Abstract
Health care provider recommendations are among the most important factors influencing parents’ decisions to vaccinate their adolescents. However, delivery of high-quality health care provider recommendations for vaccination is not universal. There is wide variation in the strength, timeliness and consistency of the delivery of recommendations for all adolescent vaccines. The factors that influence health care providers’ recommendations are multi-level and can be conceptualized in much the same way as vaccine acceptance among parents. Health care providers are influenced by their own attitudes and beliefs about a vaccine and also by the patient they are treating and by the community in which they practice as well as state and national level vaccine policy. We propose a multi-level framework for understanding the factors that influence health care providers’ recommendations at the individual, interpersonal and community level to both develop and adapt interventions to improve providers’ recommendations.
Keywords: Vaccines, Health care providers, Provider recommendations
Introduction
Immunizations are one of the most impactful public health interventions available, dramatically reducing morbidity and mortality due to infectious disease at all phases of life, including adolescence (CDC, 2020b; Centers for Disease Prevention & Control, 2011; Cohn & MacNeil, 2015; MacNeil et al., 2018; Marshall et al., 2019; Mbaeyi et al., 2019). Over the past 15 years, the Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP) has developed a robust set of vaccine recommendations for adolescents in the United States, including tetanus, diphtheria and acellular pertussis (Tdap) vaccine, meningococcal conjugate vaccine (MenACWY), meningococcal serogroup B vaccine (MenB), human papillomavirus vaccine (HPV), and a seasonal influenza vaccine (Grohskopf et al., 2020; Havers et al., 2020; Markowitz et al., 2014; Mbaeyi et al., 2020). The most recent addition in 2021 was a recommendation for adolescents (older than 12 years of age) to receive a COVID-19 vaccine (CDC, 2020a).
Since the implementation of vaccine recommendations during adolescence for Tdap and MenACWY in 2005 and HPV vaccine in 2006, coverage has climbed to approximately 90% for Tdap and MenACWY (Elam-Evans, 2020). Coverage for several adolescent vaccines lags the others. Only 72% of adolescents initiate the HPV vaccine series and even less complete the series (52%) (Elam-Evans, 2020). Coverage with the MenACWY booster and the seasonal influenza vaccine remains at about 50% (Elam-Evans, 2020). Coverage is far lower for MenB than that of the routinely recommended vaccines; only about one in four adolescents have received the MenB vaccine (Elam-Evans, 2020). In addition, coverage of all adolescent vaccines varies substantially by sociodemographic characteristics such as race/ethnicity, socioeconomic status, region and mother’s age and education (Amboree & Darkoh, 2020; Bednarczyk et al., 2019; Bernstein et al., 2016; Do et al., 2020; Elam-Evans, 2020; Kester et al., 2013; Niccolai et al., 2019; Rodriguez et al., 2020; Vu et al., 2020; Warner et al., 2017).
There is a need for a continued emphasis on understanding the drivers of uptake of vaccines by adolescents to improve coverage for all recommended vaccines. This is especially important at present; substantial disruptions in vaccine delivery have occurred in the past year due to the COVID-19 pandemic (Elam-Evans, 2020). A recent analysis from the CDC found that even following the lifting of stay at home orders and the return of in-person health care provider visits, HPV and Tdap vaccine administration was substantially lower compared to the equivalent periods in 2018 and 2019 (Patel, 2021). In addition, achieving high uptake of the COVID-19 vaccines in the adolescent population is needed to help reverse the current course of the pandemic.
Importance of health care provider recommendations
In this manuscript, we present a framework for understanding and improving the uptake of all adolescent vaccines through health care provider recommendations. A high-quality health care provider (HCP) recommendation for vaccination is an important predictor of vaccine receipt (Brewer, Chapman, et al., 2017; Brewer, Hall, et al., 2017; Oh et al., 2021). For the purposes of this discussion, a HCP is an individual in the clinical setting who may be involved in prescribing a vaccine including MDs, DOs, Physician Assistants (PA), Nurse Practitioners (NP) and other Advanced Practice Registered Nurses (APRN). Other clinical staff such as registered nurses (RN), medical assistants (MA), or front desk staff can also play an important role in vaccine promotion in the clinic; however, this is not the focus of this manuscript. A high-quality recommendation is defined as one that is strong (emphasizes the importance of vaccination), timely (recommends same day vaccination and age-appropriate vaccination) and consistent (recommended routinely for all eligible patients) (Gilkey et al., 2015, 2016). A meta-analysis by Oh et al. found that parents who received a provider recommendation were as much as ten times more likely to initiate HPV vaccination (Brewer, Chapman, et al., 2017; Brewer, Hall, et al., 2017; Gilkey & McRee, 2016; Oh et al., 2021). The role of provider recommendations has been much less extensively studied for other adolescent vaccines; however, there is evidence that receipt of a provider recommendation for the Tdap and MenACWY vaccines is also positively associated with a higher likelihood of having received the vaccine (Caldwell et al., 2020; Niccolai et al., 2019). The quality of the recommendation matters as well. In one study, parents who received a high-quality recommendation for the HPV vaccine (strong, timely and consistent) were more than nine times more likely to accept the vaccine for their child than parents who did not receive a recommendation. In comparison, parents who received a low quality recommendation were only four times as likely to accept the vaccine for their child (Gilkey et al., 2016).
Unfortunately, HCP do not universally recommend all adolescent vaccines and some may only recommend a limited number of vaccines or may not recommend any vaccines at all (Caldwell et al., 2020; Gilkey et al., 2015; Hopfer et al., 2019). In addition, even among providers who do recommend adolescent vaccines the quality of recommendation can vary substantially. In two independent national surveys of pediatricians on HPV recommendation practices, nearly 30% of respondents were categorized as “Ambivalent HPV Recommenders,” meaning that they were more likely to not recommend or to delay recommending the HPV vaccine to their patients, or that they reported that they did not strongly endorse the HPV vaccine (Gilkey et al., 2015; Hopfer et al., 2019). Further research is needed to understand what factors drive providers to recommend (or to not recommend) adolescent vaccines as well as to understand how to improve the quality of provider recommendations.
