Abstract
Background:
Acute gastroenteritis (AGE) causes a substantial burden in the United States, but its etiology frequently remains undetermined. Active surveillance within an integrated healthcare delivery system was used to estimate the prevalence and incidence of medically attended norovirus, rotavirus, sapovirus, and astrovirus.
Methods:
Active surveillance was conducted among all enrolled members of Kaiser Permanente Northwest during July 2014 – June 2016. An age-stratified, representative sample of AGE-associated medical encounters were recruited to provide a stool specimen to be tested for norovirus, rotavirus, sapovirus, and astrovirus. Medically attended AGE (MAAGE) encounters for a patient occurring within 30 days were grouped into one episode, and all-cause MAAGE incidence was calculated. Pathogen- and healthcare setting-specific incidence estimates were calculated using age-stratified bootstrapping.
Results:
The overall incidence of MAAGE was 40.6 episodes per 1000 person-years (PY), with most episodes requiring no more than outpatient care. Norovirus was the most frequently detected pathogen, with an incidence of 5.5 medically attended episodes per 1000 PY. Incidence of norovirus MAAGE was highest among children aged <5 years (20.4 episodes per 1000 PY), followed by adults aged ≥65 years (4.5 episodes per 1000 PY). Other study pathogens showed similar patterns by age, but lower overall incidence (sapovirus: 2.4 per 1000 PY, astrovirus: 1.3 per 1000 PY, rotavirus: 0.5 per 1000 PY).
Conclusions:
Viral enteropathogens, particularly norovirus, are an important contributor to MAAGE, especially among children <5 years of age. The present findings underline the importance of judicious antibiotics use for pediatric AGE and suggest that an effective norovirus vaccine could substantially reduce MAAGE.
Keywords: acute gastroenteritis, viral gastroenteritis, norovirus, rotavirus, sapovirus, astrovirus, disease burden, healthcare burden, population-based surveillance
Summary:
During two years of active surveillance within an integrated healthcare delivery system, the incidence of medically attended gastroenteritis (MAAGE) was 40.6 episodes per 1000 person-years (PY). The incidence of norovirus-associated MAAGE was 5.5 episodes per 1000 PY.
INTRODUCTION
Acute gastroenteritis (AGE) causes a substantial burden in the United States, resulting in over ten million outpatient visits and over one million hospitalizations annually [1–3]. However, due to limited use of testing, AGE etiology frequently remains undetermined, particularly in the outpatient or Emergency Department (ED) setting, where >90% of AGE encounters do not have a pathogen-specific diagnostic code [1–5]. Available data indicate that viral pathogens—especially norovirus—play a large role in medically attended AGE (MAAGE) [6–8]. Given the maturity and impact of the rotavirus vaccination program in the United States [9, 10], and the ongoing development of several norovirus vaccine candidates [11], there is a need for updated incidence estimates of MAAGE and its viral etiologies across the age and clinical severity spectrums. Population-based active surveillance platforms can provide age- and healthcare setting–specific incidence estimates, which are useful in informing appropriate clinical management of AGE patients (e.g., promoting judicious use of antibiotics) as well as in targeting interventions such as vaccines. This study used active surveillance of the Kaiser Permanente Northwest (KPNW) member population to estimate the prevalence of four viral enteropathogens (norovirus, rotavirus, sapovirus, and astrovirus) among MAAGE across the age spectrum and to generate age-, healthcare setting-, and pathogen-specific estimates of MAAGE incidence.
METHODS
Active, Population-based Surveillance for MAAGE
A detailed description of the surveillance methods in this study has been published previously [12]. Briefly, active surveillance for all MAAGE encounters (including remote encounters [email, telephone, or video visits], outpatient visits, ED visits, and hospital admissions) was conducted during April 2014 – September 2016 among all enrolled members of KPNW, an integrated health care delivery system with approximately 605,000 members in the Portland, Oregon metropolitan area. A random, age-stratified, representative sample of medical encounters with an International Classification of Disease – Clinical Modification (ICD-CM) code for AGE (in-person encounters) or a chief complaint referencing AGE (remote encounters) were recruited for study participation (Supplemental Table 1); ICD-CM version 9 (ICD-CM-9) was used during April 2014 – September 30, 2015, after which version 10 was used exclusively. Age groups <5 years and ≥75 years were oversampled to enable more precise incidence estimates. Participants who provided informed consent, completed a baseline survey and indicated willingness to complete a follow-up survey, and provided a viable stool specimen (separate from any clinical diagnostic specimen) were considered study completers. Additional information on study completers was abstracted from electronic health records and related databases. This project was reviewed and approved by the KPNW Institutional Review Board (FWA00002344).
Stool specimens were tested for norovirus, rotavirus, sapovirus, and astrovirus RNA at the Oregon State Public Health Laboratory (OSPHL) using CDC-developed TaqMan real-time reverse transcription polymerase chain reaction (RT-PCR) protocols, and positive specimens were genotyped using Sanger sequencing [12]. Rotavirus-positive specimens at OSPHL were re-tested at CDC by commercial enzyme immunoassay (EIA) and genotyped using updated methods [13–15]; only those specimens testing positive by both RT-PCR and EIA were considered positive for rotavirus, for comparability with previous studies based on EIA, and because EIA is often considered to better correlate with rotavirus-associated clinical illness [16].
All-cause and Pathogen-specific MAAGE Incidence Estimation
For each unique patient, we grouped all MAAGE encounters occurring within 30 days of each other into a single episode with an index date corresponding to the first MAAGE encounter for that episode. Unique MAAGE episodes were categorized hierarchically based on the highest level of care received (i.e., inpatient>ED>outpatient>remote). Additionally, each MAAGE episode was classified by age group (defined by the patient’s age at the index encounter), whether or not a stool specimen was tested (i.e., study completer), and the stool testing results (viral positivity). We restricted this analysis to MAAGE episodes with an index date during the 2-year study period from July 2014 through June 2016, to account for seasonal variability in all-cause and pathogen-specific MAAGE incidence. To address uncertainty introduced by the fact that not all MAAGE episodes were tested, we utilized bootstrapping [17] to generate age group-, healthcare setting-, and pathogen-specific estimates of MAAGE incidence and the corresponding 95% confidence intervals (CI). Incidence numerators were calculated in 1000 replicates (sampling with replacement); point estimates were calculated by applying the median of the replicate estimates to population denominators, and confidence limits by applying the 2.5th and 97.5th percentiles. Incidence and prevalence estimates are presented by various age groups that account for the sampling scheme. Analyses were conducted in SAS (Cary, NC, v 9.3) and the R Environment for Statistical Computing (version 3.4.2).
