Abstract
Objective:
We aimed to determine the effects of selective antegrade cerebral perfusion compared with other perfusion strategies on indices of cerebral blood flow, oxygenation, cellular stress, and mitochondrial function.
Methods:
One-week-old piglets (n = 41) were assigned to 5 treatment groups. Thirty-eight were placed on cardiopulmonary bypass. Of these, 30 were cooled to 18 °C and underwent deep hypothermic circulatory arrest (n = 10), underwent selective antegrade cerebral perfusion at 10 mL/kg/min (n = 10), or remained on continuous cardiopulmonary bypass (deep hypothermic cardiopulmonary bypass, n = 10) for 40 minutes. Other subjects remained on normothermic cardiopulmonary bypass (n = 8) or underwent sham surgery (n = 3). Novel, noninvasive optical measurements recorded cerebral blood flow, cerebral tissue oxyhemoglobin concentration, oxygen extraction fraction, total hemoglobin concentration, and cerebral metabolic rate of oxygen. Invasive measurements of cerebral microdialysis and cerebral blood flow were recorded. Cerebral mitochondrial respiration and reactive oxygen species generation were assessed after the piglets were killed.
Results:
During hypothermia, deep hypothermic circulatory arrest piglets experienced increases in oxygen extraction fraction (P < .001), indicating inadequate matching of oxygen supply and demand. Deep hypothermic cardiopulmonary bypass had higher cerebral blood flow (P = .046), oxyhemoglobin concentration (P = .019), and total hemoglobin concentration (P = .070) than selective antegrade cerebral perfusion, indicating greater oxygen delivery. Deep hypothermic circulatory arrest demonstrated worse mitochondrial function (P<.05), increased reactive oxygen species generation (P <.01), and increased markers of cellular stress (P<.01). Reactive oxygen species generation was increased in deep hypothermic cardiopulmonary bypass compared with selective antegrade cerebral perfusion (P<.05), but without significant microdialysis evidence of cerebral cellular stress.
Conclusions:
Selective antegrade cerebral perfusion meets cerebral metabolic demand and mitigates cerebral mitochondrial reactive oxygen species generation. Excess oxygen delivery during deep hypothermia may have deleterious effects on cerebral mitochondria that may contribute to adverse neurologic outcomes. We describe noninvasive measurements that may help guide perfusion strategies.
Keywords: antegrade cerebral perfusion, basic science, congenital, diffuse correlative spectroscopy, diffuse optical spectroscopy, hypothermic circulatory arrest, microdialysis, mitochondria
Graphical Abstract
SACP results in less cerebral ROS compared with DHCA and hypothermic CPB.
CENTRAL MESSAGE
SACP mitigates mitochondrial dysfunction and cellular injury that results from mismatched perfusion and metabolic demands during DHCA.
There has been significant improvement in survival among neonates undergoing cardiac surgery. Unfortunately, neurologic injury remains a persistent source of morbidity and mortality.1 The use of deep hypothermic circulatory arrest (DHCA) has been implicated as a source of neurologic injury in animal models,2 but human studies have not demonstrated superior outcomes with alternative perfusion methods, such as selective antegrade cerebral perfusion (SACP).3 Furthermore, human data have demonstrated different neurologic injury patterns among patients undergoing SACP compared with those undergoing DHCA, suggesting that both perfusion strategies can be deleterious.4 One possible explanation for the lack of superiority of one strategy over another is the variation with which SACP is practiced, even among high-volume centers. There remains debate regarding optimal flow rates, perfusate temperature, and methods of intraoperative neuromonitoring during these procedures, and such practice variation confounds current human data regarding optimal perfusion strategies and monitoring in neonatal cases requiring periods of corporeal circulatory arrest.
Matching perfusion supply to cerebral metabolic demand is the primary goal of perfusion strategies to minimize hypoxic-ischemic injury (undersupply) and cerebral edema (oversupply). Because current technology does not permit accurate, noninvasive measurements of cerebral blood flow (CBF), oxygen delivery, or the cerebral metabolic rate of oxygen (CMRO2), extant strategies of SACP are theoretical, institution-specific, and cannot be titrated to patient-specific needs. The major driver of cerebral metabolic demand is the cerebral mitochondria. Any cellular injury due to energetic mismatch originates as mitochondrial dysfunction, and mitochondrial dysfunction has been shown to be a critical inflection point for determining cellular survival or death after ischemia or other insults.5,6 Our group has previously demonstrated that DHCA is associated with decreased cerebral mitochondrial function in neonatal piglets.2 However, no studies have compared mitochondrial function in SACP and other perfusion strategies.
Using a neonatal swine model, we sought to determine the effects of SACP on CBF, oxygen delivery, and CMRO2 compared with alternative perfusion strategies, including DHCA and continuous cardiopulmonary bypass (CPB) at deep hypothermia (deep hypothermic cardiopulmonary bypass [DHCPB]) or normothermia. Novel, noninvasive frequency-domain diffuse optical spectroscopy (FD-DOS) and diffuse correlation spectroscopy (DCS) were used to continuously measure cerebral perfusion supply (ie, CBF and oxyhemoglobin concentration [HbO2]) and demand (ie, oxygen extraction fraction [OEF]). Resulting metabolic byproducts were analyzed with cerebral microdialysis (cMD) and high-resolution respirometry to quantify cerebral cellular stress and mitochondrial dysfunction associated with varying perfusion strategies. This multimodal assessment of cerebral metabolic supply and demand provides a comprehensive examination of the mechanisms underlying metabolic derangement. We hypothesized that SACP provides adequate oxygen delivery and that animals exposed to SACP would have improved cerebral mitochondrial function compared with animals exposed to DHCA.
MATERIALS AND METHODS
Animals and Study Design
All procedures were approved by the Children’s Hospital of Philadelphia Institutional Animal Care and Use Committee and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. One-week-old female piglets were premedicated with 20 mg/kg ketamine, followed by inhaled isoflurane in 100% oxygen. After endotracheal intubation, oxygen was weaned to room air. Animals were then assigned to 5 different treatment groups: DHCA (n = 10), DHCPB (n = 10), SACP (n = 10), normothermic CPB (n = 8), and sham (no bypass, n = 3). DHCA, DHCPB, and normothermic CPB animal cohorts were part of a previously published study comparing the mitochondrial effects of DHCA.2
Perioperative Monitoring
Our perioperative monitoring methods were described previously.2 Briefly, the right femoral vein and artery were cannulated for drug administration, and pressure monitoring, intravenous fentanyl (25–200 μg/kg/min), and dexmedetomidine (0.5–2 μg/kg/min) were used for anesthesia. Rectal and nasopharyngeal temperature probes were placed.
Operative Methods and Experimental Protocol
Our detailed operative methods have been described.2 Briefly, animals were assigned to normothermic bypass, DHCPB, DHCA, or SACP, and underwent median sternotomy and aortic and right atrial cannulation. CPB was initiated at 150 mL/kg/min to mimic conditions used at our institution. Animals assigned to normothermic bypass remained at normothermia for an identical duration of time as those assigned to other perfusion strategies. After establishment of stable flows, DHCPB, DHCA, and SACP animals were cooled no quicker than 1 °C/min. pH-stat was used during cooling and while cold. Alpha-stat was used at normothermia. Flow rates were adjusted to maintain mean arterial pressures 35 to 55 mm Hg (100–120 mL/kg/min) at deep hypothermia. At a nasopharyngeal temperature of 18 °C, DHCA animals underwent 40 minutes of DHCA, DHCPB animals remained at full flow CPB (100–120 mL/kg/min), and SACP animals underwent SACP (10 mL/kg/min) for 40 minutes. Forty minutes was chosen as the experimental period to mimic the duration of DHCA or SACP expected during complex procedures (eg, Norwood procedure).7,8 The SACP flow rate used is within the range used in extant neonatal swine model studies (5–50 mL/kg/min).9–15 There are no comparisons of neonatal and human flow rates during SACP, which is a limitation inherent to any such animal model. However, we chose this flow rate as analogous to what would be considered lower flow SACP (10–20 mL/kg/min) in neonatal humans.3,8,16
SACP was accomplished by advancing the aortic cannula into the right brachiocephalic artery during a brief period of circulatory arrest not exceeding 1 minute and snaring this vessel at the level of the cannula to prevent back-bleeding. After 40 minutes of SACP, the cannula was carefully withdrawn back into the ascending aorta during another brief period of circulatory arrest not exceeding 1 minute. After the experimental period, animals were rewarmed to normothermia. Sham animals had identical perioperative care and monitoring procedures, but did not undergo sternotomy or CPB cannulation, and were maintained at normothermia under general anesthesia for an identical period of time.
Measurement of Cerebral Microdialysis and Invasive Cerebral Blood Flow
Our methods have been described in detail.2 Briefly, cMD samples were collected from the left frontal cortex. Dialysate samples were collected at 20- to 30-minute intervals to correspond with initiation of CPB, cooling, deep hypothermia, rewarming, and normothermia. Sham and normothermic CPB animals had the same frequency of cMD collection. Invasive CBF was measured with laser Doppler (Periflux 5000, Perimed Inc, Las Vegas, Nev) using previously described methods.17
Measurement of Mitochondrial Respiration and Reactive Oxidative Species Generation
Our tissue extraction methods2 and our brain tissue preparation methods18 for high-resolution mitochondrial respirometry have been described in detail. Mitochondrial respiratory function was analyzed ex vivo in brain cortex homogenates using high-resolution respirometry (Oxygraph-2k, Oroboros Instruments, Innsbruck, Austria) with a substrate–uncoupler–inhibitor titration protocol as previously described.2,18 Primary variables collected included maximal oxidative phosphorylation (OXPHOS) and a measurement of the proton gradient across the inner mitochondrial membrane (inner mitochondrial membrane leak [LEAK]), as well as ex vivo measures of individual complex function along the electron transport system. Figure 1 provides a pictorial representation of the variables assessed during respirometry. In short, OXPHOS measures adenosine triphosphate (ATP) generation in mitochondria. Higher levels of OXPHOS indicate improved ATP generation and, thus, preserved mitochondrial function. LEAK is a measurement of the chemiosmotic gradient across the inner mitochondrial membrane. Increased LEAK, or increased permeability and therefore decreased chemiosmotic gradient, indicates decreased ATP generation capability of the mitochondria and, therefore, decreased mitochondrial function. A more global measurement of mitochondrial function can be derived by normalizing OXPHOS by LEAK, which generates a value of electron transport chain function (OXPHOS/LEAK) as a function of the efficiency of the inner mitochondrial membrane to allow for the most comprehensive assessment of all aspects of mitochondrial function and ATP production. This assessment is known as the respiratory control ratio (RCR) and has been used in prior studies measuring global mitochondrial function.2,5
FIGURE 1.
