Abstract
Phthalates are used in a wide range of consumer goods, resulting in exposures to specific phthalates that vary over time in accordance with changes in product use and how phthalates are utilized. We investigated trends in estimates of daily intake dose and several cumulative risk metrics, including the Hazard Quotient (HQ), Hazard Index (HI), and Maximum Cumulative Ratio (MCR) for six phthalates from 2005 to 2014 using metabolite biomonitoring data collected from spot urine samples under the National Health and Nutrition Examination Survey (NHANES). Over this period, there was a 2.2-fold decrease in the mean HI (0.34 to 0.15) and a 7.2-fold decrease in the percentage of participants with an HI > 1 (5.7% to 0.8%), indicating an overall decrease in combined exposure to these phthalates. Children (aged 6–11 years) had higher mean HI values than either adolescents (aged 12–19 years) or adults (aged 20+ years) during this period. MCR values were generally low and inversely correlated with HI. This indicated that a single phthalate usually drove the hazards for highly exposed individuals. However, the average value of MCR increased 1.2-fold (1.7–2.1) over this period indicating an increasing need to consider exposures to multiple phthalates in this group.
Graphical Abstract
Introduction
Phthalates (esters of phthalic acid) are used as plasticizers in a wide range of consumer goods including vinyl flooring, food packaging, the outer coatings of pills, cosmetics, food containers, and pipes and tubing.(1) Phthalates are not strongly bound to the polymers they plasticize and leaching of the compounds can occur in many of these products.(2,3) Over time, various phthalates gain or lose market share as a function of changes in product design, costs of production, and regulatory concerns. Such changes can result in statistically significant temporal changes in exposures to phthalates in the U.S. population.(4,5) In 2008 the National Research Council concluded that phthalates met the conditions to warrant a cumulative risk approach due to the general population’s exposure to multiple phthalates that may contribute to common adverse health outcomes including the disruption of male reproductive development.(1,6–10) It is important to explore these trends to inform changes in risk management needs. In addition, the interpretation of historical epidemiologic studies requires an understanding of the exposures that existed when the study population was investigated.(11)
The Hazard Index (HI) and Maximum Cumulative Ratio (MCR) metrics are a means to understanding risks and hazards from exposures. The HI is a measure that provides a straightforward method for quantifying cumulative risks to an individual by relating the individual’s intake of substances to the substances’ Reference Values (RfVs) assuming dose addition.(1,12–15) Examples of RfVs for oral exposures include the United States Environmental Protection Agency’s (USEPA’s) Reference Dose (RfD) and the European Union’s Tolerable Daily Intake (TDI). The application of the HI technique to individuals has been previously demonstrated in the literature.(16,17) An HI > 1 indicates that an individual’s combined exposures are potentially a concern. MCR is a measure of the relative contribution of the most dominant chemical to the risks posed by a participant’s cumulative exposures to multiple chemicals. The MCR metric has been applied to biomonitoring data of mixtures of dioxin-like chemicals,(18) exposures to mixtures of chemicals in water,(19–22) and mixtures in residential indoor air.(23) The MCR along with measures of cumulative exposures can inform risk management decisions and help identify specific combinations of chemicals that result in elevated cumulative risks.
Over the last several years, a number of researchers have investigated combined exposures to phthalates using various metrics. Many of these studies have used the biomonitoring data of phthalate metabolites in urine collected from the National Health and Nutrition Examination Survey (NHANES).(24–32) Because phthalates are nonpersistent in humans, biomonitoring data reflect phthalate exposures in surveyed participants at the time of sample collection. Data generated by continuous surveys such as NHANES can be used to characterize the temporal changes in phthalate exposures. While NHANES does not track specific participants over time, it provides snapshots of the population distribution of exposures for specific time periods.
Researchers historically have used a range of approaches for analyzing NHANES data. Zota et al. and others have looked at patterns of urinary metabolites of phthalates for one or multiple time periods.(28,31–33) Specifically, Zota et al. explored metabolites of multiple phthalate concentrations from 2001 to 2010 presented by age, race/ethnicity, gender, and household income.(31) Others have used reverse dosimetry models to estimate the daily intake of phthalates associated with observed levels of urinary metabolites. Christensen et al. and Søeborg et al. used the HI to assess cumulative exposures to phthalates.(17,30) Varshavsky et al. used a potency-weighted approach to assess combined exposures.(25) Specifically, Varshavsky et al. explored the toxicological equivalent of di-n-butyl phthalate from a group of androgen disrupting phthalates for data collected between 2001 and 2012.(25)
This work used the same approach as Reyes and Price(34) to investigate the changes in HI and the MCR on a group of six phthalates using biomonitoring data from NHANES between the years 2005–2014. Temporal trends in these values were determined population-wide and presented by different demographic groups defined by age, race/ethnicity, and gender using multivariate regression analyses. The resulting estimates of individual and cumulative risks were assessed using the MCR and related approaches.
Materials and Methods
NHANES Data Set from 2005 to 2014
Phthalate biomarker data came from five consecutive cycles of NHANES spanning 10 years from 2005 to 2014.(35) NHANES is a continuous, ongoing nationwide survey conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC) and is representative of the general, noninstitutionalized, civilian population in the United States. NHANES categorizes its data in two-year increments known as a “cycle”, with the specific number of participants varying from cycle to cycle. Spot samples of urine were collected from participants six years and older.(36) A subset of these samples were analyzed for a number of phthalate metabolites. The number of sampled participants ranged from 2527 to 2755 for the five most recent, available cycles (Supporting Information (SI), Table S2).
The six parent phthalates included in this analysis each inferred from one or more metabolites were di-n-butyl phthalate (DBP), diisobutyl phthalate (DIBP), butyl benzyl phthalate (BBP), di(2-ethylhexyl) phthalate (DEHP), diisononyl phthalate (DINP), and diisodecyl phthalate (DIDP) originating from 10 metabolites total (Table S3). All metabolite concentrations below the limit of detection (LOD) were set to , allowing the mean and associated standard deviation to have an acceptable level of bias.(37) The set of phthalates selected included all available phthalates from NHANES with four or more carbon side chains(38) and those phthalates associated with the “phthalate syndrome” measured in the five most recent cycles.(39–41) The metabolite mono (3-carboxypropyl) phthalate (MCPP) was not evaluated because it is a secondary metabolite of both DBP and DOP. A copy of the data used in this study is provided in the SI.
Unless indicated otherwise, counts, frequencies, and percentages of participants were not adjusted using NHANES survey weights; however, cycle-specific, population-wide measures (e.g., means, quantiles, confidence intervals, etc.) were adjusted. All analyses and visualizations were conducted in R (version 3.2.2) using the packages ggplot2 (version 2.2.0) and survey (version 3.31).
Daily Intake Dose and Maximum Cumulative Ratio
Daily Intake (DI) doses of phthalates for NHANES participants were calculated using metabolite concentrations in the urine, information about the metabolite and parent phthalate,(42,43) and creatinine excretion using demographic information about the participant scaled to their body weight(44,45) as described in Reyes and Price.(34) A summary of the methodology is provided in SI. The Hazard Quotient (HQ) is calculated as the ratio of an individual’s estimated exposure level to the RfV for that chemical. The chemical-specific HQs are then summed to give an individual’s HI.
