Background.
The clinical effectiveness of vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in immunosuppressed solid organ and islet transplant (SOT) recipients is unclear.
Methods.
We linked 4 national registries to retrospectively identify laboratory-confirmed SARS-CoV-2 infections and deaths within 28 d in England between September 1, 2020, and August 31, 2021, comparing unvaccinated adult SOT recipients and those who had received 2 doses of ChAdOx1-S or BNT162b2 vaccine. Infection incidence rate ratios were adjusted for recipient demographics and calendar month using a negative binomial regression model, with 95% confidence intervals. Case fatality rate ratios were adjusted using a Cox proportional hazards model to generate hazard ratio (95% confidence interval).
Results.
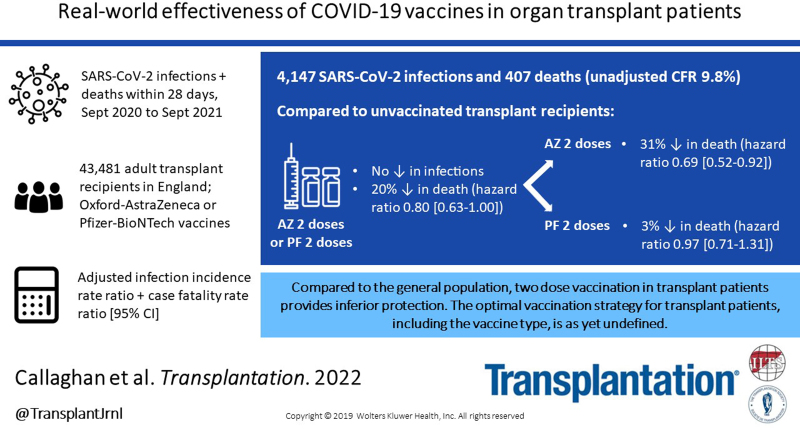
On August 31, 2021, it was found that 3080 (7.1%) were unvaccinated, 1141 (2.6%) had 1 vaccine dose, and 39 260 (90.3%) had 2 vaccine doses. There were 4147 SARS-CoV-2 infections and 407 deaths (unadjusted case fatality rate 9.8%). The risk-adjusted infection incidence rate ratio was 1.29 (1.03-1.61), implying that vaccination was not associated with reduction in risk of testing positive for SARS-CoV-2 RNA. Overall, the hazard ratio for death within 28 d of SARS-CoV-2 infection was 0.80 (0.63-1.00), a 20% reduction in risk of death in vaccinated patients (P = 0.05). Two doses of ChAdOx1-S were associated with a significantly reduced risk of death (hazard ratio, 0.69; 0.52-0.92), whereas vaccination with BNT162b2 was not (0.97; 0.71-1.31).
Conclusions.
Vaccination of SOT recipients confers some protection against SARS-CoV-2–related mortality, but this protection is inferior to that achieved in the general population. SOT recipients require additional protective measures, including further vaccine doses, antiviral drugs, and nonpharmaceutical interventions.
INTRODUCTION
The ongoing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has been responsible for >5 million deaths worldwide. The impact on solid organ transplantation has been particularly marked, with many countries reporting significantly fewer organ donors and organ transplants.1-3 Furthermore, organ transplant recipients are more likely to die after SARS-CoV-2 infection than the general population.4
It is hoped that effective vaccines may mitigate the risk of coronavirus disease 2019 (COVID-19)–associated morbidity and mortality in solid organ and islet transplant (SOT) recipients, though the systemic immunosuppression necessary to prevent allograft rejection impairs immune responses to vaccination.5,6 Thus far, data on SARS-CoV-2 vaccine clinical efficacy in immunosuppressed solid organ transplant recipients are lacking, since this patient group was not included in early trials.7,8 Many studies examining anti–SARS-CoV-2 spike protein antibody and T-lymphocyte responses after vaccination in transplant recipients show suboptimal immune responses compared with nontransplant patients,9-13 though some vaccines seem to be more immunogenic than others.14-17 More recently, reports have emerged on serological responses following a third or fourth vaccine dose.18-20
Although assays of humoral and cell-mediated responses to SARS-CoV-2 vaccines in transplant recipients have been informative, determining vaccine effectiveness (VE) in the real world using clinically relevant outcomes is essential to accurately guide vaccination and antiviral treatment policies in this vulnerable population. Several studies have reported instances of vaccine breakthrough infections in transplant recipients.21,22 Our group has reported on early, unadjusted national registry analyses showing lower death rates after SARS-CoV-2 infection in vaccinated as compared with unvaccinated transplant recipients.23
This risk-adjusted national registry study aimed to determine real-world VE in SOT recipients during a period of rising rates of SARS-CoV-2 infection in the United Kingdom. Two key outcomes were analyzed; incidence of testing positive for SARS-CoV-2 RNA and risk of death within 28 d following a positive test for SARS-CoV-2. Furthermore, VE was compared between the 2 most widely used vaccines in the United Kingdom, Pfizer-BioNTech BNT162b2 (BNT162b2) and Oxford University-AstraZeneca ChAdOx1-S (ChAdOx1-S). It should be noted our study period predates the Omicron variant–fueled surge in the United Kingdom (commenced in December 2021) and therefore it is not known whether study conclusions can be extrapolated to this new variant.
MATERIALS AND METHODS
Study Cohort and Outcome Definitions
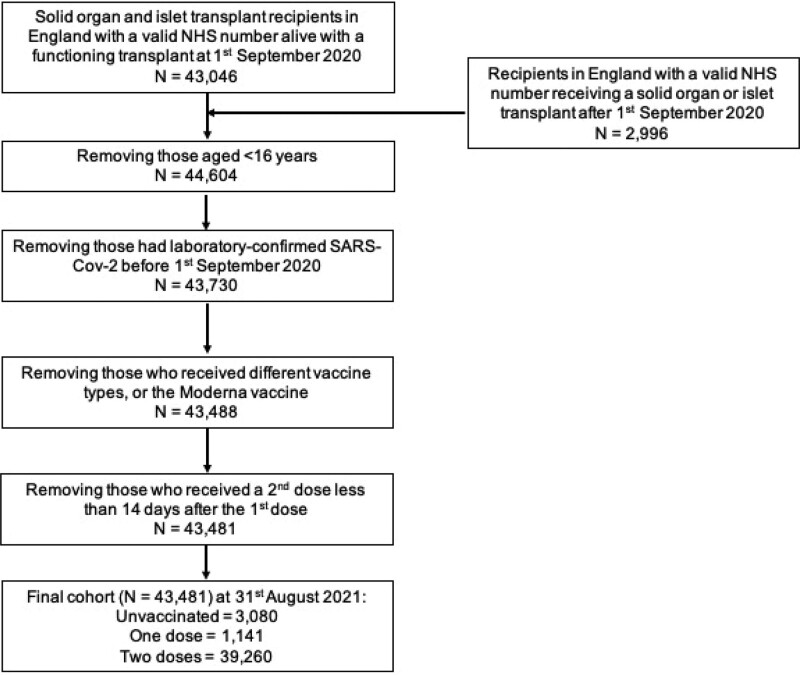
The at-risk cohorts of interest were all patients aged 16 y or more, resident in England, who were a SOT recipient from a deceased or live donor with a functioning graft on September 1, 2020, and those transplanted between that date and August 31, 2021. Outcomes of interest were the date of laboratory-confirmed SARS-CoV-2 infection and patient death within 28 d of a positive test. Cohorts were followed until August 31, 2021, for new SARS-Cov-2 infections and September 28, 2021, for patient survival to allow for 28-d exposure. This study period covered an era when there was unrestricted access to testing for SARS-CoV-2 in the United Kingdom.
