Abstract
Objective
The analysis of intraepithelial lymphocytes (IELs) by flow cytometry of duodenal biopsies—the ‘IEL’ lymphogram—has been proposed as a diagnostic test for coeliac disease. However, its clinical applicability has been limited due to variability in methods and definitions. This study set out to define useful parameters for the application of the IEL lymphogram to the diagnosis of coeliac disease.
Design
Flow cytometry was performed on 117 sets of duodenal biopsies in 107 adult patients with active coeliac disease, long-term coeliac disease on a gluten free diet and a control group. The initial 95 samples were used for hypothesis generation for the subsequent samples comprising 12 patients with coeliac disease and 10 controls.
Results
Rather than using single linear cut-offs for CD3 and T-cell receptor γδ (TCRγδ)+ve IELs, a discriminant function was identified as %CD3+ve IELs+2x(%TCRγδ+IELs)>100. This differentiated coeliac disease from control biopsies in the hypothesis generating group. These results were replicated in the validation group and found to be independent of histology in patients on long-term gluten free diet up to 12 years (combined sensitivity, 98.5%; specificity, 97.7%).
Conclusions
Flow cytometric analysis of IELs is a highly sensitive and specific adjunct to serology and histological examination for the diagnosis of coeliac disease, even in individuals with coeliac disease following a gluten free diet who exhibit normal duodenal histology.
Keywords: coeliac disease, immunology, gluten sensitive enteropathy, gluten, gluten free diet
Significance of this study.
What is already known on this topic?
Duodenal intraepithelial lymphocyte (IEL) populations are altered in coeliac disease compared with normal. This is the basis of the ‘IEL’ lymphogram using flow cytometry of fresh intestinal biopsies. It has been applied to the diagnosis of coeliac disease using specified cut offs for CD3−ve and gamma delta T cell receptor (γδ TCR)+ve cells.
What this study adds?
This study demonstrates that CD3+ve and γδ TCR+ve IELs are dependently variable such that a simple linear function combining both can discriminate coeliac from non-coeliac individuals with ~98% sensitivity and specificity. This is independent of gluten ingestion or histological appearances.
How might it impact on clinical practice in the foreseeable future?
The use of flow cytometry can strengthen the diagnosis of coeliac disease where it is not clear cut. Flow cytometry could be used on a follow-up biopsy on diet to both assess response and confirm the diagnosis on a single endoscopic procedure where the diagnosis has been made by serology alone, as occurred during the COVID-19 pandemic.
Introduction
The diagnosis of coeliac disease requires the identification of serum IgA antibodies targeting tissue-transglutaminase 2 (TTG) or deamidated gliadin peptides, confirmed by the finding of characteristic changes on histological examination of biopsies taken from the duodenum. Both tools require ongoing gluten ingestion.1–3
However, neither provides a ‘gold standard’ for the diagnosis of coeliac disease. Antibody levels may be sufficient to make the diagnosis when present in high titre in both children and adults,4 5 but low levels may be associated with marginal or absent histological changes in duodenal biopsies.6 Coeliac disease-associated antibodies may be absent altogether from the serum but detectable within the lamina propria complexed with TTG.7
Similarly, the interpretation of biopsies may be hampered by sampling error, cross-cutting of sections and minimal changes, and there is wide inter-observer variability between reporting pathologists. The characteristic histological features associated with coeliac disease are also found in other conditions.8 9 The presence or absence of symptoms is an unreliable indicator of coeliac disease,10 and assessment of human leucocyte antigen haplotype (HLA) is only helpful to rule out the condition when non-compatible.11
The increase in numbers of intestinal intraepithelial lymphocytes (IELs) is well reported in the active coeliac lesion and often persists long-term on a gluten free diet.8 Recently, it has become clear that in addition, the phenotypic composition of the IELs remains permanently altered.12 13 Studies of duodenal IELs reveal reduced CD3−ve cells and an increase in γδ T cells and it has been suggested that this could be used as a tool for the diagnosis of coeliac disease14–16 A recent study using the proportion of CD3+ve cells expressing the γδ T cell receptor to differentiate coeliac individuals from normal controls resulted in a 66.3% sensitivity with a 96.6% specificity at a cut-off of 14%.17
In this study we set out to determine whether this ‘IEL lymphogram’ could be further refined for diagnostic application in coeliac disease.
