Abstract
Objective
To determine the efficacy of repetitive transcranial magnetic stimulation vs sham stimulation on improving lower-limb functional outcomes in individuals with neurological disorders.
Data sources
PubMed, CINAHL, Embase and Scopus databases were searched from inception to 31 March 2020 to identify papers (n = 1,198). Two researchers independently reviewed studies for eligibility. Randomized clinical trials with parallel-group design, involving individuals with neurological disorders, including lower-limb functional outcome measures and published in scientific peer-reviewed journals were included.
Data extraction
Two researchers independently screened eligible papers (n = 27) for study design, clinical population characteristics, stimulation protocol and relevant outcome measures, and assessed study quality.
Data synthesis
Studies presented a moderate risk of selection, attrition and reporting bias. An overall effect of repetitive transcranial magnetic stimulation was found for outcomes: gait (effect size [95% confidence interval; 95% CI]: 0.51 [0.29; 0.74], p = 0.003) and muscle strength (0.99 [0.40; 1.58], p = 0.001) and disorders: stroke (0.20 [0.00; 0.39], p = 0.05), Parkinson’s disease (1.01 [0.65; 1.37], p = 0.02) and spinal cord injury (0.50 [0.14; 0.85], p = 0.006), compared with sham. No effect was found for outcomes: mobility and balance.
Conclusion
Supplementary repetitive transcranial magnetic stimulation may promote rehabilitation focused on ambulation and muscle strength and overall lower-limb functional recovery in individuals with stroke, Parkinson’s disease and spinal cord injury. Further evidence is needed to extrapolate these findings.
LAY ABSTRACT
Non-invasive magnetic brain stimulation can cause beneficial changes in the central nervous system of individuals with neurological disorders, which, in turn, may have a number of therapeutic qualities. This paper summarizes current knowledge about whether the technique can be used to promote recovery of leg movement function. By searching the available literature for studies on individuals with neurological disorders that have compared the effects of magnetic brain stimulation with placebo stimulation, 27 relevant studies were identified. Combined data from these studies suggested that real stimulation, compared with placebo, had positive effects specifically for recovery of walking ability and maximal leg muscle strength, as well as for improvement in overall leg movement function in individuals with stroke, Parkinson’s disease and spinal cord injury. These findings are important for patients and therapists seeking to improve rehabilitation outcomes. This research area deserves increased scientific focus.
Key words: transcranial magnetic stimulation, neurological disorders, lower extremity
Across the spectrum of neurological conditions, extensive heterogeneity exists in terms of aetiology, sequelae and clinical manifestations of disability, and, accordingly, in their management and rehabilitation approaches. Neurological disorders have common traits, however, in that they cause dynamic, plastic changes in particular neural networks. These include compensatory plastic responses, proving adaptive for the individual, but also, and to a greater extent, dysfunctional responses and deteriorative processes that contribute to further disability in the individual (1, 2). In many neurological disorders these processes contribute to impair locomotor functioning, which in turn have detrimental effects on independence and quality of life. Motor deficiencies are generally naïve to pharmacological treatment, and pharmacotherapy has other significant limitations, such as non-customizability to the individual and adverse effects, which can lead to low adherence and discontinuation (3, 4). Physical therapies, such as physiotherapy and exercise, may show some success in maintaining or promoting motor function and skills for the individual. However, successfulness of the rehabilitation effort is largely dependent on the patient’s level of corporation and on the expertise of the therapist (5); consequently, outcomes vary widely.
Neurostimulation appears as a promising tool in various neurorehabilitation settings, as it comprises a number of qualities that address many of the issues mentioned above; e.g. the ability to selectively target locomotor neural circuitries and to improve favourable, while suppressing maladaptive, patterns of neural activity. Furthermore, in particular for non-invasive brain stimulation, such as transcranial magnetic stimulation (TMS), treatment is often associated with no or only mild adverse effects (6). In TMS an electric current is discharged in an insulated coil placed on the scalp surface, which generates a brief, focal magnetic field that is capable of depolarizing neurones in the underlying cortical areas through electromagnetic induction. If the electromagnetic pulses are delivered repetitively (rTMS), it is possible to achieve persistent modulation of neuronal excitability in the targeted cortical structures (7, 8).
Since the mid-1990’s the use and efficacy of rTMS have been extensively investigated as treatment for a range of mental disorders (9), but the method has only more recently gained recognition within wider areas of neurology and neurorehabilitation. To date, a considerable amount of research has been published that investigates the effects of rTMS on chronic (10) and neuropathic (11) pain, dysphagia (12), aphasia (13), aggregate motor symptoms in Parkinson’s disease (PD) (14) and upper limb extremity function following stroke (15). Less attention has been paid to lower limb motor function, despite the fact that mobility and ambulatory function are reported as important determinants of life satisfaction in individuals with neurological disorders (16–18). One explanation for this delay in the application of rTMS for stimulation of lower limb-specific motor function may be that double-cone-shaped and Hesed coils (H-coils), which are capable of stimulating deeper cortical areas, such as the leg motor cortex, have only recently become widely available (19).
The aims of this review were therefore to: (i) perform a systematic analysis to examine study and reporting quality; and (ii) conduct a meta-analysis to evaluate the effect of rTMS on mobility- and gait-related outcome measures in individuals with neurological disorders, by summarizing evidence from published randomized trials with comparable sham-controlled group designs. Thus, the specific objectives were to: (i) systematically review studies examining rTMS on lower limb functional outcome measures (LLFO) in clinical neurorehabilitation; and (ii) compare the efficiency of real rTMS to sham stimulation in improving lower limb function.
METHODS
This review was prospectively registered (Identifier: CRD42020176837) at the International Prospective Register of Systematic Reviews (PROSPERO, Centre for Reviews and Dissemination, University of York, York, UK) and composed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) 2020 guidelines (20, 21).
Search strategy
A literature search to identify research papers examining rTMS on LLFO in individuals with neurological disorders was carried out on the following databases for the time period of inception to 31 March 2020: PubMed, CINAHL, Embase, Scopus. The title and abstract of each study were screened, and only human trials utilizing rTMS on individuals with neurological disorders were selected. The reference list of relevant papers was also examined. Key search terms were: “neurological disorder” OR “neurological disease” OR “spinal cord injury” OR “stroke” OR “multiple sclerosis” OR “cerebral palsy” OR “amyotrophic lateral sclerosis” AND “Human” for patient type; “repetitive transcranial magnetic stimulation” OR “rTMS” OR “non-invasive brain stimulation” OR “NIBS” for intervention category; and “lower limb” OR “leg” for outcome. The full search strategy is listed in Appendix SI.
Inclusion and exclusion criteria
rTMS intervention studies (minimum 5 rTMS sessions with stimulation over areas directly or indirectly involved in descending motor control) involving individuals with a neurological disorder as classified by the World Health Organization (WHO) (22), participants n ≥ 10, published in English, Danish, Swedish or Norwegian in scientific peer-reviewed journals were included in the analysis. Studies were required to include at least 1 LLFO, and only randomized controlled trials (RCTs) comparing real rTMS with sham stimulation in a comparable study population were included in the review. Acute studies, case studies, single-arm studies or reports not published in scientific peer-reviewed journals in the specified languages were excluded. RCTs involving combination treatments, where the effects of rTMS could not be isolated, and full cross-over type studies, where masking is compromised and carry-over effects are probable, were also excluded.
Study selection
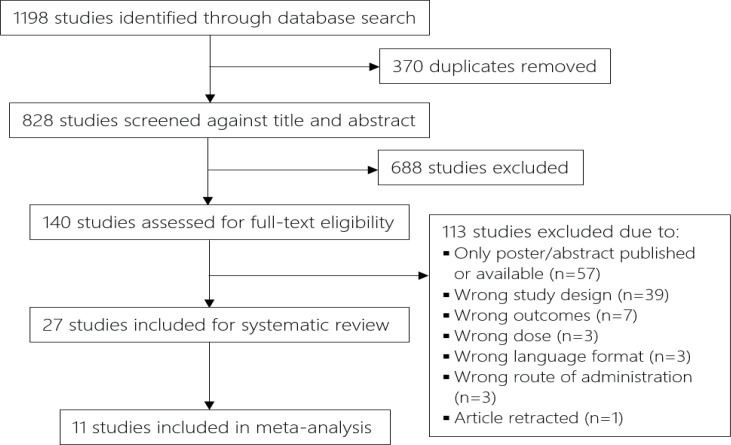
Study selection was performed using an online systematic review software (Covidence, Veritas Health Innovation, Melbourne, Australia. Available from: www.covidence.org). Two researchers (SK and ABJ) independently reviewed the entire set of studies generated from the search, and excluded duplicates and those that failed to meet the inclusion criteria (cf. Fig. 1 for a flowchart of study selection process). In case of any discrepancies a third reviewer (HK) was invited to evaluate the record.
Fig. 1.
Flowchart of the study selection process. Two reviewers independently screened each paper against title and abstract. If no information was found to cause exclusion, each individual study was categorized and would undergo full-text screening at the later stage.