The following equations were used to determine the values of HQ, HI, and MCR for participant iand phthalate j for N phthalates:
| (1) |
| (2) |
| (3) |
There were six phthalates used in this analysis (i.e., N = 6) and HQM quantifies the maximum HQ among the six phthalates for participant i. The TDIs used in this study are in line with previous works (Table S3).(30) The value of MCR for an individual i in an exposed population is defined as
| (4) |
The values of HI and the MCR can be used to evaluate cumulative exposures in several ways. A negative correlation (i.e., MCR – 1 declines as HI increases) indicates that the individuals most at risk from cumulative exposures received the majority of their risks from a single chemical.(34)
The values of HI and MCR also enable categorization of the surveyed participants into three groups (Table S4).(46,47) Group I is comprised of those participants having one or more phthalate doses which have HQ values greater than 1. Group II includes participants with values of HI ≤ 1. The remaining participants would not have been flagged under a single-chemical assessment but have HI > 1, making their combined exposures a potential concern. These participants are identified as Group III. Group III can be divided into two subgroups. Group IIIA includes those participants with an MCR < 2, whereas Group IIIB includes those with an MCR ≥ 2, indicating that a potential risk is driven by exposure to multiple chemicals.
Statistical Analyses of Demographic Groups
In line with the methodology presented in Zota et al.,(31) we calculated the association of the least squares geometric means (LSGMs) of the population-wide HI by overall NHANES cycle and by the demographic groups for age, race/ethnicity, and gender after adjusting for given covariates. Age categories include children (6–11 years of age), adolescents (12–19 years of age), and adults (20+ years of age). We explored three race/ethnicity categories (i.e., Mexican American, non-Hispanic White, and non-Hispanic Black) and gender (i.e., female and male). The category of “Other Hispanic” was excluded from the linear regression analysis because oversampling of “Other Hispanic” began in the 2007–2008 cycle and such a change prevents a comparison across the five cycles.(48) Differences in the LSGM HIs between overall cycles and within each of the three demographic groups (i.e., age, race/ethnicity, and gender) were also investigated. Depending on the interaction term, each regression was adjusted for gender, race, number of fasting hours, and poverty income ratio (PIR). PIR is a ratio which indicates by how much a participant’s household income is above or below the poverty guidelines. This varies as a function of year, location, and number of persons in a household.(49) PIR was treated as a categorical variable (i.e., PIR < 1, PIR ∈ [1,3], and PIR > 3). “Fasting hour” is the number of whole hours between when the participant last ate or drank and the time when the biomonitoring sample was taken. The full regression models can be found in the SI (Table S5). For the regression analysis, phthalate metabolite concentrations below the LOD across NHANES cycles were set to max (Table S6).
A separate analysis was made on the proportion of participants with HI > 1 within (1) a demographic group for a fixed NHANES cycle and (2) a given demographic subgroup across NHANES cycles. The analyses used χ2 tests and took into account the NHANES survey weights.
Determination of Critical Combinations of Phthalates
MCR analyses can aid in identifying which combinations of chemicals are most important to investigate for possible toxicity interactions. The critical pairs were determined by identifying the phthalates which produced the top two largest HQs for the individuals with HI > 1 in each of the five cycles. These phthalates will have the largest exposures relative to their toxicity end points and thus are likely to have an increased potential for interactions. They are also the phthalates where deviation from additivity will have the greatest impact on HI values.(13)
Sensitivity Analysis
To evaluate the robustness of these findings we investigated two alternative approaches: the urinary flow rate (UFR) methodology for estimating dose and Varshavsky et al. potency-weighting approach for assessing combined exposures.(25,50) Details on these alternative analyses are contained in the SI. Data on UFRs were not collected before 2009, therefore this method can only be applied to the three most recent NHANES cycles. The Varshavsky et al. approach allows for the integration of multiple phthalate exposures into a single metric using Benchmark Doses (BMDs). BMDs due to androgen disruption for the phthalates were obtained from the literature.(1)DBP was used as the reference because it has the lowest BMD. The relative potency factor (RPF) of each of the six phthalates were calculated using the formula RPFj = BMDreference/BMDj for phthalate j (Table S9). The potency-weighted dose (PWD) was calculated using the formula DIPWD,i = ∑j = 1NRPFj × DIi,j for participant i and phthalate j using N number of phthalates. This alternative method was used to calculate MCR and explore the relationship between MCR and the PWD. Varshavsky et al. does not provide an acceptable level of the PWDs.(25) This limits the ability of the approach to determine if the values of PWD generated here are a concern and prevents the assignment of individuals into the three Groups.
Results and Discussion
Temporal Trends in HI and HQ Values
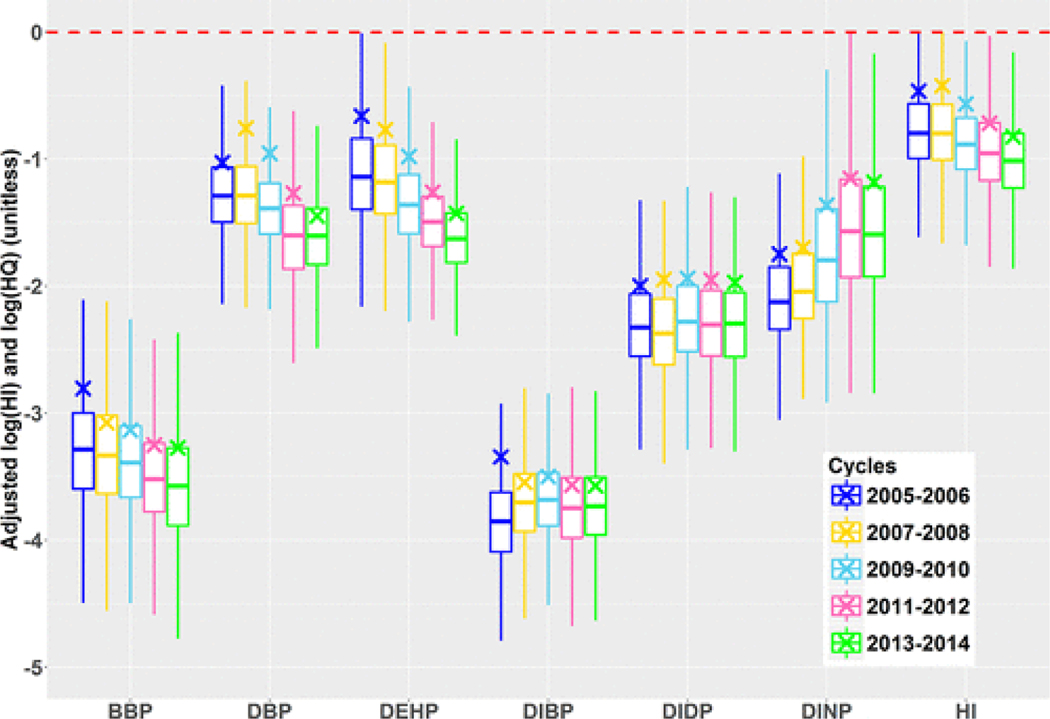
Population estimates of the mean and quantiles of HI declined overall across the five cycles. The mean (median) values declined from 0.34 to 0.15 (0.17 to 0.10). The interquartile range (IQR) of HI also declined from 0.21 to 0.10, indicating a reduction in the interindividual variation of HI values. The 95th percentile of HI estimated for the surveyed population declined from 1.1 to 0.44. During this period, only a minority of the survey participants had values of HI > 1, declining from 5.7% to 0.8%. The percentages of participants with HI > 1 for the five cycles were statistically different and a downward trend is observed in the population-wide HI values calculated from the six selected phthalates over time (Figure 1).
