Abstract
Objective
To evaluate the effects and safety of exercise training, and to determine the most effective exercise intervention for people with Duchenne muscular dystrophy. Exercise training was compared with no training, placebo or alternative exercise training. Primary outcomes were functioning and health-related quality of life. Secondary outcomes were muscular strength, endurance and lung function.
Data sources
A systematic literature search was conducted in Medline, EMBASE, CINAHL, Cochrane Central, PEDro and Scopus.
Study selection and data extraction
Screening, data extraction, risk of bias and quality assessment were carried out. Risk of bias was assessed using the Cochrane Collaborations risk of bias tools. The certainty of evidence was assessed using Grading of Recommendations Assessment, Development and Evaluation.
Data synthesis
Twelve studies with 282 participants were included. A narrative synthesis showed limited or no improvements in functioning compared with controls. Health-related quality of life was assessed in only one study. A meta-analysis showed a significant difference in muscular strength and endurance in favour of exercise training compared with no training and placebo. However, the certainty of evidence was very low.
Conclusion
Exercise training may be beneficial in Duchenne muscular dystrophy, but the evidence remains uncertain. Further research is needed on exercise training to promote functioning and health-related quality of life in Duchenne muscular dystrophy.
LAY ABSTRACT
The aim of this study was to examine the effects of all types of exercise training compared with no training, placebo or alternative exercise training programmes in people with Duchenne muscular dystrophy. The primary outcomes were functioning and health-related quality of life. Secondary outcomes were muscular strength and endurance. A further aim was to evaluate safety and, if possible, to find the most effective exercise training intervention. This review investigates existing research on this topic. The results have been systematically gathered and analysed, to give a broader view of what effect exercise training may have for people with Duchenne muscular dystrophy. The results suggest that exercise training preserves functioning, and benefits muscular strength and endurance. The study was not able to identify the best type of exercise training and prescriptions to use in Duchenne muscular dystrophy. The validity of the results was reduced by the low number of studies included, the low quality of the studies, and diversity in both the interventions and outcome measures used. The results should therefore be interpreted with caution.
Key words: Duchenne muscular dystrophy, exercise training, respiratory muscle training, rehabilitation, physiotherapy
Duchenne muscular dystrophy (DMD) is one of the most common inherited neuromuscular disorders (NMDs) in children, with an incidence of 1 in 3,500–5,000 newborn boys. DMD presents with early-life onset of progressive muscle weakness, associated motor delay, and loss of ambulation, due to absence of the structural protein dystrophin (1). Most boys become wheelchair-dependent by the age of 12 years. Thereafter, a gradual loss of arm function develops, with an increasing need for personal assistance to perform daily functions (1). DMD strongly affects longevity (2). Despite new and promising drugs, there are no curative treatments (3). Corticosteroids delay the loss of ambulation, preserve upper limb function and respiration (4), and combined with ventilation, the median survival of patients with DMD has increased to more than into their 30s (5).
Regular physical activity is essential to maintain health, functioning, quality of life and social participation (6). Exercise training is defined as a structured physical activity prescribed by the type, intensity, duration and frequency in order to improve functions of the cardiorespiratory, muscular and nervous system (7). For persons with DMD, there is uncertainty considering what type, level and intensity of exercise training are most beneficial. Regular submaximal exercise may maintain muscular strength and prevent secondary disuse atrophy (1, 8, 9). Intensive eccentric muscle exercise, where the muscle is both activated and lengthened, in addition to high-resistance exercise, may exacerbate muscle damage and should be avoided (10). Lack of dystrophin may lead to contraction-induced injuries, with ongoing cycles of degeneration and inflammation, impaired muscle tissue repair and the replacement of muscle fibres by fat and connective tissue (11).
Four systematic reviews and one meta-analysis have considered exercise interventions in mixed NMD populations (12–15), only one has focused on DMD, including solely inspiratory muscle training (15). There are no clear guidelines for exercise training in DMD. Both boys, parents and physiotherapists require exercise training that is safe and beneficial. According to the World Health Organization’s (WHO) framework, the International Classification of Functioning, Disability and Health (ICF), the term “functioning” is defined as measures of body functions and structures, in addition to activity and participation level (including interaction in the context of environmental and personal factors) (16). Health-related quality of life (HRQoL) is a broad-ranging concept affected in a complex way by a person’s physical health, psychological state, level of independence, social relationships, and relationship with salient features of their environment (17), and may be defined as subjective perceived enjoyment and well-being (18).
The aim of this systematic review was to investigate the effects of exercise training to improve functioning or decrease disability in persons with DMD. The primary outcomes were functioning and HRQoL, and secondary outcomes were surrogate measures for functioning, such as muscular strength, endurance or lung function. Further, we aimed to evaluate safety of the included exercise training interventions in DMD, and if possible, to search for the most effective exercise training intervention.
METHODS
The review protocol was registered in PROSPERO in January 2020 (CRD42020149068). The reporting of this systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.
Eligibility criteria
Study design
Randomized controlled trials (RCT), cross-over trials, quasi-RCTs and clinical controlled trials were included, regardless of publication year or language of publication.
Participants
Studies with participants with a defined DMD diagnosis (1) were included regardless of age. Studies of mixed NMD populations without separate results for DMD participants were excluded.
Interventions
Exercise training was the main intervention, including active voluntary, active assisted, endurance or muscular strength training. Exclusion criteria were: studies with whole-body vibration, facial exercises, yoga, qigong, tai chi, passive stretching or range of motion exercises, use of splints or orthoses, and studies using virtual reality to promote motor learning or task skills.
Comparisons
Studies with control groups using non-exercise, usual care, sham or alternative exercise training, were included. Control groups with pharmacological, surgical or electrotherapeutical interventions, or within-participants design using the non-exercised limb as control were excluded.
Outcomes
Primary outcomes were functioning (ICF activity and participation level, e.g. standardized functional assessments or use of questionnaires) and HRQoL (generic or disease specific validated questionnaires). Secondary outcomes were muscular strength (static or dynamic), endurance (oxygen consumption, work capacity) or lung function (ICF body functions and structure level). Furthermore, from the included studies, reported safety of exercise training interventions were of interest. Outcomes of interest were change between baseline and end of intervention.
Search strategy
A systematic search was performed (SH and MNT) in the following databases: Embase, MEDLINE, CINAHL, Cochrane Central, PEDro and Scopus, applying available thesaurus terms/subject headings and text words. The term “Duchenne” was combined with “physical activity” and/or “exercise”. The search strategy was reviewed by 2 medical science librarians and adjusted accordingly. The search was performed on 26 February 2021, in addition to searches in other sources (see Appendix SI). Reference lists of included studies and earlier similar systematic reviews were checked for other potentially eligible studies.
Study selection
After removal of duplicates, titles and abstracts were screened independently by 2 authors (SH and MT). Full-text versions were reviewed by the same authors. Disagreements were resolved through discussion or by a third author (GR).
Data collection
The extracted data were transferred to predefined summary tables (SH and MT), and transferred to Review Manager (RevMan) [computer program] Version 5.4, The Cochrane Collaboration 2020 by (SH). Data were double-checked for correct entry (MT). Extracted data included: methods (study design, duration of the study and the intervention, study locations, study settings); participants (number, mean age, age range, diagnosis criteria, functional level, inclusion criteria, exclusion criteria, withdrawals); interventions (intervention, comparison, co-interventions); outcomes (primary and secondary outcomes specified and collected, time points, values and changes in baseline and end of intervention completion) and notes (funding for trial, declared conflicts of interests by trial authors, adverse events, review authors comments or free report of outcome measures and results).
