Abstract
Neural stem/progenitor cell (NS/PC)-based therapies have shown exciting potential for regeneration of the central nervous system (CNS) and NS/PC cultures represent an important resource for disease modeling and drug screening. However, significant challenges limiting clinical translation remain, such as generating large numbers of cells required for model cultures or transplantation, maintaining physiologically representative phenotypes ex vivo and directing NS/PC differentiation into specific fates. Here, we report that culture of human NS/PCs in 3D, hyaluronic acid (HA)-rich biomaterial microenvironments increased differentiation toward oligodendrocytes and neurons over 2D cultures on laminin-coated glass. Moreover, NS/PCs in 3D culture exhibited a significant reduction in differentiation into reactive astrocytes. Many NS/PC-derived neurons in 3D, HA-based hydrogels expressed synaptophysin, indicating synapse formation, and displayed electrophysiological characteristics of immature neurons. While inclusion of integrin-binding, RGD peptides into hydrogels resulted in a modest increase in numbers of viable NS/PCs, no combination of laminin-derived, adhesive peptides affected differentiation outcomes. Notably, 3D cultures of differentiating NS/PCs were maintained for at least 70 days in medium with minimal growth factor supplementation. In sum, results demonstrate the use of 3D, HA-based biomaterials for long-term expansion and differentiation of NS/PCs toward oligodendroglial and neuronal fates, while inhibiting astroglial fates.
Keywords: hyaluronic acid, biomaterials, hydrogels, tissue engineering, neural stem/progenitor cells
INTRODUCTION
Although the central nervous system (CNS) has some innate ability to regenerate, this ability is insufficient to restore tissue function in people afflicted by injury or neurodegenerative conditions. In adults, limited regeneration is achieved by an endogenous pool of neural stem/progenitor cells (NS/PCs), which are actively recruited to damaged tissue where they have the capacity to become new neurons and glia.1–3 Glia cells include oligodendrocytes and astrocytes and have distinct roles to support neuronal function. Oligodendrocytes form an insulating myelin sheath around neuronal axons to enable faster signal transmission and longrange conduction. Astrocytes have a variety of functions, including formation and maintenance of neuronal synapses and the blood–brain barrier. However, many NS/PCs present in damaged CNS tissue differentiate into reactive astrocytes, which create a glial scar and, when in excess, likely contribute to a chronic inflammatory environment thought to prevent regeneration.4–10
Over the past two decades, many studies have evaluated the therapeutic potential of transplanting human NS/PCs or oligodendrocyte progenitor cells (OPCs), the intermediary progenitors between NS/PCs and mature oligodendrocytes (Fig. 1), to regenerate CNS tissues after injury.8,11–18 While NS/PCs can become neurons, astrocyte or oligodendrocytes, OPCs are restricted to the oligodendrocyte lineage. Differentiation of transplanted NS/PCs toward oligodendrocytes has led to significant gains in functional recovery in rodent models of spinal cord injury.8,11–18 Furthermore, transplantation of OPCs has shown some promise for spinal cord injury repair in recent clinical trials.17 Despite these positive results, differentiation of NS/PCs into OPCs is inefficient. The best in vitro yields of human OPCs remain below 30%12,19–21 and in vivo below 25% of NS/PCs become oligodendrocytes after transplantation into the injured rodent spinal cord.16,22,23 While relatively few transplanted cells differentiate and engraft into the host tissue, these few are able to induce some functional improvement.14,17,24,25
FIGURE 1.

Culture conditions and timeline. A timeline of media conditions is depicted above. The media composition is detailed in the inset table, while the expected observation timelines for markers of NS/PC, OPC, pre-oligodendrocyte, mature oligodendrocyte, astrocyte, and neuron are included below the media timeline. Differentiation marker timelines are from Miron et al.132
The potential of stem cell-based therapies to improve clinical outcomes after brain or spinal cord injury motivates development of more effective strategies to direct oligodendrocyte-lineage differentiation. Key factors in the tissue microenvironment include the extracellular matrix (ECM) and cell-secreted soluble factors.26 Compared to neuron or astrocyte differentiation, oligodendrocyte differentiation of human NS/PCs has been more difficult to achieve.21 The majority of protocols previously described for differentiation of NS/PCs toward oligodendrocytes rely on temporal application of formulations of soluble bioactive factors, a potent method of controlling differentiation.20,21,27 However, as interactions between cells and the ECM are critical for oligodendrocyte differentiation,28,29 this study aimed to evaluate whether provision of a defined, synthetic ECM could improve differentiation efficiency.
Biomaterials make excellent platforms for 3D cell culture that can provide ECM-mimetic cues in a highly modifiable, defined manner.30 Biomaterial properties have been used to direct cell fate31–33 through modulation of stiffness,34 swelling ratio,35 geometry,36 and chemistry.37 Hydrogel biomaterials are often used because of their similarities to native tissues, including high water content. They can be fabricated from biocompatible and biodegradable polymers and their mechanical properties tuned to span those found in native brain and spinal cord.38 Hydrogel scaffolds have the potential to enable large-scale production of therapeutic cells by providing control over their expansion and subsequent differentiation into defined phenotypes ex vivo prior to clinical transplantation.
Hydrogels were fabricated based on hyaluronic acid (HA), a nonsulfated glycosaminoglycan that is abundant in CNS tissue and particularly enriched during embryonic development and in adult NS/PC niches.39 HA regulates proliferation and differentiation of Sox2+ NS/PCs through the CD44 receptor.40,41 In inflammatory environments, high molecular weight HA acts as a scavenger of damaging free radical species.42 HA hydrogels can be modified to provide additional matrix-derived functionalities, such as integrin-binding and mechanical cues, in combination.43–45 Use of aqueous, cytocompatible crosslinking chemistries enables 3D encapsulation of live cells within hydrogels.46,47 HA hydrogels can be formulated with mechanical properties approximating native CNS tissues and are degraded by cell-produced hyaluronidases.39 To enable cell adhesion, integrin-binding peptides derived from ECM proteins can be included.31,39,43,47–51 Implantation of HA hydrogels into the brain or spinal cord after injury can reduce the local inflammatory response and glial scar deposition.30,52 Furthermore, previous reports found that in vitro culture or ex vivo transplantation of NS/PCs within HA hydrogels with mechanical moduli mimicking healthy brain selectively increased differentiation of NS/PCs toward neurons or oligodendrocytes while decreasing astrocyte differentiation into reactive astrocytes.53–58
In the present studies, we evaluated the effects of combinatorial presentation of laminin-derived adhesion peptides on survival, proliferation and differentiation of human NS/PCs in 3D culture. Laminins are key mediators of NS/PC progression to mature, myelinating oligodendrocytes.59 For example, when OPCs contact the laminin-rich ECM surrounding axons, engagement of integrin α6β1 switches OPCs to a non-proliferative phenotype and initiates maturation.60 Several regions of laminin I have been identified as mediators of cell adhesion, including RGD, YIGSR, and IKVAV amino acid sequences. RGD is a common integrin-binding site across several ECM proteins. YIGSR and IKVAV support neuron attachment, survival and neurite outgrowth in a synergistic manner.61,62 Inclusion of peptides from these regions, rather than whole laminin protein, in biomaterial scaffolds increases local density of the bioactive sequence, and possibly biological potency.63 As scaffolds are constructed from the “bottom-up”, adding individual bioactive regions of ECM proteins one at a time, they provide the opportunity to isolate the independent and combined effects of specific peptide-integrin interactions. Decoupling the effects of different ECM cues will enable creation of tailored, defined matrix environments containing the minimal elements necessary to efficiently direct cell fate.
Here, we characterized effects of ECM-mimetic features included in HA-based hydrogels on differentiation of 3D-cultured NS/PCs, with the ultimate goal of identifying matrix properties that can enrich differentiation toward oligodendrocytes that can support CNS repair. HA content and hydrogel modulus were chosen to best mimic properties of native CNS tissues. RGD, IKVAV and YIGSR peptides were included alone and in combination and their effects on viability and differentiation of NS/PCs were evaluated over 70 days in culture. Cultures in media formulated to maintain NS/PC “stemness” and to provide minimally necessary support for oligodendrocyte differentiation were compared. Finally, we characterized the electrophysiological behavior of the differentiated neuronal cells in hydrogels.
MATERIALS AND METHODS
All reagents were purchased from Thermo Fisher Scientific, unless otherwise stated.
Hyaluronic acid preparation and hydrogel formation
Hydrogels were formed by Michael-type addition between 4-arm polyethylene glycol-maleimide (PEG-Mal, 20 kDa, Laysan Bio) and thiolated HA (700 kDa HA, Lifecore Biomedical).64 In brief, carbodiimide chemistry (N-hydroxysuccinimide, NHS; 1-ethyl-3-(3-dimethylaminopropyl), EDC) was used to add a thiol from cystamine (Sigma-Aldrich) to the carboxylate group on the glucuronic acid moieties of HA, creating thiolated HA (HA-SH). Disulfide bridges were broken with dithiothreitol and newly synthesized HA-SH purified via dialysis (12–14 kDa molecular weight cut-off cellulose membrane, Spectrum Labs) in deionized water, adjusted to pH 4 with hydrochloric acid and changed at least twice daily for 3 days. Purified HA-SH was sterile-filtered and lyophilized for 2 days. A range of 4–6% thiolation, defined as thiol groups per HA disaccharide, was used as determined with Ellman’s reagent (5,5′-dithiobis-(2-nitrobenzoic acid), Sigma-Aldrich).65,66
NS/PC culture
Neuralized H9 human embryonic stem cells (H9 hESCs) (Life Technologies) were expanded, and stored in liquid nitrogen vapors until use. NS/PCs were cultured in StemPro® hESC serum-free media (Life Technologies) made with Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F12) containing 1× StemPro® neural supplement, 2 mM Glutamax™, 0.1× antibiotic/antimycotic, 20 ng/mL basic fibroblast growth factor (bFGF), and 20 ng/mL epidermal growth factor (EGF). Here, we will refer to this as “stem cell maintenance” medium. NS/PCs were cultured in a humidified 37° C incubator with 5% carbon dioxide. Half of the medium in each culture well was changed every 2 days. After 4 weeks in culture, NS/PCs were transitioned to pre-differentiation medium for 2 weeks, followed by an oligodendrocyte-promoting differentiation medium for 4 weeks. Compositions of pre-differentiation and differentiation media were adapted from Hu et al.67 A timeline of the culture conditions is shown in Figure 1. Both pre-differentiation and differentiation media used the stem cell maintenance medium base without bFGF or EGF. Pre-differentiation medium was supplemented with 10 ng/mL platelet-derived growth factor AA (Peprotech) and 1 μM purmorphamine (Sigma-Aldrich), a sonic hedgehog agonist to direct NS/PCs toward OPCs. Differentiation medium was supplemented with 1 μM adenosine 3′,5′-cyclic monophosphate (cAMP; Sigma-Aldrich) and 60 ng/mL triiodothyronine (T3; Sigma-Aldrich) to direct OPCs toward oligodendrocytes. Minimal growth factor supplementation was used for these studies to obtain information about how insoluble cues provided by 3D culture matrices affected differentiation.
Prior to seeding 2D cultures for assessments of viability and mRNA expression, tissue culture-treated polystyrene wells were coated with CELLstart™ (Life Technologies) diluted in phosphate buffered saline (PBS) in a humidified, 37°C incubator for at least 2 h. After aspirating the CELLstart™ solution, 25,000 NS/PCs per cm2 were seeded. TrypLE™ Express (Life Technologies) was used to dissociate adherent NS/PCs during passaging. For immunostaining experiments, cells were seeded onto laminin-coated, 12 mm-diameter glass coverslips. Prior to coating, glass coverslips were cleaned by submerging in sulfuric acid for 20 min to remove residues, rinsed three times with distilled, deionized water (ddH2O), and autoclaved. Coverslips were first coated with a 50 μg/mL solution of poly-D-lysine (Sigma-Aldrich) in sterile ddH2O in a humidified, 37°C incubator for 1 h, rinsed three times with sterile PBS (without calcium or magnesium), and then incubated with 10 μg/mL mouse laminin I in sterile ddH2O for 2 h in a humidified, 37°C incubator. Coverslips were rinsed a final three times in PBS before seeding with 25,000 NS/PCs per cm2.