Patients and methods
Patients considered likely to require mucosal biopsy were recruited into the study and gave their consent for additional biopsies to be taken from the second part of the duodenum to be used for flow cytometric analysis. Patients were categorised into three groups: control (CON)—subjects with normal duodenal histological appearances referred for gastroscopy for diverse reasons; active coeliac disease (ACD)—subjects with positive serum anti-tissue transglutaminase antibodies and characteristic duodenal biopsy features of coeliac disease at time of diagnosis or ongoing villous atrophy with or without elevated anti-TTG antibody titres; long-term coeliac disease (LTCD)—subjects diagnosed previously with coeliac disease on long-term gluten free diet and with normal mucosal appearances on duodenal histological examination.
At the time of upper gastrointestinal endoscopic examination biopsies (n=5) were taken into formalin for histological examination and additional biopsies (n=10) into normal saline for flow cytometry. IELs were isolated using an adaption of a standard technique18 19 as follows: the epithelium was separated and disaggregated by vigorous mechanical disaggregation using a vortex mixer in the presence of dithiothreitol (1 mM) and EDTA (1 mM). The cellular extract was centrifuged and washed in phosphate buffered saline supplemented with 0.45% human albumin. Washed cells were incubated at room temperature in the dark with fluorochrome-conjugated antibodies to the cell surface antigens. Following a further two wash cycles, cell permeabilisation was then performed according to manufacturers’ instructions (Fix and Perm kit Nordic-MUbio) before incubation with the intracellular CD3 conjugated antibody. The antibody panel was established for diagnostic purposes in refractory coeliac disease and included detection of intracellular (cytoplasmic) CD3 expression separately from cell surface CD3 expression. The full panel comprised the following antibody markers: CD2, CD3, CD4, CD5, CD7, CD8, CD30, CD38, CD45, CD56, CD103, CD335, T-cell receptor αβ (TCRαβ) and T-cell receptor γδ (TCRγδ). Data were acquired using a BD FACSCantoII three laser configuration flow cytometer, and analysed using BD FACS diva software (V.6.1.3). The lymphocytes were gated by CD45 and low side scatter characteristics. Cytoplasmic and surface staining of CD3 was included in the common backbone across all panels and used for selectively identify the different IEL populations—surface/cytoplasmic CD3+, surface CD3−/cytoplasmic CD3+ and CD3−.
Results from the first 95 samples were analysed to generate a hypothesis for appropriate cut-off values for subsequent lymphogram categorisation. The subsequent 22 samples were used as a ‘validation’ group to assess the validity of the discriminant parameters for the test.
A Student’s t-test was used at significance of p<0.01 between datasets.
Results
Patient details are tabulated in table 1. The most common indications for gastroscopy in the control group (CON) were iron deficiency anaemia and functional bowel symptoms (39% in each). Other indications (one or two patients each) included unexplained weight loss, unexplained diarrhoea, abnormal cross-sectional radiology, and two patients had undergone small intestinal transplantation with biopsies taken of the proximal graft.
Table 1.
Patient characteristics
| Category | Number (% male) | Median age, years (IQR) | IgA anti-TTG titre, mean IU (range) | HLA DQ 2/8? | Duodenal histology |
| CON | n=40 (40%) | 53 (40–68) | 0.62 (0.1–1.3) | Yes=8 No=2 (n=10) |
39/40 normal (1/40 showed ‘possible mild patchy increase in IELs’) |
| ACD | n=44 (57%) (n=54 biopsies) |
60 (50–68.5) | 42 (0.4–>128) | Yes=14 (n=14) |
Subtotal villous atrophy=29; partial villous atrophy=15 |
| LTCD | n=23 (17%) | 56 (42.5–62.5) | 75.5 (9.6–>128) at time of diagnosis; 2.2 (0.6–6.5) at time of follow-up biopsy |
DQ2=7 | Normal=19/23; patchy increase in IELs=4/23 |
ACD, active coeliac disease; CON, control; HLA, human leucocyte antigen; IEL, intraepithelial lymphocyte; LTCD, long-term coeliac disease; TTG, tissue-transglutaminase 2.