Risk of bias assessment
The risk of bias (ROB) of the included studies was assessed independently (SH and MNT) using the Cochrane Collaboration “Risk of Bias 2” tool (ROB2) (19) for included RCTs and RCT cross-over trials. The Risk of Bias In Non-randomized Studies – of Interventions (ROBINS-I) tool (20) was used for clinical controlled trials. The studies were not blinded to the reviewer (SH, MNT and MT). Disagreements were resolved through discussion or by a third author (HL). The certainty of evidence was assessed using GRADEpro Guideline Development Tool (SH and HL) (21).
Data synthesis and analysis
Three comparisons were made; (i) exercise training vs no exercise training; (ii) exercise training vs placebo; and (iii) exercise training vs alternative exercise training. When reporting results from multiple time-points, data closest to the end of the exercise intervention were included. In cross-over trials, effect size was extracted from the first cross-over. In studies with missing data, corresponding authors were contacted. A random effect metaanalysis was conducted, based on the variation of participants, settings, interventions and outcomes. The outcomes were calculated by standardized mean differences (SMDs). SMD effect is characterized as small when less than 0.2, moderate when between 0.21 and 0.8, and large when more than 0.8 (22). Heterogeneity was assessed by the χ2 test and I-squared statistic, p-value < 0.1 was considered statistically significant. Subgroup analysis was performed by type and duration of the exercise training for each of these outcomes. Data analysis was performed by use of RevMan Software version 5.4. In cases with heterogeneity of outcome measures or limited reporting of data on separate arms of interventions in the included studies, data were narratively synthesized. A preliminary synthesis was performed, data relationships were searched, a theory was developed for how the intervention worked, and the robustness of synthesis was assessed (23).
RESULTS
Study selection
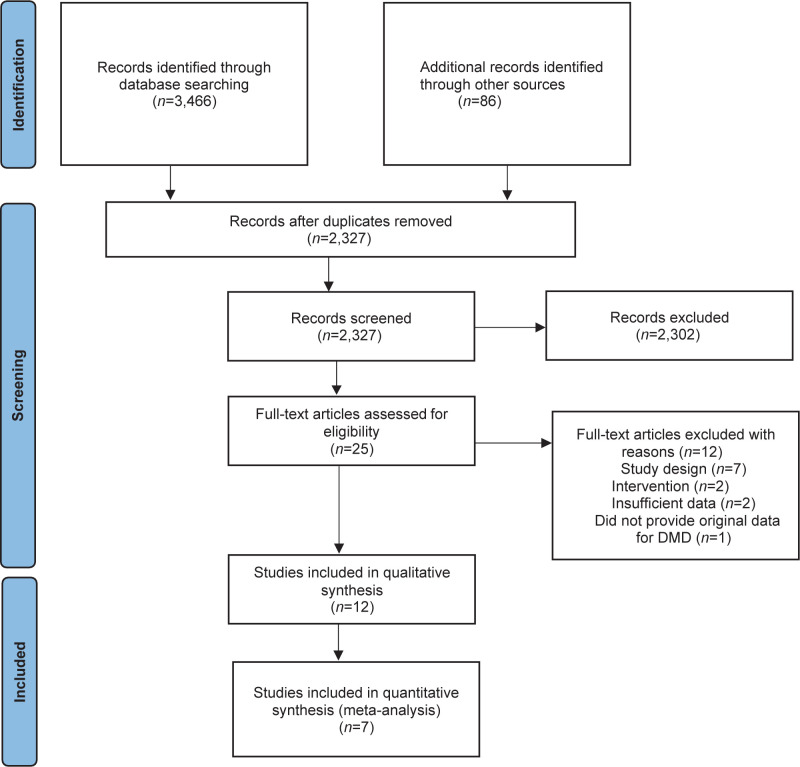
The search identified 3,466 references from 6 databases and 86 references from other sources. After screening, 25 were assessed as full-text articles. Amongst these, we identified two study reports of the same study (25, 30). Hence, the final sample included 12 studies for qualitative analysis (8, 24–35) (Fig.1).
Fig. 1.
Flow chart of identified, screened, excluded and included articles. DMD: Duchenne muscular dystrophy.
Study characteristics
All 12 studies were conducted in the Western world between 1966 and 2018. In general, the studies were diverse with respect to design, type and duration of exercise intervention, number of participants, outcome assessments and outcomes of interest. Characteristics and summary of findings of included studies are described in Table I.
Table I.
Characteristics of included studies (n = 12)
| Author, year (reference) Country Study design |
Sample size Total, IG and CG. Mean age (SD) or range And Participants functioning |
Exercise training intervention for the IG Frequency, Intensity, Time, Setting, Duration |
Intervention for the CG (Comparison) Frequency, Intensity, Time, Setting, Duration |
Outcome Measures |
|---|---|---|---|---|
| N = 24 | Arm cycling | UE ROM exercises | Functioning | |
| IG (n = 12) | F:3 days/week | F: 5 days/week | AERA. Standing from | |
| 9.5 (1.38) years IG (n = 12) 9.33 (1.37) years |
I:50% of max difficulty T:40 minutes |
I:5-10 reps depending on individual fatigue T:40 minutes |
supine. T-shirt donning/Removing NSAA |
|
| Alemdaruglo et al. 2015 (24) Turkey | All ambulant, able to sit 1 hour independently, steroid use for more than 6 months | S: Hospital, supervised by PT D: 8 weeks |
S:Home, supervised by family D: 8 weeks |
Quality-of-life NA |
| Strength | ||||
| RCT | Isometric strength by HDD UE. Grip Strength. | |||
| Endurance | ||||
| A6MCT | ||||
|
| ||||
| N = 19 | Gravity compensated | Functioning | ||
| IG (n = 9) 12.9 (2.8) years CG (n = 10) 12.6 (3.4) years |
UE training with use of 3D Sony PlayStation videogame F:5 days/week I:n/a |
Usual care | PUL, MFM ROM Abilhand-plus (questionnaire children/parents) |
|
| Heutinck et al. 2018 (26) Netherland | Ambulatory and wheelchair dependent, able to lift their hands to the head by use of elbow flexion or compensate. 100% in IG and 60% in CG used steroids. | T: 15 minutes S: Home, supervised at start and after 10 weeks, otherwise independently D: 30 weeks |
Quality-of-life Kidscreen 52 Global Health (Children and parents questionnaires) |
|
| RCT | ||||
| Strength Isometric strength by HDD UE. MVC Endurance A6MCT |
||||
|
| ||||
| N = 14 | Breathing exercises and PT | PT program | Functioning | |
| IG (n = 7) | program | FVC, FEV1, FEF25-75, PERF | ||
| 8.5-14.11 years | F: 5 days/week | F:5 days/week | ||
| CG (n = 7) 8.5-15.6 years |
I: 10 and 18 cm H20 CPAP during 12 deep insp, 4-5 cough cycles, 6 forced expirations | I: n/a T: n/a S: School, supervised by PT. |
Quality-of-life n/a |
|
| Houser et al. 1971 (27) | T: n/a | D: 12 weeks | Strength | |
| USA | All the participants were wheelchair dependent | S: School, supervised by PT. | n/a | |
| D: 12 weeks | Endurance | |||
| CCT | M | |||
|
| ||||
| N = 30 (29 analyzed) | Active assisted UE and LE cycling (KTP kinetic ergometer). | Usual care | Functioning | |
| IG (n = 17) 10.8 (2.4) years |
F: 5 days/week | MFM, ROM PEDI (self-care questionnaire). | ||
| Jansen et al. 2013 (8) Netherland RCT |
CG (n = 13) 10.5 (2.8) years Ambulatory (n = 18) and wheelchair dependent (n = 12). 23 of participants used steroids. All were able to lift both arms to the head, but unable to use wheel chair > 500 meters. |
I:65 revolutions per minute/< 6 OMNI scale) T: 15 minutes legs, 15 minutes arms. S: Home supervised by parents or PT. D: 24 weeks |
Quality-of-life n/a Strength MRC Endurance A6MCT |
|
|
| ||||
| N= 18 (17 analyzed, one died) | Respiratory strength and endurance training by use of circuit respiration device (flow limiting resistance). | Usual care | Functioning | |
| 14.2 (7-20) years IG (n = 9) CG (n = 9) |
F: 5 days/week I: Endurance; |
VC Quality-of-life n/a |
||
| Martin et al. 1986 (28) | Wheelchair dependent (n = 17), ambulatory (n = 1) | Ventilate until exhaustion within 3 minutes, 20% over | Strength | |
| Australia | VC rage in one sequence. | PeMax and | ||
| Strength: Maximal inspiratory/expiratory manoeuvers in 3-5 seconds | PiMax | |||
| RCT cross-over | S: supervised at school D: 8 weeks |