We only included those patients who received BNT162b2 or ChAdOx1-S vaccines. Recipients of any other vaccine type as a first or second dose or those who received a different vaccine type as a second dose (ie, a heterologous schedule) were excluded because of small numbers and the inability to attribute any protective effect to a particular vaccine. Patients who received >2 doses of the BNT162b2 or ChAdOx1-S vaccines (or those who received 2 doses of those vaccines and then received a third dose of a different vaccine) were censored at the time of their third dose. Recipients were also excluded if they received a second vaccine dose <14 d after their first dose. Individuals with laboratory-confirmed SARS-CoV-2 infection before September 1, 2020, were excluded, as were recipients of tissue or non–islet cell transplants (eg, cornea, sclera, bone, hematopoietic stem cells, hepatocytes), SOT recipients who were unmatched in the National Immunisation Management Service register, and those without a valid National Health Service (NHS) number, a unique identifier for all patients in England for care provided by the NHS. Those who had >1 episode of laboratory-confirmed SARS-CoV-2 infection during the study period had outcomes analyzed for the first infection only.
Throughout the study, vaccination status was defined as: “unvaccinated” if the recipient had not received any vaccine dose during the study period or was ≤14 d after a first vaccine dose, “1 dose” if >14 d after the first dose and ≤14 d after a second dose, and “2 doses” if >14 d after a second dose.8
Study Design and Data Sources
This was a national retrospective cohort study enabled by linkage of 4 national registries in England. National Health Service Blood and Transplant (NHSBT) holds the United Kingdom Transplant Registry on all patients in receipt of a SOT in the United Kingdom. The UK Health Security Agency (UKHSA, previously known as Public Health England) centrally collects and reports on all those living in England with laboratory-confirmed SARS-CoV-2 infection under the Health Protection Regulations 2010. The National Immunisation Management Service is a centralized service for the management of the SARS-CoV-2 vaccination program and records all citizens living in England who qualify for and receive vaccination against SARS-CoV-2. These data are shared with UKHSA. Finally, the NHS Digital Tracing Service records the vital status of all patients under the care of the NHS in England. The NHS Digital Tracing Service records date of death and does not collect information on cause of death.
Merger of the at-risk cohorts identified from the NHSBT data set with the UKHSA database was performed using 2 unique identifiers (NHS number and date of birth). The merged NHSBT and UKHSA data set was securely linked with the NHS Digital Tracing Service using 3 unique identifiers (NHS number, date of birth, and sex). The final data set therefore had near real-time complete mortality information on all SOT recipients in England who tested positive for SARS-CoV-2 RNA with vaccination status. In line with publications related to this pandemic, death within 28 d of laboratory-confirmed SARS-CoV-2 RNA detection was assumed to be because of COVID-19.
Vaccine roll-out for the general population in the United Kingdom, including SOT recipients, was as described previously.23,24 Recipients were not given a choice of vaccine, and, in the overwhelming majority, second doses were of the same type as the first dose. Vaccine type was determined by local availability and, other than prioritization for early receipt, SOT recipients were not assigned any particular vaccine type. National policy mandated a 10- to 12-wk gap between first and second vaccine doses, irrespective of vaccine type.24,25
SARS-CoV-2 variant information was not available for patients testing positive, though the majority of infections in England between September 2020 and May 2021 were because of the Alpha (B.1.1.7) variant and from May 2021 onward were because of the Delta (B.1.617.2) variant.26 The number of SARS-CoV-2 RNA–positive tests in the general English population was obtained from the UK Government (https://coronavirus.data.gov.uk).
Statistical Analyses
Demographic characteristics (type of organ received, time since transplant, sex, age, ethnicity, and NHS region) were summarized, stratified by vaccination status. Differences in characteristics between groups of SOT recipients were tested univariately using the chi-square test.
To account for the possibility that reducing intensity of immunosuppression and outpatient visits over time might impact on the risk of acquiring infection or subsequent mortality, 3 risk periods (<90 d, 90 d–1 y, and >1 y posttransplant) were arbitrarily selected a priori.
Incidence rates of laboratory-confirmed SARS-CoV-2 RNA detection were calculated for unvaccinated and vaccinated individuals. To minimize temporal bias and to take into account variations in community prevalence of SARS-CoV-2 infections during the study period, incidence rate was defined as the number of events divided by the person-time at risk, stratified by the 6 demographic characteristics above as well as calendar month and, in some analyses, vaccine type (BNT162b2 or ChAdOx1-S). A negative binomial regression model was used to derive incidence rate ratios (IRRs) with 95% confidence intervals (CIs) adjusted for the variables above; this approach was used because it is better suited when overdispersion of variance is identified, rather than the traditional Poisson regression method.27 IRR is the incidence rate in vaccinated recipients divided by the incidence rate for unvaccinated recipients. An IRR result of <1 would indicate reduced risk, whereas a result >1 indicates increased risk of testing positive for SARS-CoV-2 in vaccinated patients. VE in reducing the incidence of testing positive for SARS-CoV-2 was calculated as (1 – IRR) × 100. Only data from June 1, 2021, to August 31 were used for these analyses to enable comparisons over a time period where there were sufficient numbers of both unvaccinated and vaccinated individuals.
Insufficient mortality events were observed between June 1, 2021, and August 31, 2021, to undertake a similar approach to analyze death data. Therefore, the time period for the mortality analyses was broadened to include September 1, 2020, to August 31, 2021. Unadjusted Kaplan-Meier estimates of patient survival from the day of laboratory-confirmed SARS-CoV-2 RNA positive test were stratified by vaccination status and vaccine type and were compared using the log-rank test. A Cox proportional hazards model was used to estimate the hazard ratio of risk of death after laboratory-confirmed SARS-CoV-2 RNA–positive test, adjusting for type of organ received, time since transplant, sex, age, ethnicity, NHS region, vaccination status, and, in some analyses, vaccine type. VE in reducing the risk of death within 28 d of testing positive for SARS-CoV-2 was calculated as (1 – hazard ratio) × 100.
