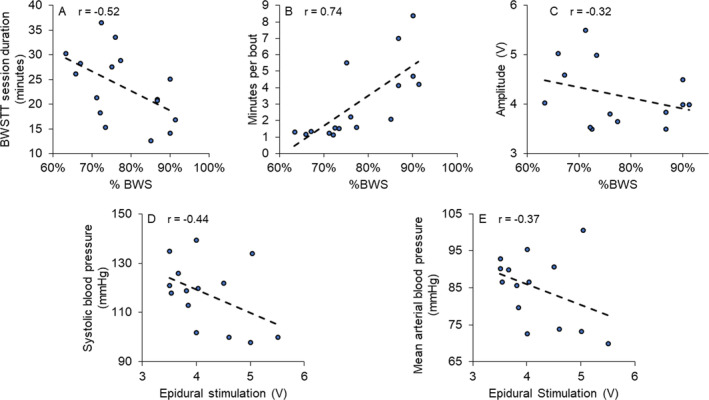
Relationships between percentage of BWS and (A) total duration; (B) minutes per bout; and (C) SCES amplitude, this negative relationship reflects the increasing energy required to maintain the corresponding decrease in percentage of BWS during training. The increase in SCES amplitude appeared to be related to the decrease in Systolic BP and mean arterial BP; (D) resting systolic BP; and (E) resting mean arterial BP. Each point represents the average of multiple visits during the course of the trial. [Colour figure can be viewed at wileyonlinelibrary.com]
Discussion
This case report demonstrated that SCES facilitates stepping in an individual with tetraplegia and the use of SCES does not exacerbate unstable BP while also mitigating symptoms of orthostatic intolerance. This suggests that SCES is safe in this population during BWSTT. The major findings indicated that following this 14‐week intervention, a person with C7 complete SCI improved resting BP and tolerance to orthostatic challenges during BWSTT. The effect on resting BP was not maintained after 4 weeks without intervention but was within a range typical of individuals with tetraplegia. 14 Additionally, coupling between systolic and diastolic BP improved over the course of the trial maintaining a safe mean arterial BP, which may have been related to the energy delivered via SCES (Fig. 4D–E).
The two configurations of SCES were used independent from one another and intended to either facilitate lower extremity muscles for restoration of mobility or activation of core muscles to manage BP during standing breaks after developing symptoms of orthostatic intolerance. Resting systolic BP and mean arterial BP appeared related to the amplitude of SCES to accommodate the decrease in the percentage of BWS. The emanation of the SCES current to the parasympathetic sacral branches may be responsible for the decrease in seated resting BP by regulating the activity of the sympathetic chain. 15 , 16 Other mechanisms may include increasing the resting membrane potential of sympathetic circuitry via the stimulation of dorsal afferent relays, modulating local efferent outflow of spinal sympathetic neurons, changes in hormonal and inflammatory profiles, or vasodilation induced by antidromic stimulation causing calcitonin gene‐related peptide release at afferent terminals. 18
It is important to note that the paddle was not placed in a typical location for clinically induced vasodilation (L2–L3). In addition, much of the prior research used a SCES paddle placed at T11‐L1 wherein a dual effect of motor control and BP control was noticed. 6 , 8 Apart from SCES, potential mechanisms underlying results of this study may include exercise‐mediated enhancement of endothelial‐dependent vasodilation, and/or arterial wall remodeling. 17 , 19
The same participant rarely reported symptoms of orthostatic intolerance during exoskeletal walking until the swing assistance was decreased to 35% of the original assistance. 7 At this level of exoskeletal assistance, the RPE increased from 10 or 11 to 16. 7 Importantly, exoskeleton training exerts a relatively low metabolic cost compared to BWSTT. 20 , 21 Previous work showed that BWSTT mitigates orthostatic intolerance in persons with tetraplegia. 22
Limitations
In the current report, it is difficult to segregate between the effects of BWSTT and SCES on mitigation of symptoms of orthostatic intolerance. Importantly, this case report does not indicate causality; however, the work may serve as the basis for future work examining the efficacy of SCES on unstable BP in persons with tetraplegia. BP was also not directly measured during standing or BWSTT to demonstrate hypotension, instead we relied on clinical symptoms of orthostatic intolerance. Furthermore, BP was measured once rather than being captured 2 or 3 times to report the average. It is possible that the first measurement is elevated and may have resulted in overestimation of the seated resting BP in the current trial; however, this is not a universal finding. 23 Another limitation is the research team was not involved in the placement of the SCES paddle which was placed higher than that typical anatomical location that covers T11‐L1 vertebrae (L1‐S2 spinal cord segments) for the purpose of restoring standing and overground locomotion. We cannot rule out other factors that may have contributed to the current findings, including potential homeostatic mechanisms and beneficial exercise effects on systemic inflammation. Furthermore, the lack of a persistent effect of the intervention on resting BP after a 4‐week break may have simply been due to intervention cessation, though other factors not controlled for during this period may be responsible for these results, such as travel‐related changes in physical activity or nutrition. Lastly, the participant's use of Midodrine HCL was constant through the training period and therefore may not have contributed to any changes seen in BP; however, the effects of any potential interaction of this medication with SCES and BWSTT cannot be inferred from this case report.