The active group (ACD) of 44 subjects included eight patients with seropositive type 1 refractory coeliac disease at diagnosis, yet despite becoming seronegative over a period of years; on follow-up still had ongoing villous atrophy. These eight patients underwent routine follow-up with flow cytometric analysis on an annual basis with the results remaining consistent on separate occasions demonstrating intra-individual reproducibility.
Of those in the LTCD group, an IgA anti-TTG antibody titre was available for only 12 individuals at initial diagnosis, although a further nine were reported from elsewhere as ‘positive’ and two were not done. Six patients underwent initial diagnostic biopsy elsewhere and were reported as showing confirmatory changes, 14 were carried out at diagnosis in Cambridge and all showed villous atrophy of which 10 were subtotal. IgA anti-TTG antibody titre at follow-up biopsy was available for 13 patients as it is not standard practice in Cambridge to use this assay during follow-up. The median duration of adherence to a gluten free diet was 5.5 years, with a range of 1–50 years. Two patients with the longest duration of gluten free diet maintained since diagnosis had been diagnosed at a time when confirmatory duodenal biopsies and antibody tests were not available (44 and 50 years, respectively). Of the remainder in this group, nine had followed a strict gluten free diet for 3 years or more, and three had done so for 10 years or more (up to 12 years).
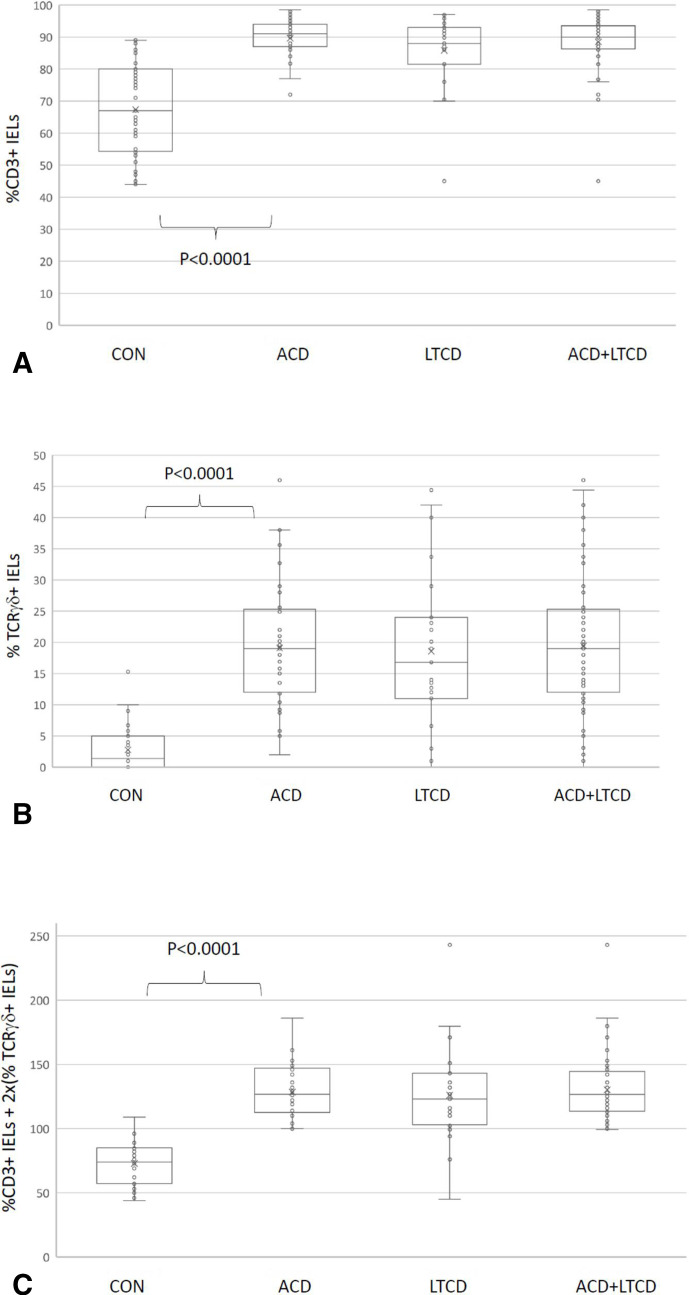
The proportions of cells by flow cytometry expressing surface CD3 and T-cell receptor γδ (TCR γδ) are shown in figure 1A, B by category. The proportions of CD3+ cells are the proportion of all gated lymphocytes—whereas the proportion of TCR γδ+ cells is the proportion of CD3+ cells expressing the TCR γδ receptor.