Figure 1.
Population-wide boxplots of the log transform of phthalate-specific hazard quotients (HQs) and the hazard index (HI) of the surveyed NHANES population presented by cycle spanning from 2005 to 2014 and adjusted for the NHANES survey weighting factors. The boxplot is marked by the median, first and third quartile, and ±1.5 × IQR (inter-quartile range). The logarithm of the arithmetic means is identified with an “X”. The dashed, horizontal, red line indicates HI = 1 (i.e., log(HI) = 0).
Figure 1 presents the temporal trends in the phthalate-specific HQ values. The decrease in HI values can mostly be attributed to decreases in HQ values from DEHP and DBP. HQs of BBP also decreased during this period; however, this compound contributed less to the overall HI declines compared to DEHP and DBP. The mean and median HQs of DIBP and DIDP remained fairly consistent over this time period. Increases in DIBP are in line with previous works(25,31) but overall contributed minimally to the HI when scaled by its TDI. DINP was the only phthalate whose HQ values notably increased during this period; however, the magnitude of the increase in HQ for DINP was mostly smaller than the corresponding magnitude of decrease of DEHP, leading to an overall decrease in HI.
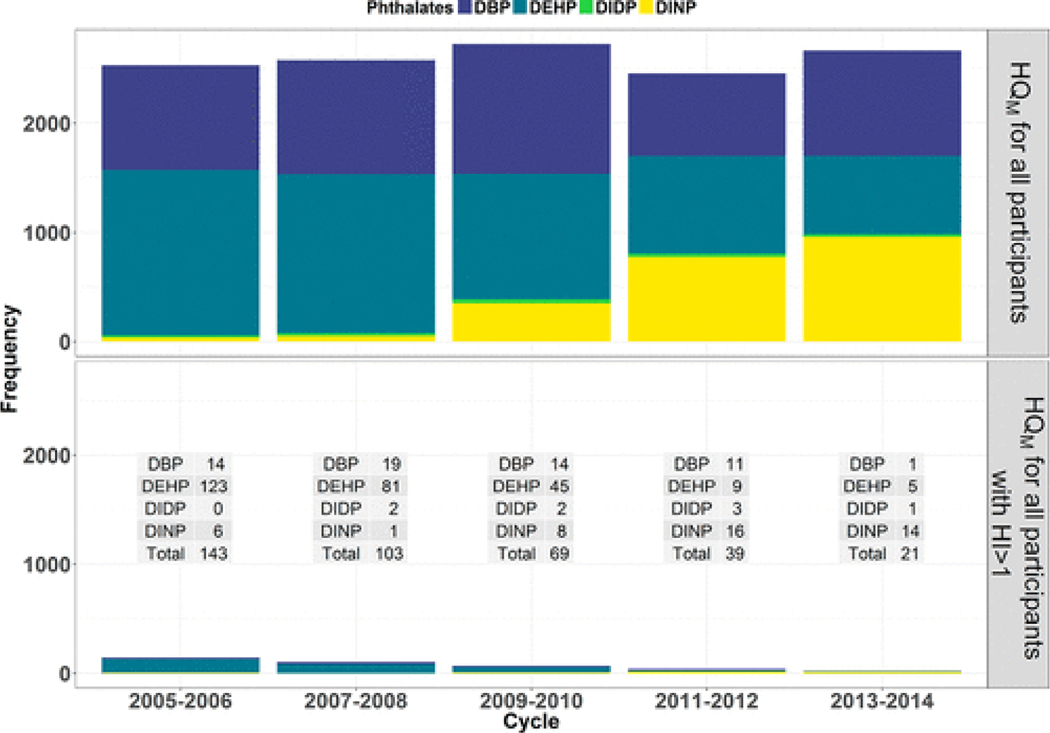
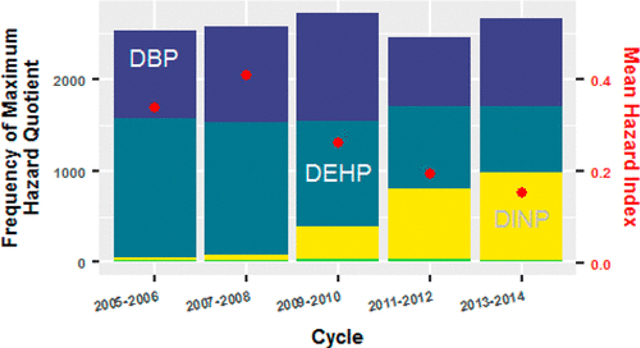
The HQM was produced by one the six phthalates selected in this work. The HQM can be calculated for all participants and the frequencies of the phthalates that produced the HQM can be tabulated (Table S1; Figure 2). This tabulation can be subset to only participants with HI > 1 (Figure 2). There was a consistent temporal decrease in the frequency of participants whose HQMwas produced by DEHP (declining from 1517 to 717 for all participants and from 123 to 5 for participants with HI > 1). This was offset by an increase in frequency of HQM produced by DINP (increasing from 37 to 961 for all participants and from 1 to 16 for participants with HI > 1). This shift in dominance of DEHP and DINP has been demonstrated in other works for specific NHANES cycles.(28,30) The frequency of HQM produced by DBP was consistent across the past five cycles except for a decline in the most recently available NHANES cycle (i.e., the 2013–2014 cycle).
Figure 2.
Stacked bar graph displaying the frequency at which each of the six phthalates produced the maximum hazard quotient (HQM) among the NHANES participants across the cycles spanning from 2005 to 2014. The top panel presents all participants and the bottom panel subsets to participants whose hazard index (HI) is greater than one with a table inset quantifying the subset, stacked bar graph.
While mean HQ for DINP has increased from 2005 to 2014, mean HI has decreased over the same time period due mainly to decreases in DEHP and DBP. Decreases in mean HQs across cycles among some of the phthalates may be attributed to regulatory or substitution strategies. In 2008, the Consumer Product Safety Commission (CPSC) banned DEHP, DBP, and BBP in children’s toys in concentrations greater than 0.1%.(51) This list was expanded in 2017 to include five additional phthalates including DINP and DIBP at the same percent concentrations.(52) Decreases in DEHP may be attributed to replacement strategies with higher molecular weight phthalates such as DINP and DIDP.(53) Changes in exposures to DEHP and DBP would have a greater impact on the HI compared to changes in exposures to DINP and DIBP because of DEHP and DBP’s lower TDIs. NHANES only offers a snapshot of population-wide exposures and does not collect samples from the same participants from cycle to cycle. Phthalate-specific HQs are determined from current TDIs and may be subject to change if TDIs are updated in the future.