Conclusion
This case report demonstrated that the energy dose of SCES combined with BWSTT safely stabilized the unstable BP in a person with tetraplegia. Unlike earlier reports that used extensive autonomic monitoring approaches, this case report used simple but reliable and accurate clinical tools to facilitate the clinical translation of the work. The findings demonstrated safety of SCES in a person with unstable BP and symptoms of orthostatic intolerance. Within the limitations of a case report design, the participant's symptoms of orthostatic intolerance improved against dynamic orthostatic challenges triggered during BWSTT. The improved resting BP was attenuated after cessation of BWSTT + SCES but was maintained in a safe range. The amplitude of SCES was increased to ensure that the participant could maintain the duration and number of exercising bouts. This change in SCES amplitude or a myriad of other factors may have played a role in the improved resting unstable BP and orthostatic intolerance. This single case report demonstrated that SCES with task‐specific training may safely stabilize BP in a person with tetraplegia. Future research should investigate the specific underlying mechanisms leading to improved BP regulation with SCES and task‐specific motor training.
Patient's perspective
Since having the stimulator implanted and then starting research with Dr. Gorgey I have seen vast improvements in my bodies' overall health. Prior to the implantation my legs had begun serious atrophy, I would estimate they were less than half of their prior injury size. The stimulator and my work with Dr. Gorgey have increased my muscle mass in my legs by quite a bit. I have noticed benefits to my cardiovascular system, when we initially started, I would struggle to be standing upright with my stimulator on, I would wear out very fast. With time my endurance grew and now I can spend long periods of time in a standing potion while having the stimulator on. I have also seen major improvement on my ability to maintain my blood pressure while standing with the stimulator on. Initially depending on the stimulator program, I could not stand for more than 2 min before my blood pressure would drop to the point where I could not continue. I believe I can now stand close to 15 min while running that same program before I need to stop due to blood pressure. They may seem like small accomplishments, but they are far from that. We have been working diligently on getting me to the point where I can stand 100% under my own power using the stimulator. I feel that by the end of the year there is a very good chance with the stimulator and Dr. Gorgey's help I will be able to stand under my own power.
Conflict of Interest
None of the authors have any conflict of interest to declare.
Funding Information
This work was supported by U.S. Department of Defense (Grant/Award Number: ‘W81XWH‐20‐1‐0845 (SC190107 CDMRP W91ZSQ)’.
Funding Statement
This work was funded by U.S. Department of Defense grant W81XWH‐20‐1‐0845 (SC190107 CDMRP W91ZSQ).
References
- 1. Frisbie JH. Unstable baseline blood pressure in chronic tetraplegia. Spinal Cord. 2007;45(1):92‐95. doi: 10.1038/sj.sc.3101920 [DOI] [PubMed] [Google Scholar]
- 2. Weaver FM, Collins EG, Kurichi J, et al. Prevalence of obesity and high blood pressure in veterans with spinal cord injuries and disorders: a retrospective review. Am J Phys Med Rehabil. 2007;86(1):22‐29. doi: 10.1097/phm.0b013e31802b8937 [DOI] [PubMed] [Google Scholar]
- 3. Carlozzi NE, Fyffe D, Morin KG, et al. Impact of blood pressure dysregulation on health‐related quality of life in persons with spinal cord injury: development of a conceptual model. Arch Phys Med Rehabil. 2013;94(9):1721‐1730. doi: 10.1016/j.apmr.2013.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hackman J, Yousak A, Wallner JJ, Gad P, Edgerton VR, Gorgey AS. Epidural spinal cord stimulation as an intervention for motor recovery after motor complete spinal cord injury. J Neurophysiol. 2021. in press. doi: 10.1152/jn.00020.2021 [DOI] [PubMed] [Google Scholar]
- 5. Wagner FB, Mignardot JB, Le Goff‐Mignardot CG, et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature. 2018;563(7729):65‐71. doi: 10.1038/s41586-018-0649-2 [DOI] [PubMed] [Google Scholar]
- 6. Angeli CA, Boakye M, Morton RA, et al. Recovery of over‐ground walking after chronic motor complete spinal cord injury. N Engl J Med. 2018;379(13):1244‐1250. doi: 10.1056/NEJMoa1803588 [DOI] [PubMed] [Google Scholar]