Figure 1.
Box and whisker plots of values of %CD3+ intraepithelial lymphocytes (IELs) as a proportion of all CD45+ lymphocytes (A), %TCRγδ+ IELs as a proportion of all CD3+ IELs (B) and ‘discriminant function’ values (%CD3+ IELs+2x(%TCRγδ+IELs)) (C) for control (CON), active coeliac disease (ACD), long-term coeliac disease (LTCD) and LTCD and ACD groups combined. TCRγδ, T-cell receptor γδ.
There is significant overlap between the CON, ACD and LTCD groups with regard to both CD3 and TCR γδ proportions. The sensitivity of using a diagnostic cut-off for TCR γδ of 14% in the ACD group was 64% and in the LTCD group, 57%. The specificity based on the one patient with a high TCR γδ proportion in the CON group is 97%.
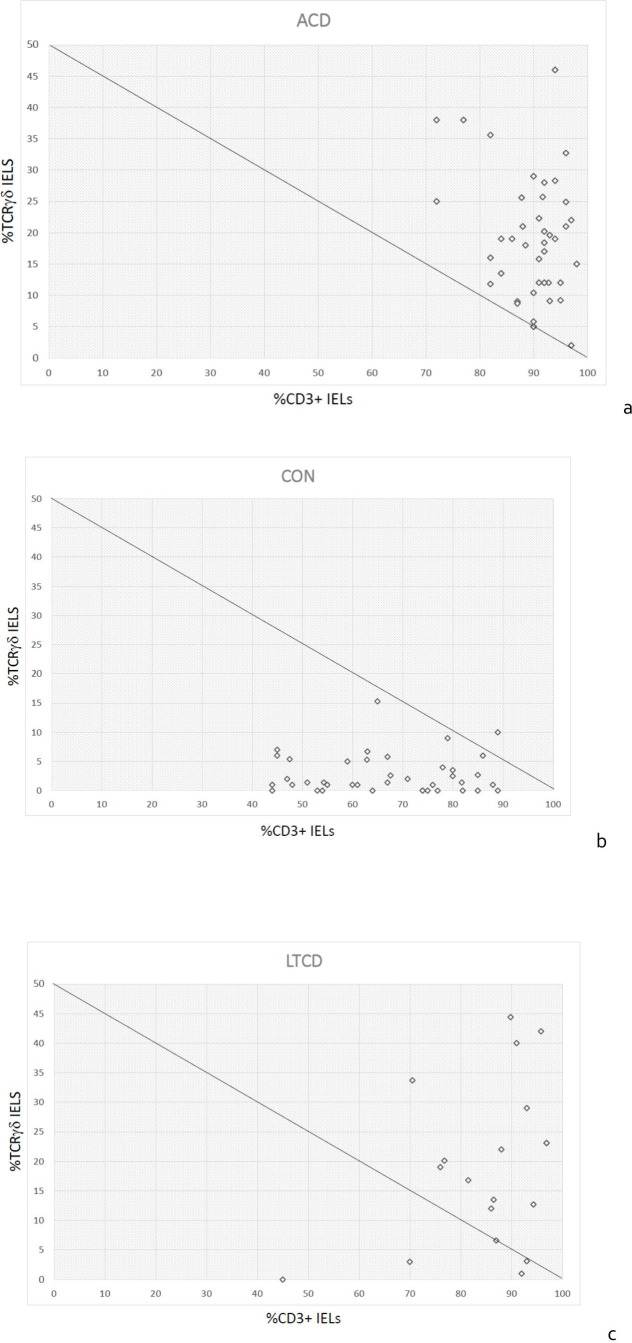
However, when surface and cytoplasmic CD3 positive cell proportions are charted against TCR γδ positive T cell proportions, there is a clear separation of ACD and CON groups as shown in figure 2A, B. The discriminant function between the non-coeliac and the coeliac groups is a simple linear equation which corresponds to (%CD3+2x(%TCR γδ)>100) (figure 1C).
