Abstract
The purpose of meiosis is to generate developmentally competent, haploid gametes with the correct number of chromosomes. For reasons not completely understood, female meiosis is more prone to chromosome segregation errors than meiosis in males, leading to an abnormal number of chromosomes, or aneuploidy, in gametes. Meiotic spindles are the cellular machinery essential for the proper segregation of chromosomes. One unique feature of spindle structures in female meiosis is spindles poles that lack centrioles. The process of building a meiotic spindle without centrioles is complex and requires precise coordination of different structural components, assembly factors, motor proteins, and signaling molecules at specific times and locations to regulate each step. In this review, we discuss the basics of spindle formation during oocyte meiotic maturation focusing on mouse and human studies. Finally, we review different factors that could alter the process of spindle formation and its stability. We conclude with a discussion of how different assisted reproductive technologies could affect spindles and the consequences these perturbations may have for subsequent embryo development.
Keywords: meiosis, spindle, aMTOC, oocyte, embryo, assisted reproductive technologies, spindle defects
This review consolidates information about how spindles form in human and mouse oocytes and how this process can be altered.
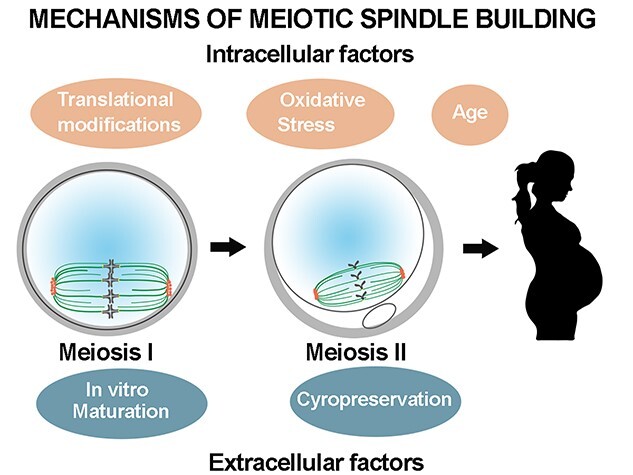
Graphical Abstract
Graphical Abstract.
Introduction
Meiosis is the developmental program that couples reducing the genome in half with gametogenesis to create egg and sperm. Chromosome segregation in meiosis occurs in two steps. First, homologous chromosomes are segregated during meiosis I (MI), which is then followed by separation of sister chromatids in meiosis II (MII). For reasons not completely understood, meiosis in females is more prone to chromosome segregation error than meiosis in males [1, 2]. These segregation errors frequently cause aneuploidy, a situation that has direct consequences on live birth outcomes if these eggs are fertilized. The spindle apparatus, in part, controls separation of chromosomes. Therefore, any defect in spindle assembly or in its interaction with chromosomes could result in chromosome errors and aneuploidy. During MI, tightly associated pairs of sister chromatid kinetochores attach to spindle microtubules (MTs) that originate from the same spindle pole (co-orientation), whereas in MII, the sister chromatid kinetochores attach to MTs from opposite poles (bi-orientation) (Figure 1). Here, we discuss the basics of spindle formation during oocyte meiotic maturation. For space considerations, we will focus on knowledge obtained from functional studies in mice, and we highlight the similarities and differences to our current knowledge of spindle building in humans. Finally, we review how assisted reproductive technologies could affect spindles and the consequences for successful embryo development.
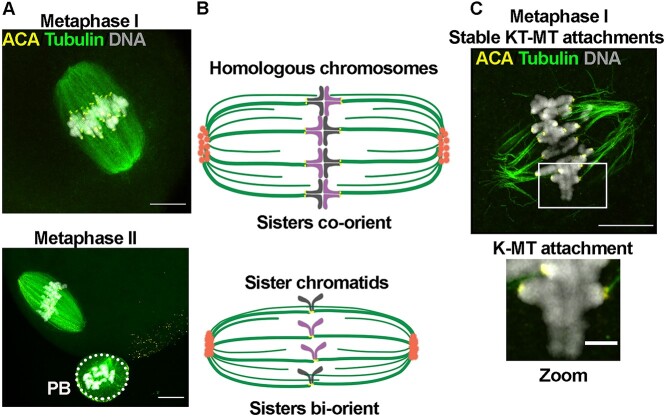
Figure 1.
Meiotic maturation in mouse oocytes. (A) Representative confocal images of mouse oocytes at metaphase I and metaphase II, (B) schematic representation of meiotic spindles in metaphase I and metaphase II, and (C) representative super-resolution confocal image of stable K-MT attachments at metaphase I. Scale bars: 10 μm and 2 μm. ACA, centromeres. PB, polar body.
Mechanism to build an acentriolar spindle in meiosis I in mammals
In mitotic cells, spindle organization relies on centrosomes. These centrosomes contain centrioles that are surrounded by pericentriolar material, and they are the primary center of MT nucleation. Mammalian oocytes lack centrioles and likely lost them during oocyte development [3]. However, some remnants of centriole components can be detected in mature oocytes, but they are not in the canonical nine-triplet form and they likely are not functional [4]. A complete understanding of the mechanism of how mammalian oocytes organize meiotic spindles is still a fundamental question. Mouse oocytes use a different strategy compared to somatic cells to build a bipolar spindle. They contain acentriolar MT organizing centers (aMTOCs) which are composed of pericentriolar materials such as, γ-tubulin [5], pericentrin [6, 7], centrosomal proteins 120 and 192 (CEP120, CEP192), and neural precursor cell expressed, developmentally down-regulated 1 (NEDD1) [8], which, together with the Ran-GTP pathway, function as main centers of MT nucleation [9, 10]. The Ran-GTP pathway initiates massive MT nucleation from aMTOCs localized around chromosomes, thereby forming a MT ball (Figure 2). Gradually, the aMTOCs sort and cluster into two poles and spindle elongation occurs.
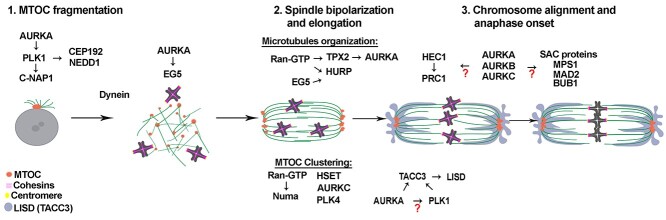
Figure 2.
Schematic of building an acentriolar spindle in MI in mouse oocytes. Key proteins involved in each step are indicated above the time in which they function.
While the spindle is forming, the MTs also influence chromosome behavior. After nuclear envelope breakdown (NEBD) occurs, chromosomes condense and move to the outside of the MT ball without any apparent organization. Concomitant with spindle bipolarization, chromosomes start to organize forming a belt with a chromosome-free center around the spindle equator. Later, chromosomes invade the center of the belt to start to form the metaphase plate. Once the chromosomes are in position, and at the same time that spindle elongation occurs, they undergo several rounds of attachment and detachment from MTs until stable, end-on kinetochore attachments are achieved [11]. To explain these processes in more detail, we divide spindle formation in three steps: (1) aMTOC fragmentation, (2) spindle bipolarization and elongation, and (3) chromosome alignment and anaphase onset (Figure 2).