Data extraction and quality assessment
Eligible papers were independently screened by the same 2 researchers for: (i) study design, (ii) clinical population characteristics, (iii) rTMS protocol, and (iv) relevant outcome measure(s). The following data were extracted: (i) level of blinding, assessment time-points. (ii) For all studies: n, sex and age for each intervention group individually, whenever possible. For stroke and amyotrophic lateral sclerosis (ALS): time since onset; spinal cord injury (SCI): level (paraplegia/tetraplegia) and degree (American Spinal Injury Association Impairment Scale) of neurological disability; PD: degree of disability (Hoehn & Yahr stage); multiple sclerosis (MS): disease phase. (iii) Number of sessions, intervention duration, area of stimulation, coil type, total number of pulses per session, stimulation frequency and intensity, procedures for sham stimulation and, if applicable, type of task performed in combination. (iv) Relevance to lower limb function.
The quality of the included studies was evaluated using the Cochrane Collaboration’s risk-of-bias guidelines (23). The results from the 2 assessors’ evaluations were compared and any discrepancies were resolved by consensus with a third researcher.
Data synthesis and meta-analysis
A meta-analysis was performed using the following comparison: pre-post change following real rTMS vs change following sham stimulation. Data were obtained in the correct format, which allowed for inclusion in the data synthesis, from 11 out of the 27 included studies, either directly from studies or via private correspondence with authors. Due to considerable heterogeneity in study designs with regard to follow-up, the first assessment available post-intervention was chosen as follow-up. First, data were sorted and analysed based on outcome: gait, mobility, muscle strength, spasticity and balance, to provide an overview of the effects of rTMS on each of the categories. Subsequently, a secondary analysis, based on patient category, was performed to assess any potential differences in neurophysiological adaptations to rTMS between populations. Due to limited data regarding maximal muscle strength, the focus of the metaanalysis was on clinical outcome measures.
Data extracted at the group level were expressed in the form of mean of difference, standard deviation (SD) and sample size. When insufficient or wrongly formatted data were provided, data were extrapolated from figures when possible; otherwise, authors were contacted and asked to provide the data. In cases of non-parametric reporting of data, data were converted using basic properties of the log normal distribution. In instances where a more negative change value was favourable, e.g. a reduction in time to complete a test, data were simply multiplied by –1. The meta-analysis was conducted using a designated review manager software (RevMan 5.4, The Cochrane Collaboration, London, UK. Available from: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman). Converted data were pooled and calculated using standardized mean differences and 95% CI; thus, a single effect size (Hedges’ g [95% confidence interval; 95% CI]) for real rTMS was expressed for each overall category and each of its sub-categories. A fixed-effect model was applied to determine heterogeneity between studies, using I2 statistics. Effect sizes were interpreted using Cohen’s guidelines (24) (0.2 = small effect, 0.5 = moderate effect, 0.8 = large effect, > 0.8 = very large effect), while heterogeneity was interpreted using Cochrane’s guidelines (25) (I2 = 0% to 40%: might not be important; I2 = 30% to 60%: may represent moderate heterogeneity; I2 = 50% to 90%: may represent substantial heterogeneity; I2 = 75% to 100%: considerable heterogeneity).
RESULTS
Study selection and study characteristics
A flowchart depicting the trial selection procedure is shown in Fig. 1. The search retrieved 1,198 studies; after removing duplicates, 828 studies were screened against title and abstract, and of these, 688 studies were excluded. A total of 140 studies were reviewed for eligibility through full-text screening, of which 113 studies were excluded due to various reasons (cf. Fig. 1). The remaining 27 studies (26, 27, 36–45, 28, 46–52, 29–35) were included in the systematic review.
Table I presents a summary of the findings. Across the included studies, the following disorders were represented: stroke (30, 33, 49, 50, 34, 36–38, 40, 42, 43, 48) (40.7%), PD (26, 28, 51, 29, 31, 32, 35, 39, 44–46) (40.7%), SCI (27, 41) (7.4%), MS (47) (3.7%) and ALS (52) (3.7%). For intervention parameters, 19 studies (27, 28, 43–48, 50, 52, 29, 30, 34, 35, 37–39, 41) (70.4%) applied high-frequency stimulation (≥ 5 Hz), 7 studies (26, 32, 33, 36, 40, 42, 49) (25.9%) applied low-frequency stimulation (≤1 Hz) while a single study (31) (3.7%) used a hybrid stimulation design. A total of 23 disparate effect measures were identified as relevant outcome parameters. Twenty-one studies (77.8%) included at least one outcome measure directly related to ambulation, 13 studies (48.1%) included at least one outcome measure related to mobility, 6 studies (22.2%) included a balance/postural stability outcome, while 4 studies (14.8%) included a lower limb muscle strength outcome and 2 studies (7.4%) included a spasticity outcome.
Table I.
Summary of relevant articles
| Study | Methods | Participants | Interventions | Relevant outcome measures | Relevant findings |
|---|---|---|---|---|---|
| Arias et al. 2010 (26) | Double-blind RCT. ATP: Baseline, postintervention, 1-week follow-up | Parkinson’s Disease. n = 18 Hoehn-Yahr stage 2–4 Sex: NA Age: NA | Active rTMS 10 sessions (Mon–Fri for 2 weeks) over the vertex with a 90-mm round coil. 100 pulses per session (1 Hz, 2 trains of 50 pulses @ 90% RMT, 50 s on/5 min off) Sham Identical treatment protocol. Stimulation parameters unavailable. Inactive coil held on the scalp. A second, active coil placed perpendicularly to the first. |
Gait velocity during non-standard gait analysis | There was no significant difference between pre- or post-intervention in either group ON or OFF medication. Similarly, there was no significant difference in effect between groups in either medication phase. |
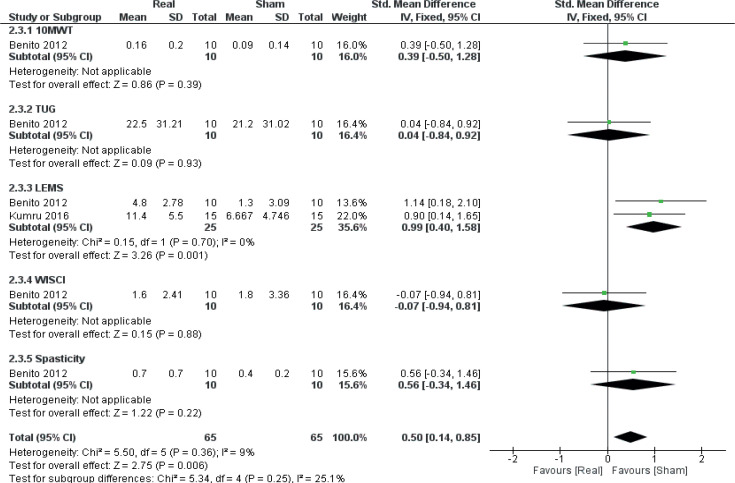
| Benito et al. 2012 (27) | Double-blind RCT. ATP: Baseline, postintervention, 2-week follow-up | Spinal cord injury. n = 17 Tetraplegia (n = 7) and paraplegia (n = 10). AIS: D. Sex (M/F): 13/4 Age: 37.3±14.1 years | Active rTMS 15 sessions (Mon–Fri for 3 weeks) over leg motor cortex with a double cone coil. 1,800 pulses per session (20 Hz, 45 trains of 40 pulses @ 90% RMT, 2 s on/28 s off) Sham Identical treatment protocol. Stimulation parameters unavailable. Inactive coil held on the scalp. A second, active coil placed below the pillow, discharging into the couch. |
LEMS, 10MWT, MAS, TUG, WISCIII | LEMS and 10MWT velocity increased and MAS decreased significantly for REAL but not for SHAM. For both groups, TUG performance increased significantly at post and at 2-week follow-up. WISCI scores did not change for either group. |
| Benninger et al. 2011 (38) | Double-blind RCT. ATP: Baseline, postintervention, 1-month follow-up | Parkinson’s Disease. n = 26 Hoehn-Yahr stage 2–4 Sex (M/F, active|sham): 7/6 | 11/2 Age (active|sham): 62.1±6.9 | 65.6±9.0 years | Active rTMS (iTBS) 8 sessions over 2 successive weeks, a session/day for 4 consecutive days/week, over hand M1 and DLPFC bilaterally with a 90 mm circular coil 600 pulses per session (3 pulses at 50 Hz repeated at 200 ms intervals (5 Hz) for 2 s (10 bursts) @ 80% AMT. These 2-s trains were repeated 20 times every 10 s) Sham Identical protocol. Sham coil. |
10MWT | rTMS had no effects on gait in ON or OFF state. |
| Benninger et al. 2012 (46) | Double-blind RCT. ATP: Baseline, postintervention, 1-month follow-up | Parkinson’s Disease. n = 26 Hoehn-Yahr stage 2–4 Sex (M/F, active|sham): 11/2 | 9/4 Age (active|sham): 62.5±9.1 | 63.7±8.3 years | Active rTMS 8 sessions over 2 successive weeks, a session/day for 4 consecutive days/week, over bilateral hand M1 with a 90-mm circular coil 600 pulses per session (50 Hz in 6-s trains @ 80% AMT) Sham Identical protocol. Inactive coil held on the scalp. A second, active coil placed perpendicularly to the first. |
10MWT | rTMS had no effects on gait in ON or OFF state. |