Prior to gelation and NS/PC encapsulation, PEG-Mal was dissolved in PBS and sterile-filtered. Peptides (Genescript, Supplementary Table 1) containing thiols (i.e., cysteines) near the N-termini were conjugated via Michael-type addition to an average of one arm of PEG-Mal prior to hydrogel formation for a total peptide concentration of 270 μM in the final hydrogels.64,68 PEG-Mal/peptide solutions were adjusted to 10 mg/mL with PBS and kept on ice. HA-SH was dissolved in DMEM/F12 (15 mg/mL). Once dissolved, the HA-SH solution was adjusted to approximately pH 6 with sterile 0.1 M NaOH. Next, 2.5 million NS/PCs per mL of HA-SH (around 25,000 NS/PCs per a 20 μL hydrogel) were added. The HA-SH solution with suspended NS/PCs was adjusted to 10 mg/mL with DMEM/F12 and kept on ice. To form cell-laden hydrogels, HA-SH with NS/PCs and PEG-Mal/peptide solutions (10 μL of each) were mixed in sterile, press-to-seal silicone isolators (12 × 4.5 mm diameter by 2 mm deep, Grace BioLabs) adhered to the bottom of a polystyrene Petri dish. Hydrogels became solid rapidly (within 30 s). Hydrogel cultures were placed in a humidified 37°C incubator for 15 min to complete gelation before they were removed from the molds, and transferred to 48-well tissue culture plates (400 μL media per well, 1 hydrogel culture per well).
Rheology
Hydrogel samples were tested for rheological stiffness using a Discovery Hybrid Rheometer (DHR2) from TA Instruments. Samples were formulated as described in the previous section, but with a final volume of 150 μL in 8 × 9 mm diameter by 2 mm deep, press-to-seal silicone isolators (Grace BioLabs). After gelation, hydrogels were allowed to equilibrate in PBS overnight at 4°C. The next morning, hydrogels were brought to room temperature and mounted onto the rheometer for testing. A 9 mm, parallel-plate geometry was used to perform an oscillation-frequency sweep with a 1% strain logarithmic sweep at a frequency range of 0.1–10 Hz. The storage and loss moduli were recorded and used to determine the complex modulus. Rheological properties of hydrogel are reported as average ± standard deviation of 15 independent experiments, each with at least three technical replicates.
Immunocytofluorescence
Neural stem/progenitor cells grown on 2D laminin-coated, coverslips or in 3D hydrogels were fixed by removing half of their medium and replacing it with fresh, 4% paraformaldehyde in PBS. After 20 min, the fixative was removed and coverslips rinsed three consecutive times in PBS. Fixed coverslips were stored at 4°C in PBS until use. Fixed hydrogel cultures were then transitioned in to a solution containing 20% sucrose in Optimal Cutting Temperature compound (OCT, Tissue-Tek) for sectioning samples using the following successive steps: 5% sucrose in PBS (1 h), 20% sucrose in PBS (2 × 30 min), 20% sucrose in PBS (overnight), 20% sucrose in OCT (2 h), 20% sucrose in OCT. In the final sucrose OCT solution, samples were frozen in a slurry of dry ice and 2-methylbutane, embedded in plastic molds, and cryo-sectioned (18 μm thick sections).
Sections were permeabilized with 0.1% Triton-X 100 and blocked using 4% normal donkey serum (Sigma-Aldrich) with 2% bovine serum albumin in PBS with 0.05% Tween-20. Triton-X 100 and Tween-20 were not used with antibodies labeling cell surface antigens. Samples were incubated with primary antibodies (Supplementary Table 2) overnight, rinsed three times in PBS, and incubated for 2 h with dye-conjugated, secondary antibodies raised in donkey (1:500; Life Technologies) and Hoechst 33342 (1:1000; ThermoFisher) to visualize nuclei. Samples were rinsed three times in PBS and mounted with Fluoromount-G® (SouthernBiotech). Widefield fluorescence images were acquired using an Axio Observer Z1 (Zeiss), Zen software (Zeiss), and an ORCA-Flash4.0V.2 CMOS (Hamamatsu). Confocal fluorescence images were acquired using a Leica TCS-SP5. Maximum projections of confocal image stacks were produced using Leica Application Suite X software.
mRNA expression
Prior to lysis, cells were isolated from hydrogels by incubating in a 1 mg/mL hyaluronidase (Sigma-Aldrich) solution in 0.01 M citrate buffer (pH 5.5) for 20 min at 37°C in a humidified incubator and then rinsed once in PBS. mRNA was isolated from lysed samples using the RNeasy and Qiashdredder kits (Qiagen) following instructions provided by the manufacturer. Real-time PCR (RT-PCR) was performed using a PCR master mix from Qiagen and an iQ5 qPCR machine (Bio-Rad). All primer/probe mixtures were purchased from Applied Biosystems (Supplementary Table 3). Samples were diluted to equivalent RNA concentrations before running RT-PCR and were normalized to GAPDH mRNA expression levels from NS/PCs at day 0.
Viability measurement
Cell viability was measured with CellTiter-Glo® 3D Cell Viability Assay (Promega) to quantify ATP present. Medium was removed from hydrogels, which were then homogenized in CellTiter-Glo® reagent and incubated for 30 min at room temperature. Hydrogel viability was determined by measuring luminescence on a plate reader (BioRad Synergy II) and comparing raw values to luminescence of a standard curve constructed from known numbers of NS/PC measured at the same time.
Electrophysiology
Electrophysiology was performed at room temperature with an Olympus BX51W1 upright microscope with optics for differential interference contrast. All recordings were performed at room temperature in an electrophysiology microscope chamber containing regular artificial cerebral spinal fluid (ACSF) composed of 130 mM NaCl, 26 mM NaHCO3, 10 mM glucose, 3 mM KCl,2 mM MgCl2, 2 mM CaCl2, and 1.25 mM NaH2PO4. Samples are oxygenated with 95% O2 and 5% CO2, maintained with a pH between 7.2 and 7.4 and osmolality between 290 and 310 mOsm/L. Whole-cell patch clamp recordings in current-clamp mode were performed using a MultiClamp 700B amplifier (Molecular Devices) and pCLAMP 10.3 acquisition software. The patch pipette (3–5 MΩ resistance) contained a solution comprised of 112.5 mM potassium-gluconate, 17.5 mM KCl, 10 mM HEPES buffer, 5 mM ATP potassium salt, 5 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 4 mM NaCl, 1 mM MgCl2, 1 mM NaGTP, 0.5mM CaCl2, and pH 7.2 with 270–280 mOsm/L. Capacitance, input resistance, decay time constant and resting membrane potential were recorded while holding the membrane potential at −70 mV and electrode access resistances were maintained at <30 MΩ.
Statistics
Statistical significance for viability differences between peptide groups was determined by evaluation with a Student’s t-test and a Holm–Šidák method for multiple comparison correction. A p < 0.05 was considered statistically significant after Holm–Šidák’s correction. Standard deviations for gene expression data were determined by propagating error from threshold cycle measurement for gene of interest and control gene (GAPDH) as well as control RNA (NS/PCs from day 0) following guidelines from Applied Biosystems.69 A two-way ANOVA was performed on gene expression levels and statistically significant differences in means were determined with a Hochberg method for multiple comparison correction (Supplementary Table 4). The false-discovery rate was set to 0.05. A p < 0.05 was considered statistically significant after Hochberg’s correction.
RESULTS
Mechanical properties
The average complex shear modulus of HA hydrogels was found to be 188 ± 42 Pa with a range of 134–282 Pa, which is consistent with native CNS tissue.55,70,71 Hydrogel storage modulus was not significantly affected by the addition of up to 500 μM peptide (Supplementary Fig. 1).
Cell viability and proliferation
There were no significant differences among groups day 7 after encapsulation (Supplementary Fig. 2). At day 28, more viable cells were detected in all conditions. Multivariable ANOVA (peptide condition, time) indicated significant differences in cell viability (assessed by ATP content) at days 28 and 70 for all conditions (p < 0.02), but not day 42, for both the stem cell maintenance and pre-oligodendrocyte medium conditions (Fig. 2A). Within these timepoints, a Holm–Šidák comparison test was used to evaluate statistical significance between peptide groups. At day 28, viability of NS/PCs cultured in hydrogels with RGD in maintenance medium was significantly higher than in hydrogels with YIGSR (p < 0.05) or CYS (p< 0.005). This difference may be attributed to some combination of improved cell survival and higher levels of proliferation being present in the RGD conditions. No significant differences in NS/PC viability among hydrogels with RGD, IKVAV, YIGSR+IKVAV, or YIGSR+IKVAV +RGD were observed. At day 70 of culture in differentiation medium, NS/PCs in hydrogels containing RGD (i.e., RGD or YIGSR+IKVAV+RGD) had significantly more viable cells than those in hydrogels without RGD (i.e., YIGSR, IKVAV, YIGSR +IKVAV, or CYS) (p < 0.05). For all peptide conditions, NS/PCs cultured in pre-differentiation or differentiation medium had decreased ATP activity compared to those in stem cell maintenance medium.
FIGURE 2.
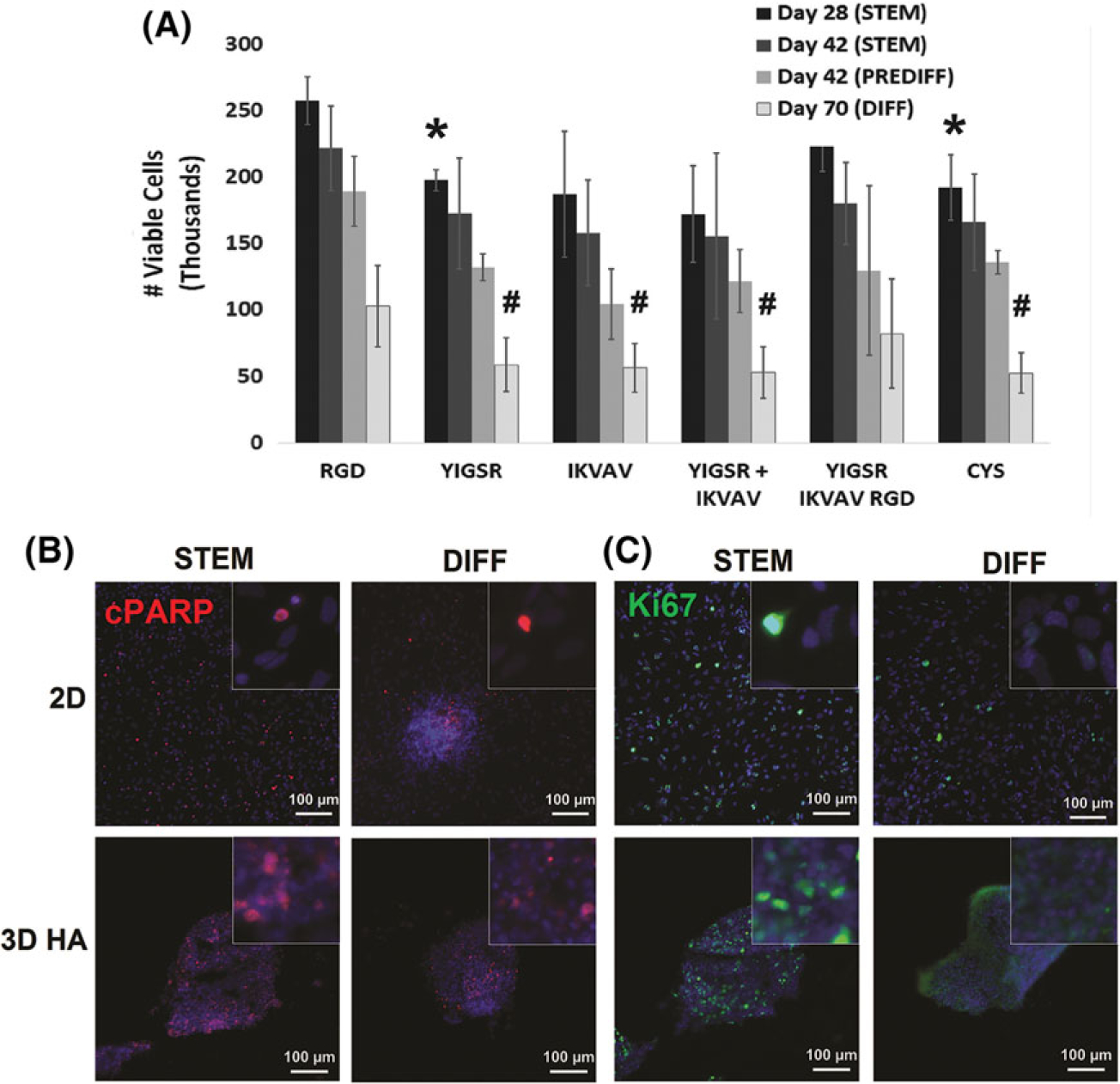
Viability in NS/PCs in 3D hydrogels. (A) The number of viable cells was quantified in each hydrogel condition using CellTiter-Glo®. Statistically significant differences were found between hydrogels with and without RGD at day 28 in STEM is represented with (*) and day 70 in DIFF is represented by (#). Statistical significance was evaluated using Student’s t-test with a Holm–Šidák sequential correction for multiple comparisons. Error bars represent standard deviation (N ≥ 4). (B, C) Immunofluorescence imaging was used to visualize apoptotic (B, cPARP, red) and proliferating (C, Ki67, green) cells, along with nuclear staining (B and C, Hoechst, blue), in 2D and 3D hydrogels with YIGSR, IKVAV, and RGD peptides at day 28 in culture. Images are magnified 200×; inset images are magnified 1000×. Media conditions were also varied: stem cell maintenance (STEM), oligodendrocyte-lineage conditioning (PRE-DIFF), and oligodendrocyte differentiation (DIFF).