Changes in HI Values in Different Demographic Groups Across Cycles
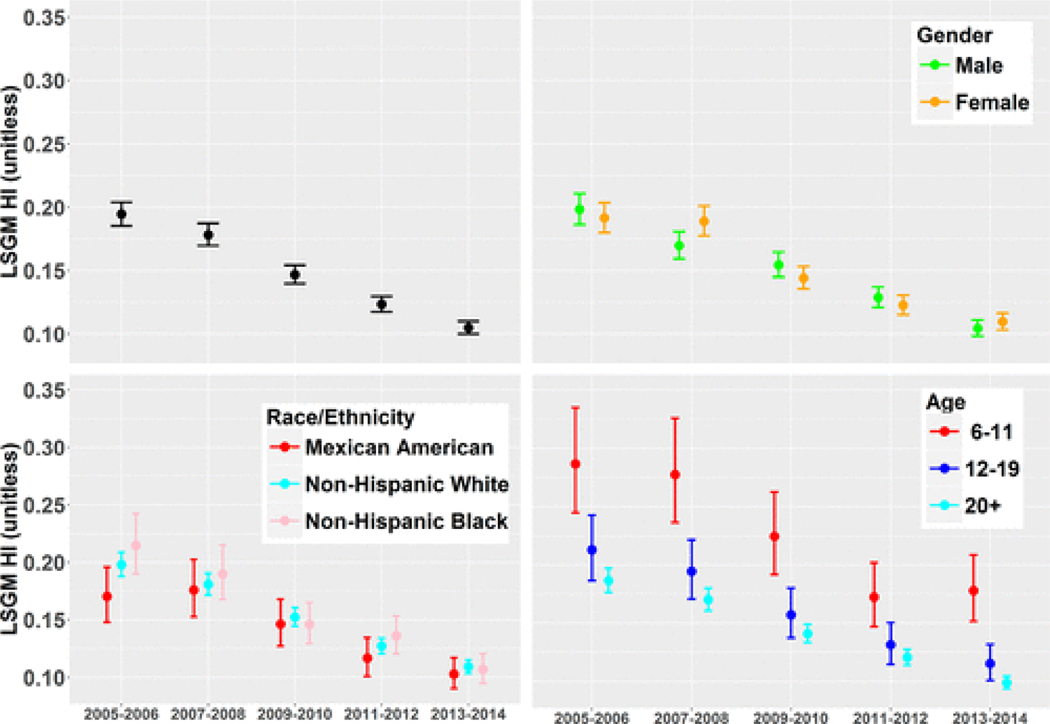
In general, the same temporal changes seen in the total population were mirrored in the demographic groups presented by age, race/ethnicity, and gender. Figure 3 shows the population-wide LSGM of HI values for NHANES participants across each cycle presented overall and by different demographic groups. Values were adjusted for selected covariates, as presented in a previous work(31) (Table S5). There was a statistically significant decrease in LSGM HI overall (Wald test, p < 0.001) and a decrease in LSGM HI in the three demographic groups over the ten years.
Figure 3.
Population least squares geometric mean (LSGM) with 95% confidence intervals of the hazard index (HI) of the surveyed NHANES population from six phthalates by cycle spanning from 2005 to 2014 presented overall (top left), by gender (top right), by race/ethnicity (bottom left), and by age (bottom right) adjusting for covariates and the NHANES survey weighting factors.
Differences in LSGM HI were explored within demographic groups for fixed NHANES cycles using pairwise Bonferroni comparisons (Table S7). Age was the only demographic group explored that had a consistent difference in LSGM HI across all five cycles. Children had a higher LSGM HI compared to adolescents and adults given a fixed NHANES cycle (p < 0.01 for all pairings). Adolescents had a slightly higher LSGM HI compared with adults; however, the difference was only statistically significant for the last NHANES cycle (p < 0.05). Elevated phthalate levels at younger ages were consistent with earlier findings. This may be due to the amount of food consumption per body weight and different metabolisms and behaviors of children compared with adults.(31,54) Currently, NHANES does not take urine samples from children under the age of six. Thus, these results may not be applicable for younger children.
There were statistically significant differences in LSGM HI values for race/ethnicity in the 2005–2006 cycle and for gender in the 2007–2008 cycle. These differences decreased over time and were not statistically significant in subsequent cycles. These results were similar to previous findings of minimal differences in exposures with race/ethnicity(25) but differ from previous findings of statistically significant gender differences as observed in Parlett et al.(55) However, when examining gender differences, Parlett et al. explored a different set of phthalates and dietary sources were outside the scope of their study.
Temporal and cycle-specific changes in the fraction of individuals with HI > 1 were also investigated. The χ2 test of proportions of individuals with HI > 1 were subset to slightly different categories than those used in the LSGM analysis.(31) Namely, all races/ethnicities were considered (including “Other Hispanic” and “Other”) and participants were not removed from the χ2testing if they had missing PIR values. The temporal changes in the proportion of individuals with HI > 1 in various demographic groups followed the same trend as the total population (Table 1). In all demographic subgroups, these trends were found to be statistically significant across cycles using a χ2 test, with the exception of the “Other” race/ethnicity subgroup. For a given fixed NHANES cycle, differences between the proportion of individuals with HI > 1 within a given demographic group were found to be statistically significant only for gender and race/ethnicity in the 2005–2006 cycle.
Table 1.
Frequency (% in Each Subgroup) Of Participants with HI > 1 Presented by Demographic Groups of Age, Race/Ethnicity, and Gender Across NHANES Cycles Spanning from 2005–2014
| Demographics | 2005–2006 | 2007–2008 | 2009–2010 | 2011–2012 | 2013–2014 | |
|---|---|---|---|---|---|---|
| Total Participants with HI>1 | 143 (5.7) | 103 (4.0) | 69 (2.5) | 39 (1.6) | 21 (0.79) | |
| Gender | Male | 81 (6.4) | 49 (3.8) | 40 (2.9) | 19 (1.5) | 10 (0.79) |
| Female | 62 (4.9) | 54 (4.2) | 29 (2.2) | 20 (1.7) | 11 (0.79) | |
| Race/Ethnicity | Mexican American | 31 (4.9) | 24 (4.6) | 15 (2.7) | 3 (0.96) | 2 (0.46) |
| Non-Hispanic White | 54 (5.2) | 35 (3.3) | 28 (2.4) | 12 (1.5) | 8 (0.82) | |
| Non-Hispanic Black | 47 (7.0) | 23 (3.9) | 12 (2.3) | 9 (1.4) | 4 (0.66) | |
| Other Hispanic | 2 (2.8) | 15 (5) | 6 (2) | 4 (1.6) | 1 (0.4) | |
| Other | 9 (7.3) | 6 (6.2) | 8 (4.8) | 11 (2.5) | 6 (1.5) | |
| Age | 6–11 years | 26 (7.3) | 27 (7.0) | 11 (2.7) | 5 (1.3) | 6 (1.5) |
| 12–19 years | 41 (5.9) | 16 (4.0) | 10 (2.4) | 5 (1.3) | 7 (1.5) | |
| 20+ years | 76 (5.1) | 60 (3.3) | 48 (2.5) | 29 (1.7) | 8 (0.45) |
Temporal Trends in MCR and Its Relationship to HI
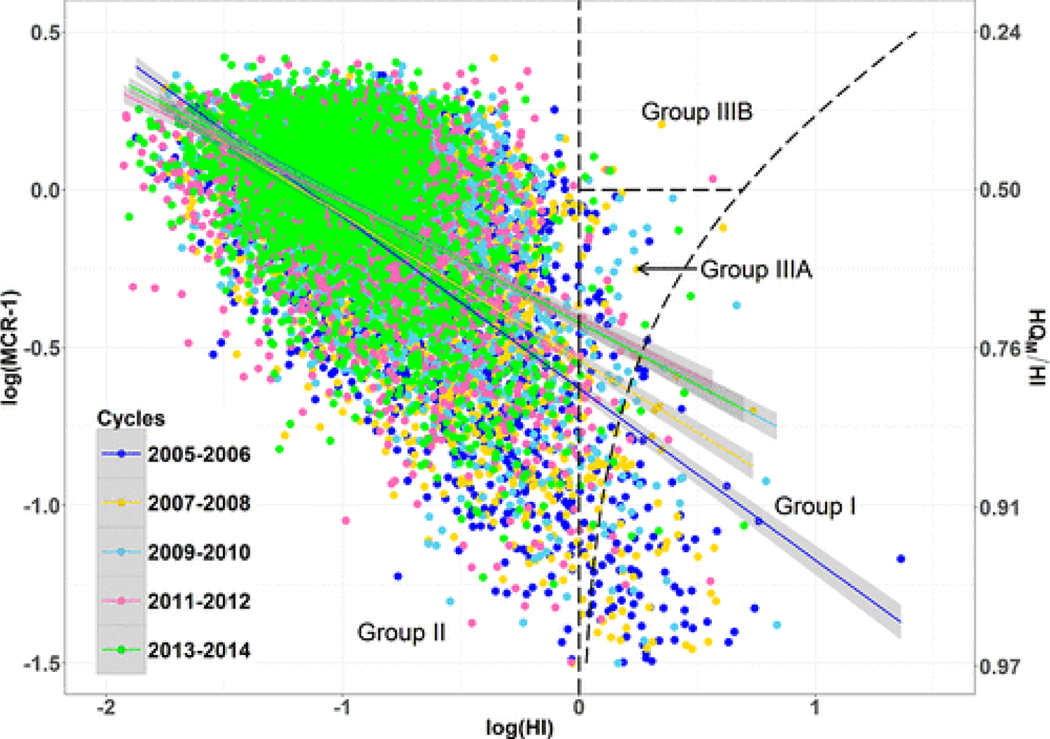
The MCR values were calculated for each participant across the five cycles. The relative frequency of participants falling in the Groups (i.e., I, II, IIIA, and IIIB) along with the ranges of HI for each Group can provide additional insight into patterns of exposure. Plotting the values of log(MCR – 1) versus log(HI) for the surveyed participants results in the three groups falling into contiguous regions (Figure 4).(22,46,47) Figure 4 shows that for all five cycles, MCR and HI values were negatively correlated and participants with the largest values of HI have values of MCR that were typically less than 1.5 (i.e., log(MCR – 1) values were typically less than −0.30). The 2005–2006 cycle contained the highest frequency of participants in Group I and III with 113 and 30 participants, respectively (Table S1; Figure 4). While mean HI values decreased over time, mean MCR values have increased from 1.7 to 2.1. Figure 4 and Table S10 also include a characterization of the relationship between MCR and HI by defining a simple linear regression between log(HI) and log(MCR – 1) in each of the five cycles. There was a consistent inverse relationship between HI and MCR; however, there was a decrease in the magnitude of the relationship over time as measured by the slope of the regression line.