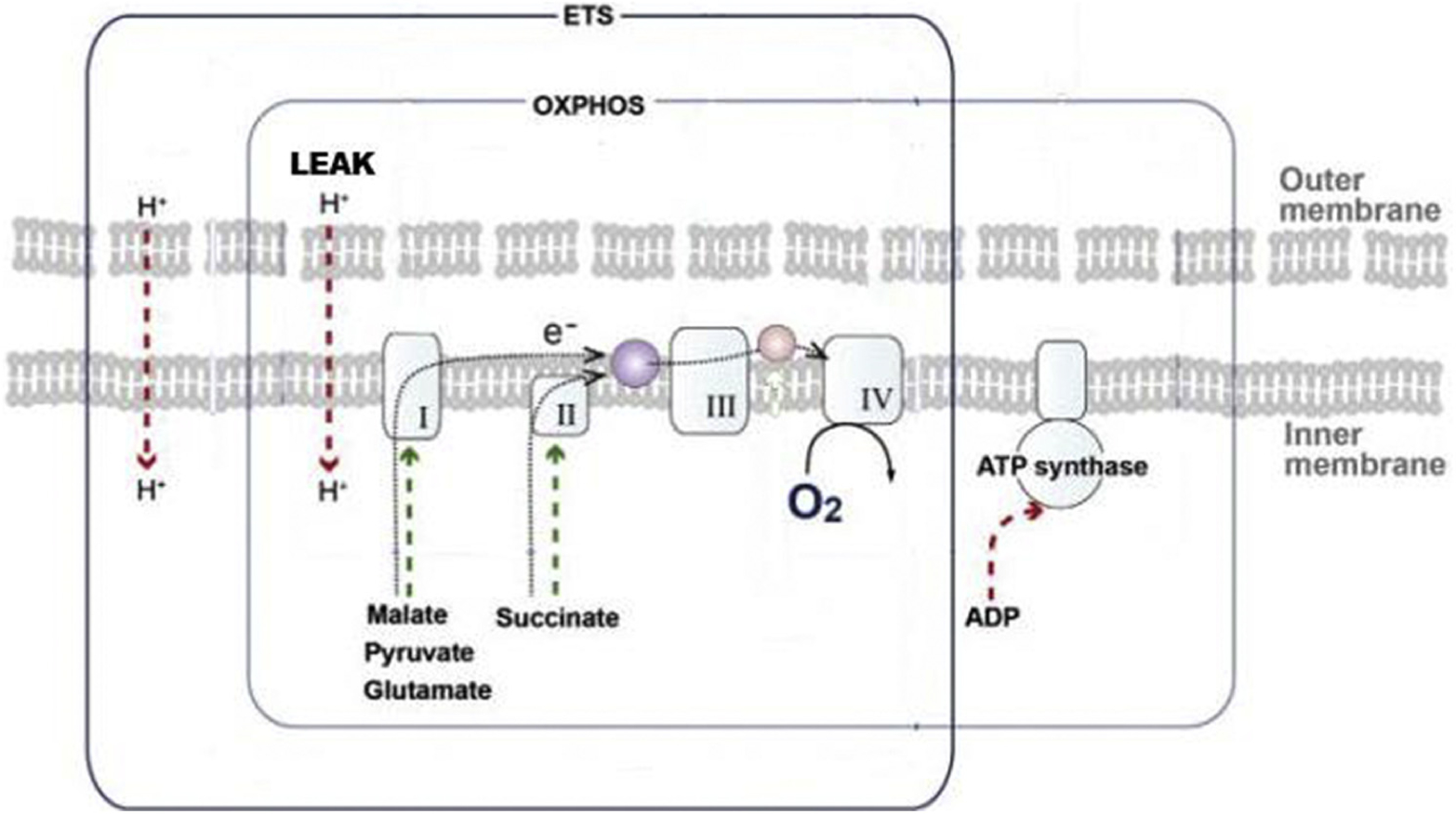
Representation of the electron transport system and its assessment via respirometry. LEAK assesses how well the chemiosmolar gradient can be maintained to drive ATP synthase, whereas maximal OXPHOS measures the ATP-generating capacity of complexes I to IVafter electron transport system substrate bombardment of mitochondria. ROS are concomitantly measured during respirometry. ETS, electron transport system; OXPHOS, oxidative phosphorylation; LEAK, inner mitochondrial membrane leak; ATP, adenosine triphosphate; ADP, adenosine diphosphate.
Reactive oxygen species (ROS) generation in brain tissue was measured using the O2k-Fluorescence LED2-Module (Oxygraph-2k, Oroboros Instruments), permitting simultaneous measurements of hydrogen peroxide (H2O2) production and mitochondrial respiration, using an Amplex UltraRed assay as previously described.2,19 Mitochondria generate the majority of ROS in the cell; at increased concentrations, ROS are highly toxic to proteins and nucleic acids and activate CASPASE-mediated apoptosis, leading to secondary neuronal death after a stressful insult.20,21
Noninvasive Measurement of Cerebral Hemodynamics
Noninvasive optical measurements of cerebral oxygenation, CBF, and CMRO2 were achieved using a combination of FD-DOS and DCS. These techniques have been validated in humans and animal models, and their methodologies have been described.17,22 Data-quality constraints were not satisfied in 3 SACP animals; therefore, 38 of 41 subjects were included in subsequent analyses of cerebral hemodynamics.
FD-DOS was performed using a customized commercial instrument (Imagent; ISS Inc Champaign, Ill). This permitted the continuous quantification of cerebral tissue [HbO2] and deoxyhemoglobin concentration from which total hemoglobin concentration (THC, μmol/L), tissue oxygen saturation (%), and OEF were derived as follows:
| (Equation 1) |
| (Equation 2) |
| (Equation 3) |
The arterial oxygen saturation (%) was sampled by arterial blood gas analysis at the start of bypass and assumed to remain constant, and the cerebral arteriovenous mixing fraction (γ) was assumed to be 0.75.23,24
DCS was performed concurrently to derive a blood flow index (BFI) using a laboratory-built system that has been described.17,25 Relative changes in BFI have been validated to correlate with relative CBF using numerous clinical gold standards.22,25 Moreover, these have been specifically validated in our animal model against invasive laser Doppler.17
Combining FD-DOS measures of OEF and DCS measures of BFI, rCMRO2 (%) was calculated using the Fick principle,17,26 assuming that the arterial blood oxygen concentration remained constant as follows:
| (Equation 4) |
Relative values were computed with respect to the mean value acquired within a 5-minute baseline period immediately before cooling in deep hypothermic animals or 25 minutes into normothermic or mild hypothermic bypass or sham exposure. With the addition of the 5-minute baseline period, this timing approximated the bypass duration before cooling in deep hypothermic animals.
Statistical Methods
Laboratory technicians analyzing mitochondrial function and cMD were blinded to treatment groups. Statistical analysis was carried out using GraphPad Prism software (GraphPad, San Diego, Calif). The Student t test was used for normally distributed data. RCRs were not normally distributed, and the Mann–Whitney nonparametric test was used. Normally distributed data are presented as mean ± standard error unless otherwise noted. For cMD intergroup comparisons, repeated-measures analysis of variance with Bonferroni correction was used to establish significant differences.
The mean value of cerebral hemodynamics during the coincident 40-minute experimental period (eg, DHCA, SACP) was compared between SACP animals and each alternative perfusion groups using the Wilcoxon rank-sum test. The sham group was omitted from comparisons due to sample size. The Benjamini–Yekutieli procedure for controlling the false discovery rate during multiple comparisons was also applied to identify parameters with adjusted P value less than .05. The false discovery rate (Q value) was specified as 0.05.
RESULTS
Baseline Characteristics, Blood Gas Chemistry, and Hemodynamic Variables
A summary of baseline characteristics (n = 41, mean weight 3.99 kg), as well as perioperative and intraoperative variables are shown in Table 1. Mean arterial blood pressure during noncirculatory arrest periods did not differ significantly between animal groups (data not shown). There were no differences in baseline blood gas measurements. Hematocrits remained at or above 30 to minimize confounding effects of hemodilution.27 Serum lactate levels were significantly elevated in DHCA animals by the end of rewarming compared with DHCPB animals.
TABLE 1.