aMTOC fragmentation
Prior to NEBD, aMTOCs localize around the nuclear envelope. Once NEBD occurs, chromosomes condense and aMTOCs concentrate in the proximity of chromosomes and are the predominant source of MT nucleation [10]. High-resolution live cell imaging of mouse oocytes allows tracking of individual aMTOCs, and this technology has increased our understanding of aMTOC behavior during meiotic maturation [12]. First, polo-like kinase 1 (PLK1) triggers aMTOC decondensation by inducing release of centrosomal protein 250 (C-NAP1), an aMTOC cross-linker protein [12, 13]. This change in structure facilitates aMTOC stretching along the NE in a dynein-dependent manner. The dynein is anchored at invaginations along the NE and the stretching force induces aMTOC fragmentation [12]. When PLK1 was specifically deleted from mouse oocytes, C-NAP1 remained associated with aMTOCs after NEBD, causing defects in the localization of aMTOC components, such as γ-Tubulin, CEP192, and NEDD1 [13]. Aurora kinase A (AURKA) is also involved in aMTOC fragmentation and their separation in two poles [10, 12, 14]. When Aurka is depleted by siRNA, oocytes have disorganized spindles, aMTOCs are not localized at spindle poles, and instead they scatter in the middle of the spindle, therefore affecting meiotic progression and chromosome segregation [15, 16]. Moreover, when Aurka was deleted specifically in oocytes (A KO), these oocytes were unable to fragment aMTOCs after meiotic resumption. AURKA is needed to phosphorylate and activate PLK1 at aMTOCs to induce the release of C-NAP1 and hence proceed with the aMTOC fragmentation process [14]. Furthermore, when AURKA is overexpressed, oocytes had excess numbers of aMTOCs [15, 17]. Therefore, AURKA is needed to determine the correct number of aMTOCs [14–17]. Finally, fragmentation of aMTOCs is regulated by a kinesin-like motor protein, Kinesin 5 (EG5) (encoded by Kif11 in mouse), a known substrate of AURKA [18], that later also drives aMTOC separation. When EG5 is inhibited by monastrol treatment [19], aMTOCs form one large cluster, thereby forming a monopolar spindle [12].
Spindle bipolarization and elongation
After MTOC fragmentation, MTOCs sort and cluster into two poles [10] (Figure 2). To make a long and bipolar MI spindle, MT nucleation first increases massively around aMTOCs and chromatin by the action of a GTPase, Ran. Ran-GTP forms a gradient from the chromosomes during MI and MII [9], releasing spindle factors, such as TPX2, nuclear mitotic apparatus protein (NuMA), and hepatoma up-regulated protein (HURP) from importins [20–22]. Interestingly, acentriolar spindles can also form without Ran-GTP in MI, but not in MII, a difference that is not yet understood [9, 10, 23]. One Ran-release factor is TPX2, a MT-binding protein that contributes to spindle formation in two ways: (1) promoting MT nucleation and (2) binding AURKA. This binding to AURKA activates the kinase and promotes regulation of spindle assembly [20, 24, 25]. Next, EG5 controls spindle elongation and stability by inducing movement of overlapping MTs in the midzone toward the spindle poles [26]. Furthermore, EG5 regulates the localization of HURP, a Ran-GTP release factor that forms a MT domain in the central region of the spindle. This MT domain is involved in the symmetrical distribution of aMTOCs between the two poles and to promote MT stability in the center of the spindle [21]. In HURP knockout oocytes, spindle bipolarity was significantly delayed by asymmetrical distribution of aMTOCs, resulting in shorter spindles. An additional mechanism in mouse oocytes from the kinetochores is reported to contribute to spindle bipolarization during MI. The kinetochore protein, HEC1 (also called Ndc80), recruits PRC1, an anti-parallel MT cross-linker protein, to kinetochores and allows the slow process of spindle bipolarization. Oocytes that lack HEC1 fail to form a bipolar spindle during MI, but this mechanism is oocyte-specific because it was not found in other cell types [27]. In determining how the kinetochore-associated MT turnover can influence spindle morphology, a recent study showed that the stabilization of the MT-kinetochore attachment via dephosphorylation of different residues in HEC1 at kinetochores is required to limit spindle elongation and to restrict aMTOCs to spindle poles. These findings suggest that the kinase(s) that phosphorylate HEC1 are critical to establishing spindle bipolarity [28].
Once aMTOCs are sorted, they need to cluster or coalesce into two poles. However, the organization of meiotic spindle poles is less focused in structure in comparison to mitotic spindle poles. The result is that an MI spindle takes on a barrel-shape configuration with broader polar ends. Several mechanisms are involved in spindle pole organization. HSET, a minus-end kinesin, contributes to aMTOC clustering [29]. When HSET is overexpressed, spindles collapse and become monopolar. However, when HSET levels are slightly increased, achievement of bipolarity occurs more rapidly and the aMTOCs become over clustered, forming a single round structure, which is more similar to the shape of mitotic spindles [29]. These data indicate that oocytes must strictly regulate the amount of HSET activity during spindle formation. Another important Ran-release factor that helps to organize spindle poles is NuMA. Upon NuMA deletion, oocyte spindles become elongated with highly focused poles, losing the barrel shape. Interestingly, these highly focused aMTOCs were not found at spindle poles but instead localized in the cytoplasm but were still close to the spindle [30]. AURKA also has a role in maintaining spindle pole structure because it phosphorylates transforming acidic coiled-coil protein 3 (TACC3) to promote the stabilization of MTs in the acentriolar poles [8, 20, 31]. A study using super-resolution microscopy showed that when AURKA is inhibited in oocytes at metaphase I and metaphase II, aMTOCs are over-clustered, generating more focused spindle poles which were characterized by small aMTOCs at each pole [32]. Although the same pattern was observed comparing the overall spindle pole structure in Aurka KO oocytes, the organization of CEP215 and Pericentrin inside aMTOCs was similar between KO and WT oocytes [14]. Consistent with a role of AURKA in spindle pole formation and maintenance, oocytes that lack pericentrin were unable to recruit AURKA to aMTOCs and hence the spindle pole structure and spindle morphology were abnormal [33]. Moreover, several MT regulatory factors are proposed to be organized in a liquid-like spindle domain (LISD) in an AURKA-dependent manner. Oocytes where AURKA was deleted, inhibited, or even mis-localized from the spindle poles, lacked localization of TACC3 around the spindle, which is a main component of the LISD [14, 33]. Importantly, when PLK1, an AURKA substrate, is deleted from oocytes, TACC3 is also mis-localized [13], suggesting that AURKA regulates the formation of LISD by phosphorylating PLK1. This LISD may allow critical spindle regulator proteins to concentrate around MTs and to change quickly, adapting to the rapid and dynamic changes of the MTs (see [8] for details). This LISD is conserved in different species of mammals, however, whether human oocytes use the same strategy to compartmentalize spindle factors remains unknown [8]. More studies are needed to confirm the presence and the function of the LISD on spindle formation. Complementary with AURKA-localized functions, PLK4 localizes to aMTOCs along with a pericentriolar protein, CEP192, and regulates MT nucleation from aMTOCs. These two kinases have independent functions in early MT growth because the inhibition of each one separately reduces MT nucleation and delays spindle formation. However, PLK4 also regulates the kinetics of initial MT growth by phosphorylating AURKA and enhancing its activity [31]. Therefore, further investigation into how these two kinases control MT nucleation and spindle formation is warranted.
In addition to AURKA, a population of Aurora kinase C (AURKC) localizes to spindle poles and has two roles in regulating spindle formation: (1) an indirect role, the chromosome-localized population competes with AURKA for binding the chromosome docking protein INCENP, thereby maintaining high levels of AURKA at spindles poles [34] and (2) a direct role in controlling clustering aMTOCs [35]. How AURKC is accomplishing this clustering role is an outstanding question. One hypothesis is that antagonistic functions occur between AURKA and AURKC to balance and control spindle formation. Consistent with this idea, AURKC cannot compensate for loss of AURKA, although AURKC is localized to aMTOCs in Aurka KO oocytes [14]. However, further studies are needed to understand the role of AURKC at spindle poles.
