Abstract
Objective
To develop an algorithm for the selection of adults with disabling spasticity for treatment with intrathecal baclofen (ITB) and/or botulinum toxin type A (BoNT A).
Methods
A European Advisory Board of 4 neurologists and 4 rehabilitation specialists performed a literature review on ITB and BoNT A treatment for disabling spasticity. An online survey was sent to 125 physicians and 13 non-physician spasticity experts. Information on their current clinical practice and level of agreement on proposed selection criteria was used to inform algorithm design. Consensus was considered reached when ≥75% of respondents agreed or were neutral.
Results
A total of 79 experts from 17 countries completed the on-line survey (57%).
Agreement was reached that patients with multi-segmental or generalized disabling spasticity refractory to oral drugs are the best candidates for ITB (96.1% consensus), while those with focal/segmental disabling spasticity are ideal candidates for BoNT A (98.7% consensus). In addition the following are good candidates for ITB (% consensus): bilateral disabling spasticity affecting lower limbs only (97.4%), bilateral (100%) or unilateral (90.9%) disabling spasticity affecting lower limbs and trunk, and unilateral or bilateral disabling spasticity affecting upper and lower extremities (96.1%).
Conclusion
This algorithm will support the management of adult patients with disabling spasticity by aiding patient selection for ITB and/or BoNT A treatments.
LAY ABSTRACT
Involuntary muscle overactivity or spasticity is the increase in muscle tone caused as a consequence of a brain or spine lesion. The muscle overactivity can become disabling spasticity and adequate treatment or combination of treatments are essential to reduce or eliminate the problems and disability caused by the involuntary muscle overactivity. An European expert consensus on the treatment of disabling spasticity, with Intrathecal Baclofen (an implanted pump that delivers baclofen directly near the spinal cord) or injection of Botulinum Toxin into muscles, was made using an online survey with 79 experts completing the survey. This algorithm supports the future management of adult patients with disabling muscle overactivity by aiding patient selection for Intrathecal Baclofen and/or Botulinum Toxin treatment.
Key words: intrathecal baclofen, botulinum toxin, spasticity, selection criteria, focal, generalized, segmental
Involuntary muscle overactivity or spasticity is an increase in muscle tone caused by a neurological insult that creates an upper motor neurone lesion from either a cerebral (e.g. cerebral palsy, acute brain injury, stroke) or spinal cord (e.g. spinal cord injury, multiple sclerosis) aetiology (1, 2).
Development of spasticity is a complex process, occurring over time, caused by changes in afferent central and peripheral input to the spinal motor neurones, changes in tonic and phasic stretch reflexes that affect spinal excitability, and changes in the intrinsic properties of motor neurones (3). Depending on the location of the lesion, the muscle overactivity can be focal, multi-focal, segmental, multi-segmental or generalized and can become disabling spasticity. The correct treatment, or combination of treatments, is essential to reduce or eliminate the problems and disability caused by the involuntary muscle overactivity, to optimize function, and to prevent secondary complications, such as muscle and soft-tissue shortening (contractures) or skin breakdown.
Many parameters can influence the clinician’s choice of treatment for disabling spasticity; whilst physiotherapy and an effective physical management programme remain pivotal to effective management, pharmacological agents are often required and oral medication is frequently utilized first line; however, side-effects or poor efficacy are commonly reported.
Other treatment options may then be considered, including intrathecal baclofen (ITB) or botulinum toxin type A (BoNT A).
ITB is administered by a programmable, subcutaneously implanted drug delivery system with a reservoir and catheter, delivering low doses of baclofen (< 1% of the oral dose) directly to the spinal cord, where Gamma-aminobutyric acid (GABA) receptors are expressed at high density. In long-term follow-up studies, ITB has proven to be safe and its effect sustainable over time (4–7), with many individuals demonstrating high levels of satisfaction and continuing to benefit for many years. However, despite the increasing body of evidence documenting the usefulness of ITB in managing spasticity, there is a lack of patient selection tools to aid the clinician in deciding which patients are most likely to benefit from ITB (8). On the basis of current evidence, the best-established treatment effect of ITB is in reducing spasticity in the lower limbs of patients with spasticity of cerebral or spinal origin who have failed to respond to maximum tolerated or recommended doses of oral antispasmodics (9–11).
Botulinum toxin (BoNT) is an extremely powerful naturally occurring neurotoxin produced by Clostridium botulinum, a Gram-negative anaerobic bacterium. BoNT type A products are licensed for the treatment of upper and lower limb spasticity in adults and children. BoNT A is injected directly into muscles and causes inhibition of release of acetylcholine at the neuromuscular junction, the clinical effects of which last some months before functional recovery of the injected muscle. Current treatment guidelines for BoNT A recommend that injections should be offered for focal spasticity of the upper and lower limbs. Such recommendations are based largely on extensive safety and efficacy data from well-designed clinical trials in adults with upper-limb spasticity, while there are fewer data reporting the efficacy of BoNT A in clinical trials in adults and children with lower-limb spasticity (12–15). Furthermore, treating multi-focal or multi-segmental upper- or lower-limb spasticity may require higher total doses per session than those currently approved for products available in Europe, in order to meet individual clinical needs and goals of rehabilitation therapy (16). The safety of BoNT A treatment is well established in both adults (17) and children (18), across a variety of indications (19) and also at higher dosages for one BoNT A product (incobotulinumtoxinA, Xeomin, Merz Pharmaceuticals, Germany) (20). However, there are some concerns that the administration of higher doses of BoNT A can increase the risk of systemic diffusion, with the development of clinically evident adverse effects and neutralizing antibodies (21).
When faced with a patient with disabling spasticity, however, there remains uncertainty in how to select the most appropriate treatment. The objective of this study was therefore, through a review and consensus statement, to develop an algorithm to aid clinicians in the management of adult patients with disabling spasticity who are potential candidates for treatment with ITB, BoNT A or both in combination.
METHODS
An Advisory Board of 8 specialists (4 neurologists and 4 physical medicine and rehabilitation (PM&R) physicians) from 8 European countries, with experience in spasticity management, were assembled to evaluate current knowledge and to share experience on patient selection and the optimal treatment pathway for patients with disabling spasticity.
A 4-step approach was implemented, as described below.
Step 1
A literature review on recent evidence of ITB and BoNT A treatments for patients with disabling spasticity was conducted. PubMed and EMBASE databases were searched from January 2010 to November 2020 using the following search strategies:
(‘botulinum toxin a’ OR ‘ btxa’ OR ‘abobotulinum’ OR ‘incobotulinum’ OR ‘onabotulinum’ OR ‘intrathecal drug delivery systems’ OR ‘ITB’) AND (‘spasticity’ OR spasticit*) AND ((‘cerebrovascular accident’ OR ‘brain ischaemic attack*’ OR ‘brain injury’ OR ‘stroke’ OR ‘brain hemorrhage’ OR ‘multiple sclerosis’ OR ‘cerebral palsy’ OR ‘spinal cord injury’) OR (brain OR cerebral OR spinal) NEAR/5 ‘origin’)).
This resulted in 555 abstracts. Following an in-depth review of the abstracts by the advisory board members, a final total of 29 ITB papers, 29 BoNT papers, and 6 guidelines reporting information on patient selection criteria and outcomes of ITB and BoNT for disabling spasticity treatment were selected and summarized as pre-reading material. The list of selected studies along with their main characteristics is shown in Appendix I. The included papers were selected based on study type (non-systematic reviews, books, comments, abstracts, letters, and editorials were excluded), sample size (papers containing fewer than 10 patients analysed were excluded), and main outcomes of safety and/or effectiveness reported. Non-human studies and non-English publications were not considered for inclusion.
Step 2
The results of the literature review were debated by the board members in a virtual meeting in December 2020. Based on the discussion a survey was designed and finalized at the second virtual meeting in January 2021 (Appendix II reports the survey questions). During that meeting, an algorithm for the management of adult patients with disabling spasticity, who are potential candidates for ITB or BoNT, was proposed, based on the evidence and the expert opinion of the advisory board. Further survey questions were also developed in order to gather information on parameters relevant for treatment selection (ITB or BoNT), treatment timing, and evaluation of patient outcomes, including pain, spasticity/muscle tone, spasms, individual and motor function, quality of life and goal attainment.
The survey consisted of a total of 27 questions: 6 were introductory questions aimed at stratifying the sample of responders, 9 focused on clinical practice, and 12 on patient selection designed to seek their level of agreement on the proposed algorithm. Nine of the 27 questions were multiple-choice questions. Most questions allowed responders to enter comments (Appendix II).
Definitions of disabling spasticity and of the distribution of disabling spasticity were agreed upon by the advisory board and included in the survey for the external experts (Table I).
Table I.
