Abstract
Background:
Midlife cardiovascular risk factors (CVRF) increase dementia risk. Less is known about whether CVRF identified before midlife impact late-life cognition in diverse populations.
Methods:
Linear regression models examined hypertension, hyperlipidemia, and overweight/obesity at ages 30–59 with late-life executive function, semantic memory, verbal episodic memory and global cognition in a cohort of Asians, Blacks, Latinos, and Whites (n=1,127; mean age=75.8, range 65–98). Models adjusted for age at CVRF, age at cognitive assessment, gender, race/ethnicity, participant education and parental education.
Results:
Thirty-four percent had 1 CVRF at ages 30–59; 19% had 2+. Blacks (26%) and Latinos (23%) were more likely to have 2+ CVRF than Asians (14%) or Whites (13%). Having 2+ CVRF was associated with lower global cognition (β=−0.33; 95% CI=−0.45, −0.21), executive function (β=−0.26; 95% CI=−0.39, −0.13), verbal episodic memory (β=−0.34; 95% CI=−0.48, −0.20), and semantic memory (β=−0.20; 95% CI=−0.33, −0.07). Interaction by age (p=0.06) indicated overweight/obesity was negatively associated with executive function at ages 30–39 but not at ages 40–59. Race/ethnic-specific effects showed disparities in CVRF prevalence impacts population disparities in late-life cognition.
Conclusions:
Being overweight/obese in early adulthood and having 2+ CVRF in early adulthood/midlife are modifiable targets to redress racial/ethnic disparities in cognitive impairment and dementia.
Introduction
Prior studies have observed marked racial/ethnic disparities in dementia.1,2 One possible hypothesis is that these disparities are partially due to differential exposure to cardiovascular risk factors (CVRF) associated with lower late-life cognition and higher dementia incidence.3–7 Specifically, disparities in late-life cognitive outcomes could result from higher prevalence of CVRF,8–11 an increased sensitivity to the impacts of CVRF on cognition among some groups,4 and/or earlier onset of CVRF.12–14
While there is a growing, body of literature examining the role of racial/ethnic differences in midlife CVRF on late-life cognition, most studies are limited to Black/White differences.4,15–19 and less is known about other racial/ethnic groups such as Asians and Latinos though there are disparities in rates of dementia.1 Other studies with more diverse samples have not explored racial/ethnic differences in associations between midlife CVRF and late-life cognitive outcomes.20–25 Additionally, the age at which CVRF were observed and the duration between CVRF measures and cognitive assessment is highly variable in prior studies. Many studies examining the CVRF-cognition association broadly define midlife at ages 40 to 64,19,26,27 though some have included measures of CVRF assessed at age 3019 or after age 65.4,15–18,28–30 Few have examined race/ethnic differences in associations of CVRF identified prior to midlife and late-life cognitive domains in cohorts that include Asians, Blacks. Latinos and Whites.
In this study, we aim to address these gaps by examining the association between three CVRF (hypertension, hyperlipidemia and overweight/obesity) measured between 1977 and 1985 when participants were between the ages of 30 and 59 and three cognitive domains (executive function, verbal episodic memory and semantic memory) assessed in 2017 and 2018 when participants were age 65+. We hypothesize that CVRF burden will be negatively associated with late-life cognition for all racial/ethnic groups, but that the pattern and magnitude of these associations will vary by race/ethnicity in our diverse cohort of Asian, Black, Latino and White older adults. We also hypothesize that the impact of CVRF on late-life cognition will be most strongly associated with adverse outcomes when CVRF are present earlier in mid-life (30s).
Methods
Study Participants
We used Wave 1 data from the Kaiser Healthy Aging and Diverse Life Experiences (KHANDLE) cohort, comprised of community-dwelling older adults residing in the San Francisco Bay area and Sacramento valley. KHANDLE aims to evaluate how life course and sociocultural factors influence late-life brain health and cognitive decline and may contribute to racial/ethnic disparities. Individuals eligible for KHANDLE were long-term members of Kaiser Permanente Northern California (KPNC) who were age 65 or older on January 1, 2017 and who previously participated in one or more Kaiser Permanente Multiphasic Health Checkups (MHC). MHC were a series of wellness assessments offered between1964 and 1992 as part of routine care to members in northern California that collected data on lifestyle and risk factors through questionnaires and clinical assessments. Stratified random sampling by race/ethnicity and educational attainment was used to recruit approximately equal proportions of Asian, Black, Latino, and White participants with diversity in educational attainment. Eligible participants were excluded if they had an electronic medical record diagnosis of dementia or other neurodegenerative disease (frontotemporal dementia, Lewy body disease, Pick’s disease, Parkinson’s disease with dementia, Huntington’s disease); or presence of health conditions that would impede participation in KHANDLE study interviews, defined by hospice activity in the past 12 months, history of severe chronic obstructive pulmonary disease in the past 6 months, congestive heart failure hospitalizations in the past 6 months, and history of end stage renal disease or dialysis in the past 12 months. Participants were interviewed in their homes or at KPNC clinics from March 2017 to December 2018, totaling 1,712 enrolled participants. The study was approved by the KPNC and UC Davis Institutional Review Boards and all enrolled participants provided informed consent.