RESULTS
Incidence of All-Cause MAAGE
During July 2014 – June 2016, there were a total of 42,030 unique MAAGE episodes presenting to KPNW, corresponding to an overall incidence of 40.6 MAAGE episodes per 1000 person-years (PY). Based on the highest level of care received, most MAAGE episodes were classified as outpatient visits (35,068 episodes, 83.4%, 33.9 per 1000 PY); 3,169 (7.5%, 3.1 per 1000 PY) were ED visits; 2,922 (7.0%, 2.8 per 1000 PY) were remote encounters; and 871 (2.1%, 0.84 per 1000 PY) were hospitalizations. Children aged <5 years and adults aged ≥65 made up a disproportionately large share of MAAGE episodes (children <5: 10.3% of episodes vs. 4.8% of KPNW population; adults ≥65: 24.8% vs. 16.6%). Other age groups were under-indexed: children aged 5 – 17 made up 14.9% of the population and 9.6% of MAAGE episodes, while adults 18 – 64 made up 63.7% of the population and 24.75% of MAAGE episodes.
The greatest incidence of MAAGE was observed among children aged <2 years, specifically 6–11-month-olds (167 per 1000 PY) and 12–17-month-olds (164 per 1000 PY) (Figure 1). Similarly, when stratifying by healthcare setting, the highest rates of outpatient and ED visits were seen in children aged 6–11 months (150 per 1000 PY and 14.2 per 1000 PY, respectively) and those aged 12–17 months (148 per 1000 PY and 11.4 per 1000 PY, respectively) (Figure 1). In contrast, incidence of hospitalizations and remote encounters were greatest among adults aged ≥65 years (2.7 per 1000 PY and 5.9 per 1000 PY, respectively), and rates increased with increasing age within this group (Figure 1).
Figure 1:
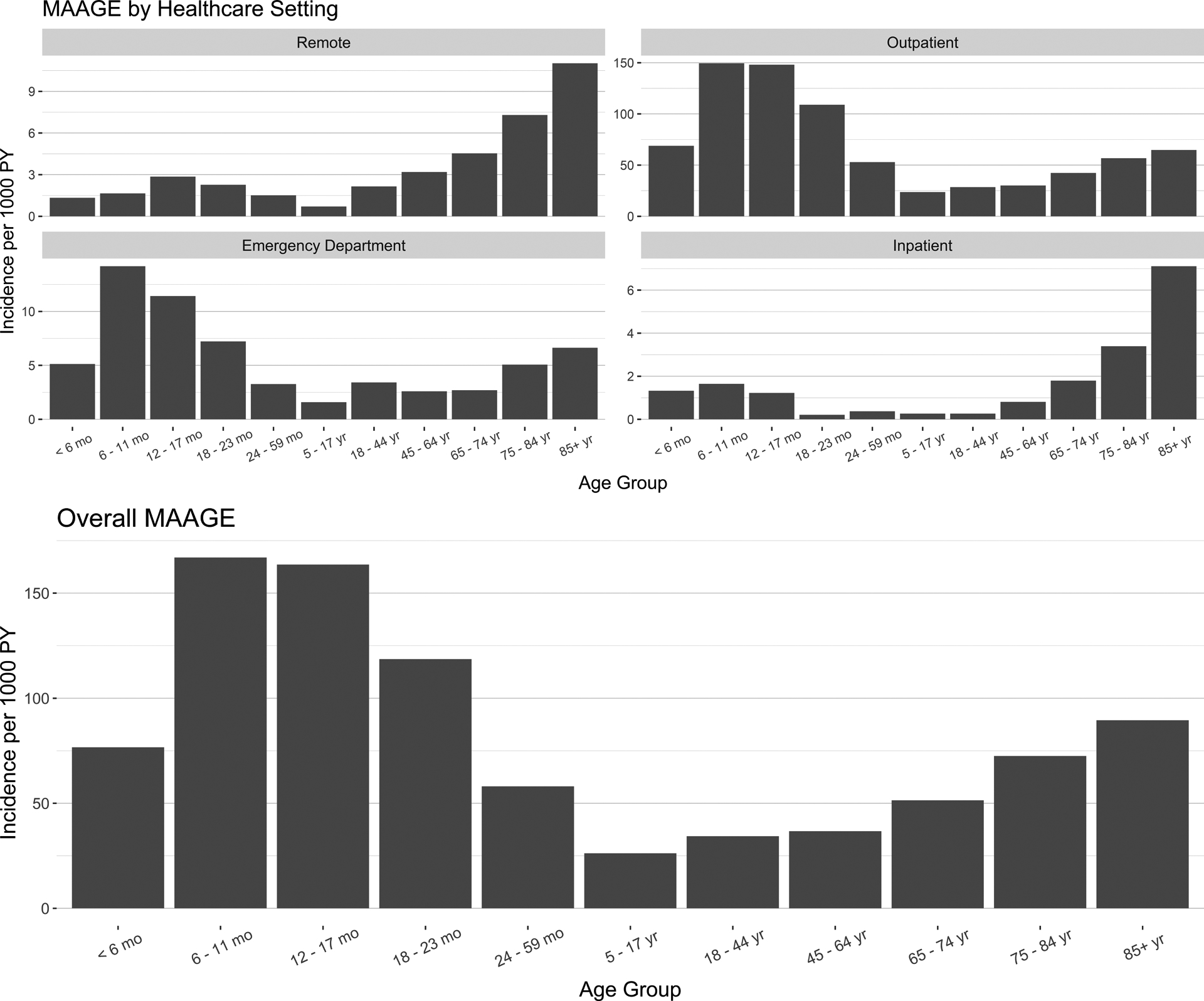
Incidence of medically attended acute gastroenteritis by age group and healthcare setting, Kaiser Permanente Northwest, July 2014–June 2016. Remote healthcare setting is defined as a phone, email, or video encounter.