Analyses were undertaken using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Ethical Approval
NHSBT is reliant on the General Data Protection Regulation Article 6(1)(e) – Performance of a public task. Under Article 9(2)(h), (i), and (j), NHSBT is allowed to use patient identifiable information for service evaluation and safety monitoring without the consent of patients.
RESULTS
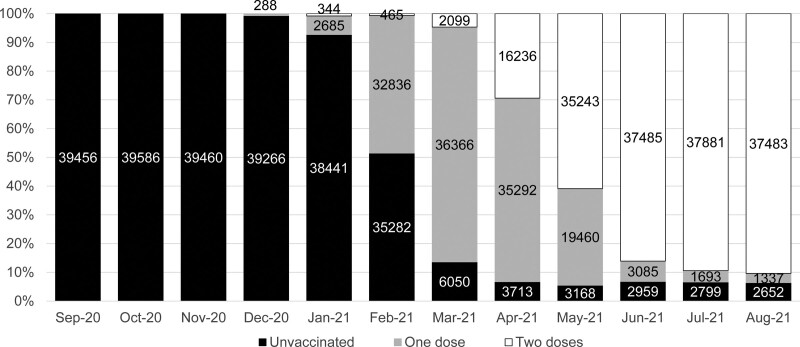
On August 31, 2021, it was found that 3080 (7.1%) SOT recipients meeting study criteria were unvaccinated, 1141 (2.6%) had 1 vaccine dose, and 39 260 (90.3%) had 2 vaccine doses, according to the definitions above (Figure 1). Vaccination in SOT recipients began in late December 2020, with the majority of patients having received 2 doses by June 2021 (Figure 2). The median (interquartile range) interval between first and second vaccine doses was 77 (70–79) d, with no difference between vaccine types (data not shown).
FIGURE 1.
Study inclusion and exclusion criteria and patient flow. NHS, National Health Service; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
FIGURE 2.
Percentage of unvaccinated and vaccinated solid organ and islet transplant recipients through the study period. Numbers are shown on the graph. Recipients can be counted more than once in each month as they receive a vaccine. Numbers in each month don’t add up to summary data by the end of the study date because of patient deaths, incident transplants, and censoring.
Demographic characteristics of unvaccinated SOT recipients and those that had received 1 or 2 doses at August 31 are shown in Table 1. The proportion of SOT recipients that received 2 doses was similar between males and females and time since transplant. Double vaccination rates were lower in recipients of intestinal and multiorgan transplants, younger patients, in non-White recipients, and in those living in London.
TABLE 1.
Demographic characteristics of unvaccinated and vaccinated solid organ and islet transplant recipients at August 31, 2021
| Variable | Vaccination status | ||||||
|---|---|---|---|---|---|---|---|
| Unvaccinated | One dose | Two doses | |||||
| N | % | N | % | N | % | P | |
| Total | 3080 | 7.1 | 1141 | 2.6 | 39 260 | 90.3 | |
| Transplant type | 0.01 | ||||||
| Kidneya | 2146 | 6.9 | 770 | 2.5 | 28 016 | 90.6 | |
| SPKb | 110 | 6.6 | 40 | 2.4 | 1518 | 91 | |
| Liver | 588 | 7.8 | 218 | 2.9 | 6747 | 89.3 | |
| Heart | 127 | 6.8 | 58 | 3.1 | 1690 | 90.1 | |
| Lungc | 83 | 7 | 44 | 3.7 | 1062 | 89.3 | |
| Intestinal and multiorgand | 26 | 9.8 | 11 | 4.2 | 227 | 86 | |
| Ethnicity | <0.0001 | ||||||
| Asian | 472 | 9.5 | 189 | 3.8 | 4318 | 86.7 | |
| Black | 478 | 19.4 | 119 | 4.8 | 1868 | 75.8 | |
| Other | 126 | 10.6 | 41 | 3.4 | 1025 | 86 | |
| Unknown | 120 | 5.5 | 52 | 2.4 | 2002 | 92.1 | |
| White | 1884 | 5.8 | 740 | 2.3 | 30 047 | 92 | |
| Age (y) | <0.0001 | ||||||
| 16–49 | 1368 | 9.3 | 465 | 3.2 | 12 908 | 87.6 | |
| 50+ | 1712 | 6 | 676 | 2.4 | 26 352 | 91.7 | |
| Sex | 0.13 | ||||||
| Male | 1913 | 7.2 | 697 | 2.6 | 23 848 | 90.1 | |
| Female | 1167 | 6.9 | 444 | 2.6 | 15 412 | 90.5 | |
| Time from transplant | 0.52 | ||||||
| <90 d | 51 | 7.7 | 22 | 3.3 | 593 | 89 | |
| 90 d–1 y | 126 | 6.4 | 95 | 4.8 | 1752 | 88.8 | |
| >1 y | 2903 | 7.1 | 1024 | 2.5 | 36 915 | 90.4 | |
| Region | <0.0001 | ||||||
| East of England | 302 | 5.8 | 107 | 2 | 4817 | 92.2 | |
| London | 868 | 11.3 | 252 | 3.3 | 6533 | 85.4 | |
| Midlands | 502 | 6.4 | 213 | 2.7 | 7151 | 90.9 | |
| North East and Yorkshire | 424 | 6.2 | 161 | 2.3 | 6287 | 91.5 | |
| North West | 390 | 7.5 | 159 | 3.1 | 4648 | 89.4 | |
| South East | 369 | 5.7 | 146 | 2.3 | 5946 | 92 | |
| South West | 225 | 5.3 | 103 | 2.4 | 3878 | 92.2 | |
aIncludes single kidney, en-bloc kidney, and double kidney transplants.
bSimultaneous pancreas-kidney transplants, including pancreas only, islet only, and simultaneous islet-kidney transplants.
cIncludes single lung, double lung, partial lung, and heart-lung transplants.
dIncludes if any other organ was transplanted with the intestine as well as liver-kidney, heart-lung-liver, liver-pancreas, heart-kidney, heart-liver, and lung-liver transplants.
SPK, simultaneous pancreas-kidney transplant.
Of those who had 2 vaccine doses, 22 788 (58%) received ChAdOx1-S vaccines and 16 472 (42%) received BNT162b2 (Supplementary Data Table 1, SDC, http://links.lww.com/TP/C360). Vaccination with ChAdOx1-S was more frequent in liver, heart, and lung recipients and also varied by region and ethnicity.