Measures
Cognition.
Three cognitive domains (verbal episodic memory, semantic memory, and executive function) were derived from the Spanish and English Neuropsychological Assessment Scales (SENAS). The SENAS is a battery of cognitive tests that has undergone extensive development using item response theory (IRT) methodology for valid comparisons of cognition and cognitive change across racially/ethnically and linguistically diverse groups. The SENAS was explicitly developed for research purposes and is designed to detect small variations in cognitive capacities that are typically missed with clinical assessments and less comprehensive cognitive tests. The SENAS was administered during wave 1 interviews in either English or Spanish, with language of administration determined by an algorithm that considered preferred language and everyday language usage in a variety of settings. IRT methods were used to derive a verbal episodic memory score from a multi-trial word-list-learning test. A semantic memory composite score was calculated from IRT based verbal (object-naming) and nonverbal (picture association) scores. An executive function composite score was calculated from IRT based component measures of category fluency, phonemic (letter) fluency, and working memory (digit-span backward, visual-span backward, list sorting). Details of the administration procedures, development, and psychometric characteristics have been described in detail elsewhere.31,32 Global cognition was defined as the mean of verbal episodic memory, semantic memory and executive function scores. Each domain was z-standardized using the Wave 1 sample mean and standard deviation.
Cardiovascular Risk Factors.
These analyses leverage CVRF measured between 1977 and 1985 through questionnaires and clinical measurements conducted by KPNC medical staff during MHC as part of routine care. For this study, midlife hypertension was defined as having systolic blood pressure >140 mm/Hg or diastolic blood pressure >90 mm/Hg, reflecting the diagnostic criteria of the period of assessment (capturing 66% of hypertension cases); or participant report of having been told by a physician they had hypertension and/or self-report of taking blood pressure medication (capturing 34% of hypertension cases). Midlife hyperlipidemia was defined as having total cholesterol >240 mg/dL. Being overweight/obese at midlife was defined as having a BMI ≥25 kg/m2. We summed our dichotomous variables for hypertension, hyperlipidemia and being overweight/obese to create a CVRF variable indicative of participants having 0, 1 or 2+ midlife CVRF.
Demographics.
Race/ethnicity was self-reported at participant interview, or obtained from the participant’s medical record when missing, and classified as non-Latino White, non-Latino Black, non-Latino Asian, or Latino. Gender was recorded as male or female. Age at KHANDLE interview was derived from the participant’s date of birth obtained from medical records. Age at MHC was calculated based on the date of the exam. Highest level of education/degree completed was captured through interview questions for the participant and their parents. Participant education, maternal education and paternal education were each converted to a continuous measure of years of education.
Analytic Sample
We restricted participants to those who were ages 30–59 when attending MHC between 1977 and 1985. The types of data collected during MHC varied the 28 years they were conducted. We selected this time period as it provided the largest possible sample size with uniformly collected data for our exposures of interest, while also allowing us to reduce bias caused by secular trends in the diagnosis and treatment of CVRF of interest, such as the introduction of statins in 1987 to treat hyperlipidemia.33 Of the 1,712 KHANDLE participants, 1,221 completed at least one MHC during the 1977–1985 phase and, of these, 1,140 completed a MHC between the ages of 30 and 59 and had available measures for one or more of the CVRF of interest. About 4% were missing 1 CVRF and another 4% missing 2 CVRF but were retained in the overall sample to provide information where CVRF were present. If participants had more than one MHC from 1977–1985 at which they met age criteria, we used the first visit to prioritize the younger age of exposure for this analysis. Ninety-six percent of our sample met age criteria at their first or only MHC during the selected time frame; the remaining 4% aged into our sample at subsequent MHC completed during the same time frame. Participants were additionally excluded if they were missing scores on all cognitive domains (n=13), resulting in an analytic sample of 1,127.
Analysis
Prevalence of CVRF and participant demographics were compared by race/ethnicity using ANOVA or Chi-square tests. Using a series of linear regression models, we separately examined the relationship between each midlife CVRF and late-life cognition (overall and domain specific). We also examined the relationship between the number of midlife CVRF (0, 1 or 2+) and overall and domain specific cognition in late life. Effect modification by race/ethnicity or midlife age group was examined using interaction terms (i.e., the interaction of each CVRF and age of assessment, or race/ethnic group). We stratified all analyses by race/ethnicity or midlife age group category (ages 30–39; 40–49; 50–59) to further examine sub-group variability.