Figure 2.
Scatter plots of %CD3+ intraepithelial lymphocytes (IELs) as a proportion of all CD45+ lymphocytes (x-axis) charted against %TCRγδ+ IELs as a proportion of CD3+ IELs (y-axis), showing the discriminant function (%CD3+ IELs+2x(%TCRγδ+IELs)) as a line. (A) Active coeliac disease (ACD), (B) control (CON), (C) long-term coeliac disease on diet (LTCD). TCRγδ, T-cell receptor γδ.
The validation cohort comprised 12 samples from patients with ACD and 10 without. None of these samples were from the individuals in the ACD cohort that had previously undergone flow cytometric analysis. Of those with coeliac disease in the validation group, 58% were male with a median age of 56 years (compared with 56% and 60 years in the hypothesis generating group). Of those without coeliac disease, 40% were men with median age 34 years (compared with 40% and 59.5 years in the hypothesis generating group). The average discriminant function was respectively 131 (range 104–151) for the active coeliac patients and 65.5 (range 54–96) for non-coeliac patients, thereby correctly identifying all subsequent patients in each group. The validation and hypothesis-generating groups have been combined in subsequent data analysis.
It can be noted that (due to the long-term persistence of altered IEL phenotypes in coeliac disease) the IEL lymphogram is indistinguishable between the LTCD (figure 2C) and the ACD (figure 2A) groups, of which 63/67 (94%) show a discriminant function of ≥100. However, on closer inspection of the four outlying cases, one was borderline (99.2) and two were those that had been diagnosed before any diagnostic tests were available, 44 and 50 years previously. Both of these were challenged by prolonged (>3 months) gluten ingestion and re-biopsied. Both remained symptom free, seronegative and with normal repeat duodenal biopsies and chose to eat gluten thereafter. One further patient had been diagnosed 10 years previously in a children’s hospital on the basis of anti-gliadin antibody positivity alone, but did not undergo histological confirmation and was negative for both anti-TTG and endomysial antibodies. This patient chose to continue a gluten free diet. Therefore, on the assumption that two of these three patients did not have coeliac disease, and the evidence for the third patient having the condition was extremely weak, the sensitivity of flow cytometry in the remaining 64 cases increases to 98.44% (95% CI 91.60% to 99.96%).
In terms of specificity, 1/40 non-coeliac/control patients had a discriminant function >100. This patient had no reported symptoms or family history and was seronegative for anti-TTG antibodies. The HLA DQ status was not known. This gives a specificity of 97% for flow cytometry in this setting (97.67% including the three deemed unlikely to have coeliac disease as above, with 95% CI 87.7% to 99.94%).