SARS-CoV-2 Infections
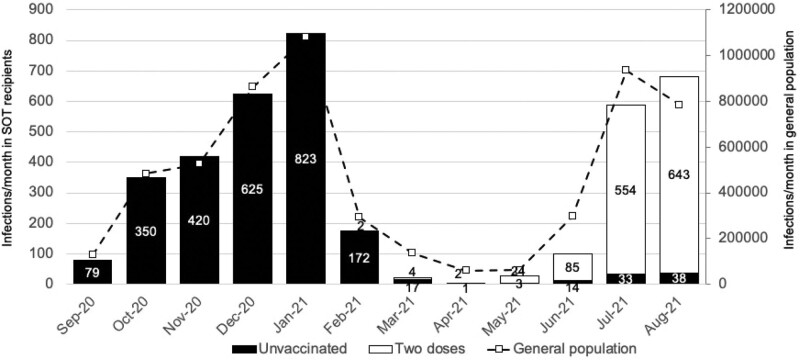
Cases of laboratory-confirmed SARS-CoV-2 infections peaked in January and again in August 2021 (Figure 3), coinciding with Alpha and Delta variant surge periods in the national population. The first peak occurred in unvaccinated patients; the second predominantly affected those who had received 2 vaccine doses. Overall, there were 4147 cases of first SARS-CoV-2 infections in SOT recipients within the study period. Demographic characteristics of recipients with SARS-CoV-2 infections are shown in Table 2, with data by vaccine type shown in Supplementary Data Table 2 (SDC, http://links.lww.com/TP/C360). The median (interquartile range) interval between the second vaccine dose (defined above) and SARS-CoV-2 infection was 90 (69–109) d (Supplementary Data Figure 1, SDC, http://links.lww.com/TP/C360; BNT162b2 95 [76–118] d versus ChAdOx1-S 86 [65–105] d).
FIGURE 3.
Number of laboratory-confirmed SARS-CoV-2 infections per month in solid organ and islet transplant recipients by vaccination status, compared with the general population in England, September 1, 2020, to August 31, 2021. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOT, solid organ and islet transplant.
TABLE 2.
Demographic characteristics of solid organ and islet transplant recipients with laboratory-confirmed SARS-CoV-2 infection and death within 28 d, by vaccination status
| Variable | Unvaccinated | One vaccine dose | Two vaccine doses | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Deaths | Cases | Deaths | Cases | Deaths | P | ||||
| N | N | % | N | N | % | N | N | % | ||
| Total | 2575 | 269 | 10.4 | 258 | 30 | 11.6 | 1314 | 108 | 8.2 | |
| Transplant type | 0.52 | |||||||||
| Kidneya | 1985 | 209 | 10.5 | 191 | 22 | 11.5 | 960 | 78 | 8.1 | |
| SPKb | 84 | 7 | 8.3 | 10 | 0 | 0 | 61 | 2 | 3.3 | |
| Liver | 370 | 29 | 7.8 | 33 | 4 | 12.1 | 174 | 12 | 6.9 | |
| Heart | 72 | 9 | 12.5 | 12 | 2 | 16.7 | 74 | 5 | 6.8 | |
| Lungc | 58 | 15 | 25.9 | 8 | 1 | 12.5 | 41 | 11 | 26.8 | |
| Intestinal and multiorgand | 6 | 0 | 0 | 4 | 1 | 25 | 4 | 0 | 0 | |
| Ethnicity | 0.10 | |||||||||
| Asian | 567 | 57 | 10.1 | 61 | 9 | 14.8 | 167 | 15 | 9 | |
| Black | 214 | 30 | 14 | 16 | 2 | 12.5 | 79 | 10 | 12.7 | |
| Other | 82 | 7 | 8.5 | 11 | 1 | 9.1 | 24 | 0 | 0 | |
| Unknown | 114 | 14 | 12.3 | 9 | 0 | 0 | 50 | 4 | 8 | |
| White | 1598 | 161 | 10.1 | 161 | 18 | 11.2 | 994 | 79 | 7.9 | |
| Age group (y) | 0.22 | |||||||||
| 16–49 | 1019 | 30 | 2.9 | 96 | 2 | 2.1 | 593 | 17 | 2.9 | |
| 50+ | 1556 | 239 | 15.4 | 162 | 28 | 17.3 | 721 | 91 | 12.6 | |
| Sex | 0.96 | |||||||||
| Male | 1540 | 175 | 11.4 | 157 | 20 | 12.7 | 799 | 70 | 8.8 | |
| Female | 1035 | 94 | 9.1 | 101 | 10 | 9.9 | 515 | 38 | 7.4 | |
| Time from transplant | 0.02 | |||||||||
| <90 d | 76 | 14 | 18.4 | 8 | 1 | 12.5 | 12 | 0 | 0 | |
| 90 d–1 y | 144 | 12 | 8.3 | 18 | 0 | 0 | 62 | 2 | 3.2 | |
| >1 y | 2355 | 243 | 10.3 | 232 | 29 | 12.5 | 1240 | 106 | 8.5 | |
| Region | 0.26 | |||||||||
| East of England | 220 | 38 | 17.3 | 22 | 2 | 9.1 | 128 | 10 | 7.8 | |
| London | 608 | 50 | 8.2 | 41 | 6 | 14.6 | 192 | 13 | 6.8 | |
| Midlands | 533 | 50 | 9.4 | 62 | 10 | 16.1 | 246 | 26 | 10.6 | |
| North East and Yorkshire | 378 | 37 | 9.8 | 56 | 5 | 8.9 | 298 | 23 | 7.7 | |
| North West | 383 | 43 | 11.2 | 43 | 5 | 11.6 | 206 | 16 | 7.8 | |
| South East | 322 | 34 | 10.6 | 21 | 2 | 9.5 | 131 | 12 | 9.2 | |
| South West | 131 | 17 | 13 | 13 | 0 | 0 | 113 | 8 | 7.1 | |
aIncludes single kidney, en-bloc kidney, and double kidney transplants.
bSimultaneous pancreas-kidney transplants, including pancreas only, islet only, and simultaneous islet-kidney transplants.
cIncludes single lung, double lung, partial lung, and heart-lung transplants.
dIncludes if any other organ was transplanted with the intestine as well as liver-kidney, heart-lung-liver, liver-pancreas, heart-kidney, heart-liver, and lung-liver transplants.
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SPK, simultaneous pancreas-kidney transplant.
During the Delta variant dominant period between June and August 2021, the incidence rate of SARS-CoV-2 infection was 34.4 per 100 000 person-days for unvaccinated and 39.2 per 100 000 person-days for vaccinated SOT recipients (Table 3). After risk adjustment, the overall IRR (95% CI) was 1.29 (1.03-1.61), indicating that vaccination was not associated with reduction in risk of testing positive for SARS-CoV-2. When vaccine type was analyzed, neither vaccine showed a protective effect from SARS-CoV-2 infection (ChAdOx1-S: IRR, 1.37 [1.10-1.72]; BNT162b2: IRR, 1.18 [0.93-1.48]). Risk-adjusted IRRs by demographic variable are shown in Supplementary Data Table 3 (SDC, http://links.lww.com/TP/C360).
TABLE 3.