Baseline group characteristics and intraoperative blood gas variables
| Variables | DHCA (n = 10) | DHCPB (n = 10) | SACP (n = 10) | CPB (n = 8) | Sham (n = 3) | P value DHCA vs DHCPB | P value DHCA vs SACP | P value DHCPB vs SACP |
|---|---|---|---|---|---|---|---|---|
| Weight (kg) | 3.79 (0.7) | 4.13 (0.5) | 4.01 (0.2) | 4.07 (0.4) | 3.5, 3.8, 3.9 (0.1) | .0580 | .0608 | .5643 |
| Cooling duration (min) | 27.92 (5.0) | 25.75 (5.7) | 22.98 (3.0) | n/a | .3826 | .0162 | .1943 | |
| Rewarming duration (min) | 28.46 (5.6) | 29.04 (6.8) | 28.58 (6.9) | n/a | .8364 | .9672 | .8802 | |
| Baseline Hct (%) | 32.64 (4.1) | 31.14 (3.0) | 31.60 (3.1) | 34.63 (3.9) | 21, 25, 29 (4.0) | .364 | .529 | .743 |
| Hct cooling (%) | 32.41 (6.5) | 32.07 (5.6) | 34.53 (3.2) | 35.33 (4.0) | .902 | .367 | .246 | |
| Hct hypothermia (%) | 30.25 (4.1) | 29.92 (5.9) | 33.81 (4.3) | 34.64 (5.1) | 20, 27, 27 (4.0) | .885 | .074 | .109 |
| Hct rewarming (%) | 31.32 (7.1) | 33.40 (5.6) | 33.47 (5.1) | 32.67 (2.5) | .478 | .447 | .978 | |
| Hct end (%) | 33.64 (4.0) | 31.58 (5.5) | 34.47 (4.5) | 33.50 (5.7) | 20, 21, 26 (5.7) | .352 | .668 | .213 |
| Baseline pH | 7.46 (0.1) | 7.48 (0.1) | 7.45 (0.1) | 7.48 (0.1) | 7.4, 7.42, 7.51 (0.1) | .694 | .683 | .390 |
| pH cooling | 7.45 (0.1) | 7.42 (0.1) | 7.47 (0.1) | 7.47 (0.04) | .357 | .553 | .170 | |
| pH hypothermia | 7.36 (0.2) | 7.33 (0.2) | 7.37 (0.1) | 7.41 (0.16) | 7.4, 7.46, 7.49 (0.05) | .756 | .826 | .529 |
| pH rewarming | 7.48 (0.2) | 7.49 (0.1) | 7.43 (0.1) | 7.43 (0.04) | .817 | .449 | .215 | |
| pH end | 7.36 (0.1) | 7.30 (0.1) | 7.42 (0.1) | 7.43 (0.1) | 7.38, 7.4, 7.46 (0.05) | .166 | .207 | .012 |
| Baseline PaO2 (mm Hg) | 240.6 (45.5) | 240.6 (69.1) | 299.2 (118.5) | 304.9 (58.1) | 463, 515, 525 (33.3) | .998 | .162 | .193 |
| PaO2 cooling (mm Hg) | 206.4 (59.5) | 262.9 (85.9) | 238.5 (61.6) | 304.3 (66.0) | .104 | .252 | .474 | |
| PaO2 hypothermia (mm Hg) | 241.0 (66.4) | 267.6 (65.4) | 269.4 (71.3) | 268.6 (82.6) | 109, 483, 556 (239.8) | .379 | .369 | .954 |
| PaO2 rewarming (mm Hg) | 139.4 (85.1) | 200.7 (65.3) | 206.9 (77.6) | 305.0 (6.1) | .087 | .080 | .848 | |
| PaO2 end (mm Hg) | 200.3 (71.5) | 214.7 (105.9) | 221.8 (92.2) | 257.4 (69.5) | 84, 124, 444 (160.4) | .726 | .567 | .874 |
| Baseline PaCO2 (mm Hg) | 38.27 (6.5) | 40.36 (5.7) | 47.60 (11.6) | 42.75 (8.0) | 38, 39, 49 (6.1) | .454 | .039 | .092 |
| PaCO2 cooling (mm Hg) | 38.47 (8.8) | 44.93 (14.6) | 39.82 (39.8) | 43.33 (2.1) | .245 | .714 | .337 | |
| PaCO2 hypothermia (mm Hg) | 55.92 (33.4) | 53.39 (30.8) | 48.85 (13.9) | 50.43 (25.3) | 39, 41, 51 (6.4) | .862 | .544 | .676 |
| PaCO2 rewarming (mm Hg) | 39.14 (20.0) | 34.60 (10.1) | 41.93 (8.1) | 41.33 (0.6) | .530 | .687 | .090 | |
| PaCO2 end (mm Hg) | 49.45 (16.8) | 57.83 (14.8) | 43.13 (6.8) | 47.62 (11.7) | 38, 43, 48 (4.5) | .252 | .284 | .011 |
| Baseline lactate (mmol/L) | 3.78 (2.1) | 3.71 (1.4) | 2.70 (0.9) | 2.38 (0.8) | 0.6, 0.9, 1.2 (0.3) | .933 | .148 | .062 |
| Lactate cooling (mmol/L) | 3.91 (1.1) | 3.14 (0.8) | 3.85 (1.1) | 4.40 (1.0) | .087 | .906 | .122 | |
| Lactate hypothermia (mmol/L) | 3.83 (1.3) | 3.98 (1.2) | 3.74 (1.0) | 3.43 (1.1) | 0.6, 0.9, 1 (0.2) | .800 | .853 | .627 |
| Lactate rewarming (mmol/L) | 5.95 (1.4) | 5.65 (2.1) | 5.36 (1.0) | 6.03 (1.3) | .707 | .296 | .702 | |
| Lactate end (mmol/L) | 6.84 (1.8) | 5.11 (1.8) | 5.71 (1.5) | 4.54 (1.8) | 0.5, 0.7, 0.7 (0.07) | .045 | .151 | .426 |
Data presented as mean (standard error of the mean). Bold indicates P value<.05. CPB, Cardiopulmonary bypass; DHCA, deep hypothermic circulatory arrest; DHCPB, deep hypothermic cardiopulmonary bypass; SACP, selective antegrade cerebral perfusion; Hct, hematocrit; PaO2, partial pressure of oxygen; PaCO2, partial pressure of carbon dioxide.
Cerebral Hemodynamics
Noninvasive and invasive measurements of CBF during the 40 minutes of the experimental period are shown in Figure 2. These parameters and their distributions are reported using the median and interquartile range. Relative parameters are reported as a percentage of the precooling baseline value. There is a cooling related decrease in CBF and CMRO2 at hypothermia, likely a reflection of decreased metabolic demand in the setting of intact flow-metabolism coupling.28 DHCA animals go to zero CBF, whereas normothermic animals retain baseline levels of CBF throughout. Invasive measurements show significantly higher CBF in DHCPB animals compared with SACP animals (Table 2). Noninvasive CBF measurements demonstrate comparable median values to invasive measurements in all groups. However, greater measurement variability, evidenced by a wider within-group distribution, limited the power of comparisons between DHCPB and SACP groups.
FIGURE 2.
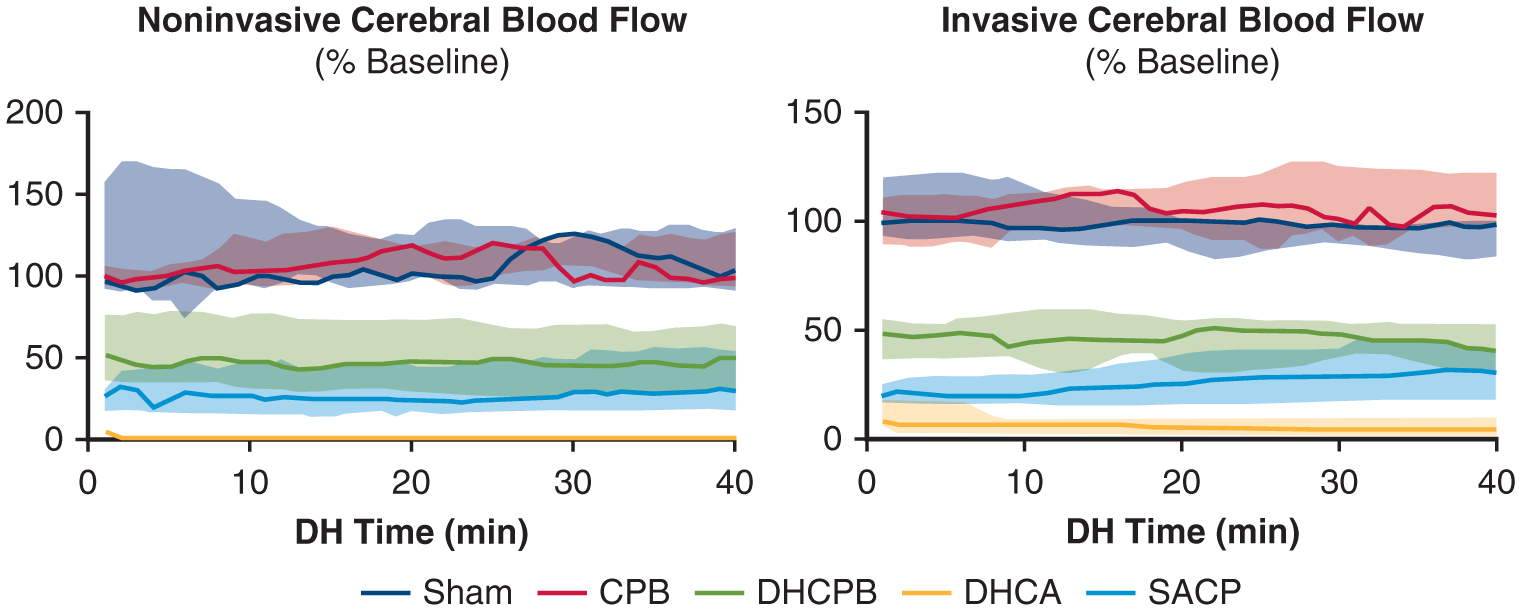
Noninvasive (DCS, left) and invasive (laser Doppler, right) measurements of CBF relative to precooling, baseline values during the 40-minute experimental period. Lines indicate mean values, and the shaded area indicates the interquartile range. Note the decrease in CBF noted in all hypothermic animals not seen in sham and normothermic animals. DHCA animals, expectedly, have zero flow. There is a significant difference between DHCPB and SACP animals in invasive CBF (P = .046) that does not reach significance in noninvasive measurements (P = .108). CBP, Cardiopulmonary bypass; DHCPB, deep hypothermic cardiopulmonary bypass; DHCA, deep hypothermic circulatory arrest; SACP, selective antegrade cerebral perfusion; DH, deep hypothermia.
TABLE 2.
