The immunogenicity of the mRNA coronavirus disease 2019 vaccine in thoracic organ transplant recipients is poor.1,2 Early reports provided evidence of increased immunogenicity after the third mRNA vaccine dose in solid organ transplant recipients.3,4 However, the antibody and cellular responses after the third dose of the BNT162b2 vaccine (Pfizer-BioNTech) and its safety in lung transplant recipients (LTRs) are unknown to date.
We included 15 LTRs without a history of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection who received 2 doses of the BNT162b2 vaccine 21 d apart with no antibody response. In this cohort, we assessed the antibody and cellular responses immediately before and 3 wk after the third dose administered 3 mo after the second dose. Anti–SARS-CoV-2 immunoglobulin (Ig) G levels were tested by Microblot-Array coronavirus disease 2019 IgG against a mix of recombinant antigens (TestLine Clinical Diagnostics, Brno, Czech Republic). SARS-CoV-2–specific T cells were assessed by detecting intracellular cytokines after a 4-h stimulation of patients’ peripheral blood mononuclear cells with 51 overlapping 11mer peptides of the spike receptor–binding domain protein (JPT Peptide Technologies, Berlin, Germany) as we described previously.2
The study was approved by the Motol University Hospital institutional review board and the participants provided written informed consent.
The median age was 56.2 y (interquartile range [IQR], 54–60), 87% were male, the median time from transplant to the first dose was 1277 d (IQR, 889–2496), the median time from the second to third dose was 96 d (IQR, 95–97), and the median time from the third dose to SARS-CoV-2 IgG and specific T-cell detection was 21 d (IQR, 20–21). The maintenance immunosuppression included calcineurin inhibitors (100%), mycophenolate (93%), and corticosteroids (100%).
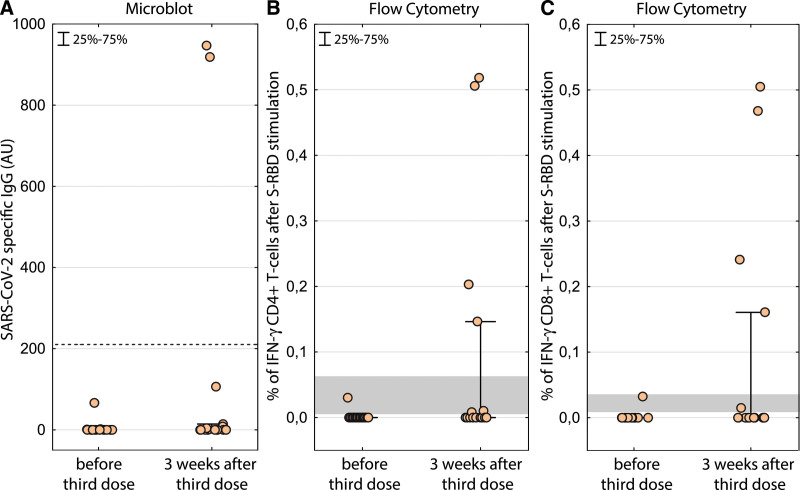
Before the third vaccine dose, we detected cellular response in 2 out of 15 patients (13%), albeit at low frequency. SARS-CoV-2–specific IgG levels were not detected in any of the vaccinated LTRs. Three weeks after the third dose, we detected cellular response in 7 patients (47%) and humoral response in 2 patients (13%). The frequencies of the SARS-CoV-2–specific T cells were above 0.1% in 4 of the 7 responders; levels never achieved after the second dose in our previous cohort (Figure 1).2
FIGURE 1.
SARS-CoV-2–specific IgG response before and after the third BNT162b2 mRNA vaccine. The dotted line shows the positivity threshold (A). SARS-CoV-2 S-RBD–specific response of CD4+ (B) and CD8+ T cells (C). The magnitude of the response is calculated as percent of interferon-γ–responding T cells after S-RBD stimulation less percent of interferon-γ without any stimulation. The gray area depicts the levels of positive response measured after second dose in a previous cohort by the same technique.2 IFN-γ, interferon-γ; IgG, immunoglobulin G; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; S-RBD, spike receptor–binding domain.
The significantly lower antibody response in LTRs as compared to patients after other organ transplantations is probably related to higher immunosuppression in this group, specifically to the dose of mycophenolate.3,5 One of the 2 patients with humoral response was the only one without mycophenolate, and the second one had the lowest dose of mycophenolate of all patients.
We did not observe any systemic adverse events, rejection episodes, or decline in allograft function in any patient within 3 mo after the third dose.
In conclusion, in the absence of humoral response, we detected emergence of cellular response in 47% of LTRs after the third vaccine dose, which might have a clinical benefit; however, the measurable response is low, dominantly cellular, and only detectable in half of the patients. Together with no evidence of rejection episodes, the third dose should be recommended in LTRs but with the caution that protection by the vaccine may not be more than partial.
Footnotes
J.H. and T.K. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis, and they participated in drafting of the article, statistical analysis, and data visualization. J.H., T.K., P.H., E.D., A.Se., and R.L. participated in concept and design. J.H., T.K., J.L., and A.Sk. participated in acquisition, analysis, and interpretation of data. All authors participated in critical revision of the article for important intellectual content. A.Sk., J.L., M.S., and E.D. participated in administrative or technical support. T.K., J.H., A.Se., P.H., and R.L. participated in supervision.
The authors declare no conflicts of interest.
This work was supported by the grant of the Ministry of Health of the Czech Republic for conceptual development of research organization (No. 0064203) (Motol University Hospital, Prague, Czech Republic). A.Sk. was supported by grant 534120 from Charles University.
The support was used for covering the costs of the detection kits used in the study. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; and the decision to submit the article for publication.
REFERENCES
- 1.Peled Y, Ram E, Lavee J, et al. BNT162b2 vaccination in heart transplant recipients: clinical experience and antibody response. J Heart Lung Transplant. 2021;40:759–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Havlin J, Svorcova M, Dvorackova E, et al. Immunogenicity of BNT162b2 mRNA COVID-19 vaccine and SARS-CoV-2 infection in lung transplant recipients. J Heart Lung Transplant. 2021;40:754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peled Y, Ram E, Lavee J, et al. Third dose of the BNT162b2 vaccine in heart transplant recipients: immunogenicity and clinical experience. J Hear Lung Transplant. 2022;41:148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamar N, Abravanel F, Marion O, et al. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kantauskaite M, Müller L, Kolb T, et al. Intensity of mycophenolate mofetil treatment is associated with an impaired immune response to SARS-CoV-2 vaccination in kidney transplant recipients. Am J Transplant. 2022;22:634–639. [DOI] [PMC free article] [PubMed] [Google Scholar]