Background.
Heart transplant (HT) recipients may be at higher risk of acquiring SARS-CoV-2 infection and developing critical illness. The aim of this study is to describe characteristics and outcomes of HT recipients infected by SARS-COV-2, from a high-volume transplant center.
Methods.
We have described data of all adult HT recipients with confirmed coronavirus disease 2019 by RT-PCR in nasopharyngeal samples from April 5, 2020, to January 5, 2021. Outcomes and follow-up were recorded until February 5, 2021.
Results.
Forty patients were included. Twenty-four patients (60%) were men; the median age was 53 (40–60) y old; median HT time was 34 mo; and median follow-up time 162 d. The majority needed hospitalization (83%). Immunosuppressive therapy was reduced/withdrawn in the majority of patients, except from steroids, which were maintained. Seventeen patients (42.5%) were classified as having severe disease according to the ordinal scale developed by the World Health Organization Committee. They tended to have lower absolute lymphocyte count (P < 0.001) during follow-up when compared with patients with mild disease. Thirty-day mortality was 12.5%. However, a longer follow-up revealed increased later mortality (27.5%), with median time to death around 35 d. Bacterial nosocomial infections were a leading cause of death. Cardiac allograft rejection (10%) and ventricular dysfunction (12.5%) were also not negligible.
Conclusions.
Major findings of this study corroborate other cohorts’ results, but it also reports significant rate of later events, suggesting that a strict midterm surveillance is advisable to HT recipients with coronavirus disease 2019.
INTRODUCTION
Coronavirus disease 2019 (COVID-2019) is already considered the most important pandemic of the 21st century. Since the first reports from Wuhan, China, COVID-2019 has rapidly spread throughout the world with >100 million cases and 2.4 million deaths until February 2021.1,2 Healthcare systems have been deeply disrupted particularly in low- to mid- income countries and regions with large community transmission. Brazil is one of the worst hotspots in the world with >10 million cases and approximately 240 000 deaths until February 2021.3
Heart transplant (HT) recipients represent a unique population that often presents advanced age and comorbidities such as hypertension, diabetes, and chronic kidney disease, which are well-recognized risk factors for worse outcomes in COVID-19.4,5 In addition, differently from the general population, HT recipients are chronically immunosuppressed and its impact on COVID-19 infection is still a subject of debate.6 Although immunosuppression could facilitate viral replication at the early infection phase, at this point we also acknowledge the benefits of steroids in COVID-19 patients with hypoxemia or requiring ventilatory support, as shown in the Randomized Evaluation of COVID-19 therapy trial.7-9 As such, a certain degree of immunosuppression might be protective in severe forms of COVID-19.9-11
Best evidence on outcomes of solid-organ transplant (SOT) recipients infected by COVID-19 comes from moderate sized multicenter cohort studies mainly from Europe and the United States.12-15 Nevertheless, follow-up in these studies was usually short, with most of them ranging from 20 to 30 d, so that late consequences of COVID-19 infection in SOT recipients are still unknown.
The aim of this study is to describe characteristics and outcomes of HT recipients infected by the SARS-CoV-2, from a high-volume transplant center (about 50 adult HT/y) in São Paulo, Brazil. In addition, we also report extended follow-up data focused on late events.
MATERIALS AND METHODS
Study Population
In this case series, we prospectively included all adult HT recipients (>18 y old), who received the diagnosis of COVID-19 at Heart Institute, University of Sao Paulo, Brazil. The enrollment was carried out from April 5, 2020 to January 5, 2021. The study was conducted according to principles expressed in the Declaration of Helsinki and approved by the local Ethics Committee (CAAE: 42622215.9.0000.0068, SDC 4180/15/007).
Inclusion criteria for this case series were adult HT recipients with 1 or more clinical symptoms of SARS-CoV-2 infection in the last 7 d (fever, dry cough, malaise, and/or dyspnea) and positive SARS-CoV-2 RT-PCR in nasopharyngeal samples, following local hospital testing protocols based on the Charité protocol and World Health Organization recommendations.16 HT recipients with suspected symptoms of COVID-19 whose diagnostic test was not performed, were not included in this analysis.