Definitions of disabling spasticity and location used in the current paper
| Disabling spasticity | |
|---|---|
| Term | Definition |
| Disabling spasticity | Spasticity which is perceived by the individual or caregivers as hindering body function, activities, and/or participation. |
| This definition is based on clinical expertise and conceptually incorporates the domains of the International Classification of Functioning, Disability and Health (ICF). | |
| Disabling spasticity location | |
| Term | Definition |
| Focal spasticity | Spasticity limited to muscles in a close anatomical region, including only 1 or 2 joints (excluding finger and toe joints, e.g. hand and forearm or foot and ankle) (19). |
| Segmental spasticity | Spasticity limited to several adjacent anatomical regions (e.g. hand, forearm, elbow and/or shoulder) (19). |
| Multi-segmental spasticity | Spasticity distributed to anatomically separate and distant sites and affecting at least 2 limbs, including the trunk (e.g. arm and leg, leg and trunk, or arm and trunk) (19). |
| Generalized spasticity | Spasticity diffused in more than 2 limbs. |
| Multi-focal spasticity* | Spasticity affecting multiple joints that are not adjacent (e.g. ankle and hip or wrist and shoulder). |
This definition was proposed by the advisory board.
Step 3
Each advisory board member was asked to send the on-line survey to at least 10 physicians with expertise in spasticity treatment, as well as additional non-physician experts (e.g. physiotherapists and specialist nurses), in his/her geographical area with a 4-week deadline for completion and a weekly reminder. The survey was sent to 138 external experts in total (125 European physicians and 13 non-physician specialists) via Qualtrics electronic platform (22).
In line with previous consensus reports (23), the advisory board members defined that consensus was reached when ≥75% of respondents agreed with or were neutral on the question response (<25% disagreed).
Step 4
A third virtual meeting was held in April 2021 to present and discuss the results of the survey and revise the algorithm based on external expert responses.
Statistical analysis
Descriptive statistics were used to summarize responder characteristics and survey results. Excel Office 365 (Microsoft, California, CA, USA) was used to perform statistical analyses.
RESULTS
Survey results
A total of 105 of 138 surveys were received. Twenty-six surveys were excluded as they were incomplete and a further two were excluded because the physicians were solely treating children. Therefore, a final total of 77 surveys were analysed and contributed to the development of the algorithm; these 77 were completed in full by external experts who manage adults with disabling spasticity from 17 European countries (56% response rate). Respondents were mainly physicians (54% PM&R specialists, 30% neurologists, 4% neurosurgeons, and 1% anaesthetists), but also included physical therapists (7%), specialist nurses (3%), and clinical scientists (1%).
The estimated number of annually implanted ITB pumps in total for all respondents was 1,007, and the estimated total number of new patient treatments with BoNT in at least 2 limbs each year was 4,177 (Table II).
Table II.
Survey introduction results
| Survey questions | n (%) |
|---|---|
| Country | |
| Italy | 13 (16.9) |
| Germany | 12 (15.6) |
| UK | 12 (15.6) |
| Iberia | 8 (10.4) |
| France | 7 (9.1) |
| Eastern Europe | 5 (6.5) |
| Austria | 4 (5.2) |
| Slovenia | 4 (5.2) |
| BeNeLux | 3 (3.9) |
| Denmark | 3 (3.9) |
| Norway | 3 (3.9) |
| Sweden | 3 (3.9) |
| Role | |
| PM&R (physical medicine & rehabilitation physician) | 42 (54.5) |
| Neurologist | 23 (29.9) |
| Physical therapist | 5 (6.5) |
| Neurosurgeon | 3 (3.9) |
| Other | 4 (5.2) |
| Mean number of NEW patients treated/year for spasticity with ITB pump implant during the last 3 years in your hospital (adults; subjects > 18 years): | |
| 0 implant/year | 11 (14.3) |
| 1–5 implants/year | 28 (36.4) |
| 6–10 implants/year | 23 (29.9) |
| 11–50 implants/year | 13 (16.9) |
| 51–100 implants/year | 2 (2.6) |
| >100 implants/year | 0 (0.0) |
| Mean number of NEW patients treated/year for spasticity with botulinum neurotoxin injections in at least two limbs during the last 3 years in your hospital (adults; subjects > 18 years): | |
| 0 patients/year | 6 (7.8) |
| 1–5 patients/year | 5 (6.5) |
| 6–10 patients/year | 7 (9.1) |
| 11–50 patients/year | 38 (49.4) |
| 51–100 patients/year | 16 (20.8) |
| > 100 patients/year | 5 (6.5) |
| In your opinion, the level of evidence on ITB therapy is: (select all that apply) | |
| Appropriate | 22 (28.6) |
| Not appropriate: | 55 (71.4) |
| Low, more evidence and guide are needed on characteristics of patients who could benefit more from this treatment | 37 (48.1) |
| Low, more evidence is needed on functional improvement in ambulatory patients | 35 (45.5) |
| Low, more evidence is needed on patient’s QoL improvement | 29 (37.7) |
| Low, more evidence is needed on health economics | 25 (32.5) |
| Low, more evidence is needed on functional improvement in non-ambulatory patients | 19 (24.7) |
| Low, more evidence is needed on burden of care improvement for patients and caregivers | 13 (16.9) |
| Low, more evidence is needed on therapy safety | 4 (5.2) |
| Other | 3 (3.9) |
| In your opinion, the level of evidence on BoNT therapy is: (select all that apply) | |
| Appropriate | 34 (44.2) |
| Not appropriate: | 43 (55.8) |
| Low, more evidence is needed on the role of BoNT therapy in combination with other treatments | 27 (35.1) |
| Low, more evidence is needed on efficacy in patients with multisegmental disabling spasticity | 24 (31.2) |
| Low, more evidence and guide are needed on characteristics of patients who could benefit more from this treatment | 22 (28.6) |
| Low, more evidence is needed on patient’s functional improvement | 17 (22.1) |
| Low, more evidence is needed on patient’s QoL improvement | 17 (22.1) |
| Low, more evidence is needed on immunogenicity | 8 (10.4) |
| Low, more evidence is needed on injection techniques | 4 (5.2) |
| Low, more evidence is needed on therapy safety | 2 (2.6) |
| Other | 2 (2.6) |
QoL: quality of life; ITB: intrathecal baclofen; BoNT: botulinum toxin.
The six introductory survey questions addressed the external expert’s opinion on the current level of evidence for ITB therapy and BoNT treatment in patients with disabling spasticity (Table II). The majority of responders (55/77; 71.4%) agreed that the evidence on ITB is still lacking. Specifically, they agreed that more evidence is needed on the characteristics of patients who could benefit most from this treatment (37/77; 48.1%) as well as on its efficacy in ambulatory patients (35/77; 45.5%).
Concerning the available literature on BoNT, over half of responders (43/77; 55.8%) consider it is not conclusive, particularly with regard to the role of BoNT therapy in combination with other treatments (e.g. ITB, rehabilitation, orthosis) (27/77; 35.1%) and on its efficacy in patients with multi-segmental disabling spasticity (24/77; 31.2%).
For both treatments, the current evidence on safety aspects was deemed adequate by responders.
Patient management
The survey enabled information to be gathered on the current clinical practice of patients with disabling spasticity treated with BoNT or ITB. A question concerning the earliest optimal timing of treatment of BoNT demonstrated the majority of external experts recommended BoNT immediately (24/77; 31.2%), or not more than 3 months (38/77; 49.4%) from disabling spasticity onset in order to prevent secondary complications of spasticity. The same question for ITB revealed that this treatment is proposed later than BoNT in clinical practice, with the respondents recommending waiting at least 4–6 months (22/77; 28.6%) or more than 6 months (20/77; 26.0%), depending on the neurological condition causing spasticity.
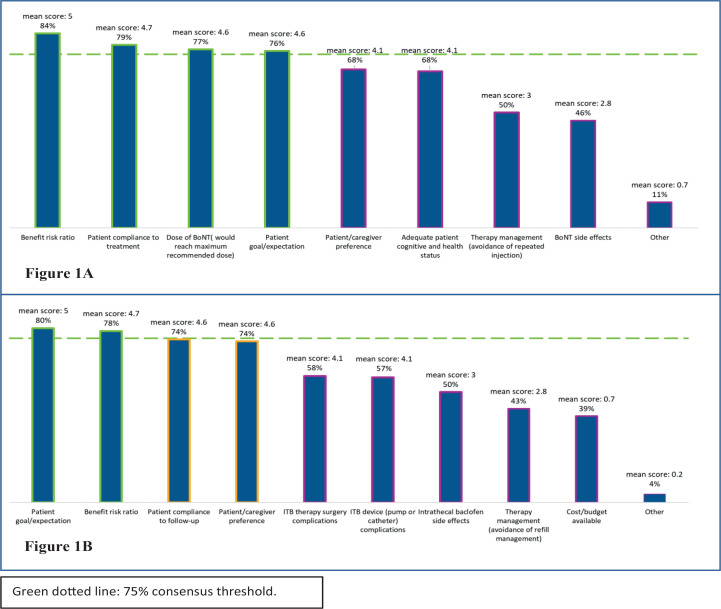
When respondents were asked which parameters are taken into consideration when selecting a candidate for either ITB or BoNT treatment, consensus regarding parameters for choosing ITB treatment (defined as level of agreement ≥75%) was achieved for four parameters: “benefit/risk ratio” (84%), “patient compliance to treatment” (79%), “dose of BoNT (i.e. BoNT would reach maximum recommended dose)” (77%), and “patient goal/expectation” (76%) (Fig. 1A).