Immunofluorescent images of cleaved poly (ADP-ribose) polymerase (cPARP) and Ki67 were used to indicate NS/PC apoptosis and proliferation, respectively (Fig. 2B). There were no observable differences in numbers of cPARP+ cells among any conditions. However, there was a clear, qualitative decrease in the numbers of proliferative cells (i.e., Ki67+ nuclei) when cultured in differentiation medium (days 42 and 70), compared to stem cell maintenance medium (day 28), in agreement with results from ATP assays (Fig. 2A). Cells in stem cell maintenance medium continued to proliferate throughout their culture period. By day 42, these cultures became overconfluent within scaffolds. Thus, cultures in stem cell maintenance medium were discontinued at day 42. As cells cultured in differentiation medium had slowed proliferation, they were continued to day 70.
Stem cell maintenance.
Effects of peptides on NS/PC fate were assessed based on mRNA (RT-PCR) and protein (immunostaining) expression of lineage-specific markers. SOX2, a transcription factor indicative of multipotent NS/PCs, was not significantly altered by 3D culture as measured by mRNA (Fig. 3A). Protein levels of SOX2 and nestin, another commonly used marker for NS/PCs,72 were evaluated using immunofluorescence (Fig. 3B). Qualitatively, expression of SOX2, in particular nuclear SOX2, and nestin protein appeared to decrease after culture in differentiation medium. Together, results suggest that only a few NS/PCs maintain stemness throughout the 70-day culture period.
FIGURE 3.
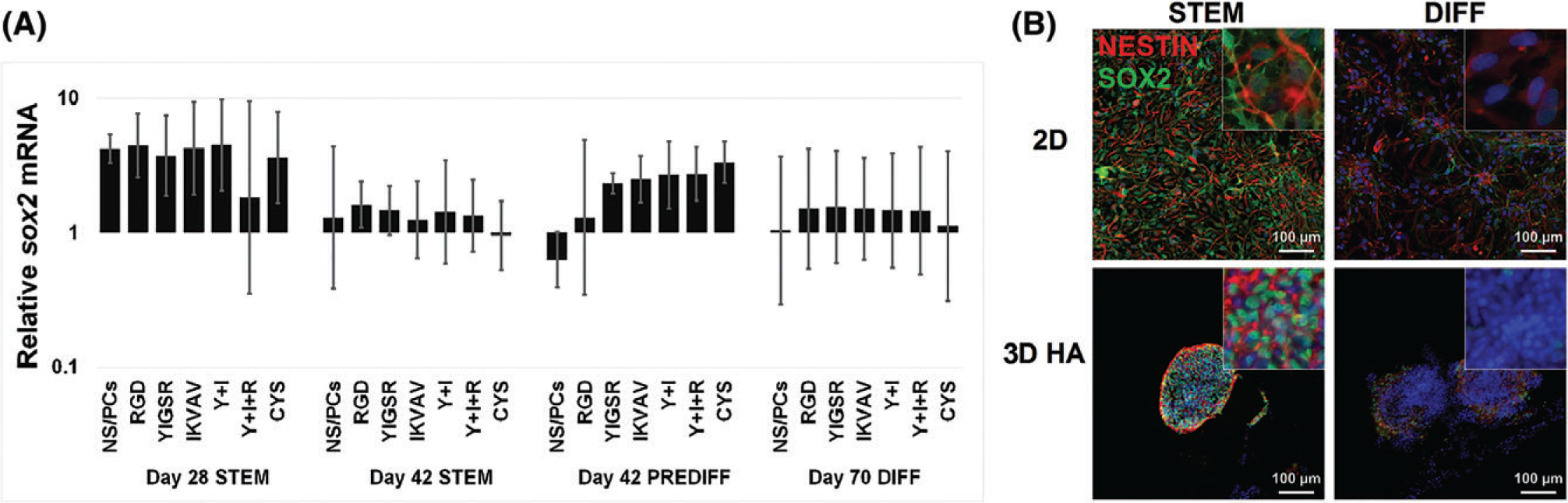
Maintenance of “stemness” in cultured NS/PCs. (A) Levels of SOX2 mRNA were evaluated using RT-PCR in 2D and 3D cultures under varying media conditions: stem cell maintenance (STEM), oligodendrocyte-lineage conditioning (PRE-DIFF), and oligodendrocyte differentiation (DIFF). Error bars show the standard deviation (N ≥ 4). (B) NS/PCs cultured in 2D (top) or 3D with YIGSR, IKVAV, and RGD peptides (bottom) were immunofluorescently labeled for nestin (red) and SOX2 (green). Cells cultured in STEM (42 days, left) or in differentiation conditions (PREDIFF, days 28–42 + DIFF, days 43–70; right) both showed expression of nestin and SOX2. Images are 200×; inset images are 1000×.
Levels of CD44, a marker of NS/PCs and receptor for HA, were evaluated. Compared to 2D culture, NS/PCs in 3D culture HA hydrogels displayed a trend toward reduced levels of cd44 mRNA in all peptide and media conditions at all time points (Fig. 4A). However, this difference was only statistically significant at day 28 in stem cell maintenance medium and day 42 in pre-differentiation medium (for all peptide conditions). Immunofluorescent labeling in 3D HA hydrogels indicated sporadic CD44 expression in few cells (Fig. 4B).
FIGURE 4.

CD44 expression by NS/PCs decreases when cultured in 3D, HA-based hydrogels. (A) CD44 mRNA was evaluated with RT-PCR in 2D and 3D cultures with varying peptides under varying media conditions: stem cell maintenance (STEM), oligodendrocyte-lineage conditioning (PRE-DIFF), and oligodendrocyte differentiation (DIFF). All 3D culture conditions showed statistically significant decreases in CD44 expression for day 28 STEM and day 42 PREDIFF compared to 2D controls (*). Error bars show the standard deviation (N ≥ 4). (B) Cells cultured in either 2D (top) or 3D with YIGSR, IKVAV, and RGD peptides (bottom) were immunofluorescently labeled for CD44. NS/PCs showed very little discernable CD44 expression in all conditions Images are 200×; inset images are 1000×.
Oligodendrocyte-lineage differentiation.
In all 3D cultures independently of peptide or media conditions, mRNA levels of olig2, a transcription factor present in CNS glial and motor neuron progenitors,73,74 significantly increased (Fig. 5A).75 Furthermore, increased levels of OLIG2 protein was observed in 3D hydrogel cultures in all media conditions (Fig. 5B). Qualitatively, more NS/PCs appeared positive for nuclear OLIG2 protein after culture in differentiation medium in both 2D and in 3D conditions. This shift from cytoplasmic to nuclear localization of OLIG2 protein indicates functional differentiation away from glial fibrillary acidic protein (GFAP)-positive astrocytes and toward progenitors of either oligodendrocytes, motor neurons, or fibrous astrocytes.58,76–78 Oligodendrocyte-lineage differentiation was characterized at days 42 (stem cell maintenance medium) and 70 (differentiation medium) using immunofluorescence staining for platelet-derived growth factor-α (PDGFR-α, OPCs), O4 (pre-oligodendrocytes), and 2′,3′-cyclic-nucleotide 3′-phosphodiesterase (CNPase, pre-oligodendrocytes, and mature oligodendrocytes).67,79,80(Fig. 5C–E). In 2D, NS/PCs in differentiation medium tended to form rosette-like clusters positive for OPC markers, similar to previous reports.81 Cells cultured in 3D, HA hydrogels consistently showed robust labeling of oligodendrocyte-lineage markers, which was qualitatively more abundant when compared to culture in stem cell maintenance medium in 2D or 3D and on laminin-coated, 2D substrates coverslips in any medium. However, no immunolabeling of myelin basic protein (MBP) was observed, indicated that oligodendrocyte-lineage cells did not produce myelin (data not shown).
FIGURE 5.
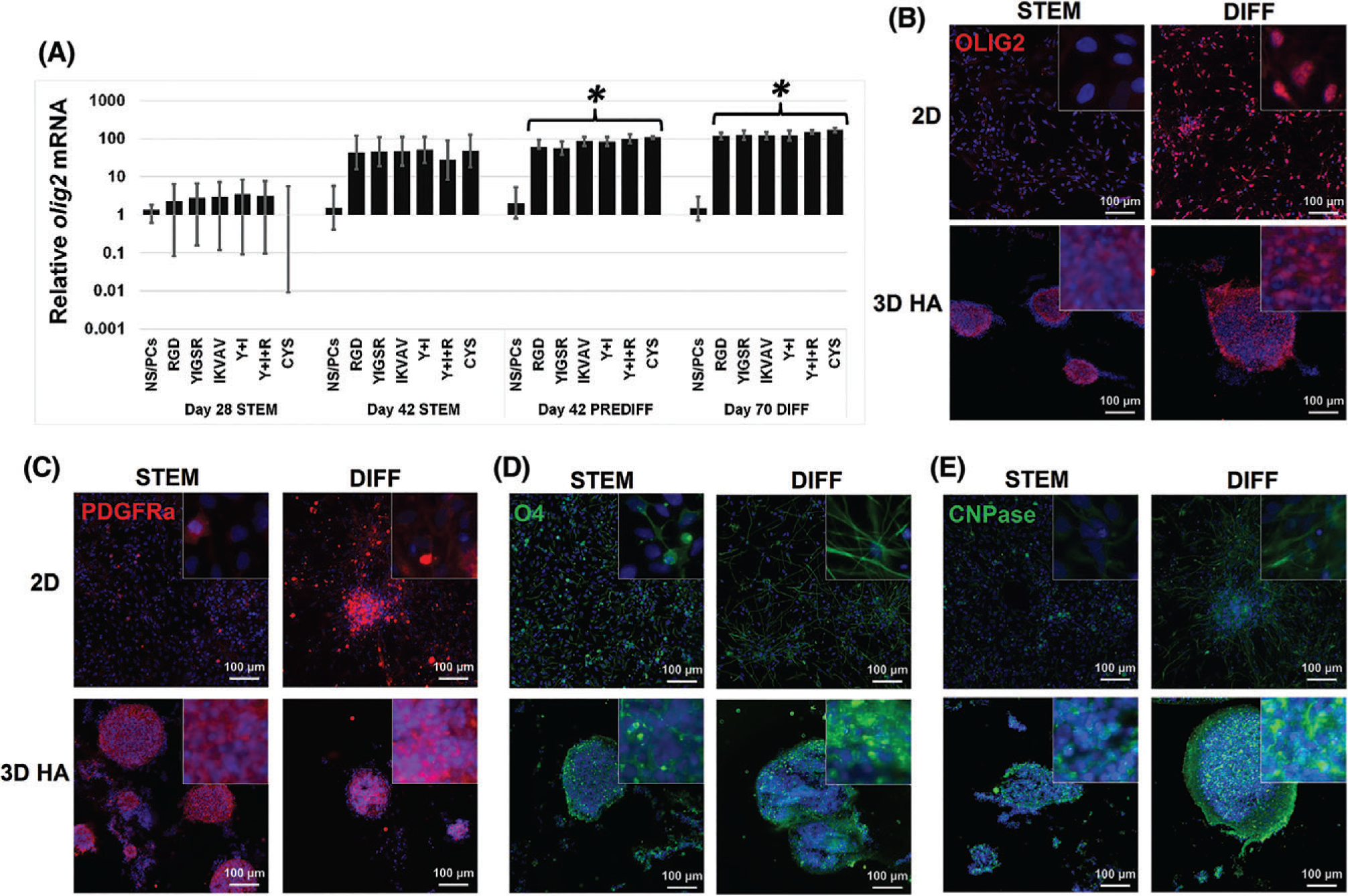
Oligodendrocyte-lineage differentiation increases when NS/PC are cultured in 3D under differentiation conditions. (A) OLIG2 mRNA was evaluated with RT-PCR in NS/PCs cultured in 2D and 3D with various peptides exposed to different media conditions: stem cell maintenance (STEM), oligodendrocyte-lineage conditioning (PRE-DIFF), and oligodendrocyte differentiation (DIFF). NS/PCs grown in 3D HA with PREDIFF (day 42) and with full differentiation conditions (PREDIFF, days 28–42 + DIFF, days 43–70) showed a statistically significant increase in OLIG2 expression compared to 2D NS/PCs treated with the same media (*). Error bars show the standard deviation (N ≥ 4). (B) NS/PCs cultured in either 2D (top) or 3D with YIGSR, IKVAV, and RGD peptides (bottom) were immunofluorescently labeled for OLIG2 expression (red). Fewer NS/PCs expressed OLIG2 in 2D, compared to 3D, when cultured in STEM for 42 days. In 2D, OLIG2 expression OLIG2 increased when cultured in differentiation conditions (day 70) compared to STEM medium (42 days). (C–E) NS/PCs showed an increase in expression of the OPC marker PDGFRα (C), pre-oligodendrocyte marker O4 (D), and oligodendrocyte marker CNPase (E) when cultured in 3D, HA hydrogels with YIGSR, IKVAV, and RGD peptides (bottom) compared to NS/PCs cultured in 2D (top) in both STEM and DIFF media conditions. Images are 200×; inset images are 1000×.