Figure 4.
Plot of log Hazard Index (HI) versus log(MCR – 1) (with HQM/HI) for all participants from the five NHANES cycles spanning from 2005 to 2014. Regions corresponding to Groups I, II, IIIA, and IIIB and linear regression between log HI with log(MCR – 1) with 95% confidence interval are presented. Regressions for cycles 2009–2010 and 2013–2014 overlap.
Increasing MCR values and decreasing (in magnitude) slope between MCR and HI, along with the declining HI values, indicated a change in the pattern of cumulative exposures to phthalates over time. As discussed above, the combined impacts of this set of phthalates declined. This decline was most sizable among the participants with the larger exposures. This leads to a reduction in the variation of participants’ HQ values, such that the HI values have become less dominated by a single chemical. These changes increased the impacts of combined exposures.(20) This trend was reflected in the increase in the percentage of participants with HI > 1 that fell into Group III over the five cycles (a more than doubling from 21% to 43%). This trend, however, was not sufficient to change the inverse relationship between MCR and HI. Notably, in the most recent cycle the participants with the largest values of HI still had MCR values less than two. This indicates that although mean MCR has increased over time, those with the highest cumulative exposures were most likely consistently and primarily driven by one chemical within the mixture.(34)
Christensen et al. calculated the HQ and HI for a mixture of phthalates from NHANES, but did not investigate temporal trends.(30) Zota et al. investigated trends in urinary concentration of phthalate metabolites and looked at trends in various demographic groups. Varshavsky et al. examined temporal trends in the general population and in various demographic groups using a potency-weighted approach but did not determine estimates of hazard.(25) All of these previous works determined changes in phthalate exposures over multiple years. This work demonstrates that the trends presented from previous cycles of NHANES continued in the most recent cycle for which there were available phthalate data (i.e., the 2013–2014 cycle). Likewise, this is the first work to investigate population-wide temporal trends of phthalates using the hazard index. Lastly, this is the first work to investigate the relative contributions of phthalates to a mixture over time as seen through the MCR metric and the quantification of the frequency at which particular phthalates contributed to HQM within a given mixture.
Between 2005 and 2014 for the six phthalates selected in this work, NHANES data showed a population-wide push from exposure to phthalates with lower TDI values (i.e., phthalates which were more toxic) to phthalates with higher TDI values (i.e., phthalates which were less toxic), resulting in declines in estimates of aggregate screening risks over this period. Mean HI values decreased 2.2-fold, and the proportion of NHANES participants with HI > 1 decreased 7.2-fold. While these measures of cumulative risk declined, there is an increased need for cumulative exposure assessments to accurately characterize risk. In the most recent cycle, 43% of individuals with HI > 1 would have been missed by performing separate assessments of each phthalate. If the individual exposures from phthalates continue to decline, the collection and analysis of biomonitoring data for cumulative exposures from these compounds will become increasingly more important. Researchers should continue to monitor changes in population exposures and track the entry of new phthalates and plasticizers into the market.
Temporal Trends in Participants’ Top Two Maximum HQs
An individual with MCR < 2 indicates that a pair of phthalates were responsible for the majority of a given HI value; therefore, when investigating interactions of phthalates, the focus should be on this pair. The frequency at which the phthalates that produced the top two HQs in each of the participants with HI > 1 across the five consecutive cycles of NHANES were determined (Table 2). While there are 15 unique pairs of phthalates, only seven pairs occurred in the participants and the frequency of occurrence of the pairs changed over time. The most notable temporal decrease in pairings were in the pairs DEHP/DBP and DEHP/DIDP. DEHP/DINP constitute both a consistent and high frequency of pairs. The largest increase of pairs was for DIDP/DINP. This change in frequency of pairs may speak to the substitution of DEHP with DINP over time.(11,56) DINP was in 21 (15%) pairs in the earliest cycle and 18 (86%) in the most recent cycle, whereas DIDP was in 6 (4.2%) pairs in the earliest cycle and 7 (33%) in the most recent cycle. This analysis implies that toxicological investigation of DEHP/DINP pairing continues to be important and that the DIDP/DINP pairing may be important going forward. Exploring the frequencies of the phthalates which produced the top two HQM among participants with HI > 1 allow researchers to prioritize the toxicological interactions of phthalates in a mixtures analysis. This work found that the mean HI was cut by over half during a 10-year time period. This speaks to how quickly replacement strategies and population-wide exposures can change. The size of the observed changes suggests that effects of the phthalates in the general population could have changed over this time period. Epidemiologic studies of the effects of phthalates should take care to use measures of exposures that are concurrent to observed effects and the application of epidemiologic findings to current and future populations should take into account temporal changes in phthalate exposures.