Chromosome alignment and anaphase onset
Once the spindle is bipolar and chromosomes occupy the metaphase plate, chromosomes start to oscillate with several rounds of kinetochore-microtubule (K-MT) error-correction until final co-orientation is achieved [11] (Figure 2). The Aurora kinase proteins are involved in the K-MT error-correction pathway in oocytes [36, 37]. Aurora kinase B and AURKC phosphorylate specific residues in HEC1 at kinetochores to reduce MT affinity, thereby allowing new attachments to occur [37]. Moreover, AURKA is involved in K-MT error correction by destabilizing attachments when chromosomes are close to the spindle poles [38]. PLK1 is also needed to stabilize K-MT attachments [39]. During the process of error correction, unattached kinetochores are generated and the spindle assembly checkpoint (SAC) is activated to prevent anaphase onset until sister kinetochores are stably attached and co-oriented [40]. The unoccupied kinetochores recruit MPS1, a protein kinase that triggers the SAC signaling cascade. First, mitotic arrest deficiency MAD1/MAD2 are recruited to unoccupied kinetochores and act as an assembly platform for the meiotic checkpoint complex that consists of MAD1, MAD2, BUB1, and BUB3 complexes. This complex diffuses into the cytoplasm and sequesters CDC20, thereby preventing anaphase-promoting complex/cyclosome (APC/C) activation and preventing the degradation of securin and cyclin B1. The result of keeping securin and cyclin B1 levels high prevents the cleavage of cohesin, the “protein glue” that keeps homologous chromosome together and delays anaphase onset, respectively. What the SAC senses is an intense debate in the field. One proposal is that the SAC detects improper K-MT attachments and/or inter kinetochore tension when chromosomes are oriented in a bipolar fashion [41]. In mouse oocytes, the SAC is weaker than it is in mitotic cells and appears to tolerate more than one misaligned bivalent [30, 42, 43]. Defects in spindle building can activate the SAC and delay or prevent meiotic progression. Once most kinetochores are properly attached, MAD2 is released from kinetochores and SAC signaling is silenced. Once the SAC is silent, CDC20 is free to activate the APC/C and induce homologous chromosome separation.
Mechanism to build a MII spindle
One difference between MI and MII is that Ran-GTP is essential for spindle formation in MII [9]. When Ran was inhibited by expression of Ran dominant-negative mutants in oocytes, MII spindles were disorganized, causing delay in completing MII after parthenogenic activation [9]. The importance of the Ran-GTP pathway during spindle formation in MII could depend on the speed of spindle formation. MII spindle assembly is fast; taking around 1 h in contrast to the 6 h it takes for the MI spindle to form. Therefore, Ran-GTP could enhance MT nucleation from aMTOCs, allowing the spindle to assemble rapidly. Consistent with the rapid spindle assembly and chromosome alignment during MII, cyclin A2 has a specific role during MII to promote spindle formation. This protein accumulates during oocyte maturation with the highest levels in MII where it is involved in destabilization of abnormal K-MT attachments [44]. In oocyte-specific cyclin A2 KOs, MI occurred normally but the eggs had abnormal metaphase II spindles. These defects included multipolar spindles, astral MTs in the cytoplasm, and chromosome misalignment. These eggs take longer to convert merotelic attachments to normal K-MT attachments because the K-MTs have increased stability [44]. Another difference between MI and MII is that protein regulator of cytokinesis (PRC1) is enriched at kinetochores and contributes to spindle bipolarization during MI, whereas in MII, PRC1 is mostly cytoplasmic and promotes the rapid spindle building in a kinetochore-independent manner [27].
Spindles at metaphase II require stability, sometimes for several hours, while awaiting fertilization. Spindle stabilization requires a specific mechanism that involves the MOS-mitogen-activated protein kinase (MAPK) pathway to control MT organization [45, 46]. MAP kinase–interacting and spindle-stabilizing protein (MISS) and deleted in oral cancer one related (DOC1R) are substrates of MAPK, which have specific roles to maintain spindle integrity during MII. MISS accumulates during meiotic maturation and localizes on the spindle. After depletion of MISS, MII spindles lose bipolarity and the eggs contain cytoplasmic asters of MTs [45]. By contrast, DOC1R is present in all stages of meiotic maturation and is enriched around the spindle at metaphase I and metaphase II. However, similar to MISS depletion, DOC1R depletion does not impair MI, but the eggs have serious spindle defects at metaphase II with spindles poles connected to cytoplasmic aMTOC asters [46]. Therefore, both proteins are important for MII MT organization. Importantly, EG5 also contributes to spindle stability at metaphase II in vivo [26] and after in vitro maturation [47]. EG5 is highly enriched on MII spindle poles and has an essential role in maintaining spindle bipolarity during the MII arrest by maintaining the movement of the MTs to the poles (poleward MT flux) [26]. When EG5 is inhibited in metaphase II-arrested eggs, spindles shorten and become monopolar [26]. Consistent with the role of the Ran pathway during MII, TPX2 is also needed to assemble a bipolar spindle and to maintain polar structure [20].
Actin function in spindle assembly
Along with tubulin-based spindle formation, mammalian oocytes also rely on actin filaments for spindle formation [48]. Actin filaments enter the MT spindle and their formation depends on MT polymerization. When the actin spindle was disrupted, oocytes were prone to chromosome segregation errors, showing slower movement and lagging chromosomes during anaphase I. Moreover, when actin was acutely removed in metaphase II eggs by cytochalasin D treatment, chromosomes lost their alignment at the metaphase plate. Therefore, the actin spindle is important to achieve and to maintain chromosome alignment during metaphase and in inducing formation of K-MT fibers [48].
Because oocytes undergo asymmetric cell division, the position of the meiotic spindle within the oocyte is critical. Actin filaments are important to move the MI spindle to the cortex via polymerization of F-actin filaments [49, 50]. During MII, the spindle forms close to the cortex in a parallel orientation while awaiting fertilization, an event that can occur much later. The maintenance of spindle position here is also dependent upon actin filaments [51]. This topic was extensively reviewed in other studies (see [51–53]).
Key differences in spindle formation between mouse and human oocytes
How the meiotic spindle is formed in human oocytes is less understood for several reasons. It is difficult to obtain significant numbers of human oocytes, and they can be of lower quality because they are discarded material from patients undergoing in vitro fertilization (IVF) treatment. There are obvious ethical limitations in the type of studies that can be conducted, such as the inability to genetically alter the human genome, and in the USA, the inability to fertilize or activate eggs to study impacts on embryonic development. Nevertheless, through studies using discarded human oocytes, we have discovered important biology. For example, MI spindle formation is slower when compared to mouse oocytes, taking around 16 h when compared to 8 h [54]. This spindle-building kinetics difference may stem from a lack of aMTOC-driven MT nucleation and more predominant role for Ran-GTP dependent spindle formation [54]. However, more studies are needed to elucidate this mechanism and other differences. One important aspect to highlight in human oocytes is the inherent instability of the spindle. Once a spindle is bipolar, some oocytes fail to maintain it, oscillating through multipolar or monopolar morphologies for several hours. This spindle instability is correlated with increased errors in chromosome segregation [54]. The reason why spindles are unstable in human oocytes remains unknown, but, human oocytes may lack a kinetochore-dependent pathway spindle bipolarization, thereby explaining why transient multipolar spindles occurs [27]. Importantly, the detection of spindle instability could be particularly relevant in improving outcomes in IVF treatments.
Causes of spindle alterations
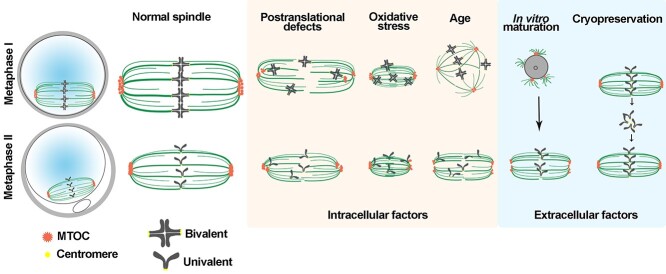
As can be appreciated by the mechanisms discussed here, the formation and maintenance of a bipolar spindle is complex and requires the coordination of different spindle assembly factors, motor proteins, and signaling molecules at precise times in the precise location. Any alteration of these factors could cause spindle defects and generate aneuploid eggs and embryos. We next discuss some causes of spindle alterations and how the routine techniques used during IVF procedures could affect the formation and/or stability of oocyte spindles (Figure 3).
Figure 3.
Schematic to illustrate changes in spindle morphology caused by intracellular and extracellular factors.