A multilevel framework for factors influencing provider recommendations
The vaccine decision making process among parents and patients is influenced by factors at multiple levels including the individual, interpersonal, community and policy levels (Brewer, Chapman, et al., 2017; Brewer, Hall, et al., 2017). Multi-level frameworks have been employed successfully in understanding the determinants of vaccine hesitancy (Betsch et al., 2018; Brewer, Chapman, et al., 2017; Brewer, Hall, et al., 2017; The SAGE Vaccine Hesitancy Working Group, 2013) and have also been applied to designing tiered interventions to improve vaccine uptake (Bednarczyk et al., 2018). We can shift the perspective to HCP and conceptualize the factors that influence provider recommendations in much the same way – HCPs are not only influenced by their own attitudes and beliefs about the vaccine but also by the parent and patient they are treating and the community and the environment in which they practice. We propose a multi-level framework for understanding the factors that influence provider recommendations that includes individual, interpersonal, community and policy levels that both interact with each other and influence the quality of provider recommendations (Fig. 1). To our knowledge this is the first application of such a framework to provider recommendations specifically. While HCP recommendations are not the only driver of vaccine uptake among adolescents, further examination of the factors that drive provider recommendations will help us strengthen intervention or intervention-components that focus on health care providers.
Fig. 1.
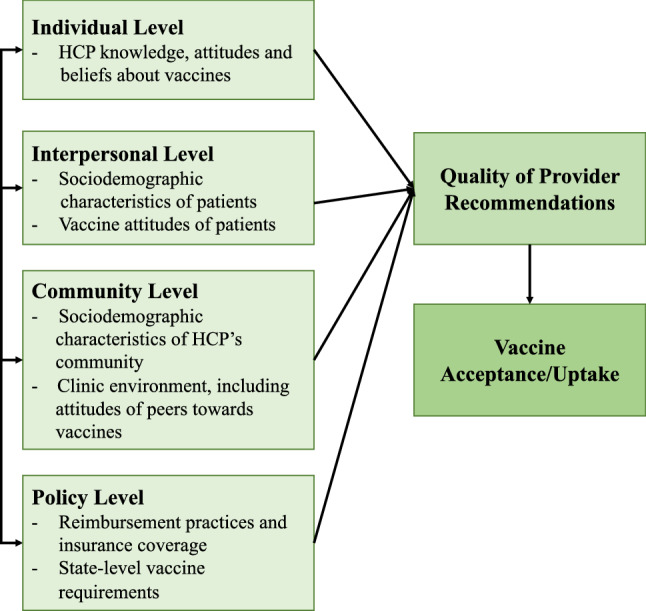
A multi-level framework for understanding the factors that influence provider recommendations of adolescent vaccines
Individual level factors
We define the “individual level” as factors that pertain to the HCPs knowledge, attitudes and beliefs regarding adolescent vaccines. Numerous studies have found that a perceived lack of knowledge among HCP about HPV vaccines can lead to a lack of confidence in discussing the vaccine (Farias et al., 2017; Leung et al., 2019; Suryadevara et al., 2015). Concerns about vaccine safety or efficacy appear to be decreasing among physicians; however, some concerns linger—such as a misconception that administering the HPV vaccine at the same time as the Tdap and MenACWY vaccines is “too many” vaccines in one visit (Cataldi et al., 2021). For physicians who expressed concerns about either the HPV vaccine’s efficacy or its safety, vaccine initiation among their patients was lower, perhaps as a result of the physician either not recommending the vaccine or providing a low quality recommendation (Farias et al., 2017). Although most research has focused on the HPV vaccine, Huang et al. found a wide variety in knowledge and interpretation of recommendations for meningococcal vaccines (MenACWY and MenB) and for prescribing habits among a sample of HCP (Huang et al., 2020). Another study found that only 50% of pediatricians surveyed reported initiating a conversation about the MenB vaccine for 16–18 year old patients and their parents and that initiating a discussion was significantly associated with being aware of MenB vaccinations and the risk of MenB outbreaks for their patients (Kempe et al., 2018).
In addition to knowledge about the vaccines, personal beliefs and biases can influence provider recommendation practices, both consciously and subconsciously. Cognitive biases, mental shortcuts that can result in errors in uncertain situations, have also been proposed as a framework for understanding HCP hesitancy (Hansen et al., 2020; Niccolai & Pettigrew, 2016). In a qualitative study, clinicians discussed how the anticipation of an uncomfortable discussion around the HPV vaccine or perceptions about the lack of urgency for the HPV vaccine influenced decisions either to delay recommending the vaccine or to recommending the vaccine less enthusiastically, examples of optimism bias and present bias (Hansen et al., 2020). Similar findings have been confirmed in quantitative studies—two national surveys of pediatricians found that about one third of respondents anticipated uncomfortable conversations about the HPV vaccine and one found that about half of respondents did not recommend same day vaccination for the HPV vaccine (Gilkey et al., 2015; Kempe et al., 2019).
Interpersonal level factors
The “interpersonal level” encompasses how the characteristics and attitudes of the patient and/or parent can influence a provider’s recommendation practices. HCP may recommend vaccines differently based on perceived parent attitudes and concerns. For example, in a study by Cunningham-Erves et al., perceived parent vaccine hesitancy by providers was significantly associated with lower provider confidence in vaccine safety, lower outcome expectations for the discussion and lower self-efficacy (Cunningham-Erves et al., 2019). Parental concerns about the safety of the HPV vaccine appear to be increasing – an analysis of NIS-Teen data from 2015 to 2018 found that the proportion of parents citing “Safety Concerns” as the primary reason for not vaccinating their child increased significantly from 13.0% in 2015 to 23.4% in 2018 (Sonawane et al., 2021). There is evidence that this increase in parental hesitancy is impacting providers as well. A longitudinal study of provider recommendation practices for the HPV vaccine found that provider reports of parental concerns of vaccine safety or moral/religious concerns increased from 5% in 2008 to 35% and 25% respectively in 2018 (Cataldi et al., 2021). When parents express a desire to reject the HPV vaccine providers may in turn recommend delaying the vaccine to a later visit (Dang et al., 2020). In a survey of pediatricians by Kempe et al., physicians reporting a refusal or deferral rate for the HPV vaccine of greater than 50% among their patient/parents was associated with anticipating an uncomfortable conversation and expecting less resistance to the vaccine from the parents for older adolescents (Kempe et al., 2019).