Astrocyte-lineage differentiation.
Glial fibrillary acidic protein is an intermediate filament protein whose expression is associated with activated astrocytes. GFAP is upregulated in response to inflammation and in the glial scar that forms to isolate injured tissue from surrounding healthy tissue in the CNS.21,82,83 Levels of GFAP mRNA were significantly decreased in NS/PCs cultured in 3D, compared to 2D, in pre-differentiation and differentiation media conditions (Fig. 6A). In 2D cultures, immunofluorescent staining confirmed a substantial increase in GFAP levels in differentiation medium, compared to minimal expression in stem cell maintenance medium. In 3D cultures, NS/PCS in all conditions had no detectable GFAP (Fig. 6B).
FIGURE 6.
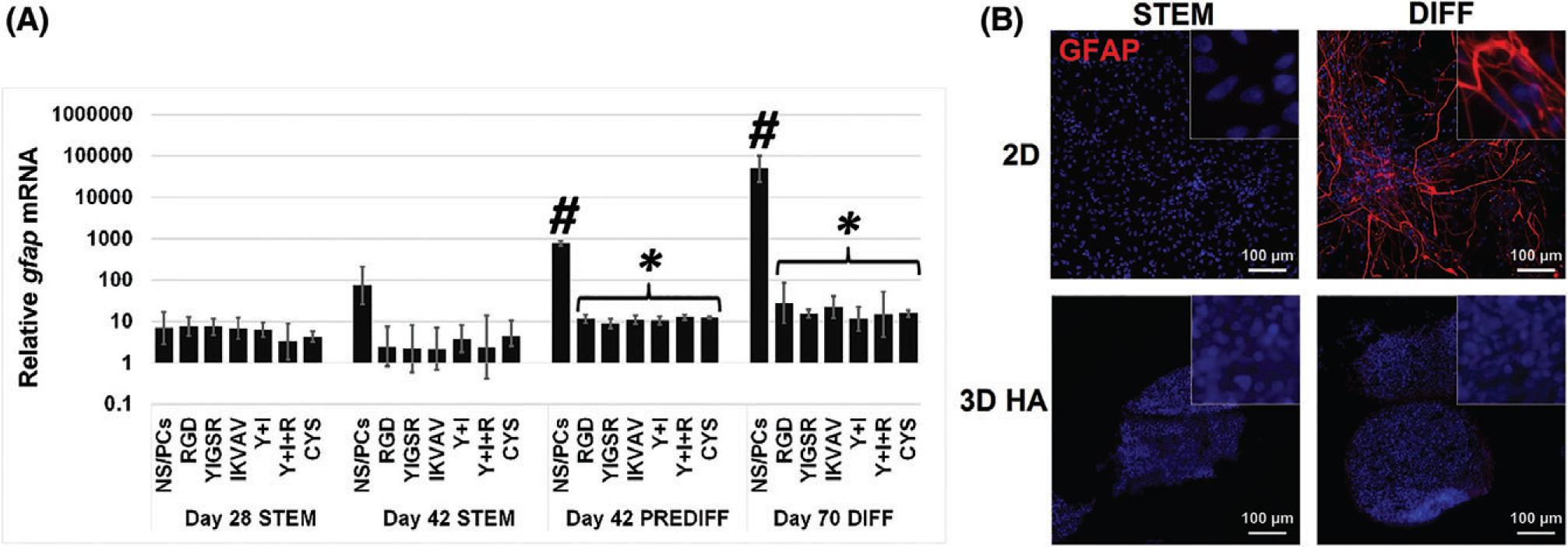
GFAP expression by NS/PCs increases with 2D culture and differentiation conditions. (A) GFAP mRNA was evaluated with RT-PCR in 2D and 3D hydrogels. In 2D, both PREDIFF (day 42) and full differentiation (PREDIFF, days 28–42 + DIFF, days 43–70) conditions significantly increased GFAP expression compared to 2D STEMRPO (day 28) (#). Both PREDIFF (day 42) and differentiation (day 70) conditions in 3D cultures showed a statistically significant decrease in GFAP expression compared to NS/PCs cultured in the same media conditions in 2D (*). Error bars show the standard deviation of multiple experiments. Error bars show the standard deviation (N ≥ 4). (B) NS/PCs cultured in either 2D (top) or 3D with YIGSR, IKVAV, and RGD peptides (bottom) were immunofluorescently labeled for GFAP (red), a marker of activated astrocytes. Images are 200×; inset images are 1000×. Media conditions: stem cell maintenance (STEM), oligodendrocyte-lineage conditioning (PRE-DIFF), and oligodendrocyte differentiation (DIFF).
While GFAP expression indicates activated astrocytes, it is possible that NS/PCs in 3D culture differentiated toward non-activated astrocytes. Thus, we assessed levels of S100β, a marker of astroglial differentiation that is not specific to activated or reactive astrocytes (Supplementary Fig. 3).21,82,84
While immunofluorescence staining revealed the presence of a few S100β+ cells in both 2D and 3D cells in all conditions, overall low levels of S100β mRNA expression, which is often present even on fibrous astrocytes derived from olig2+ progenitors,58 in 3D culture provides evidence that the OLIG2+ cells observed at days 42 and 70 in 3D, differentiation culture are likely OPCs (Fig. 5A, B). NS/PC culture in HA hydrogels with CYS peptides in stem cell maintenance medium at day 28 was the only condition with a statistically significant difference in S100β mRNA levels compared to 2D cultures at the same time point.
Neuron-lineage differentiation.
Next, we evaluated expression of βIII-tubulin (TUBB3) and doublecortin (DCX), which both indicate a neuronal or neuronal precursor phenotype (Fig. 7).21,85 A decrease in TUBB3 mRNA was observed for 2D-cultured NS/PCs in maintenance medium at day 28 compared to those in pre-differentiation medium at day 42 (Fig. 7A). No other statistically significant differences in levels of TUBB3 mRNA were observed. Immunofluorescence staining for βIII-tubulin protein showed no obvious differences in expression, in agreement with mRNA results (Fig. 7B). Immunofluorescence staining for DCX indicated the presence of more DCX+ cells in 3D culture and an additional increase when cultured in differentiation medium (Fig. 7B).
FIGURE 7.
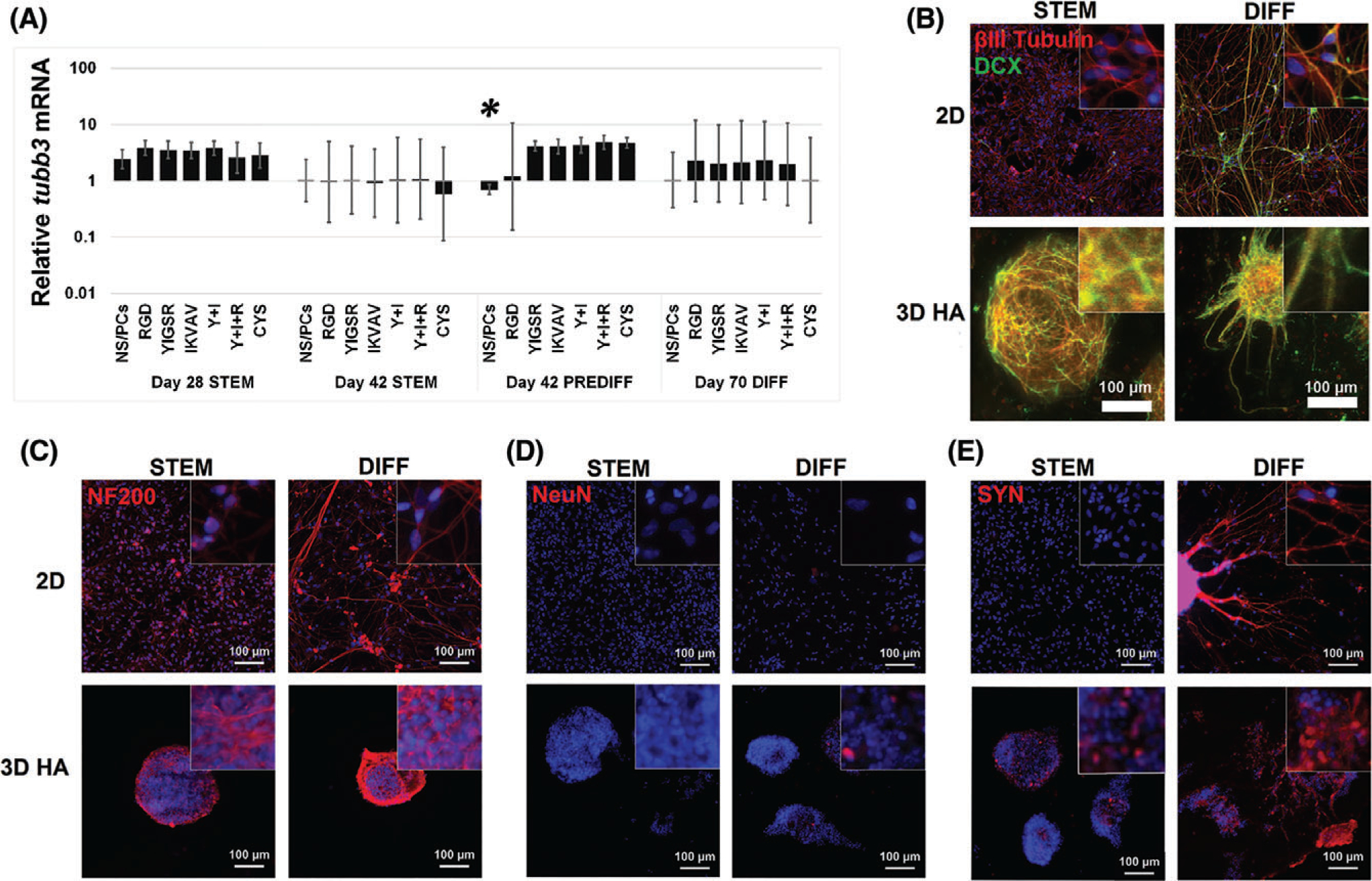
3D culture increased neuronal differentiation of NS/PCs. (A) βIII-tubulin (TUBB3) mRNA was evaluated with RT-PCR in 2D and 3D HA with varying adhesive peptides. In STEM (day 42), all 3D conditions showed a statistically significant decrease in TUBB3 compared to 2D NSPC at day 28 in STEM (*). Error bars show the standard deviation (N ≥ 4). (B–E) NS/PCs cultured in either 2D (top) or 3D with YIGSR, IKVAV, and RGD peptides (bottom) were immunofluorescently labeled for neuronal markers. Neuronal differentiation of NS/PCs in 3D cultures increases under oligodendrocyte-promoting differentiation conditions. (B) Expression of TUBB3 (red) and doublecortin (DCX, green) were observed in all conditions. DCX expression appeared to increase after undergoing the full, 70-day differentiation protocol (PREDIFF, days 28–42 + DIFF, days 43–70) and in 3D cultures. For 3D cultures, maximum projections of stacked images acquired using confocal microscopy are shown. (C–E) NS/PCs showed an increase in expression of mature neuronal markers neurafilament 200 (NF200, C), NeuN (D) synaptophysin (SYN, E) after undergoing the full, 70-day differentiation protocol. NS/PCs grown in 3D hydrogels with YIGSR, IKVAV, and RGD peptides (bottom) showed increased NF200 compared to those cultured in 2D in both STEM (day 42) and DIFF (day 70) conditions. (D) NS/PCs grown in 3D in DIFF conditions (day 70) exhibited more cells with nuclear NeuN than 3D cultures in STEM (day 42) or 2D cultures. (E) In 2D, SYN expression increased between STEM (day 42) and DIFF (day 70). In 3D cultures, SYN was observed in both STEM (day 42) and DIFF (day 70) conditions. Images are 200×; inset images are 1000×. Media conditions: stem cell maintenance (STEM), oligodendrocyte-lineage conditioning (PRE-DIFF), and oligodendrocyte differentiation (DIFF).