Discussion
The utility of the IEL lymphogram in the diagnosis of coeliac disease has been described in a recent meta-analysis.20 Of the six studies included, only five reported an ‘IEL lymphogram’ based on proportions of CD3−ve and TCRγδ+ve IELs.12 14–16 21 Two of these studies were specifically in children12 16 and the other three were mixed paediatric and adult populations. Methods varied between studies including gating strategies: three additionally gated cell populations for CD103 positivity, and one for CD7 positive cells. There is great diversity of IEL phenotype, especially within the CD3−ve population which also comprises a subset expressing cytoplasmic CD3 but lacking surface CD3 and this may add to the variability between studies relying on CD3−ve populations.
In addition, of those five studies using measurement of CD3−ve IELs by flow cytometry (by various definitions), only one provided the relevant cut-offs applied for their ‘lymphogram’.14 In this case a lymphogram comprising ≥8.5% TCRγδ and ≤10% CD3−ve IELs gave a sensitivity and specificity of 85% and 100%, respectively. This demonstrates a better sensitivity than the use of TCRγδ proportions alone.20 Applying these ‘IEL lymphogram’ criteria to our data would provide a sensitivity of 64% and a specificity of 92.5%. Despite lacking information on the cut-offs applied, a further study reported the sensitivity and specificity of the IEL lymphogram in adults as 89% and 96%, respectively.15
In our study, in order to simplify the IEL lymphogram and to remove possible confounding variables, we selected lymphocytes by their CD45 high/low side scatter properties, measuring proportions of CD45+CD3+ and CD45+CD3+TCR γδ IELs. The plot of %CD3+ve IELs against %TCRγδ+ve IELs was able to differentiate the samples from controls and those with ACD very effectively according to whether they lay above or below a line corresponding to the discriminant function: %CD3+ve+2x(%TCRγδ)>100. This gave a high sensitivity of 100% and a specificity of 97%. The discriminant function in this instance was derived through charting and identification of separate populations. With larger datasets it may be possible to define regions of interest mathematically with greater accuracy.
Application of our discriminant function to the LTCD group with normal histology on gluten free diet showed that four patients would have an IEL lymphogram considered incompatible with coeliac disease. However, one of these was borderline, and on examination of diagnostic records and subsequent gluten challenge and biopsy, the other three were highly unlikely to have the condition. The overall specificity and sensitivity of this test after combining the ACD and LTCD groups were 98.3% and 97.5%, respectively. The results from the LTCD group would suggest that this is an effective way of making—or refuting—the diagnosis of coeliac disease in individuals following a gluten free diet over many years without any changes on microscopic examination of duodenal biopsies, and without the need for undergoing a gluten challenge.
The use of a separate validation cohort following the generation of a hypothesis ensured that the discriminant function used for diagnosis was reproducible within the single centre. However, the main weakness of this study is that it is from only one centre and laboratory and the findings will require corroboration. Of note, transferability of results from studies of the IEL lymphogram between sites has not been possible to date given the different methods and definitions of IEL lymphogram applied. It is hoped that this simplified test will provide the basis for comparison with results from other centres. However, it is notable that using the same cut-off just for TCR γδ+ cells in this study as those from another recent study17 gave equivalent values of sensitivity and specificity suggesting a degree of transferability of results between sites.
Intra-individual reproducibility was also demonstrated in this study by the eight patients who underwent repeated flow cytometry analyses—the discriminant function differed by less than 10% between tests (data not shown) and did not result in a change of diagnosis in any case.
In this study we have demonstrated that the greatest utility of the IEL lymphogram is when a discriminant function is used that provides for adjustable, mutually dependent cut offs to be applied rather than simple independent linear cut-off levels for each variable as used in other IEL lymphograms. It is unclear why many patients with coeliac disease do not exhibit an increase in TCRγδ+ve IELs and why this should be compensated by TCRαβ cells, such that the combination of proportions of CD3+ve and TCRγδ+ve cells becomes diagnostic rather than either alone. In our data we were unable to find any difference in IEL subsets (using a variety of different cell surface markers) between those patients with coeliac disease in whom TCRγδ+ proportions were low and those in which they were high. It has previously been postulated that the age of the patient may dictate the TCRγδ response, however we were unable to demonstrate any such association, in either the ACD or LTCD groups. There were notable differences in gender distribution between the study groups. It is unlikely that this skewed the data in this study as IEL subtypes are not thought to differ between sexes.