SARS-CoV-2 infection incidence rates and risk-adjusted incidence rate ratios in solid organ and islet transplant recipients, June 1, 2021, to August 31, 2021
| Unvaccinated | Two vaccine doses | ||||||
|---|---|---|---|---|---|---|---|
| Cases | Incidence rate per 100 000 person-days | Cases | Incidence rate per 100 000 person-days | Risk-adjusted incidence rate ratio (95% CI) | P | Vaccine efficacy (95% CI) | |
| Total | 85 | 34.4 | 1283 | 39.2 | 1.29 (1.03-1.61) | 0.02 | –29% (–61 to –3) |
| By vaccine type | |||||||
| AZ | 793 | 41.9 | 1.37 (1.10-1.72) | 0.006 | –37% (–72 to –10) | ||
| PF | 490 | 35.5 | 1.18 (0.93-1.48) | 0.17 | –18% (–48 to 7) | ||
AZ, Oxford University-AstraZeneca ChAdOx1-S; CI, confidence interval; PF, Pfizer-BioNTech BNT162b2; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Deaths Within 28 d of a Positive SARS-CoV-2 Test
Of the 4147 SOT recipients with laboratory-confirmed SARS-CoV-2 infection, 407 (9.8%) died within 28 d. Demographic characteristics of recipients dying after SARS-CoV-2 infection are shown in Table 2, with graphical representation of unadjusted case fatality rates in Supplementary Data Figure 2 (SDC, http://links.lww.com/TP/C360). Overall, of those who were unvaccinated, 10.4% (269/2575) died within 28 d of SARS-CoV-2 infection, compared with 8.2% (108/1314) of SOT recipients who had received 2 doses of vaccine. Unadjusted case fatality rates were high in unvaccinated recipients <90 d from transplant (14/76; 18.4%).
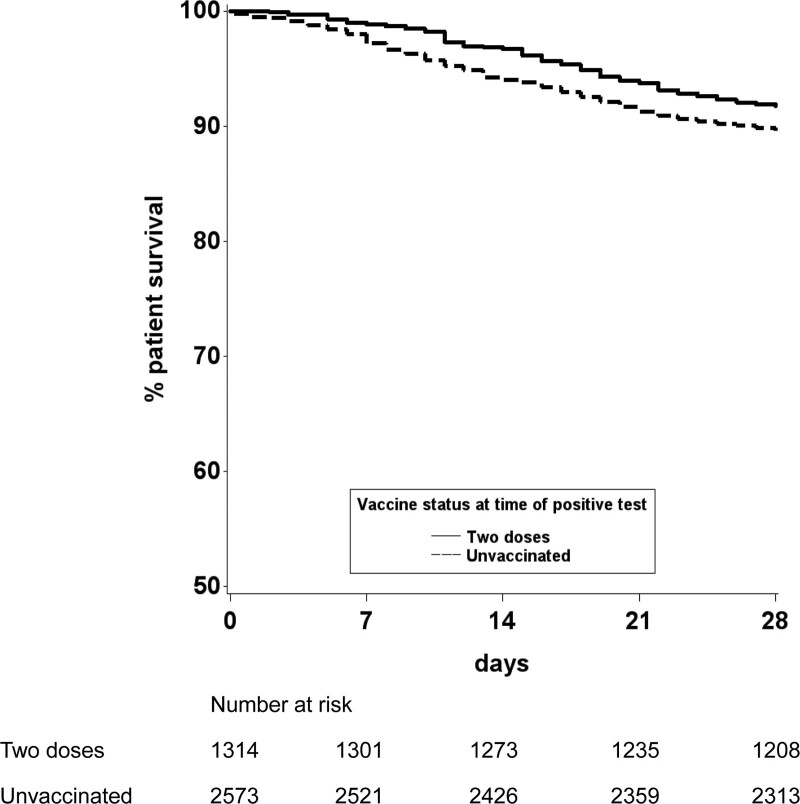
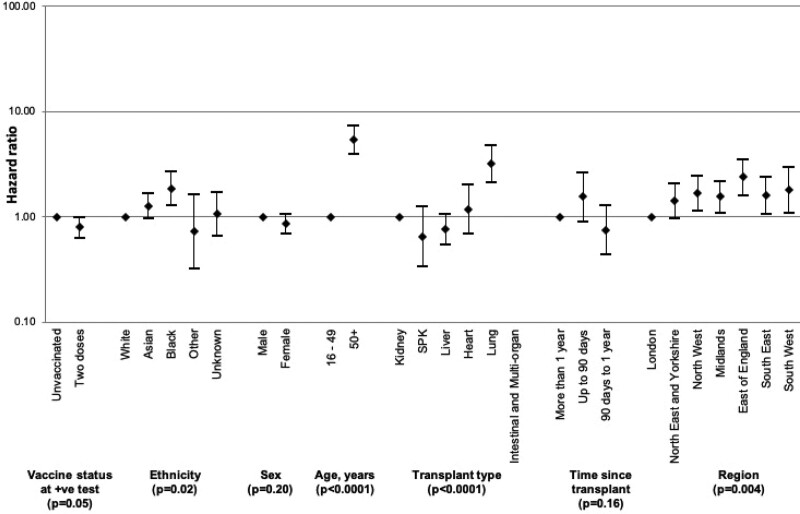
Patient survival from the day of SARS-CoV-2 laboratory diagnosis were plotted using an unadjusted Kaplan-Meier analysis, stratifying by vaccination status (Figure 4). This showed that vaccinated recipients had a higher chance of survival at 28 d when compared with those that were unvaccinated (91.8% versus 88.8%; P = 0.0028). After risk adjustment, a statistically significantly increased chance of death within 28 d of SARS-CoV-2 infection was found in those that were age ≥50 y, of Black ethnicity, a recipient of a lung transplant, and those living outside London except for the North East of England and Yorkshire (Figure 5; Supplementary Data Table 4, SDC, http://links.lww.com/TP/C360). Overall, the hazard ratio for death within 28 d of SARS-CoV-2 infection was 0.80 (0.63-1.00), a 20% reduction in risk of death in vaccinated patients (P = 0.05).
FIGURE 4.
Patient survival from date of laboratory-confirmed SARS-CoV-2 infection, by vaccination status. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
FIGURE 5.
Hazard ratios with 95% confidence intervals of risk of death within 28 d of laboratory-confirmed SARS-CoV-2 infection in solid organ and islet transplant recipients, by vaccination status and demographic characteristics. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SPK, simultaneous pancreas-kidney transplant.