| Chang et al. 2010 (47) | Double-blind RCT. ATP: Baseline, postintervention, 3-month follow-up | Stroke. n = 28 Post-onset duration <1 month Sex (M/F, active|sham): 11/7 | 6/4 Age (active|sham): 56.4±11.2 | 57.0±14.5 years | Active rTMS 10 sessions over 2 weeks over lesional hemisphere hand M1 with a figure-of-eight coi 1,000 pulses per session (10 Hz, 50 trains @ 90% RMT, 5 s on/55 s off). Hand motor training between trains (50 s active/5 s rest). Sham Identical protocol. Active coil rotated perpendicularly to the scalp. | MI-L, FMA-LL, FAC | MI-L, FMA-LL and FAC scores increased significantly for both groups, with no significant difference in effect between groups. |
| Cohen et al. 2018 (48) | Double-blind RCT. ATP: Baseline, postintervention | Parkinson’s Disease. n = 42 Hoehn-Yahr stage 2–4 Sex (M/F, active|sham): 17/4 | 15/6 Age (active|sham): 64.4±6.8 | 66.8±8.1 years | Active rTMS 24 sessions over 3 months (3/week in the first month, 2/week in the second, and 1/week in the third) over hand M1 and PFC with an H-coil 1,700 pulses per session [900 for M1 and then 800 for PFC] (M1: 1 Hz for 900 s @ 110% RMT | PFC: 10 Hz, 40 trains of 20 pulses @ 100% RMT, 2 s on/20 s off). Sham Identical protocol. Sham coil. |
TUG, Foot tapping | TUG and foot tapping (most affected side) performance increased significantly for both groups, with no significant difference in effect between groups. |
| El-Tamawy et al. 2013 (49) | Double-blind RCT. ATP: Baseline, 2-month follow-up | Parkinson’s Disease. n = 16 Hoehn-Yahr stages 2.5–4 Sex (M/F): 11/5 Age: 67±7.32 years | Active rTMS 12 sessions over 4 weeks over leg M1 contralateral to the more affected side with a figure-of-eight coil 500 pulses per session (1 Hz, 10 trains of 50 pulses @ 90% RMT, 50 s on/20 s off) Sham Identical protocol. Active coil rotated perpendicularly to the scalp. |
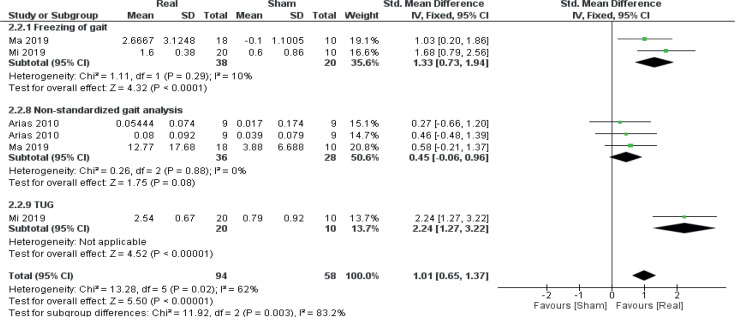
Frequency of FOG episodes, FOG-Q, Turn time | ”Freezing episodes statistically decreased in patients subjected to the active rTMS stimulation relative to the placebo arm. FOG Q showed marked improvement after the sessions. Significant decrease in turn time was detected.” |
| Forogh et al. 2017 (50) | Double-blind RCT. ATP: Baseline, postintervention, 3-week, 12-week follow-up | Stroke. n = 26 Post-onset duration >1 month Sex (M/F): 16/10 Age: range 53–79 years | Active rTMS 5 sessions over 5 consecutive days over hand contralesional motor cortex with a figure-of-eight coil 1,200 pulses per session (1 Hz for 1,200 s) Sham Identical protocol. A speaker replayed stimulation sound from the handle of the coil. |
MRC scale, BBS, Postural stability | For all relevant outcome measures, no improvement was seen at post-intervention; only REAL showed significant improvements at 3- and 12- week follow-up, compared with baseline, and the improvements were significantly greater compared with SHAM. |
| Guan et al. 2017 (51) | Double-blind RCT. ATP: Baseline, post-intervention, 1-month, 3-month, 6-month, 1-year follow-up | Stroke. n = 42 Post-onset duration <1 week Sex (M/F, active|sham): 16/5 | 14/7 Age (active|sham): 59.7±6.8 | 57.4±14.0 years | Active rTMS 10 sessions over 10 consecutive days over ipsilesional motor cortex with a figure-of-eight coil 1,000 pulses per session (5 Hz, 50 trains of 20 pulses @ 120% contralesional RMT, 2 s on/2 s off) Sham Identical protocol. Active coil rotated perpendicularly to the scalp. |
FMA-LL | There was no significant difference from baseline at any time-point in either group. Similarly, there was no significant difference in effect between groups at any time-point. |
| Hamada et al. 2009 (52) | Double-blind RCT. ATP: Baseline, 4-week follow-up | Parkinson’s Disease. n = 99 Hoehn-Yahr stage 2.8±0.6 (active) and 2.9±0.7 (sham) Sex (M/F, active|sham): 29/26 | 25/18 Age (active|sham): 65.3±8.9 | 67.4±8.5 years | Active rTMS 8 sessions over 8 consecutive weeks over SMA with a figure-of-eight coil 1,000 pulses per session (5 Hz, 20 trains of 50 pulses @ 110% AMT, 10 s on/50 s off) Sham Identical protocol. Inactive coil held over the scalp. Active coil discharged near the participants head, while scalp electrodes produced cutaneous electrical currents. |
UPDRS-Gait, UPDRS-Chair rise, Postural stability | For all relevant outcome measures, no significant differences in improvement were seen between groups. |
| Huang et al. 2018 (28) | Double-blind RCT. ATP: Baseline, postintervention, 3-month follow-up | Stroke. n = 38 Post-onset duration 10–90 days Sex (M/F, active|sham): 10/8 | 13/7 Age (active|sham): 62.2±10.4 | 61.2±9 years | Active rTMS 15 sessions over 3 weeks over contralesional leg M1 with a double-cone coil 900 pulses per session (1 Hz continuously for 15 min @ 120% AMT), followed by 45 min physical therapy Sham Identical protocol. Sham coil. |
TUG, FMA-LL, PASS | Both groups achieved significant improvement in all relevant outcome measures. No differences between groups were seen. |
| Ji et al. 2014 (29) | RCT. ATP: Baseline, post-intervention | Stroke. n = 29 Post-onset duration <1 year Sex (M/F, active|sham): 9/6 | 8/6 Age (active|sham): 49.0±11.0 | 44.28±8.5 years | Active rTMS 18 sessions over 6 weeks (3 sessions/week) over the hotspot of the ipsilesional hemisphere with a figure-of-eight coil 1,500 pulses per session (10 Hz, 15 trains of 100 pulses, 10 s on/50 s off. Stimulation intensity information unavailable) and 15 min motor imagery practice Sham Identical protocol. Sham procedure information unavailable. |
Gait velocity during non-standard gait analysis | Both groups achieved significant improvement. REAL improved significantly more than SHAM. |
| Ji et al. 2015 (30) | RCT. ATP: Baseline, post-intervention | Stroke. n = 39 Post-onset duration <3 months Sex (M/F, active|sham): 11/9 | 12/7 Age (active|sham): 55.7±9.0 | 56.4±10.4 years | Active rTMS 20 sessions over 4 weeks (5 sessions/week) over the hotspot of the ipsilesional hemisphere with a figure-of-eight coil 2,000 pulses per session (10 Hz, 20 trains of 100 pulses, 10 s on/50 s off. Stimulation intensity information unavailable) Sham Identical protocol. Sham procedure information unavailable. |
Gait velocity during non-standard gait analysis | Both groups achieved significant improvement. REAL improved significantly more than SHAM. |
| Khedr et al. 2003 (31) | Double-blind RCT. ATP: Baseline, after the first, fifth, 10th session, 1-month follow-up | Parkinson’s Disease. n = 36 Hoehn-Yahr stage 2-3 Sex (M/F, active|sham): 14/5 | 10/7 Age (active|sham): 57.8±9.2 | 57.5±8.4 years | Active rTMS 10 sessions over 10 consecutive days over bilateral leg and hand motor cortex with a figure-of-eight coil 2000 pulses per session [1,000 pulses per hemisphere, 500 pulses per location] (5 Hz, 1000 trains of 2 pulses @ 120% RMT) Sham Identical protocol. Active coil elevated and angled away from the head. |
TUG-25 m | Only REAL increased walking speed at post-intervention, and the change was significantly greater than for SHAM. |
| Kim et al. 2014 (32) | Double-blind RCT. ATP: Baseline, postintervention, 1-month follow-up | Ataxic stroke. n = 32 Post-onset duration <3 months Sex (M/F, active|sham): 11/11 | 6/4 Age (active|sham): 67.4±7.8 | 64.8±11.7 years | Active rTMS 5 sessions over 5 consecutive days over cerebellum ipsilateral to the ataxic side 900 pulses per session (1 Hz continuously for 15 min @ 100% RMT) Sham Identical protocol. Active coil rotated perpendicularly to the scalp. |
10MWT, BBS | Gait velocity increased significantly for REAL but not for SHAM. Both groups increased BBS scores significantly. There were no differences between groups in either outcome. |
| Kumru et al. 2016 (33) | Double-blind RCT. ATP: Baseline, postintervention, 1-month follow-up | Spinal cord injury. n = 31 Tetraplegia (n = 14) and paraplegia (n = 17). All AIS C or D Sex (M/F): 24/7 Age: range 23–68 years | Active rTMS 20 sessions over 4 weeks (Mon–Fri) over the vertex with a double cone coil 1,800 pulses per session (20 Hz, 45 trains of 40 pulses @ 90% RMT, 2 s on/28 s off) followed by 30–45 min body-weight-assisted treadmill training Sham Identical protocol. Inactive coil held on the scalp. A second, active coil placed below the pillow, discharging into the couch. |
10MWT, WISCI-II, LEMS | For 10MWT, a trend for more participants being able to complete the test was seen in REAL compared with SHAM at post-intervention and at followup. WISCI change scores were similar between groups at both time-points. LEMS increased significantly for both groups at both time-points, but change score for REAL was significantly greater than for SHAM at both time-points. |