Viral Prevalence among MAAGE
Following age-stratified sampling for recruitment, screening for eligibility, and consenting [12], there were 2,633 (6.3%) unique MAAGE episodes within our analysis period for which the patient was a study completer (Supplemental Figure 1). Across all encounter types and age groups (except ≥85 years), norovirus was the most frequently identified study pathogen (detected in 7 – 32% of tested samples), followed by sapovirus, astrovirus, and rotavirus (Supplemental Table 2). Among norovirus-positive samples, GII.4 (most often GII.4 Sydney) was the most prevalent genotype, particularly in the youngest (<5 years) and oldest (≥75 years) age groups (Supplemental Table 3).
All four viruses were detected more frequently among children than adults, although infections occurred throughout the age spectrum (Supplemental Table 2). The highest prevalence of norovirus and astrovirus was observed among children aged 18–23 months (32% and 8.3%, respectively), whereas sapovirus was most prevalent among infants aged 6–11 months (12%) and rotavirus was most prevalent among children aged 5–17 years (3.8%). In most but not all age groups, the prevalence of norovirus was higher in the ED compared with the outpatient setting (Supplemental Table 2). When viral prevalence was plotted by month, a clear peak was evident for rotavirus in the spring of 2015, but trends were less clear for the other viruses (Figure 2). Stratifying MAAGE episodes by patient survey-reported symptoms (vomiting and diarrhea, diarrhea only, or vomiting only), the prevalence of norovirus was much higher among patients reporting vomiting, compared with those reporting only diarrhea (Table 1), especially among children <5 years of age. Diarrhea-only MAAGE episodes were less likely to test positive for one of the study viruses compared with MAAGE episodes where the patient reported vomiting (with or without diarrhea).
Figure 2:
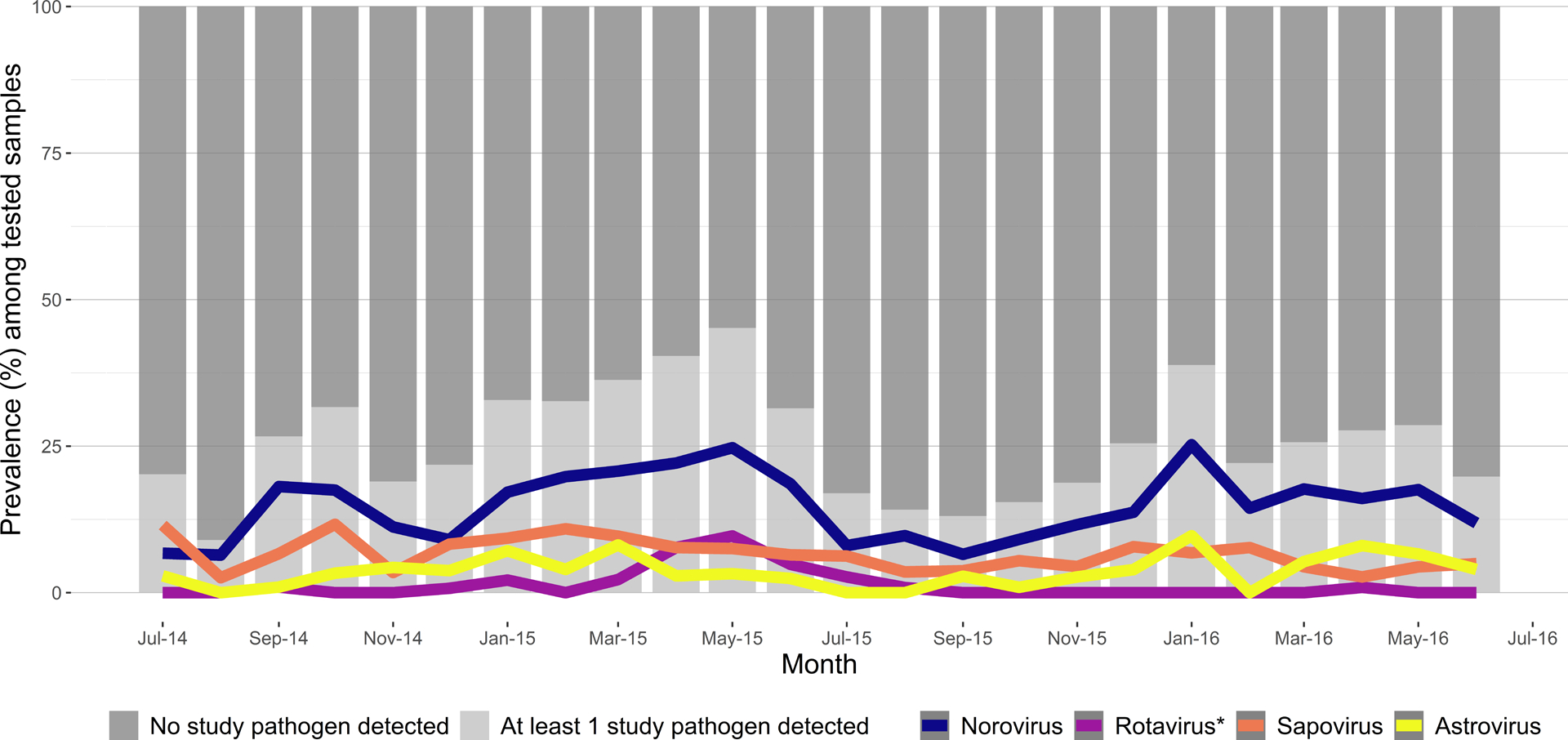
Prevalence of viruses among 2,633 patients with medically attended acute gastroenteritis by month of encounter, Kaiser Permanente Northwest, July 2014–June 2016. *Rotavirus positivity defined by both RT-PCR and EIA positivity.