Post-translational tubulin modification
Tubulin, the main component of meiotic spindles, is subject to post-translational modifications that regulate MT activity, localization, and molecular interactions. MTs are dynamic structures that assemble and disassemble continuously. Tubulin acetylation is considered a stabilizing event [55]. This mark creates a pattern (landmarks) on MTs which allows motor proteins to bind to and to regulate MT motor function and MT stability [56]. In oocytes, tubulin acetylation is regulated by lysine acetyltransferase and histone deacetylases (HDAC) to ensure normal spindle formation [57, 58]. Some members of the HDAC family, such as HDAC3 [57], HDAC6 [58], and HDAC11 [59], are expressed in oocytes and localize around the meiotic spindle. They decrease the levels of tubulin acetylation to allow MT turnover and the formation of a normal bipolar spindle. Oocytes, where these HDAC proteins were depleted or inhibited, contained longer and disorganized spindles, which was associated with an increase in tubulin acetylation. These spindle defects are hypothesized to lead to chromosome misalignment and aneuploidy [57–59]. Moreover, sirtuin proteins (SIRTs), most well-known for deacetylating histones, also target non-histone proteins such as tubulin. At least two members of this family, SIRT1 [60] and SIRT2 [61], localize around the spindle in mouse oocytes and have an important role in regulating spindle integrity by reducing tubulin acetylation levels. However, these SIRTs seem to act at different times during meiosis because SIRT2 localizes to spindles during MI, whereas SIRT1 localizes to MII spindles. This temporal division could reflect differences in the mechanism of regulation of tubulin acetylation between MI and MII. Further studies need to be conducted to fully understand the molecular mechanism behind tubulin acetylation during spindle building and its function in oocytes.
Oxidative stress
Oxidative stress (OS) occurs when the balance between pro-oxidant and anti-oxidant substances is altered in a cell. Reactive oxygen species (ROS) are products generated from normal cellular metabolic processes and from the cellular environment. The most common ROS are superoxide anion, hydrogen peroxide, and hydroxyl radicals. At physiological levels, ROS are important for the regulation of several processes in the ovary, such as folliculogenesis and oocyte maturation [62]. However, if this balance is altered, ROS are toxic for oocytes. In fact, the levels of ROS in follicular fluid can be used as a predictor of oocyte quality and potential embryo development [63]. Moreover, there are several factors that contribute to OS in female reproduction, such as age, polycystic ovary syndrome, endometriosis, and lifestyle factors [63, 64]. Here, we focus on how ROS could affect the spindle structure.
In mouse oocytes, exposure to oxidizing agents during meiotic maturation causes spindle shrinkage which is characterized by a decrease in spindle length and width at metaphase I and metaphase II and is associated with elevated aneuploidy rates [65]. Furthermore, when spindles at metaphase II were exposed to different concentrations of hydrogen peroxide (H2O2), they became shorter and even disassembled when exposed to the highest concentration. This disassembly causes chromosome misalignment, suggesting that ROS affects MT stability [66, 67]. Several explanations are proposed regarding the mechanism by which ROS can affect spindle structure. One possible reason is that exposure to ROS affects mitochondrial function. For instance, if hydrogen peroxide decreases ATP production by mitochondria, then ROS could affect the amount of energy available to organize and maintain spindle structure during metaphase II arrest [67]. Moreover, OS also produces lipid peroxidation, generating secondary products, such as 4-hydroxynonenal (4-HNE). Elevated levels of these electrophilic aldehyde secondary products are toxic for oocytes because they can alter the structure, activity, and the levels of key proteins by inducing covalent modifications of the nucleophile residues of amino acids (Cys, His, and Lys), a process which is called as adduction. In mouse oocytes, exposure to OS causes increased levels of 4-HNE. This elevation was associated with formation of spindle asters and abnormal organization of aMTOCs and chromosome misalignment at metaphase II [68]. Furthermore, the authors showed that 4-HNE colocalizes with α-, β-, and γ- tubulin, suggesting that these proteins are being modified, thereby causing the spindle defects observed under OS [68].
Age
Oocyte quality decreases with maternal age, which is reflected in the increased percentage of aneuploid eggs in women >35 years [1]. Oocyte aging is not only associated with maternal age, but eggs can age after ovulation. After ovulation, there is an optimal window of time where fertilization must occur (8–12 h in mouse and 24 h in humans). If fertilization does not occur during this time, gamete quality starts to decline [69]. Postovulatory aging involves a wide range of changes, such as a decrease in metabolism and ATP production, decrease in organelle activity; destabilization of cytoskeletal fibers, and loss of cohesin proteins that maintain sister chromatid together [56, 62, 69]. For the purposes of this review, we focus on the changes that affect spindle structure during oocyte aging.
It is well documented that, in mouse and human oocytes, the frequency of abnormal and disorganized spindles increases with age [69–71]. Some of the characteristic features of aged eggs at metaphase II is the instability of spindle pole organization. This instability is observed by cytoplasmic NUMA and γ-tubulin foci and declustered spindle poles [56, 72, 73]. Why spindle poles fail to organize properly in aged oocytes remains unknown; one hypothesis is that a reduction in dynein and kinesin activity over time impairs centrosomal protein transport to spindle poles [56]. Moreover, there are reportedly decreased levels of kinesins in aged oocytes, consistent with causing age-related spindle abnormalities [74]. However, a recent single-cell transcriptome analysis of human eggs from young and old women found upregulation of several aMTOC- and MT-associated genes with maternal age [75]. More studies are therefore needed to fully understand the importance of this gene upregulation on spindle morphology.
Complementary to these studies, live imaging analyses showed that maternal age influences the dynamics and organization of MTs during spindle formation MI of mouse oocytes from young and old females. Fifty percent of aged oocytes had a transient multipolar spindle state that eventually became bipolar. Although bipolar, these oocytes contained a high frequency of abnormal K-MT attachments which caused chromosome misalignment at metaphase I and lagging chromosomes in anaphase I [76]. These data are consistent with a previous study where oocytes from aged mice presented high levels of abnormal merotelic and lateral K-MT attachments [77]. Why these errors in K-MT attachments are generated is an important question. Oocytes from older females frequently have reduced levels of cohesin proteins on chromosome arms and at centromeres, which contributes to premature separation of sister chromatids generating aneuploid eggs [78–80]. Therefore, one hypothesis that explains the high level of abnormal K-MT attachments in aged oocytes could be the loss of cohesin that allows the separation of sister kinetochores, thereby permitting bi-orientation. Notably, in oocytes from aged mice, chromosomes with sister kinetochores separated establish abnormal K-MT attachment more frequently than chromosomes with sister kinetochores working as a unit, suggesting a role of cohesin contributing to normal K-MT attachments [77]. A close evaluation of kinetochore structure during MI and MII in oocytes from old mice and older women shows that loss of cohesin with age facilitates kinetochore fragmentation [81]. The fragments of kinetochore can establish independent attachments with MTs and increase the chances of merotelic K-MT attachments and lead to errors in chromosome segregation during anaphase [81]. Further studies are needed for complete understanding of the molecular mechanisms of how MT dynamics and organization become deregulated during oocyte aging.
In vitro maturation
Oocytes need to grow and mature inside follicles to be fully competent to support embryo development after fertilization. Changes in the cytoskeleton, reorganization of organelles, cytoplasmic maturation, and chromosome condensation are some of the events that must be organized and coordinated for high gamete quality. Oocytes can resume meiosis and mature ex vivo once they are released from follicles. This ability to mature oocytes in vitro (IVM) gained particular attention of IVF clinics as a mechanism to prevent the overstimulation of ovaries. However, the rate of success of implantation and development of embryos from in vitro matured oocytes is low across mammalian species, including humans [82]. Protocols have been improved over the years, incorporating information obtained from basic research. For example, oocytes matured in vitro benefit from culture within follicle cells because of the cell-to-cell communication required for cytoplasmic maturation and proper timing for meiotic resumption. The use of follicle stimulating hormone in culture media also benefits the health of the follicle [82]. Importantly, studies that evaluate the impact of components, such as the composition of culture media, hormone concentrations, duration of culture, and environmental conditions of incubation on oocyte quality, are lacking. Besides the culture media, handling oocytes involves exposure to light and an altered concentration of oxygen which can cause OS [63]. Therefore, it is perhaps not surprising that IVM still report low maturation success rates, around 77–62%, depending on whether cumulus cells are present [83].