| Lin 2015 (34) | Double-blind RCT. ATP: Baseline, postintervention | Stroke. n = 32 Post-onset duration 10–90 days Sex (M/F, active|sham): 10/6 | 11/5 Age (active|sham): 58.3±10.8 | 62.3±11.7 years | Active rTMS 15 sessions over 15 consecutive days over contralesional leg motor cortex with a figure-of-eight coil 900 pulses per session (1 Hz continuously for 15 min @ 130% AMT) followed by 45 min physical therapy Sham Identical protocol. Sham coil. |
TUG, PASS, FMA-LL | Significantly more participants were able to complete the TUG at post-intervention in REAL compared with SHAM. For PASS and FMA-LL, both groups improved significantly, but for PASS there was significantly greater improvement in REAL. |
| Lin et al. 2019 (35) | Double-blind RCT. ATP: Baseline, postintervention | Stroke. n = 20 Post-onset duration >6 months Sex (M/F, active|sham): 9/1 | 8/2 Age (active|sham): 60.8±8.1 | 61.1±9.7 years | Active rTMS (iTBS) 10 sessions over 5 weeks (2 sessions/week) over the midline of the scalp with a figure-of-eight coil 1,200 pulses per session (10 pulses at 35 Hz repeated at 200 ms intervals (5 Hz) for 2 s @ 100% AMT. These 2-s trains were repeated every 10 s for a total of 40 trains.) Sham Identical protocol. Sham coil. |
TUG, 10MWT, FMALL, BBS | There was no change in TUG or 10MWT for both groups. In FMA-LL only REAL improved significantly, but there was no difference between groups. Both groups improved significantly in BBS, with no difference between groups. |
| Lomarev et al. 2006 (36) | Double-blind RCT. ATP: Baseline, after each session, 1-month follow-up | Parkinson’s disease. n = 18 Hoehn-Yahr stage 2–4 Sex (M/F, active|sham): 7/2 | 8/1 Age (active|sham): 63±10 | 66±10 years | Active rTMS 8 sessions over 4 weeks over bilateral hand motor cortex and DLPFC with a solid core coil 1,200 pulses per session [300 pulses each location] (25 Hz @ 100% RMT. No other information given.) Sham Identical protocol. Active coil rotated 180°. | 10MWT | Only REAL significantly improved time-to-complete, and the improvement was significantly greater than for SHAM. |
| Ma 2019 et al. (37) | Double-blind RCT. ATP: Baseline, after the 1st, 5th session, post-intervention, 2-week, 4-week follow-up | Parkinson’s Disease. n = 28 Sex (M/F, active|sham): 8/10 | 5/5 Age (active|sham): 59.9±9.2 | 66.0±8.6 years | Active rTMS 10 sessions over 2 weeks (Mon–Fri) over bilateral leg SMA with a figure-of-eight coil 1,000 pulses per session (10 Hz, 20 trains of 50 pulses @ 90% RMT, 5 s on/55 s off) Sham Identical protocol. Active coil rotated perpendicularly to the scalp. |
Gait velocity during non-standard gait analysis, FOG-Q | Only REAL showed significant improvements for both outcome measures, and the improvements were significantly greater than for SHAM. |
| Mi 2019 et al. (39) | Double-blind RCT. ATP: Baseline, after the 5th session, postintervention, 2-week, 4-week follow-up | Parkinson’s Disease. n = 30 Hoehn-Yahr stage 2.60±0.85 (active) and 2.35±0.91 (sham) Sex (M/F, active|sham): 9/11 | 5/5 Age (active|sham): 62.7±10.6 | 65.6±8.7 years | Active rTMS 10 sessions over 2 weeks (Mon–Fri) over bilateral leg SMA with a figure-of-eight coil 1,000 pulses per session (10 Hz, 20 trains of 50 pulses @ 90% RMT, 5 s on/55 s off) Sham Identical protocol. Active coil rotated perpendicularly to the scalp. |
TUG-7 m, FOG-Q | For TUG, time-to-complete significantly improved at all time-points for REAL, and REAL improved significantly more compared with SHAM. Similar developments were seen for FOG-Q. |
| Mor et al. 2010 (40) | Double-blind RCT. ATP: Baseline, after the 7th session, postintervention, 1-week, 2-week, 3-week, 4-week follow-up | Multiple Sclerosis. n = 20 Relapsing-remitting MS and spasticity Sex (M/F): 7/13 Age: 44.3±12.5 years | Active rTMS (iTBS) 14 sessions over 14 consecutive days over leg M1 contralateral to the affected side with a figure-of-eight coil 600 pulses per session (10 bursts, each burst composed of 3 stimuli at 50 Hz, repeated at a theta frequency of 5 Hz every 10 s @ 80% AMT) Sham Identical protocol. Active coil rotated perpendicularly to the scalp. |
MAS, H-reflex | Compared with baseline, REAL displayed significant decreases in (a) MAS scores on the stimulated target limb at postintervention and 1-week follow-up and (b) H/M ratio at post-7th session, postintervention and 1- and 2-week follow-up. There was no change in SHAM. |
| Sasaki et al. 2017 (41) | Double-blind RCT. ATP: Baseline, postintervention | Stroke. n = 21 Post-onset duration 10.9±6.6 days Sex (M/F, active|sham): 8/3 | 5/5 Age (active|sham): 66.5±16.6 | 62.4±10.3 years | Active rTMS 10 sessions over 5 consecutive days (2 sessions/day) over bilateral leg motor cortex with a double-cone coil 1000 pulses per session (10 Hz, 10 trains of 100 pulses @ 90% RMT, 10 s on/50 s off) Sham Inactive coil held over scalp. Speaker playing recording from 10 Hz stimulation. |
BRS-LL | BRS for the lower limb significantly improved in REAL but did not change in the sham stimulation group. |
| Wang et al. 2012 (42) | Double-blind RCT. ATP: Baseline, postintervention | Stroke. n = 24 Post-onset duration >6 months Sex (M/F, active|sham): 7/5 | 8/4 Age (active|sham): 64.9±12.4 | 63.0±10.9 years | Active rTMS 10 sessions over 10 consecutive days over contralesional leg motor cortex with a figure-of-eight coil 600 pulses per session (1 Hz continuously for 10min @ 90% RMT) followed by 30 min taskoriented training Sham Identical protocol. Active coil rotated perpendicularly to the scalp. |
Gait velocity during non-standard gait analysis, FMA-LL | Gait velocity and FMA-LL scores increased significantly more for REAL compared with SHAM. |
| Wang et al. 2019 (43) | Double-blind RCT. ATP: Baseline, postintervention, 1-month follow-up | Stroke. n = 14 Post-onset duration >6 months Sex (M/F, active|sham): 7/1 | 4/2 Age (active|sham): 53.5±13.7 | 54.7±12.2 years | Active rTMS 9 sessions over 3 weeks (3 sessions/week) over ipsilesional leg motor cortex with a figure-of-eight coil 900 pulses per session (5 Hz, 15 trains of 60 pulses @ 90% RMT, 12 s on/48 s off) followed by treadmill training Sham Identical protocol. Active coil rotated perpendicularly to the scalp. |
Gait velocity during non-standard gait analysis, FMA-LL | Gait velocity and FMA-LL scores increased significantly for REAL at post-intervention and follow-up (only gait), and increased significantly more than for SHAM. |
| Yang et al. 2013 (44) | Double-blind RCT. ATP: Baseline, postintervention | Parkinson’s Disease. n = 20 Hoehn-Yahr stage 2.30±0.42 (active) and 2.35±0.41 (sham) Sex (M/F, active|sham): 5/5 | 7/3 Age (active|sham): 65.2±11.1 | 67.0±13.2 years | Active rTMS 12 sessions over 4 weeks (3 sessions/week) over leg motor cortex contralaterally to the more affected side with a figure-ofeight coil 1200 pulses per session (5 Hz, 24 trains of 50 pulses @ 100% RMT, 10 s on/5 s off) followed by treadmill training Sham Identical protocol. Active coil rotated 45° to the scalp. |
10MWT, TUG | Gait velocity for ”fast” and ”comfortable” walking speed in the 10MWT increased significantly in both groups, but ”fast” increased significantly more for REAL. Time-to-complete the TUG decreased significantly in both groups, but decreased significantly more in REAL. |
| Zanette et al. 2008 (45) | RCT ATP: Baseline, post-intervention, 2-week follow-up | Amyotrophic lateral sclerosis. n = 10 Disease duration 11.4±3.0 (active) and 12.2±4.0 (sham) months Sex (M/F, active|sham): 4/1 | 3/2 Age (active|sham): 59.4±9.2 | 60.2±8.7 years | Active rTMS 10 sessions over 2 weeks over left and right hand and bilateral leg motor cortex with a figure-of-eight (hand) and circular (leg) coil 900 pulses per session [300 pulses each location] (5 Hz, 20 trains of 15 pulses @ 110% RMT, 3 s on/60 s off (each location)) Sham Identical protocol. Sham coil. |
MRC scale, Isokinetic dynamometry | There were no changes in MRC scores in either group. Lower limb muscle power increased significantly for REAL at post-intervention (but not at follow-up), and the increase was significantly greater compared with SHAM. |
10MWT: 10-metre walking test; AIS: ASIA Impairment Scale; AMT: active motor threshold; ATP: assessment time-points; BBS: Berg Balance Scale; BRS-LL: Brunnstrom Recovery Stages Lower Limb; DLPFC: dorsolateral prefrontal cortex; FAC: Functional Ambulatory Category; FMA-LL: Fugl-Meyer Assessment Lower Limb; FOG-Q: Freezing of Gait Questionnaire; iTBS: Intermittent Theta Burst Stimulation; LEMS: Lower Extremity Motor Score; MAS: Modified Ashworth Scale; MBI: Modified Barthel Index; MI-L: Motricity Index Leg; MRC: Medical Research Council; PASS: Postural Assessment Scale for Stroke Patients; RMT: Resting Motor Threshold; SMA: Supplementary Motor Area; TUG: Timed Up-and-Go test; WISCI: Walking Index for Spinal Cord Injury.