Table 1:
Viral prevalence by clinical presentation and age group, Kaiser Permanente Northwest, July 2014–June 2016
| Diarrhea & Vomiting | Diarrhea Only | Vomiting Only | |
|---|---|---|---|
| Under 5 years of age | |||
| N | 437 | 262 | 36 |
| Pathogen Results | |||
| Norovirus | 125 (28.6) | 24 (9.2) | 13 (36.1) |
| Rotavirus* | 13 (3.0) | 0 (0.0) | 0 (0.0) |
| Sapovirus | 52 (11.9) | 15 (5.7) | 4 (11.1) |
| Astrovirus | 30 (6.9) | 11 (4.2) | 0 (0.0) |
| Mixed viral | 9 (2.1) | 4 (1.5) | 0 (0.0) |
| No virus detected in study testing | 208 (47.6) | 208 (79.4) | 19 (52.8) |
| 5 to 74 years | |||
| N | 627 | 760 | 41 |
| Pathogen Results | |||
| Norovirus | 129 (20.6) | 28 (3.7) | 13 (31.7) |
| Rotavirus* | 19 (3.0) | 1 (0.1) | 0 (0.0) |
| Sapovirus | 41 (6.5) | 25 (3.3) | 5 (12.2) |
| Astrovirus | 15 (2.4) | 26 (3.4) | 0 (0.0) |
| Mixed viral | 5 (0.8) | 3 (0.4) | 0 (0.0) |
| No virus detected in study testing | 418 (66.7) | 677 (89.1) | 23 (56.1) |
| 75 years and up | |||
| N | 130 | 327 | 9 |
| Pathogen Results | |||
| Norovirus | 21 (16.2) | 10 (3.1) | 4 (44.4) |
| Rotavirus* | 2 (1.5) | 0 (0.0) | 0 (0.0) |
| Sapovirus | 9 (6.9) | 13 (4.0) | 0 (0.0) |
| Astrovirus | 2 (1.5) | 4 (1.2) | 0 (0.0) |
| Mixed viral | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| No virus detected in study testing | 96 (73.8) | 300 (91.7) | 5 (55.6) |
RT-PCR and EIA positive.
One third (886; 33%) of MAAGE episodes that underwent study testing, of which 118 (13%) were positive for ≥1 study virus, also had data from clinical diagnostic testing. Inconsistent results were rare (n = 2 [<1%], both rotavirus). Co-infection with a non-study pathogen (viral or non-viral) was uncommon (n = 7 [6%]; 5 C. difficile and 2 Campylobacter).
Incidence of MAAGE Associated with Norovirus, Rotavirus, Sapovirus, or Astrovirus
Of the four viral enteropathogens assessed, norovirus had the highest estimated MAAGE incidence (5.5 per 1000 PY, 95% CI: 4.8–6.1), followed by sapovirus (2.4 per 1000 PY, 95% CI: 2.0–2.8), astrovirus (1.3 per 1000 PY, 1.0–1.6), and rotavirus (0.5 per 1000 PY, 95% CI: 0.3–0.7) (Figure 3). This pattern was similar across all four healthcare settings (Figure 3). MAAGE incidence for all four viruses was greatest among children aged <2 years, with peak incidence for norovirus and sapovirus occurring in 6–11-month-olds (47.7 per 1000 PY and 20.0 per 1000 PY, respectively), for astrovirus in 12–17-month-olds (10.0 per 1000 PY), and for rotavirus in 18–23-month-olds (3.1 per 1000 PY) (Figure 4). Incidence of viral MAAGE generally declined with increasing age, although somewhat elevated incidence of MAAGE associated with norovirus, sapovirus, and rotavirus was observed among adults aged ≥65 years (Figure 4). Viral incidence estimates stratified by age group and healthcare setting were limited by small cell sizes; thus, estimates are only available for age–setting strata with at least one positive stool specimen (Supplemental Tables 2 and 4).
Figure 3:
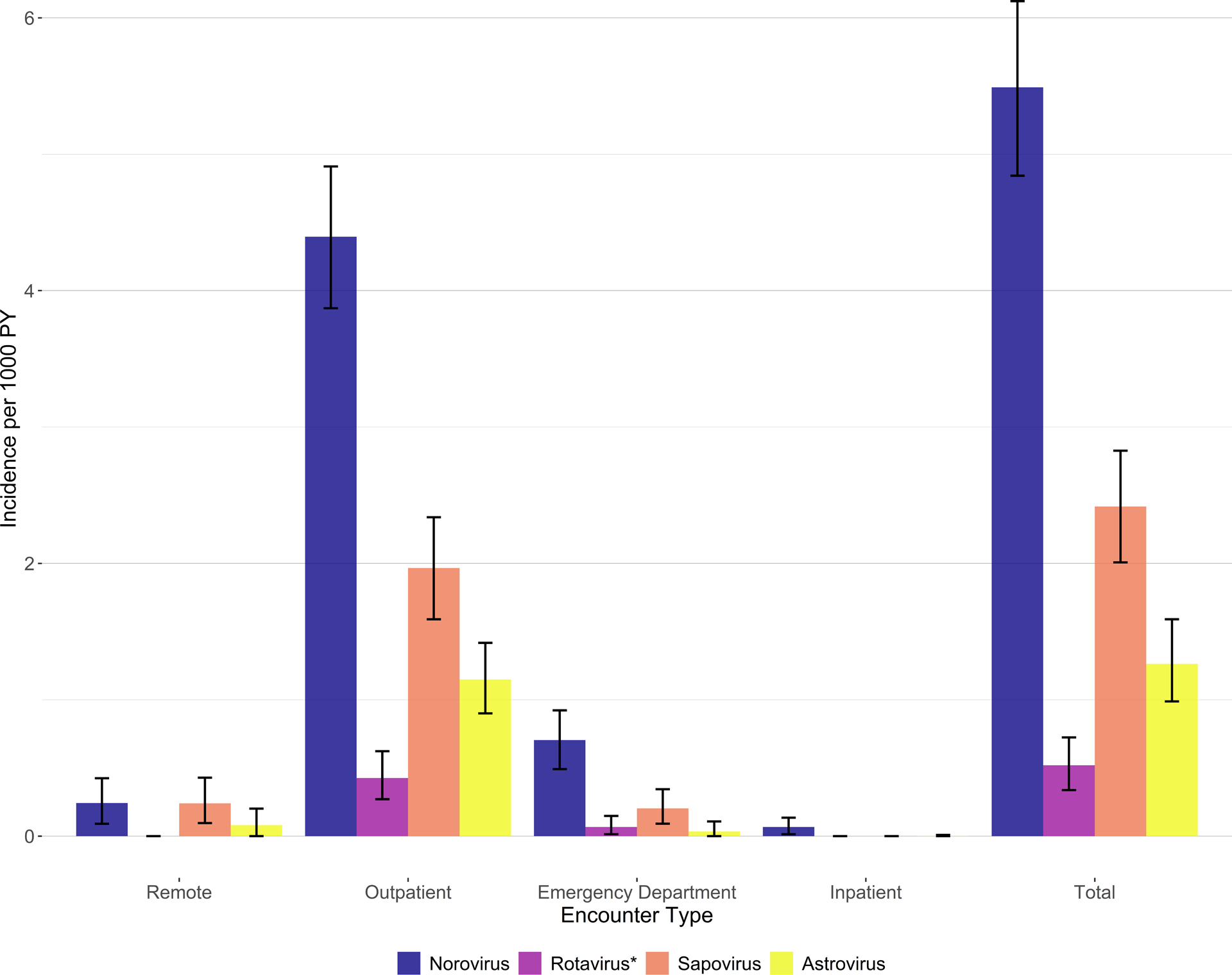
Incidence of medically attended acute gastroenteritis by virus and healthcare setting, Kaiser Permanente Northwest, July 2014–June 2016. *Rotavirus positivity defined by both RT-PCR and EIA positivity.