Fig. 1.
Parameters considered by experts when selecting (a) intrathecal baclofen (ITB) or (b) botulinum toxin (BoNT) treatment for the management of disabling spasticity.
External expert agreement was reached on two parameters that need to be considered when selecting candidates for BoNT treatment: “patient goal/expectation” (80%), and “benefit/risk ratio” (78%) (Fig. 1B).
In the on-line survey, the external experts were provided with a list of scales and questionnaires based on the results of the literature review and asked which of them they use to monitor patient-reported outcomes in their practice. Respondents rated the visual analogue scale (VAS) and Numeric Pain Rating Scale (NPRS) as the preferred scales for measuring pain (73/77; 94.8%), the Ashworth scale (or modified Ashworth scale) for the measurement of spasticity/muscle tone/spasm outcomes (70/77; 90.9%), and the Barthel Index (or modified Barthel Index) for the measurement of individual and motor functions (38/77; 49.4%).
In terms of health-related quality of life (HRQoL) almost half of respondents stated that they do not routinely measure QoL in their clinical practice (34/77; 44.2%), and although the most selected option for measuring goal attainment was the Goal Attainment Scale (GAS) (40/77; 51.9%), nearly a quarter of respondents said they do not routinely record goal attainment (Table III).
Table III.
Survey results on patient outcomes measurement
| Survey questions | n (%) |
|---|---|
| If you monitor Pain in your service, which scales do you generally use? (select all that apply) | |
| VAS (visual analogue scale)/NPRS (Numeric Pain Rating Scale) | 73 (94.8) |
| I don’t usually measure Pain | 3 (3.8) |
| Other | 7 (8.9) |
| If you monitor health-related QoL (Quality of Life) in your service, which generic or spasticity-specific questionnaires do you generally use? (select all that apply) | |
| I don’t usually measure health-related QoL | 34 (44.2) |
| SF-36 (Short Form Health Survey) | 25 (32.5) |
| EuroQol-EQ5D (5 dimensions of health) | 18 (23.4) |
| Life Satisfaction Index | 5 (6.5) |
| Other | 10 (13) |
| If you monitor Individual and Motor function in your service, which scales do you generally use? (select all that apply) | |
| Barthel Index or Modified Barthel Index | 38 (49.4) |
| GAS (Global Assessment Scale) | 32 (41.6) |
| FIM (Functional Independence Measure) | 22 (28.6) |
| DAS (Disability Assessment Scale) | 10 (13.0) |
| I don’t usually measure function | 8 (10.4) |
| Other | 21 (27.3) |
| If you monitor Spasticity/Muscle tone/Spasm outcomes in your service, which scales do you generally use? (select all that apply) | |
| Ashworth Scale or Modified Ashworth Scale | 70 (90.9) |
| PSS (Penn Spasm Scale) | 30 (39.0) |
| Tardieu Scale or Modified Tardieu Scale | 22 (28.6) |
| I don’t usually measure Spasm outcomes | 2 (2.6) |
| I don’t usually measure Muscle tone | 1 (1.3) |
| Other | 1 (1.3) |
| If you monitor Goal Attainment in your service, which scales do you generally use? (select all that apply) | |
| GAS (Goal Attainment Scale) | 40 (51.9) |
| Personalized goal achievement questionnaire | 19 (24.7) |
| I don’t usually measure Goal Attainment | 19 (24.7) |
| Other | 7 (9.1) |
Algorithm development
The main goal of the advisory board was, on the basis of current evidence and expert opinion, to develop an algorithm to aid the clinical decision-making process for treatment of adult patients with disabling spasticity, who are potential candidates for ITB or BoNT.
Table IV summarizes the results of the survey in terms of consensus on the algorithm. Even though consensus was reached for all the questions addressed in the algorithm, for three of the questions the percentage of neutral respondents was crucial to reach the consensus threshold of 75%.
Table IV.
Survey results in terms of agreement with the proposed algorithm
| Survey questions | Agree, n (%) | Neutral, n (%) | Disagree, n (%) | Reasons for disagreement |
|---|---|---|---|---|
| Distribution of disabling spasticity | ||||
| I consider patients with multi-segmental or generalized disabling spasticity refractory to oral drug treatment the best candidates for ITB treatment. | 67 (87.0) | 7 (9.1) | 3 (3.9) | Some responders stated that in patients with generalized spasticity the high doses of baclofen could lead to listlessness, severe motor slowdown and weakness, as well as to some side-effects; others consider ITB the therapy of choice for patients with spasticity in both legs, but not necessarily multi-segmental or generalized. |
| I consider patients with focal or segmental disabling spasticity the best candidates for BoNT treatment. | 74 (96.1) | 2 (2.6) | 1 (1.3) | One responder stated that other treatments could be considered. |
| I consider patients with unilateral disabling spasticity affecting lower extremities only as good candidates for ITB treatment. | 10 (13.0) | 29 (37.7) | 38 (49.4) | Some responders stated that this case is a focal/segmental spasticity, therefore ITB is not the therapy of choice as per current guidelines; others confirmed that they treat these patients with BoNT or other therapeutic alternative than ITB. |
| I consider patients with bilateral disabling spasticity affecting lower extremities only as good candidates for ITB treatment. | 64 (83.1) | 11 (14.3) | 2 (2.6) | One responder stated that the decision on treatment depends on ambulatory capacity of patients. |
| I consider patients with unilateral or bilateral disabling spasticity affecting both upper and lower extremities as good candidates for ITB treatment. | 52 (67.5) | 22 (28.6) | 3 (3.9) | Some responders expressed concerns about negative effects of ITB on the healthy extremity and the trunk/respiratory muscles; others would recommend ITB only in case of bilateral spasticity. |
| I consider patients with unilateral disabling spasticity affecting lower extremities and back and/or abdominal muscles as good candidates for ITB treatment. | 35 (45.5) | 35 (45.5) | 7 (9.1) | Some responders expressed concerns about negative effects of ITB on the healthy side and postural muscles (worsening balance); others recommend BoNT as first option in case of unilateral spasticity. |
| I consider patients with bilateral disabling spasticity affecting lower extremities and back and/or abdominal muscles as good candidates for ITB treatment. | 74 (96.1) | 3 (3.9) | 0 (0.0) | None. |
| I consider patients with bilateral disabling spasticity affecting upper extremities only more appropriate candidates for BoNT treatment than ITB. | 69 (89.6) | 8 (10.4) | 0 (0.0) | None. |
| Combined therapy | ||||
| Combined ITB and BoNT treatments should be proposed: in case of generalized or multi-segmental disabling spasticity and patient’s goals not fully achieved with ITB only. | 69 (89.6) | 0 (0.0) | 8 (10.4) | None reported. |
| Combined ITB and BoNT treatments should be proposed: in case of generalized or multi-segmental disabling spasticity affecting lower limbs and jaw and/or neck. | 44 (57.1) | 0 (0.0) | 33 (42.9) | None reported. |
| If a patient doesn’t fully reach their rehabilitation goals with BoNT therapy plus rehabilitation/orthosis, I consider him/her for ITB. | 40 (51.9) | 30 (39.0) | 7 (9.1) | Some responders stated that in these cases they would prefer oral medications or other conservative measure first; others stated that they would consider surgical procedures if contracture was evident. |
| Patient preference | ||||
| I consider patient/caregiver preferences regarding treatment option at every stage of the treatment decision process (shared decision approach). | 74 (96.1) | 3 (3.9) | 0 (0.0) | None. |
ITB: intrathecal baclofen; BoNT: botulinum toxin.