To assess differentiation into more mature neurons, we evaluated expression of neurofilament 200 (NF200), nuclear antigen (NeuN), and synaptophysin (SYN). Presence of NF200, an intermediate filament protein associated with mature axons, increased with both 3D culture and differentiation medium at day 7086 (Fig. 7C). NeuN is a transcription factor active in the nucleus of many mature neurons. Nuclear NeuN was observed only in 3D cultures in differentiation medium, indicative of active transcriptional regulation of genes associated with mature neurons86 (Fig. 7D). SYN is a protein found in pre-synaptic vesicles associated with early stages of synapse maturation.87 In 2D cultures, SYN was only present when cultured in differentiation medium at day 70 (Fig. 7E). In 3D cultures, SYN was present in all conditions and increased with differentiation medium at day 70, suggesting that 3D HA hydrogels are conducive to maturation of functional neurons. However, markers for more specific mature neuronal subtypes, including serotonin, tyrosine hydroxylase, and choline acetyltransferase, were not present (data not shown).
Electrophysiology
Previous reports have demonstrated that the ECM provided to NS/PCs in culture can alter their electrophysiological characteristics.88,89 Thus, we performed electrophysiology on NS/PCs cultured in 3D HA hydrogels with YIGSR+IKVAV+RGD for the full 70-day differentiation period (Fig. 8). Recorded NS/PCs had membrane channels that conducted potassium and sodium, but were too immature to determine an electrophysiological phenotype associated with any mature neuronal subtype. Measured values for capacitance, input resistance, and resting membrane potential were similar to values measured in embryonic stem cells89 and induced pluripotent stem cells90 undergoing neuronal differentiation but not yet fully matured. While this data is insufficient to definitively characterize the differentiation of NS/PCs, the observed electrophysiological characteristics suggest that NS/PCs were in an intermediate stage of maturation.
FIGURE 8.
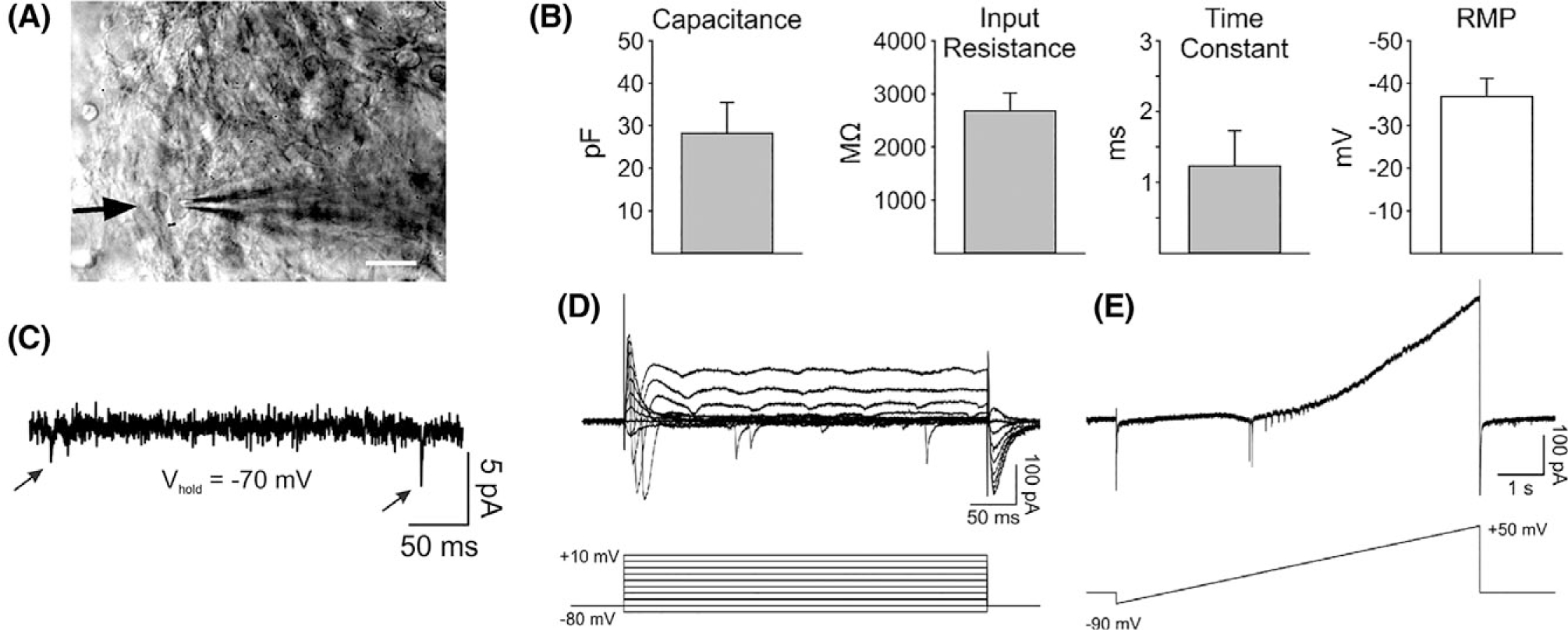
NS/PCs differentiated in 3D HA hydrogels exhibit electrophysiological properties of immature neurons. (A) Infrared differential interference contrast microscopy (IR-DIC) image taken from a recorded cell (arrow). Scale bar = 20 μm. (B) Membrane properties of recorded NS/PCs (N = 5). (C) Whole-cell patch clamp recording at a holding potential of −70 mV shows the presence of occasional synaptic inputs (see arrows) in a recorded NS/PC. (D) Sample recording from an NS/PC at different voltage steps (−80 mV to +10 mV, 10 mV increments) shows the presence of transient voltage-gated Na+ inward currents K+ outward currents. (E) Recording of voltage-gated currents from the same NS/PC as shown in D that are activated during a slowly depolarizing ramp (8 s).
DISCUSSION
Hydrogel biomaterials have immense potential to enable successful stem cell-based therapies for CNS regeneration. This study investigated whether 3D culture in an HA-rich environment incorporating laminin-derived, adhesive peptides could direct NS/PC differentiation more efficiently and in a more controlled manner than is currently possible with 2D cultures. Results demonstrated that HA-based hydrogels support viable, 3D cultures of human NS/PCs for at least 70 days and that culture conditions can be used to promote NS/PC expansion or differentiation, as desired. In addition, culture in 3D, HA hydrogels increased differentiation toward both neurons and oligodendrocytes. Very few, if any, astrocytes expressing S100β or GFAP were present in 3D cultures. In contrast, both astrocyte markers were widely observed in 2D culture. As GFAP expression is typically associated with inflammatory astrocytes in vivo, this result agrees with previous reports that HA hydrogels can reduce the presence of GFAP+ astrocytes in the spinal cord after injury.52,91
The ECM acts through integrins to regulate NS/PC phenotype, including maintenance of a stem-like phenotype and differentiation toward specific mature cell types.92,93 Pinkstaff and colleagues identified a specific set of integrins are expressed in the rat brain.94 Of these, integrin subunits α1, α3, α6, αV, and β1 are abundant and are known to interact with laminin and other ECM proteins bearing the RGD sequence. Integrin dimers αV β1 and αV β5 have been linked to proliferation of NS/PCs.95 For OPCs, various integrin dimers with the β1 subunit have been found to promote survival,96 regulate a switch from proliferation to differentiation29 and mediate myelination by mature oligodendrocytes.97,98 Several studies have found that inclusion of IKVAV peptides in hydrogel-type biomaterials increased neuronal differentiation of transplanted NS/PCs after traumatic brain injury.99 Inclusion of IKVAV has also been reported to reduce astrogliosis after injury.100,101 In vitro, IKVAV-bearing hydrogels are reported to promote differentiation of neurons while reducing differentiation of astrocytes.102,103 YIGSR has also been reported to support survival and neurogenesis of transplanted NS/PCs104 and has been widely found to promote neurite extension.105,106 YIGSR and IKVAV are known to synergistically improve neurite attachment and outgrowth.61,62 Their combination has been reported to maximize neurogenesis of NS/PCs cultured in 3D HA hydrogels.44 Collectively, these results and prior studies confirm that RGD, YIGSR, and IKVAV are supportive of NS/PC survival and differentiation into oligodendrocytes and neurons.
Despite progress in our understanding, integrin regulation of NS/PC differentiation is understudied and has potential to be a valuable tool for developing and implementing stem cell-based therapies. While this study demonstrated that RGD peptides likely increases cell proliferation in HA hydrogels, no other peptides or combinations investigated significantly altered NS/PC maturation compared to non-bioactive cysteine. However, higher cell viability was observed in 3D hydrogels including RGD peptide, either exclusively or in combination with YIGSR and IKVAV, after 70 days in culture. Given that NS/PCs cultured in hydrogels with IKVAV and/or YIGSR without RGD had equivalent viabilities to those with cysteine, which served as a negative control as it does not bind integrins, higher viability with RGD may be because of the abilities of RGD to interact with many different integrins and bind more strongly to integrins, thereby improving cell adhesion. The decrease in numbers of viable NS/PCs between days 28 and 42 likely can be attributed to the cells filling, or overgrowing, hydrogel cultures by the later time point. After day 28, NS/PC spheroids continued to grow but often breached the edges of the hydrogel and were released into the surrounding medium. These spheroids were not included in analysis of cell numbers and were rinsed away before viability and phenotype experiments.
As anticipated, removal of the mitogenic factors bFGF and EGF from culture medium after day 28 in the PREDIFF and DIFF media reduced proliferation and initiated differentiation of NS/PCs.107,108 A subset of NS/PCs in a population are expected to undergo apoptosis in response to the differentiation stimuli,108–110 which helps to explain the reduced numbers of viable cells in differentiation medium at day 70. However, many NS/PCs were still viable at day 70. Notably, apoptotic cells were identified throughout the 3D culture volume, indicating that cell death was likely not caused by inadequate diffusion through the hydrogels.
Although CD44 has been reported widely as a marker for NS/PCs,41,111,112 immunofluorescence labeling in 3D HA hydrogels indicated sporadic CD44 presence in only a few cells in all 2D and 3D conditions. Moreover, there was a trend toward less CD44 expression in 3D compared to 2D cultures. While the implications of this result remain unclear, it is possible that the increased HA binding of CD44 may lead to reduced CD44 expression. Given that CD44 is associated with stemness41 and our data showing that NS/PCs cultured in 3D, HA-based hydrogels are more mature than those in 2D, differentiation in 3D cultures may lead to reductions in CD44 expression.41 Another study reported that during differentiation, CD44 remains high in astrocyte progenitors, but is substantially reduced in OPCs.112 CD44 expression is also seen in developing and mature, branched fibrous astrocytes.112 In this study, we observed no astrocyte-lineage differentiation, but significant oligodendrocyte-lineage differentiation. Thus, the reduction in CD44 expression in 3D cultures may correlate with an increased oligodendrocyte-lineage differentiation. However, further investigation is warranted in future studies.