Figure 4:
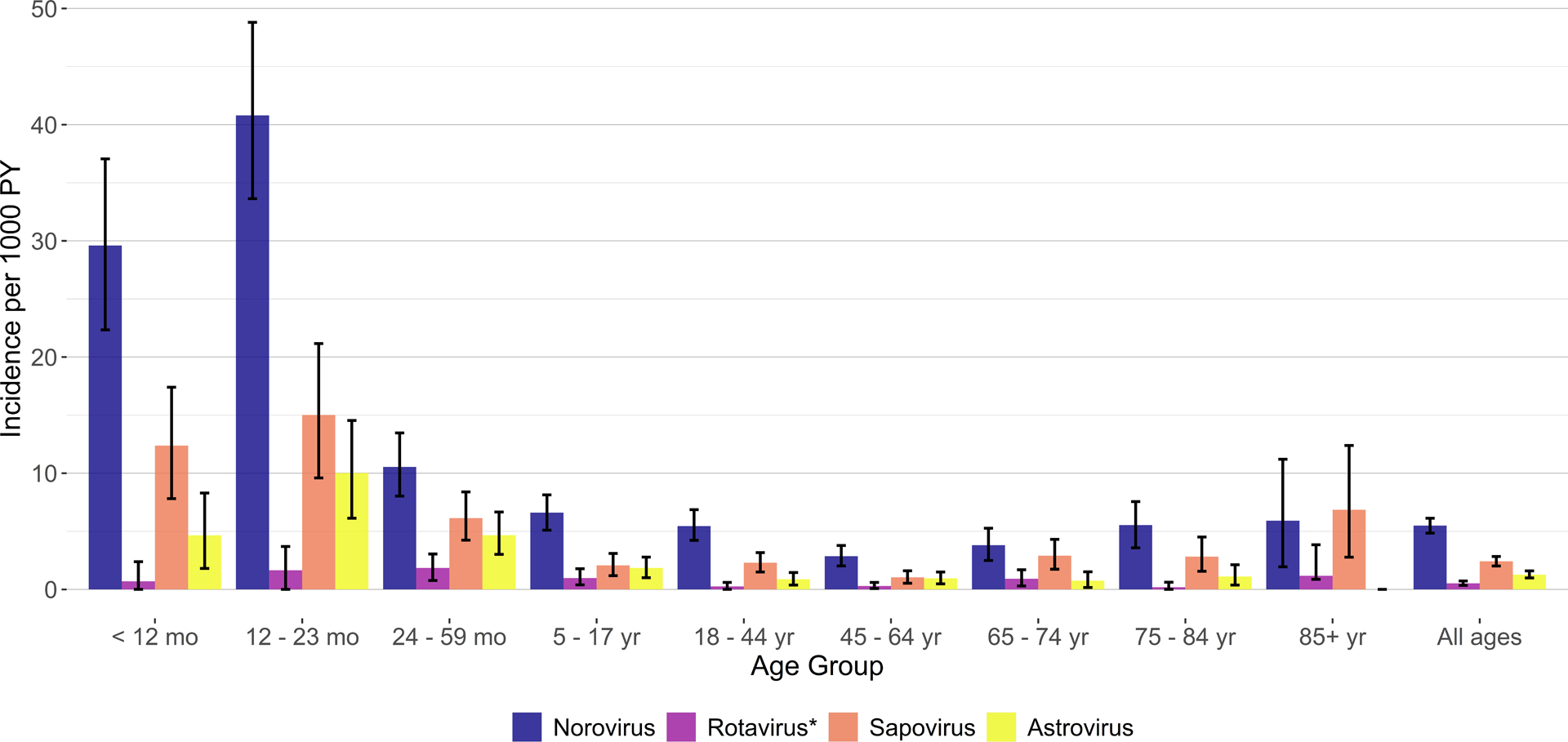
Incidence of medically attended acute gastroenteritis by virus and age group, Kaiser Permanente Northwest, July 2014–June 2016. *Rotavirus positivity defined by both RT-PCR and EIA positivity.
DISCUSSION
This is the first population-based, prospective cohort study for MAAGE across the age spectrum in the United States and demonstrates the key role played by four viral enteropathogens—particularly norovirus. In this study population, approximately 1 in 25 people experience a medically attended episode of AGE each year, approximately 1 in 4 children will have a MAAGE episode by the end of the second year of life, and approximately 1 in 10 children will have a medically attended episode of norovirus by the time they turn 5.
Our estimates of the overall, all-ages incidence of MAAGE are similar to other U.S. estimates. In our cohort, AGE hospitalizations occurred at a rate of 0.9 per 1000 PY, similar to, though somewhat lower than, previously estimated rates of 1.2–4.0 per 1000 PY [2, 3]. Similarly, our outpatient MAAGE rate of 38 per 1000 PY was slightly lower than estimates from other U.S.-based studies, which ranged from 47 to 54 per 1000 PY [1, 3, 18]. However, previous estimates of AGE rates may represent different subsets of the U.S. population than our KPNW cohort. Further, we excluded diarrheal episodes from patients with a history of chronic gastrointestinal disease and grouped AGE-related encounters occurring within 30 days into single episodes. Both of these practices likely contributed to a lower overall MAAGE incidence compared with analyses based solely on administrative data, which often apply no restrictions based on chronic disease and typically use AGE encounters to form rate numerators. Additionally, KPNW encourages remote consultations, which may have lowered outpatient rates as compared to other healthcare networks. Nonetheless, the overall patterns of AGE observed in our study, wherein the highest outpatient burden was observed in children and the highest hospitalization rates observed in the elderly, are consistent with other analyses [1–3].