Demographic features, clinical characteristics, modes of transmission, laboratory data such as renal function, and other known prognosis markers at admission and through at least 30 d of follow-up were recorded. Nosocomial transmission was considered in patients who had been hospitalized for other reasons than COVID-19 and developed the first symptoms after at least 7 d of hospitalization. Laboratory and treatment data were also recorded.
Patients were categorized according to the ordinal scale developed by World Health Organization Committee.17 Mild disease was defined as outpatient treatment or hospitalization without oxygen need or oxygen by mask or nasal prongs (score <5) and severe disease was defined as hospitalization with noninvasive ventilation, high-flow oxygen, or mechanical ventilation; use of inotropes, vasopressors, intraaortic balloon pump, or extracorporeal membrane oxygenation; or death (score ≥5).
Outcomes
Thirty-day mortality, overall mortality, and allograft rejection rates were computed. Outcomes and follow-up were recorded until February 5, 2021. Extended follow-up data focused on late events were also reported.
Statistical Analysis
Categorical variables were expressed as absolute (n) and relative frequencies (%). Continuous variables were expressed by mean ± SD or median (interquartile range, p25th –p75th) according to its normality distribution (Kolmogorov–Smirnov test). Baseline characteristics were compared using chi-square test for categorical variables and the Student test T or Mann–Whitney test for continuous variables. Kaplan–Meier curves were built to show the survival rate after COVID-19 according to illness severity. The SPSS version 20.0 (Software Statistical Package for the Social Science) was used for statistical analysis.
RESULTS
Patient Characteristics
Forty HT recipients tested positive for SARS-CoV-2 between April 5, 2020 and January 5, 2021 and were included in this case series. Twenty-four patients (60%) were men; the median age was 53 (42–60) y old; most of them were white (80%) and Chagas disease was the main etiology of previous cardiomyopathy (38%). Comorbidities related to worse prognosis in patients with COVID-19, such as hypertension (35%) and diabetes (25%), were common in this population. At admission, immunosuppressive therapy included tacrolimus in 60% and sodium mycophenolate in 83% of patients and steroids were part of immunosuppressive therapy for 88% of them. The median time of transplant at the moment of infection diagnosis was about 33 mo. Table 1 gives detailed information on patients` features and immunosuppressive treatment.
TABLE 1.
Baseline characteristics, clinical, and laboratory data at admission of heart transplant recipients with COVID-19: all cohort (n = 40) and hospitalized patients (n = 33)
| Characteristics | All cohort (n = 40) | Hospitalized patients (n = 33) |
|---|---|---|
| Male, n (%) | 24 (60) | 18 (54.5) |
| Age (y) | 53 (42–60) | 53 (46–60) |
| White, n (%) | 32 (80) | 25 (75.8) |
| Etiology | ||
| Ischemic, n (%) | 2 (5) | 2 (6.1) |
| Chagas, n (%) | 16 (40) | 12 (36.4) |
| Idiopathic, n (%) | 1 (2.5) | 1 (3) |
| Valvar, n (%) | 10 (25) | 10 (30.3) |
| Other, n (%) | 9 (22.5) | 6 (18.2) |
| Comorbidities | ||
| Hypertension, n (%) | 14 (35) | 13 (39.4) |