Our method involved taking 10 additional biopsies. This resulted in a prolongation of the procedure by under 3 min as the biopsies were taken as ‘double bites’—there being no requirement for architectural interpretation. This is a much larger amount of tissue than is strictly necessary as we applied our standard immunostaining protocols used for analysis of biopsies for refractory coeliac disease and to look for additional potential biomarkers. However, in the longer term, the number of additional biopsies could be reduced to just one or two for flow cytometry if limited to analysis of surface CD3 and TCR γδ markers for diagnostic purposes, as used in other centres.15
The potential clinical utility of the IEL lymphogram has been demonstrated in this study but in view of the relatively small sample sizes will require larger scale studies to validate. Many cases of coeliac disease are ‘challenging’ to diagnose on the basis of weak seropositivity and low-grade changes in the biopsies. The addition of flow cytometry as an additional tool can strengthen the diagnosis. It is notoriously difficult for patients to undergo gluten challenge for re-biopsy and results may not be definitive due to poor compliance with the challenge protocol. The use of flow cytometry also obviates this requirement. This may be of particular relevance in the COVID-19 period when gluten free diets were started on the basis of seropositivity alone. Indeed, for those centres where routine practice includes a confirmatory diagnostic biopsy and a subsequent follow-up biopsy for assessment of response, the use of flow cytometry could abolish the requirement for a diagnostic biopsy and be carried out on the follow-up biopsy alone. The laboratory cost of flow cytometry in our institution is equivalent to that of a gastroscopy and therefore this would be a cost-effective pathway to both confirm the diagnosis and to assess the response to diet in one procedure.
Footnotes
Contributors: KB contributed to the writing and laboratory work. HC, NB, JS and DB carried out the cell separation and flow cytometry and contributed intellectual input. JMW conceived the project, carried out the gastroscopies and duodenal biopsies and contributed to the writing.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. Deidentified participant data available from the author on reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Ethical permission for this study was granted by the research ethics service (14/WA/1270, January 2015) and the local research and development department.
References
- 1. Al-Toma A, Volta U, Auricchio R, et al. European Society for the study of Celiac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol J 2019;7:583–613. 10.1177/2050640619844125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hujoel IA, Reilly NR, Rubio-Tapia A. Celiac disease: clinical features and diagnosis. Gastroenterol Clin North Am 2019;48:19–37. 10.1016/j.gtc.2018.09.001 [DOI] [PubMed] [Google Scholar]
- 3. Ludvigsson JF, Bai JC, Biagi F, et al. Diagnosis and management of adult coeliac disease: guidelines from the British Society of gastroenterology. Gut 2014;63:1210–28. 10.1136/gutjnl-2013-306578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Husby S, Koletzko S, Korponay-Szabo IR. ESPGHAN gastroenterology Committee; European Society for pediatric coeliac disease diagnosis, hepatology and nutrition. European Society for pediatric gastroenterology, hepatology and nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 2012;54:136–60. [DOI] [PubMed] [Google Scholar]
- 5. Holmes G, Ciacci C. The serological diagnosis of coeliac disease – a step forward. Gastroenterol Hepatol Bed Bench 2018;11:209–15. [PMC free article] [PubMed] [Google Scholar]
- 6. Donaldson MR, Firth SD, Wimpee H, et al. Correlation of duodenal histology with tissue transglutaminase and endomysial antibody levels in pediatric celiac disease. Clin Gastroenterol Hepatol 2007;5:567–73. 10.1016/j.cgh.2007.01.003 [DOI] [PubMed] [Google Scholar]