Providers may also make judgments based on perceived patient and/or parent characteristics. A common judgment made by providers in the context of the HPV vaccine is about the sexual maturity of the adolescent. In some cases, providers may perceive an adolescent as not sexually active and therefore not at risk for HPV and not a candidate. Conversely, providers may also preferentially recommend the HPV vaccine to adolescents who they perceive as sexually active. In both qualitative and quantitative studies, providers who make judgments about sexual maturity of the adolescent are less likely to make strong recommendations for the HPV vaccine (Hansen et al., 2020; Henrikson et al., 2016; Hopfer et al., 2019). Additionally, reports of provider recommendations vary by sociodemographic characteristics, such as poverty status, sex of the adolescent, and maternal education (Mohammed et al., 2016).
Community level factors
The environment and community in which the HCP practices is also likely to influence provider recommendation practices. This includes both clinic-level factors, such as the ability of the clinic to stock the vaccine, the presence of tools like provider prompts and reminder-recall systems and peer influences as well as the characteristics of the broader community in which the provider practices. HCP peers may influence provider attitudes about vaccines or recommendation practices (P. W. Lake et al., 2019a, 2019b). Providers who were characterized as “Ambivalent HPV recommenders”—those who did not consistently or strongly recommend the HPV vaccine—were also less likely to perceive their peers as thinking the HPV vaccine was important for adolescents or strongly recommending the vaccine (Hopfer et al., 2019). The characteristics of the community or environment in which an adolescent resides, such as the socioeconomic status of the neighborhood or racial/ethnic make-up of the area, are associated with vaccine receipt (Do et al., 2020). However, the exact mechanisms of these associations have not been fully explored nor has the potential variation of provider recommendations by such area-level factors.
Policy level factors
There is substantial evidence that policy can influence provider recommendation practices. This includes financial policies such as insurance coverage, initial vaccine costs and reimbursement practices as well as vaccine requirement policies, such as school entry requirements or vaccine mandates. All ACIP recommended vaccines are required to be covered by insurance; however, providers may have to cover the initial costs of purchasing vaccine prior to being reimbursed by patients’ insurance. Many physicians still perceive financial burdens among their patients/parents or for their own practice as a major barrier to administering the HPV vaccine (Cataldi et al., 2021; Farias et al., 2017; Tsai et al., 2018). Improving reimbursement policies for the HPV vaccine has been associated with an increase in both initiation and completion of the series (Tsai et al., 2018). In a study of physicians in Florida, many cited financial barriers such as the high cost of purchasing vaccine or inadequate reimbursement for administered vaccines from insurance companies as primary reasons for not vaccinating patients or referring patients/parents to Departments of Health (P. W. Lake et al., 2019a, 2019b). However, providers who participated in the Vaccines for Children (VFC), which provides vaccines free of charge to children who are not insured, have Medicaid or whose private insurance does not cover the vaccine, were not only more likely to recommend the HPV vaccine to their patient and/or their parents but also more likely to provide a strong vaccine recommendation (P. W. Lake et al., 2019a, 2019b). This indicates that removing financial barriers can improve provider recommendation practices. One potential policy avenue to address this is the implementation of Universal Purchase programs which are policies where states purchase vaccines for all children (privately and publicly insured) at VFC prices (Mulligan et al., 2018). There is some evidence a more expansive purchase programs is associated with higher state-level HPV vaccine coverage, however the impact that these kind of programs have on health care provider attitudes has not been examined (Gowda & Dempsey, 2012). Another potential policy avenue that could impact provider recommendation practices is the addition of insurance codes for vaccine counseling separate from vaccine administration, such that providers can be incentivized to spend time during patient visits discussing the vaccine (American Academy of Pediatrics, 2021). However, the potential impact of this policy on provider recommendations needs to be further explored.
The presence or absence of vaccine requirements can also impact provider recommendation practices. Only four states currently have HPV vaccine mandates in place, but a vast majority have school entry requirements for the Tdap and MenACWY vaccines (Elam-Evans, 2020). One qualitative study found that lack of school entry requirements for the HPV vaccine influenced provider’s framing of the HPV vaccine as “non-urgent” (Niccolai et al., 2018). Another study conducted among stakeholders in Florida found a belief among stakeholders that physicians view the HPV vaccine as less important because there is no mandate in place (P. Lake et al., 2019a, 2019b). State-level up-to-date coverage with the MenACWY vaccine (two doses by age 17 or one dose at age 16 or 17) was higher in states with either one or two-dose school entry requirements for the MenACWY vaccine. (Niccolai et al., 2019) MenACWY coverage is higher among college students than non-college students with many colleges requiring students be up-to-date with MenACWY prior to entry (Schaffer DeRoo et al., 2021). Further work is needed to fully understand how different vaccine requirement policies influence provider recommendation practices and how these attitudes can be addressed.
Improving health care provider recommendations
Given the strong correlation between a provider recommendation and vaccine receipt, identifying or improving existing interventions to improve provider recommendations has been emphasized by many experts as a priority in adolescent health (Dempsey & O’Leary, 2018; Markowitz et al., 2018; Stokley & Szilagyi, 2018). The model presented in this commentary provides a framework for understanding how provider recommendations vary at all levels which in turn would allow us to identify targets for intervention to improve both the frequency and quality of provider recommendations for adolescent vaccines. Interventions that address HCP can be a vital component of any intervention designed to improve vaccine uptake among adolescents and incorporated into multi-level intervention packages that also focus on other potential drivers of vaccine uptake.