| Diabetes, n (%) | 10 (25) | 9 (27.3) |
| Obesity, n (%) | 2 (5) | 2 (6.1) |
| Chronic obstructive pulmonary disease, n (%) | 2 (5) | 2 (6.1) |
| Chronic renal failure, n (%) | 15 (37.5) | 13 (39.4) |
| Immunosuppressive medication at admission | ||
| Calcineurin inhibitor, n (%) | ||
| Tacrolimus, n (%) | 24 (60) | 19 (57.6) |
| Cyclosporine, n (%) | 15 (37.5) | 13 (39.4) |
| Antimetabolite, n (%) | ||
| Sodium mycophenolate, n (%) | 33 (82.5) | 27 (81.8) |
| Azathioprine, n (%) | 4 (10) | 3 (9.1) |
| Mechanistic target of rapamycin inhibitor, n (%) | ||
| Sirolimus, n (%) | 1 (2.5) | 1 (3.0) |
| Everolimus, n (%) | 0 | 0 |
| Steroids, n (%) | 35 (87.5) | 31 (93.9) |
| Prednisone, n (%) | 33 (82.5) | 29 (87.9) |
| Hydrocortisone, n (%) | 1 (2.5) | 1 (3.0) |
| Methylprednisolone, n (%) | 1 (2.5) | 1 (3.0) |
| Previous pulse therapy (last 3 mo), n (%) | 9 (22.5) | 9 (27.3) |
| Time of transplant (median [IQR]), mo | 33.7 (2.0–70.8) | 22.3 (1.7–64.6) |
| Clinical data | ||
| Tomographic result | ||
| Normal, n (%) | 3 (7.5) | 2 (6.1) |
| Unspecific changes | 7 (17.5) | 6 (18.2) |
| <50%, n (%) | 23 (57.5) | 21 (63.6) |
| ≥50%, n (%) | 4 (10) | 4 (12.1) |
| Nosocomial transmission, n (%) | 12 (30) | 12 (36.4) |
| Symptoms | ||
| Fever, n (%) | 18 (45) | 14 (42.4) |
| Headache, n (%) | 20 (50) | 14 (42.4) |
| Respiratory symptoms, n (%) | 26 (65) | 22 (66.7) |
| Dyspnea, n (%) | 16 (40) | 13 (39.4) |
| Anosmia, n (%) | 2 (5) | 1 (3) |
| Dysgeusia, n (%) | 6 (15) | 3 (9.1) |
| Gastrointestinal symptoms, n (%) | 11 (27.5) | 9 (27.3) |
| Thrombosis at admission, n (%) | 2 (5) | 2 (6.1) |
| Venous thrombosis or thromboembolism, n (%) | 1 (2.5) | |
| Arterial thrombosis, n (%) | 1 (2.5) | |
| Left ventricular dysfunction at admission n% (n = 38) | 5 (12.5) | 5 (15.2) |
| Left ventricular ejection fraction (%) (n = 38) | 60 (52–64) | 60 (55–62) |
| Systolic blood pressure (mm Hg) | 120 (110–130) | 120 (109–132) |
| Diastolic blood pressure (mm Hg) | 80 (70–86) | 80 (70–85) |
| Heart rate (bpm) | 96 (88–104) | 97 (87–105) |
| Oxygen saturation (%) | 95 (89–96) | 93(89–97) |
| Laboratory data (at admission) | ||
| Lymphocytes count (per mm3) | 796 (589–1226) | 707 (552–904) |
| D-dimer (ng/mL) | 1035 (447–2067) | 1251 (497–2157) |
| Urea (mg/dL) | 61 (44–82) | 64 (44–91) |
| Creatinine (mg/dL) | 1.72 (1.18–2.08) | 1.88 (1.23–2.27) |
| High sensitivity C-reactive protein (mg/dL) | 56 (19–104) | 68 (36–118) |
Continuous values are presented as median (interquartile range) and categorical ones as n (%).
COVID-19, coronavirus disease 2019; IQR, interquartile range.
Clinical Presentation at Admission
Respiratory symptoms were the most common symptoms reported (65%), including dyspnea in 40% of patients. Interestingly, thrombosis was the first manifestation of infection in 2 HT recipients, one of them with deep venous thrombosis and the other with aortic and renal artery thrombosis. Radiological changes were reported in 67.5% of patients and left ventricular dysfunction in 5 patients (12.5%) at admission. Laboratory data at admission revealed low lymphocytes count (796 [589–1226]/mm3) and high levels of D-dimer (1035 [447–2067] ng/mL). Laboratory data at admission are depicted in Table 1. Nosocomial transmission occurred in 12 patients (30%) and half of them had severe disease.