We also examined the relative impact of racial/ethnic specific CVRF prevalence on late-life cognition. To do so, we multiplied the race/ethnic prevalence with the race/ethnic CVRF β coefficient and calculated bootstrapped 95% confidence intervals (500 estimates). This approach demonstrates the potential impact of race/ethnic disparities in prevalence of risk factors for race/ethnic disparities in cognitive outcomes, which is a more sensitive and prudent approach to investigating disparities than interaction-term p-values alone.34 In exploratory analyses, we stratified our analyses by both race/ethnicity and age-group. Pooled models adjust for participant education, parental education, gender, indicators for race/ethnicity, and linear terms for age at KHANDLE Wave I, and age at MHC. All analyses were performed in 2020 using Stata 14.2 (StataCorp, College Station, TX).
Results
Participants had a mean age of 75.8 (SD 6.5) years at KHANDLE Wave 1 (2017–2018) and a mean age of 38.7 (SD 6.5) at MHC, with no significant differences in MHC age by race/ethnicity (Table 1). Among those assessed when they were ages 30 to 39 years, 47.9% had at least one CVRF, while 62.0% and 72.5% of those assessed when ages 40 to 49 or 50 to 59 years had at least one CVRF, respectively. About 21% of the sample were classified as hypertensive at their mid-life visit with the highest prevalence among Blacks (30.1%) and the lowest among Whites (14.8%). Hyperlipidemia was present among 22.4% of our sample, with similar prevalence across racial/ethnic groups. About 38% of participants were overweight/obese at midlife, though substantially higher prevalence was observed among African Americans (53.4%) and Latinos (49.3%) compared to Asians (17.3%) or Whites (30.2%). Prevalence of all CVRF increased with age.
Table 1.
Demographic Characteristics of the KHANDLE Sample.
| Pooled (n=1127) | Asian (n=289) | Black (n=332) | Latino (n=229) | White (n=277) | |
|---|---|---|---|---|---|
| Mean (SD) or n (column %) | |||||
|
| |||||
| KHANDLE Age (range= 65–98), mean (SD) | 75.8 (6.5) | 75.5 (6.2) | 75.6 (6.6) | 75.7 (6.2) | 76.4 (6.9) |
| MHC Age, mean (sd) | 38.7 (6.5) | 38.3 (6.3) | 38.4 (6.6) | 38.6 (6.1) | 39.6 (6.9) |
| Ages 30–39, n (%) | 721 (64.0) | 191 (66.1) | 215 (65.0) | 150 (65.5) | 165 (59.6) |
| Ages 40–49, n (%) | 326 (28.9) | 86 (29.8) | 91 (27.3) | 65 (28.4) | 84 (30.3) |
| Ages 50–59, n (%) | 80 (7.1) | 12 (4.2) | 26 (7.8) | 14 (6.1) | 28 (10.1) |
| Follow-up time (range=30–41 years), mean (SD) | 37.1 (2.2) | 37.2 (2.2) | 37.2 (2.3) | 37.1 (2.2) | 36.8 (2.2) |
| Female, n (%) | 668 (59.3) | 152 (52.6) | 220 (66.3) | 139 (60.7) | 157 (56.7) |
| Participant Education, mean (SD) | 14.7 (3.2) | 15.6 (2.5) | 14.3 (2.8) | 13.1 (4.0) | 15.7 (2.7) |
| Maternal Education, mean (SD) | 8.4 (5.5) | 7.8 (5.8) | 8.2 (5.2) | 6.3 (5.2) | 10.9 (4.9) |
| Paternal Education, mean (SD) | 8.2 (6.4) | 9.4 (6.7) | 6.1 (5.6) | 6.2 (6.2) | 11.2 (5.6) |
| Systolic BP, mean (SD) | 119.0 (15.6) | 117.2 (16.2) | 121.6 (15.5) | 118.0 (15.0) | 118.9 (15.3) |
| Diastolic BP, mean (SD) | 73.1 (10.1) | 72.5 (10.4) | 75.2 (10.2) | 72.9 (9.8) | 71.5 (9.5) |
| Hypertension, n (%) | 240 (21.3) | 56 (19.4) | 100 (30.1) | 43 (18.8) | 41 (14.8) |
| HTN Ages 30–39: | 125 (17.3) | 30 (15.7) | 53 (24.7) | 23 (15.3) | 19 (11.5) |
| HTN Ages 40–49: | 84 (25.8) | 21 (24.4) | 35 (38.5) | 14 (21.5) | 14 (16.7) |
| HTN Ages 50–59: | 31 (38.8) | 5 (41.7) | 12 (46.2) | 6 (42.9) | 8 (28.6) |
| Total Cholesterol, mean (SD) | 210.7 (39.3) | 211.6 (41.3) | 210.4 (38.6) | 210.1 (41.0) | 210.5 (36.7) |