Culture conditions could affect the process of cytoplasmic maturation and spindle formation. This evidence comes from the observed differences between gametes matured in vivo (IVO) to IVM. These differences could affect the capability to sustain embryo development, implantation, and pregnancy. For example, IVM mouse oocytes produce longer spindles with less focused aMTOCs (i.e., there were increased distances between aMTOCs within each pole) [84, 85] and an abnormal localization pattern of aMTOC components, such as γ-tubulin in mouse [85, 86] and NUMA in human [72]. Moreover, IVM mouse oocytes showed elevated levels of the kinesin EG5, thereby increasing sister kinetochore distances. It appears that IVM creates conditions where oocytes recruit more EG5 to form spindles compared to the IVO setting. This difference highlights the profound changes in spindle assembly and maintenance mechanisms which can be caused by in vitro conditions [47]. By contrast, a study using confocal microscopy to evaluate IVO and IVM human oocyte spindles reports no differences in sizes or tubulin abundance. These species-specific differences could reflect the differences in spindle formation mechanisms, such as the degree of dependency on the Ran-GTP pathway in human [54] and aMTOCs in mouse [10, 87].
Cryopreservation
Cryopreservation is the freezing of gametes or embryos; a procedure increasingly used for fertility preservation as part of oncofertility treatment or for personal reasons [62, 88, 89]. There are two main methods of cryopreservation: a slow cooling method and a vitrification method. The slow cooling method was the first cryopreservation technique developed and was extensively modified and improved over the years [88]. Briefly, this method uses a low concentration of cryoprotectant solutions and a gradual reduction of temperature (0.3–1°C/min) at the beginning of the process until around −30°C. Eighty percent of gametes survive after thawing; however, the success of a live birth is lower in comparison with cycles using fresh gametes [90]. Vitrification is now the most common technique used. This technique uses a high concentration of cryoprotectants and the gametes are rapidly frozen in liquid nitrogen [91, 92]. An extensive meta-analysis that compared slow freezing versus vitrification success rates found that vitrification is currently the best method for human gamete preservation [89, 90].
Although cryopreservation is widely used in the clinic, the impact of this technique on gamete and embryo quality is still under intense study. There are several biological processes that could be altered [88]. Here, we focus on how this technique can affect the spindle. It is well known that exposure of oocytes to low temperatures can cause spindle defects [62]. For example, brief exposure of human oocytes to room temperature conditions reduced spindle size, caused spindle disorganization, and MT depolymerization [93]. Longer exposure (up to 10 min and down to 0 °C) caused complete spindle disassembly and loss [94]. During cryopreservation, the spindle depolymerizes and is required to reassemble during thawing [95, 96]. To understand better the process of spindle disassembly and reassembly after vitrification, a study in mouse oocytes evaluated MT polymerization and aMTOCs during this procedure [97]. First, MTs gradually depolymerize until they completely disappear. When the de-vitrification and dehydration process starts, the spindle MTs are absent and then they start to re-polymerize at the end of the thawing process. Full recovery occurs at the final incubation in culture medium (KSOM). KSOM incubation for at least 2 h appears essential to give the oocytes time to fully recover the spindle and to acquire competence to finish meiosis. This dynamic change of MT polymerization and depolymerization during vitrification was also associated with changes in aMTOC appearance and reassembly. However, MT depolymerization and aMTOC disappearance occurs by different mechanisms because aMTOCs are still present after MT depolymerization [97].
It is postulated that cryopreservation of human oocytes has similar effects on MTs dynamics as in mouse [95], but understanding how spindle reassembly occurs in human oocytes is more challenging. Using confocal microscopy, a study described how the spindle behaves in human oocytes during slow-cooling method of cryopreservation [98]. Human oocytes show disorganized spindles after thawing and, after 1 h of incubation in culture media, restored spindle bipolarity and chromosome alignment. Moreover, the study found that the time of incubation after the procedure is crucial because shorter incubation periods are not long enough to allow the spindle to organize, but longer incubation periods produce chromosome misalignment [98]. As basic studies have carefully evaluated these types of procedures, protocols are modified to mitigate negative effects. Another important point to highlight is that the results of slow cooling method or vitrification depends on the use of specialized equipment or expertise of the operator, respectively [62]. Therefore, variability of the efficiency of these techniques could be due to extrinsic factors that must also be considered.
Spindle visualization as IVF outcome predictor
Early evaluation of spindle morphology could be informative of the gamete/embryo quality and success upon transfer to the uterus. Polarized light microscopy (Polscope) is a non-invasive method, which uses polarized light to visualize and measure birefringent cellular structures in living cells [99]. Because the spindle consists of highly organized fibers of tubulin with birefringent properties, this technique allows evaluation of the meiotic spindle without apparent effect on gamete quality and potential for embryo development [100]. There is extensive agreement that the presence of a spindle evaluated by Polscope is a good predictor of higher rate of fertilization and embryo development [101–103]. Not only is the presence of the spindle detected but also the spindle retardance of light can be used as a quantitative approach to diagnose gamete quality and predict success [103]. Importantly, one study demonstrates that the presence of a normal spindle evaluated by this technique is associated with the production of euploid embryos [104]. All these data suggest that this non-invasive tool is useful for gamete selection based on the presence and morphology of the spindle as a marker of quality.
Conclusions
We have highlighted specific defects in the spindle apparatus which are associated with errors in chromosome segregation during meiosis. Much knowledge about spindle assembly in oocytes comes from mouse studies that allow us to have a detailed mechanistic understanding about the most important steps needed to generate an acentriolar bipolar spindle. Spindle assembly mechanisms in human oocytes are not as well understood as they are in model organisms, in part, because of the challenges in obtaining these precious cells. However, the access to human samples for collaboration between basic and clinical scientists will create opportunities for deeper our understanding. Importantly, further studies are needed to fully comprehend what are the molecular mechanisms behind the spindle alterations in oocytes by in vitro manipulations during IVF treatments and the consequences during embryogenesis and pregnancy.
Footnotes
† Grant Support: This work is supported by grants from the National Institutes of Health (NIH) (R01-GM112801 and R01-HD091331) to KS.
Contributor Information
Cecilia S Blengini, Department of Genetics, Human Genetics Institute of New Jersey, Rutgers University, Piscataway, NJ, USA.
Karen Schindler, Department of Genetics, Human Genetics Institute of New Jersey, Rutgers University, Piscataway, NJ, USA.