Current state of provider-focused interventions to improve uptake
Most interventions or intervention components designed to target HCP have focused on either the individual level (educational interventions focused on improving provider knowledge about the vaccine) or the community level with clinic-focused interventions such as reminder-recall systems, electronic decision support etc. (Abdullahi et al., 2020; Leung et al., 2019; Niccolai & Hansen, 2015). Educational interventions alone focused on HCP have had a positive effect on both provider knowledge about the HPV vaccine as well as vaccine uptake; however, the effect is generally moderate with increases ranging from 0 to 6 percentage points (Abdullahi et al., 2020; Leung et al., 2019). Similarly, studies evaluating the impact of implementing clinic-level measures such as reminder-recall systems or electronic decision support, which both provide a nudge to the HCP to recommend a vaccine at the appropriate visit or time, have had a more limited impact, although it is difficult to completely disentangle the impact of provider prompts from other concurrently delivered interventions (Abdullahi et al., 2020; Niccolai & Hansen, 2015). A promising class of provider-focused interventions are those that in addition to individual level and community level aspects incorporate provider communication training at the interpersonal level. For illustrative purposes, we will describe three such interventions. As with much of the existing literature on adolescent vaccine uptake, the focus of all three of these interventions is on improving HPV vaccine uptake.
First, in a study conducted by Brewer et al., primary care clinics were randomized to receive announcement training, conversation training or to be a control (Brewer, Hall et al., Brewer, Hall, et al., 2017). In the announcement training, providers at the clinic were instructed to begin immunization encounters with parents by announcing that the child is due for three vaccines recommended for their age and only engage in further conversation if the parent expresses any hesitancy. This approach has also been called presumptive communication (Opel et al., 2013). In the conversation training, providers were instructed to start an open conversation with parents about the three recommended vaccines, listening and responding to parent concerns before recommending the vaccines. Brewer et al. found that clinics that received the announcement training had a 5.4% increase (95% Confidence interval [CI] 1.1%–9.7%) in HPV vaccine initiation coverage six months after the training. The conversation training did not result in a statistically significant improvement in coverage (Brewer, Hall, et al., Brewer, Hall, et al., 2017).
Dempsey et al. also evaluated the impact of training health care providers at primary care practices in presumptive communication combined with motivational interviewing techniques in addition to providing practices with a set of educational resources about HPV (Dempsey et al., 2018). Motivational interviewing is a technique where providers are instructed to identify patient/parents’ underlying motivations to engage in a health behavior and utilize that to encourage compliance (Dempsey & O’Leary, 2018). In the communication training providers were instructed to open conversations with parents using a presumptive approach (similar to the announcement approach utilized by Brewer et al.) and proceeding to motivational interviewing techniques (e.g., asking the parent if it is OK to share what they know about the recommended vaccines) if the parent presents any resistance to the recommendation (Dempsey et al., 2018, 2019). Among intervention clinics, there was a 9.5 percentage point increase in the proportion of adolescents initiating the HPV vaccine series (p < 0.001) (Dempsey et al., 2018).
In both the Brewer et al. and Dempsey et al. trials, at least part of the provider training was delivered through in-person training sessions. A potential limitation of many of these interventions is the challenge of implementing and potentially scaling up interventions that include in-person training sessions. In contrast, Szilagyi et al. evaluated the impact of an online asynchronous training consisting of three modules that focused on (1) delivering an effective recommendation, (2) confidence in vaccine recommendations and (3) communicating with hesitant parents including the incorporation of motivational interviewing techniques (Szilagyi et al., 2021). In this study, primary care clinics were randomized to either receive access to the online training (intervention) or not (control). Among intervention clinics, there was a 3.4 percentage point increase (95% CI 0.6%–6.2%) in HPV vaccine initiation among adolescents compared to control clinics (Szilagyi et al., 2021). All three of these interventions are among some of the more impactful interventions to improve adolescent vaccine coverage proving that a focus on provider communication is a promising route forward (Vaccination—Healthy People 2030 | Health.Gov, n.d.). To date these approaches have only been applied to HPV vaccine promotion. If we are to increase adolescent vaccine coverage for all recommended adolescent vaccines to levels sufficient to protect this population, we must continue to improve upon existing interventions.
Applying the multi-level framework to intervention design
One potential avenue for increasing the effectiveness of provider communications like those outlined above is to view them through the framework proposed in this commentary, including identifying areas where a multilevel intervention could be implemented. Using this framework, we can identify factors that are adequately addressed by the intervention as well as those that may be contributing to disparities in provider recommendations but are not fully addressed in the intervention. As an illustration, we will evaluate the three interventions described above through this lens.
At the individual level, a lack of knowledge about HPV or the HPV vaccine can contribute to lack of confidence in provider recommendations of the vaccines (Leung et al., 2019). This applies to other vaccines as well; a lack of clarity around MenB recommendations and shared clinical decision making has been associated with variation in MenB vaccination recommendation patterns (Huang et al., 2020). Both the Szilagyi et al. and Brewer et al. interventions incorporate elements aimed at improving provider knowledge about HPV and the HPV vaccine—in both this comprises a portion of the first section or module of the training (Brewer, Hall, et al., Brewer, Hall, et al., 2017; Szilagyi et al., 2021). Dempsey et al. do not describe provider education about HPV and the HPV vaccine as an explicit part of the training, but intervention practices were provided with various fact sheets and decision aids that could be construed as provider education in addition to the patient/parent-focused aspect (Dempsey et al., 2019). However, knowledge about HPV is only one aspect of the individual factors that might influence a provider’s recommendation—underlying cognitive biases may also be driving recommendation practices. Portions of these three interventions could potentially be addressing these biases, even if that was not the intention of the intervention. For example, all three interventions emphasize same-day vaccination either as part of the presumptive/announcement communication approach or as part of providing an effective recommendation, which could combat present bias (i.e., the HPV vaccine is not urgent, it could be delayed) (Brewer, Hall, et al., Brewer, Hall, et al., 2017; Dempsey et al., 2018; Hansen et al., 2020; Niccolai & Pettigrew, 2016; Szilagyi et al., 2021). In future studies, it may be beneficial to incorporate training that is more targeted towards identifying and combatting cognitive biases in provider recommendation practices.