- 7. Gorgey AS, Gill S, Holman ME, et al. The feasibility of using exoskeletal‐assisted walking with epidural stimulation: a case report study. Ann Clin Transl Neurol. 2020;7(2):259‐265. doi: 10.1002/acn3.50983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aslan SC, Legg Ditterline BE, Park MC, et al. Epidural spinal cord stimulation of lumbosacral networks modulates arterial blood pressure in individuals with spinal cord injury‐induced cardiovascular deficits. Front Physiol. 2018;9:565. doi: 10.3389/fphys.2018.00565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harkema SJ, Legg Ditterline B, Wang S, et al. Epidural spinal cord stimulation training and sustained recovery of cardiovascular function in individuals with chronic cervical spinal cord injury. JAMA Neurol. 2018;75(12):1569‐1571. doi: 10.1001/jamaneurol.2018.2617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Legg Ditterline BE, Wade S, Ugiliweneza B, et al. Beneficial cardiac structural and functional adaptations after lumbosacral spinal cord epidural stimulation and task‐specific interventions: a pilot study. Front Neurosci. 2020;14:554018. doi: 10.3389/fnins.2020.554018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. West CR, Phillips AA, Squair JW, et al. Association of epidural stimulation with cardiovascular function in an individual with spinal cord injury. JAMA Neurol. 2018;75(5):630‐632. doi: 10.1001/jamaneurol.2017.5055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dolbow D, Gorgey AS, Sutor TW, Bochkezanian V, Musselman K. Invasive and non‐invasive approaches of electrical stimulation to improve physical functioning after spinal cord injury. J Clin Med. 2021;10:5356. doi: 10.3390/jcm10225356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gorgey AS, Caudill C, Khalil RE. Effects of once weekly NMES training on knee extensors fatigue and body composition in a person with spinal cord injury. J Spinal Cord Med. 2016;39(1):99‐102. doi: 10.1179/2045772314Y.0000000293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Katzelnick CG, Weir JP, Jones A, et al. Blood pressure instability in persons with SCI: evidence from a 30‐day home monitoring observation. Am J Hypertens. 2019;32(10):938‐944. doi: 10.1093/ajh/hpz089 [DOI] [PubMed] [Google Scholar]
- 15. Wu M, Linderoth B, Foreman RD. Putative mechanisms behind effects of spinal cord stimulation on vascular diseases: a review of experimental studies. Auton Neurosci. 2008;138(1–2):9‐23. doi: 10.1016/j.autneu.2007.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Squair JW, Gautier M, Mahe L, et al. Neuroprosthetic baroreflex controls haemodynamics after spinal cord injury. Nature. 2021;590(7845):308‐314. [DOI] [PubMed] [Google Scholar]
- 17. Dela F, Mohr T, Jensen CM, et al. Cardiovascular control during exercise: insights from spinal cord‐injured humans. Circulation. 2003;107(16):2127‐2133. doi: 10.1161/01 [DOI] [PubMed] [Google Scholar]
- 18. Croom JE, Foreman RD, Chandler MJ, Barron KW. Cutaneous vasodilation during dorsal column stimulation is mediated by dorsal roots and CGRP. Am J Phys. 1997;272(2 Pt 2):H950‐H957. [DOI] [PubMed] [Google Scholar]
- 19. Rowley NJ, Dawson EA, Birk GK, et al. Exercise and arterial adaptation in humans: uncoupling localized and systemic effects. J Appl Physiol. 2011;110(5):1190‐1195. doi: 10.1152/japplphysiol.01371.2010 [DOI] [PubMed] [Google Scholar]
- 20. Gorgey AS, Poarch H, Harnish C, Miller JM, Dolbow D, Gater DR. Acute effects of locomotor training on neuromuscular and metabolic profile after incomplete spinal cord injury. NeuroRehabilitation. 2011;29(1):79–83. doi: 10.3233/NRE-2011-0680. PMID: 21876299 [DOI] [PubMed] [Google Scholar]
- 21. Gorgey AS, Wade R, Sumrell R, Villadelgado L, Khalil RE, Lavis T. Exoskeleton training may improve level of physical activity after spinal cord injury: a case series. Top Spinal Cord Inj Rehabil. 2017;23(3):245‐255. doi: 10.1310/sci16-00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harkema SJ, Ferreira CK, van den Brand RJ, Krassioukov AV. Improvements in orthostatic instability with stand locomotor training in individuals with spinal cord injury. J Neurotrauma. 2008;25(12):1467‐1475. doi: 10.1089/neu.2008.0572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Salazar MR, Espeche WG, Aizpurúa M, et al. Should the first blood pressure reading be discarded? J Hum Hypertens. 2015;29(6):373‐378. doi: 10.1038/jhh.2014.98 [DOI] [PubMed] [Google Scholar]