References
- 1. Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet 2001; 2:280–291. [DOI] [PubMed] [Google Scholar]
- 2. Gruhn JR, Zielinska AP, Shukla V, Blanshard R, Capalbo A, Cimadomo D, Nikiforov D, Chan AC-H, Newnham LJ, Vogel I, Scarica C, Krapchev Met al. . Chromosome errors in human eggs shape natural fertility over reproductive life span. Science 2019; 365:1466–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Manandhar G, Schatten H, Sutovsky P. Centrosome reduction during gametogenesis and its significance. Biol Reprod 2005; 72:2–13. [DOI] [PubMed] [Google Scholar]
- 4. Simerly C, Manil-Segalen M, Castro C, Hartnett C, Kong D, Verlhac MH, Loncarek J, Schatten G. Separation and loss of centrioles from primordidal germ cells to mature oocytes in the mouse. Sci Rep 2018; 8:12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Combelles CM, Albertini DF. Microtubule patterning during meiotic maturation in mouse oocytes is determined by cell cycle-specific sorting and redistribution of gamma-tubulin. Dev Biol 2001; 239:281–294. [DOI] [PubMed] [Google Scholar]
- 6. Ma W, Viveiros MM. Depletion of pericentrin in mouse oocytes disrupts microtubule organizing center function and meiotic spindle organization. Mol Reprod Dev 2014; 81:1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baumann C, Wang X, Yang L, Viveiros MM. Error-prone meiotic division and subfertility in mice with oocyte-conditional knockdown of pericentrin. J Cell Sci 2017; 130:1251–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. So C, Seres KB, Steyer AM, Monnich E, Clift D, Pejkovska A, Mobius W, Schuh M. A liquid-like spindle domain promotes acentrosomal spindle assembly in mammalian oocytes. Science 2019; 364:eaat9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dumont J, Petri S, Pellegrin F, Terret ME, Bohnsack MT, Rassinier P, Georget V, Kalab P, Gruss OJ, Verlhac MH. A centriole- and RanGTP-independent spindle assembly pathway in meiosis I of vertebrate oocytes. J Cell Biol 2007; 176:295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schuh M, Ellenberg J. Self-organization of MTOCs replaces centrosome function during acentrosomal spindle assembly in live mouse oocytes. Cell 2007; 130:484–498. [DOI] [PubMed] [Google Scholar]
- 11. Kitajima TS, Ohsugi M, Ellenberg J. Complete kinetochore tracking reveals error-prone homologous chromosome biorientation in mammalian oocytes. Cell 2011; 146:568–581. [DOI] [PubMed] [Google Scholar]
- 12. Clift D, Schuh M. A three-step MTOC fragmentation mechanism facilitates bipolar spindle assembly in mouse oocytes. Nat Commun 2015; 6:7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Little TM, Jordan PW. PLK1 is required for chromosome compaction and microtubule organization in mouse oocytes. Mol Biol Cell 2020; 31:1206–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blengini CS, Ibrahimian P, Vaskovicova M, Drutovic D, Solc P, Schindler K. Aurora kinase A is essential for meiosis in mouse oocytes. PLOS Genetics 2021; 17:e1009327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saskova A, Solc P, Baran V, Kubelka M, Schultz RM, Motlik J. Aurora kinase A controls meiosis I progression in mouse oocytes. Cell Cycle 2008; 7:2368–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ding J, Swain JE, Smith GD. Aurora kinase-A regulates microtubule organizing center (MTOC) localization, chromosome dynamics, and histone-H3 phosphorylation in mouse oocytes. Mol Reprod Dev 2011; 78:80–90. [DOI] [PubMed] [Google Scholar]
- 17. Solc P, Baran V, Mayer A, Bohmova T, Panenkova-Havlova G, Saskova A, Schultz RM, Motlik J. Aurora kinase A drives MTOC biogenesis but does not trigger resumption of meiosis in mouse oocytes matured in vivo. Biol Reprod 2012; 87:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Crane R, Gadea B, Littlepage L, Wu H, Ruderman JV. Aurora A, meiosis and mitosis. Biol Cell 2004; 96:215–229. [DOI] [PubMed] [Google Scholar]
- 19. Maliga Z, Mitchison TJ. Small-molecule and mutational analysis of allosteric Eg5 inhibition by monastrol. BMC Chem Biol 2006; 6:2–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brunet S, Dumont J, Lee KW, Kinoshita K, Hikal P, Gruss OJ, Maro B, Verlhac MH. Meiotic regulation of TPX2 protein levels governs cell cycle progression in mouse oocytes. PLoS One 2008; 3:e3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Breuer M, Kolano A, Kwon M, Li CC, Tsai TF, Pellman D, Brunet S, Verlhac MH. HURP permits MTOC sorting for robust meiotic spindle bipolarity, similar to extra centrosome clustering in cancer cells. J Cell Biol 2010; 191:1251–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Forbes DJ, Travesa A, Nord MS, Bernis C. Nuclear transport factors: global regulation of mitosis. Curr Opin Cell Biol 2015; 35:78–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Drutovic D, Duan X, Li R, Kalab P, Solc P. RanGTP and importin beta regulate meiosis I spindle assembly and function in mouse oocytes. EMBO J 2020; 39:e101689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eyers PA, Erikson E, Chen LG, Maller JL. A novel mechanism for activation of the protein kinase Aurora A. Curr Biol 2003; 13:691–697. [DOI] [PubMed] [Google Scholar]
- 25. Tsai MY, Wiese C, Cao K, Martin O, Donovan P, Ruderman J, Prigent C, Zheng Y. A ran signalling pathway mediated by the mitotic kinase Aurora A in spindle assembly. Nat Cell Biol 2003; 5:242–248. [DOI] [PubMed] [Google Scholar]
- 26. Fitzharris G. A shift from kinesin 5-dependent metaphase spindle function during preimplantation development in mouse. Development 2009; 136:2111–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yoshida S, Nishiyama S, Lister L, Hashimoto S, Mishina T, Courtois A, Kyogoku H, Abe T, Shiraishi A, Choudhary M, Nakaoka Y, Herbert Met al. . Prc1-rich kinetochores are required for error-free acentrosomal spindle bipolarization during meiosis I in mouse oocytes. Nat Commun 2020; 11:2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Courtois A, Yoshida S, Takenouchi O, Asai K, Kitajima TS. Stable kinetochore–microtubule attachments restrict MTOC position and spindle elongation in oocytes. EMBO reports 2021; 22:e51400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bennabi I, Queguiner I, Kolano A, Boudier T, Mailly P, Verlhac MH, Terret ME. Shifting meiotic to mitotic spindle assembly in oocytes disrupts chromosome alignment. EMBO Rep 2018; 19:368–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kolano A, Brunet S, Silk AD, Cleveland DW, Verlhac MH. Error-prone mammalian female meiosis from silencing the spindle assembly checkpoint without normal interkinetochore tension. Proc Natl Acad Sci U S A 2012; 109:E1858–E1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bury L, Coelho PA, Simeone A, Ferries S, Eyers CE, Eyers PA, Zernicka-Goetz M, Glover DM. Plk4 and Aurora A cooperate in the initiation of acentriolar spindle assembly in mammalian oocytes. J Cell Biol 2017; 216:3571–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang X, Baumann C, De La Fuente R, Viveiros MM. CEP215 and AURKA regulate spindle pole focusing and aMTOC organization in mouse oocytes. Reproduction 2020; 159:261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang X, Baumann C, De La Fuente R, Viveiros MM. Loss of acentriolar MTOCs disrupts spindle pole Aurora A and assembly of the liquid-like meiotic spindle domain in oocytes. J Cell Sci 2021;134:jcs256297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nguyen AL, Drutovic D, Vazquez BN, El Yakoubi W, Gentilello AS, Malumbres M, Solc P, Schindler K. Genetic interactions between the aurora kinases reveal new requirements for AURKB and AURKC during oocyte meiosis. Curr Biol 2018; 28:3458.−+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Balboula AZ, Nguyen AL, Gentilello AS, Quartuccio SM, Drutovic D, Solc P, Schindler K. Haspin kinase regulates microtubule-organizing center clustering and stability through Aurora kinase C in mouse oocytes. J Cell Sci 2016; 129:3648–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Balboula AZ, Schindler K. Selective disruption of aurora C kinase reveals distinct functions from aurora B kinase during meiosis in mouse oocytes. PLoS Genet 2014; 10:e1004194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vallot A, Leontiou I, Cladiere D, El Yakoubi W, Bolte S, Buffin E, Wassmann K. Tension-induced error correction and not kinetochore attachment status activates the SAC in an aurora-B/C-dependent manner in oocytes. Curr Biol 2018; 28:130–139.e133. [DOI] [PubMed] [Google Scholar]