| Hyperlipidemia, n (%) | 241 (22.4) | 64 (22.7) | 69 (22.4) | 47 (21.5) | 61 (22.9) |
| Hyperlip. Ages 30–39: | 121 (17.4) | 32 (17.3) | 33 (16.2) | 25 (17.5) | 31 (19.1) |
| Hyperlip. Ages 40–49: | 84 (27.5) | 25 (29.4) | 23 (28.4) | 16 (25.8) | 20 (25.6) |
| Hyperlip. Ages 50–59: | 36 (47.4) | 7 (58.3) | 13 (56.5) | 6 (42.9) | 10 (37.0) |
| BMI, mean (SD) | 24.5 (4.4) | 22.6 (3.2) | 26.1 (5.3) | 25.5 (4.3) | 24.0 (3.6) |
| Overweight/Obese, n (%) | 389 (37.7) | 45 (17.3) | 159 (53.4) | 107 (49.3) | 78 (30.2) |
| Ages 30–39: | 235 (35.1) | 27 (15.7) | 95 (47.5) | 66 (46.5) | 47 (30.1) |
| Ages 40–49: | 117 (40.3) | 16 (20.8) | 48 (63.2) | 33 (54.1) | 20 (26.3) |
| Ages 50–59: | 37 (50.7) | 2 (18.2) | 16 (72.7) | 8 (57.1) | 11 (42.3) |
| 1 CV Risk | 383 (33.9) | 71 (24.6) | 130 (38.9) | 80 (34.9) | 102 (36.8) |
| Ages 30–39: | 227 (31.4) | 37 (19.4) | 78 (35.9) | 48 (32.0) | 64 (38.8) |
| Ages 40–49: | 134 (41.1) | 33 (38.4) | 45 (49.5) | 26 (40.0) | 30 (35.7) |
| Ages 50–59: | 22 (27.5) | 1 (8.3) | 7 (26.9) | 6 (42.9) | 8 (28.6) |
| 2+ CV Risks | 214 (19.1) | 41 (14.2) | 87 (26.1) | 51 (22.6) | 35 (12.7) |
| Ages 30–39: | 119 (16.5) | 24 (12.6) | 49 (22.6) | 31 (20.7) | 15 (9.1) |
| Ages 40–49: | 68 (20.9) | 13 (15.1) | 27 (29.7) | 17 (26.2) | 11 (13.1) |
| Ages 50–59: | 36 (45.0) | 6 (50.0) | 14 (53.9) | 6 (42.9) | 10 (35.7) |
In linear regression models, the presence of each CVRF was significantly and negatively associated with all cognitive domains, except for hyperlipidemia with semantic memory (Table 2). For hypertension, we observed significant negative associations of about 1/5th standard deviation lower cognition in all domains except semantic memory. Midlife hyperlipidemia was associated with a −0.18 (95% CI:−0.29, −0.06) standard deviation difference in late-life global cognition, while having a midlife BMI ≥25 was associated with a −0.14 (95% CI:−0.25, −0.04) to −0.23 (95% CI:−0.34, −0.11) standard deviation difference in late-life cognition across all cognitive domains. Compared with having no CVRF, having any 1 or 2+ CVRF was associated with lower cognitive function across all cognitive domains (global cognition: 1 CVRF β =−0.18; 95% CI: −0.28, −0.08; 2+ CVRF β =−0.33; 95% CI: −0.45, −0.21). We also identified a stepwise gradient where having 2+ CVRF was associated with significantly lower verbal episodic memory (Wald test p=0.01) and global cognition (Wald test p=0.02) than having 1 CVRF.
Table 2.
Linear regression estimating the association of mid-life cardiovascular risk factors and late-life cognition, KHANDLE Wave 1 (2017–2018).
| Executive Function | Semantic Memory | Verbal Episodic Memory | Global Cognition | |
|---|---|---|---|---|
| Hypertension (ref= no) | −0.18 (−0.29, −0.06) | −0.11 (−0.22, −0.001) | −0.19 (−0.32, −0.07) | −0.19 (−0.30, −0.08) |
| Hyperlipidemia (ref=no) | −0.15 (−0.26, −0.03) | −0.11 (−0.23, 0.001) | −0.16 (−0.28, −0.03) | −0.18 (−0.29, −0.06) |
| BMI>25 (ref: BMI<25) | −0.15 (−0.26, −0.04) | −0.14 (−0.25, −0.04) | −0.23 (−0.34, −0.11) | −0.21 (−0.31, −0.11) |
| CVRF | ||||
| 0 CVRF | Ref | Ref | Ref | Ref |
| 1 CVRF | −0.15 (−0.25, −0.04) | −0.16 (−0.26, −0.05) | −0.15 (−0.26, −0.03) | −0.18 (−0.28, −0.08) |
| 2 CVRF | −0.26 (−0.39, −0.13) | −0.20 (−0.33, −0.07) | −0.34 (−0.48, −0.20) | −0.33 (−0.45, −0.21) |
All models adjusted for age at KHANDLE, age at MHC, gender, race/ethnicity, participant education and parental education. Cognitive domain scores were z-standardized to the full KHANDLE Wave 1 sample (mean=0; SD=1).