Overall, results from this study demonstrate the potential utility of HA-based hydrogels for biomanufacturing of stem cell-based therapies for CNS regeneration. First, NS/PCs could be expanded for 42 days when cultured in hydrogels in the presence of mitogens. 3D cultures provide a larger space for culture expansion than 2D cultures. Thus, more new cells are generated without the need for passaging, which may lead to significant phenotypic drift affecting therapeutic potential. Furthermore, as relatively high numbers of NS/PCs are required for clinical use, a 3D culture method for cell expansion could address this significant challenge to translation. HA hydrogels maintained high levels of SOX2 mRNA and immunostaining consistent with observations that high expression of SOX2 is conserved throughout embryogenesis in vertebrate NS/PCs.113
Second, with mitogen withdrawal and progressive addition of soluble factors intended to promote oligodendrocyte-lineage differentiation, 3D culture in HA-based hydrogels generally increased differentiation toward OPCs over monolayer culture on CELLstart™, evidenced by increased olig2 expression and nuclear localization coinciding with an increase in expression of PDGFR-α and followed by increased expression of O4 and CNPase, early markers of differentiating oligodendrocytes. The goal of this study was to establish a baseline for how these 3D scaffolds affect differentiation. Thus, minimal soluble growth factor support was provided. However, there have been several reports detailing how progression addition of soluble factor cocktails can drive oligodendrocyte differentiation.20,21,27 In future work, we expect that combining 3D scaffolds with these soluble factor protocols will likely increase numbers of oligodendrocytes generated.
While many NS/PCs in 3D culture progressed down the oligodendrocyte lineage, others expressed the neuronal lineage markers βIII-tubulin and DCX. After 70 days, many cells in differentiation medium exhibited signs of more mature neurons, including nuclear NeuN and synaptophysin expression. However, electrophysiological measurements and immunostaining for markers of mature synapses showed that these cells did not differentiate completely. As the differentiation protocol used here was designed to promote differentiation into oligodendrocytes, and not neurons, use of a neuron-promoting protocol may further synaptic maturation in 3D cultures.
Notably, NS/PC differentiation into astrocytes (assessed via GFAP or S100β expression) was only observed in 2D and not in 3D cultures. As GFAP expression is associated astrocyte activation, NS/PC culture on substrates with non-physiological stiffness, like glass, may promote an inflammatory response from differentiating astrocytes.114–116 High molecular weight HA, as used here for hydrogel fabrication, has also been shown to reduce astrocyte proliferation and activation, both of which may contribute to inhibition of axon regeneration by the glial scar after injury.91,117
Schwann cells also express S100β and can promote regeneration of damaged CNS tissue.118,119 Schwann cells normally myelinate peripheral nerve axons, but have been observed to remyelinate CNS axons after injury. However, Schwann cell myelination reduces axon density to a pattern more similar to peripheral nerves than CNS, limiting the overall effectiveness of Schwann cells in CNS repair.119,120 As NS/PCs are unlikely to differentiate into Schwann cells due to differences in their developmental origins,118,121 it is likely S100β+ cells in these studies, even those not expressing GFAP, were astroglial cells.
Hyaluronic acid hydrogel stiffness and degradability are areas of particular importance for future studies. Hydrogel stiffness affects differentiation of cultured NS/PCs, with peak differentiation toward neurons when stiffness is closest to that of normal brain tissue.122 Previous work found that NS/PCs respond to stiffness in a time-dependent manner, typically on a time scale of seconds to minutes.123 This finding suggests that NS/PCs may “remember” a previous mechanical stimulus that primes them toward a particular lineage. Thus, it is possible that stiffness of a culture material could be transiently altered to create an environment selecting for differentiation to a specific phenotypic fate.
In general, HA found in the normal CNS tissues is high molecular weight (>500 kDa), where it is more thought to help maintain NS/PC stemness124,125 and quiescence.39,126 However, low molecular weight HA (<30 kDa) may promote differentiation,127 while mid-sized HA (~65 kDa) has been reported to inhibit OPC maturation and myelination.128 Thus, the presence of high molecular weight HA (~700 kDa) in the 3D cultures may prevent complete maturation. While HA may be a beneficial material to initiate NS/PC proliferation and early OPC differentiation, it may also create an inhibitory “valley” that prevents full maturation. In the future, it will be beneficial to evaluate effects of lower molecular weight HA in hydrogel cultures, either by using smaller HA to form hydrogels or by digesting larger HA molecules with hyaluronidase after cell encapsulation. In future studies, incorporating additional regions that can be enzymatically degraded by cells cultured 3D hydrogels may provide an extra parameter to direct NS/PC fate.129
Hyaluronic acid hydrogels are a useful scaffolding material for 3D culture of NS/PCs and as bioactive carriers of therapeutic cells for CNS regeneration. HA hydrogels have beneficial effects on recovery after brain or spinal cord injury in rodents as they decrease inflammation52,101 and improve NS/PC cell survival following transplantion.130,131 The tunable hydrogel platforms reported here can be used to characterize how stiffness, peptide concentration, ECM content, degradability, and soluble factors interact to dynamically coordinate NS/PC differentiation into specific fates, helping to inform the optimal design of regenerative scaffolds. As the hydrogel chemistry used in this study is compatible with injection and gelation in situ, which is an advantage to avoid excessive tissue damage when delivered to the CNS, the same scaffolds found to direct NS/PC fate in vitro can be easily translated in vivo to evaluate their therapeutic effects.
CONCLUSIONS
This study demonstrates that 3D culture in HA-based hydrogels can be used to expand or differentiate human NS/PCs with improved efficiency over standard 2D cultures. Inclusion of the RGD adhesive peptide increased cell viability in the early growth phase and through the 70-day differentiation period. Differentiation toward neurons and oligodendrocytes was increased significantly in 3D over 2D cultures, while differentiation into reactive astrocytes was reduced. However, differentiation into fully mature oligodendrocytes or neurons was not observed. The laminin I-derived adhesive peptides included had no significant effects on NS/PC fate. Taken together, these results establish that 3D, HA-based culture platforms can improve viability, expansion and differentiation of human NS/PCs and underscore the exciting potential of 3D, HA-based culture system for biomanufacturing of therapeutic NS/PCs.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to acknowledge financial support for this work from a National Science Foundation CAREER Award 1653730 (SKS), a UCLA Henry Samueli School of Engineering and Applied Sciences (HSSEAS) Faculty Research Grant (SKS), a UCLA Faculty Career Development Award (SKS), an NIH Training Grant in Genomic Analysis and Interpretation T32HG002536 (JL) and the Eugene V. Cota Robles Fellowship provided by the UCLA Graduate Division (JK). The authors would like to thank Dr. Michael Levine for his generous help with electrophysiology experiments, the UCLA Statistical Consulting Center and the UCLA Translational Pathology Core Laboratory for hydrogel sectioning. Confocal laser scanning microscopy was performed at the California NanoSystems Institute (CNSI) Advanced Light Microscopy/Spectroscopy Shared Resource Facility at UCLA.
Contract grant sponsor:
Division of Chemical, Bioengineering, Environmental, and Transport Systems; contract grant number: CAREER Award 1653730
Contract grant sponsor:
National Science Foundation; contract grant number: T32HG002536
Footnotes
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell 2002;110:429–441. [DOI] [PubMed] [Google Scholar]
- 2.Grégoire CA, Goldenstein BL, Floriddia EM, Barnabé-Heider F, Fernandes KJL. Endogenous neural stem cell responses to stroke and spinal cord injury. Glia 2015;63:1469–1482. [DOI] [PubMed] [Google Scholar]
- 3.Kimura A, Ohmori T, Ohkawa R, Madoiwa S, Murakami T, Kobayashi E, Hoshino Y, Yatomi Y, Sakata Y. Essential roles of sphingosine 1-phosphate/S1P1 receptor axis in the migration of neural stem cells toward a site of spinal cord injury. Stem Cells 2007;25:115–124. [DOI] [PubMed] [Google Scholar]
- 4.Sabelström H, Stenudd M, Réu P, Dias DO, Elfineh M, Zdunek S, Damberg P, Göritz C, Frisén J. Resident neural stem cells restrict tissue damage and neuronal loss after spinal cord injury in mice. Science 2013;342:637–640. [DOI] [PubMed] [Google Scholar]
- 5.Barnabé-Heider F, Göritz C, Sabelström H, Takebayashi H, Pfrieger FW, Meletis K, Frisén J. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell 2010;7:470–482. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Floriddia EM, Toskas K, Fernandes KJL, Guérout N, Barnabé-Heider F. Regenerative potential of ependymal cells for spinal cord injuries over time. EBioMedicine 2016;13:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russo I, Barlati S, Bosetti F. Effects of neuroinflammation on the regenerative capacity of brain stem cells. J Neurochem 2011;116: 947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings BJ, Uchida N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, Gage FH, Anderson AJ. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci U S A 2005;102:14069–14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hooshmand MJ, Ngyuen HX, Piltti KX, Benvente F, Hong S, Flanagan L, Uchida N, Cummings BJ, Anderson AJ. Neutrophils induce astroglial differentiation and migration of human neural stem cells via C1q and C3a synthesis. J Immunol 2017;199:1069–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson RE, Pardieck J, Smith L, Kenny P, Crawford L, Shoichet M, Sakiyama-Elbert S. Effect of hyaluronic acid hydrogels containing astrocyte-derived extracellular matrix and/or V2a interneurons on histologic outcomes following spinal cord injury. Biomaterials 2018;162:208–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci 2005;25:4694–4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead HS. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia 2005;49:385–396. [DOI] [PubMed] [Google Scholar]
- 13.Cloutier F, Siegenthaler MM, Nistor G, Keirstead HS. Transplantation of human embryonic stem cell-derived oligodendrocyte progenitors into rat spinal cord injuries does not cause harm. Regen Med 2006;1:469–479. [DOI] [PubMed] [Google Scholar]
- 14.Hooshmand MJ, Sontag CJ, Uchida N, Tamaki S, Anderson AJ, Cummings BJ. Analysis of host-mediated repair mechanisms after human CNS-stem cell transplantation for spinal cord injury: Correlation of engraftment with recovery. PLoS One 2009;4(6):e5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marques SA, Almeida FM, Fernandes AM, dos Santos SC, Cadilhe DV, Rehen SK, Martinez AM. Predifferentiated embryonic stem cells promote functional recovery after spinal cord compressive injury. Brain Res 2010;1349:115–128. [DOI] [PubMed] [Google Scholar]