Endurance Pe time and Pi time |
||
|
| ||||
| N = 22 (20 analyzed) | Inspiratory muscle training | Placebo | Functioning | |
| 11.6 (9-14) years | (Triflow II) | Forced expirations (Peak expiratory flow meter). | FVC, FEV1, PERF | |
| F: 5 days/week | ||||
| Radillo et al. 1989 (29) United Kingdom |
n/a | I: 20 inspirations with increased resistance flow T: n/a S: School, supervised by PT D: 18 days |
F: 5 days/week I: 10 expirations T: n/a S: School, supervised by PT D: 18 days |
Quality-of-life n/a Strength PiMax |
| RCT cross-over | Endurance n/a |
|||
|
| ||||
| N = 18 | LE exercise program and passive stretching. | Series of oral instructed free exercises for the LE and passive stretching. | Functioning | |
| Scott et al. 1989 (30) United Kingdom | 6.9 (1.17) years (5-9) years IG (n = 9) CG (n = 11) All were fully ambulatory, with anticipated compliance to the intervention. |
F: 7 days/week I: Manual resistance (n/a) T: 15 minutes S: Home, supervised by the parents D: 6 months |
F: 7 days/week I: n/a T: 15 minutes S: Home, supervised by the parents D: 6 months |
Locomotor ability, ROM ankle dorsiflexion. Vignos Scale, 8.4 and 45 meter timed test. Quality-of-life n/a |
| RCT | Strength MRC, Myometric and torque force output Endurance n/a |
|||
|
| ||||
| N = 18 (12 analyzed, four died). | Inspiratory muscle training | Usual care | Functioning | |
| 15 (range 10.4 – 23.4) years IG (n = 7) |
with flow resistance to play a video game with visual audio feedback. | FVC (% pred) | ||
| CG (n = 11) Ambulatory (n = 2) and wheelchair dependent (n = 2). | F: 5 days/week I: Exceed a pre-set level of resistance, 6.25, 4.76, 3.18 or 2.38 mm restrictors. |
Quality-of-life n/a |
||
| Stern et al. 1989 (31) Australia | S: School, supervised | Strength PeMax |
||
| D: 6 months IP, 12 months SP. | (% pred), PiMax (% pred) | |||
| RCT cross-over | Endurance | |||
| Endurance (mmHg) | ||||
|
| ||||
| N = 16 | Inspiratory resistive muscle training (Triflow) | Placebo | Functioning | |
| IG (n = 8) | F: 2times/5 days | Inspiratory muscle training (Triflow) | VC, FRC, TLC, FEV1, FEV1/ | |
| 14.7 (4.5) years | I: 30% of PiMax | FVC | ||
| Topin et al. 2002 (32) | CG (n = 8) | T: 10 minutes | F:2times/5 days | |
| France | 12.63 (1.8) years | S: Home, supervised by parents, D: 6 weeks | I: 5% of PiMax | Quality-of-life |
| T: 10 minutes | n/a | |||
| RCT | All were wheelchair dependent, clinically stable and free of medication and dyspnea | S: Home, supervised by parents | Strength | |
| D: 6 weeks | PiMax/MIP | |||
| Endurance | ||||
| Tlim | ||||
|
| ||||
| N = 28 | Resistance muscle training (active/active assisted) of LE, UE and abdominal muscles. | Usual care | Functioning | |
| Vignos et al. 1966 (33) | IG (n = 14) 7.4 years CG (n = 14) 7.7 years |
F: 7 days/week first 6 months, 3-5 days/week next 6 months | Timed tests by Stair climbing, rising from floor, rising from chair, 23 feet walking. | |
| USA | Fully ambulating with good functional status | I: 10 reps with maximal resistance/lowest degree of assistance by antigravity pulley. | Quality-of-life n/a |
|
| CCT | T: 30 minutes S: Home, initial supervised by PT. D: 12 months |
Strength Overall muscle strength %-of normal (MRC). Weight lifted in each exercise. Endurance n/a |
||
|
| ||||
| N = 30 (22 analyzed) IG (n = 15) 13.6 (4.5) years CG (n = 15) 14.5 (3.8) years |
Inspiratory muscle training with special constructed training device. F: 2 times/day 5 days/week |
Usual care | Functioning VC, FEV1, 12 s MVV Quality-of-life n/a |
|
| Wanke et al. 1994 (34) Australia | Both ambulatory (n = 7) and wheelchair depended (n = 23). | I: Endurance; 10 cycles of 1-minute duration with variable resistance, 20 second rest. |
Strength PesMax, Pdi | |
| Strength; | ||||
| 10 maximal inspirations. | Endurance | |||
| RCT | Endurance time (Te) | |||
| T:n/a | ||||
| S: Home, supervised by clinicians or parents | ||||
| D: 6 months | ||||
|
| ||||
| N = 45 | Breathing exercises and a PT program as CG. | PT program | Functioning | |
| IG (n=24) | F: 2 times/day | VC, FVC, FEV1, Mobility of thorax (circumference measured at maximal inspiration (FVC level), neutral (functional residual capacity- level) and maximal expiration (residual volume-level) at three defined anatomical reference points | ||
| Zileili et al. 1999 (35) Turkey | 12.08 (1.79) years CG (n = 21) 23.43 (2.04) years |
F: 3 times/day 7 days/week I: 10 reps a) isolated chest breathing b) respiratory exercise combined with other exercises c) Breathing cycles with Triflow device T: n/a S: Home, supervised by parents |
7 days/week I: 10 reps active or active assisted UE and LE exercises isotonic exercise for abdominal muscles. Passive stretching of LE (hip, flexors, hamstrings, tensor facia latae, gastro soleus, lumbar extensors) T: n/a S: Home, supervised and help from parents |
Quality-of-life n/a |
| CCT | Participants with early scoliosis, able to cooperate and without affected respiration and use of respiratory assistive devices | D: 4 weeks | D: 4 weeks | |
| Strength | ||||
| n/a | ||||
| Endurance | ||||
| n/a | ||||
IG : Intervention group; CG: Control Group; SD : Standard deviation; F: Frequency; I: Intensity; T: time; S : Setting; D: Duration (intervention period); RCT: Randomized Controlled Trial; HHD: Hand Held Dynamometer; MMDT: Minnesota Manual Dexterity Test; AREA: Arm elevation assessment; NSAA: North Star Ambulatory Assessment; A6MCT: Assisted 6 Minutes Cycling Test; PUL: Performance Of Upper Limb; MFM: Motor Function Measure; ROM : Range Of Motion; HRQoL: Health Related Quality of Life; CCT : Clinical Controlled Trial; MVV: Maximal Voluntary Ventilation; FVC: Forced Vital Capasity; FEV1 : Forced Expiratory Volume first second; FEF: Forced Expiratory Flow; PEFR: Peak Expiratory Flow Rate; MRC: Medical Research Council (scale); PEDI: Pediatric Evaluation of Disability Inventory; MEP: Maximal Expiratory Pressure; MIP and Pi Max : Maximal Inspiratory Pressure; Pe and Pi time: Expiratory or Inspiratory Pressure sustained over time; Tlim: Time limit, maximal time a subject was able to sustain breathing against a predetermined inspiratory load without fatigue; VC: Vital Capacity; TLC: Total Lung Capacity; Pesmax: Maximal Sniff assessed Esophageal; Pdi: Trans diaphragmatic Pressure; n/a: not available
Study design
Six studies were randomized controlled trials (8, 24, 26, 30, 32, 34), 3 were randomized cross-over trials (28, 29, 31), and 3 were clinically controlled trials (27, 33, 35).