CVRF Associations by Race/Ethnicity
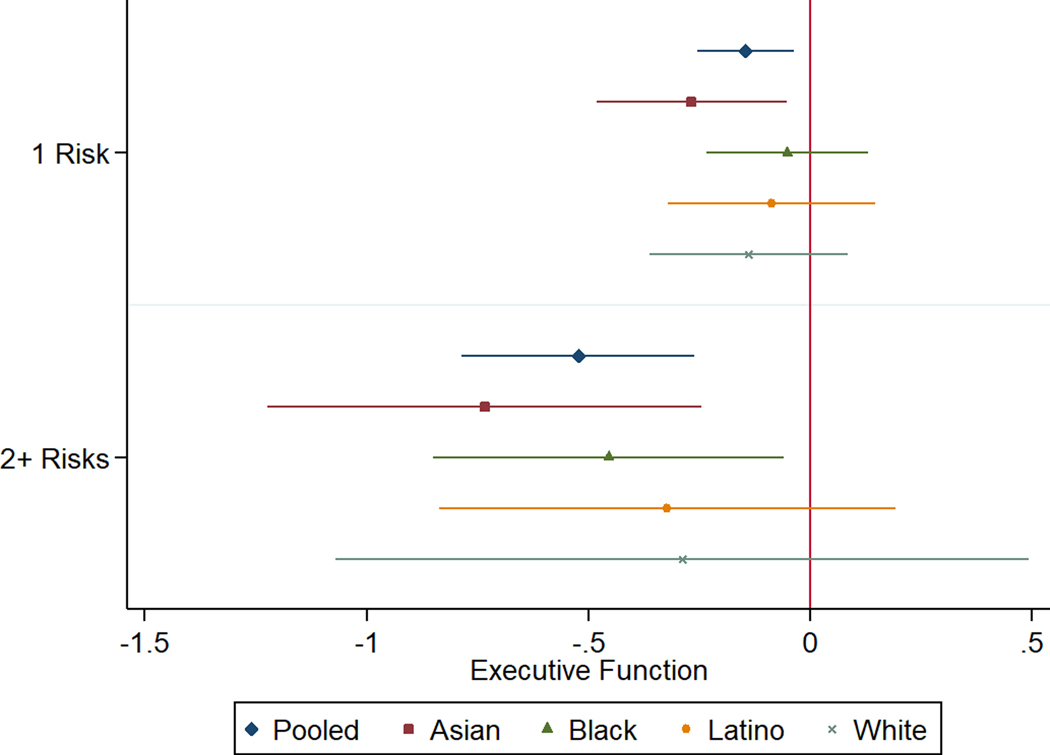
We did not observe any statistically significant interactions between CVRF and race/ethnicity on late-life cognition, though point estimates often differed between racial/ethnic groups to an extent that would be substantively important if confirmed (Supplemental Table 1). Differences in prevalence of CVRF across racial/ethnic groups imply a differential total impact of CVRF on average cognitive scores. Combining race/ethnicity-specific prevalence of each CVRF with race/ethnicity-specific regression coefficients for the individual cognitive impact of each CVRF, we can estimate the potential impact of CVRF on cognition for each race/ethnic group. For example, the prevalence of BMI ≥25 was 49.3% in Latinos and the impact of BMI ≥25 on verbal episodic memory for Latinos was −0.29 (95% CI: −0.53, −0.06), resulting in a total impact of BMI ≥25 among Latinos of −0.27 (95% CI: −0.40, −0.14) on verbal episodic memory. In contrast for Asian participants, the prevalence of BMI ≥25 was only 17.3%; the estimated effect of BMI ≥25 on verbal episodic memory for Asian participants was −0.16 (−0.42, 0.10); in combination this suggests a net effect of BMI ≥25 on Asian participants overall of −0.06 (95% CI: −0.12, −0.003), (Figure 1). Overall, for verbal episodic memory, eliminating overweight/obesity would have the greatest benefit for Black, Latino, and White participants, but eliminating hypercholesterolemia would have the greatest benefit for Asian participants. For semantic memory and executive function, patterns were similar, with overweight/obesity having the largest effect for Black, Latino, and White participants (though hypercholesterolemia effects on semantic memory was very similar for Whites) and hypercholesterolemia the largest effect for Asian participants (though hypertension for Asians had a nearly identical effect on executive function (Figures 2, 3). Overall, eliminating any two or more CVRF was anticipated to have a large effect on cognition for all race/ethnic groups (global cognition = −1.50, 95% CI: −1.81, −1.19), (Supplemental Figure 1).
FIGURE 1.
Estimated population effect of each CVRF on verbal episodic memory within each racial/ethnic group, based on the race/ethnic-specific prevalence of the risk factor and the race/ethnic-specific estimated impact of the risk factor on verbal episodic memory (from linear regression). 95% confidence intervals were bootstrapped. These estimates indicate the total impact of each CVRF on the average verbal episodic memory scores of each racial/ethnic group, comparing observed CVRF prevalence to eliminating the CVRF.
FIGURE 2.