Table 2.
Frequency of Pairs of Phthalates That Produced the Top Two Hazard Quotients among the Participants with HI > 1 across NHANES Cycles Spanning from 2005–2014
| 2005–2006 | 2007–2008 | 2009–2010 | 2011–2012 | 2013–2014 | ||
|---|---|---|---|---|---|---|
|
| ||||||
| DEHP | DBP | 115 | 78 | 35 | 13 | 1 |
| DEHP | DINP | 21 | 17 | 32 | 17 | 12 |
| DEHP | DIDP | 6 | 5 | 2 | 1 | 2 |
| DBP | BBP | 1 | 0 | 0 | 0 | 0 |
| DBP | DIDP | 0 | 2 | 0 | 0 | 0 |
| DBP | DINP | 0 | 1 | 0 | 4 | 1 |
| DIDP | DINP | 0 | 0 | 0 | 4 | 5 |
The results of the sensitivity analyses indicated that while alternative approaches changed the specific values of the results, the above findings were robust when considering alternative methods of dose reconstruction and combined hazards. The treatment of below-detects of metabolites did not measurably influence the magnitude of corresponding phthalate estimates (Figure S1). The work presented here calculated DI values using a creatinine correction methodology (eq S1) due to the missingness of UFR in earlier cycles.(30) However, there were several notable limitations to calculating DI. Phthalates have short half-lives and maximum urinary concentration of a phthalate metabolite likely occurs a few hours after exposure.(29) This concern was partially addressed by adjusting the HI by “fasting hour” in the LSGM regression analysis. However, this would only address dietary exposures. An alternative to the creatinine correction method of estimating DI is to use measures of UFR (eq S3). There are limitations to the creatinine-correction approach and the UFR may be preferable in certain scenarios.(57,58) However, because reporting of UFR began in the 2009–2010 cycle, creatinine-correction was the primary method used in this work. UFR was only available for a subset of participants due to missingness (92%, 94%, and 90%, for the three cycles, respectively). Mean HIs across the three available NHANES cycles for UFR (creatinine correction) were 0.25, 0.18, and 0.14 (0.27, 0.19, and 0.15), respectively (Figure S2). Although the mean HI for UFR was generally lower than the creatinine correction methods, there was only a statistically significant difference between mean HI in the 2009–2010 cycle (p < 0.01). The UFR generally produced more participants with HI > 1 in the three cycles: 61, 55, and 35 compared with 62, 35, and 19 (Table S8), respectively. Using a χ2test, there was a difference in the proportion of participants with HI > 1 across the three most recent cycles using the creatinine correction method (p < 0.01), but not the UFR method (p ≥ 0.05). The majority of participants still fell into Group I (Table S8). It was notable that the differences between the two methods changed as a function of quantile of HI (Figure S3).
MCR based on the total potency-weighted dose for a participant was an alternative to the calculation that used HI (Table S9).(25) The log of total potency-weighted doses plotted against log(MCR – 1) from the five cycles showed similar trends as the plot of log(HI) versus log(MCR – 1) (Figure S4). The slopes for the potency-weighted approach were generally larger in magnitude compared to the HI approach and the mean MCR across the five cycles for the potency-weighted approach was slightly but consistently lower compared to the HI approach (Table S10). The alternative approaches confirmed that there was an increasing dominance of a single phthalate for participants with elevated measures of hazard.
Supplementary Material
Acknowledgments
Disclaimer: This research was supported in part by an appointment of Jeanette Reyes to the Postdoctoral Research Program at the National Center for Environmental Assessment, Office of Research and Development, USEPA administered by the Oak Ridge Institute for Science and Education through Interagency Agreement No. DW-89-92298301 between the U.S. Department of Energy and the USEPA. This manuscript has been reviewed by the U.S. Environmental Protection Agency and approved for publication. The views expressed in this manuscript are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency.
Footnotes
Supporting Information
• Files include: displaying raw data and all final data sets (XLSX)
• Detailed equations for calculating the daily dose using the creatinine correction and UFR, figures comparing the HI between the creatinine correction and UFR, figures and tables comparing temporal trends in the MCR between the HI and potency-weighted approach, tables describing frequency of participants by cycle, temporal limit of detection by metabolite, tolerable daily intakes by phthalate, Group designation of the MCR, complete regression equations used in the regression analysis, comparison of Group counts by creatinine correction and UFR, and relative potency factors by phthalate
The authors declare no competing financial interest.
References
- 1.NRC. Phthalates and Cumulative Risk Assessment: The Task Ahead; Committee on the Health Risks of Phthalates, National Research Council: Washington, DC, 2008; http://www.nap.edu/catalog/12528.html [Google Scholar]
- 2.Sathyanarayana S. Phthalates and Children’s Health. Curr. Probl. Pediatr. Adolesc. Health Care 2008, 38(2), 34–49, DOI: 10.1016/j.cppeds.2007.11.001 [DOI] [PubMed] [Google Scholar]
- 3.Colacino JA; Harris TR; Schecter A. Dietary intake is associated with phthalate body burden in a nationally representative sample. Environ. Health Perspect 2010, 118 (7), 998–1003, DOI: 10.1289/ehp.0901712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koch HM; Rüther M; Schütze A; Conrad A; Pälmke C; Apel P; Brüning T; Kolossa-Gehring M Phthalate metabolites in 24-h urine samples of the German Environmental Specimen Bank (ESB) from 1988 to 2015 and a comparison with US NHANES data from 1999 to 2012. Int. J. Hyg. Environ. Health 2017, 220(2), 130–141, DOI: 10.1016/j.ijheh.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 5.Gyllenhammar I; Glynn A; Jönsson BAG; Lindh CH; Darnerud PO; Svensson K; Lignell S Diverging temporal trends of human exposure to bisphenols and plastizisers, such as phthalates, caused by substitution of legacy EDCs?. Environ. Res 2017, 153 (November 2016), 48–54, DOI: 10.1016/j.envres.2016.11.012 [DOI] [PubMed] [Google Scholar]
- 6.Jurewicz J; Hanke W. Exposure to phthalates: Reproductive outcome and children health. A review of epidemiological studies. Int. J. Occup. Med. Environ. Health 2011, 24 (2), 115–141, DOI: 10.2478/s13382-011-0022-2 [DOI] [PubMed] [Google Scholar]
- 7.Lyche JL; Gutleb AC; Bergman A; Eriksen GS; Murk AJ; Ropstad E; Saunders M;Skaare JU Reproductive and developmental toxicity of phthalates. J. Toxicol. Environ. Health, Part B2009, 12 (4), 225–249, DOI: 10.1080/10937400903094091 [DOI] [PubMed] [Google Scholar]