In this case series, 33 (82.5%) patients were hospitalized. The baseline characteristics of hospitalized patients may be seen in Table 1. The mean time of hospitalization was 15 (7–43) d. During hospitalization, recorded laboratory data revealed that lymphocytes count nadir was (376 [186–601]/mm3) and D-dimer peak was (2146 [684–5837] ng/mL); the peak of urea (101 [59–233] mg/dL), creatinine (2.00 [1.63–3.43] mg/dL), and high sensitivity C-reactive protein (123 [72–194] mg/dL) levels were also computed (Table S1, SDC, http://links.lww.com/TP/C200).
Noteworthy, 2 patients presented COVID-19 pneumonia and allograft rejection simultaneously. The first presented himself at the hospital with respiratory symptoms and severe biventricular dysfunction. Endomyocardial biopsy showed a grade 2 cellular rejection without evidence of SARS-CoV-2 virus in the myocardial tissue by the immunohistochemical analysis and PCR test. human leucocyte antigen (HLA) donor-specific antibodies were not detected, but different non-HLA antibodies were present at admission. After treatment with intravenous corticosteroids, thymoglobulin, intravenous immunoglobulin, and plasmapheresis, the non-HLA antibodies disappeared, the inotrope was withdrawn, and the patient was discharged. The second patient was hospitalized because of the heart transplantation surgery, which had been performed 20 d before. He was submitted to routine endomyocardial biopsy, which showed a grade 2 cellular rejection. On the same day of biopsy, he presented a temperature spike, thorax cardiac tomography scan showed ground glass opacities and his nasopharyngeal swab was positive to COVID-19. There was also no evidence of COVID-19 myocarditis on immunohistochemical analysis, neither on PCR test.
Treatment Approach
The majority of HT recipients received antibiotics (82%), 1 was treated with hydroxychloroquine, and no one received tocilizumab. Immunosuppressive therapy was reduced or withdrawn in most of them. Thirty-one (78%) HT recipients had at least 1 class of drugs withdrawn; among hospitalized patients, 91% had immunosuppressive therapy withdrawn. Calcineurin inhibitors were withdrawn in 24 patients (60%) and antimetabolite drugs were stopped in 28 patients (70%). Intravenous steroid therapy (hydrocortisone; methylprednisolone, or dexamethasone) was used in 27 cases (67.5% of all cohort) and 3 patients used 2 different types of intravenous steroids during hospitalization. Inotropes were needed in 5 (12.5%) and vasopressors in 13 (32.5%) patients (Table 2).
TABLE 2.
Pharmacological therapy and clinical outcomes during follow-up after COVID-19 (n = 40)
| Follow-up | All cohort (n = 40) |
|---|---|
| Median time of follow-up, d | 162 (46–257) |
| Pharmacological therapy | |
| Immunosuppressive therapy withdrawn, n (%) | 31 (78) |
| Calcineurin inhibitor | 24 (60) |
| Antimetabolite | 28 (70) |
| Mechanistic target of rapamycin inhibitor | 1 (2.5) |
| Intravenous steroids use, n (%) | 27 (67.5) |
| Hydrocortisone, n (%) | 14 (35) |
| Methylprednisolone, n (%) | 13 (32) |
| Dexamethasone, n (%) | 3 (7.5) |
| Antibiotics use, n (%) | 32 (80) |
| Hydroxychloroquine, n (%) | 1 (2.5) |
| Tocilizumab, n (%) | 0 |
| Therapeutic anticoagulation, n (%) | 11 (27.5) |
| Prophylactic anticoagulation, n (%) | 16 (40) |
| Vasoactive drug use, n (%) | |
| Inotrope | 5 (12.5) |
| Vasopressor | 13 (32.5) |
| Outcomes | |
| Ordinal scale for clinical improvement | |
| Mild disease (score < 5) | 23 (57.5) |