- 38. Chmatal L, Yang K, Schultz RM, Lampson MA. Spatial regulation of kinetochore microtubule attachments by destabilization at spindle poles in meiosis I. Curr Biol 2015; 25:1835–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Solc P, Kitajima TS, Yoshida S, Brzakova A, Kaido M, Baran V, Mayer A, Samalova P, Motlik J, Ellenberg J. Multiple requirements of PLK1 during mouse oocyte maturation. PLOS ONE 2015; 10:e0116783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Musacchio A. The molecular biology of spindle assembly checkpoint signaling dynamics. Curr Biol 2015; 25:R1002–R1018. [DOI] [PubMed] [Google Scholar]
- 41. Maresca TJ, Salmon ED. Welcome to a new kind of tension: translating kinetochore mechanics into a wait-anaphase signal. J Cell Sci 2010; 123:825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nagaoka SI, Hodges CA, Albertini DF, Hunt PA. Oocyte-specific differences in cell-cycle control create an innate susceptibility to meiotic errors. Curr Biol 2011; 21:651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lane SI, Yun Y, Jones KT. Timing of anaphase-promoting complex activation in mouse oocytes is predicted by microtubule-kinetochore attachment but not by bivalent alignment or tension. Development 2012; 139:1947–1955. [DOI] [PubMed] [Google Scholar]
- 44. Zhang Q-H, Yuen WS, Adhikari D, Flegg JA, FitzHarris G, Conti M, Sicinski P, Nabti I, Marangos P, Carroll J. Cyclin A2 modulates kinetochore–microtubule attachment in meiosis II. J Cell Biol 2017; 216:3133–3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lefebvre C, Terret ME, Djiane A, Rassinier P, Maro B, Verlhac MH. Meiotic spindle stability depends on MAPK-interacting and spindle-stabilizing protein (MISS), a new MAPK substrate. J Cell Biol 2002; 157:603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Terret ME, Lefebvre C, Djiane A, Rassinier P, Moreau J, Maro B, Verlhac MH. DOC1R: a MAP kinase substrate that control microtubule organization of metaphase II mouse oocytes. Development 2003; 130:5169–5177. [DOI] [PubMed] [Google Scholar]
- 47. Kovacovicova K, Awadova T, Mikel P, Anger M. In vitro maturation of mouse oocytes increases the level of Kif11/Eg5 on meiosis II spindles. Biol Reprod 2016; 95:18. [DOI] [PubMed] [Google Scholar]
- 48. Mogessie B, Schuh M. Actin protects mammalian eggs against chromosome segregation errors. Science 2017; 357:eaal1647. [DOI] [PubMed] [Google Scholar]
- 49. Brunet S, Verlhac MH. Positioning to get out of meiosis: the asymmetry of division. Hum Reprod Update 2010; 17:68–75. [DOI] [PubMed] [Google Scholar]
- 50. Clift D, Schuh M. Restarting life: fertilization and the transition from meiosis to mitosis. Nat Rev Mol Cell Biol 2013; 14:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chaigne A, Terret M-E, Verlhac M-H. Asymmetries and symmetries in the mouse oocyte and zygote. In: Tassan J-P, Kubiak JZ (eds.), (eds.)Asymmetric Cell Division in Development, Differentiation and Cancer. Cham: Springer International Publishing; 2017: 285–299. [DOI] [PubMed] [Google Scholar]
- 52. Mogessie B, Scheffler K, Schuh M. Assembly and positioning of the oocyte meiotic spindle. Annu Rev Cell Dev Biol 2018; 34:381–403. [DOI] [PubMed] [Google Scholar]
- 53. Uraji J, Scheffler K, Schuh M. Functions of actin in mouse oocytes at a glance. J Cell Sci 2018; 131:jcs218099. [DOI] [PubMed] [Google Scholar]
- 54. Holubcová Z, Blayney M, Elder K, Schuh M. Human oocytes. Error-prone chromosome-mediated spindle assembly favors chromosome segregation defects in human oocytes. Science (New York, N.Y.) 2015; 348:1143–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wloga D, Joachimiak E, Fabczak H. Tubulin post-translational modifications and microtubule dynamics. Int J Mol Sci 2017; 18:2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schatten H, Sun QY. Centrosome and microtubule functions and dysfunctions in meiosis: implications for age-related infertility and developmental disorders. Reprod Fertil Dev 2015; 27:934–943. [DOI] [PubMed] [Google Scholar]
- 57. Li X, Liu X, Gao M, Han L, Qiu D, Wang H, Xiong B, Sun SC, Liu H, Gu L. HDAC3 promotes meiotic apparatus assembly in mouse oocytes by modulating tubulin acetylation. Development 2017; 144:3789–3797. [DOI] [PubMed] [Google Scholar]
- 58. Ling L, Hu F, Ying X, Ge J, Wang Q. HDAC6 inhibition disrupts maturational progression and meiotic apparatus assembly in mouse oocytes. Cell Cycle 2018; 17:550–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sui L, Zhang S, Huang R, Li Z. HDAC11 promotes meiotic apparatus assembly during mouse oocyte maturation via decreasing H4K16 and alpha-tubulin acetylation. Cell Cycle 2020; 19:354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nevoral J, Landsmann L, Stiavnicka M, Hosek P, Moravec J, Prokesova S, Rimnacova H, Koutna E, Klein P, Hoskova K, Zalmanova T, Fenclova Tet al. . Epigenetic and non-epigenetic mode of SIRT1 action during oocyte meiosis progression. J Anim Sci Biotechnol 2019; 10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang L, Hou X, Ma R, Moley K, Schedl T, Wang Q. Sirt2 functions in spindle organization and chromosome alignment in mouse oocyte meiosis. FASEB J 2014; 28:1435–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sharma RK, Azeem A, Agarwal A. Spindle and chromosomal alterations in metaphase II oocytes. Reprod Sci 2013; 20:1293–1301. [DOI] [PubMed] [Google Scholar]
- 63. Combelles CMH, Gupta S, Agarwal A. Could oxidative stress influence the in-vitro maturation of oocytes? Reprod Biomed Online 2009; 18:864–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol 2012; 10:49–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tarín JJ, Vendrell FJ, Ten J, Blanes R, Van Blerkom J, Cano A. The oxidizing agent tertiary butyl hydroperoxide induces disturbances in spindle organization, c-meiosis, and aneuploidy in mouse oocytes. Mol Hum Reprod 1996; 2:895–901. [DOI] [PubMed] [Google Scholar]
- 66. Choi W-J, Banerjee J, Falcone T, Bena J, Agarwal A, Sharma RK. Oxidative stress and tumor necrosis factor–α–induced alterations in metaphase II mouse oocyte spindle structure. Fertil Steril 2007; 88:1220–1231. [DOI] [PubMed] [Google Scholar]
- 67. Zhang X, Wu XQ, Lu S, Guo YL, Ma X. Deficit of mitochondria-derived ATP during oxidative stress impairs mouse MII oocyte spindles. Cell Research 2006; 16:841–850. [DOI] [PubMed] [Google Scholar]
- 68. Mihalas BP, De Iuliis GN, Redgrove KA, McLaughlin EA, Nixon B. The lipid peroxidation product 4-hydroxynonenal contributes to oxidative stress-mediated deterioration of the ageing oocyte. Sci Rep 2017; 7:6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Miao Y-L, Kikuchi K, Sun Q-Y, Schatten H. Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Hum Reprod Update 2009; 15:573–585. [DOI] [PubMed] [Google Scholar]
- 70. Liu L, Keefe DL. Ageing-associated aberration in meiosis of oocytes from senescence-accelerated mice. Hum Reprod 2002; 17:2678–2685. [DOI] [PubMed] [Google Scholar]
- 71. Battaglia DE, Goodwin P, Klein NA, Soules MR. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod 1996; 11:2217–2222. [DOI] [PubMed] [Google Scholar]