Risk of bias assessment
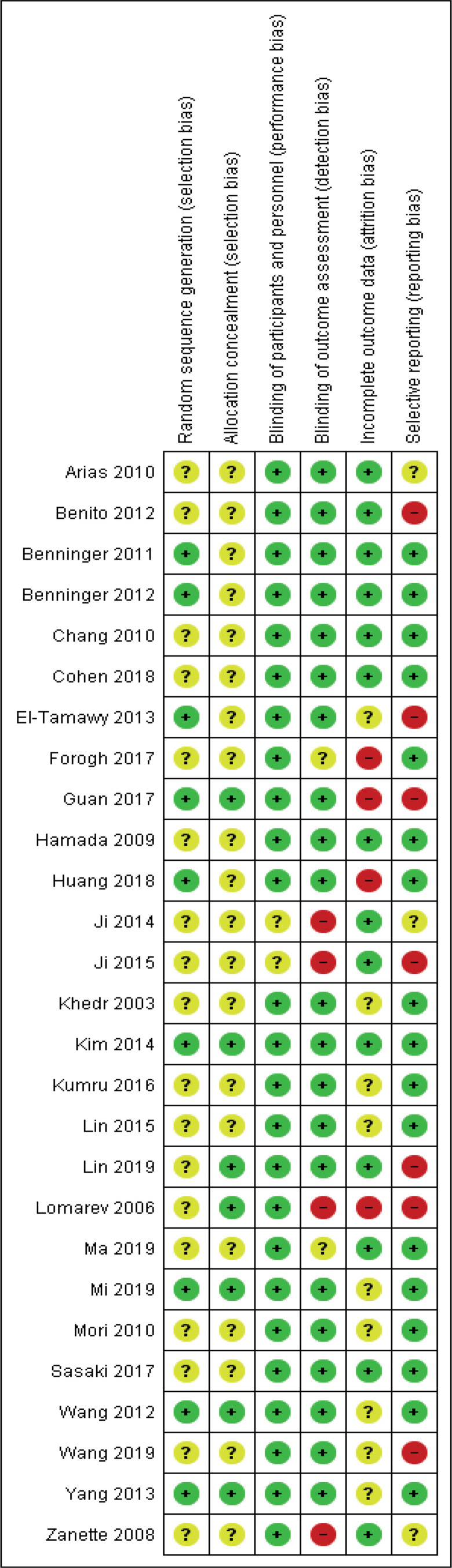
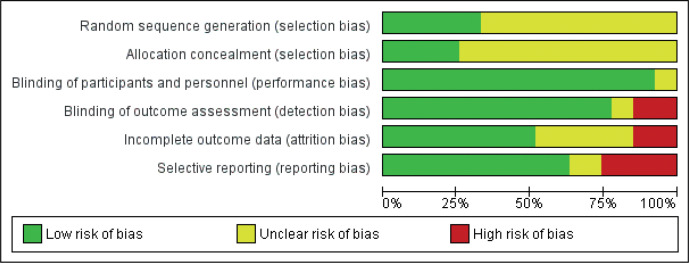
Figs 2 and 3 provide an overview of the risk of bias in the included trials. Overall, a low risk of bias was found for reporting related to blinding. All studies blinded participants to allocation, as per inclusion criteria, and only 2 studies failed to report of blinding of intervention personnel (37, 38). In 6 studies (22.2%), blinding of outcome assessors were either not performed or not concisely reported (33, 37, 38, 44, 45, 52). A majority of studies (66.7%) failed to adequately report randomization sequence generation and allocation concealment procedures. Typically, while information was provided that participant allocation was performed using a randomized sequence method, it was not specified how the specific randomization sequence was generated. In addition, most studies (74.1%) did not specify how allocation concealment was achieved. Therefore, a moderate to high risk of selection bias was deemed to be present in the presented findings. Approximately half (51.9%) of the studies sufficiently disclosed data related to drop-outs and loss-to-follow-ups, while most studies (63.0%) provided detailed results, which appeared free of potential reporting bias.
Fig. 2.
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study (26–52).
Fig. 3.
Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
Effect on selected outcome parameters
A qualitative summary of the findings for each individual outcome measure at each follow-up period is provided in Table I.
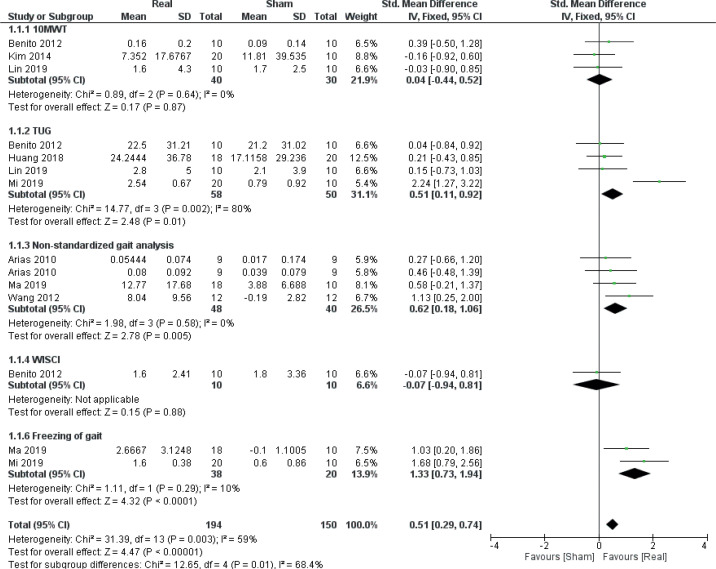
Gait
Data from 8 RCTs (26, 27, 36, 40, 43, 45, 46, 49) (14 comparisons, n = 344) were included for meta-analysis to evaluate the effect of real rTMS on gait function (Fig. 4). A moderate effect on overall gait function, which was found to be moderately to substantially heterogeneous, was found for real rTMS compared with sham stimulation (effect size (ES) 0.51 [0.29; 0.74], p < 0.00001, I2 = 59%). For sub-groups, a very large and homogenous effect of real rTMS was found for self-reported scores of freezing-of-gait (ES: 1.33 [0.73; 1.94], p < 0.00001, I2 = 10%) while a moderate effect was found for time-to-complete the Timed Upand-Go (TUG) test (ES: 0.51 [0.11; 0.92], p = 0.01, I2 = 80%) and for gait improvement assessed with nonstandardized gait tests (ES: 0.62 [0.18; 1.06], p = 0.005, I2 = 0%). No effect was found in 10-metre walking test (10MWT) performance or for Walking Index for Spinal Cord Injury II (WISCI II) scores. A test for homogeneity revealed significant and substantial heterogeneity between subgroups (I2 = 68.4%, p = 0.01).
Fig. 4.
Forest plot of comparison: outcome type – gait. 10MWT: 10-metre walking test: Change in gait speed (Benito 2012: m/s; Kim 2014, Lin 2019: reduction in time-to-complete). TUG: Timed Up-and-Go test: Reduction in time to complete (s). WISCI: Walking Index for Spinal Cord Injury: change in scores. Freezing of gait: change in scores (FOG Questionnaire). 95% CI: 95% confidence interval; SD: standard deviation (26, 27, 36, 40, 43, 45, 46, 49)
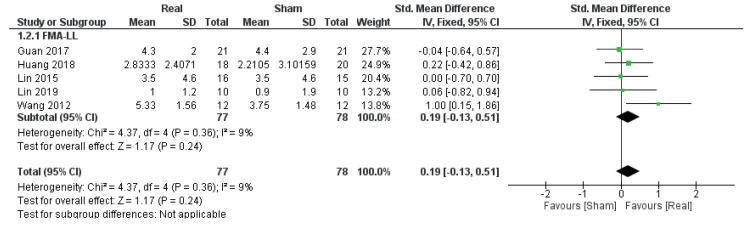
Mobility
Data from 5 RCTs (34, 36, 42, 43, 49) (5 comparisons, n = 155) were obtained in order to evaluate the effect of real rTMS on improvements in mobility. Only data from studies using lower limb sub-scores of the Fugl-Meyer Assessment, a stroke-specific, performancebased motor recovery index, were available. Here, no effect of real rTMS was found (ES: 0.19 [–0.13; 0.51], p = 0.24, I2 = 9 %) (Fig. 5).