| Score ≤ 3 (outpatient or hospitalized, no oxygen therapy) | 19 (47.5) |
| Score = 4 (hospitalized, oxygen by mask or nasal prongs) | 4 (10) |
| Severe disease (score ≥ 5) | 17 (42.5) |
| Score = 5 (hospitalized, noninvasive ventilation or high-flow oxygen) | 4 (10) |
| Score = 6 (hospitalized, mechanical ventilation) | 0 (0) |
| Score = 7 (hospitalized, mechanical ventilation, organ support such as inotropes, vasopressors, intraaortic balloon pump or ECMO) | 2 (5.0) |
| Score = 8 (death) | 11 (27.5) |
| Hospitalization, n (%) | 33 (82.5) |
| Post–COVID-19 diagnosis hospitalization time, d (n = 33) | 15 (7–43) |
| Intensive care unit hospitalization | 17 (42.5) |
| Noninvasive ventilation or high-flow oxygen | 6 (15) |
| Invasive mechanical ventilation | 9 (22.5) |
| Hemodynamic instability (shock) | 11 (27.5) |
| Intraaortic balloon pump need | 3 (7.5) |
| Extracorporeal membrane oxygenation | 0 |
| Acute allograft rejection, n (%) | 4 (10) |
| Thrombotic events, n (%) | 5 (12.5) |
| Arterial thrombosis | 2 (5) |
| Venous thrombosis or pulmonary thromboembolism | 3 (7.5) |
| 30-d mortality, n (%) | 5 (12.5) |
| Overall mortality, n (%) | 11 (27.5) |
| Mortality cause | |
| Severe acute respiratory syndrome, n (%) | 3 (7.5) |
| Septic shock, n (%) | 6 (15) |
| Hemorrhagic shock, n (%) | 1 (2.5) |
| Sudden death (cardiac allograft vasculopathy), n (%) | 1 (2.5) |
| Time to death (d) | 34 (13–48) |
Continuous values are presented as median (interquartile range) and categorical ones as n (%).
COVID-19, coronavirus disease 2019; ECMO, extracorporeal membrane oxygenation.
Characteristics According to Illness Severity During the First 30 d of Follow-up
Twenty-three patients (57.5%) required no oxygen therapy or oxygen by mask/nasal cannula and were classified as mild disease. Seventeen other patients (43.5%) were classified as severe disease as depicted in Table S2, SDC, http://links.lww.com/TP/C200.
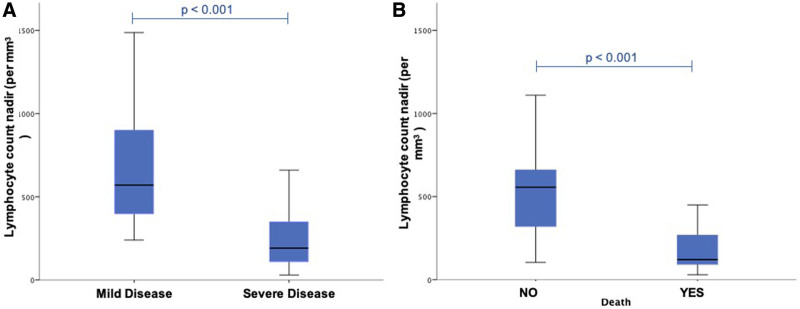
There was no difference in baseline characteristics, immunosuppressive therapy, and clinical presentation at admission of HT recipients who had developed severe or mild disease. Calcineurin inhibitors, antimetabolites, and mechanistic target of rapamycin inhibitors were withdrawn or reduced in 94% of patients with severe disease and 65% of patients with mild disease (P = 0.030). Intravenous steroid therapy use was more frequent in patients with severe disease (94% versus 48%; P = 0.002). Also, the median time of hospitalization was longer in patients with severe disease (35 [16–50] d) than in mild disease (9 [5–14] d), P < 0.001. At admission, patients with severe disease tended to have higher levels of creatinine (2.02 [1.63–2.53] versus 1.50 [1.09–1.94] mg/dL, P = 0.011). During 30-d follow-up, patients with severe disease showed higher median levels of urea (233 [103–295] versus 63 [48–95] mg/dL, P < 0.001) and creatinine (3.29 [2.00–4.03] versus 1.73 [1.18–2.08] mg/dL, P < 0.001). They also presented lower median absolute lymphocytes count (191 [107–375] versus 570 [387–920] /mm3, P < 0.001). Detailed information may be seen in Table S2, SDC, http://links.lww.com/TP/C200. Figure 1A depicts the absolute lymphocyte count nadir in HT recipients who developed mild and severe disease. Also, the midterm follow-up showed greater survival rates among patients with mild disease when compared to patients with severe disease (log rank test, P < 0.0001) as depicted in Kaplan-Meier curves in Figure S1B, SDC, http://links.lww.com/TP/C200.