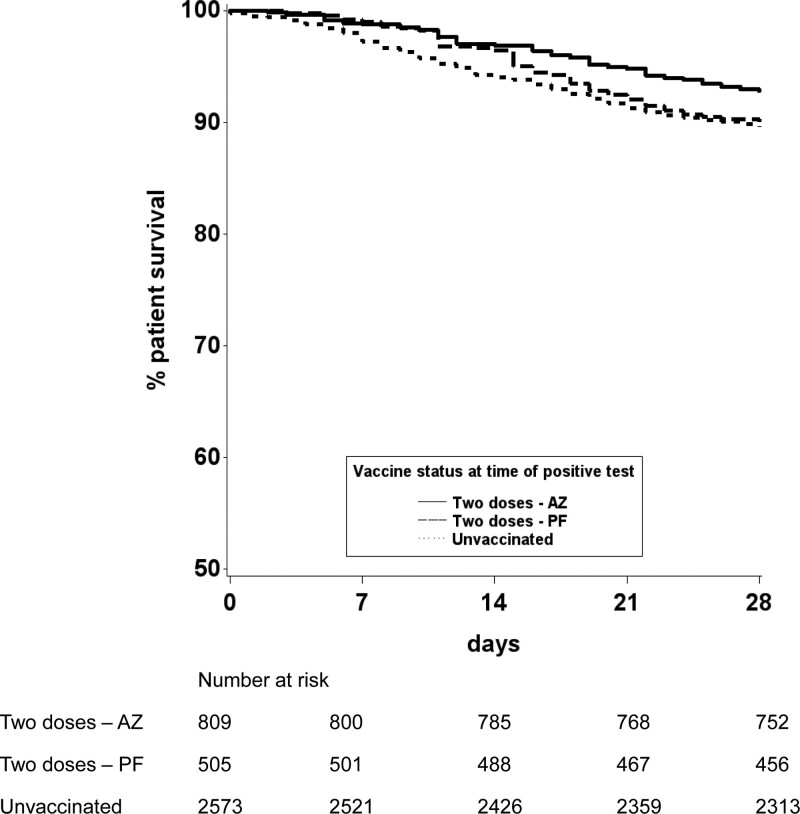
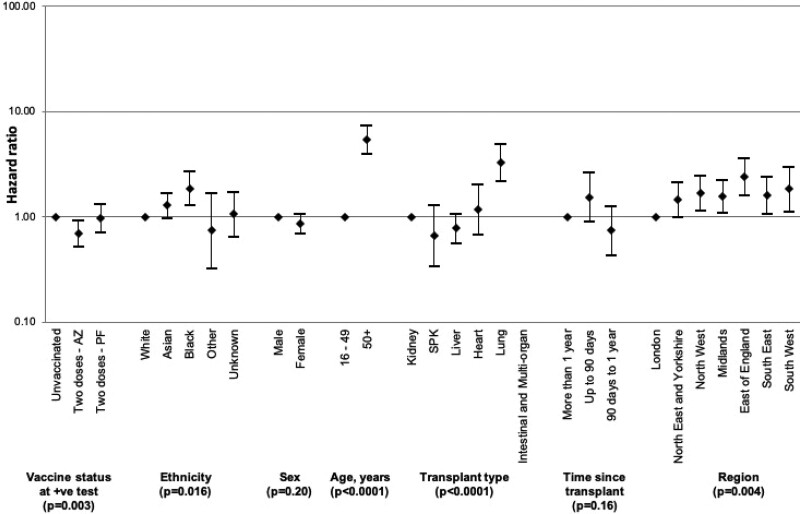
Differences in effectiveness between vaccine types were also investigated with unadjusted and risk-adjusted analyses. Kaplan-Meier survival curves suggested that rates of death within 28 d following SARS-CoV-2 infection were lower after vaccination with 2 doses of ChAdOx1-S versus those receiving BNT162b2 (7.2% versus 9.9%; Figure 6). Inclusion of vaccine type as a variable in the Cox model showed that those SOT recipients who had been vaccinated with 2 doses of the BNT162b2 vaccine had no statistically significant protective effect from death within 28 d of SARS-CoV-2 infection when compared with those who were unvaccinated (hazard ratio, 0.97; 95% CI, 0.71-1.31; Figure 7; Supplementary Data Table 5, SDC, http://links.lww.com/TP/C360). Recipients who had been vaccinated with 2 doses of the ChAdOx1-S vaccine had a hazard ratio (95% CI) for death of 0.69 (0.52-0.92), indicating a 31% reduction in risk of death compared with unvaccinated recipients. Restricting analyses to patient inclusion from December 1, 2020 (coinciding with the Alpha variant surge and the start of vaccine roll-out in the United Kingdom) did not significantly change these findings (data not shown).
FIGURE 6.
Patient survival from date of laboratory-confirmed SARS-CoV-2 infection, by vaccination status and vaccine type. AZ, Oxford University-AstraZeneca ChAdOx1-S; PF, Pfizer-BioNTech BNT162b2; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
FIGURE 7.
Hazard ratios with 95% confidence intervals of risk of death within 28 d of laboratory-confirmed SARS-CoV-2 infection in solid organ and islet transplant recipients, by vaccination status, vaccine type, and demographic characteristics. AZ, Oxford University-AstraZeneca ChAdOx1-S; PF, Pfizer-BioNTech BNT162b2; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SPK, simultaneous pancreas-kidney transplant.
DISCUSSION
In these first, national registry-based analyses, we describe real-world, risk-adjusted VE in SOT recipients following 2 widely used SARS-CoV-2 vaccines. Compared with unvaccinated SOT patients, a 2-dose homologous vaccine course does not appear to reduce the risk of testing positive for SARS-CoV-2. ChAdOx1-S, but not BNT162b2, 2-dose vaccine course was associated with a reduction in risk of death in SOT recipients who tested positive for SARS-CoV-2. Older age, Black ethnicity, lung transplantation, and care location were associated with a higher risk of death. In patients who had received 2 doses of the vaccine, the median time to test positive was 90 d.
Both Israel and the United Kingdom have reported VE for the general population, with both countries deploying early national vaccination programs and benefitting from linked national registries to identify cases in unvaccinated and vaccinated citizens. In Israel, a 2-dose program in the general population, with a 3-wk gap between doses using the BNT162b2 vaccine, was associated with 95% and 96% reduction in risk of infection and death, respectively, during an Alpha variant–dominant period.19 In the United Kingdom, a 2-dose program with a 12-wk gap between doses, predominantly with ChAdOx1-S or BNT162b2 vaccines, was reported as showing 79% reduction in risk of infection during the Alpha-dominant and 67% risk reduction during the Delta variant–dominant periods,28 with the extended dosing schedule associated with superior VE.25 Lopez Bernal et al29 reported a modest reduction in VE against the Delta variant in a test-negative, case–control study in the general UK population. Direct comparisons of VE between different studies are challenging since VE wanes with time and at variable rates in different populations, and therefore, follow-up period and demographic characteristics must be considered. However, the 60% to 90% reduction in the risk of SARS-CoV-2 infection demonstrated in the vaccinated general population, even taking into account the Delta variant and the issues above, is in stark comparison with the apparent absence of protection from infection seen in double-vaccinated SOT recipients in our analysis.