Fig. 5.
Forest plot of comparison: outcome type – mobility. FMA-LL: Fugl-Meyer Assessment Scale Lower limb sub-scores: change in scores. 95% CI: 95% confidence interval; SD: standard deviation (34, 36, 42, 43, 49).
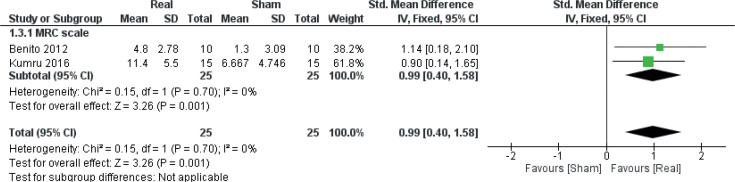
Maximal muscle strength
Data were obtained from 2 studies (27, 41) (2 comparisons, n = 50), both o-f which had individuals with SCI as their study population, to investigate the effect of real rTMS on recovery of lower limb muscle strength (Fig. 6). A large and homogenous effect of real rTMS on the SCI-specific variant of the Medical Research Council (MRC) scale for lower limb muscle strength (LEMS, Lower Extremity Motor Score) was found ((ES: 0.99 [0.40; 1.58], p = 0.001, I2 = 9%).
Fig. 6.
Forest plot of comparison: outcome type – muscle strength. MRC Scale: Medical Research Council Scale: change in leg motor scores. 95% CI: 95% confidence interval; SD: standard deviation (27, 41).
Spasticity
Data from a single study (27) (n = 20) investigating the effect of rTMS on spasticity (Modified Ashworth Scale; MAS) was obtained (ES: 0.56 [–0.34; 1.46], p = 0.22). Therefore, meta-analysis could not be performed.
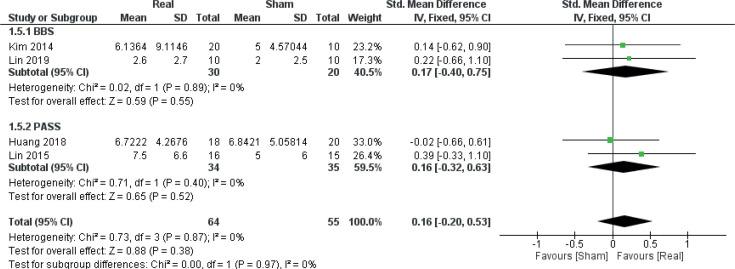
Balance
Data from 4 RCTs (36, 40, 42, 43) (4 comparisons, n = 119) were obtained in order to evaluate the effect of real rTMS on outcomes related to balance. No clear effect of real rTMS was found for this category (ES: 0.16 [–0.20; 0.53], p = 0.38, I2 = 0%) (Fig. 7).
Fig. 7.
Forest plot of comparison: Outcome type – balance. BBS: Berg’s Balance scale: change in scores. PASS: Postural Assessment Scale for Stroke Patients: change in scores. 95% CI: 95% confidence interval; SD: standard deviation (36, 40, 42, 43).
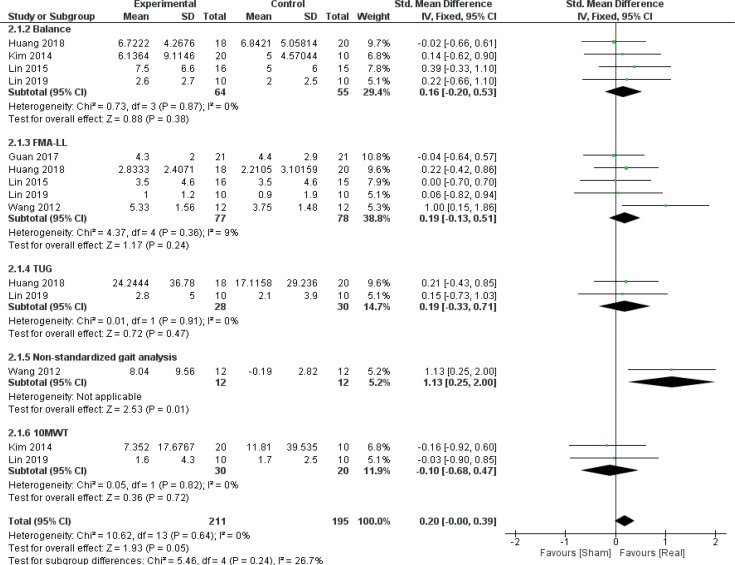
Disorder type
A secondary meta–analysis was performed on the identified outcome parameters in order to evaluate whether the adaptive effects of rTMS differed among the included disorders. The results are shown in Figs 8–10. A small and homogeneous increase in overall lower limb function was found for real rTMS compared with sham in individuals with stroke (14 comparisons, n = 406, ES: 0.20 [0.00; 0.39], p = 0.05, I2 = 26.7%) (Fig. 8), while a very large, but moderately to substantially heterogeneous effect was observed in individuals with PD (6 comparisons, n = 152, ES: 1.01 [0.65; 1.37], p<0.00001, I2 = 62%) (Fig. 9). In addition, a moderate and homogenous overall effect on LLFO was found following real rTMS in individuals with SCI (6 comparisons, n = 130, ES: 0.50 [0.14; 0.85], p = 0.006, I2 = 9%) (Fig. 10). No data could be obtained from studies investigating MS or ALS.
Fig. 8.
Forest plot of comparison: disorder type – stroke. 10MWT: 10-metre walking test: Change in gait speed (reduction in time-to-complete, s). Balance: change in test scores (BBS, PASS). FMA-LL: Fugl-Meyer Assessment Scale Lower limb sub-scores: change in scores. Non-standardized gait analysis: reduction in time-to-complete. TUG: Timed Up-and-Go test: reduction in time to complete (s). 95% CI: 95% confidence interval; SD: standard deviation (34, 36, 40, 42, 43, 49).
Fig. 9.
Forest plot of comparison: disorder type – Parkinson’s disease. Freezing of gait: change in scores (FOG Questionnaire). Non-standardized gait analysis: change in gait speed (Arias 2010 ON medication state (first entry), m/s; Arias 2010 OFF medication state (second entry), m/s; Ma 2019, reduction in time-to-complete (s)). TUG: Timed Up-and-Go test: reduction in time to complete (s). 95% CI: 95% confidence interval; SD: standard deviation (26, 45, 46).
Fig. 10.
Forest plot of comparison: disorder type – spinal cord injury. 10MWT: 10-metre walking test: change in gait speed (m/s). LEMS: Lower Extremity Motor Score: change in scores. Spasticity: change in Modified Ashworth Scale scores. TUG: Timed Up-and-Go test: reduction in time to complete (s). WISCI: Walking Index for Spinal Cord Injury: change in scores. 95% CI: 95% confidence interval; SD: standard deviation (27, 41).
Risk of bias due to missing results
Table II gives an overview of the number of comparisons included in the present meta-analysis in relation to the total number of comparisons identified through study screening. In the primary analyses, data could not be obtained for more than half of the identified comparisons in all subgroups, except for “Balance” (inclusion ratio (IR): 0.33–0.57). As for the secondary analyses, data completeness was moderate for the Stroke (IR 0.56), low for the PD (IR 0.29) and high for the SCI (IR 0.75) subgroups. Therefore, with the exception of the SCI subgroup, a considerable risk of bias due to non-reporting exists for the results from the data syntheses.
Table II.
Identified vs included comparisons for the data synthesis
| Outcome | Total number of comparisons identified | Total number of comparisons included for meta-analysis | Inclusion ratio |
|---|---|---|---|
| Gait | 31 | 14 | 0.45 |
| Mobility | 11 | 5 | 0.45 |
| Muscle strength | 5 | 2 | 0.40 |
| Spasticity | 3 | 1 | 0.33 |
| Balance/posture | 7 | 4 | 0.57 |
| Total | 57 | 26 | 0.46 |
| Stroke | 25 | 14 | 0.56 |
| Parkinson’s disease | 21 | 6 | 0.29 |
| Spinal cord injury | 8 | 6 | 0.75 |
| Multiple sclerosis | 1 | 0 | 0.00 |
| Amyotrophic lateral sclerosis | 2 | 0 | 0.00 |
| Total | 57 | 26 | 0.46 |
DISCUSSION
This study reviewed 27 RCTs examining the effects of rTMS vs sham stimulation on lower limb functional outcome measures in individuals with neurological disorders. The included studies were heterogeneous, had small to medium sample sizes and presented a moderate risk of selection, attrition and reporting bias. A statistically significant effect of real rTMS compared with sham stimulation was found for outcome parameters: gait and muscle strength and for disorder types: stroke, PD and SCI. No effect of real rTMS was found for outcomes related to mobility or balance compared with sham stimulation, while meta-analysis of outcome parameters: spasticity and disorders: MS and ALS could not be performed due to a lack of data. A considerable risk of bias due to non-reporting was present in all categories except for disorder: SCI.