FIGURE 1.
Absolute lymphocyte count and outcomes in heart transplant recipients with COVID-19. Absolute lymphocyte count nadir in heart transplanted recipients who developed mild and severe disease (A) and who died or not (B). COVID-19, coronavirus disease 2019.
Outcomes
All HT recipients were followed for at least 30 d after the diagnosis of COVID-19. Seventeen (43%) patients required intensive care unit (ICU) admission; 6 (15%) required noninvasive ventilation or high-flow oxygen and 9 (23%) required mechanical ventilation. Hemodynamic instability was observed in 11 patients (28%), who received inotropes or/and vasopressors. Intraaortic balloon pump was required in 3 patients (7.5%). Thromboembolic events were reported in 5 patients: 2 with deep venous thrombosis; 1 with pulmonary thromboembolism; 2 with arterial thrombosis; 1 with aortic and right renal artery thrombosis; and another 1 with stroke. Acute rejection was reported in 4 (10%) patients.
Five HT recipients (12.5%) died in 30 d of follow-up and 6 more patients died until January 5, 2021 (overall mortality of 27.5%). The median time to death was 34 (13–48) d. All deaths occurred in patients classified as having severe disease at admission (n = 11; 65%). Three patients died because of severe acute respiratory syndrome (10%) associated with COVID-19; 6 patients (15%) died because of septic shock, 2 of them because of carbapenem-resistant Enterobacteriaceae; 1 patient died because of hemorrhagic shock and another one, who had previous diagnosis of cardiac allograft vasculopathy, died after discharge from sudden death. Figure S1, SDC, http://links.lww.com/TP/C200 depicts the Kaplan-Meier overall survival rate curves after COVID-19 for all HT recipient’s cohort (A) and also according to illness severity (B).
During in-hospital follow-up, patients who died tended to have higher levels of creatinine peak (3.6 [3.0–4.3] versus 1.9 [1.3–2.1] mg/dL, P < 0.001) and urea peak (256 [161–305] versus 75 [50–102] mg/dL, P < 0.001) and lower absolute lymphocyte count nadir (121 [73–325] versus 556 [310–710]/mm3, P < 0.001) when compared to patients who have survived. Figure 1B depicts the absolute lymphocyte count nadir in HT recipients during follow-up.
DISCUSSION
In this case series of HT recipients infected by SARS-CoV-2 from a high-volume transplant center, 30-d mortality was 12.5%. In a longer follow-up, with median time of 162 d, the overall mortality was 27.5%. Similar to other cohorts, lymphopenia was a marker of disease severity.12,15 Median time to death was 34 d, and septic shock was the cause of death in 6 of 11 patients (54%). Also, cardiac allograft rejection and ventricular disfunction rates were also not negligible: 10% and 12.5%, respectively. To the best of our knowledge, this is the longest follow-up in HT recipients reported to date.