Participants
The total number of participants was 282, of whom 264 (94%) completed the studies. The sample size range was 14–45 participants, mean age was 10.7 (range 5–24) years, 108 participants were wheelchairdependent, 86 were able to walk, and this information was lacking for 88 participants. Withdrawals occurred due to illness during the intervention period (29) or motivational problems (26). Five participants died (mean age 17.8 years) due to superimposed infection and respiratory failure or respiratory failure alone; all had severely restricted lung capacity (28, 31).
Interventions
Five studies used exercise training for limbs, and 7 applied respiratory muscle training (RMT). For limb exercise, 2 studies used cycling (arms or arms and legs), 1 used videogames for arms, 1 used resistance training for legs, and 1 used resistance training for arms and legs. For RMT, inspiratory muscle training was used in 5, inspiratory and expiratory muscle training in 1 and breathing exercises in 2 studies. Exercise training was performed at home (7 studies), at school (4 studies) or in the hospital (1 study). Half of the studies had a short-term training intervention (range 36 days to 12 weeks), the other half had a long-term training intervention (range 5–12 months).
Comparison
Eight studies used usual care for comparison (8, 26-28, 31, 33–35), 2 used placebo (29, 32) and 2 used alternative training (24, 30).
Outcomes
The studies did not report functioning outcomes uniformly (8, 24, 26, 33), and only one reported HRQoL (26). Overall, studies with RMT as exercise training intervention assessed respiratory muscle strength (28, 29, 31, 32, 34) or endurance (27, 28, 31, 32, 34), and/or lung function parameters ((27–29, 31, 32, 34, 35). Limb exercise training interventions assessed muscular strength of the trained extremities (8, 24, 26, 30, 33), endurance (8, 24, 26), and functioning measures (e.g. timed physical tests or range of motion (ROM)) (8, 24, 26, 30, 33).
Risk of bias
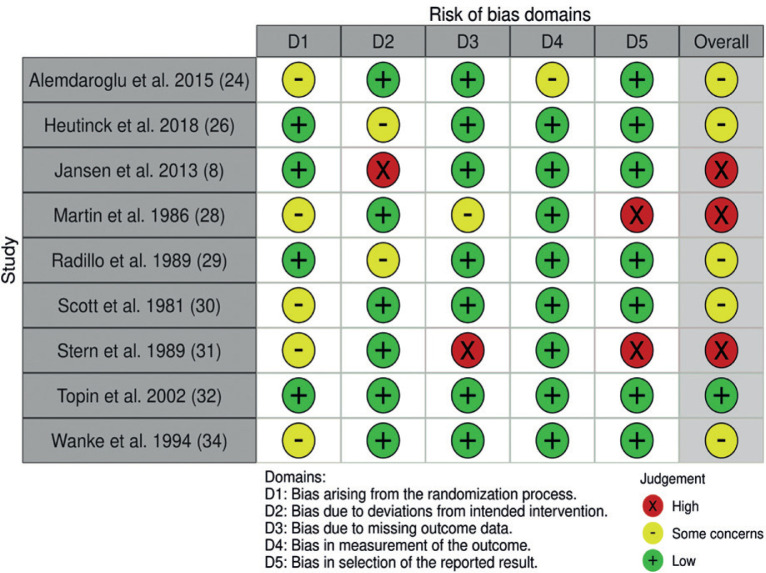
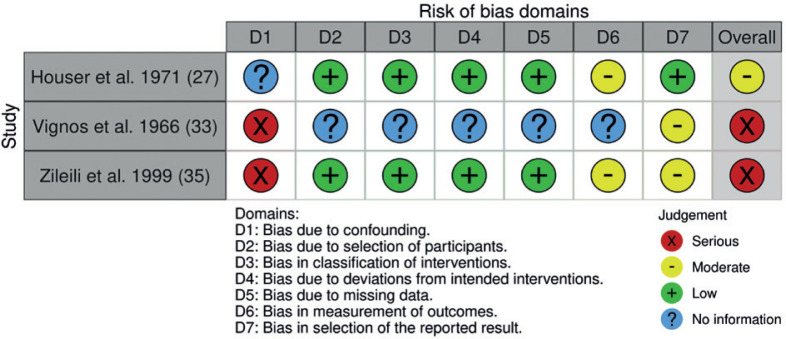
ROB is summarized by outcome level of the RCT studies in the meta-analysis (Fig. 2), and by the study level for the non-randomized interventional studies (Fig. 3). All but one of the included studies (32) presented unclear or high ROB factors.
Fig. 2.
Risk of bias of the included randomized controlled studies.
Fig. 3.
Risk of bias by study level for the non-randomized studies.
Missing description of the allocation process (8, 24, 28, 30, 31, 34) led to “some concern” regarding risk of bias. No intention-to-treat analysis (26) and no washout time (29) led to “some concern” regarding risk of bias. One study was judged “high risk” as participants were moved between groups after randomization, with no intention-to-treat analysis (8). Another study was also considered to have high risk of bias due to missing data from 7 participants (31). No intention-to-treat analysis caused “some concern” (28), as did bias in outcome measurement without assessor blinding (24), and biased results reporting was judged “high” in 2 cross-over studies with lack of separate results for specific time-points (28, 31) (Fig. 2).