The majority of the study population received their second vaccine dose by June 2021, and most of the infections in the vaccinated cohort occurred approximately 90 d later, coinciding with relaxation of nationally mandated nonpharmaceutical interventions (NPIs) such as the use of face-coverings in public places. A possible reason for the apparent increased incidence of infection in vaccinated versus unvaccinated SOT recipients may be risk compensation with reduced adherence to NPI in the vaccinated population.30 Modeling has shown that risk compensation is likely to disproportionately reduce VE in high-risk populations.31
UKHSA reported on >90% protection against mortality with both ChAdOx1-S and BNT162b2 vaccines during both Alpha and Delta variant surges in the United Kingdom.32 This compares with the 31% reduction in mortality risk in SOT recipients seen only with the ChAdOx1-S vaccine in our study. These results indicate that vaccinated SOT recipients, compared with the general population, have significantly less protection against SARS-CoV-2 infection or mortality. However, it is important to emphasize that SOT recipients who received 2 vaccine doses had a better chance of survival compared with unvaccinated SOT recipients. Further doses may increase VE in this patient population, but until this can be demonstrated, SOT recipients may be advised to adhere to NPI to minimize risk, though we recognize the significant psychosocial and economic consequences of stringent NPI. Given the low VE and high case fatality rates in SOT recipients, this patient group should be considered for priority treatment with novel antiviral agents,33-35 according to up-to-date evidence.
We note the risk associations with demographic and organ-type variables. Although the association with lung transplantation may be intuitively explained from a biological perspective as the target organ of the virus is the allograft, within the available data set, we are unable to speculate on the underlying medical or socioeconomic bases for other associations. Similarly, Hippisley-Cox et al36 have shown that COVID-19–related deaths in vaccinated patients in the general UK population are higher in non-White versus White ethnic groups, and in those aged >50 y, even after adjustment for many other variables. Of note, we did not find that time posttransplant was independently associated with increased risk of death.
Our study has the strengths of scale and minimal ascertainment bias. The IRR and hazard ratio methodologies significantly reduce the risk of temporal bias influencing the results and provides a more accurate estimate of VE. Linkage of the 4 national registries that host data on immunization, infection, organ transplantation, and survival allows near real-time complete identification of new SARS-CoV-2 infections or related mortality in this patient cohort. Inclusion and comprehensive follow-up of the entire at-risk population in the country provides an accurate effect estimate when comparing vaccinated versus unvaccinated SOT patients in the real world and is therefore likely to be translatable to similar patient populations in other countries.
Because of the registry-based retrospective methodology, it is not possible to account for asymptomatic infections that were not laboratory confirmed in any of the cohorts. Similar to published VE trials, it is not possible to disaggregate the protective influence of NPI from any vaccine-derived protection. The implementation, adherence, and subsequent relaxation of UK government–mandated NPI varied significantly between September 2020 and August 2021. The study period also covers wild-type (September–November 2020), Alpha (December 2020–May 2021), and Delta (June–August 2021) variant population surge periods in the United Kingdom, and therefore, there is difficulty in controlling for differing infection and mortality risks associated with these variants. We aimed to assess VE in the transplant waitlisted population in England for the purposes of this study but were unable to complete the analysis because of insufficient events in this relatively small patient cohort. We were not able to analyze VE by immunosuppressant regimen because of incomplete data. Finally, it is not possible to rule out residual confounding because of factors that we were unable to control for or were unknown to the study team.
Adenovirus vector and mRNA vaccines promote substantially different innate responses that can lead to different adaptive immune responses.37 A previous report has described stronger serological responses to the BNT162b2 vaccine compared with ChAdOx1-S in SOT recipients, with similar T-cell responses.16 Studies of other immunocompromised cohorts have similarly detailed stronger antispike protein immunoglobulin G responses following BNT162b2 vaccination when compared with ChAdOx1-S.38 Our finding that only the ChAdOx1-S vaccine was associated with a statistically significant reduction in risk of death is therefore unexpected and merits closer consideration. It is possible that our study was underpowered to detect a protective effect of the BNT162b2 vaccine in this study cohort or that those with additional risk factors for COVID-19–related death that we were unable to adjust for were more likely to receive the BNT162b2 vaccine. It seems unlikely that transplant recipients receiving the BNT162b2 vaccine exhibited different behavioral risk modifications than those receiving the ChAdOx1-S vaccine, and the demographics of the populations receiving the 2 vaccines were similar (Supplementary Data Table 1, SDC, http://links.lww.com/TP/C360). One possible explanation is that stronger T-cell immunity in ChAdOx1-S–vaccinated individuals offers protection even in the absence of a detectable humoral response. Confirmation whether the survival advantage of ChAdOx1-S seen in our study is a true biological finding will require further research, including comparison with nontransplant immunosuppressed cohorts and longer follow-up in SOT patients in the United Kingdom and other countries.
Further research is needed to understand whether addition of further vaccine doses confers improved protection in SOT patients. Roll-out of a UK government–mandated third primary vaccine dose to this patient population with BNT162b2 or mRNA-1273 (Moderna) vaccines commenced in September 2021. With >80% of patients waitlisted for an organ transplant vaccinated in the United Kingdom, we expect to see an increasing number of this patient group receive a transplant soon. Analyzing this group may clarify whether pretransplant vaccination is associated with better protection from SARS-CoV-2 infection and death as compared to posttransplant vaccination.5 With the 4 linked registries in place, we aim to report on such outcomes in early summer 2022.
In conclusion, this first, national, real-world VE study in SOT patients demonstrates that 2 SARS-CoV-2 vaccine doses reduce risk of death from COVID-19 compared with unvaccinated SOT recipients, though the level of vaccine-enabled protection in SOT recipients is markedly less than that observed in the general population. In light of the above findings, the risk of vaccine escape predicted to be associated with the Omicron variant and pending evidence of the effectiveness of third and subsequent vaccine doses, NPI continues to be essential risk mitigation for SOT recipients along with other antiviral strategies.
ACKNOWLEDGMENTS
Members of the Organ and Tissue Donation and Transplantation Clinical Team other than the authors already named are Mr John Asher, FRCS; Dr Richard Baker, FRCP; Mr Marius Berman, FRCS; Ms Lisa Burnapp, MA; Mr Andrew Butler, FRCS; Mr John Casey, FRCS; Mr Ian Currie, FRCS; Dr Jan Dudley, FRCPCH; Dr Dale Gardiner, FFICM; Dr Dan Harvey, FFICM; Professor Derek Manas, FRCS; Dr Jasvir Parmar, FRCP; Professor Steven White, FRCS; and Mr Rajamiyer Venkateswaran, FRCS. All these authors are also affiliated to NHSBT. The authors would also like to acknowledge and thank colleagues in UKHSA and NHS Digital Tracing Service for sharing data, as well as James Thomas, Tariq Malik, Anne Marie O’Connell (UKHSA), and Richard Little (NHSBT) for enabling database linkages.
Supplementary Material
Footnotes
C.J.C. and L.M. are joint first authors.
J.L.R.F. and R.R. are joint senior authors.
A full list of members of the Organ and Tissue Donation and Transplantation Clinical Team is included under Acknowledgments.