- 8.Martino-Andrade AJ; Chahoud I. Reproductive toxicity of phthalate esters. Mol. Nutr. Food Res 2009, 54(1), 148–157, DOI: 10.1002/mnfr.200800312 [DOI] [PubMed] [Google Scholar]
- 9.Meeker JD; Ferguson KK Relationship between urinary phthalate and bisphenol a concentrations and serum thyroid measures in u.s. adults and adolescents from the national health and nutrition examination survey (NHANES) 2007–2008. Environ. Health Perspect 2011, 119 (10), 1396–1402, DOI: 10.1289/ehp.1103582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pak VM; McCauley LA; Pinto-Martin J. Phthalate Exposures and Human Health Concerns: A Review and Implications for Practice. AAOHN J. 2011, 59 (5), 228–235, DOI: 10.3928/08910162-20110426-01 [DOI] [PubMed] [Google Scholar]
- 11.Sarigiannis DA Assessing the impact of hazardous waste on children’s health: The exposome paradigm.Environ. Res 2017, 158 (January), 531–541, DOI: 10.1016/j.envres.2017.06.031 [DOI] [PubMed] [Google Scholar]
- 12.EPA. Framework for Cumulative Risk Assessment; Washington Office, Washington, DC, 2003.https://www.epa.gov/sites/production/files/2014-11/documents/frmwrk_cum_risk_assmnt.pdf. [Google Scholar]
- 13.EPA. Concepts, Methods, and Data Sources for Cumulative Health Risk Assessment of Multiple Chemicals, Exposures and Effects: A Resource Document (Final Report); Washington, DC, 2007;http://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=190187. [Google Scholar]
- 14.Teuschler LK; Hertzberg RC Current and future risk assessment guidelines, policy, and methods development for chemical mixtures. Toxicology 1995, 105 (2–3), 137–144, DOI: 10.1016/0300-483X(95)03207-V [DOI] [PubMed] [Google Scholar]
- 15.EPA. Guidelines for the Health Risk Assessment of Chemical Mixtures; Washington Office: Washington, DC,1986; Vol. 51. https://www.epa.gov/sites/production/files/2014-11/documents/chem_mix_1986.pdf. [Google Scholar]
- 16.Kortenkamp A; Faust M. No TitleCombined exposures to anti-androgenic chemicals: steps towards cumulative risk assessment. Int. J. Androl 2010, 33 (2), 463–474, DOI: 10.1111/j.1365-2605.2009.01047.x [DOI] [PubMed] [Google Scholar]
- 17.Søeborg T; Frederiksen H; Andersson AM Cumulative risk assessment of phthalate exposure of Danish children and adolescents using the hazard index approach. Int. J. Androl 2012, 35 (2), 245–252,DOI: 10.1111/j.1365-2605.2011.01240.x [DOI] [PubMed] [Google Scholar]
- 18.Han X; Price PS Applying the maximum cumulative ratio methodology to biomonitoring data on dioxin-like compounds in the general public and two occupationally exposed populations. J. Exposure Sci. Environ. Epidemiol 2013, 23 (4), 343–349, DOI: 10.1038/jes.2012.74 [DOI] [PubMed] [Google Scholar]
- 19.Han X; Price PS Determining the maximum cumulative ratios for mixtures observed in ground water wells used as drinking water supplies in the United States. Int. J. Environ. Res. Public Health 2011, 8 (12),4729–4745, DOI: 10.3390/ijerph8124729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price PS; Han X. Maximum cumulative ratio (MCR) as a tool for assessing the value of performing a cumulative risk assessment. Int. J. Environ. Res. Public Health 2011, 8 (6), 2212–2225, DOI: 10.3390/ijerph8062212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva E; Cerejeira MJ Concentration addition-based approach for aquatic risk assessment of realistic pesticide mixtures in Portuguese river basins. Environ. Sci. Pollut. Res 2015, 22 (9), 6756–6765, DOI: 10.1007/s11356-014-3857-9 [DOI] [PubMed] [Google Scholar]
- 22.Vallotton N; Price PS Use of the Maximum Cumulative Ratio As an Approach for Prioritizing Aquatic Coexposure to Plant Protection Products: A Case Study of a Large Surface Water Monitoring Database.Environ. Sci. Technol 2016, 50 (10), 5286–5293, DOI: 10.1021/acs.est.5b06267 [DOI] [PubMed] [Google Scholar]
- 23.De Brouwere K; Cornelis C; Arvanitis A; Brown T; Crump D; Harrison P; Jantunen M; Price P;Torfs R. Application of the maximum cumulative ratio (MCR) as a screening tool for the evaluation of mixtures in residential indoor air. Sci. Total Environ 2014, 479–480 (1), 267–276, DOI: 10.1016/j.scitotenv.2014.01.083 [DOI] [PubMed] [Google Scholar]
- 24.Silva MJ; Barr DB; Reidy JA; Malek NA; Hodge CC; Caudill SP; Brock JW; Needham LL;Calafat AM Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ. Health Perspect 2004, 112 (3), 331–338, DOI: 10.1289/ehp.6723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varshavsky JR; Zota AR; Woodruff TJ A Novel Method for Calculating Potency-Weighted Cumulative Phthalates Exposure with Implications for Identifying Racial/Ethnic Disparities among U.S. Reproductive-Aged Women in NHANES 2001–2012. Environ. Sci. Technol 2016, 50 (19), 10616 DOI: 10.1021/acs.est.6b00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christensen K; Sobus J; Phillips M; Blessinger T; Lorber M; Tan YM Changes in epidemiologic associations with different exposure metrics: A case study of phthalate exposure associations with body mass index and waist circumference. Environ. Int 2014, 73, 66–76, DOI: 10.1016/j.envint.2014.07.010 [DOI] [PubMed] [Google Scholar]
- 27.Meeker JD; Ferguson KK Urinary phthalate metabolites are associated with decreased serum testosterone in men, women, and children from NHANES 2011–2012. J. Clin. Endocrinol. Metab 2014, 99(11), 4346–4352, DOI: 10.1210/jc.2014-2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qian H; Chen M; Kransler KM; Zaleski RT Assessment of chemical coexposure patterns based upon phthalate biomonitoring data within the 2007/2008 National Health and Nutrition Examination Survey. J. Exposure Sci. Environ. Epidemiol 2015, 25 (3), 249–255, DOI: 10.1038/jes.2014.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aylward LL; Lorber M; Hays SM Urinary DEHP metabolites and fasting time in NHANES. J. Exposure Sci. Environ. Epidemiol 2011, 21 (6), 615–624, DOI: 10.1038/jes.2011.28 [DOI] [PubMed] [Google Scholar]
- 30.Christensen KLY; Makris SL; Lorber M. Generation of hazard indices for cumulative exposure to phthalates for use in cumulative risk assessment. Regul. Toxicol. Pharmacol 2014, 69 (3), 380–389, DOI: 10.1016/j.yrtph.2014.04.019 [DOI] [PubMed] [Google Scholar]