Notably, our study provides the first active surveillance-based estimates of norovirus incidence in the United States. As reported by several other U.S.-based surveillance platforms, norovirus was the most commonly detected pathogen, and GII.4 was the most commonly detected norovirus genotype [19–22]. Our norovirus estimates are broadly similar to other U.S.-based estimates, albeit slightly lower than most: we estimated 0.1 norovirus-associated hospitalizations per 1000 PY, as compared to previous estimates of 0.09–0.4 per 1000 PY [2–4, 23]; 0.7 ED visits per 1000 PY, as compared to previous estimates of 0.6–1.5 per 1000 PY [1, 3, 23]; and 4.4 norovirus-associated outpatient visits per 1000 PY, as compared to previous estimates of 1.0–7.5 per 1000 PY [1, 3, 18, 19, 23]. Our estimates of norovirus-associated hospitalizations and ED visits are closest to those of Karve, et al. (0.09 hospitalizations and 0.6 ED visits per 1000 PY), who used a different methodology but also grouped MAAGE encounters occurring within a short time period into a single episode [23]. However, none of the other U.S. studies grouped encounters into episodes, and passive surveillance can be biased by clinician testing practices [1–4, 18, 19]. Norovirus incidence may also vary geographically within the U.S. [21, 22]. We found that norovirus was more prevalent among persons reporting a history of vomiting, compared with those reporting only diarrhea; this pattern was especially pronounced in children <5 years of age and consistent with research among Canadian children <5 years old [24]. The high prevalence of norovirus among vomiting-only presentations has implications for AGE surveillance as well as clinical diagnosis of AGE. Mirroring overall patterns of MAAGE, and consistent with other studies, we found the highest norovirus incidence among young children, followed by older adults [1–3, 18, 19, 25]. We also confirmed the expanded role of norovirus (relative to rotavirus) in MAAGE in children <5 years old in the setting of a mature rotavirus vaccine program [7, 8, 26].
After norovirus, sapovirus was the most frequently detected viral pathogen in our cohort, followed by astrovirus, with outpatient incidence estimates of 2.0 per 1000 PY and 1.1 per 1000 PY, respectively. Previously, passive surveillance in two Kaiser Permanente networks, including KPNW, also found sapovirus and astrovirus to play an important role, though with slightly lower incidence estimates of 1.6 per 1000 PY and 0.6 per 1000 PY, respectively [19]. Other U.S. studies in children have also suggested an increased relative prevalence of sapovirus following universal rotavirus vaccine introduction [7, 27, 28]. Among children <5, our estimate of astrovirus outpatient incidence (5.5 per 1000 PY) was highly comparable to that reported by the New Vaccine Surveillance Network (NVSN; ranging 3.2 – 8.7 per 1000 PY for the years 2013–2015) [28].
Rotavirus demonstrated the lowest incidence out of all the study viruses (0.5 per 1000 PY) but was also the only virus for which we required both RT-PCR and EIA positivity. Our estimates of outpatient rotavirus incidence (1.3 per 1000 PY) and ED rotavirus incidence (0.3 per 1000 PY) among children <5 were somewhat lower than estimates reported from NVSN (outpatient incidence of 5.5–17.7 per 1000 PY for the years 2013–2015; ED incidence ranging 0.8 – 11.1 per 1000 PY for the years 2009–2010) [8, 28]. However, these reports include earlier time periods, and rotavirus circulation in the U.S. has been decreasing since vaccine introduction in 2006, with a biennial pattern wherein even years demonstrate even further dampened activity [9, 29, 30]; we observed this pattern in our data, with estimated rotavirus prevalence and incidence for the seasonal year 2014–2015 far exceeding that in 2015–2016. High rotavirus vaccine coverage in this population may also have contributed to lower incidence [13].
The present study should be considered in relation to the following limitations. Not all MAAGE episodes were tested for the study viruses. Further, small sample sizes in some strata limited our ability to precisely estimate pathogen-specific incidence in certain age group-setting combinations. However, study participants were recruited using age-stratified random sampling, and submission of stool samples was high among those enrolled (76%) [12]. The present study made no adjustment for the possibility of detection of non-causative viral pathogens in stool samples (e.g., detection of viral RNA from an earlier or asymptomatic infection unrelated to the present illness). However, clinical testing of samples from this study identified a low prevalence of co-infection with non-study pathogens. Further, in a separate study of the KPNW population during a similar time period, the prevalence of these four pathogens among persons not experiencing AGE was much lower than among persons experiencing AGE (Schmidt et al. in preparation). As in many other studies, we identified MAAGE episodes using ICD codes. However, ICD codes have been found to have only moderate sensitivity for AGE in some settings, even when including vomiting-only codes [31]. This lack of sensitivity could have affected not only our overall MAAGE incidence estimates (biasing them downwards), but also our pathogen-specific MAAGE estimates, especially if episodes missed by our ICD code set or chief complaint text were more or less likely to have been attributable to one of the study pathogens. Additionally, our study period spanned the transition from the 9th version to the 10th version of ICD-CM; this could have affected our estimates if the sensitivity of the ICD code set was different in each version. Because study testing lagged symptom onset by more than a few days in some episodes, estimates could have been biased downwards if participants were no longer shedding a study virus that had caused their illness. However, the vast majority of stool samples for this cohort were collected within 10 days of the encounter (median 6 days) [12]. Further, fecal shedding ≥14 days has been documented as common among children infected with sapovirus [32] or rotavirus [33, 34], as well as among norovirus infections across the age spectrum [35–38]. Finally, because a suitable assay was not available at OSPHL at the time of the study, enteric adenoviruses were not included in the study virus panel. This may have caused an underestimation of the role of viral pathogens in MAAGE, since adenoviruses can be non-negligible causes of AGE, particularly in pediatric populations [39–41].
Our study, using active, population-based surveillance of a well-defined cohort over a two-year period, demonstrated the substantial role played by viral enteropathogens, particularly norovirus, in MAAGE—a role most prominent among children <5 years old and among persons experiencing vomiting. Consistent with other analyses, this study also showed a higher rate of AGE-related hospitalizations among elderly persons. The present findings highlight the importance of appropriate use of diagnostic testing and judicious use of antibiotics in MAAGE (particularly among young children). These findings further suggest that an effective norovirus vaccine, particularly one effective against GII.4 viruses, could alleviate healthcare burden and morbidity across the age and severity spectrum. Future active surveillance in a larger, nationally representative population could provide generalizable estimates with more precision, which may be especially informative as norovirus vaccines proceed in clinical development.