Mortality of SOT recipients infected by SARS-CoV-2 has ranged from 3% to 50% in different case series.18-30 Heterogeneity of patient populations, threshold for testing, follow-up duration, and overburden of healthcare resources probably account for these differences. Now, larger multicenter cohorts have shown more consistent mortality rates around 20%–30%.12-15 In these studies, main drivers of mortality and severe infections were age, comorbidities, overweight, and dyspnea at presentation.12-15 In addition, similar to the general population, lymphopenia has also been independently associated with higher mortality.4,5 This is particularly relevant for SOT recipients, since most of them already have lymphopenia due to antimetabolite drugs use. For instance, in our series median admission lymphocyte count was 796 (589–1226) per mm3 compared to admission lymphocyte of 1100 (1100–1200) per mm3 in a large meta-analysis of COVID-19 in general population.5 In most studies of SOT recipients with COVID-19, including our own, antimetabolite drugs were frequently held at presentation.10,11 On the other hand, multicenter cohorts have reported calcineurin inhibitors withdrawn in only 36.5%, 28.7%, and 23%, and of patients, respectively.13-15 In our series, calcineurin inhibitors were suspended in 78% of patients at admission. A novel finding from our study was the not negligible rates of cardiac allograft rejection (10%) and ventricular dysfunction (12.5%). This finding must be considered with caution: a single-center case series precludes us to make definitive casual inferences. However, it generates the hypothesis that too aggressive immunosuppression reduction may not be innocuous for SOT recipients because of allograft dysfunction and rejection. In addition, maintaining some level of immunosuppression could protect during progression to the hyperinflammation phase.10,11 These questions should be addressed in larger studies. Furthermore, the fact that 2 patients presented simultaneously with COVID-19 and rejection episodes raises the question whether the immune dysregulation caused by SARS-COV-2 itself could be a trigger for rejection episodes.
Another important finding from our study was nosocomial bacterial infections as important drivers of mortality (6 of 11 patients, 15% of all cohorts). Although bacterial coinfections are not a prominent feature of COVID-19 at presentation, secondary bacterial infections have been found in 23% of SOT recipients and up to 45% of SOT recipients admitted to ICUs.9,31 In addition, in a case-control study, COVID-19 was shown to increase the risk of ICU-acquired bloodstream infections especially after 7 d of ICU admission.32 In our study, median length of hospital stay was 15 d and 42.5% of patients developed severe disease requiring ICU admission, which could explain these high rates of nosocomial infections. Although some transplant centers have recommended prophylactic use of antibiotics in high-risk patients, the benefits must be balanced with the harms of increasing antimicrobial resistance.9,33 In our report, for example, 2 patients died because of septic shock caused by carbapenem-resistant Enterobacteriaceae.
Some limitations of the present study should be highlighted. First, it is a single-center observational case series, which precludes definitive causality assumptions. In addition, despite being a large transplant center, our results reflect outcomes of a specific patient population and may not apply to different populations. Furthermore, the small number of patients included may undermine the external validation of our results. On the other hand, we present detailed information of HT recipients with COVID-19 from one of the hotspots in Brazil. We also report long-term follow-up, late causes of death and allograft rejection rates.
In summary, in this case series, in the longest follow-up, we found an overall mortality of 27.5% with a median time to death of 35 d. Markers of disease severity corroborate other cohorts’ findings. In addition, midterm complications such as nosocomial infections and allograft failure were significant. These findings suggest the need for strict midterm follow-up of these patients.
Supplementary Material
Footnotes
The authors declare no funding or conflicts of interest.
F.G.M.-B. was involved with study design and ethical approval; F.G.M.-B., C.M.M., D.S.P.B., R.C.T.D., M.T.S.S.L., and C.A.S.A. contributed to patient data acquisition; F.G.M.-B. and R.F.S. made the statistical analysis; F.G.M.-B. and C.M.M. wrote the manuscript; and R.F.S., S.M., L.F.B.C.S., I.W.C., M.S.A., M.V.O.B., F.B.A.d.S., T.M.V.S., F.A.G., and F.B. revised the manuscript.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO coronavirus disease (COVID-19) dashboard. 2021. Available at https://covid19.who.int/. Accessed February 19, 2021. [PubMed]
- 3.Ministério da Saúde. Painel coronavirus. 2021. Available at https://covid.saude.gov.br/. Accessed February 19, 2021.