One way to conceptualize these interventions is to view the goal as eliminating the influence of interpersonal factors by instructing physicians to recommend the HPV vaccine the same way every time, regardless of the patient and/or parent. In addition to a universal recommendation, both Dempsey et al. and Szilagyi et al. incorporate training in motivational interviewing as a way of addressing more hesitant parents. Providing health care providers with techniques to address hesitant parents can increase confidence and self-efficacy—Szilagyi et al. found that in a post-intervention survey of participating clinicians 85.1% felt confident answering questions about the HPV vaccine and 72.3% felt confident talking with hesitant parents (Szilagyi et al., 2021). Whether or not physicians were differentially implementing the provided recommendation practices based on patient and/or parent characteristics is unknown and difficult to measure. In post-intervention surveys of participating health care providers, both Dempsey et al. and Szilagyi et al. did find substantial uptake of the communication techniques by health care providers (72%–90% and 86% respectively), but this does not necessarily indicate that providers were universally utilizing these techniques (Dempsey et al., 2018; Szilagyi et al., 2021).
All three studies evaluated differential treatment effects by sex and found no differences, perhaps indicating that providers were not recommending vaccination differently based on the adolescent’s sex, which is encouraging given previous studies that have identified providers as less likely to recommend the HPV vaccine to adolescent boys (Brewer, Hall, et al., Brewer, Hall, et al., 2017; Dempsey et al., 2018; Szilagyi et al., 2021). Dempsey et al. did find a difference in HPV vaccine series initiation between patients with private insurance and those with public insurance, although as the authors discuss, this may be a consequence of the specific circumstances of the public practices included in the study (Dempsey et al., 2018). A potential method for determining whether or not providers are universally implementing effective recommendations is to survey parents as done by Dempsey et al. In a random sample of parents from participating clinics, the authors found that 80% of parents at participating clinics recalled receiving a presumptive recommendation, but only 53% recalled a “very strong” recommendation. Interestingly, there was also no difference in recollection of a presumptive recommendation or a strong recommendation between parents from intervention and control clinics (Dempsey et al., 2019). Future exploration of the disconnect between provider report of recommendations and parental recollection of recommendations and the potential association between provider recommendations and patient/parent characteristics could provide information on how to better achieve universal strong recommendations.
Both the interventions designed by Szilagyi et al. and Brewer et al. contain elements for involving office staff and others in the clinic in communication around the HPV vaccine or adapting the communication strategy to their specific clinical practice, which may allow recommendation practices to be tailored to the environment or community in which the clinic exists (Brewer, Hall, et al., Brewer, Hall, et al., 2017; Szilagyi et al., 2021). Brewer et al. and Dempsey et al. both conducted their study among clinics in a single geographic area, limiting conclusions that can be drawn about the potential application of such a provider-focused intervention to different geographical regions. The online-only nature of the study by Szilagyi et al. allowed the authors to include clinics across the US; however, there is no discussion in the manuscript of how the effectiveness of the intervention may have varied by setting. Adolescent vaccine coverage, particularly HPV vaccine coverage, varies substantially geographically and further investigation is needed to evaluate how provider recommendations, and subsequently how the effectiveness of provider communication interventions, may vary geographically as well.
Conclusions
Improving uptake of recommended adolescent vaccines is an important public health priority and optimizing provider communication and incorporating provider-focused interventions into multi-level intervention packages is one of the most promising paths forward. While research has primarily focused on the HPV vaccine, it will be important to expand our understanding of the factors influencing provider recommendations of adolescent vaccines to other recommended vaccines as well. This is especially important at present as the ongoing pandemic continues to negatively impact the delivery of adolescent vaccinations. Health care providers will play an essential role in ensuring that those adolescents who missed opportunities for routine administration of vaccines during the pandemic are brought up to date. Additionally, with the expansion of COVID-19 vaccine recommendations to include 5–11-year-olds COVID-19 vaccines are being administered in pediatric offices increasing the importance of understanding provider recommendation practices for the COVID-19 vaccines in the adolescent population. By utilizing the framework proposed within, we can not only expand our understanding the of the factors that influence provider recommendations of adolescent vaccinations but also apply this model to designing or improving provider communication interventions.
Author contributions
MKE and LMN conceived of the framework and wrote the initial draft. RAB, STO, JLS and EDS assisted in refinement of the framework and provided feedback on the manuscript. All authors reviewed and approved of the final submitted manuscript.
Funding
This publication was made possible by CTSA Grant Number TL1 TR001864 (MKE) and CTSA Grant Number KL2 TR001862 (EDS) from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH). RAB is supported by NIH Grant R37 CA234119. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH.
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
Dr. Niccolai serves as a scientific advisor for Merck and Moderna. Drs. Schwartz, Bednarczyk, Shapiro, O’Leary and Ms. Ellingson declare that they have no competing interests.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Consent for publication
Not applicable.