Supplementary Material
Acknowledgments
We would like to acknowledge the contributions of the MAAGE study recruiters, as well as Elizabeth Esterberg, Kevin Moua, and Holly Groom (KPNW) for assisting with data collection, management, and analysis. Initial testing for viral gastroenteritis agents was performed by Laura Tsaknaridis and Vladimir Yamshchikov (OSPHL), with confirmatory testing for rotavirus conducted by Eric Katz and Mike Bowen (CDC).
Funding
This work was supported by the CDC Foundation (institutional research funding to the Kaiser Permanente Center for Health Research) and Takeda Vaccines, Inc. (investigator-initiated research grants IISR-2015-101015 and IISR-2017-101938 to the Kaiser Permanente Center for Health Research). Takeda had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The Centers for Disease Control and Prevention received no funding from Takeda.
Conflicts of Interest Statements:
Rachel M. Burke, PhD, MPH: No conflicts
Claire Mattison, MPH: No conflicts
Zachary Marsh, MPH: No conflicts
Kayoko Shioda, DVM, MPH: No conflicts
Judy Donald, MPH: No conflicts
S. Bianca Salas, MPH: No conflicts
Allison L. Naleway, PhD has received research funding from Pfizer, Merck, MedImmune (now AstraZeneca) for unrelated studies.
Christianne Biggs, PhD: No conflicts
Mark A. Schmidt, PhD: No conflicts
Aron J. Hall, DVM, MSPH: No conflicts
Abbreviations Used:
- AGE
Acute gastroenteritis
- CDC
US Centers for Disease Control and Prevention
- CI
Confidence interval
- ED
Emergency department
- EIA
Enzyme immunoassay
- ICD-9-CM
International Classification of Diseases, 9th Revision, Clinical Modification
- ICD-10-CM
International Classification of Diseases, 10th Revision, Clinical Modification
- KPNW
Kaiser Permanente Northwest
- MAAGE
Medically attended acute gastroenteritis
- NVSN
New Vaccine Surveillance Network
- OSPHL
Oregon State Public Health Laboratory
- PY
Person years
- RT-PCR
Reverse transcription polymerase chain reaction
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention (CDC).
This is a pre-copyedited, author-produced version of an article accepted for publication in Clinical Infectious Diseases following peer review. The version of record, Rachel M Burke, Claire P Mattison, Zachary Marsh, Kayoko Shioda, Judy Donald, S Bianca Salas, Allison L Naleway, Christianne Biggs, Mark A Schmidt, Aron J Hall, Norovirus and Other Viral Causes of Medically Attended Acute Gastroenteritis Across the Age Spectrum: Results from the Medically Attended Acute Gastroenteritis Study in the United States, Clinical Infectious Diseases, Volume 73, Issue 4, 15 August 2021, Pages e913–e920, is available online at: https://doi.org/10.1093/cid/ciab033.
REFERENCES
- 1.Gastanaduy PA, Hall AJ, Curns AT, Parashar UD, Lopman BA. Burden of norovirus gastroenteritis in the ambulatory setting--United States, 2001–2009. J Infect Dis 2013; 207(7): 1058–65. [DOI] [PubMed] [Google Scholar]
- 2.Lopman BA, Hall AJ, Curns AT, Parashar UD. Increasing rates of gastroenteritis hospital discharges in US adults and the contribution of norovirus, 1996–2007. Clin Infect Dis 2011; 52(4): 466–74. [DOI] [PubMed] [Google Scholar]
- 3.Burke RM, Mattison C, Pindyck T, et al. The Burden of Norovirus in the United States, as Estimated Based on Administrative Data: Updates for Medically Attended Illness and Mortality, 2001 – 2015. Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scallan E, Hoekstra RM, Angulo FJ, et al. Foodborne illness acquired in the United States--major pathogens. Emerg Infect Dis 2011; 17(1): 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scallan E, Griffin PM, Angulo FJ, Tauxe RV, Hoekstra RM. Foodborne illness acquired in the United States--unspecified agents. Emerg Infect Dis 2011; 17(1): 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bresee JS, Marcus R, Venezia RA, et al. The etiology of severe acute gastroenteritis among adults visiting emergency departments in the United States. J Infect Dis 2012; 205(9): 1374–81. [DOI] [PubMed] [Google Scholar]
- 7.Hassan F, Kanwar N, Harrison CJ, et al. Viral Etiology of Acute Gastroenteritis in <2-Year-Old US Children in the Post-Rotavirus Vaccine Era. J Pediatric Infect Dis Soc 2019; 8(5): 414–21. [DOI] [PubMed] [Google Scholar]
- 8.Payne DC, Vinje J, Szilagyi PG, et al. Norovirus and medically attended gastroenteritis in U.S. children. N Engl J Med 2013; 368(12): 1121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hallowell BD, Parashar UD, Curns A, DeGroote NP, Tate JE. Trends in the Laboratory Detection of Rotavirus Before and After Implementation of Routine Rotavirus Vaccination - United States, 2000–2018. MMWR Morb Mortal Wkly Rep 2019; 68(24): 539–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pindyck T, Tate JE, Parashar UD. A decade of experience with rotavirus vaccination in the United States - vaccine uptake, effectiveness, and impact. Expert Rev Vaccines 2018; 17(7): 593–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattison CP, Cardemil CV, Hall AJ. Progress on norovirus vaccine research: public health considerations and future directions. Expert Rev Vaccines 2018; 17(9): 773–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt MA, Groom HC, Naleway AL, et al. A model for rapid, active surveillance for medically-attended acute gastroenteritis within an integrated health care delivery system. PLoS One 2018; 13(8): e0201805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burke RM, Groom HC, Naleway AL, et al. Rotavirus Vaccine is Effective Against Rotavirus Gastroenteritis Resulting in Outpatient Care: Results from the MAAGE Study. Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katz EM, Esona MD, Betrapally NS, et al. Whole-gene analysis of inter-genogroup reassortant rotaviruses from the Dominican Republic: Emergence of equine-like G3 strains and evidence of their reassortment with locally-circulating strains. Virology 2019; 534: 114–31. [DOI] [PubMed] [Google Scholar]