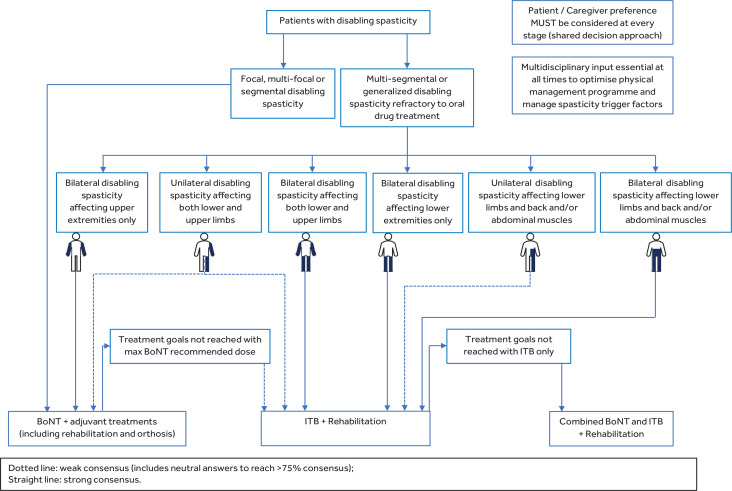
In terms of the distribution of disabling spasticity, there was high-level agreement on the fact that patients with multi-segmental or generalized disabling spasticity refractory to oral drug treatment are the best candidates for ITB (96.1% consensus), while patients with focal or segmental disabling spasticity are the ideal candidates for BoNT (98.7% consensus). During the final meeting the advisory board debated and agreed to include patients with multi-focal disabling spasticity in the algorithm, although this sub-category was not addressed in the survey. The advisory board defined multi-focal as “spasticity affecting multiple joints that are not adjacent (e.g. ankle and hip, or wrist and shoulder)” (see Table I), and they were unanimous in indicating BoNT as the preferential treatment option for these patients.
In terms of spasticity location, negative consensus (87.0%) was reached on proposing ITB to patients with unilateral disabling spasticity affecting lower extremities only. The main reason indicated in the external experts’ comments was that this represents a case of focal/segmental spasticity; therefore BoNT would be first-line treatment in combination with walking aids, splints, and rehabilitation, and there was concern that ITB may decrease muscle strength/tone in the non-spastic side. With regards to bilateral disabling spasticity affecting lower extremities only, there was a high-level agreement (97.4% consensus) on the fact that these patients could be good candidates for ITB treatment. Patients with bilateral disabling spasticity affecting lower limbs and back and/or abdominal muscles were similarly considered as good candidates for ITB by all the respondents (100% consensus), while a weaker consensus (90.9%) with 45% of respondents being neutral was achieved for unilateral spasticity and back and/or abdominal muscle involvement.
Weak consensus (96.1% including 28.6% of neutral answers) was achieved for patients with unilateral or bilateral disabling spasticity affecting both upper and lower extremities as good candidates for ITB treatment. It was felt by the advisory board members that the large number of neutrals in this case was probably due to the fact that this question considered both unilateral (hemiplegia) and bilateral (tetraplegia) distribution together. During the final advisory board meeting it was considered appropriate to represent this question as two separate boxes in the algorithm, one for unilateral, and one for bilateral spasticity: for unilateral spasticity ITB treatment is therefore recommended through a weak consensus (as found in the survey), but for bilateral spasticity affecting both upper and lower extremities ITB should be the treatment of choice, in line with current guidelines that strongly recommended ITB for such patients (24) (Fig. 2).
Fig. 2.
Algorithm developed by the expert panel for the management of adult patients with disabling spasticity who are potential candidates for intrathecal baclofen (ITB) or botulinum toxin (BoNT)
All external and advisory board experts agreed that patients with bilateral disabling spasticity affecting only the upper extremities are more appropriate candidates for BoNT treatment than ITB, while those with unilateral disabling spasticity affecting only the upper extremities are already included in the focal, multi-focal and segmental spasticity arm of the algorithm, with BoNT as the first-choice treatment (Fig. 2).
More than 75% of experts agreed on proposing combined therapy (ITB together with BoNT) in cases of generalized or multi-segmental disabling spasticity where the patient’s goals are not fully achieved with ITB alone. Consensus on utilizing combined therapy was not reached for patients with generalized or multi-segmental spasticity affecting the lower limbs and jaw/neck; therefore this section was removed from the algorithm. During the final meeting the advisory board debated and agreed that non-consensus on this latter statement is probably due to the rarity of this presentation; they, however, suggest combined therapy (plus rehabilitation) is most appropriate, based on their experience.
For patients previously managed with BoNT, external experts agreed that ITB should be considered as a second-line treatment option (90.9% consensus) if rehabilitation goals are not fully reached following a trial of oral medication.
All respondents agreed (96.1%) or were neutral (3.9%) with respect to a “shared decision approach” in the patient management strategy, where patient’ and caregiver’ preferences are taken into consideration at every stage of the treatment decision process.
The final algorithm for patient selection and management is shown in Fig. 2.
Given the high level of agreement reached in the first survey round, the algorithm was not tested further.
DISCUSSION
To increase the likelihood that patients with disabling involuntary muscle overactivity/spasticity due to upper motor neurone (UMN) lesions receive the correct treatment at the correct time, a treatment algorithm is warranted. In the clinical setting, spasticity is often associated with pain, sleep disorders, feeding problems and difficulties with positioning, transfers, dressing and personal care (10). As spasticity is not always disabling and, if not problematic, should not be pharmacologically treated, it was important for the advisory board to agree on the definition of disabling spasticity. After discussion it was agreed to use the definition on disabling spasticity published by “The Ability Network”: “Spasticity which is perceived by the individual or caregivers as hindering body function, activities, and/or participation (25)” (Table I ). This definition is based on clinical expertise and conceptually incorporates the domains of the International Classification of Functioning, Disability and Health (ICF) and is therefore helpful when describing goals related to spasticity treatment (26). Likewise, advisory board members found it relevant to use only the definition of disabling spasticity in this context and not the terms “mild, moderate and severe spasticity”, since all the severity grades can become disabling and therefore warrant treatment. The level of functioning should be considered as the base to set the goals of an individual rehabilitation programme.
First-line management of spasticity is always through a multidisciplinary approach of non-pharmacological measures, predominantly by establishing an effective physical management programme, which will include optimizing posture, positioning (with the use of equipment and splints), stretching, standing, etc., with input from physiotherapy and occupational therapy. Effective management of any spasticity trigger factors, such as bladder and bowel dysfunction or reduced skin integrity, are also essential. When considering pharmacological measures many factors influence the choice of treatment of disabling spasticity; often spasticity distribution is highlighted as one of the most important features, as illustrated by the algorithm (Fig. 2). Patients considered for ITB tend to have predominantly lower limb spasticity (8); however, ITB is known to have some impact in the upper limbs and trunk (often considered to be approximately one-third of the effect in lower limbs), particularly at higher doses and perhaps depending on the placement of the tip of the catheter (7, 27–29).
The current study found high-level agreement that patients with multi-segmental beside those patients with only bilateral affected upper extremities or generalized disabling spasticity refractory to oral drug treatment are the best candidates for ITB. Furthermore, there was a high level of consensus in the current study that combined ITB and BoNT treatments should be proposed in cases of generalized or multi-segmental disabling spasticity if the patient’s goals are not fully achieved with ITB alone.
With regards to treatment of multi-segmental unilateral disabling spasticity affecting both upper and lower limb, the current study cannot draw a firm conclusion on the level of consensus, because the survey question combined “unilateral or bilateral disabling spasticity affecting both upper and lower extremities”. However, the advisory board agreed that, in bilateral spasticity affecting both upper and lower extremities, ITB should be the treatment of choice, which is in accordance with current guidelines (24). The advisory board also agreed that if treatment goals for the upper extremities are not reached after initiating ITB treatment, BoNT should be considered early for management of upper limb spasticity. Comparing these results with other studies on unilateral disabling spasticity affecting both the upper and lower limb, the “Spasticity In Stroke – Randomised Study” (SISTERS) showed a significant superior effect of ITB therapy compared with conventional medical management with oral antispasmodic medication, in reducing muscle tone in the lower limb of the affected side (27). The study showed a significant beneficial treatment effect for ITB over conventional management for spasticity reduction in the upper limb; however, the study was not powered to detect these treatment differences. More patients reported adverse events while receiving ITB (24/25 patients, 96%; 149 events) compared with conventional medical management (22/35, 63%; 77 events) (27). Similarly, the cohort study by Ivanhoe et al. demonstrated significant improvement in function, QoL, and spastic hypertonia after ITB implant in stroke patients, without adversely affecting muscle strength of the unaffected limbs (30). In the current study we report a weak consensus for selecting ITB for unilateral disabling spasticity, affecting both upper and lower limbs. The advisory board agreed that such patients (multi-segmental unilateral disabling spasticity affecting both upper and lower limbs) may also benefit from BoNT treatment alone. Therefore, even though this aspect was not part of the survey, during the final advisory board expert meeting it was agreed to also add a dotted line to BoNT in the treatment algorithm (Fig. 2).