Human and animal rights statement
This manuscript is a narrative review of the existing literature and did not include any human or animal studies and therefore did not require ethical clearance.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mallory K. Ellingson, Email: Mallory.ellingson@yale.edu
Robert A. Bednarczyk, Email: Robert.a.bednarczyk@emory.edu
Sean T. O’Leary, Email: Sean.oleary@cuanschutz.edu
Jason L. Schwartz, Email: Jason.schwartz@yale.edu
Eugene D. Shapiro, Email: Eugene.shapiro@yale.edu
Linda M. Niccolai, Email: Linda.niccolai@yale.edu
References
- Abdullahi LH, Kagina BM, Ndze VN, Hussey GD, Wiysonge CS. Improving vaccination uptake among adolescents. Cochrane Database of Systematic Reviews. 2020 doi: 10.1002/14651858.CD011895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amboree TL, Darkoh C. Barriers to human papillomavirus vaccine uptake among racial/ethnic minorities: A systematic review. Journal of Racial and Ethnic Health Disparities. 2020 doi: 10.1007/s40615-020-00877-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Pediatrics Reporting encounters for immunization safety counseling. AAP Pediatric Coding Newsletter. 2021;16(12):5–6. doi: 10.1542/pcco_book209_document002. [DOI] [Google Scholar]
- Bednarczyk RA, Chamberlain A, Mathewson K, Salmon DA, Omer SB. Practice-, provider-, and patient-level interventions to improve preventive care: Development of the P3 Model. Preventive Medicine Reports. 2018;11:131–138. doi: 10.1016/j.pmedr.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarczyk RA, Ellingson MK, Omer SB. Human papillomavirus vaccination before 13 and 15 years of age: Analysis of national immunization survey teen data. The Journal of Infectious Diseases. 2019;220(5):730–734. doi: 10.1093/infdis/jiy682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein S, North A, Schwartz J, Niccolai LM. State-level voting patterns and adolescent vaccination coverage in the United States, 2014. American Journal of Public Health. 2016;106(10):1879–1881. doi: 10.2105/AJPH.2016.303381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsch C, Schmid P, Heinemeier D, Korn L, Holtmann C, Böhm R. Beyond confidence: Development of a measure assessing the 5C psychological antecedents of vaccination. PLoS ONE. 2018 doi: 10.1371/journal.pone.0208601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer NT, Chapman GB, Rothman AJ, Leask J, Kempe A. Increasing vaccination: Putting psychological science into action. Psychological Science in the Public Interest. 2017;18(3):149–207. doi: 10.1177/1529100618760521. [DOI] [PubMed] [Google Scholar]
- Brewer NT, Hall ME, Malo TL, Gilkey MB, Quinn B, Lathren C. Announcements versus conversations to improve HPV vaccination coverage: A randomized trial. Pediatrics. 2017 doi: 10.1542/peds.2016-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell AC, Madden CA, Thompson DM, Garbe MC, Roberts JR, Jacobson RM, Darden PM. The impact of provider recommendation on human papillomavirus vaccine and other adolescent vaccines. Human Vaccines & Immunotherapeutics. 2020 doi: 10.1080/21645515.2020.1817713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldi JR, O’Leary ST, Markowitz LE, Allison MA, Crane LA, Hurley LP, Brtnikova M, Beaty BL, Gorman C, Meites E, Lindley MC, Kempe A. Changes in strength of recommendation and perceived barriers to human papillomavirus vaccination: Longitudinal analysis of primary care physicians, 2008–2018. The Journal of Pediatrics. 2021;234:149–157. doi: 10.1016/j.jpeds.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. (2020a, February 11). COVID-19 vaccination. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/adolescents.html [PubMed]
- CDC. (2020b, November 17). HPV cancers. Centers for Disease Control and Prevention. https://www.cdc.gov/hpv/parents/cancer.html
- Centers for Disease Prevention and Control Ten great public health achievements—worldwide, 2001–2010. MMWR. Morbidity and Mortality Weekly Report. 2011;60(24):814–818. [PubMed] [Google Scholar]
- Cohn A, MacNeil J. The changing epidemiology of meningococcal disease. Infectious Disease Clinics of North America. 2015;29(4):667–677. doi: 10.1016/j.idc.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Cunningham-Erves J, Koyama T, Huang Y, Jones J, Wilkins CH, Harnack L, McAfee C, Hull PC. Providers’ perceptions of parental human papillomavirus vaccine hesitancy: Cross-sectional study. JMIR Cancer. 2019 doi: 10.2196/13832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang JHT, Stewart SL, Blumberg DA, Rodriguez HP, Chen MS. “There’s Always Next Year”: Primary care team and parent perspectives on the human papillomavirus vaccine. Human Vaccines & Immunotherapeutics. 2020;16(8):1814–1823. doi: 10.1080/21645515.2019.1710410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey AF, O’Leary ST. Human papillomavirus vaccination: Narrative review of studies on how providers’ vaccine communication affects attitudes and uptake. Academic Pediatrics. 2018;18(2S):S23–S27. doi: 10.1016/j.acap.2017.09.001. [DOI] [PubMed] [Google Scholar]
- Dempsey AF, Pyrzanowski J, Campagna EJ, Lockhart S, O’Leary ST. Parent report of provider HPV vaccine communication strategies used during a randomized, controlled trial of a provider communication intervention. Vaccine. 2019;37(10):1307–1312. doi: 10.1016/j.vaccine.2019.01.051. [DOI] [PubMed] [Google Scholar]
- Dempsey AF, Pyrznawoski J, Lockhart S, Barnard J, Campagna EJ, Garrett K, Fisher A, Dickinson LM, O’Leary ST. Effect of a health care professional communication training intervention on adolescent human papillomavirus vaccination: A cluster randomized clinical trial. JAMA Pediatrics. 2018;172(5):e180016. doi: 10.1001/jamapediatrics.2018.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do EK, Rossi B, Miller CA, Ksinan AJ, Wheeler DC, Chukmaitov A, Cyrus JW, Fuemmeler BF. Area-level variation and human papillomavirus vaccination among adolescents and young adults in the United States: A systematic review. Cancer Epidemiology and Prevention Biomarkers. 2020 doi: 10.1158/1055-9965.EPI-20-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elam-Evans, L. D. (2020). National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2019. MMWR. Morbidity and Mortality Weekly Report. 10.15585/mmwr.mm6933a1 [DOI] [PMC free article] [PubMed]