- 15.Katz EM, Gautam R, Bowen MD. Evaluation of an Alternative Recombinant Thermostable Thermus thermophilus (rTth)-Based Real-Time Reverse Transcription-PCR Kit for Detection of Rotavirus A. J Clin Microbiol 2017; 55(5): 1585–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tate JE, Mijatovic-Rustempasic S, Tam KI, et al. Comparison of 2 assays for diagnosing rotavirus and evaluating vaccine effectiveness in children with gastroenteritis. Emerg Infect Dis 2013; 19(8): 1245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Efron B. Bootstrap Methods: Another Look at the Jackknife. Ann Statist 1979; 7(1): 1–26. [Google Scholar]
- 18.Hall AJ, Rosenthal M, Gregoricus N, et al. Incidence of acute gastroenteritis and role of norovirus, Georgia, USA, 2004–2005. Emerg Infect Dis 2011; 17(8): 1381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grytdal SP, DeBess E, Lee LE, et al. Incidence of Norovirus and Other Viral Pathogens That Cause Acute Gastroenteritis (AGE) among Kaiser Permanente Member Populations in the United States, 2012–2013. PLoS One 2016; 11(4): e0148395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grytdal SP, Rimland D, Shirley SH, et al. Incidence of Medically-Attended Norovirus-Associated Acute Gastroenteritis in Four Veteran’s Affairs Medical Center Populations in the United States, 2011–2012. PLoS One 2015; 10(5): e0126733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kambhampati AK, Vargas B, Mushtaq M, et al. Active Surveillance for Norovirus in a US Veterans Affairs Patient Population, Houston, Texas, 2015–2016. Open Forum Infect Dis 2019; 6(4): ofz115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grytdal S, Browne H, Collins N, et al. Trends in Incidence of Norovirus-associated Acute Gastroenteritis in 4 Veterans Affairs Medical Center Populations in the United States, 2011–2015. Clin Infect Dis 2020; 70(1): 40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karve S, Krishnarajah G, Korsnes JS, Cassidy A, Candrilli SD. Burden of acute gastroenteritis, norovirus and rotavirus in a managed care population. Hum Vaccin Immunother 2014; 10(6): 1544–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanderkooi OG, Xie J, Lee BE, et al. A prospective comparative study of children with gastroenteritis: emergency department compared with symptomatic care at home. Eur J Clin Microbiol Infect Dis 2019; 38(12): 2371–9. [DOI] [PubMed] [Google Scholar]
- 25.Burke RM, Shah MP, Wikswo ME, et al. The Norovirus Epidemiologic Triad: Predictors of Severe Outcomes in US Norovirus Outbreaks, 2009–2016. J Infect Dis 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McAtee CL, Webman R, Gilman RH, et al. Burden of Norovirus and Rotavirus in Children After Rotavirus Vaccine Introduction, Cochabamba, Bolivia. Am J Trop Med Hyg 2016; 94(1): 212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bucardo F, Reyes Y, Svensson L, Nordgren J. Predominance of norovirus and sapovirus in Nicaragua after implementation of universal rotavirus vaccination. PLoS One 2014; 9(5): e98201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halasa N, Piya B, Stewart LS, et al. The Changing Landscape of Pediatric Viral Enteropathogens in the Post-Rotavirus Vaccine Era. Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Staat MA, Payne DC, Halasa N, et al. Continued Evidence of the Impact of Rotavirus Vaccine in Children Less than 3 Years of Age from the US New Vaccine Surveillance Network- a Multi-Site Active Surveillance Program, 2006–2016. Clin Infect Dis 2020. [DOI] [PubMed] [Google Scholar]
- 30.Sederdahl BK, Yi J, Jerris RC, et al. Trends in rotavirus from 2001 to 2015 in two paediatric hospitals in Atlanta, Georgia. Epidemiol Infect 2018; 146(4): 465–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pindyck T, Hall AJ, Tate JE, et al. Validation of Acute Gastroenteritis-related International Classification of Diseases, Clinical Modification Codes in Pediatric and Adult US Populations. Clin Infect Dis 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanchez GJ, Mayta H, Pajuelo MJ, et al. Epidemiology of Sapovirus Infections in a Birth Cohort in Peru. Clin Infect Dis 2018; 66(12): 1858–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukhopadhya I, Sarkar R, Menon VK, et al. Rotavirus shedding in symptomatic and asymptomatic children using reverse transcription-quantitative PCR. J Med Virol 2013; 85(9): 1661–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye S, Whiley DM, Ware RS, Kirkwood CD, Lambert SB, Grimwood K. Multivalent Rotavirus Vaccine and Wild-type Rotavirus Strain Shedding in Australian Infants: A Birth Cohort Study. Clin Infect Dis 2018; 66(9): 1411–8. [DOI] [PubMed] [Google Scholar]
- 35.Kirby AE, Shi J, Montes J, Lichtenstein M, Moe CL. Disease course and viral shedding in experimental Norwalk virus and Snow Mountain virus infection. J Med Virol 2014; 86(12): 2055–64. [DOI] [PubMed] [Google Scholar]
- 36.Aoki Y, Suto A, Mizuta K, Ahiko T, Osaka K, Matsuzaki Y. Duration of norovirus excretion and the longitudinal course of viral load in norovirus-infected elderly patients. J Hosp Infect 2010; 75(1): 42–6. [DOI] [PubMed] [Google Scholar]
- 37.Atmar RL, Opekun AR, Gilger MA, et al. Norwalk virus shedding after experimental human infection. Emerg Infect Dis 2008; 14(10): 1553–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murata T, Katsushima N, Mizuta K, Muraki Y, Hongo S, Matsuzaki Y. Prolonged norovirus shedding in infants <or=6 months of age with gastroenteritis. Pediatr Infect Dis J 2007; 26(1): 46–9. [DOI] [PubMed] [Google Scholar]
- 39.Operario DJ, Platts-Mills JA, Nadan S, et al. Etiology of Severe Acute Watery Diarrhea in Children in the Global Rotavirus Surveillance Network Using Quantitative Polymerase Chain Reaction. J Infect Dis 2017; 216(2): 220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Redli PM, Wanzenried A, Huder JB, et al. Evaluation of the RIDA(R)GENE RT-PCR assays for detection of sapovirus, astrovirus, adenovirus, and rotavirus in stool samples of adults in Switzerland. Diagn Microbiol Infect Dis 2020; 96(2): 114924. [DOI] [PubMed] [Google Scholar]
- 41.Pabbaraju K, Tellier R, Pang XL, et al. A Clinical Epidemiology and Molecular Attribution Evaluation of Adenoviruses in Pediatric Acute Gastroenteritis: a Case-Control Study. J Clin Microbiol 2020; 59(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