Amongst non-randomized studies (Fig. 3), no information regarding confounders (e.g. age, training supervision) (33, 35) led to serious ROB. Lack of information regarding participant selection, retrospectively assigned intervention classification, deviation from intended intervention and missing data led to moderate ROB in one study (33). Three studies had moderate ROB in outcome measurement due to no information regarding assessor blinding (27, 33, 35). As for the reported results, ROB was considered moderate in 2 studies, which were judged to report “no information” due to insufficient description (33, 35).
Synthesis of results
Due to the low number of studies included and the large heterogeneity in outcome measures and comparisons, it was not possible to perform a meta-analysis for the primary outcomes, functioning and HRQoL. A metaanalysis was performed for the secondary outcomes, muscular strength and endurance following any exercise intervention.
Narrative synthesis
Functioning
Two studies reported on multiple domains of functioning at the ICF activity and participation level, with no significant differences evaluated by the ABIL-Hand and PEDI-questionnaire (8, 26). When measuring functioning using standardized functional assessments at ICF-activity level, the 2 studies using arm-cycling revealed improved or maintained functioning measured by arm elevation assessment and motor function measure (8, 24). There were no improvements in the other functioning outcome variables reported (8, 26, 30, 33). RMT did not improve lung function parameters (27–29, 32, 35), but breathing exercises improved chest mobility (35).Vital capacity decreased in all but one study (28) regardless of participants underwent training or not (see Table II).
Table II.
Certainty of evidence
| GRADE domain | Judgement according to outcomes of interest | Concerns about certainty domains |
|---|---|---|
| Methodological limitations of the studies | Functioning: Three studies had some concerns regarding ROB (22, 24, 28), and 2 had high risk of bias (7, 31), conservatively the trials were judged to have very serious methodological limitations. In the studies investigating lung function, 1 study was judged to have low ROB (30), 3 to have some concern (25, 27, 32), and 3 to have high risk of bias (26, 29, 33). Conservatively the trials were judged to have very serious methodological limitations Quality of life: Only 1 study reported this outcome (24). The study was judged to have some concerns regarding ROB. The trial was judged to have serious methodological limitations. |
Very serious Serious |
| Indirectness | The patients, interventions and comparators in the studies all provided direct evidence to the clinical question at hand. | Not serious |
| Imprecision | Functioning: Five studies reported on function, with a total of 119 participants (very low) (7, 22, 24, 28, 31). Two studies reported small improvements (7, 22), and 3 with non-significant results likely because of enrolling a small number of participants, and presence of clinical heterogeneity (age, progression) (24, 28, 31). The evidence was judged to have serious imprecision. Seven studies reported in lung function parameters as outcome (25-27, 29, 30, 32, 33). One study reported improvements in lung function (32), while in 6 studies lung function remained unchanged or declined with non-significant changes (25-27, 29, 30, 33). The evidence was judged to have serious imprecision. Quality of life: The only study including this outcome reported non-significant improvement in favour of intervention (24). The evidence was judged to have serious or very serious imprecision. |
Serious |
| Inconsistency | Functioning: The direction and magnitude of effect varied across the different trials. Overall the results showed either small or no change in functioning in favour exercise training. The evidence was judged to have serious inconsistency. In the studies who investigated change in lung function, the direction and magnitude were similar across all except 1 of the studies with no change in lung function (32). The evidence was judged to not serious inconsistency. |
Serious/very serious Serious Not serious |
| Publication bias | Functioning: Publication bias was not strongly suspected because both negative and positive trials were published, and search for studies were comprehensive. Publication bias was not strongly suspected with respect to lung function, except in 2 studies without reported outcome data for the time-points and separate arms for the groups of intervention (26, 29). In addition to this, publication bias was not strongly suspected, because both negative and positive trials were published, and search for studies were comprehensive. Quality of life: Publication bias was not strongly suspected, because a non-significant improvement in favour intervention was reported (24). |
Not suspicious Not suspicious |
Grade evidence by ROB judgements was considered as; low to be no serious or serious, unclear to be equal to serious or very serious and high ROB to be very serious. If GRADE domains were judged as serious, they were downgraded by 1 point, and very serious, certainty of evidence was downgraded by 2 points.
Health related quality-of-life
HRQoL was reported in only one study (applying videogame exercise with gravity compensation), no significant improvements were reported following the intervention (26) (see Table II).
Meta-analysis
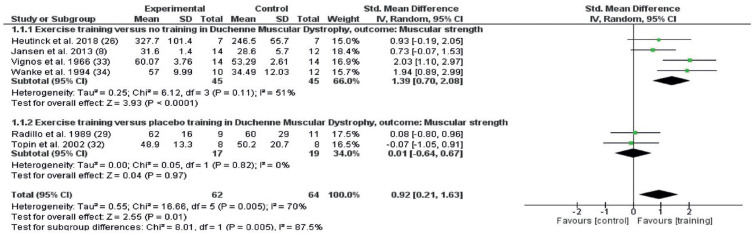
Muscular strength
The muscular strength outcomes of studies that included any exercise training intervention are shown in Fig. 4. Random effects meta-analysis included 6 studies with 126 participants. Muscular strength was improved by the interventions (SMD 0.92; 95% confidence interval (95% CI) 0.21–1.63, I2 70%) (Fig. 4). When comparing exercise vs placebo, no effect on muscular strength was found (SMD 0.01; 95% CI –0.64 to 0.67, I 0%), but a large effect was found for the comparison exercise vs no exercise (SMD 1.39; 95% CI 0.7–2.08, I2 70%).
Fig. 4.
Forest plot of the effect on muscular strength of any exercise vs no exercise (1.1.1) and any exercise vs placebo (1.1.2) in persons with Duchenne muscular dystrophy (DMD), with pooled effects of these 2 comparisons (Total). 95% CI: 95% confidence interval; df: degrees of freedom; I2: measure of heterogeneity; Tau2: measure of variance; SD: standard deviation.
Endurance
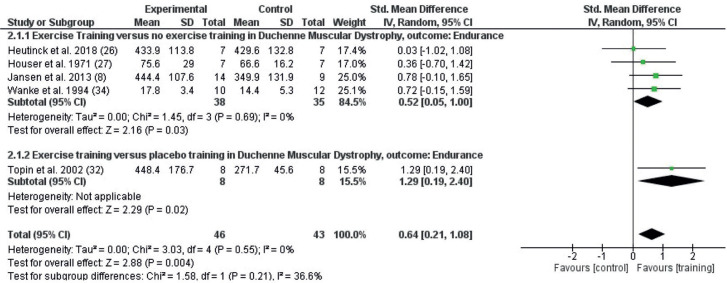
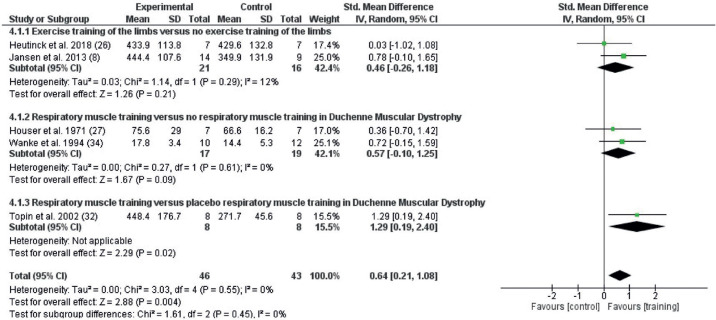
The endurance outcomes from studies that included any exercise training intervention are shown in Fig. 5. Random effects meta-analysis included 5 studies with 89 participants. Endurance was improved by the exercise training interventions (SMD 0.64; 95% CI = 0.21–1.08, I2 0%). When comparing exercise vs placebo, the study found significant differences in favour of exercise training vs placebo (SMD 1.29; 95% CI 0.19–2.40) and exercise training vs no exercise (SMD 0.52; 95% CI 0.05–1.00, I2 0%)
Fig. 5.