- 31.Zota AR; Calafat AM; Woodruff TJ Temporal trends in phthalate exposures: Findings from the national health and nutrition examination survey, 2001–2010. Environ. Health Perspect 2014, 122 (3), 235–241, DOI: 10.1289/ehp.1306681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hernández-Díaz S; Mitchell AA; Kelley KE; Calafat AM; Hauser R. Medications as a Potential Source of Exposure to Phthalates in the U.S. Population. Environ. Health Perspect 2009, 117 (2), 185–189, DOI: 10.1289/ehp.11766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Branch F; Woodruff TJ; Mitro SD; Zota AR Vaginal douching and racial/ethnic disparities in phthalates exposures among reproductive-aged women: National Health and Nutrition Examination Survey 2001–2004. Environ. Health 2015, 14, 57, DOI: 10.1186/s12940-015-0043-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reyes JM; Price PS An analysis of cumulative risks based on biomonitoring data for six phthalates using the Maximum Cumulative Ratio. Environ. Int 2018, 112, 77–84, DOI: 10.1016/j.envint.2017.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.CDC. National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Hyattsville, MD, 2016. (accessed 3 January 2017). [Google Scholar]
- 36.CDC. 2013–2014 Laboratory Data Overview. National Health and Nutrition Examination Survey, 2016.https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/overviewlab.aspx?BeginYear=2013 (accessed 31 May 2017). [Google Scholar]
- 37.Hornung RW; Reed LD; Hornung RW; Reed LD Estimation of Average Concentration in the Presence of Nondetectable Values. Appl. Occup. Environ. Hyg 1990, 5 (1), 46–51, DOI: 10.1080/1047322X.1990.10389587 [DOI] [Google Scholar]
- 38.Howdeshell KL; Rider CV; Wilson VS; Gray LE Mechanisms of action of phthalate esters, individually and in combination, to induce abnormal reproductive development in male laboratory rats.Environ. Res 2008, 108 (2), 168–176, DOI: 10.1016/j.envres.2008.08.009 [DOI] [PubMed] [Google Scholar]
- 39.CHAP (Chronic Hazard Advisory Panel on Phthalates and Phthalate Alternatives). U.S. Consumer Product Safety Commission, Directorate for Health Sciences: Bethesda, MD, 2014;https://www.cpsc.gov/PageFiles/169876/CHAP-REPORT-FINAL.pdf. [Google Scholar]
- 40.Koch HM; Christensen KLY; Harth V; Lorber M; Brüning T. Di-n-butyl phthalate (DnBP) and diisobutyl phthalate (DiBP) metabolism in a human volunteer after single oral doses. Arch. Toxicol 2012, 86(12), 1829–1839, DOI: 10.1007/s00204-012-0908-1 [DOI] [PubMed] [Google Scholar]
- 41.Lorber M; Koch HM Development and application of simple pharmacokinetic models to study human exposure to di-n-butyl phthalate (DnBP) and diisobutyl phthalate (DiBP). Environ. Int 2013, 59, 469–477,DOI: 10.1016/j.envint.2013.07.010 [DOI] [PubMed] [Google Scholar]
- 42.David RM Exposure to phthalate esters. Environ. Health Perspect 2000, 108 (10), A440, DOI: 10.1289/ehp.108-a440a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kohn MC; Parham F; Masten SA; Portier CJ; Shelby MD; Brock JW; Needham LL Human Exposure Estimates for Phthalates. Environ. Health Perspect 2000, 108 (10), A440–A443, DOI: 10.1289/ehp.108-a440b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mage DT; Allen RH; Gondy G; Smith W; Barr DB; Needham LL Estimating pesticide dose from urinary pesticide concentration data by creatinine correction in the Third National Health and Nutrition Examination Survey (NHANES-III). J. Exposure Sci. Environ. Epidemiol 2004, 14 (6), 457–465, DOI: 10.1038/sj.jea.7500343 [DOI] [PubMed] [Google Scholar]
- 45.Mage DT; Allen RH; Kodali A. Creatinine corrections for estimating children’s and adult’s pesticide intake doses in equilibrium with urinary pesticide and creatinine concentrations. J. Exposure Sci. Environ. Epidemiol 2008, 18 (4), 360–368, DOI: 10.1038/sj.jes.7500614 [DOI] [PubMed] [Google Scholar]
- 46.Price P; Han X; Junghans M; Kunz P; Watts C; Leverett D. An application of a decision tree for assessing effects from exposures to multiple substances to the assessment of human and ecological effects from combined exposures to chemicals observed in surface waters and waste water effluents. Environ. Sci. Eur 2012, 24 (1), 34, DOI: 10.1186/2190-4715-24-34 [DOI] [Google Scholar]
- 47.Price P; Dhein E; Hamer M; Han X; Heneweer M; Junghans M; Kunz P; Magyar C; Penning H;Rodriguez C. A decision tree for assessing effects from exposures to multiple substances. Environ. Sci. Eur 2012, 24 (1), 26, DOI: 10.1186/2190-4715-24-26 [DOI] [Google Scholar]
- 48.CDC. 2007–2008 Data Documentation, Codebook, and Frequencies https://wwwn.cdc.gov/Nchs/Nhanes/2007-2008/DEMO_E.htm (accessed September 10, 2018).
- 49.HHS. Annual Update of the HHS Poverty Guidelines, Federal Register 79:14 (January 22, 2014) p 3593–3594. http://www.gpo.gov/fdsys/pkg/FR-2014-01-22/pdf/2014-01303.pdf (accessed September 15, 2018). [Google Scholar]
- 50.Koch HM; Drexler H; Angerer J. An estimation of the daily intake of di(2-ethylhexyl)phthalate (DEHP) and other phthalates in the general population. Int. J. Hyg. Environ. Health 2003, 206 (2), 77–83, DOI: 10.1078/1438-4639-00205 [DOI] [PubMed] [Google Scholar]
- 51.CPSIA. Consumer Product Safety Improvement Act of 2008, Public Law 110–314, 110th Congress, 2008.https://www.cpsc.gov/s3fs-public/pdfs/blk_pdf_cpsia.pdf (accessed 21 February 2018).
- 52.CPSC. Prohibition of Children’s Toys and Child Care Articles Containing Specified Phthalates, Federal Register 82:207 (October 27, 2017) p 49938–49982. https://www.gpo.gov/fdsys/pkg/FR-2017-10-27/pdf/2017-23267.pdf (accessed February 21, 2018). [PubMed] [Google Scholar]
- 53.ECHA. Evaluation of New Scientific Evidence Concerning the Restrictions Contained in Annex XVII to Regulation (EC) No 1907/2006 (REACH): Review of New Available Information for bis (2-ethylhexyl) phthalate (DEHP); 2010. [Google Scholar]
- 54.Xia M; Ouyang X; Wang X; Shen X; Zhan Y. Occupational exposure assessment of phthalate esters in indoor and outdoor microenvironments. J. Environ. Sci 2018, 72, 1–14, DOI: 10.1016/j.jes.2017.12.013 [DOI] [PubMed] [Google Scholar]
- 55.Parlett LE; Calafat AM; Swan SH Women’s exposure to phthalates in relation to use of personal care products. J. Exposure Sci. Environ. Epidemiol 2013, 23 (2), 197–206, DOI: 10.1038/jes.2012.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abb M; Heinrich T; Sorkau E; Lorenz W. Phthalates in house dust. Environ. Int 2009, 35 (6), 965–970,DOI: 10.1016/j.envint.2009.04.007 [DOI] [PubMed] [Google Scholar]
- 57.Middleton DRS; Watts MJ; Lark RM; Milne CJ; Polya DA Assessing urinary flow rate, creatinine, osmolality and other hydration adjustment methods for urinary biomonitoring using NHANES arsenic, iodine, lead and cadmium data. Environ. Health 2016, 15 (1), 1–13, DOI: 10.1186/s12940-016-0152-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aylward L; Hays SM; Vezina A; Deveau M; St-Amand A; Nong A. Biomonitoring Equivalents for interpretation of urinary iodine. Regul. Toxicol. Pharmacol 2015, 72 (1), 158–167, DOI: 10.1016/j.yrtph.2015.04.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.