- Farias AJ, Savas LS, Fernandez ME, Coan SP, Shegog R, Healy CM, Lipizzi E, Vernon SW. Association of physicians perceived barriers with human papillomavirus vaccination initiation. Preventive Medicine. 2017;105:219–225. doi: 10.1016/j.ypmed.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilkey MB, Calo WA, Moss JL, Shah PD, Marciniak MW, Brewer NT. Provider communication and HPV vaccination: The impact of recommendation quality. Vaccine. 2016;34(9):1187–1192. doi: 10.1016/j.vaccine.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilkey MB, Malo TL, Shah PD, Hall ME, Brewer NT. Quality of physician communication about human papillomavirus vaccine: Findings from a national survey. Cancer Epidemiology, Biomarkers & Prevention. 2015;24(11):1673–1679. doi: 10.1158/1055-9965.EPI-15-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilkey MB, McRee A-L. Provider communication about HPV vaccination: A systematic review. Human Vaccines & Immunotherapeutics. 2016;12(6):1454–1468. doi: 10.1080/21645515.2015.1129090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowda C, Dempsey AF. Medicaid reimbursement and the uptake of adolescent vaccines. Vaccine. 2012;30(9):1682–1689. doi: 10.1016/j.vaccine.2011.12.097. [DOI] [PubMed] [Google Scholar]
- Grohskopf LA, Alyanak E, Broder KR, Blanton LH, Fry AM, Jernigan DB, Atmar RL. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization practices—United States, 2020–21 influenza season. MMWR Recommendations and Reports. 2020;69(8):1–24. doi: 10.15585/mmwr.rr6908a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen CE, North A, Niccolai LM. Cognitive Bias in clinicians’ communication about human papillomavirus vaccination. Health Communication. 2020;35(4):430–437. doi: 10.1080/10410236.2019.1567439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havers FP, Moro PL, Hunter P, Hariri S, Bernstein H. Use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccines: Updated recommendations of the Advisory Committee on Immunization Practices—United States, 2019. Morbidity and Mortality Weekly Report. 2020;69(3):77–83. doi: 10.15585/mmwr.mm6903a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrikson NB, Tuzzio L, Gilkey MB, McRee A-L. “You’re never really off time”: Healthcare providers’ interpretations of optimal timing for HPV vaccination. Preventive Medicine Reports. 2016;4:94–97. doi: 10.1016/j.pmedr.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfer S, Wright ME, Pellman H, Wasserman R, Fiks AG. HPV vaccine recommendation profiles among a national network of pediatric practitioners: Understanding contributors to parental vaccine hesitancy and acceptance. Human Vaccines & Immunotherapeutics. 2019;15(7–8):1776–1783. doi: 10.1080/21645515.2018.1560771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Goren A, Lee LK, Li VW, Dempsey A, Srivastava A. Disparities in healthcare providers’ interpretations and implementations of ACIP’s meningococcal vaccine recommendations. Human Vaccines & Immunotherapeutics. 2020;16(4):933–944. doi: 10.1080/21645515.2019.1682845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempe A, Allison MA, MacNeil JR, O’Leary ST, Crane LA, Beaty BL, Hurley LP, Brtnikova M, Lindley MC, Albert AP. Adoption of serogroup B meningococcal vaccine recommendations. Pediatrics. 2018;142(3):e20180344. doi: 10.1542/peds.2018-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempe A, O’Leary ST, Markowitz LE, Crane LA, Hurley LP, Brtnikova M, Beaty BL, Meites E, Stokley S, Lindley MC. HPV vaccine delivery practices by primary care physicians. Pediatrics. 2019;144(4):e20191475. doi: 10.1542/peds.2019-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kester LM, Zimet GD, Fortenberry JD, Kahn JA, Shew ML. A national study of HPV vaccination of adolescent girls: Rates, predictors, and reasons for non-vaccination. Maternal and Child Health Journal. 2013;17(5):879–885. doi: 10.1007/s10995-012-1066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake P, Kasting ML, Malo T, Giuliano AR, Vadaparampil ST. An environmental scan to examine stakeholder perspectives on human papillomavirus vaccination: A mixed methods study. Vaccine. 2019;37(1):187–194. doi: 10.1016/j.vaccine.2018.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake PW, Kasting ML, Christy SM, Vadaparampil ST. Provider perspectives on multilevel barriers to HPV vaccination. Human Vaccines & Immunotherapeutics. 2019;15(7–8):1784–1793. doi: 10.1080/21645515.2019.1581554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung SOA, Akinwunmi B, Elias KM, Feldman S. Educating healthcare providers to increase human papillomavirus (HPV) vaccination rates: A qualitative systematic review. Vaccine X. 2019;3:100037. doi: 10.1016/j.jvacx.2019.100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil JR, Blain AE, Wang X, Cohn AC. Current epidemiology and trends in meningococcal disease—United States, 1996–2015. Clinical Infectious Diseases. 2018;66(8):1276–1281. doi: 10.1093/cid/cix993. [DOI] [PubMed] [Google Scholar]
- Markowitz, L. E., Dunne, E. F., Saraiya, M., Chesson, H. W., Curtis, C. R., Gee, J., Bocchini, J. A., Unger, E. R., & Centers for Disease Control and Prevention (CDC). (2014). Human papillomavirus vaccination: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommendations and Reports,63(RR-05), 1–30. [PubMed]