- 16.Salazar DL, Uchida N, Hamers FPT, Cummings BJ, Anderson AJ. Human neural stem cells differentiate and promote locomotor recovery in an early chronic spinal cord injury NOD-scid mouse model. PLoS One 2010;5:e12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Priest CA, Manley NC, Denham J, Wirth ED 3rd, Lebkowski JS. Preclinical safety of human embryonic stem cell-derived oligodendrocyte progenitors supporting clinical trials in spinal cord injury. Regen Med 2015;10:939–958. [DOI] [PubMed] [Google Scholar]
- 18.Manley NC, Priest CA, Denham J, Wirth ED 3rd, Lebkowski JS. Human embryonic stem cell-derived oligodendrocyte progenitor cells: Preclinical efficacy and safety in cervical spinal cord injury. Stem Cells Transl Med 2017;6:1917–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sundberg M, Hyysalo A, Skottman H, Shin S, Vemuri M, Suuronen R, Narkilahti S. A xeno-free culturing protocol for pluripotent stem cell-derived oligodendrocyte precursor cell production. Regen Med 2011;6:449–460. [DOI] [PubMed] [Google Scholar]
- 20.Alsanie WF, Niclis JC, Petratos S. Human embryonic stem cell-derived oligodendrocytes: Protocols and perspectives. Stem Cells Dev 2013;22:2459–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen HX, Nekanti U, Haus DL, Funes G, Moreno D, Kamei N, Cummings BJ, Anderson AJ. Induction of early neural precursors and derivation of tripotent neural stem cells from human pluripotent stem cells under xeno-free conditions. J Comp Neurol 2014; 522:2767–2783. [DOI] [PubMed] [Google Scholar]
- 22.Anderson AJ, Piltti KM, Hooshmand MJ, Nishi RA, Cummings BJ. Preclinical efficacy failure of human CNS-derived stem cells for use in the pathway study of cervical spinal cord injury. Stem Cell Rep 2017;8:249–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sontag CJ, Uchida N, Cummings BJ, Anderson AJ. Injury to the spinal cord niche alters the engraftment dynamics of human neural stem cells. Stem Cell Rep 2014;2:620–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenzweig ES, Brock JH, Lu P, Kumamaru H, Salegio E, Kadoya K, Weber JL, Liang JJ, Moseanko R, Hawbecker S, Huie JR, Havton LA, Nout-Lomas YS, Ferguson AR, Beattie MS, Bresnahan JC, Tuszynski MH. Restorative effects of human neural stem cell grafts on the primate spinal cord. Nat Med 2018;24:484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao Q, He Q, Wang Y, Cheng X, Howard RM, Zhang Y, DeVries WH, Shields CB, Magnuson DSK, Xu X, Kim DH, Whittemore SR. Transplantation of CNTF-expressing adult oligodendrocyte precursor cells promotes remyelination and functional recovery after spinal cord injury. J Neurosci 2010;30:2989–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faissner A, Reinhard J. The extracellular matrix compartment of neural stem and glial progenitor cells. Glia 2015;63:1330–1349. [DOI] [PubMed] [Google Scholar]
- 27.Hu BY, Du ZW, Zhang SC. Differentiation of human oligodendrocytes from pluripotent stem cells. Nat Protoc 2009;4:1614–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colognato H, ffrench-Constant C, Feltri ML. Human diseases reveal novel roles for neural laminins. Trends Neurosci 2005;28:480–486. [DOI] [PubMed] [Google Scholar]
- 29.ffrench-Constant C, Colognato H. Integrins: Versatile integrators of extracellular signals. Trends Cell Biol 2004;14:678–686. [DOI] [PubMed] [Google Scholar]
- 30.Teixeira AI, Duckworth JK, Hermanson O. Getting the right stuff: Controlling neural stem cell state and fate in vivo and in vitro with biomaterials. Cell Res 2007;17:56–61. [DOI] [PubMed] [Google Scholar]
- 31.Murphy WL, McDevitt TC, Engler AJ. Materials as stem cell regulators. Nat Mater 2014;13:547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel AK, Tibbitt MW, Celiz AD, Davies MC, Langer R, Denning C, Alexander MR, Anderson DG. High throughput screening for discovery of materials that control stem cell fate. Curr Opin Solid State Mater Sci 2016;20:202–211. [Google Scholar]
- 33.Fisher OZ, Khademhosseini A, Langer R, Peppas NA. Bioinspired materials for controlling stem cell fate. Acc Chem Res 2010;43: 419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 2006;126:677–689. [DOI] [PubMed] [Google Scholar]
- 35.Park H, Guo X, Temenoff JS, Tabata Y, Caplan AI, Kasper FK, Mikos AG. Effect of swelling ratio of injectable hydrogel composites on chondrongenic differentiation of encapsculated rabbit marrow mesenchymal stem cells in vitro. Biomacromolecules 2010;10: 541–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee J, Abdeen AA, Zhang D, Kilian KA. Directing stem cell fate on hydrogel substrates by controlling cell geometry, matrix mechanics and adhesion ligand composition. Biomaterials 2013;34: 8140–8148. [DOI] [PubMed] [Google Scholar]
- 37.Benoit DSW, Schwartz MP, Durney AR, Anseth KS. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat Mater 2008;7(10):816–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seidlits SK, Khaing ZZ, Petersen RR, Nickels JD, Vanscoy JE, Shear JB, Schmidt CE. The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials 2010;31(14):3930–3940. [DOI] [PubMed] [Google Scholar]
- 39.Preston M, Sherman LS. Neural stem cell niches: Roles for the hyaluronan-based extracellular matrix. Front Biosci (Schol Ed) 2011;3:1165–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ponta H, Sherman L, Herrlich PA. CD44: From adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol 2003;4:33–45. [DOI] [PubMed] [Google Scholar]
- 41.Su W, Foster SC, Xing R, Feistel K, Olsen RH, Acevedo SF, Raber J, Sherman LS. CD44 transmembrane receptor and hyaluronan regulate adult hippocampal neural stem cell quiescence and differentiation. J Biol Chem 2017;292:4434–4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cowman MK, Lee HG, Schwertfeger KL, McCarthy JB, Turley EA. The content and size of hyaluronan in biological fluids and tissues. Front Immunol 2015;6:261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Segura T, Anderson BC, Chung PH, Webber RE, Shull KR, Shea LD. Crosslinked hyaluronic acid hydrogels: A strategy to functionalize and pattern. Biomaterials 2005;26:359–371. [DOI] [PubMed] [Google Scholar]
- 44.Lam J, Truong NF, Segura T. Design of cell-matrix interactions in hyaluronic acid hydrogel scaffolds. Acta Biomater 2014;10: 1571–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seidlits SK, Drinnan CT, Petersen RR, Shear JB, Suggs LJ. Schmidt. Fibronectin-hyaluronic acid composite hydrogels for three-dimensional endothelial cell culture. Acta Biomater 2011;7: 2401–2409. [DOI] [PubMed] [Google Scholar]
- 46.Nair DP, Podgórski M, Chatani S, Gong T, Xi W, Fenoli CR, Bowman CN. The thiol-Michael addition click reaction: A powerful and widely used tool in materials chemistry. Chem Mater 2004;26: 724–744. [Google Scholar]
- 47.Burdick JA, Prestwich GD. Hyaluronic acid hydrogels for biomedical applications. Adv Mater 2011;23:H41–H56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X, He J, Wang Y, Cui FZ. Hyaluronic acid-based scaffold for central neural tissue engineering. Interface Focus 2012;2:278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Addington CP, Heffernan JM, Millar-Haskel CS, Tucker EW, Sirianni RW, Stabenfeldt SE. Enhancing neural stem cell response to SDF-1alpha gradients through hyaluronic acid-laminin hydrogels. Biomaterials 2015;72:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cui FZ, Tian WM, Hou SP, Xu QY, Lee IS. Hyaluronic acid hydrogel immobilized with RGD peptides for brain tissue engineering. J Mater Sci Mater Med 2006;17:1393–1401. [DOI] [PubMed] [Google Scholar]
- 51.Lam J, Carmichael ST, Lowry WE, Segura T. Hydrogel design of experiments methodology to optimize hydrogel for iPSC-NPC culture. Adv Healthc Mater 2014;4(4):534–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Isa IL, Srivastava A, Tiernan D, Owens P, Rooney P, Dockery P, Pandit A. Hyaluronic acid based hydrogels attenuate inflammatory receptors and neurotrophins in interleukin-1β induced inflammation model of nucleus pulposus cells. Biomacromolecules 2015;16: 1714–1725. [DOI] [PubMed] [Google Scholar]
- 53.Aurand E, Wagner JL, Shandas R, Bjugstad KB. Hydrogel formulation determines cell fate of fetal and adult neural progenitor cells. Stem Cell Res 2014;12:11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hopkins AM, DeSimone E, Chwalek K, Kaplan DL. 3D in vitro modeling of the central nervous system. Prog Neurobiol 2015; 125:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farrell K, Joshi J, Kothapalli CR. Injectable uncrosslinked biomimetic hydrogels as candidate scaffolds for neural stem cell delivery. J Biomed Mater Res Part A 2017;105:790–805. [DOI] [PubMed] [Google Scholar]
- 56.Matsui M, Tabata Y. Enhanced angiogenesis by multiple release of platelet-rich plasma contents and basic fibroblast growth factor from gelatin hydrogels. Acta Biomater 2012;8:1792–1801. [DOI] [PubMed] [Google Scholar]
- 57.Tabata H Diverse subtypes of astrocytes and their development during corticogenesis. Front Neurosci 2015;9:114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tatsumi K, Isonishi A, Yamasaki M, Kawabe Y, Morita-Takemura S, Nakahara K, Terada Y, Shinjo T, Okuda H, Tank T, Wanaka A. Olig2-lineage astrocytes: A distinct subtype of astrocytes that differs from GFAP astrocytes. Front Neuroanat 2018;12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gardiner NJ. Integrins and the extracellular matrix: Key mediators of development and regeneration of the sensory nervous system. Dev Neurobiol 2011;71:1054–1072. [DOI] [PubMed] [Google Scholar]
- 60.Baron W, Colognato H, ffrench-Constant C. Integrin-growth factor interactions as regulators of oligodendroglial development and function. Glia 2005;49:467–479. [DOI] [PubMed] [Google Scholar]
- 61.Dhoot NO, Tobias CA, Fischer I, Wheatley MA. Peptide-modified alginate surfaces as a growth permissive substrate for neurite outgrowth. J Biomed Mater Res Part A 2004;71:191–200. [DOI] [PubMed] [Google Scholar]
- 62.Shaw D, Shoichet MS. Toward spinal cord injury repair strategies: Peptide surfacemodificationofexpanded poly(tetrafluoroethylene) fibers for guided neurite outgrowth in vitro. J Craniofac Surg 2003;14:308–316. [DOI] [PubMed] [Google Scholar]
- 63.Haggerty AE, Marlow MM, Oudega M. Extracellular matrix components as therapeutics for spinal cord injury. Neurosci Lett 2017; 652:50–55. [DOI] [PubMed] [Google Scholar]
- 64.Xiao W, Zhang R, Sohrabi A, Ehsanipour A, Sun S, Liang J, Walthers CM, Ta L, Nathanson DA, Seidlits SK. Brain-mimetic 3D culture platforms allow investigation of cooperative effects of extracellular matrix features on therapeutic resistance in glioblastoma. Cancer Res 2018;78(5):1358–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shu X, Liu Y, Palumbo F, Prestwich GD. Disulfide-crosslinked hyaluronan-gelatin hydrogel films: A covalent mimic of the extracellular matrix for in vitro cell growth. Biomaterials 2003;24: 3825–3834. [DOI] [PubMed] [Google Scholar]
- 66.Shu XZ, Liu Y, Luo Y, Roberts MC, Prestwich GD. Disulfide crosslinked hyaluronan hydrogels. Biomacromolecules 2002;3: 1304–1311. [DOI] [PubMed] [Google Scholar]
- 67.Hu BY, Du ZW, Zhang SC. Differentiation of human oligodendrocytes from pluripotent stem cells. Nat Protoc 2009;4:1614–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shikanov A, Smith RM, Xu M, Woodruff TK, Shea LD. Hydrogel network design using multifunctional macromers to coordinate tissue maturation in ovarian follicle culture. Biomaterials 2011;32: 2524–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Biosystems Applied. Guide to performing relative quantitation of gene expression using real-time quantitative PCR; 2004.