- 72. Alvarez Sedo C, Schatten H, Combelles CM, Rawe VY. The nuclear mitotic apparatus (NuMA) protein: localization and dynamics in human oocytes, fertilization and early embryos. Mol Hum Reprod 2011; 17:392–398. [DOI] [PubMed] [Google Scholar]
- 73. Shimoi G, Tomita M, Kataoka M, Kameyama Y. Destabilization of spindle assembly checkpoint causes aneuploidy during meiosis II in murine post-ovulatory aged oocytes. J Reprod Dev 2019; 65:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tang F, Pan MH, Lu Y, Wan X, Zhang Y, Sun SC. Involvement of Kif4a in spindle formation and chromosome segregation in mouse oocytes. Aging Dis 2018; 9:623–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Llonch S, Barragán M, Nieto P, Mallol A, Elosua-Bayes M, Lorden P, Ruiz S, Zambelli F, Heyn H, Vassena R, Payer B. Single human oocyte transcriptome analysis reveals distinct maturation stage-dependent pathways impacted by age. Aging Cell 2021; 20:e13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nakagawa S, FitzHarris G. Intrinsically defective microtubule dynamics contribute to age-related chromosome segregation errors in mouse oocyte meiosis-I. Curr Biol 2017; 27:1040–1047. [DOI] [PubMed] [Google Scholar]
- 77. Shomper M, Lappa C, FitzHarris G. Kinetochore microtubule establishment is defective in oocytes from aged mice. Cell cycle (Georgetown, Tex.) 2014; 13:1171–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chiang T, Duncan FE, Schindler K, Schultz RM, Lampson MA. Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes. Curr Biol 2010; 20:1522–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lister LM, Kouznetsova A, Hyslop LA, Kalleas D, Pace SL, Barel JC, Nathan A, Floros V, Adelfalk C, Watanabe Y, Jessberger R, Kirkwood TBet al. . Age-related meiotic segregation errors in mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr Biol 2010; 20:1511–1521. [DOI] [PubMed] [Google Scholar]
- 80. Duncan FE, Hornick JE, Lampson MA, Schultz RM, Shea LD, Woodruff TK. Chromosome cohesion decreases in human eggs with advanced maternal age. Aging cell 2012; 11:1121–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zielinska AP, Bellou E, Sharma N, Frombach A-S, Seres KB, Gruhn JR, Blayney M, Eckel H, Moltrecht R, Elder K, Hoffmann ER, Schuh M. Meiotic kinetochores fragment into multiple lobes upon cohesin loss in aging eggs. Curr Biol 2019; 29:3749–3765.e3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Coticchio G, Dal-Canto M, Guglielmo MC, Mignini-Renzini M, Fadini R. Human oocyte maturation in vitro. Int J Dev Biol 2012; 56:909–918. [DOI] [PubMed] [Google Scholar]
- 83. Virant-Klun I, Bauer C, Ståhlberg A, Kubista M, Skutella T. Human oocyte maturation in vitro is improved by co-culture with cumulus cells from mature oocytes. Reprod Biomed Online 2018; 36:508–523. [DOI] [PubMed] [Google Scholar]
- 84. Sakai C, Hoshino Y, Sato Y, Sato E. Evaluation of maturation competence of metaphase II oocytes in mice based on the distance between pericentriolar materials of meiotic spindle: distance of PCM during oocyte maturation. J Assist Reprod Genet 2011; 28:157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sanfins A, Lee GY, Plancha CE, Overstrom EW, Albertini DF. Distinctions in meiotic spindle structure and assembly during in vitro and in vivo maturation of mouse oocytes1. Biol Reprod 2003; 69:2059–2067. [DOI] [PubMed] [Google Scholar]
- 86. Sanfins A, Plancha CE, Overstrom EW, Albertini DF. Meiotic spindle morphogenesis in in vivo and in vitro matured mouse oocytes: insights into the relationship between nuclear and cytoplasmic quality. Hum Reprod 2004; 19:2889–2899. [DOI] [PubMed] [Google Scholar]
- 87. Coticchio G, Guglielmo MC, Dal Canto M, Fadini R, Mignini Renzini M, De Ponti E, Brambillasca F, Albertini DF. Mechanistic foundations of the metaphase II spindle of human oocytes matured in vivo and in vitro. Hum Reprod 2013; 28:3271–3282. [DOI] [PubMed] [Google Scholar]
- 88. Iussig B, Maggiulli R, Fabozzi G, Bertelle S, Vaiarelli A, Cimadomo D, Ubaldi FM, Rienzi L. A brief history of oocyte cryopreservation: arguments and facts. Acta Obstet Gynecol Scand 2019; 98:550–558. [DOI] [PubMed] [Google Scholar]
- 89. Chen SU, Yang YS. Slow freezing or vitrification of oocytes: their effects on survival and meiotic spindles, and the time schedule for clinical practice. Taiwan J Obstet Gynecol 2009; 48:15–22. [DOI] [PubMed] [Google Scholar]
- 90. Rienzi L, Gracia C, Maggiulli R, LaBarbera AR, Kaser DJ, Ubaldi FM, Vanderpoel S, Racowsky C. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update 2017; 23:139–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kuwayama M, Vajta G, Kato O, Leibo SP. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod Biomed Online 2005; 11:300–308. [DOI] [PubMed] [Google Scholar]
- 92. Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology 2007; 67:73–80. [DOI] [PubMed] [Google Scholar]
- 93. Pickering SJ, Braude PR, Johnson MH, Cant A, Currie J. Transient cooling to room temperature can cause irreversible disruption of the meiotic spindle in the human oocyte**supported by program grant PG 8302273 to P.R.B, and M.H.J, from the Medical Research Council of Great Britain, Cambridge, England. Fertil Steril 1990; 54:102–108. [DOI] [PubMed] [Google Scholar]
- 94. Zenzes MT, Bielecki R, Casper RF, Leibo SP. Effects of chilling to 0°C on the morphology of meiotic spindles in human metaphase II oocytes. Fertil Steril 2001; 75:769–777. [DOI] [PubMed] [Google Scholar]
- 95. Rienzi L, Martinez F, Ubaldi F, Minasi MG, Iacobelli M, Tesarik J, Greco E. Polscope analysis of meiotic spindle changes in living metaphase II human oocytes during the freezing and thawing procedures. Hum Reprod 2004; 19:655–659. [DOI] [PubMed] [Google Scholar]
- 96. Gomes CM, Silva CA, Acevedo N, Baracat E, Serafini P, Smith GD. Influence of vitrification on mouse metaphase II oocyte spindle dynamics and chromatin alignment. Fertil Steril 2008; 90:1396–1404. [DOI] [PubMed] [Google Scholar]
- 97. Tamura AN, Huang TTF, Marikawa Y. Impact of vitrification on the meiotic spindle and components of the microtubule-organizing center in mouse mature oocytes1. Biol Reprod 2013; 89:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bromfield JJ, Coticchio G, Hutt K, Sciajno R, Borini A, Albertini DF. Meiotic spindle dynamics in human oocytes following slow-cooling cryopreservation. Hum Reprod 2009; 24:2114–2123. [DOI] [PubMed] [Google Scholar]
- 99. Keefe D, Liu L, Wang W, Silva C. Imaging meiotic spindles by polarization light microscopy: principles and applications to IVF. Reprod Biomed Online 2003; 7:24–29. [DOI] [PubMed] [Google Scholar]
- 100. Liu L, Oldenbourg R, Trimarchi JR, Keefe DL. A reliable, noninvasive technique for spindle imaging and enucleation of mammalian oocytes. Nat Biotechnol 2000; 18:223–225. [DOI] [PubMed] [Google Scholar]
- 101. Madaschi C, Souza Bonetti TC, Almeida Ferreira Braga DP, Pasqualotto FF, Iaconelli A Jr, Borges E Jr. Spindle imaging: a marker for embryo development and implantation. Fertil Steril 2008; 90:194–198. [DOI] [PubMed] [Google Scholar]
- 102. Kilani S, Cooke S, Kan A, Chapman M. Are there non-invasive markers in human oocytes that can predict pregnancy outcome? Reprod Biomed Online 2009; 18:674–680. [DOI] [PubMed] [Google Scholar]
- 103. Garcia-Oro S, Rey MI, Rodriguez M, Duran A, Devesa R, Valverde D. Predictive value of spindle retardance in embryo implantation rate. J Assist Reprod Genet 2017; 34:617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Tilia L, Venetis C, Kilani S, Cooke S, Chapman M. Is oocyte meiotic spindle morphology associated with embryo ploidy? A prospective cohort study. Fertil Steril 2016; 105:1085, e1087–1092. [DOI] [PubMed] [Google Scholar]