C.J.C. and R.R. participated in research design, data analysis, and cowrote the article. L.M. and R.M.K.C. participated in research design, coled the data analysis, and participated in the writing of the article. S.V.W., H.W., N.A., J.L.B., I.U.-L., G.J.P., D.T., and J.L.R.F. participated in research design, data analysis, and the writing of the article.
The authors declare no funding or conflicts of interest.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Aubert O, Yoo D, Zielinski D, et al. COVID-19 pandemic and worldwide organ transplantation: a population-based study. Lancet Public Health. 2021;6:e709–e719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loupy A, Aubert O, Reese PP, et al. Organ procurement and transplantation during the COVID-19 pandemic. Lancet. 2020;395:e95–e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manara AR, Mumford L, Callaghan CJ, et al. Donation and transplantation activity in the UK during the COVID-19 lockdown. Lancet. 2020;396:465–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danziger-Isakov L, Kumar D; AST ID Community of Practice. Vaccination of solid organ transplant candidates and recipients: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33:e13563. [DOI] [PubMed] [Google Scholar]
- 6.Chong PP, Avery RK. A comprehensive review of immunization practices in solid organ transplant and hematopoietic stem cell transplant recipients. Clin Ther. 2017;39:1581–1598. [DOI] [PubMed] [Google Scholar]
- 7.Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voysey M, Clemens SAC, Madhi SA, et al. ; Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sattler A, Schrezenmeier E, Weber UA, et al. Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J Clin Invest. 2021;131:150175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itzhaki Ben Zadok O, Shaul AA, Ben-Avraham B, et al. Immunogenicity of the BNT162b2 mRNA vaccine in heart transplant recipients—a prospective cohort study. Eur J Heart Fail. 2021;23:1555–1559. [DOI] [PubMed] [Google Scholar]
- 11.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rabinowich L, Grupper A, Baruch R, et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021;75:435–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crespo M, Barrilado-Jackson A, Padilla E, et al. Negative immune responses to two-dose mRNA COVID-19 vaccines in renal allograft recipients assessed with simple antibody and interferon gamma release assay cellular monitoring. Am J Transplant. [Epub ahead of print. September 22, 2021]. doi:10.1111/ajt.16854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stumpf J, Siepmann T, Lindner T, et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg Health Eur. 2021;9:100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt T, Klemis V, Schub D, et al. Cellular immunity predominates over humoral immunity after homologous and heterologous mRNA and vector-based COVID-19 vaccine regimens in solid organ transplant recipients. Am J Transplant. 2021;21:3990–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prendecki M, Thomson T, Clarke CL, et al. ; Imperial Renal COVID-19 vaccine study group in collaboration with the OCTAVE Study Consortium. Immunological responses to SARS-CoV-2 vaccines in kidney transplant recipients. Lancet. 2021;398:1482–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonald I, Murray SM, Reynolds CJ, et al. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. NPJ Vaccines. 2021;6:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamar N, Abravanel F, Marion O, et al. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall VG, Ferreira VH, Ku T, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385:1244–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alejo JL, Mitchell J, Chiang TP, et al. Antibody response to a fourth dose of a SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Transplantation. 2021;105:e280–e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ali NM, Alnazari N, Mehta SA, et al. Development of COVID-19 infection in transplant recipients after SARS-CoV-2 vaccination. Transplantation. 2021;105:e104–e106. [DOI] [PubMed] [Google Scholar]
- 22.Chenxi Song C, Christensen J, Kumar D, et al. Early experience with SARs-CoV-2 mRNA vaccine breakthrough among kidney transplant recipients. Transpl Infect Dis. 2021;23:e13654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravanan R, Mumford L, Ushiro-Lumb I, et al. ; OTDT Clinical Team. Two doses of SARS-CoV-2 vaccines reduce risk of death due to COVID-19 in solid organ transplant recipients: preliminary outcomes from a UK registry linkage analysis. Transplantation. 2021;105:e263–e264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.UK Department of Health & Social Care Joint Committee on Vaccination and Immunisation: advice on priority groups for COVID-19 vaccination, 30 December 2020. 2020. Available at https://www.gov.uk/government/publications/priority-groups-for-coronavirus-covid-19-vaccination-advice-from-the-jcvi-30-december-2020/joint-committee-on-vaccination-and-immunisation-advice-on-priority-groups-for-covid-19-vaccination-30-december-2020. Accessed December 8, 2021.
- 25.Amirthalingam G, Bernal JL, Andrews NJ, et al. Serological responses and vaccine effectiveness for extended COVID-19 vaccine schedules in England. Nat Commun. 2021;12:7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Twohig KA, Nyberg T, Zaidi A, et al. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study. Lancet Infect Dis. 2022;22:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Office of National Statistics. Coronavirus (COVID-19) infection survey technical article: impact of vaccination on testing positive in the UK, October 2021. 2021. Available at https://www.ons.gov.uk/releases/coronaviruscovid19infectionsurveytechnicalarticleimpactofvaccinationontestingpositiveintheukoctober2021. Accessed December 8, 2021.
- 29.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385:585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iyengar KP, Ish P, Botchu R, Jain VK, Vaishya R. Influence of the Peltzman effect on the recurrent COVID-19 waves in Europe. Postgrad Med J. [Epub ahead of print. April 29, 2021]. doi:10.1136/postgradmedj-2021-140234 [DOI] [PubMed] [Google Scholar]
- 31.Ioannidis JPA. Benefit of COVID-19 vaccination accounting for potential risk compensation. NPJ Vaccines. 2021;6:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.UK Health Security Agency. COVID-19 vaccine surveillance report. Week 47. 2021. Available at https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1036047/Vaccine_surveillance_report_-_week_47.pdf. Accessed December 8, 2021.
- 33.Sidebottom DB, Gill D. Ronapreve for prophylaxis and treatment of covid-19. BMJ. 2021;374:n2136. [DOI] [PubMed] [Google Scholar]
- 34.Mahase E. Covid-19: UK becomes first country to authorise antiviral molnupiravir. BMJ. 2021;375:n2697. [DOI] [PubMed] [Google Scholar]
- 35.U.S. Food and Drugs Administration. Coronavirus (COVID-19) update: FDA authorizes new long-acting monoclonal antibodies for pre-exposure prevention of COVID-19 in certain individuals. 2021. Available at https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-new-long-acting-monoclonal-antibodies-pre-exposure. Accessed December 15, 2021.
- 36.Hippisley-Cox J, Coupland CA, Mehta N, et al. Risk prediction of Covid-19 related death and hospital admission in adults after Covid-19 vaccination: national prospective cohort study. BMJ. 2021;374:n2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heinz FX, Stiasny K. Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines. 2021;6:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prendecki M, Clarke C, Edwards H, et al. Humoral and T-cell responses to SARS-CoV-2 vaccination in patients receiving immunosuppression. Ann Rheum Dis. 2021;80:1322–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.