- 7. Salmi TT, Collin P, Korponay-Szabó IR, et al. Endomysial antibody-negative coeliac disease: clinical characteristics and intestinal autoantibody deposits. Gut 2006;55:1746–53. 10.1136/gut.2005.071514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walker MM, Woodward J. A clinicopathological approach to the diagnosis of coeliac disease. Diag Histopath 2012;18:402–10. 10.1016/j.mpdhp.2012.08.011 [DOI] [Google Scholar]
- 9. Lagana SM, Bhagat G. Biopsy diagnosis of celiac disease: the pathologist's perspective in light of recent advances. Gastroenterol Clin North Am 2019;48:39–51. 10.1016/j.gtc.2018.09.003 [DOI] [PubMed] [Google Scholar]
- 10. Downey L, Houten R, Murch S, et al. Recognition, assessment, and management of coeliac disease: summary of updated NICE guidance. BMJ 2015;351:h4513. 10.1136/bmj.h4513 [DOI] [PubMed] [Google Scholar]
- 11. Clouzeau-Girard H, Rebouissoux L, Taupin J-L, et al. HLA-DQ genotyping combined with serological markers for the diagnosis of celiac disease: is intestinal biopsy still mandatory? J Pediatr Gastroenterol Nutr 2011;52:729–33. 10.1097/MPG.0b013e31820a724d [DOI] [PubMed] [Google Scholar]
- 12. Camarero C, Eiras P, Asensio A, et al. Intraepithelial lymphocytes and coeliac disease: permanent changes in CD3-/CD7+ and T cell receptor gammadelta subsets studied by flow cytometry. Acta Paediatr 2000;89:285–90. [PubMed] [Google Scholar]
- 13. Mayassi T, Ladell K, Gudjonson H, et al. Chronic inflammation permanently reshapes tissue-resident immunity in celiac disease. Cell 2019;176:967–81. 10.1016/j.cell.2018.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fernández-Bañares F, Carrasco A, García-Puig R, et al. Intestinal intraepithelial lymphocyte cytometric pattern is more accurate than subepithelial deposits of anti-tissue transglutaminase IgA for the diagnosis of celiac disease in lymphocytic enteritis. PLoS One 2014;9:e101249. 10.1371/journal.pone.0101249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Valle J, Morgado JMT, Ruiz-Martín J, et al. Flow cytometry of duodenal intraepithelial lymphocytes improves diagnosis of celiac disease in difficult cases. United European Gastroenterol J 2017;5:819–26. 10.1177/2050640616682181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saborido R, Martinón N, Regueiro A, et al. Intraepithelial lymphocyte immunophenotype: a useful tool in the diagnosis of celiac disease. J Physiol Biochem 2018;74:153–8. 10.1007/s13105-017-0586-9 [DOI] [PubMed] [Google Scholar]
- 17. Nijeboer P, van Gils T, Reijm M, et al. Gamma-delta T lymphocytes in the diagnostic approach of coeliac disease. J Clin Gastroenterol 2019;53:e208–13. 10.1097/MCG.0000000000001060 [DOI] [PubMed] [Google Scholar]
- 18. Leon F. Flow cytometry of intestinal intraepithelial lymphocytes in celiac disease. J Immunol Methods 2011;363:177–86. 10.1016/j.jim.2010.09.002 [DOI] [PubMed] [Google Scholar]
- 19. Raine T, Liu JZ, Anderson CA, et al. Generation of primary human intestinal T cell transcriptomes reveals differential expression at genetic risk loci for immune-mediated disease. Gut 2015;64:250–9. 10.1136/gutjnl-2013-306657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fernández-Bañares F, Carrasco A, Martín A, et al. Systematic review and meta-analysis: accuracy of both gamma Delta+ intraepithelial lymphocytes and coeliac Lymphogram evaluated by flow cytometry for coeliac disease diagnosis. Nutrients 2019;11:1992–2007. 10.3390/nu11091992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Calleja S, Vivas S, Santiuste M, et al. Dynamics of non-conventional intraepithelial lymphocytes-NK, NKT, and γδ T-in celiac disease: relationship with age, diet, and histopathology. Dig Dis Sci 2011;56:2042–9. 10.1007/s10620-010-1534-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request. Deidentified participant data available from the author on reasonable request.