The TOWER study of patients with upper and lower-limb post-stroke spasticity found that the mean improvement in spasticity was greater with treatment with incobotulinumtoxinA in both the upper and lower limbs vs the upper limb only, concluding that a combined upper and lower limb treatment may optimize treatment results (12). Furthermore, the study by Hara et al. demonstrates that greater improvement in motor function was achieved by administering BoNT A concurrently to the upper and lower limbs in the context of multidisciplinary rehabilitation (31). At present, there is limited clinical evidence supporting the efficacy of all BoNT A products in the management of multi-focal or multi-segmental spasticity. Contributing factors to this may be BoNT A dose limitations in current product licences and the lack of general consensus on the maximum safe dose per injection site and injection session for all BoNT A products (32, 33), as patients may benefit from BoNT A treatment with higher total doses per session than currently recommended (20). This is in accordance with the high level of agreement in the current study on choosing ITB over BoNT, due to “the dose of BoNT would reach maximum recommended dose”.
No randomized controlled trials comparing ITB with BoNT A injection were identified in the current review, although it is acknowledged in one study that patients not responding to oral medications and BoNT A treatment showed a significant reduction in generalized spasticity following ITB pump implantation (34). Likewise, no studies were found examining the role of combining ITB and BoNT A treatment.
Another factor that might influence the clinician’s choice of treatment is the patient’s functional level, particularly if they are able to walk. ITB is traditionally reserved for non-ambulatory patients (35), based on the assumption that improvement in walking function should not be expected with ITB therapy (36). However, there is a growing body of evidence that this is not the case and early ITB treatment may keep people walking for longer, particularly in progressive disorders, such as multiple sclerosis (29, 35), although a higher rate of catheter-related complications has been observed in this cohort (35, 37).
Concerns about adverse events with ITB may also influence the choice of treatment due to the relatively high rate of complications, particularly infections, cerebrospinal fluid (CSF) leakage, and catheter-related problems (1, 6, 37–41). That the ITB risk profile is important when considering the choice of treatment is illustrated by the high agreement on the “benefit–risk ratio” as a parameter to be considered when choosing ITB in the current study. In the same way, there is consensus on the parameter “patient compliance with treatment”, which is highly likely to be dependent on the parameter “patient goal/expectation”. It is therefore of paramount importance that, before embarking on ITB treatment, patients/relatives and care-givers must be engaged, suitable and willing to undergo a trial with a test dose of ITB to assess efficacy, a surgical procedure for implantation and commit to regular follow-up for pump refills (29).
In accordance with other guidelines (15), patients with focal or multi-focal disabling spasticity are ideal candidates for BoNT treatment (98.7% consensus).
Interestingly, in the UK National guideline of spasticity treatment in adults (15), ITB is proposed for segmental or regional disabling spasticity, whereas BoNT is recommended for focal and multi-focal spasticity, while the current study among European experts indicates a high agreement for BoNT treatment in segmental disabling spasticity. This disparity is probably due to differences in definitions, in that segmental spasticity in the UK guideline is combined with “regional spasticity”, which, in our definition, might be regarded as multi-segmental spasticity (e.g. lower limb with back/abdominal muscle involvement). This illustrates the importance of definitions with clear descriptions of their meanings (Table I). The earliest optimal timing of treatment might also influence the management pathway chosen due to a deteriorating patient, and suggests that BoNT may be chosen over ITB in this situation, perhaps without considering all other factors. The current study confirmed the results of the recent Delphi consensus paper by Baricich et al. (41), with the majority of external experts proposing BoNT immediately (24/77; 31.2%), or not more than 3 months (38/77; 49.4%) after onset of disabling spasticity, in order to prevent negative consequences of spasticity. The same question for ITB revealed that ITB is proposed later than BoNT in clinical practice, with the respondents recommending waiting at least 4–6 months (22/77; 28.6%) or more than 6 months (20/77; 26.0%), depending on the cause of spasticity, before treating patients with ITB. This is clearly inappropriate if patients are losing range, particularly in the context of dysautonomia following brain injury, where ITB has been shown to be beneficial (42).
Although helpful, it is important to recognize that a treatment algorithm cannot stand alone when selecting the appropriate spasticity treatment for the specific patient. As described above, many other parameters can influence the choice of treatment and need to be considered.
Reassuringly, there is a high level of agreement in the current study with respect to a “shared decision approach” in the patient management strategy, where patient’ and caregiver’ preferences are taken into consideration at every stage of the treatment decision process. The patient or caregiver might have other concerns or expectations than the clinician regarding the choice of treatment, and if these concerns or expectations are not taken into consideration and explored, a successful outcome for the physician, patient/relative or caregiver is unlikely.
Similar to many other studies, the Ashworth scale (or modified Ashworth scale) remains the most commonly used objective measure of spasticity in current clinical practice for the evaluation of patients with disabling spasticity treated with BoNT or ITB (43); however, patient-reported outcomes (visual analogue scales) and goal attainment are also commonly reported, reflecting the importance of patient-reported outcomes.
The choice of treatment may also be influenced by external factors, such as healthcare funding systems or the availability of treatments/expertise in the treating centre and the consequent need to refer the patient to another centre for treatment. The current study did not explore this dilemma, and our high level of consensus on many parameters probably illustrates that many of the participating centres in the current study are able to offer both ITB and BoNT treatment. This is, of course, a possible confounding factor and weakness of the current study, as the advisory board members selected experts known to them in their country/region of origin. Furthermore, the current study did not explore concerns or perceptions regarding treatment costs or cost-effectiveness (although participants could have raised this in free-text answers), so this could also be considered a study weakness/limitation.
The algorithm produced by this panel review is not a substitute for clinical judgment and is not intended to define a standard of practice or requirement for ITB or BoNT treatment. No single document can categorically define appropriate practice in this setting, as every patient with disabling spasticity is unique; however, we hope that it is a useful tool to guide treatment choice and help our patients and their families understand and engage in shared decision-making.
In conclusion, based on the current literature, a comprehensive survey of European expert opinion, and the consensus process, this study provides an algorithm for the management of adult patients with disabling spasticity who are potential candidates for treatment with ITB and/or BoNT A (Fig. 2). Further studies focusing on goal attainment, functional benefit, QoL, and the role of combined ITB and BoNT treatment are needed to accurately define which patients could benefit most.
ACKNOWLEDGEMENTS
The authors thank Teodora Bellone (Medtronic), Aafje tom Dieck (Medtronic), Lorenza Mangoni (Medtronic), and Alessandra Calabrese for technical and methodological support to the project. They collated responses from the surveys and helped produce the figure, but were not involved in the writing or conclusions of the manuscript.
The authors also thank all the participating external experts from all over Europe for completing the survey and thus helping to develop a consensus algorithm.
Conflicts of interest
The Advisory Board and the publication fees were supported by Medtronic.