- Markowitz LE, Gee J, Chesson H, Stokley S. Ten years of human papillomavirus vaccination in the United States. Academic Pediatrics. 2018;18(2S):S3–S10. doi: 10.1016/j.acap.2017.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall GS, Dempsey AF, Srivastava A, Isturiz RE. US college students are at increased risk for serogroup B meningococcal disease. Journal of the Pediatric Infectious Diseases Society. 2019;9(2):244–247. doi: 10.1093/jpids/piz024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbaeyi SA, Bozio CH, Duffy J, Rubin LG, Hariri S, Stephens DS, MacNeil JR. Meningococcal vaccination: recommendations of the advisory committee on immunization practices, United States, 2020. MMWR Recommendations and Reports. 2020;69(9):1–41. doi: 10.15585/mmwr.rr6909a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbaeyi SA, Joseph SJ, Blain A, Wang X, Hariri S, MacNeil JR. Meningococcal disease among college-aged young adults: 2014–2016. Pediatrics. 2019 doi: 10.1542/peds.2018-2130. [DOI] [PubMed] [Google Scholar]
- Mohammed KA, Geneus CJ, Osazuwa-Peters N, Adjei Boakye E, Tobo BB, Burroughs TE. Disparities in provider recommendation of human papillomavirus vaccination for US adolescents. Journal of Adolescent Health. 2016;59(5):592–598. doi: 10.1016/j.jadohealth.2016.06.005. [DOI] [PubMed] [Google Scholar]
- Mulligan K, Snider JT, Arthur P, Frank G, Tebeka M, Walker A, Abrevaya J. Examination of universal purchase programs as a driver of vaccine uptake among US States, 1995–2014. Vaccine. 2018;36:4032–4038. doi: 10.1016/j.vaccine.2018.05.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niccolai LM, Hansen CE. Practice- and community-based interventions to increase human papillomavirus vaccine coverage: A systematic review. JAMA Pediatrics. 2015;169(7):686–692. doi: 10.1001/jamapediatrics.2015.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niccolai LM, North AL, Footman A, Hansen CE. Lack of school requirements and clinician recommendations for human papillomavirus vaccination. Journal of Public Health Research. 2018;7(1):1324. doi: 10.4081/jphr.2018.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niccolai LM, Pettigrew MM. The role of cognitive bias in suboptimal HPV vaccine uptake. Pediatrics. 2016 doi: 10.1542/peds.2016-1537. [DOI] [PubMed] [Google Scholar]
- Niccolai LM, Yakely AE, Hansen CE. Up-to-date coverage with meningococcal vaccine among adolescents age 17 years: Patterns and correlates in the United States, 2017. Vaccine. 2019;37(40):5934–5938. doi: 10.1016/j.vaccine.2019.08.015. [DOI] [PubMed] [Google Scholar]
- Oh NL, Biddell CB, Rhodes BE, Brewer NT. Provider communication and HPV vaccine uptake: A meta-analysis and systematic review. Preventive Medicine. 2021 doi: 10.1016/j.ypmed.2021.106554. [DOI] [PubMed] [Google Scholar]
- Opel DJ, Heritage J, Taylor JA, Mangione-Smith R, Salas HS, Devere V, Zhou C, Robinson JD. The architecture of provider-parent vaccine discussions at health supervision visits. Pediatrics. 2013;132(6):1037–1046. doi: 10.1542/peds.2013-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, B. (2021). Impact of the COVID-19 Pandemic on administration of selected routine childhood and adolescent vaccinations—10 U.S. Jurisdictions, March–September 2020. MMWR. Morbidity and Mortality Weekly Report. 10.15585/mmwr.mm7023a2 [DOI] [PMC free article] [PubMed]
- Rodriguez SA, Mullen PD, Lopez DM, Savas LS, Fernández ME. Factors associated with adolescent HPV vaccination in the US: A systematic review of reviews and multilevel framework to inform intervention development. Preventive Medicine. 2020;131:105968. doi: 10.1016/j.ypmed.2019.105968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer DeRoo S, Torres RG, Fu LY. Meningococcal disease and vaccination in college students. Human Vaccines & Immunotherapeutics. 2021;17(11):4675–4688. doi: 10.1080/21645515.2021.1973881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonawane K, Lin Y-Y, Damgacioglu H, Zhu Y, Fernandez ME, Montealegre JR, Cazaban CG, Li R, Lairson DR, Lin Y, Giuliano AR, Deshmukh AA. Trends in human papillomavirus vaccine safety concerns and adverse event reporting in the United States. JAMA Network Open. 2021;4(9):e2124502. doi: 10.1001/jamanetworkopen.2021.24502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokley S, Szilagyi PG. Improving human papillomavirus vaccination in the United States: Executive summary. Academic Pediatrics. 2018;18(2S):S1–S2. doi: 10.1016/j.acap.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryadevara M, Handel A, Bonville CA, Cibula DA, Domachowske JB. Pediatric provider vaccine hesitancy: An under-recognized article in immunizing children. Vaccine. 2015;33:6629–6634. doi: 10.1016/j.vaccine.2015.10.096. [DOI] [PubMed] [Google Scholar]
- Szilagyi PG, Humiston SG, Stephens-Shields AJ, Localio R, Breck A, Kelly MK, Wright M, Grundmeier RW, Albertin C, Shone LP, Steffes J, Rand CM, Hannan C, Abney DE, McFarland G, Kominski GF, Seixas BV, Fiks AG. Effect of training pediatric clinicians in human papillomavirus communication strategies on human papillomavirus vaccination rates: A cluster randomized clinical trial. JAMA Pediatrics. 2021 doi: 10.1001/jamapediatrics.2021.0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The SAGE Vaccine Hesitancy Working Group. (2013). What influences vaccine acceptance: A model of determinants of vaccine hesitancy. Geneva: WHO. https://www.who.int/immunization/sage/meetings/2013/april/1_Model_analyze_driversofvaccineConfidence_22_March.pdf
- Tsai Y, Lindley MC, Zhou F, Stokley S. Provider payments and the receipt of human papillomavirus vaccine among privately insured adolescents. Health Affairs. 2018;37(10):1587–1595. doi: 10.1377/hlthaff.2018.0545. [DOI] [PubMed] [Google Scholar]
- Vaccination—Healthy People 2030 | health.gov. (n.d.). Retrieved March 24, 2021, from https://health.gov/healthypeople/objectives-and-data/browse-objectives/vaccination
- Vu M, Berg CJ, Escoffery C, Jang HM, Nguyen TT, Travis L, Bednarczyk RA. A systematic review of practice-, provider-, and patient-level determinants impacting Asian-Americans’ human papillomavirus vaccine intention and uptake. Vaccine. 2020;38(41):6388–6401. doi: 10.1016/j.vaccine.2020.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner EL, Ding Q, Pappas LM, Henry K, Kepka D. White, affluent, educated parents are least likely to choose HPV vaccination for their children: A cross-sectional study of the National Immunization Study—teen. BMC Pediatrics. 2017;17(1):200. doi: 10.1186/s12887-017-0953-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.