Invasive and noninvasive cerebral hemodynamic parameters by group comparison with selective antegrade cerebral perfusion group
| Median [IQR] | Sham | CPB | DHCPB | DHCA | SACP |
|---|---|---|---|---|---|
| Noninvasive | |||||
| StO2 (%) | n = 3 | n = 8 | n = 10 | n = 10 | n = 7 |
| 60.0 [59.6, 61.3] | 55.5 [50.8, 59.0] | 73.5 [65.6, 75.4] | 37.0 [27.1, 42.2] | 64.4 [54.7, 67.7] | |
| P = .833 | P = .152 | P = .033 | P < .001 * | - | |
| r[HbO2] (%) | n = 3 | n = 8 | n = 10 | n = 10 | n = 7 |
| 102.1 [98.1, 104.4] | 101.2 [98.1, 105.2] | 136.8 [120.7, 151.0] | 53.1 [38.4, 65.6] | 110.5 [87.5, 117.6] | |
| P = .667 | P = .536 | P = .019 | P < .001 * | - | |
| rTHC (%) | n = 3 | n = 8 | n = 10 | n = 10 | n = 7 |
| 98.5 [96.7, 102.2] | 103.6 [97.7, 105.8] | 111.2 [101.2, 116.3] | 80.0 [74.9, 87.1] | 98.3 [95.6, 102.1] | |
| P = .833 | P = .397 | P = .070 | P < .001 * | - | |
| OEF | n = 3 | n = 8 | n = 10 | n = 10 | n = 7 |
| 0.5 [0.5, 0.5] | 0.6 [0.5, 0.6] | 0.3 [0.3, 0.4] | 0.8 [0.8, 1.0] | 0.5 [0.4, 0.6] | |
| P = .833 | P = .152 | P = .033 | P < .001 * | - | |
| rCBF DCS (%) | n = 3 | n = 7 | n = 8 | n = 9 | n = 6 |
| 102.7 [96.4, 134.0] | 105.0 [93.9, 120.2] | 46.1 [30.1, 72.3] | 1.5 [0.8, 1.9] | 26.3 [17.2, 48.5] | |
| P = .024 | P = .001 * | P = .108 | P < .001 * | - | |
| rCMRO2 (%) | n = 3 | n = 7 | n = 8 | n = 9 | n = 6 |
| 105.5 [97.0, 124.9] | 107.0 [91.6, 114.4] | 32.2 [24.2, 41.6] | 2.3 [1.1, 2.4] | 23.7 [13.6, 34.4] | |
| P = .024 | P = .001 * | P = .228 | P< .001 * | - | |
| Invasive | |||||
| rCBF LD (%) | n = 3 | n = 7 | n = 9 | n = 10 | n = 8 |
| 99.6 [88.5, 106.5] | 103.4 [94.5, 115.6] | 46.1 [34.2, 53.5] | 7.0 [2.4, 10.1] | 26.3 [17.1, 36.4] | |
| P = .012 * | P < .001 * | P = .046 | P = .002 * | - |
P value result from nonparametric Wilcoxon rank-sum test comparing each perfusion group with the SACP group. Relative (r) values computed with respect to mean baseline values before study intervention. Animals in which noninvasive optical data did not meet predetermined data quality criteria were excluded. These criteria have been described by Ko and colleagues.17 Bold indicates P value<.05. IQR, Interquartile range; CPB, normothermic cardiopulmonary bypass; DHCPB, deep hypothermic cardiopulmonary bypass; DHCA, deep hypothermic circulatory arrest; SACP, selective antegrade cerebral perfusion; StO2, tissue oxygen saturation; [HbO2], oxyhemoglobin concentration; THC, total hemoglobin concentration; OEF, oxygen extraction fraction; CBF, cerebral blood flow; rCBF, relative CBF; DCS, diffuse correlation spectroscopy; CMRO2, cerebral metabolic rate; rCMRO2, relative CMRO2; LD, laser Doppler.
P<.05 following false-positive rate repeated-comparisons correction.
Noninvasive measurements of [HbO2], THC, OEF, and CMRO2 during the 40 minutes of the experimental period are shown in Figure 3. Comparisons of the mean value during this period in the normothermic, DHCA, and DHCPB groups versus SACP are tabulated in Table 2. The consistent OEF demonstrated in all non-DHCA animal groups indicates that oxygen metabolism remained coupled with oxygen supply (ie, CBF) throughout the experimental period. SACP animals maintained CMRO2 levels commensurate with DHCPB animals. DHCA animals had zero calculated CMRO2 but had significantly increased oxygen extraction. Such a discrepancy is a limitation of the steady-state approximation of the Fick CMRO2 model, which relies on CBF (Equation 4).28
FIGURE 3.
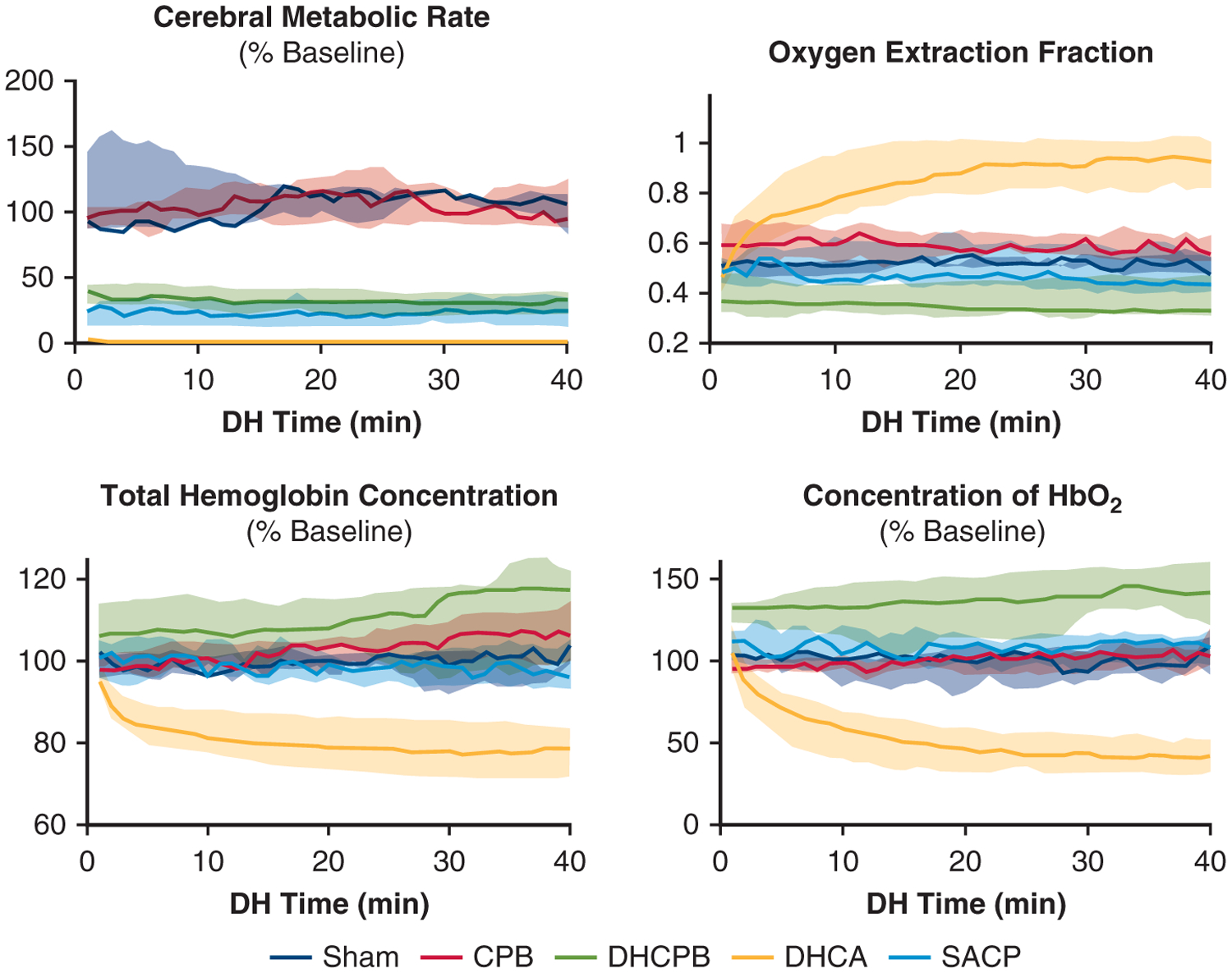
Noninvasive measurements of cerebral metabolic rate (CMRO2, top left), OEF (top right), THC (bottom left), and [HbO2] (bottom right). Lines indicate mean values, and the shaded area indicates the interquartile range. % baseline refers to the relative values compared with precooling, baseline values for animals. Note the stabilization of OEF and CMRO2 in all non-DHCA animal groups. There is a significant increase in [HbO2] among DHCPB animals that is well above baseline values (P = .019), indicating potential overdelivery of oxygen. There is a trend toward increased THC in DHCPB animals as well, but this does not reach significance (P = .070). CPB, Cardiopulmonary bypass; DHCPB, deep hypothermic cardiopulmonary bypass; DHCA, deep hypothermic circulatory arrest; SACP, selective antegrade cerebral perfusion.
Additionally, DHCPB animals trended toward increased THC and [HbO2] compared with normothermic and sham animals during the experimental period. This trend was not present in SACP animals in which both THC and [HbO2] were maintained at precooling baseline values. Such marked elevations in [HbO2] and THC indicate delivery that is significantly higher than baseline levels in DHCPB animals.
Cerebral Mitochondrial Respiration
The cortical RCR (described as the maximal OXPHOS normalized by mitochondrial inner membrane potential, OXPHOS/LEAK)2,5 for each cohort is shown in Figure 4. Higher levels indicate improved OXPHOS capacity of the mitochondria. DHCA animals had significantly worse function than any other perfusion strategy. Sham animals had significantly better function than all animal groups who were exposed to CPB. There were no differences among the non-DHCA perfusion strategies.
FIGURE 4.