The findings of a positive effect of rTMS on gait function and lower limb muscle strength are in line with results from a recent review on rTMS in stroke survivors, where the authors reported improvements in walking speed, lower limb body function and FMALL scores following rTMS compared with control interventions (53). Notably, improvements were also found for outcomes related to lower limb activities and participation following rTMS, indicating that rTMSinduced improvements in lower limb function can lead to meaningful functional recovery in individuals with neurological impairment. In addition, another recent review investigated the effect of rTMS on ambulatory function in PD (54). Here, walking speed was reported to improve following rTMS interventions compared with sham stimulation, while a trend for improved freezing-of-gait (questionnaire scores) was found following rTMS compared with control interventions (54).
The neurophysiological basis for the beneficial adaptations observed following systematic rTMS has not yet been clearly identified. A moderate body of evidence suggests that long-term rTMS can alter synaptic plasticity within the corticospinal tract, i.e. by potentiating excitatory post-synaptic potentials, which is thought to occur through a regulation of Ca2+, NO and glutamate availability and sensitivity (8, 55). Therefore, and particularly for individuals with CNS impairment, it is possible that initiation and control of volitional movement are promoted by rTMS due to an increased strength of the descending corticospinal volleys projecting onto the spinal motoneurones.
However, the findings compiled in the current study are associated with a number of potential limitations. Firstly, extensive heterogeneity was observed for several outcome parameters, indicating that treatment effects varied between the included studies. This may in part be explained by heterogeneous study protocols (study population, length of intervention, total number of sessions, follow-up) and stimulation paradigms (frequency, area of stimulation, total number of pulses per session), although it appears that substantial differences were also present in response to treatment between individuals within the same patient category. This is supported by the large variance observed in the change score values, but also by recent findings in patients with depression (56) and consciousness disorder (57), where electroencephalography was applied to clearly discriminate rTMS responders from non-responders within their study population. This observation underlines a need for further investigations of the inter-individual variability in response to rTMS treatment in individuals with neurological motor impairments. Identifying clinical and non-clinical predictors of responsiveness to rTMS treatment holds particular importance, as it could increase clinical efficacy by personalizing and adapting rTMS protocols to optimize the rehabilitation of neurological patients.
Limitations in the present findings are also present due to considerable non-reporting, as over half of the identified comparisons were unavailable for analyses. Missing results are problematic in all data syntheses, because available results may differ systematically from missing results (25); therefore, it is possible that the treatment effects reported in the present study are either under- or over-estimated. Furthermore, the observed rTMS treatment effects were based on analyses of pre- to post-intervention data. We chose to analyse post-intervention changes instead of follow-up changes due to considerations about data availability and underlying homogeneity of the findings (because the included studies employed disparate follow-up time-points). However, it is possible that an analysis on changes from pre-intervention to last available follow-up would have yielded different results. A post-hoc qualitative analysis of studies employing follow-up beyond the rTMS intervention revealed that the improvements induced by real rTMS at post-intervention were generally maintained during follow-up, except for 1 study (52). An additional study reported that improvements were seen only in real rTMS during follow-up assessments, compared with sham stimulation (33).
In conclusion, the findings of the current study suggest that implementing rTMS as an adjunct therapy to neurorehabilitation may be a viable strategy to promote ambulation and lower limb muscle strength. Specifically for rehabilitation of individuals with stroke, PD and SCI, rTMS appears to enhance recovery of overall lower limb motor function. However, treatment effects vary widely between the included studies and the results are synthesized from heterogeneous studies, which employed moderate sample sizes and presented with risk of bias. In addition, the results may be influenced by bias due to considerable non-reporting. Therefore, further data from large RCTs are needed in order to provide unambiguous recommendations for the clinical use of rTMS in neurorehabilitation. In addition, further studies on the use of rTMS on individuals with other neurological conditions are needed before these findings can be extrapolated to other patient groups.
ACKNOWLEDGEMENTS
The authors thank the original study researchers who kindly provided us with relevant data in relation to the current study.
Footnotes
The authors have no conflicts of interest to declare.
Funding
This study was carried out with internal research funding from the Department of Neurology, Regional Hospital Viborg.
REFERENCES
- 1.Alia C, Spalletti C, Lai S, Panarese A, Lamola G, Bertolucci F, et al. Neuroplastic changes following brain ischemia and their contribution to stroke recovery: Novel approaches in neurorehabilitation. Front Cell Neurosci 2017; 11: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thickbroom GW, Mastaglia FL. Plasticity in neurological disorders and challenges for noninvasive brain stimulation (NBS). J Neuroeng Rehabil 2009; 6: 2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halpern R, Agarwal S, Dembek C, Borton L, Lopez-Bresnahan M. Comparison of adherence and persistence among multiple sclerosis patients treated with disease-modifying therapies: a retrospective administrative claims analysis. Patient Prefer Adherence 2011; 5: 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Straka I, Minár M, Gažová A, Valkovič P, Kyselovič J. Clinical aspects of adherence to pharmacotherapy in Parkinson disease: a PRISMA-compliant systematic review. Medicine (Baltimore) 2018; 97: e10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr J, Shepherd R. The changing face of neurological rehabilitation. Rev Bras Fisioter 2006; 10: 147–156. [Google Scholar]
- 6.Maizey L, Allen CPG, Dervinis M, Verbruggen F, Varnava A, Kozlov M, et al. Comparative incidence rates of mild adverse effects to transcranial magnetic stimulation. Clin Neurophysiol 2013; 124: 536–544. [DOI] [PubMed] [Google Scholar]
- 7.Hallett M. Transcranial magnetic stimulation and the human brain. Nature 2000; 406: 147–150. [DOI] [PubMed] [Google Scholar]
- 8.Klomjai W, Katz R, Lackmy-Vallée A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann Phys Rehabil Med 2015; 58: 208–213. [DOI] [PubMed] [Google Scholar]
- 9.Slotema CW, Blom JD, Hoek HW, Sommer IEC. Should we expand the toolbox of psychiatric treatment methods to include repetitive transcranial magnetic stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. J Clin Psychiatry 2010; 71: 873–884. [DOI] [PubMed] [Google Scholar]
- 10.Giannoni-Luza S, Pacheco-Barrios K, Cardenas-Rojas A, Mejia-Pando PF, Luna-Cuadros MA, Barouh JL, et al. Noninvasive motor cortex stimulation effects on quantitative sensory testing (QST) in healthy and chronic pain subjects. Pain 2020; 161: 1955–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leung A, Donohue M, Xu R, Lee R, Lefaucheur JP, Khedr EM, et al. rTMS for suppressing neuropathic paIn: a metaanalysis. J Pain 2009; 10: 1205–1216. [DOI] [PubMed] [Google Scholar]
- 12.Papadopoulou SL, Ploumis A, Exarchakos G, Theodorou SJ, Beris A, Fotopoulos AD. Versatility of repetitive transcranial magnetic stimulation in the treatment of poststroke dysphagia. J Neurosci Rural Pract 2018; 9: 391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naeser MA, Martin PI, Treglia E, Ho M, Kaplan E, Bashir S, et al. Research with rTMS in the treatment of aphasia. Restor Neurol Neurosci 2010; 28: 511–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benninger DH, Hallett M. Non-invasive brain stimulation for Parkinson’s disease: Current concepts and outlook 2015. NeuroRehabilitation 2015; 37: 11–24. [DOI] [PubMed] [Google Scholar]
- 15.van Lieshout ECC, van der Worp HB, Visser-Meily JMA, Dijkhuizen RM. Timing of repetitive transcranial magnetic stimulation onset for upper limb function after stroke: a systematic review and meta-analysis. Front Neurol 2019; 10: 1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broe GA, Jorm AF, Creasey H, Grayson D, Edelbrock D, Waite LM, et al. Impact of chronic systemic and neurological disorders on disability, depression and life satisfaction. Int J Geriatr Psychiatry 1998; 13: 667–173. [DOI] [PubMed] [Google Scholar]