Appendix 1. Characteristics of included studies
| First Author, Publication Year (Ref) | Study Design | Size (n) | Spasticity Origin |
|---|---|---|---|
| Abbatemarco, 2020 (35) | Retrospective | 256 | Spinal (Multiple Sclerosis) |
| Barney, 2020 (8) | Prospective non-RCT | 32 | Cerebral (Cerebral Palsy) |
| Bayhan, 2016 (39) | Retrospective | 294 | Cerebral (Cerebral Palsy) |
| Borowski, 2010 (S1) | Retrospective | 174 | Cerebral (Cerebral Palsy) |
| Borrini, 2014 (S2) | Prospective non-RCT | 158 | Multiple (Cerebral and Spinal) |
| Creamer, 2018 (27, S3) | RCT | 60 | Cerebral (Stroke) |
| Dvorak, 2010 (S4) | Retrospective | 167 | Multiple (Cerebral and Spinal) |
| Imerci, 2019 (40) | Retrospective | 341 | Cerebral (Cerebral Palsy) |
| Khan, 2010 (S5) | Retrospective | 40 | Spinal (Multiple Sclerosis) |
| Lee, 2018 (36) | Retrospective | 47 | Spinal (Multiple Sclerosis) |
| Morton, 2011 (S6) | Prospective non-RCT | 38 | Cerebral (Cerebral Palsy) |
| Motta, 2016 (38) | Retrospective | 508 | Multiple (Cerebral and Spinal) |
| Motta, 2014 (S7) | Retrospective | 430 | Multiple (Cerebral and Spinal) |
| Motta, 2011 (S8) | Retrospective | 30 | Cerebral (Cerebral Palsy) |
| Natale, 2012 (4, S9) | Prospective non-RCT | 112 | Multiple (Cerebral and Spinal) |
| Pucks-Faes, 2018 (37) | Retrospective | 116 | Multiple (Cerebral and Spinal) |
| Ramstad, 2010 (S10) | Prospective non-RCT | 38 | Cerebral (Cerebral Palsy) |
| Reis, 2019 (S11) | Retrospective | 155 | Multiple (Cerebral and Spinal) |
| Sammaraiee, 2020 (29) | Prospective non-RCT | 30 | Spinal (Multiple Sclerosis) |
| Sammaraiee, 2019 (7) | Prospective non-RCT | 106 | Spinal (Multiple Sclerosis) |
| Schiess, 2020 (1) | Prospective non-RCT | 1,743 | Multiple (Cerebral and Spinal) |
| Schiess, 2011 (28) | Prospective non-RCT | 30 | Cerebral (Stroke) |
| ITB Studies | |||
| Taira, 2013 (S12) | Retrospective | 400 | Multiple (Cerebral and Spinal) |
| Vles, 2013 (S13–S15) | RCT (through 6-month) then Prospective non-RCT | 17 | Cerebral (Cerebral Palsy) |
| Yoon, 2017 (34) | Prospective non-RCT | 37 | Cerebral (Cerebral Palsy and Brain Injury) |
| BoNT A Studies | |||
| Baricich, 2015 (32) | Retrospective | 26 | Cerebral (Stroke) |
| Chaleat-Valayer, 2011 (S16) | Prospective non-RCT | 282 | Cerebral (Cerebral Palsy) |
| Choi, 2019 (S17) | Retrospective | 591 | Cerebral (Cerebral Palsy) |
| Clemenzi, 2012 (S18) | Prospective non-RCT | 21 | Cerebral (Brain Injury) |
| Copeland, 2014 (S19) | RCT | 41 | Cerebral (Cerebral Palsy) |
| Delgado, 2017 (S20) | Prospective non-RCT (extension of a previous RCT) | 216 | Cerebral (Cerebral Palsy) |
| Demetrios, 2014 (S21) | Prospective non-RCT | 59 | Cerebral (Stroke) |
| Dressler, 2015 (33) | Prospective non-RCT | 54 | Multiple (Cerebral and Spinal) |
| Esquenazi, 2019 (17) | RCT | 468 | Cerebral (Stroke) |
| Fheodoroff, 2020 (12, 16, 20) | Prospective non-RCT | 155 | Cerebral (Cerebral Palsy, Stroke, Brain Injury, Brain Tumour, Cerebral Vascular Disorders) |
| Gonnade, 2018 (18) | RCT | 61 | Cerebral (Cerebral Palsy) |
| Hara, 2018 (13) | Retrospective | 35 | Cerebral (Stroke) |
| Hara, 2017 (31) | Retrospective | 51 | Cerebral (Stroke) |
| Ianieri, 2018 (S22) | Retrospective | 120 | Multiple (Cerebral and Spinal) |
| Intiso, 2014 (S23) | Prospective non-RCT | 22 | Cerebral (Cerebral Palsy and Brain Injury) |
| Invernizzi, 2015 (21) | Prospective non-RCT | 11 | Cerebral (Stroke) |
| Juneja, 2012 (S24) | Retrospective | 29 | Cerebral (Cerebral Palsy) |
| BoNT A Studies | |||
| Jung, 2011 (S25) | Prospective non-RCT | 27 | Cerebral (Cerebral Palsy) |
| Kerzoncuf, 2020 (S26) | RCT | 40 | Cerebral (Stroke) |
| Lannin, 2018 (S27) | Prospective non-RCT (feasibility randomized study) | 37 | Cerebral (Stroke) |
| Maanum, 2011 (S28) | RCT | 66 | Cerebral (Cerebral Palsy) |
| Roche, 2015 (S29) | RCT | 35 | Cerebral (Stroke) |
| Safer, 2016 (S30) | Prospective non-RCT | 24 | Cerebral (Cerebral Palsy) |
| Santamato, 2017 (S31) | Prospective non-RCT | 20 | Cerebral (Stroke) |
| Santamato, 2013 (S32) | Prospective non-RCT | 25 | Cerebral (Stroke) |
| Schramm, 2014 (S33) | Prospective non-RCT | 508 | Multiple (Cerebral and Spinal) |
| Unlu, 2010 (S34) | Retrospective | 71 | Cerebral (Cerebral Palsy) |
RCT: randomized controlled trial
SUPPLEMENTARY REFERENCES
- S1.Borowski A, Littleton AG, Borkhuu B, Presedo A, Shah S, Dabney KW, et al. Complications of intrathecal baclofen pump therapy in pediatric patients. J Pediatr Orthop 2010; 30: 76–81. [DOI] [PubMed] [Google Scholar]
- S2.Borrini L, Bensmail D, Thiebaut JB, Hugeron C, Rech C, Jourdan C. Occurrence of adverse events in long-term intrathecal baclofen infusion: a 1-year follow-up study of 158 adults. Arch Phys Med Rehabil 2014; 95: 1032–1038. [DOI] [PubMed] [Google Scholar]
- S3.Creamer M, Cloud G, Kossmehl P, Yochelson M, Francisco GE, Ward AB, et al. Effect of intrathecal baclofen on pain and quality of life in poststroke spasticity: a Randomized Trial (SISTERS). Stroke 2018; 49: 2129–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.Dvorak EM, McGuire JR, Nelson MES. Incidence and identification of intrathecal baclofen catheter malfunction. PM R 2010; 2: 751–756. [DOI] [PubMed] [Google Scholar]
- S5.Khan AA, Birks-Agnew I, Bullock P, Rushton D. Clinical outcome and complications of intrathecal baclofen pump in multiple sclerosis patients: a retrospective study. NeuroRehabilitation 2010; 27: 117–120. [DOI] [PubMed] [Google Scholar]
- S6.Morton RE, Gray N, Vloeberghs M. Controlled study of the effects of continuous intrathecal baclofen infusion in nonambulant children with cerebral palsy. Dev Med Child Neurol 2011; 53: 736–741. [DOI] [PubMed] [Google Scholar]
- S7.Motta F, Antonello CE. Analysis of complications in 430 consecutive pediatric patients treated with intrathecal baclofen therapy: 14-year experience. J Neurosurg Pediatr 2014; 13: 301–306. [DOI] [PubMed] [Google Scholar]
- S8.Motta F, Antonello CE, Stignani C. Intrathecal baclofen and motor function in cerebral palsy. Dev Med Child Neurol 2011; 53: 443–448. [DOI] [PubMed] [Google Scholar]
- S9.Natale M, D’Oria S, Nero VV, Squillante E, Gentile M, Rotondo M. Long-term effects of intrathecal baclofen in multiple sclerosis. Clin Neurol Neurosurg 2016; 143: 121–125. [DOI] [PubMed] [Google Scholar]
- S10.Ramstad K, Jahnsen R, Lofterod B, Skjeldal OH. Continuous intrathecal baclofen therapy in children with cerebral palsy – when does improvement emerge? Acta Paediatr Int J Paediatr 2010; 99: 1661–1665. [DOI] [PubMed] [Google Scholar]
- S11.Reis PV, Vieira CR, Midões AC, Rebelo V, Barbosa P, Gomes A. Intrathecal baclofen infusion pumps in the treatment of spasticity: a retrospective cohort study in a Portuguese centre. Acta Med Port 2019; 32: 754–759. [DOI] [PubMed] [Google Scholar]
- S12.Taira T, Ueta T, Katayama Y, Kimizuka M, Nemoto A, Mizusawa H, et al. Rate of complications among the recipients of intrathecal baclofen pump in Japan: a multicenter study. Neuromodulation 2013; 16: 266–272. [DOI] [PubMed] [Google Scholar]
- S13.Vles GF, Soudant DL, Hoving MA, Vermeulen RJ, Bonouvrié LA, Van Oostenbrugge RJ, et al. Long-term follow-up on continuous intrathecal baclofen therapy in non-ambulant children with intractable spastic Cerebral Palsy. Eur J Paediatr Neurol 2013; 17: 639–644. [DOI] [PubMed] [Google Scholar]
- S14.Hoving MA, van Raak EPM, Spincemaille GHJJ, van Kranen-Mastenbroek VHJM, van Kleef M, Gorter JW, et al. Safety and one-year efficacy of intrathecal baclofen therapy in children with intractable spastic cerebral palsy. Eur J Paediatr Neurol 2009; 13: 247–256. [DOI] [PubMed] [Google Scholar]
- S15.Hoving MA, van Raak EPM, Spincemaille GHJJ, Palmans LJ, Becher JG, Vles JSH. Efficacy of intrathecal baclofen therapy in children with intractable spastic cerebral palsy: a randomised controlled trial. Eur J Paediatr Neurol 2009; 13: 240–246. [DOI] [PubMed] [Google Scholar]