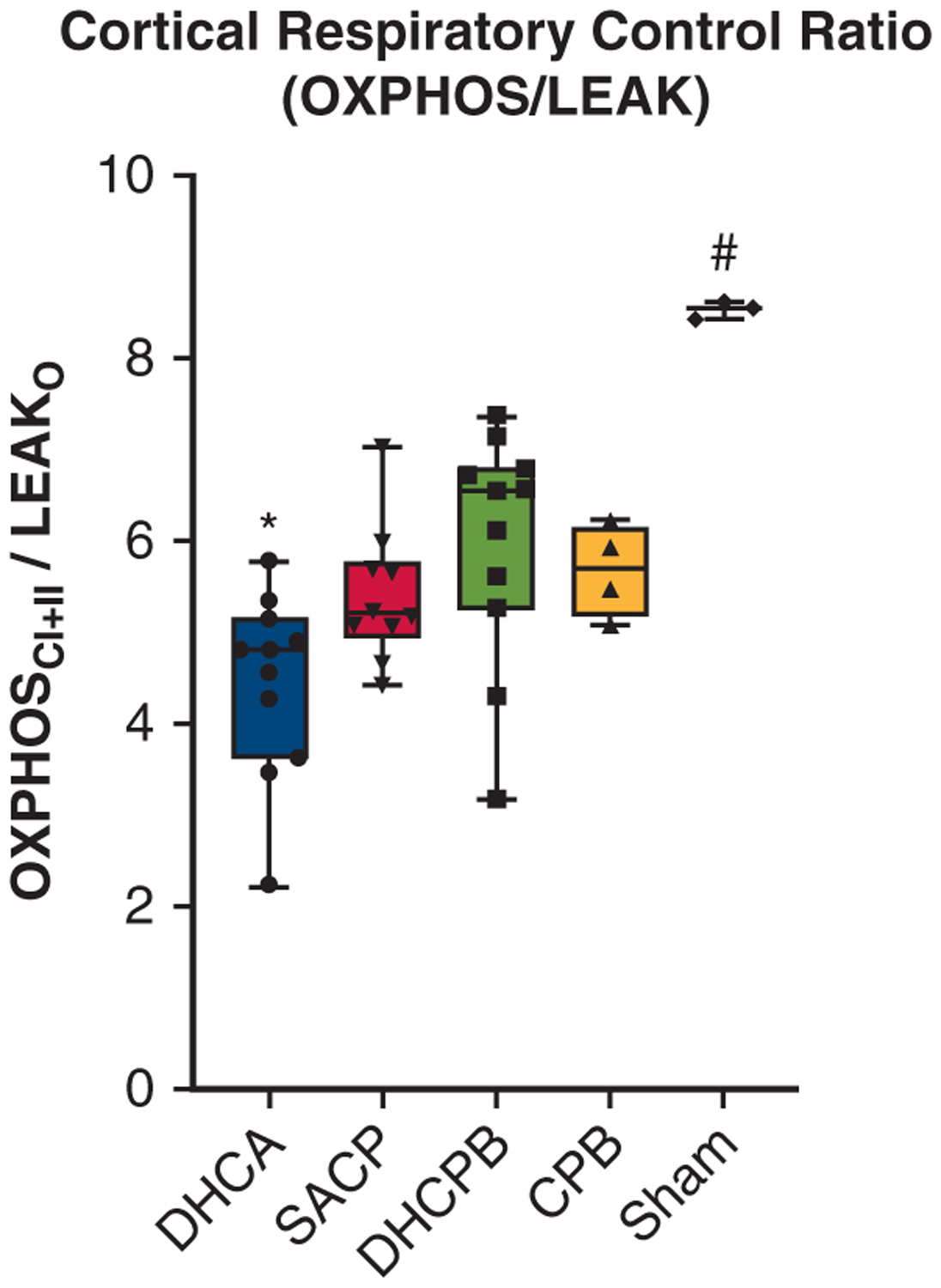
RCRs are derived from normalized measurements (maximal OXPHOS/LEAK) and globally assess cortical electron transport chain function. Data are presented for different groups. DHCA animals had significantly decreased values compared with all other perfusion strategies (*P<.05), whereas sham animals had significantly higher values than any group exposed to CPB (#P <.01). There were no significant differences among the non-DHCA groups exposed to CPB. OXPHOS, Oxidative phosphorylation; LEAK, inner mitochondrial membrane leak; DHCA, deep hypothermic circulatory arrest; SACP, selective antegrade cerebral perfusion; DHCPB, deep hypothermic cardiopulmonary bypass; CPB, cardiopulmonary bypass.
Cerebral Mitochondrial Reactive Oxygen Species Generation
Mitochondrial ROS generation data are shown in Figure 5. Higher levels indicate less efficient mitochondrial respiration and the potential for cellular injury and death. Among non-DHCA perfusion strategies, DHCPB animals had significantly higher ROS generation. Sham animals had the least ROS generation and DHCA animals had significantly higher ROS generation than any other group.
FIGURE 5.
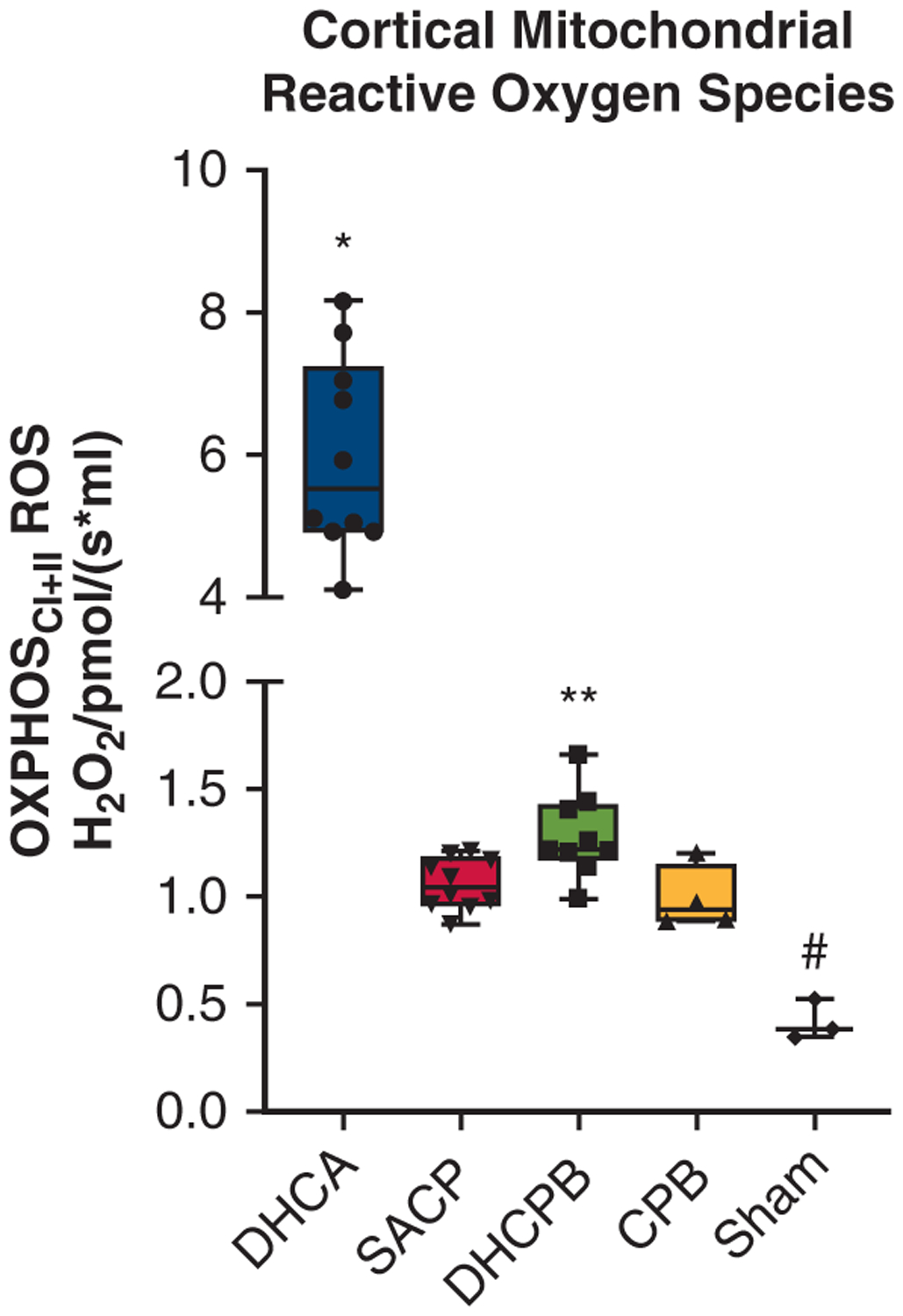
Cerebral mitochondrial ROS generation for the different groups. DHCA animals had significantly higher ROS generation than all other groups (*P <.01), and sham animals had significantly lower ROS generation compared with all groups (#P <.05). Among the non-DHCA groups, DHCPB had significantly increased ROS generation compared with both normothermic CPB and SACP animals (**P <.05). OXPHOS, Oxidative phosphorylation; ROS, reactive oxygen species; DHCA, deep hypothermic circulatory arrest; SACP, selective antegrade cerebral perfusion; DHCPB, deep hypothermic cardiopulmonary bypass; CPB, cardiopulmonary bypass.
Cerebral Microdialysis
Microdialysis data (Figure 6) demonstrated progressive increases in lactate:pyruvate ratio (LPR) and glycerol levels throughout the experimental period in DHCA animals relative to other perfusion strategies. The spike in LPR in DHCA animals was not seen in other perfusion strategies. There were no significant differences among the non-DHCA strategies. Sham animals had significantly lower LPR. Glycerol levels in DHCA animals significantly increased throughout the experiment and by comparison with all other non-DHCA groups. There were no significant different among non-DHCA groups. Sham animals had the lowest glycerol levels.
FIGURE 6.
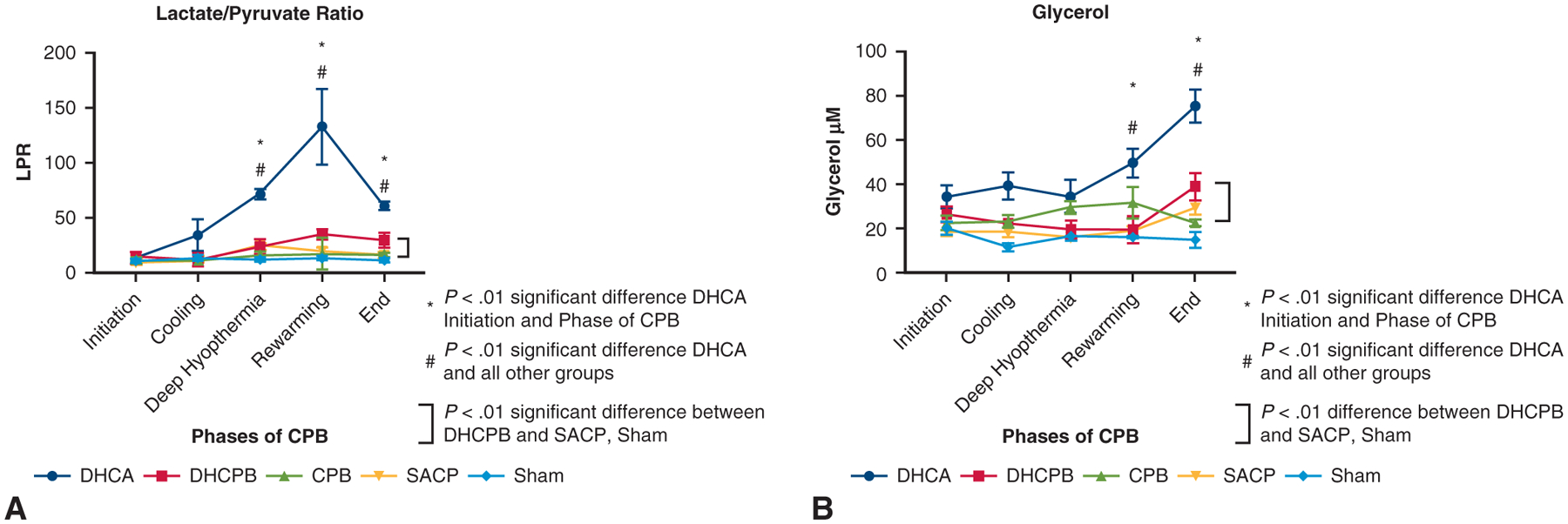
Longitudinal assessment of cMD measurements of (A) LPR and (B) glycerol levels among the different groups. DHCA animals had significantly increased LPR and glycerol levels compared with all other groups, sham animals had significantly lower LPR and glycerol levels compared with all other groups, and there were no significant differences among the non-DHCA animal groups that were exposed to CPB. LPR, Lactate:pyruvate ratio; DHCA, deep hypothermic circulatory arrest; DHCPB, deep hypothermic cardiopulmonary bypass; CPB, cardiopulmonary bypass; SACP, selective antegrade cerebral perfusion.