Estimated population effect of each CVRF on semantic memory within each racial/ethnic group, based on the race/ethnic-specific prevalence of the risk factor and the race/ethnic-specific estimated impact of the risk factor on semantic memory (from linear regression). 95% confidence intervals were bootstrapped. These estimates indicate the total impact of each CVRF on the average semantic memory scores of each racial/ethnic group, comparing observed CVRF prevalence to eliminating the CVRF.
FIGURE 3.
Estimated effect of each CVRF on executive function within each racial/ethnic group, based on the race/ethnic-specific prevalence of the risk factor and the race/ethnic-specific effect of the risk factor on executive function (from linear regression). 95% confidence intervals were bootstrapped. These estimates indicate the total impact of each CVRF on the average executive function scores of each racial/ethnic group, comparing observed CVRF prevalence to eliminating the CVRF.
CVRF Associations by Age
We observed a significant interaction between age at MHC and having a BMI ≥25 (p=0.03). Supplemental Figure 2 shows the predicted executive function values for having low versus high BMI among each age group when all other covariates are at their means. Those with high BMI at ages 30–39 had the strongest association with executive function (β=−0.19, 95% CI: −0.33, −0.06), while the effect was non-significant at ages 40–49 (β=−0.13, 95% CI: −0.33, 0.08) and non-significant albeit positively associated with executive function at ages 50–59 (β=0.25, 95% CI: −0.26, 0.75). This pattern was not seen for other cognitive domains or CVRFs (Supplemental Table 2).
Discussion
We observed markedly higher prevalence of young to mid adulthood hypertension among Black participants and higher prevalence of young to mid-adulthood overweight/obesity among Blacks and Latino participants, while Asians had the lowest prevalence. There were no differences in young to mid-adulthood prevalence of hyperlipidemia by race/ethnicity. In linear regression models, we found that hypertension, hyperlipidemia, and being overweight assessed at ages 30–59 was associated with poorer late-life cognition in this diverse cohort. We did not observe any significant interactions by race/ethnicity for the estimated effects of any CVRF, though differences in the prevalence of the CVRFs implied that the adverse impact of CVRFs on cognition varied by group. For Black, Latino, and White participants, BMI ≥25 had the largest net impact on cognition. Among Asian participants, hypercholesterolemia had a bigger impact, primarily because of the low prevalence of BMI ≥25 among Asian participants. It is possible that using different BMI cut-offs for Asians or an alternative measure such as body fat percentage would yield different results.35,36 Our findings lend support to the hypothesis that racial/ethnic differences in CVRF prevalence may contribute to racial/ethnic disparities in late-life cognitive outcomes, even when the effect of CVRF on late-life cognition is stable across groups. We also observed one age-trend: BMI ≥25 at ages 30–39 was associated with worse executive function, but this was not the case for BMI ≥25 after age 50. Because of the higher prevalence of BMI ≥25 among Blacks and Latinos, which we observe is prominent by ages 30–39, earlier onset of high BMI may help to explain some of the disparities in late-life cognition in these groups.
This study supports and extends prior research findings. Several studies have found that CVRF among Blacks and/or Latinos are associated with late-life cognition,4,15,16,37,38 and some have found that CVRF have larger effects on late-life cognition for some race/ethnic groups than others.17–19,37 Our study adds to this literature as the only study to our knowledge that examines racial/ethnic differences in the association of CVRF and late-life cognition in a sample of similar proportions of Asians, Blacks, Latinos and Whites. Our study is also unique in its ability to leverage CVRF that were measured more than three decades prior to late-life cognitive assessment, reducing potential bias from reverse causality.
A key limitation of this study was the inability to thoroughly examine how CVRF age differences may vary by race/ethnicity due to power restrictions and the single measurement of cognition. Additionally, our study defined CVRF from a singular dichotomous measure, rather than using continuous and/or multiple measures of exposure during the age period of interest. We also defined age of CVRF onset based on the participant’s first MHC, which could have misclassified some participants who may have developed the condition prior to attending a MHC. Cohort participants were not excluded for history of stroke or psychiatric conditions, though all were community dwelling which likely limited participation among those with severe cognitive impacts from these conditions. Lastly, too few individuals had blood glucose measures from MHC that would classify them as diabetic, precluding analysis of this important risk factor. However, as additional waves of data are collected, future research will explore associations of CVRF, including blood glucose, as continuous and repeated exposures with both baseline cognition and rate of cognitive change.
Nonetheless, our findings highlight the public health importance of CVRF developed earlier in the lifecourse than has been previously studied for late-life cognition across racially/ethnically diverse populations. CVRF identified prior to midlife may contribute to poorer late-life cognition and the accumulation of multiple CVRF may increase the magnitude of this effect. Future studies should continue to examine how racial/ethnic differences in the prevalence of CVRF at different ages are associated with differential effects on late-life cognition, cognitive decline, and incident dementia. Although our study is the first to support harmonized analyses of Black, White, Latino, and Asian participants, more research in this area is needed. Evaluating how both the prevalence of CVRF and the effect of exposure to CVRF are associated with late-life cognition in diverse racial/ethnic groups can help prioritize population health interventions to reduce racial/ethnic disparities. Assumptions that CVRF contribute in the same way to cognitive aging outcomes across diverse populations may miss important opportunities for interventions among vulnerable populations. By contrast, fully exploring and understanding these patterns could point to key mechanisms that provide opportunities for targeted intervention to reduce racial/ethnic disparities in age-related cognitive outcomes among our increasingly diverse aging population.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING: This work was funded by the National Institute on Aging, National Institutes of Health under grant number 7RF1AG052132-02.