- S16.Chaléat-Valayer E, Parratte B, Colin C, Denis A, Oudin S, Bérard C, et al. A French observational study of botulinum toxin use in the management of children with cerebral palsy: BOTULOSCOPE. Eur J Paediatr Neurol 2011; 15: 439–448. [DOI] [PubMed] [Google Scholar]
- S17.Choi JY, Kim SK, Park ES. The effect of botulinum toxin injections on gross motor function for lower limb spasticity in children with cerebral palsy. Toxins (Basel) 2019; 11: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S18.Clemenzi A, Formisano R, Matteis M, Gallinacci L, Cochi G, Savina P, et al. Care management of spasticity with botulinum toxin-A in patients with severe acquired brain injury: a 1-year follow-up prospective study. Brain Inj 2012; 26: 979–983. [DOI] [PubMed] [Google Scholar]
- S19.Copeland L, Edwards P, Thorley M, Donaghey S, Gascoigne-Pees L, Kentish M, et al. Botulinum toxin a for nonambulatory children with cerebral palsy: a double blind randomized controlled trial. J Pediatr 2014; 165: 140-146. [DOI] [PubMed] [Google Scholar]
- S20.Delgado MR, Bonikowski M, Carranza J, Dabrowski E, Matthews D, Russman B, et al. Safety and efficacy of repeat open-label abobotulinumtoxina treatment in pediatric cerebral palsy. J Child Neurol 2017; 32: 1058–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S21.Demetrios M, Gorelik A, Louie J, Brand C, Baguley IJ, Khan F. Outcomes of ambulatory rehabilitation programmes following botulinum toxin for spasticity in adults with stroke. J Rehabil Med 2014; 46: 730–737. [DOI] [PubMed] [Google Scholar]
- S22.Ianieri G, Marvulli R, Gallo GA, Fiore P, Megna M. “Appropriate treatment” and therapeutic window in spasticity treatment with IncobotulinumtoxinA: from 100 to 1000 units. Toxins (Basel) 2018; 10: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S23.Intiso D, Simone V, Di Rienzo F, Iarossi A, Pazienza L, Santamato A, et al. High doses of a new botulinum toxin type A (NT-201) in adult patients with severe spasticity following brain injury and cerebral palsy. NeuroRehabilitation 2014; 34: 515–522. [DOI] [PubMed] [Google Scholar]
- S24.Juneja M, Jain R, Gautam A, Khanna R, Narang K. Clinical & immunological erythematosus patients characteristics in systemic lupus Maryam. J Dent Educ 2012; 76: 1532–1539.23144490 [Google Scholar]
- S25.Jung NH, Heinen F, Westhoff B, Doederlein L, Reissig A, Berweck S, et al. Hip lateralisation in children with bilateral spastic cerebral palsy treated with botulinum toxin type A: a 2-year follow-up. Neuropediatrics 2011; 42: 18–23. [DOI] [PubMed] [Google Scholar]
- S26.Kerzoncuf M, Viton JM, Pellas F, Cotinat M, Calmels P, Milhe de Bovis V, et al. Poststroke postural sway improved by botulinum toxin: a multicenter randomized double-blind controlled trial. Arch Phys Med Rehabil 2020; 101: 242–248. [DOI] [PubMed] [Google Scholar]
- S27.Lannin NA, Ada L, Levy T, English C, Ratcliffe J, Sindhusake D, et al. Intensive therapy after botulinum toxin in adults with spasticity after stroke versus botulinum toxin alone or therapy alone: a pilot, feasibility randomized trial. Pilot Feasibility Stud 2018; 4: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S28.Maanum G, Jahnsen R, Stanghelle JK, Sandvik L, Keller A. Effects of botulinum toxin a in ambulant adults with spastic cerebral palsy: a randomized double-blind placebo-controlled trial. J Rehabil Med 2011; 43: 338–347. [DOI] [PubMed] [Google Scholar]
- S29.Roche N, Zory R, Sauthier A, Bonnyaud C, Pradon D, Bensmail D. Effect of rehabilitation and botulinum toxin injection on gait in chronic stroke patients: a randomized controlled study. J Rehabil Med 2015; 47: 31–37. [DOI] [PubMed] [Google Scholar]
- S30.Safer VB, Demir SO, Ozkan E, Guneri FD. Effects of botulinum toxin serotype a on sleep problems in children with cerebral palsy and on mothers’ sleep quality and depression. Neurosciences 2016; 21: 331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S31.Santamato A, Panza F, Intiso D, Baricich A, Picelli A, Smania N, et al. Long-term safety of repeated high doses of incobotulinumtoxinA injections for the treatment of upper and lower limb spasticity after stroke. J Neurol Sci 2017; 378: 182–186. [DOI] [PubMed] [Google Scholar]
- S32.Santamato A, Panza F, Ranieri M, Frisardi V, Micello MF, Filoni S, et al. Efficacy and safety of higher doses of botulinum toxin type A NT 201 free from complexing proteins in the upper and lower limb spasticity after stroke. J Neural Transm 2013; 120: 469–476. [DOI] [PubMed] [Google Scholar]
- S33.Schramm A, Ndayisaba JP, Auf Dem Brinke M, Hecht M, Herrmann C, Huber M, et al. Spasticity treatment with onabotulinumtoxin A: data from a prospective German real-life patient registry. J Neural Transm 2014; 121: 521–530. [DOI] [PubMed] [Google Scholar]
- S34.Unlu E, Cevikol A, Bal B, Gonen E, Celik O, Kose G. Multilevel botulinum toxin type a as a treatment for spasticity in children with cerebral palsy: a retrospective study. Clinics 2010; 65: 613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
REFERENCES
- 1.Schiess M, Eldabe S, Konrad P, Molus L, Spencer R, Stromberg K, et al. Intrathecal baclofen for severe spasticity: longitudinal data from the product surveillance registry. Neuromodulation 2020; 23: 996–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pandyan AD, Gregoric M, Barnes MP, Wood D, Van Wijck F, Burridge J, et al. Spasticity: clinical perceptions, neurological realities and meaningful measurement. Disabil Rehabil 2005; 27: 2–6. [DOI] [PubMed] [Google Scholar]
- 3.Opheim A, Danielsson A, Alt Murphy M, Persson H, Sunnerhagen K. Upper-limb spasticity during the first year after stroke: stroke arm longitudinal study at the University of Gothenburg. Am J Phys Med Rehabil 2014; 93: 884–896. [DOI] [PubMed] [Google Scholar]
- 4.Natale M, Mirone G, Rotondo M, Moraci A. Intrathecal baclofen therapy for severe spasticity: Analysis on a series of 112 consecutive patients and future prospectives. Clin Neurol Neurosurg 2012; 114: 321–325. [DOI] [PubMed] [Google Scholar]
- 5.Draulans N, Vermeersch K, Degraeuwe B, Meurrens T, Peers K, Nuttin B, et al. Intrathecal baclofen in multiple sclerosis and spinal cord injury: complications and long-term dosage evolution. Clin Rehabil 2013; 27: 1137–1143. [DOI] [PubMed] [Google Scholar]
- 6.Mathur S, Chu S, McCormick Z, Chang Chien G, Marciniak C. Long-term intrathecal baclofen: outcomes after more than 10 years of treatment. Phys Med Rehabil 2014; 6: 506–513. [DOI] [PubMed] [Google Scholar]
- 7.Sammaraiee Y, Yardley M, Keenan L, Buchanan K, Stevenson V, Farrell R. Intrathecal baclofen for multiple sclerosis related spasticity: a twenty year experience. Mult Scler Relat Disord 2019; 27: 95–100. [DOI] [PubMed] [Google Scholar]
- 8.Barney CC, Merbler AM, Stansbury J, Krach LE, Partington M, Graupman P, et al. Musculoskeletal pain outcomes pre- and post intrathecal baclofen pump implant in children with cerebral palsy: a prospective cohort study. Arch Rehabil Res Clin Transl 2020; 2: 100049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiess MC, Eldabe S, Konrad P, Molus L, Spencer R, Stromberg K, et al. Intrathecal baclofen for severe spasticity: longitudinal data from the product surveillance registry. Neuromodulation 2020; 23: 996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dan B, Motta F, Vles J, Vloeberghs M, Becher J, Eunson P, et al. Consensus on the appropriate use of intrathecal baclofen (ITB) therapy in paediatric spasticity. Eur J Paediatr Neurol 2010; 14: 19–28. [DOI] [PubMed] [Google Scholar]
- 11.Saulino M, Ivanhoe C, McGuire J, Ridley B, Shilt J, Boster A. Best practices for intrathecal baclofen therapy: patient selection. Neuromodulation 2016; 19: 607–615. [DOI] [PubMed] [Google Scholar]