DISCUSSION
Our data provide evidence of ongoing derangements in cerebral metabolism during DHCA that lead to a mismatch of CBF and OEF, cerebral cellular injury, and mitochondrial dysfunction. We demonstrated that such mismatch of perfusion supply with cerebral metabolic demand can be attenuated with SACP, which results in less evidence of downstream cellular injury and mitochondrial dysfunction. Matching supply and demand is crucial to avoid secondary oxidative mitochondrial injury (increased ROS generation) associated with higher flow perfusion strategies at deep hypothermia (ie, DHCPB) compared with lower flow strategies (ie, SACP). By assessing cerebral supply and demand on the hemodynamic, cellular, and mitochondrial level, our data provide important and novel insights into the mechanisms underlying neurologic injury during neonatal cardiac surgery (Figure 7).
FIGURE 7.
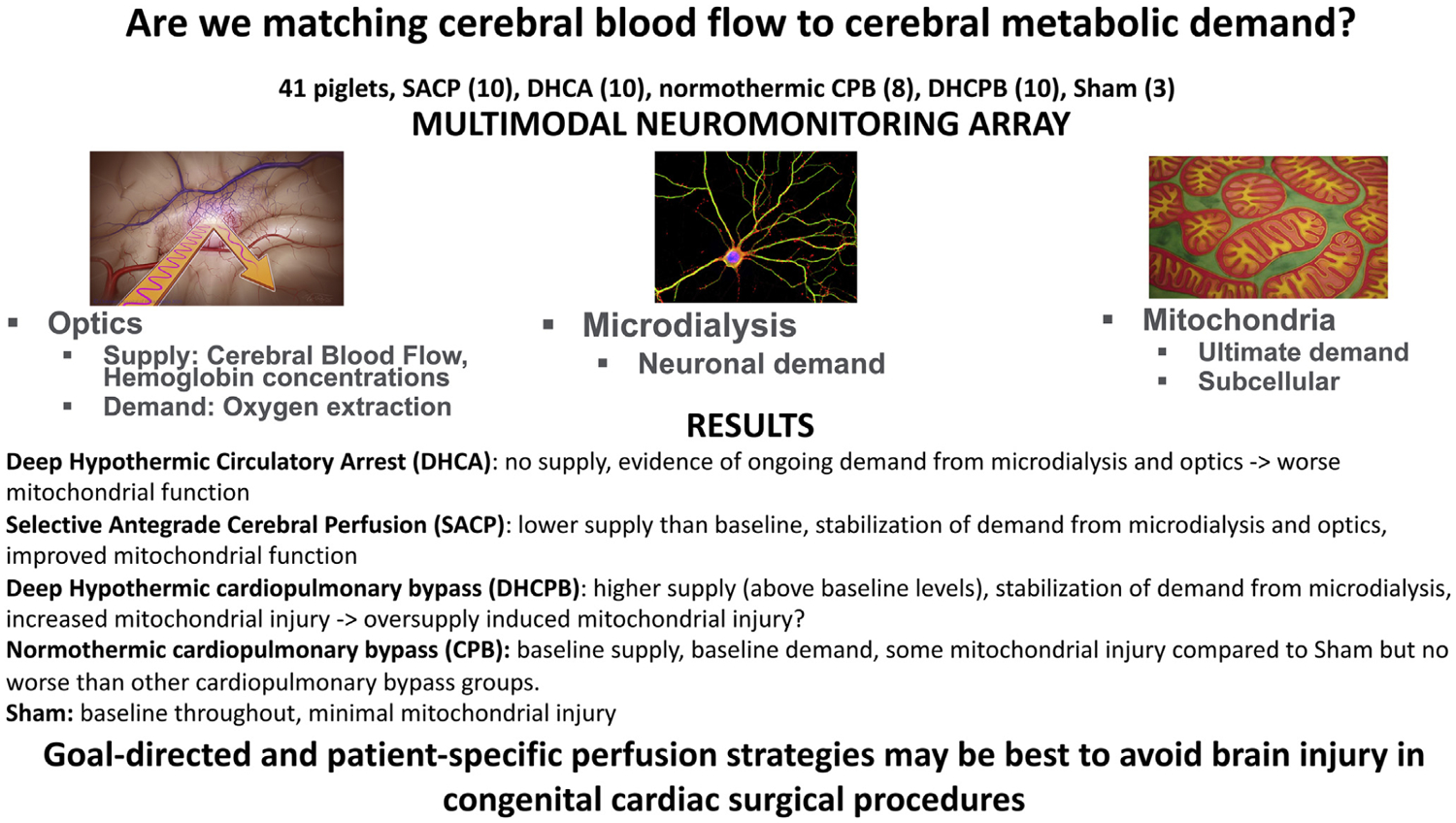
This investigation provides important and novel insights into the mechanisms underlying neurologic injury during neonatal cardiac surgery by assessing cerebral perfusion supply cerebral metabolic demand on the hemodynamic, cellular, and mitochondrial level. These data provide evidence of ongoing derangements in cerebral metabolism during DHCA that leads to a mismatch of CBF and oxygen extraction as demonstrated by cerebral cellular injury and mitochondrial dysfunction. However, this can be attenuated with SACP. Matching supply and demand is crucial to avoid oxidative mitochondrial injury associated with higher flow perfusion strategies, such as DHCPB compared with lower flow strategies (ie, SACP).
The importance of matching CBF with CMRO2 has been demonstrated in both animal and human models of traumatic brain injury and stroke.29,30 To date, it has been difficult to track the presence of metabolic mismatch during cardiac surgery. Although the specific implications of matched CMRO2 with CBF and stable OEF remain un-proven in this population, mismatch remains a harbinger of autoregulation failure and neurologic injury. Further research is needed to elucidate the role that such measurements will have as potentials for goal-directed therapy.
The potentially deleterious consequences of hyperoxia have been well described in patients undergoing congenital heart surgery.31,32 Among cyanotic and single-ventricle patients, Caputo and colleagues31 demonstrated significantly decreased serum markers of myocardial and neurologic injury when comparing normoxic to hyperoxic CPB. Hyperoxia has also been associated with worse outcomes among pediatric patients supported with venoarterial extracorporeal membrane oxygenation.32 We have previously demonstrated that increased oxygen during cardiopulmonary resuscitation is associated with increased cerebral mitochondrial ROS generation33 and helped characterize a mechanism by which cerebral hyperoxia might affect neurologic function after cardiac arrest. Although the insult of CPB is not as significant as cardiac arrest, the mechanisms of secondary brain injury due to overdelivery of oxygen or disruptions in autoregulation may be similar in CPB. These mechanisms likely involve mitochondrial dysfunction and require additional inquiry. Further studies are needed to discover critical values of oxygen delivery in critical illness and during surgery to minimize oxidative injury in the developing brain.
In this investigation, we leveraged FD-DOS and DCS measurements of CBF, tissue oxygenation, and CMRO2 to generate values that indicate brain-specific oxygen delivery. Despite no difference in arterial PaO2 among groups, DHCPB animals had significantly increased tissue oxygenation that was above baseline compared with SACP animals. Although not measured directly, increased oxygen delivery can be inferred from increases in THC and [HbO2]. Our work is the first to demonstrate a corresponding excess in oxygen delivery to the brain at hypothermia and changes in mitochondrial function that may deleteriously affect neurologic health. We also demonstrated evidence of ongoing cerebral metabolism during DHCA. The dependence of Fick-derived measurements of CMRO2 on CBF limits these measurements in detecting ongoing metabolism during ischemia. In such cases, OEF is a more accurate representation of tissue metabolism.28,34 Thus, cerebral metabolism persisted in DHCA animals, and this metabolic demand was not met during ischemia. Further research is needed to improve understanding of the pathophysiology of oxidative injury associated with levels of oxygen delivery and to establish critical criteria for cerebral-specific oxygen delivery during the conduct of CPB.
Our microdialysis findings are in accordance with our prior demonstrations of the associated deleterious effects of DHCA and cMD evidence of cellular injury.2,14 That there was no difference among the non-DHCA perfusion strategies strongly suggests that all non-DHCA perfusion strategies provided adequate substrate to maintain aerobic metabolism. This suggests that injury detected by cMD or other modalities was not due to tissue ischemia but, rather, such injury is caused by a different mechanism such as increased tissue oxygen tension, as has been postulated in both human and animal studies.31,35 The increased markers of cellular stress found in all animals exposed to CPB is interesting, and further research is needed to understand how exposure to CPB may lead to neurologic injury.
In human subjects, ideal flow rates during SACP have not been established and vary considerably among centers. Although flow rates are among the more important variables to ensure proper perfusion supply, equally important variables such as oxygen content, temperature, and blood gas management of the perfusate have not been determined. Persistence of this equipoise represents a critical knowledge gap and a hindrance to optimization and personalization of cerebral perfusion strategies during cardiac surgery. Our data provide proof of concept for how perfusion supply and cerebral metabolic demand can be monitored noninvasively during cardiac surgery. Further studies are needed to ascertain critical perfusion thresholds that meet cerebral metabolic demand and to correlate these theoretical concepts with clinical measurements of neurologic injury. Noninvasive measurements of oxygen extraction and targeting oxygen extraction stability may prove to be important metrics in goal-directed perfusion during neonatal cardiac surgery.