Footnotes
No conflicts of interest were reported by the authors of this paper.
References
- 1.Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimer’s Dement. 2016;12(3):216–224. doi: 10.1016/j.jalz.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayeda ER, Karter AJ, Huang ES, Moffet HH, Haan MN, Whitmer RA. Racial/ethnic differences in dementia risk among older type 2 diabetic patients: the diabetes and aging study. Diabetes Care. 2014;37(4):1009–1015. doi: 10.2337/dc13-0215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–2734. doi: 10.1016/S0140-6736(17)31363-6 [DOI] [PubMed] [Google Scholar]
- 4.Gottesman R, Fornage M, Knopman D, Mosley T. Brain Aging in African-Americans: The Atherosclerosis Risk in Communities (ARIC) Experience. Curr Alzheimer Res. 2015;12(7):607–613. doi: 10.2174/1567205012666150701102445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: A meta-analysis of prospective studies. Obes Rev. 2011;12(5):e426–e437. doi: 10.1111/j.1467-789X.2010.00825.x [DOI] [PubMed] [Google Scholar]
- 6.Tolppanen AM, Solomon A, Soininen H, Kivipelto M. Midlife vascular risk factors and Alzheimer’s disease: Evidence from epidemiological studies. J Alzheimer’s Dis. 2012;32(3):531–540. doi: 10.3233/JAD-2012-120802 [DOI] [PubMed] [Google Scholar]
- 7.Anstey KJ, Lipnicki DM, Low LF. Cholesterol as a risk factor for dementia and cognitive decline: A systematic review of prospective studies with meta-analysis. Am J Geriatr Psychiatry. 2008;16(5):343–354. doi: 10.1097/01.JGP.0000310778.20870.ae [DOI] [PubMed] [Google Scholar]
- 8.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health and Nutrition Examination Survey 1999–2002. Diabetes Care. 2006;29(6):1263–1268. doi: 10.2337/dc06-0062 [DOI] [PubMed] [Google Scholar]
- 9.Hajjar I, Kotchen TA. Trends in Prevalence, Awareness, Treatment, and Control of Hypertension in the United States, 1988–2000. J Am Med Assoc. 2003;290(2):199–206. doi: 10.1001/jama.290.2.199 [DOI] [PubMed] [Google Scholar]
- 10.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284. doi: 10.1001/jama.2016.6458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borrell LN. Race, ethnicity, and self-reported hypertension: Analysis of data from the national health interview survey, 1997–2005. Am J Public Health. 2009;99(2):313–319. doi: 10.2105/AJPH.2007.123364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skinner AC, Skelton JA. Prevalence and trends in obesity and severe obesity among children in the united states, 1999–2012. JAMA Pediatr. 2014;168(6):561–566. doi: 10.1001/jamapediatrics.2014.21 [DOI] [PubMed] [Google Scholar]
- 13.Dabelea D, Bell RA, D’Agostino RB, et al. Incidence of diabetes in youth in the United States. J Am Med Assoc. 2007;297(24):2716–2724. doi: 10.1001/jama.297.24.2716 [DOI] [PubMed] [Google Scholar]
- 14.Muntner P, He J, Cutler JA, Wildman RP, Whelton PK. Trends in Blood Pressure among Children and Adolescents. J Am Med Assoc. 2004;291(17):2107–2113. doi: 10.1001/jama.291.17.2107 [DOI] [PubMed] [Google Scholar]
- 15.Levine DA, Gross AL, Briceño EM, et al. Association Between Blood Pressure and Later-Life Cognition Among Black and White Individuals. JAMA Neurol. April 2020. doi: 10.1001/jamaneurol.2020.0568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noble JM, Manly JJ, Schupf N, Tang MX, Luchsinger JA. Type 2 diabetes and ethnic disparities in cognitive impairment. Ethn Dis. 2012;22(1):38–44. doi: 10.13016/gt2j-7why [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arvanitakis Z, Capuano AW, Bennett DA, Barnes LL. Body mass index and decline in cognitive function in older black and white persons. Journals Gerontol - Ser A Biol Sci Med Sci. 2018;73(2):198–203. doi: 10.1093/gerona/glx152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson RS, Rajan KB, Barnes LL, Weuve J, Evans DA. Factors Related to Racial Differences in Late-Life Level of Cognitive Function. Neuropsychology. 2016;30(5):517–524. doi: 10.1037/neu0000290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dore GA, Waldstein SR, Evans MK, Zonderman AB. Associations Between Diabetes and Cognitive Function in Socioeconomically Diverse African American and White Men and Women. Psychosom Med. 2015;77(6):643–652. doi: 10.1097/PSY.0000000000000196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64(2):277–281. doi: 10.1212/01.WNL.0000149519.47454.F2 [DOI] [PubMed] [Google Scholar]