- 17.Putzke JD, Richards JS, Hicken BL, DeVivo MJ. Predictors of life satisfaction: A spinal cord injury cohort study. Arch Phys Med Rehabil 2002; 83: 555–561. [DOI] [PubMed] [Google Scholar]
- 18.Soh S-E, McGinley JL, Watts JJ, Iansek R, Murphy AT, Menz HB, et al. Determinants of health-related quality of life in people with Parkinson’s disease: a path analysis. Qual Life Res 2013; 22: 1543–1553. [DOI] [PubMed] [Google Scholar]
- 19.Zangen A, Roth Y, Voller B, Hallett M. Transcranial magnetic stimulation of deep brain regions: evidence for efficacy of the H-coil. Clin Neurophysiol 2005; 116: 775–779. [DOI] [PubMed] [Google Scholar]
- 20.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med 2021; e 1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol 2021; 134: 103–112. [DOI] [PubMed] [Google Scholar]
- 22.WHO . Neurological disorders: public health challenges. Geneva (Switzerland). WHO Library Cataloguing; 2006. [Google Scholar]
- 23.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen J. Statistical power analysis for the behavioral sciences. New York: Academic Press; 2013. [Google Scholar]
- 25.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. 2nd edition. Chichester, UK: John Wiley & Sons; 2019. [Google Scholar]
- 26.Arias P, Vivas J, Grieve KL, Cudeiro J. Controlled trial on the effect of 10 days low-frequency repetitive transcranial magnetic stimulation (rTMS) on motor signs in Parkinson’s disease. Mov Disord 2010; 25: 1830–1838. [DOI] [PubMed] [Google Scholar]
- 27.Benito J, Kumru H, Murillo N, Costa U, Medina J, Tormos JM, et al. Motor and gait improvement in patients with incomplete spinal cord injury induced by high-frequency repetitive transcranial magnetic stimulation. Top Spinal Cord Inj Rehabil 2012; 18: 106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benninger DH, Berman BD, Houdayer E, Pal N, Luckenbaugh DA, Schneider L, et al. Intermittent theta-burst transcranial magnetic stimulation for treatment of Parkinson disease. Neurology 2011; 76: 601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benninger DH, Iseki K, Kranick S, Luckenbaugh DA, Houdayer E, Hallett M. Controlled study of 50-Hz repetitive transcranial magnetic stimulation for the treatment of parkinson disease. Neurorehabil Neural Repair 2012; 26: 1096–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang WH, Kim YH, Bang OY, Kim ST, Park YH, Lee PKW. Long-term effects of RTMS on motor recovery in patients after subacute stroke. J Rehabil Med 2010; 42: 758–764. [DOI] [PubMed] [Google Scholar]
- 31.Cohen OS, Rigbi A, Yahalom G, Warman-Alaluf N, Nitsan Z, Zangen A, et al. Repetitive deep TMS for Parkinson disease: a 3-month double-blind, randomized shamcontrolled study. J Clin Neurophysiol 2018; 35: 159–165. [DOI] [PubMed] [Google Scholar]
- 32.El-Tamawy MS, Shehata HS, Shalaby NM, Nawito A, Esmail EH. Can repetitive transcranial magnetic stimulation help on-freezers with Parkinson’s disease? Egypt J Neurol Psychiatry Neurosurg 2013; 50: 355–360. [Google Scholar]
- 33.Forogh B, Ahadi T, Nazari M, Sajadi S, Latif LA, Akhavan Hejazi SM, et al. The effect of repetitive transcranial magnetic stimulation on postural stability after acute stroke: a clinical trial. Basic Clin Neurosci 2017; 8: 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guan YZ, Li J, Zhang XW, Wu S, Du H, Cui LY, et al. Effectiveness of repetitive transcranial magnetic stimulation (rTMS) after acute stroke: a one-year longitudinal randomized trial. CNS Neurosci Ther 2017; 23: 940–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamada M, Ugawa Y, Tsuji S. High-frequency rTMS over the supplementary motor area improves bradykinesia in Parkinson’s disease: subanalysis of double-blind shamcontrolled study. J Neurol Sci 2009; 287: 143–146. [DOI] [PubMed] [Google Scholar]
- 36.Huang YZ, Lin LF, Chang KH, Hu CJ, Liou TH, Lin YN. Priming with 1-Hz repetitive transcranial magnetic stimulation over contralesional leg motor cortex does not increase the rate of regaining ambulation within 3 months of stroke: a randomized controlled trial. Am J Phys Med Rehabil 2018; 97: 339–345. [DOI] [PubMed] [Google Scholar]
- 37.Ji S-G, Cha H-G, Kim K-J, Kim M-K. Effects of motor imagery practice in conjunction with repetitive transcranial magnetic stimulation on stroke patients. J Magn 2014; 19: 181–184. [Google Scholar]
- 38.Ji S-G, Kim M-K. The effects of repetitive transcranial magnetic stimulation on the gait of acute stroke patients. J Magn 2015; 20: 129–132. [Google Scholar]
- 39.Khedr EM, Farweez HM, Islam H. Therapeutic effect of repetitive transcranial magnetic stimulation on motor function in Parkinson’s disease patients. Eur J Neurol 2003; 10: 567–572. [DOI] [PubMed] [Google Scholar]
- 40.Kim WS, Jung SH, Oh MK, Min YS, Lim JY, Paik NJ. Effect of repetitive transcranial magnetic stimulation over the cerebellum on patients with ataxia after posterior circulation stroke: a pilot study. J Rehabil Med 2014; 46: 418–423. [DOI] [PubMed] [Google Scholar]
- 41.Kumru H, Benito-Penalva J, Valls-Sole J, Murillo N, Tormos JM, Flores C, et al. Placebo-controlled study of rTMS combined with Lokomat® gait training for treatment in subjects with motor incomplete spinal cord injury. Exp Brain Res 2016; 234: 3447–3455. [DOI] [PubMed] [Google Scholar]
- 42.Lin YN, Hu CJ, Chi JY, Lin LF, Yen TH, Lin YK, et al. Effects of repetitive transcranial magnetic stimulation of the unaffected hemisphere leg motor area in patients with subacute stroke and substantial leg impairment: a pilot study. J Rehabil Med 2015; 47: 305–310. [DOI] [PubMed] [Google Scholar]
- 43.Lin LF, Chang KH, Huang YZ, Lai CH, Liou TH, Lin YN. Simultaneous stimulation in bilateral leg motor areas with intermittent theta burst stimulation to improve functional performance after stroke: a feasibility pilot study. Eur J Phys Rehabil Med 2019; 55: 162–168. [DOI] [PubMed] [Google Scholar]
- 44.Lomarev MP, Kanchana S, Bara-Jimenez W, Iyer M, Wassermann EM, Hallett M. Placebo-controlled study of rTMS for the treatment of Parkinson’s disease. Mov Disord 2006; 21: 325–331. [DOI] [PubMed] [Google Scholar]
- 45.Ma J, Gao L, Mi T, Sun J, Chan P, Wu T. Repetitive transcranial magnetic stimulation does not improve the sequence effect in freezing of gait. Parkinsons Dis 2019; 2196195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mi TM, Garg S, Ba F, Liu AP, Wu T, Gao LL, et al. Highfrequency rTMS over the supplementary motor area improves freezing of gait in Parkinson’s disease: a randomized controlled trial. Park Relat Disord 2019; 68: 85–90. [DOI] [PubMed] [Google Scholar]
- 47.Mori F, Codecà C, Kusayanagi H, Monteleone F, Boffa L, Rimano A, et al. Effects of intermittent theta burst stimulation on spasticity in patients with multiple sclerosis. Eur J Neurol 2010; 17: 295–300. [DOI] [PubMed] [Google Scholar]
- 48.Sasaki N, Abo M, Hara T, Yamada N, Niimi M, Kakuda W. High-frequency rTMS on leg motor area in the early phase of stroke. Acta Neurol Belg 2017; 117: 189–194. [DOI] [PubMed] [Google Scholar]
- 49.Wang RY, Tseng HY, Liao KK, Wang CJ, Lai KL, Yang YR. RTMS combined with task-oriented training to improve symmetry of interhemispheric corticomotor excitability and gait performance after stroke: a randomized trial. Neurorehabil Neural Repair 2012; 26: 222–230. [DOI] [PubMed] [Google Scholar]
- 50.Wang RY, Wang FY, Huang SF, Yang YR. High-frequency repetitive transcranial magnetic stimulation enhanced treadmill training effects on gait performance in individuals with chronic stroke: a double-blinded randomized controlled pilot trial. Gait Posture 2019; 68: 382–387. [DOI] [PubMed] [Google Scholar]
- 51.Yang YR, Tseng CY, Chiou SY, Liao KK, Cheng SJ, Lai KL, et al. Combination of rTMS and treadmill training modulates corticomotor inhibition and improves walking in parkinson disease: a randomized trial. Neurorehabil Neural Repair 2013; 27: 79–86. [DOI] [PubMed] [Google Scholar]
- 52.Zanette G, Forgione A, Manganotti P, Fiaschi A, Tamburin S. The effect of repetitive transcranial magnetic stimulation on motor performance, fatigue and quality of life in amyotrophic lateral sclerosis. J Neurol Sci 2008; 270: 18–22. [DOI] [PubMed] [Google Scholar]
- 53.Tung YC, Lai CH, Liao C De, Huang SW, Liou TH, Chen HC. Repetitive transcranial magnetic stimulation of lower limb motor function in patients with stroke: a systematic review and meta-analysis of randomized controlled trials. Clin Rehabil 2019; 33: 1102–1012. [DOI] [PubMed] [Google Scholar]
- 54.Xie YJ, Gao Q, He CQ, Bian R. Effect of repetitive transcranial magnetic stimulation on gait and freezing of gait in Parkinson disease: a systematic review and meta-analysis. Arch Phys Med Rehabil 2020; 101: 130–140. [DOI] [PubMed] [Google Scholar]
- 55.Hoogendam JM, Ramakers GMJ, Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul 2010; 3: 95–118. [DOI] [PubMed] [Google Scholar]
- 56.Bailey NW, Hoy KE, Rogasch NC, Thomson RH, McQueen S, Elliot D, et al. Differentiating responders and nonresponders to rTMS treatment for depression after one week using resting EEG connectivity measures. J Affect Disord 2019; 242: 68–79. [DOI] [PubMed] [Google Scholar]
- 57.He R, Fan J, Wang H, Zhong Y, Ma J. Differentiating responders and non-responders to rTMS treatment for disorder of consciousness using EEG after-effects. Front Neurol 2020; 11: 583268. [DOI] [PMC free article] [PubMed] [Google Scholar]