Study Limitations
Our work has several limitations. Although this is a previously validated swine model, it is not possible to replicate the complexity of human neonates that require cardiac surgery using previously healthy piglets. Our studies were meant to assess the acute injury associated with CPB and cardiac surgery, and the chronicity of such mitochondrial damage. Its clinical correlation has yet to be elucidated. Although we hypothesize that increased oxygen delivery in DHCPB animals compared with SACP is due to increased CBF, our data do not unequivocally demonstrate this. We have previously published validation studies comparing DCS and laser Doppler measurements of CBF on CPB during deep hypothermia,17 and were expecting to see continued correlation and agreement at low-flow SACP. There is a trend toward this, and future experiments with improved statistical power may allow for more definitive determination. Although our group has correlated cerebral mitochondrial dysfunction with clinical evidence of neurologic injury in swine, no such correlation has been undertaken in humans. Being a preliminary study in cerebral perfusion strategies, our data are not able to demonstrate critical perfusion parameters. Further studies are needed to delineate such thresholds.
CONCLUSIONS
Our work is a comprehensive, multimodal assessment of noninvasive measurements of cerebral hemodynamics and metabolic activity across different perfusion strategies in neonatal swine, and the first to correlate such findings with measurements of oxidative and mitochondrial injury. Although DHCA resulted in the most significant disruption to cerebral hemodynamics, cellular injury, and mitochondrial function, maintaining near full flow CPB during deep hypothermia also led to mitochondrial evidence of oxidative injury that was ameliorated by the use of SACP. Such oxidative injury occurred without microdialysis evidence of ischemia, indicating overdelivery of oxygen that was supported through noninvasive measurements. Although this work is an important step in advancing understanding of mechanisms of neurologic injury during neonatal cardiac surgery, further research is necessary to establish the role that noninvasive measurements of oxygen delivery may have in the diagnosis and treatment of potentially devastating neurologic injury.
Webcast
You can watch a Webcast of this AATS meeting presentation by going to: https://aats.blob.core.windows.net/media/20AM/Presentations/Low%20Flow%20Selective%20Antegrade%20Cerebra.mp4.
PERSPECTIVE.
This is the first study correlating cerebral hemodynamics and metabolic activity with oxidative and mitochondrial injury in a swine model of neonatal cardiac surgery using different perfusion strategies. We identify a potentially novel mechanism of neurologic injury. Further studies are needed to determine critical values of oxygen delivery while minimizing oxidative injury in the developing brain.
Acknowledgments
Institutional support was provided by the Children’s Hospital of Philadelphia, Department of Anesthesiology and Critical Care, and funds from the Alice Langdon Warner and Daniel S. Tabas Endowed Chairs in Pediatric Cardiac Surgery at the Children’s Hospital of Philadelphia.
Abbreviations and Acronyms
- ATP
adenosine triphosphate
- BFI
blood flow index
- CBF
cerebral blood flow
- cMD
cerebral microdialysis
- CMRO2
cerebral metabolic rate of oxygen
- CPB
cardiopulmonary bypass
- DCS
diffuse correlation spectroscopy
- DHCA
deep hypothermic circulatory arrest
- DHCPB
deep hypothermic cardiopulmonary bypass
- FD-DOS
frequency-domain diffuse optical spectroscopy
- [HbO2]
oxyhemoglobin concentration
- LEAK
inner mitochondrial membrane leak
- LPR
lactate:pyruvate ratio
- OEF
oxygen extraction fraction
- OXPHOS
oxidative phosphorylation
- RCR
respiratory control ratio
- ROS
reactive oxygen species
- THC
total hemoglobin concentration
- SACP
selective antegrade cerebral perfusion
Biographies
Discussion
Presenter: Dr Constantine D. Mavroudis

Dr James S. Tweddell (Cincinnati, Ohio). Your group is attempting to address the question of optimal flow rate for antegrade cerebral perfusion during operations when deep hypothermia and altered perfusion are required, and you’ve conducted an acute animal study using a neonatal swine model. You looked at a number of end points, specifically, mitochondrial function, CBF, and oxygenation. The results are internally consistent, which suggests that the data are high quality and these studies can be challenging, so I commend you on that accomplishment.
How did you determine the flow rate for SACP in this study? My colleagues and I have used the strategy for almost 2 decades now, and the flow rates we typically use are higher than in your study. As you know, there are human studies in which flow rates of 10 mL/kg/min resulted in an undetectable internal carotid artery flow. It appears that if I were to begin this kind of work, I guess I would have tried to match the flow rate achieved with continuous flow with deep hypothermia, rather than the arbitrary value that you chose. What was the justification for the SACP flow rates you used in the study?

Dr Constantine D. Mavroudis (Philadelphia, Pa). I know this issue is a matter of choice. If one does an assessment of the swine literature as we did before attempting this study, the median flow rates that groups have used to assess perfusion in swine has been approximately 10 mL/kg/min with a range from 5 to 45, which is a considerable range. That said, of course, it’s lower than one sees in the human literature. But when taken together, we chose this primarily as the median of the piglet literature but also as a way to highlight something of low flow, thinking that it would give us the most contrast between a higher flow model (which is full bypass) and lower flow (ie, 10 mL/kg/min).
Of course, the comparative literature between humans and piglets in this matter is not exactly robust. So one does not know what a pig necessarily needs and how different it is from a human. One might assume that it would be less, but from a post hoc standpoint, because we showed that there is a consistency or a stabilization of oxygen extraction despite being low flow, it certainly appears by all measures to have been adequate for the piglets.
Dr Tweddell. Yes, “What does a pig need?”—always a good question! But I guess I would have anticipated that one group might have had CBF equivalent to the CBF in the deep hypothermic animals that were continuously perfused. But I get your point here. So you hypothesize that overflowing and over-delivery during deep hypothermia may be linked with increased production of cerebral mitochondrial-ROS.
How do you differentiate injury from too much flow from that caused by too little flow and associated ischemia–reperfusion injury? Certainly, looking at the micro-dialysis data, it looks as though the glycerol in the DHCA animals continued to increase, sort of suggesting that they experienced ischemia–reperfusion injury, whereas in your experimental group and control animals, there really wasn’t much change there.
Dr Mavroudis. That’s an excellent question, and it can be answered really by this slide a little bit and also by the optical data that don’t suggest any evidence of ischemic stress—that is to say, increasing oxygen extraction. Additionally, these are markers of extracellular data of stress and ischemia. If your lactate-pyruvate ratio goes up, there are a few things that cause that besides ischemia and lack of aerobic metabolism. So because we don’t have any evidence of ischemia on the lactate-pyruvate ratio, it strongly suggests there not being a significant ischemic (therefore, reperfusion) injury. Additionally, it’s a matter of scale. The reactive oxidative species will be turbocharged in an ischemia-reperfusion injury because of the various reactions that occur at the mitochondrial level. There will be fission and fusion of mitochondria. Complex 1 will be disrupted and then have to ramp up to deal with this influx of metabolites that are not being made. This creates a much higher concentration of reactive oxidative species, than in a typical overdelivery or different mechanism of injury that allow for increased levels of reactive oxidative species in the cell.
Dr Tweddell. I was intrigued by the frequency domain near-infrared spectroscopy. Is there any potential for a clinical application of this technology? In particular, the ability to be able to measure absolute values of oxygenated and deoxygenated hemoglobin would be extremely valuable. How far we are away from a clinical version of this device.
Dr Mavroudis. Absolutely, and this is something that our group is primarily focused on. One of the co-investigators of this received a grant to make this ready for clinical use, because at its current state, it requires specialized engineers and technicians to run these machines and the data streams are complicated. But the future of this—what we envision—is being able to have an easily digestible, continuous, high-fidelity variables of oxygen delivery in any kind of tissue to which this probe is affixed. If one is interested in the kidney, if one is interested in the brain, one could place this probe in such a way and titrate the source-detector frequencies to get the tissue in question.
We’ve already seen in the adult literature looking at global oxygen delivery and its effect on acute kidney injury and postoperative delirium. Our hope for this is that high-fidelity, tissue specific indicators of oxygen delivery could allow for more targeted measurements of perfusion adequacy. The idea that a brain that is exposed to fetal hypoxia is going to behave the same way and have the same oxygen delivery needs as a brain that has not been exposed to fetal hypoxia in the varying types of patients who we operate on in the neonatal period is something that warrants further investigation. This is one among many other questions that this continuous assessment of both supply and demand will be able to give us in the future. So the short answer to your question is: absolutely, and we’re just looking for the best ways to go about doing so.
Dr Tweddell. What’s the future direction of your research? Are you going to do more acute studies looking at different flow rates, or survival studies, looking at intermediate-term outcome of the animals?
Dr Mavroudis. There are many different directions that we are hoping that this goes, the first of which is a survival model that will allow us to do neuroimaging and a longitudinal assessment of the pathway and the mechanism of mitochondrial injury—because as of now, we just have the single timepoint. In addition, this would allow for a more complete neuro-assessment of animals after they’re weaned from CPB.
From an acute standpoint, I would like to see more data with different flow rates to assess where the inflection point is, between under-flowing and overflowing in this model and, of course, furthering the methods and device development of this frequency domain near infrared spectroscopy and DCS technology.
Dr Tweddell. This is fascinating work, and it looks pretty clearly as though a DHCA is the worst of all the possible experimental conditions. Is this work having any impact on the clinical practice at the Children’s Hospital of Philadelphia? Your institution has been a stalwart advocate for the use of DHCA. I know this is 40 minutes and somewhat of an exaggerated model to achieve ischemia, but has this kind of work altered the practice at the Children’s Hospital of Philadelphia?
Dr Mavroudis. Well, it’s hard to think that anything that we’re doing is particularly novel. While the technology we’re using and the focus on mitochondria are both novel, our research really joins a tradition of about 30 years or so of people demonstrating the various deleterious effects of deep hypothermic circulatory arrest in animal models, be it CASPASE formation, cerebral edema, or various other types of markers of, brain injury that are clear in these animal studies.