Forest plot of the effect on endurance after any exercise vs no exercise (2.1.1) and any exercise vs placebo (2.1.2) in persons with Duchenne muscular dystrophy (DMD), with pooled effects of these 2 comparisons (Total). 95% CI: 95% confidence interval; df: degrees of freedom; I2: measure of heterogeneity; Tau2: measure of variance; SD: standard deviation.
Subgroup analysis
Due to study heterogeneity, subgroup analysis by type and duration of exercise training intervention was performed.
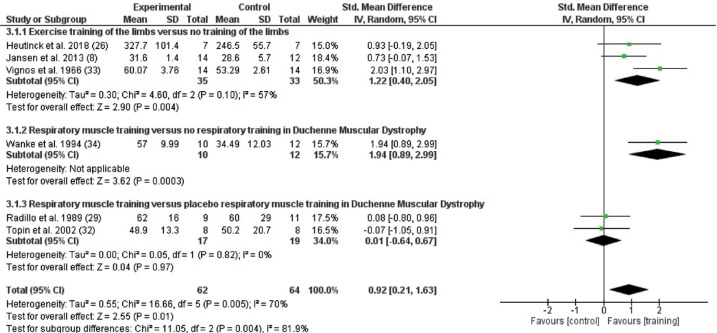
For muscular strength, effects were identified of limb exercise training (SMD 1.23; 95% CI 0.42–2.05, I2 55%) and RMT (SMD 1.94; 95% CI 0.89–2.99, I2: not applicable) compared with no exercise. No effect was seen of RMT compared with placebo (SMD 0.01; 95% CI –0.64 to 0.67, I2 0%) (Fig. 6).
Fig. 6.
Forest plot of the effect on muscular strength of the different types of exercise, exercise of limbs and postural muscle vs no exercise (3.1.1), respiratory muscle training (RMT) and breathing exercises vs no exercise (3.1.2), and RMT vs placebo (3.1.3) in persons with Duchenne muscular dystrophy (DMD). 95% CI: 95% confidence interval; df: degrees of freedom; I2: measure of heterogeneity; Tau2: measure of variance; SD: standard deviation.
For endurance, no significant effects were identified for limb exercise training (SMD 0.46; 95% CI –0.26 to 1.18, I2 12%) or RMT (SMD 0.57; 95% CI –0.10 to 1.25, I2 0%) compared with no exercise, whereas there was an effect for RMT vs placebo (SMD 1.29; 95% CI 0.19–2.40) (Fig. 7).
Fig. 7.
Forest plot of effects on endurance of the different types of exercise training, exercises of limb and postural muscle vs no exercise (4.1.1), respiratory muscle training (RMT) and breathing exercises vs no exercise (4.1.2), and RMT vs placebo (4.1.3) in persons with DMD. 95% CI: 95% confidence interval; df: degrees of freedom; I2: measure of heterogeneity; Tau2: measure of variance; SD: standard deviation.
Safety of exercise training
Regarding the safety of exercise training intervention, no studies systematically reported adverse events. In 2 studies, symptoms of fatigue or pain or blood serum creatine kinase levels were monitored for safety assessment (8, 34). Most studies provided careful supervision.
Certainty of results
The certainty of results was assessed by the GRADE approach. Due to high study heterogeneity, few participants and imprecision by large confidence intervals for both muscular strength and endurance, the certainty of evidence was downgraded 3 steps (Table III).
Table III.
Summary of findings
| Outcomes | Results from narrative synthesis or meta-analyses with the effect size Standardized mean difference (95% confidence interval) | Number of participants (studies) | Certainty of the evidence* |
|---|---|---|---|
| Functioning | ⊗OOO | ||
| Functional assessments | The studies showed small or no effect in functioning | 119 participants (4 randomized controlled trials and 1 clinical controlled trial) | Very low Due to very serious ROB, serious inconsistency, serious imprecision (variance in reported results and low numbers of participants) |
| Lung function | The studies showed no effect on lung function | 163 participants (2 randomized controlled trials, 3 cross-over trials and 2 clinical controlled trials) | ⊗OOO Very low Due to very serious ROB, serious inconsistency, serious imprecision. |
| Health-related Quality of life | One study showed non-significant improvement, the mean HRQoL improved 2.4 (SD 3.3) in intervention group and 1.4 (SD 2.4) in the control group by Kidscreen 52. | 19 participants (1 randomized controlled trial) | ⊗OOO Very low Due to serious ROB, serious to very serious inconsistency and imprecision (1 study, few participants) |
| Muscular strength | 0.92 (0.21, 1.63) | 126 (5 randomized controlled trials and 1 cross-over trial) | ⊗OOO Very low Due to very serious ROB, serious imprecision (e.g. low number of participants), very serious inconsistency (large CI). |
| Endurance | 0.64 (0.21, 1.08) | 89 participants (4 randomized controlled trials, 1 clinical controlled trial) | ⊗OOO Very low Due to very serious ROB, serious inconsistency (broad CI), and serious indirectness (low numbers of participants and variance in reported results). |
The primary outcomes were functioning and health-related quality of life for which a narrative synthesis of the evidence was provided. For the secondary outcomes by muscular strength and endurance, a pooled effect estimate was possible.
Commonly used symbols to describe certainty of evidence profiles: high certainty ⊗⊗⊗⊗, moderate certainty ⊗⊗⊗O, low certainty ⊗⊗OO and very low certainty ⊗OOO.
DISCUSSION
This systematic review included 12 studies with 282 participants with DMD. It was only possible to conduct a narrative synthesis for the primary outcomes of functioning and HRQoL, and this indicated no clear effect of exercise training interventions. Data from 126 participants were included in meta-analyses, with findings suggesting that any exercise training intervention may improve muscular strength and endurance in persons with DMD. Subgroup analyses to evaluate the specific type of exercise intervention suggests that limb exercise training improved limb muscular strength and RMT improved respiratory muscular strength, both compared with no training. For endurance, RMT improved respiratory muscular endurance compared with placebo. No study reported signs of overuse or injuries during the intervention period; thus, long-term effects and possible adverse effects of exercise training intervention remain uncertain. Two studies reported the death of 5 participants due to superimposed infections and respiratory failure. The certainty of evidence was very low, due to low quality studies and to large heterogeneity between the included studies.
This systematic review identified few studies, with small sample sizes and a wide range of interventions and outcomes. This resulted in low evidence and high risks of bias. Comparisons were challenging and did not allow subgroup analyses by age or disease stage (e.g. ambulatory or non-ambulatory). The majority of study participants were children. As the severity of impairment increases during the disease course, the results of this systematic review may not be generalizable to all persons with DMD. In addition, exercise training effects may be influenced by the different phenotypes of DMD (1), which are not covered in this review.