- 4.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, Huang DQ, Zou B, et al. Epidemiology of COVID-19: a systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J Med Virol. 2021;93:1449–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman JR, Baan CC, Bromberg J, et al. COVID-19: a year on. Transplantation. 2021;105:1–3. [DOI] [PubMed] [Google Scholar]
- 7.Horby P, Lim WS, Emberson JR, et al. ; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azzi Y, Bartash R, Scalea J, et al. COVID-19 and solid organ transplantation: a review article. Transplantation. 2021;105:37–55. [DOI] [PubMed] [Google Scholar]
- 10.Avery RK. COVID-19 therapeutics for solid organ transplant recipients; 6 months into the pandemic: where are we now? Transplantation. 2021;105:56–60. [DOI] [PubMed] [Google Scholar]
- 11.Zaidan M, Legendre C. Solid organ transplantation in the era of COVID-19: lessons from France. Transplantation. 2021;105:61–66. [DOI] [PubMed] [Google Scholar]
- 12.Kates OS, Haydel BM, Florman SS, et al. COVID-19 in solid organ transplant: a multi-center cohort study. Clin Infect Dis. 2021;73:e4090–e4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coll E, Fernández-Ruiz M, Sánchez-Álvarez JE, et al. COVID-19 in transplant recipients: the Spanish experience. Am J Transplant. 2020.00:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caillard S, Anglicheau D, Matignon M, et al. ; French SOT COVID Registry. An initial report from the French SOT COVID Registry suggests high mortality due to COVID-19 in recipients of kidney transplants. Kidney Int. 2020;98:1549–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cravedi P, Mothi SS, Azzi Y, et al. COVID-19 and kidney transplantation: results from the TANGO International Transplant Consortium. Am J Transplant. 2020;20:3140–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO R&D Blueprint. Novel Coronavirus COVID-19. Therapeutic trial synopsis. 2020. Available at https://www.who.int/publications/i/item/covid-19-therapeutic-trial-synopsis. Accessed August 28, 2020.
- 18.Latif F, Farr MA, Clerkin KJ, et al. Characteristics and outcomes of recipients of heart transplant with coronavirus disease 2019. JAMA Cardiol. 2020;5:1165–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tschopp J, L’Huillier AG, Mombelli M, et al. ; Swiss Transplant Cohort Study (STCS). First experience of SARS-CoV-2 infections in solid organ transplant recipients in the Swiss Transplant Cohort Study. Am J Transplant. 2020;20:2876–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20:1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernández-Ruiz M, Andrés A, Loinaz C, et al. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant. 2020;20:1849–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crespo M, Pérez-Sáez MJ, Redondo-Pachón D, et al. COVID-19 in elderly kidney transplant recipients. Am J Transplant. 2020;20:2883–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alberici F, Delbarba E, Manenti C, et al. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020;97:1083–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akalin E, Azzi Y, Bartash R, et al. COVID-19 and kidney transplantation. N Engl J Med. 2020;382:2475–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yi SG, Rogers AW, Saharia A, et al. Early experience with COVID-19 and solid organ transplantation at a US high-volume transplant center. Transplantation. 2020;104:2208–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ali T, Al-Ali A, Fajji L, et al. Coronavirus disease-19: disease severity and outcomes of solid organ transplant recipients: different spectrums of disease in different populations? Transplantation. 2021;105:121–127. [DOI] [PubMed] [Google Scholar]
- 27.Felldin M, Søfteland JM, Magnusson J, et al. Initial report from a Swedish high-volume transplant center after the first wave of the COVID-19 pandemic. Transplantation. 2021;105:108–114. [DOI] [PubMed] [Google Scholar]
- 28.Kutzler HL, Poulos CM, Cheema F, et al. COVID-19 in solid organ transplant recipients: observations from Connecticut. Transplantation. 2021;105:e6–e8. [DOI] [PubMed] [Google Scholar]
- 29.Bottio T, Bagozzi L, Fiocco A, et al. COVID-19 in heart transplant recipients: a multicenter analysis of the Northern Italian outbreak. JACC Heart Fail. 2021;9:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rivinius R, Kaya Z, Schramm R, et al. COVID-19 among heart transplant recipients in Germany: a multicenter survey. Clin Res Cardiol. 2020;109:1531–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26:1622–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buetti N, Ruckly S, de Montmollin E, et al. COVID-19 increased the risk of ICU-acquired bloodstream infections: a case-cohort study from the multicentric OUTCOMEREA network. Intensive Care Med. 2021;47:180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clancy CJ, Buehrle DJ, Nguyen MH. PRO: COVID-19 pandemic will result in increased antimicrobial resistance rates. JAC-Antimicrobial Resistance. 2020;2:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.