That having been said, these animal studies haven’t changed clinical practice that much for surgical groups who can still perform procedures using DHCA safely. As we’re all aware, there are multiple randomized controlled trials for the use of SACP versus DHCA, and the deleterious effects that we see in animal models have not rung true in the human models. One potential hypothesis on this is just that we haven’t gotten the flow rates correct for antegrade cerebral perfusion. The antegrade cerebral perfusion subjects have displayed different types of brain injury as opposed to the DHCA subjects. So I think this question is far from answered. These data certainly suggest a more deleterious effect in this animal model, but whether that’s going to change clinical practice, I hope that it will at some point when we have a more targeted and personalized approach to SACP and perfusion in general. Until that time, the use of DHCA remains another institution-specific issue and is a matter of what one can accomplish in the safest possible manner.
Dr Tweddell. That was a nice, thought-provoking study and excellent work.
Footnotes
Read at the 100th Annual Meeting of The American Association for Thoracic Surgery: A Virtual Learning Experience, May 22–23, 2020.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
References
- 1.Gaynor JW, Stopp C, Wypij D, Andropoulos DB, Atallah J, Atz AM, et al. Neurodevelopmental outcomes after cardiac surgery in infancy. Pediatrics. 2015;135: 816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mavroudis CD, Karlsson M, Ko T, Hefti M, Gentile JI, Morgan RW, et al. Cerebral mitochondrial dysfunction associated with deep hypothermic circulatory arrest in neonatal swine. Eur J Cardiothorac Surg. 2018;54:162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldberg CS, Bove EL, Devaney EJ, Molleen E, Schwartz E, Tindall S, et al. A randomized clinical trial of regional cerebral perfusion versus deep hypothermic circulatory arrest: out- comes for infants with functional single ventricle. J Thorac Cardiovasc Surg. 2007;133:880–7. [DOI] [PubMed] [Google Scholar]
- 4.Algra SO, Jansen NJ, van der Tweel I, Schouten AN, Groenendaal F, Toet M, et al. Neurological injury after neonatal cardiac surgery: a randomized, controlled trial of 2 perfusion techniques. Circulation. 2014;129:224–33. [DOI] [PubMed] [Google Scholar]
- 5.Kilbaugh TJ, Bhandare S, Lorom DH, Saraswati M, Robertson CL, Margulies SS. Cyclosporin A preserves mitochondrial function after traumatic brain injury in the immature rat and piglet. J Neurotrauma. 2011;28:763–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kilbaugh TJ, Sutton RM, Karlsson M, Hansson MJ, Naim MY, Morgan RW, et al. Persistently altered brain mitochondrial bioenergetics after apparently successful resuscitation from cardiac arrest. J Am Heart Assoc. 2015;4:e002232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuller S, Rajagopalan R, Jarvik GP, Gerdes M, Bernbaum J, Wernovsky G, et al. Deep hypothermic circulatory arrest does not impair neurodevelopmental outcome in school-age children after infant cardiac surgery. Ann Thorac Surg. 2010;90:1985–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spielvogel D, Kai M, Tang GH, Malekan R, Lansman SL. Selective cerebral perfusion: a review of the evidence. J Thorac Cardiovasc Surg. 2013;145:S59–62. [DOI] [PubMed] [Google Scholar]
- 9.Khaladj N, Peterss S, Oetjen P, von Wasielewski R, Hauschild G, Karack M, et al. Hypothermic circulatory arrest with moderate, deep or profound hypothermic selective antegrade cerebral perfusion: which temperature provides best brain protection? Eur J Cardiothorac Surg. 2006;30:492–8. [DOI] [PubMed] [Google Scholar]
- 10.Meybohm P, Hoffmann G, Renner J, Boening A, Cavus E, Steinfath M, et al. Measurement of blood flow index during antegrade selective cerebral perfusion with near-infrared spectroscopy in newborn piglets. Anesth Analg. 2008;106:795–803. [DOI] [PubMed] [Google Scholar]
- 11.Salazar JD, Coleman RD, Griffith S, McNeil JD, Steigelman M, Young H, et al. Selective cerebral perfusion: real-time evidence of brain oxygen and energy metabolism preservation. Ann Thorac Surg. 2009;88:162–9. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki T, Tsuda S, Riemer RK, Ramamoorthy C, Reddy VM, Hanley FL. Optimal flow rate for antegrade cerebral perfusion. J Thorac Cardiovasc Surg. 2010;139:530–5. [DOI] [PubMed] [Google Scholar]
- 13.Li B, Zhu Y, Liu A, Lu W, Su J, Zhang J, et al. Selective antegrade cerebral perfusion reduces brain injury following deep hypothermic circulatory arrest in the piglets’ model by decreasing the levels of protein SUMO2/3-ylation. Int J Clin Exp Med. 2014;7:4562–71. [PMC free article] [PubMed] [Google Scholar]
- 14.Liang MY, Tang ZX, Chen GX, Rong J, Yao JP, Chen Z, et al. Is selective antegrade cerebral perfusion superior to retrograde cerebral perfusion for brain protection during deep hypothermic circulatory arrest? Metabolic evidence from microdialysis. Crit Care Med. 2014;42:e319–28. [DOI] [PubMed] [Google Scholar]
- 15.Tang Z, Chen G, Liang M, Rong J, Yao J, Yang X, et al. Selective antegrade cerebral perfusion attenuating the TLR4/NF-κB pathway during deep hypothermia circulatory arrest in a pig model. Cardiology. 2014;128:243–50. [DOI] [PubMed] [Google Scholar]
- 16.Pigula FA, Nemoto EM, Griffith BP, Siewers RD. Regional low-flow perfusion provides cerebral circulatory support during neonatal aortic arch reconstruction. J Thorac Cardiovasc Surg. 2000;119:331–9. [DOI] [PubMed] [Google Scholar]
- 17.Ko TS, Mavroudis CD, Baker WB, Morano VC, Mensah-Brown K, Boorady TW, et al. Non-invasive optical neuromonitoring of the temperature-dependence of cerebral oxygen metabolism during deep hypothermic cardiopulmonary bypass in neonatal swine. J Cereb Blood Flow Metab. 2020;40:187–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kilbaugh TJ, Karlsson M, Duhaime AC, Hansson MJ, Elmer E, Margulies SS. Mitochondrial response in a toddler-aged swine model following diffuse non-impact traumatic brain injury. Mitochondrion. 2016;26:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karlsson M, Pukenas B, Chawla S, Ehinger JK, Plyler R, Stolow M, et al. Neuro-protective effects of cyclosporine in a porcine pre-clinical trial of focal traumatic brain injury. J Neurotrauma. 2018;36:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pastuszko P, Pirzadeh A, Reade E, Kubin J, Mendoza A, Schears GJ, et al. The effect of hypothermia on neuronal viability following cardiopulmonary bypass and circulatory arrest in newborn piglets. Eur J Cardiothorac Surg. 2009;35:577–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain V, Buckley EM, Licht DJ, Lynch JM, Schwab PJ, Naim MY, et al. Cerebral oxygen metabolism in neonates with congenital heart disease quantified by MRI and optics. J Cereb Blood Flow Metab. 2014;34:380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito H, Kanno I, Iida H, Hatazawa J, Shimosegawa E, Tamura H, et al. Arterial fraction of cerebral blood volume in humans measured by positron emission tomography. Ann Nucl Med. 2001;15:111–6. [DOI] [PubMed] [Google Scholar]
- 24.Culver JP, Durduran T, Furuya D, Cheung C, Greenberg JH, Yodh AG, et al. Diffuse optical tomography of cerebral blood flow, oxygenation, and metabolism in rat during focal ischemia. J Cereb Blood Flow Metab. 2003;23:911–24. [DOI] [PubMed] [Google Scholar]
- 25.Durduran T, Zhou C, Buckley EM, Kim MN, Yu G, Choe R, et al. Optical measurement of cerebral hemodynamics and oxygen metabolism in neonates with congenital heart defects. J Biomed Opt. 2010;15:037004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kety SS, Schmidt CF. The nitrous oxide method for the quantitative determination of cerebral blood flow in man: theory, procedure and normal values. J Clin Invest. 1948;27:476–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miura T, Sakamoto T, Kobayashi M, Shin’oka T, Kurosawa H. Hemodilutional anemia impairs neurologic outcome after cardiopulmonary bypass in a piglet model. J Thorac Cardiovasc Surg. 2007;133:29–36. [DOI] [PubMed] [Google Scholar]
- 28.Harris NG, Mironova YA, Chen SF, Richard HK, Pickard JD. Preventing flow-metabolism uncoupling acutely reduces axonal injury after traumatic brain injury. J Neurotrauma. 2012;29:1469–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lenzi GL, Frackowiak RSJ, Jones T. Cerebral oxygen metabolism and blood flow in human cerebral ischemic infarction. J Cereb Blood Flow Metab. 1982;2:321–35. [DOI] [PubMed] [Google Scholar]
- 30.Soustiel JF, Sviri GE. Monitoring of cerebral metabolism: non-ischemic impairment of oxidative metabolism following severe traumatic brain injury. Neurol Res. 2007;29:654–60. [DOI] [PubMed] [Google Scholar]
- 31.Caputo M, Mokhtari A, Miceli A, Ghorbel MT, Angelini GD, Parry AJ, et al. Controlled reoxygenation during cardiopulmonary bypass decreases markers of organ damage, inflammation, and oxidative stress in single-ventricle patients undergoing pediatric heart surgery. J Thorac Cardiovasc Surg. 2014;148:792–801. [DOI] [PubMed] [Google Scholar]
- 32.Sznycer-Taub NR, Lowery R, Yu S, Owens ST, Hirsch-Romano JC, Owens GE. Hyperoxia is associated with poor outcomes in pediatric cardiac patients supported on venoarterial extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2016;17:350–8. [DOI] [PubMed] [Google Scholar]
- 33.Marquez AM, Morgan RW, Ko T, Landis WP, Hefti MM, Mavroudis CD, et al. Oxygen exposure during cardiopulmonary resuscitation is associated with cerebral oxidative injury in a randomized, blinded, controlled, preclinical trial. J Am Heart Assoc. 2020;9:e015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boas DA, Franceschini MA. Haemoglobin oxygen saturation as a biomarker: the problem and a solution. Philos Trans A Math Phys Eng Sci. 2011;369:4407–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng YW, Mohammed A, Deatrick KB, Major T, Cheng D, Charpie I, et al. Differential effects of normoxic and hyperoxic reperfusion on global myocardial ischemia-reperfusion injury. Semin Thorac Cardiovasc Surg. 2019;31:188–98. [DOI] [PubMed] [Google Scholar]