No previous reviews have examined the effects of exercise training specific to persons with DMD. The only other systematic review in DMD reported solely on RMT intervention, indicating effects on muscular strength and endurance (15); however, with similar limitations.
The effect of exercise training in persons with DMD has been controversial for a long time. DMD is characterized by dystrophic muscle with enhanced fragility, and exercise training was for years considered harmful, due to the potential for increasing muscle damage and injury (36, 37). In addition to a general recommendation for submaximal exercise (1), there is a lack of guidelines regarding exercise training in DMD. As such, the included studies evaluated a broad range of exercise interventions in DMD. It was not possible to point out any specific type of exercise training being more appropriate, but exercise training might have potential benefits, specifically for the secondary outcomes muscular strength and endurance. Subgroup analyses, although they should be interpreted with caution due to the above-mentioned limitations, indicated a larger effect size in favour of RMT compared with limb exercise training. The RMT studies generally reported significant improvements in inspiratory muscle endurance (28, 32, 34), while inspiratory muscle strength improved only after a prolonged training period (31, 34). Moreover, studies with exercise for limbs aimed to prevent disuse of dystrophic muscles and maintain or optimize the participants’ functioning. The only study reporting HRQoL found non-significant improvements after the intervention compared with the control group. Thus, no change after intervention may in fact be beneficial in a group of patients with a progressive disease characterized by the gradual development of muscle degeneration and weakness.
This systematic review included studies that compared exercise training intervention with no training or usual care, placebo exercise training or alternative exercise training programmes. The term “usual care” was not uniformly described in the individual studies, and one cannot exclude the possibility that persons in this group in fact participated in various forms of exercise training. Placebo studies are impossible to perform in traditional exercise training studies. In one of the 2 RMT studies that used placebo treatment, the only difference in exercise training intervention was the intensity grading, while the other compared non-resistance expiration training by use of a peak flow meter with inspiratory muscle resistance training, which, in fact, might represent 2 different exercise training interventions.
The results of this systematic review are very uncertain and should be interpreted with caution. The certainty of evidence was very low for key outcomes for all comparisons. The study limitations indirectness, imprecision or combinations of these were the main reason for downgrading evidence. Five studies were at high ROB in at least 1 domain, 4 were at some concerns regarding ROB in at least 1 domain, and only 1 had low ROB. The major limitation with regard to validity of evidence was the small number of studies, as well as the small sample sizes. None of the RCTs described power analyses, intention-totreat analyses were not performed in 2, while 2 RCT cross-overs had insufficient data. Overestimation of treatment effect is more likely to occur with smaller studies (38). The small samples may be explained by the rarity of the disease. DMD causes early disability, cognitive and behavioural problems, and comorbidities such as cardiorespiratory limitation and joint contractures represent important barriers for interventions. Blinding of participants is not possible in exercise studies, but blinding of outcome assessors is recommended (19).
By supplementing our systematic database search with searches of reference lists, study registers and grey literature, and by approaching authors by mail, we probably identified most relevant studies. Given the nearly complete consensus between the 2 review authors responsible for study selection, the risks of selection bias were probably low. However, none of the study authors contacted responded to our request for data; hence, these studies could not be included in the meta-analysis. We encountered challenges in performing and interpreting comparisons due to substantial differences between the studies, including designs, population, exercise prescription, outcomes and data presentation. In order to minimize heterogeneity between studies, we performed 3 comparisons; hence there were few studies for each comparison. This may explain the high variance in reported results. We still chose to perform a meta-analysis, acknowledging these important obstacles to obtaining a valid result, as this field of medicine is in a developing phase, and more research is needed to guide clinical decisions in this vulnerable group of patients with a devastating disease.
Further research is needed. Ideally, studies should include large groups of participants stratified by disease severity. Due to the rarity and nature of DMD, it will require international multicentre studies to include sufficient numbers. A more realistic approach would be to plea for pragmatic trials as they better correspond to real practice and the willingness to participate is greater. A relevant aim for the study could be to increase the overall physical activity level that can be measured objectively by the use of accelerometers or smart watches, and with self-reported participation in daily activities. Possible influence on functioning could be investigated by using multivariate analyses due to disease stages. Qualitative research may capture how participants experience exercise training, their potential wellbeing or enjoyment, and evaluate changes in functioning and HRQoL. A sedentary or active lifestyle, based on the level of physical activity at baseline, should be acknowledged, as it may influence outcomes, such that untrained persons may respond with larger gains. In addition to a well-described exercise training intervention, adherence should be reported with respect to dose-response. Finally, safety should be systematically addressed, in order to reveal any negative effects in this vulnerable population.
CONCLUSION
This systematic review was performed to evaluate the effects of exercise training interventions to improve functioning and HRQoL in persons with DMD. It was not possible to determine whether exercise training improves functioning and HRQoL. However, the meta-analysis indicated that exercise training improves muscular strength and endurance in persons with DMD. Given that these secondary outcomes are important surrogate measures for functioning, this might represent an effect of exercise intervention. It was also not possible to conclude whether exercise training is safe in persons with DMD. Due to the low number of studies included and large heterogeneity, it was not possible to identify the most effective exercise training intervention in DMD. The certainty of evidence was very low, and more research is needed.