- 70.Lu YB, Franze K, Seifert G, Steinhäuser C, Kirchhoff F, Wolburg H, Guck J, Janmey P, Wei EQ, Käs J, Reichenbach A. Viscoelastic properties of individual glial cells and neurons in the CNS. Proc Natl Acad Sci U S A 2006;103(47):17759–17764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Canovic EP, Qing B, Mijailovic AS, Jagielska A, Whitfield MJ, Kelly E, Turner D, Sahin M, Van Vliet KJ. Characterizing multiscale mechanical properties of brain tissue using atomic force microscopy, impact indentation, and rheometry. J Vis Exp 2016;155: e54201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goodus MT, Guzman AM, Calderon F, Jiang Y, Levison SW. Neural stem cells in the immature, but not the mature, subventricular zone respond robustly to traumatic brain injury. Dev Neurosci 2015;37:29–42. [DOI] [PubMed] [Google Scholar]
- 73.Yang N, Zuchero JB, Ahlenius H, Marro S, Ng YH, Vierbuchen T, Hawkins JS, Geissler R, Barres BA, Wernig M. Generation of oligodendroglial cells by direct lineage conversion. Nat Biotechnol 2013;31:434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang J, Pol SU, Haberman AK, Wang C, O’Bara M, Sim FJ. Transcription factor induction of human oligodendrocyte progenitor fate and differentiation. Proc Natl Acad Sci U S A 2014;111(28): e2885–e2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marshall CA, Novitch BG, Goldman JE. Olig2 directs astrocyte and oligodendrocyte formation in postnatal subventricular zone cells. J Neurosci 2005;25:7289–7298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fukuda S, Kondo T, Takebayashi H, Taga T. Negative regulatory effect of an oligodendrocytic bHLH factor OLIG2 on the astrocytic differentiation pathway. Cell Death Differ 2004;11:196–202. [DOI] [PubMed] [Google Scholar]
- 77.Setoguchi T, Kondo T. Nuclear export of OLIG2 in neural stem cells is essential for ciliary neurotrophic factor-induced astrocyte differentiation. J Cell Biol 2004;166:963–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun T, Dong H, Wu L, Kane M, Rowitch DH, Stiles CD. Cross-repressive interaction of the Olig2 and Nkx2.2 transcription factors in developing neural tube associated with formation of a specific physical complex. J Neurosci 2003;23:9547–9556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rivers LE, Young KM, Rizzi M, Jamen F, Pschoulia K, Wade A, Kessaris N, Richardson WD. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci 2008;11:1392–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Raff MC, Mirsky R, Fields KL, Lisak RP, Dorfman SH, Silberberg DH, Gregson NA, Leibowitz S, Kennedy MC. Galactocerebroside is a specific cell-surface antigenic marker for oligodendrocytes in culture. Nature 1978;274:813–816. [PubMed] [Google Scholar]
- 81.Kim DS, Jung SJ, Lee JS, Lim BY, Kim H, Yoo JE, Kim DW, Leem JW. Rapid generation of OPC-like cells from human pluripotent stem cells for treating spinal cord injury. Exp Mol Med 2017; 49(7):e361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khakh BS, Sofroniew MV. Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci 2015;18:942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sofroniew MV. Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci 2015;16:249–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Krencik R, Weick JP, Liu Y, Zhang ZJ, Zhang SC. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat Biotechnol 2011;29:528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gleeson JG, Peter TL, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron 1999;23:257–271. [DOI] [PubMed] [Google Scholar]
- 86.Wolf HK, Buslei R, Schmidt-Kastner R, Schmidt-Kastner PK, Pietsch T, Wiestler OD, Blümcke I. NeuN: A useful neuronal marker for diagnostic histopathology. J Histochem Cytochem 1996;44: 1167–1171. [DOI] [PubMed] [Google Scholar]
- 87.Wiedenmann B, Franke WW, Kuhn C, Moll R, Gould VE. Synaptophysin: A marker protein for neuroendocrine cells and neoplasms. Proc Natl Acad Sci U S A 1986;83:3500–3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goetz AK, Scheffler B, Chen HX, Wang S, Suslov O, Xiang H, Brüstle O, Roper SN, Steindler DA. Temporally restricted substrate interactions direct fate and specification of neural precursors derived from embryonic stem cells. Proc Natl Acad Sci U S A 2006;103:11063–11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu BY, Weick JP, Yu J, Ma LX, Zhang XQ, Thomson JA, Zhang SC. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci U S A 2010;107:4335–4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Prè D, Nestor MW, Sproul AA, Jacob S, Koppenstiner P, Chinchalongporn V, Zimmer M, Yamamoto A, Noggle SA, Arancio O. A time course analysis of the electrophysiological properties of neurons differentiated from human induced pluripotent stem cells (iPSCs). PLoS One 2014;9(7):e103418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Khaing ZZ, Milman BD, Vansoy JE, Seidlits SK, Grill RJ, Schmidt CE. High molecular weight hyaluronic acid limits astrocyte activation and scar formation after spinal cord injury. J Neural Eng 2011;8(4):046033. [DOI] [PubMed] [Google Scholar]
- 92.Ariza CA, McHugh KP, White SJ, Sakaguchi DS, Mallapragada SK. Extracellular matrix proteins and astrocyte-derived soluble factors influence the differentiation and proliferation of adult neural progenitor cells. J Biomed Mater Res Part A 2010;94:816–824. [DOI] [PubMed] [Google Scholar]
- 93.Hu J, Deng L, Wang X, Xu XM. Effects of extracellular matrix molecules on the growth properties of oligodendrocyte progenitor cells in vitro. J Neurosci Res 2009;87:2854–2862. [DOI] [PubMed] [Google Scholar]
- 94.Pinkstaff JK, Detterich J, Lynch G, Gall C. Integrin subunit gene expression is regionally differentiated in adult brain. J Neurosci 1999;19:1541–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jacques TS, Relvas JB, Nishimur S, Pytel R, Edwards GM, Streuli CH, ffrench-Constant C. Neural precursor cell chain migration and division are regulated through different beta1 integrins. Development 1998;125:3167–3177. [DOI] [PubMed] [Google Scholar]
- 96.Buttery PC, ffrench-Constant C. Laminin-2/integrin interactions enhance myelin membrane formation by oligodendrocytes. Mol Cell Neurosci 1999;14:199–212. [DOI] [PubMed] [Google Scholar]
- 97.Barros CS, Nguyen T, Spencer KSR, Nishiyama A, Colognato H, Müller U. Beta-1 integrins are required for normal CNS myelination and promote AKT-dependent myelin outgrowth. Development 2009;136:2717–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gudz TI, Komuro H, Macklin WB. Glutamate stimulates oligodendrocyte progenitor migration mediated via an αv integrin/myelin proteolipid protein complex. Cell Motil 2006;26:2458–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sahab Negah S, Khooei A, Samini F, Gorji A. Laminin-derived Ile-Lys-Val-ala-Val: A promising bioactive peptide in neural tissue engineering in traumatic brain injury. Cell Tissue Res 2018;371:223–236. [DOI] [PubMed] [Google Scholar]
- 100.Robel S, Mori T, Zoubaa S, Schlegel J, Sirko S, Faissner A, Goebbels S, Dimou L, Götz M. Conditional deletion of β1-integrin in astroglia causes partial reactive gliosis. Glia 2009;57:1630–1647. [DOI] [PubMed] [Google Scholar]
- 101.Wei YT, Tian WM, Yu X, Cui FZ, Hou SP, Xu QY, Lee IS. Hyaluronic acid hydrogels with IKVAV peptides for tissue repair and axonal regeneration in an injured rat brain. Biomed Mater 2007;2(3): S142–S146. [DOI] [PubMed] [Google Scholar]
- 102.Cheng TY, Chen MH, Chang WH, Huang MY, Wang TW. Neural stem cells encapsulated in a functionalized self-assembling peptide hydrogel for brain tissue engineering. Biomaterials 2013;34: 2005–2016. [DOI] [PubMed] [Google Scholar]
- 103.Farrukh A, Ortega F, Fan W, Marichal N, Paez JI, Berninger B, del Campo A, Salierno MJ. Bifunctional hydrogels containing the laminin motif IKVAV promote neurogenesis. Stem Cell Rep 2017;9: 1432–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cui GH, Shao SJ, Yang JJ, Liu JR, Guo HD. Designer self-assemble peptides maximize the therapeutic benefits of neural stem cell transplantation for Alzheimer’s disease via enhancing neuron differentiation and paracrine action. Mol Neurobiol 2016; 53:1108–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Smith Callahan LA, Xie S, Barker IA, Zheng J, Reneker DH, Dove AP, Becker ML. Directed differentiation and neurite extension of mouse embryonic stem cell on aligned poly(lactide) nanofibers functionalized with YIGSR peptide. Biomaterials 2013;34 (36):9089–9095. [DOI] [PubMed] [Google Scholar]
- 106.Masuda T, Sakuma C, Kobayashi K, Kikuchi K, Soda E, Shiga T, Kobayashi K, Yaginuma H. Laminin peptide YIGSR and its receptor regulate sensory axonal response to the chemoattractive guidance cue in the chick embryo. J Neurosci Res 2009;87(2):353–359. [DOI] [PubMed] [Google Scholar]
- 107.Zhang SC, Wernig M, Duncan ID, Brüstle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol 2001;19:1129–1133. [DOI] [PubMed] [Google Scholar]
- 108.Rodrigues GMC, Gaj T, Adil MM, Wahba J, Rao AT, Lorbeer FK, Kulkarni RU, Diogo MM, Cabral JMS, Miller EW, Hockemeyer D, Schaffer DV. Defined and scalable differentiation of human oligodendrocyte precursors from pluripotent stem cells in a 3D culture system. Stem Cell Rep 2017;8(6):1770–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang J, O’Bara MA, Pol SU, Sim FJ. CD133/CD140a-based isolation of distinct human multipotent neural progenitor cells and oligodendrocyte progenitor cells. Stem Cells Dev 2013;22:2121–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Luo Y, Cai J, Liu Y, Xue H, Chrest FJ, Wersto RP, Rao M. Microarray analysis of selected genes in neural stem and progenitor cells. J Neurochem 2002;83(6):1481–1497. [DOI] [PubMed] [Google Scholar]
- 111.Yuan SH, Martin J, Elia J, Flippin J, Paramban RI, Hefferan MP, Vidal JG, Mu Y, Killian RL, Israel MA, Emre N, Marsala S, Marsala M, Gage FH, Goldstein LSB, Carson CT. Cell-surface marker signatures for the isolation of neural stem cells, glia and neurons derived from human pluripotent stem cells. PLoS One 2011;6(3):e17540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Naruse M, Shibasaki K, Yokoyama S, Kurachi M, Ishizaki Y. Dynamic changes of CD44 expression from progenitors to subpopulations of astrocytes and neurons in developing cerebellum. PLoS One 2013;8(1):e53109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ellis P, Fagan BM, Magness ST, Hutton S, Taranova O, Hayashi S, McMahon A, Rao M, Pevny L. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci 2004;26 (2–4):148–165. [DOI] [PubMed] [Google Scholar]
- 114.Lee G, Kim H, Elkabetz Y, Al Shamy G, Panagiotakos G, Barberi T, Tabar V, Studer L. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat Biotechnol 2007;25(12):1468–1475. [DOI] [PubMed] [Google Scholar]
- 115.Mao Y, Nguyen T, Sutherland T, Gorrie C. Endogenous neural progenitor cells in the repair of the injured spinal cord. Neural Regen Res 2016;11(7):1075–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mothe A, Tator C. Proliferation, migration, and differentiation of endogenous ependymal region stem/progenitor cells following minimal spinal cord injury in the adult rat. Neuroscience 2005;131: 177–187. [DOI] [PubMed] [Google Scholar]
- 117.Struve J, Maher PC, Li YQ, Kinney S, Fehlings MG, Kuntz C 4th, Sherman LS. Disruption of the hyaluronan-based extracellular matrix in spinal cord promotes astrocyte proliferation. Glia 2005; 52(1):16–24. [DOI] [PubMed] [Google Scholar]
- 118.Mirsky R, Jessen KR. Schwann cell development, differentiation and myelination. Curr Opin Neurobiol 1996;6:89–96. [DOI] [PubMed] [Google Scholar]
- 119.Papastefanaki F, Chen J, Lavdas AA, Thomaidou D, Schachner M, Matsas R. Grafts of Schwann cells engineered to express PSA-NCAM promote functional recovery after spinal cord injury. Brain 2007;130:2159–2174. [DOI] [PubMed] [Google Scholar]
- 120.Lankford KL, Imaizumi T, Honmou O, Kocsis JD. A quantitative morphometric analysis of rat spinal cord remyelination following transplantation of allogenic Schwann cells. J Comp Neurol 2002; 443:259–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jessen KR, Mirsky R. The repair Schwann cell and its function in regenerating nerves. J Physiol 2016;594:3521–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, Healy KE. Substrate modulus directs neural stem cell behavior. Biophys J 2008;95(9):4426–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rammensee S, Kang MS, Georgiou K, Kumar S, Schaffer DV. Dynamics of mechanosensitive neural stem cell differentiation. Stem Cells 2017;2:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Khaing ZZ, Seidlits SK. Hyaluronic acid and neural stem cells: Implications for biomaterial design. J Mater Chem B 2015;3:7850–7866. [DOI] [PubMed] [Google Scholar]
- 125.Zimmermann DR, Dours-Zimmermann MT. Extracellular matrix of the central nervous system: From neglect to challenge. Histochem Cell Biol 2008;130:635–653. [DOI] [PubMed] [Google Scholar]
- 126.Moshayedi P, Carmichael ST. Hyaluronan, neural stem cells and tissue reconstruction after acute ischemic stroke. Biomatter 2013;3 (1):e23863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Back SA, Tuohy TM, Chen H, Wallingford N, Craig A, Struve J, Luo NL, Banine F, Liu Y, Chang A, Trapp BD, Bebo BF Jr, Rao MS, Sherman LS. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med 2005;11(9):966–972. [DOI] [PubMed] [Google Scholar]
- 128.Preston M, Gong X, Su W, Matsumoto SG, Banine F, Winkler C, Foster S, Xing R, Struve J, Dean J, Baggenstoss B, Weigel PH, Montine TJ, Sherman LS. Digestion products of the PH20 hyaluronidase inhibit remyelination. Ann Neurol 2013;73(2):266–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Madl CM, LeSavage BL, Dewi RE, Dinh CB, Stowers RS, Hariton M, Lampe KJ, Nguyen D, Chaudhuri O, Enejder A, Heilshorn SC. Maintenance of neural progenitor cell stemness in 3D hydrogels requires matrix remodelling. Nat Mater 2017;16:1233–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liang Y, Walcza P, Bulte JWM. The survival of engrafted neural stem cells within hyaluronic acid hydrogels. Biomaterials 2013;34: 5521–5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lam J, Lowry WE, Carmichael ST, Segura T. Delivery of iPS-NPCs to the stroke cavity within a hyaluronic acid matrix promotes the differentiation of transplanted cells. Adv Funct Mater 2014;24: 7053–7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Miron VE, Kuhlmann T, Antel JP. Cells of the oligodendroglial lineage, myelination, and remyelination. Biochim Biophys Acta Mol Basis Dis 2011;1812(2):184–193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