- 21.Gilsanz P, Mayeda ER, Glymour MM, et al. Female sex, early-onset hypertension, and risk of dementia. Neurology. 2017;89(18):1886–1893. doi: 10.1212/WNL.0000000000004602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Exalto LG, Quesenberry CP, Barnes D, Kivipelto M, Biessels GJ, Whitmer RA. Midlife risk score for the prediction of dementia four decades later. Alzheimer’s Dement. 2014;10(5):562–570. doi: 10.1016/j.jalz.2013.05.1772 [DOI] [PubMed] [Google Scholar]
- 23.Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA. Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement Geriatr Cogn Disord. 2009;28(1):75–80. doi: 10.1159/000231980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitmer R, Gunderson E, Quesenberry C, Zhou J, Yaffe K. Body Mass Index in Midlife and Risk of Alzheimer Disease and Vascular Dementia. Curr Alzheimer Res. 2007;4(2):103–109. doi: 10.2174/156720507780362047 [DOI] [PubMed] [Google Scholar]
- 25.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Yaffe K. Obesity in middle age and future risk of dementia: A 27 year longitudinal population based study. Br Med J. 2005;330(7504):1360–1362. doi: 10.1136/bmj.38446.466238.E0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aljondi R, Szoeke C, Steward C, Gorelik A, Desmond P. The effect of midlife cardiovascular risk factors on white matter hyperintensity volume and cognition two decades later in normal ageing women. Brain Imaging Behav. 2020;14(1):51–61. doi: 10.1007/s11682-018-9970-5 [DOI] [PubMed] [Google Scholar]
- 27.Kazlauskaite R, Janssen I, Wilson RS, et al. Is Midlife Metabolic Syndrome Associated With Cognitive Function Change? The Study of Women’s Health Across the Nation. J Clin Endocrinol Metab. 2020;105(4). doi: 10.1210/clinem/dgaa067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai MMY, Sharman MJ, Ames DJ, et al. Relationship of established cardiovascular risk factors and peripheral biomarkers on cognitive function in adults at risk of cognitive deterioration. J Alzheimer’s Dis. 2020;74(1):163–171. doi: 10.3233/JAD-190953 [DOI] [PubMed] [Google Scholar]
- 29.Khezrian M, Waymont JMJ, Myint PK, et al. Aspirin moderates the association between cardiovascular risk, brain white matter hyperintensity total lesion volume and processing speed in normal ageing. Maturitas. 2020;133:49–53. doi: 10.1016/j.maturitas.2020.01.001 [DOI] [PubMed] [Google Scholar]
- 30.Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62(10):1556–1560. doi: 10.1001/archneur.62.10.1556 [DOI] [PubMed] [Google Scholar]
- 31.Mungas D, Reed BR, Haan MN, Gonzalez H. Spanish and English Neuropsychological Assessment Scales: Relationship to demographics, language, cognition, and independent function. Neuropsychology. 2005;19(4):466–475. doi: 10.1037/0894-4105.19.4.466 [DOI] [PubMed] [Google Scholar]
- 32.Mungas D, Reed BR, Crane PK, Haan MN, González H. Spanish and English Neuropsychological Assessment Scales (SENAS): Further Development and Psychometric Characteristics. Psychol Assess. 2004;16(4):347–359. doi: 10.1037/1040-3590.16.4.347 [DOI] [PubMed] [Google Scholar]
- 33.Endo A. A historical perspective on the discovery of statins. Proc Japan Acad Ser B Phys Biol Sci. 2010;86(5):484–493. doi: 10.2183/pjab.86.484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ward JB, Gartner DR, Keyes KM, Fliss MD, McClure ES, Robinson WR. How do we assess a racial disparity in health? Distribution, interaction, and interpretation in epidemiological studies. Ann Epidemiol. 2019;29:1–7. doi: 10.1016/j.annepidem.2018.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishida C, Barba C, Cavalli-Sforza T, et al. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–163. doi: 10.1016/S0140-6736(03)15268-3 [DOI] [PubMed] [Google Scholar]
- 36.Kapoor N, Furler J, Paul T V, Thomas N, Oldenburg B. The BMI-adiposity conundrum in South Asian populations: Need for further research. J Biosoc Sci. 2019;51(4):619–621. doi: 10.1017/S0021932019000166 [DOI] [PubMed] [Google Scholar]
- 37.Arvanitakis Z, Bennett DA, Wilson RS, Barnes LL. Diabetes and cognitive systems in older black and white persons. Alzheimer Dis Assoc Disord. 2010;24(1):37–42. doi: 10.1097/WAD.0b013e3181a6bed5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65(4):545–551. doi: 10.1212/01.wnl.0000172914.08967.dc [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.