ACKNOWLEDGEMENT
This project have been made possible by the funding received from Dam Foundation
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Birnkrant DJ, Bushby K, Bann CM, Apkon SD, Blackwell A, Brumbaugh D, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol 2018; 17: 251–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mendell JR, Lloyd-Puryear M. Report of MDA muscle disease symposium on newborn screening for Duchenne muscular dystrophy. Muscle Nerve 2013; 48: 21–26. [DOI] [PubMed] [Google Scholar]
- 3.Matthews E, Brassington R, Kuntzer T, Jichi F, Manzur AY. Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst Rev 2016: CD003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lebel DE, Corston JA, McAdam LC, Biggar WD, Alman BA. Glucocorticoid treatment for the prevention of scoliosis in children with Duchenne muscular dystrophy: long-term follow-up. J Bone Joint Surg Am 2013; 95: 1057–1061. [DOI] [PubMed] [Google Scholar]
- 5.Eagle M, Bourke J, Bullock R, Gibson M, Mehta J, Giddings D, et al. Managing Duchenne muscular dystrophy--the additive effect of spinal surgery and home nocturnal ventilation in improving survival. Neuromuscul Disord 2007; 17: 470–475. [DOI] [PubMed] [Google Scholar]
- 6.World Health O . WHO guidelines on physical activity and sedentary behaviour. Geneva: World Health Organization, 2020. [Google Scholar]
- 7.Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, et al. A tutorial on pilot studies: the what, why and how. BMC Medical Research Methodology 2010; 10: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jansen M, van Alfen N, Geurts AC, de Groot IJ. Assisted bicycle training delays functional deterioration in boys with Duchenne muscular dystrophy: the randomized controlled trial ”no use is disuse”. Neurorehabilitation and neural repair 2013; 27: 816–827. [DOI] [PubMed] [Google Scholar]
- 9.Markert CD, Case LE, Carter GT, Furlong PA, Grange RW. Exercise and Duchenne muscular dystrophy: where we have been and where we need to go. Muscle Nerve 2012; 45: 746–751. [DOI] [PubMed] [Google Scholar]
- 10.Birnkrant DJ, Bushby K, Bann CM, Alman BA, Apkon SD, Blackwell A, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol 2018; 17: 347–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deconinck N, Dan B. Pathophysiology of duchenne muscular dystrophy: current hypotheses. Pediatr Neurol 2007; 36: 1–7. [DOI] [PubMed] [Google Scholar]
- 12.Gianola S, Castellini G, Pecoraro V, Monticone M, Banfi G, Moja L. Effect of Muscular Exercise on Patients With Muscular Dystrophy: A Systematic Review and Meta-Analysis of the Literature. Front Neurol 2020; 11: 958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Human A, Corten L, Jelsma J, Morrow B. Inspiratory muscle training for children and adolescents with neuromuscular diseases: A systematic review. Neuromuscul Disord 2017; 27: 503–517. [DOI] [PubMed] [Google Scholar]
- 14.Silva IS, Pedrosa R, Azevedo IG, Forbes AM, Fregonezi GA, Dourado Junior ME, et al. Respiratory muscle training in children and adults with neuromuscular disease. Cochrane Database Syst Rev 2019; 9: CD011711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williamson E, Pederson N, Rawson H, Daniel T. The Effect of Inspiratory Muscle Training on Duchenne Muscular Dystrophy: A Meta-analysis. Pediatr Phys Ther 2019; 31: 323–330. [DOI] [PubMed] [Google Scholar]
- 16.Prodinger B, Reinhardt JD, Selb M, Stucki G, Yan T, Zhang X, et al. Towards system-wide implementation of the International Classification of Functioning, Disability and Health (ICF) in routine practice: Developing simple, intuitive descriptions of ICF categories in the ICF Generic and Rehabilitation Set. Journal of rehabilitation medicine 2016; 48: 508–514. [DOI] [PubMed] [Google Scholar]
- 17.Pangalila RF, van den Bos GA, Bartels B, Bergen MP, Kampelmacher MJ, Stam HJ, et al. Quality of life of adult men with Duchenne muscular dystrophy in the Netherlands: implications for care. Journal of rehabilitation medicine 2015; 47: 161–166. [DOI] [PubMed] [Google Scholar]
- 18.McDougall J, Wright V, Schmidt J, Miller L, Lowry K. Applying the ICF framework to study changes in quality-oflife for youth with chronic conditions. Dev Neurorehabil 2011; 14: 41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JPT, Higgins JPT. Cochrane handbook for systematic reviews of interventions 2019; Hoboken, New Jersey: Cochrane; 2019. [Google Scholar]
- 20.Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355: i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of clinical epidemiology 2011; 64: 380–382. [DOI] [PubMed] [Google Scholar]
- 22.Takeshima N, Sozu T, Tajika A, Ogawa Y, Hayasaka Y, Furukawa TA. Which is more generalizable, powerful and interpretable in meta-analyses, mean difference or standardized mean difference? BMC Med Res Methodol 2014; 14: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Popay J, Roberts H, Sowden A, Petticrew M, Arai L, Rodgers M, et al. Guidance on the conduct of narrative synthesis in systematic Reviews. A Product from the ESRC Methods Programme. Version 1. Lancaster University; 2006. [Google Scholar]
- 24.Alemdaroglu I, Karaduman A, Yilmaz OT, Topaloglu H. Different types of upper extremity exercise training in Duchenne Muscular Dystrophy: effects on functional performance, strength, endurance, and ambulation. Muscle & Nerve 2015; 51: 697–705. [DOI] [PubMed] [Google Scholar]
- 25.Dubowitz V, Hyde SA, Scott OM, Goddard C. Controlled trrial of exercise in Duchenne muscular dystrophy. Neuromuscular diseases 1984: 571–575. [Google Scholar]
- 26.Heutinck L, Jansen M, van den Elzen Y, van der Pijl D, de Groot IJM. Virtual Reality Computer Gaming with Dynamic Arm Support in Boys with Duchenne Muscular Dystrophy. Journal of neuromuscular diseases 2018; 5: 359–372. [DOI] [PubMed] [Google Scholar]
- 27.Houser CR, Johnson DM. Breathing exercises for children with pseudohypertrophic muscular dystrophy. Phys Ther 1971; 51: 751–759. [DOI] [PubMed] [Google Scholar]
- 28.Martin AJ, Stern L, Yeates J, Lepp D, Little J. Respiratory muscle training in Duchenne muscular dystrophy. Developmental medicine and child neurology 1986; 28: 314–318. [DOI] [PubMed] [Google Scholar]
- 29.Rodillo E, Noble-Jamieson CM, Aber V, Heckmatt JZ, Muntoni F, Dubowitz V. Respiratory muscle training in Duchenne muscular dystrophy. Archives of disease in childhood 1989; 64: 736–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scott OM, Hyde SA, Goddard C, Jones R, Dubowitz V. Effect of exercise in Duchenne muscular dystrophy. A controlled six-month study of the effects of two different regimes of exercises in children with Duchenne muscular dystrophy. Physiotherapy 1981; 67: 174–176. [PubMed] [Google Scholar]
- 31.Stern LM, Martin AJ, Jones N, Garrett R, Yeates J. Training inspiratory resistance in Duchenne dystrophy using adapted computer games. Developmental medicine and child neurology 1989; 31: 494–500. [DOI] [PubMed] [Google Scholar]
- 32.Topin N, Matecki S, Le Bris S, Rivier F, Echenne B, Prefaut C, et al. Dose-dependent effect of individualized respiratory muscle training in children with Duchenne muscular dystrophy. Neuromuscular disorders : NMD 2002; 12: 576–583. [DOI] [PubMed] [Google Scholar]
- 33.Vignos PJ, Watkins MP. The effect of exercise in muscular dystrophy. Jama 1966; 197: 843–848. [PubMed] [Google Scholar]
- 34.Wanke T, Toifl K, Merkle M, Formanek D, Lahrmann H, Zwick H. Inspiratory muscle training in patients with Duchenne muscular dystrophy. Chest 1994; 105: 475–482. [DOI] [PubMed] [Google Scholar]
- 35.Zileli İ, Bilir M, Sipahi S, Zileli F, Karayel T. The Effects Of Respiratory Exercises On Pulmonary Functions In Patients With Duchenne Muscular Dystrophy. Eurasian Journal of Pulmonology 1999; 1: 59–65. [Google Scholar]
- 36.Lott DJ, Taivassalo T, Cooke KD, Park H, Moslemi Z, Batra A, et al. Safety, feasibility, and efficacy of strengthening exercise in Duchenne muscular dystrophy. Muscle Nerve 2021; 63: 320–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spaulding HR, Selsby JT. Is Exercise the Right Medicine for Dystrophic Muscle? Med Sci Sports Exerc 2018; 50: 1723–1732. [DOI] [PubMed] [Google Scholar]
- 38.Dechartres A, Trinquart L, Boutron I, Ravaud P. Influence of trial sample size on treatment effect estimates: meta– epidemiological study. BMJ 2013; 346: f2304. [DOI] [PMC free article] [PubMed] [Google Scholar]