- 12.Bensmail D, Wissel J, Laffont I, Simon O, Scheschonka A, Flatau-Baqué B, et al. Efficacy of incobotulinumtoxinA for the treatment of adult lower-limb post-stroke spasticity, including pes equinovarus. Ann Phys Rehabil Med 2020; 0–6. [DOI] [PubMed] [Google Scholar]
- 13.Hara T, Abo M, Hara H, Sasaki N, Yamada N, Niimi M, et al. The effect of repeated botulinum toxin a therapy combined with intensive rehabilitation on lower limb spasticity in post-stroke patients. Toxins (Basel) 2018; 10: 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francisco G, Balbert A, Bavikatte G, Bensmail D, Carda S, Deltombe T, et al. A practical guide to optimizing the benefits of post-stroke spasticity interventions with botulinum toxin A: an international group consensus. J Rehabil Med 2021; 53: jrm00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashford S, Turner Stokes L, Allison R, Duke L, Bavikatte G, Kirker S, et al. Spasticity in adults: management using botulinum toxin – National Guidelines 20 March 2018. [cited 2021 Jul 7]. Available from: https://www.rcplondon.ac.uk/guidelines-policy/spasticity-adults-management-using-botulinum-toxin
- 16.Fheodoroff K, Rekand T, Medeiros L, Koßmehl P, Wissel J, Bensmail D, et al. Quality of life in subjects with upper- and lower-limb spasticity treated with incobotulinumtoxinA. Health Qual Life Outcomes 2020; 18: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esquenazi A, Wein TH, Ward AB, Geis C, Liu C, Dimitrova R. Optimal muscle selection for onabotulinumtoxina injections in poststroke lower-limb spasticity: a randomized trial. Am J Phys Med Rehabil 2019; 98: 360–368. [DOI] [PubMed] [Google Scholar]
- 18.Gonnade N, Lokhande V, Ajij M, Gaur A, Shukla K. Phenol versus botulinum toxin a injection in ambulatory cerebral palsy spastic diplegia: a comparative study. Pediatr Neurosci 2018; 13: 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wissel J, Ward AB, Erztgaard P, Bensmail D, Hecht MJ, Lejeune TM, et al. European consensus table on the use of botulinum toxin type a in adult spasticity. J Rehabil Med 2009; 41: 13–25. [DOI] [PubMed] [Google Scholar]
- 20.Wissel J, Bensmail D, Ferreira JJ, Molteni F, Satkunam L, Moraleda S, et al. Safety and efficacy of incobotulinumtoxinA doses up to 800 U in limb spasticity the TOWER study. Neurology 2017; 88: 1321–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Invernizzi M, Carda S, Molinari C, Stagno D, Cisari C, Baricich A. Heart rate variability (HRV) modifications in adult hemiplegic patients after botulinum toxin type A (NT-201) injection. Eur J Phys Rehabil Med 2015; 51: 353–359. [PubMed] [Google Scholar]
- 22.Qualtrics Surveys - Qualtrics XM Software. [cited 2021 Jul 7]. Available from: https://www.qualtrics.com/qualtrics/xm%0A
- 23.Diamond IR, Grant RC, Feldman BM, Pencharz PB, Ling SC, Moore AM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol 2014; 67: 401–409. [DOI] [PubMed] [Google Scholar]
- 24.Guideline for the treatment of cerebral and/or spinal spasticity in adults (2017). Last review 01.01.2016 Federatie Medisch Specialisten: Netherlands Society of Rehabilitation Medicine (NSRM), The Netherlands. [cited 2021 May 13]. Available from: https://revalidatiegeneeskunde.nl/article/richtlijn-behandeling-van-cerebrale-enof-spinale-spasticiteit-bij-volwassenen-gepubliceerd [Google Scholar]
- 25.Burns AS, Lanig I, Grabljevec K, New PW, Bensmail D, Ertzgaard P, et al. Optimizing the management of disabling spasticity following spinal cord damage: The Ability Network – an international initiative. Arch Phys Med Rehabil 2016; 97: 2222–2228. [DOI] [PubMed] [Google Scholar]
- 26.Fheodoroff K, Scheschonka A, Wissel J. Goal analysis in patients with limb spasticity treated with incobotulinumtoxinA in the TOWER study. Disabil Rehabil 2020; 17: 1–7. [DOI] [PubMed] [Google Scholar]
- 27.Creamer M, Cloud G, Kossmehl P, Yochelson M, Francisco GE, Ward AB, et al. Intrathecal baclofen therapy versus conventional medical management for severe poststroke spasticity: results from a multicentre, randomised, controlled, open-label trial (SISTERS). J Neurol Neurosurg Psychiatry 2018; 89: 642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schiess MC, Oh IJ, Stimming EF, Lucke J, Acosta F, Fisher S, et al. Prospective 12-month study of intrathecal baclofen therapy for poststroke spastic upper and lower extremity motor control and functional improvement. Neuromodulation 2011; 14: 38–45. [DOI] [PubMed] [Google Scholar]
- 29.Sammaraiee Y, Stevenson VL, Keenan E, Buchanan K, Lee H, Padilla H, et al. Evaluation of the impact of intrathecal baclofen on the walking ability of people with Multiple Sclerosis related spasticity. Mult Scler Relat Disord 2020; 46: 102503. [DOI] [PubMed] [Google Scholar]
- 30.Ivanhoe C, Francisco G, McGuire J, Subramanian T, Grissom S. Intrathecal baclofen management of poststroke spastic hypertonia: implications for function and quality of life. Arch Phys Med Rehabil 2006; 87: 1509–1515. [DOI] [PubMed] [Google Scholar]
- 31.Hara T, Abo M, Hara H, Kobayashi K, Shimamoto Y, Samizo Y, et al. Effects of botulinum toxin A therapy and multidisciplinary rehabilitation on upper and lower limb spasticity in post-stroke patients. Int J Neurosci 2017; 127: 469–478. [DOI] [PubMed] [Google Scholar]
- 32.Baricich A, Grana E, Carda S, Santamato A, Cisari C, Invernizzi M. High doses of onabotulinumtoxinA in post-stroke spasticity: a retrospective analysis. J Neural Transm 2015; 122: 1283–1287. [DOI] [PubMed] [Google Scholar]
- 33.Dressler D, Adib Saberi F, Kollewe K, Schrader C. Safety aspects of incobotulinumtoxinA high-dose therapy. J Neural Transm 2015; 122: 327–333. [DOI] [PubMed] [Google Scholar]
- 34.Yoon YK, Lee KC, Cho HE, Chae M, Chang JW, Chang WS, et al. Outcomes of intrathecal baclofen therapy in patients with cerebral palsy and acquired brain injury. Med (United States) 2017; 96: e7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abbatemarco JR, Griffin A, Jones NG, Hartman J, McKee K, Wang Z, et al. Long-term outcomes of intrathecal baclofen in ambulatory multiple sclerosis patients: a single-center experience. Mult Scler J 2020; 1–9. [DOI] [PubMed] [Google Scholar]
- 36.Lee BS, Jones J, Lang M, Achey R, Dai L, Lobel DA, et al. Early outcomes after intrathecal baclofen therapy in ambulatory patients with multiple sclerosis. J Neurosurg 2018; 129: 1056–1062. [DOI] [PubMed] [Google Scholar]
- 37.Pucks-Faes E, Hitzenberger G, Matzak H, Fava E, Verrienti G, Laimer I, et al. Eleven years’ experience with intrathecal baclofen – complications, risk factors. Brain Behav 2018; 8: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Motta F, Antonello CE. Comparison between an Ascenda and a silicone catheter in intrathecal baclofen therapy in pediatric patients: analysis of complications. J Neurosurg Pediatr 2016; 18: 493–498. [DOI] [PubMed] [Google Scholar]
- 39.Bayhan IA, Sees JP, Nishnianidze T, Rogers KJ, Miller F. Infection as a complication of intrathecal baclofen treatment in children with cerebral palsy. J Pediatr Orthop 2016; 36: 305–309. [DOI] [PubMed] [Google Scholar]
- 40.Imerci A, Rogers KJ, Pargas C, Sees JP, Miller F. Identification of complications in paediatric cerebral palsy treated with intrathecal baclofen pump: a descriptive analysis of 15 years at one institution. J Child Orthop 2019; 13: 529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baricich A, Wein T, Cinone N, Bertoni M, Picelli A, Chisari C, et al. BoNT-A for post-stroke spasticity: guidance on unmet clinical needs from a Delphi panel approach. Toxins (Basel) 2021; 13: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pucks-Faes E, Hitzenberger G, Matzak H, Verrienti G, Schauer R, Saltuari L. Intrathecal baclofen in paroxysmal sympathetic hyperactivity: Impact on oral treatment. Brain Behav 2018; 8: e01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harb A, Kishner S. Modified Ashworth Scale. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. Jan. 2021 May 9. [PubMed] [Google Scholar]