Abstract
More children with single-sided deafness (SSD) are receiving cochlear implants (CIs) due to the expansion of CI indications. This unique group of pediatric patients has different needs than the typical recipient with bilateral deafness and requires special consideration and care. The goal of cochlear implantation in these children is to provide bilateral input to encourage the development of binaural hearing. Considerations for candidacy and follow-up care should reflect and measure these goals. The purpose of this document is to review the current evidence and provide guidance for CI candidacy, evaluation, and management in children with SSD.
Keywords: Candidacy, Children, Cochlear implant, Guidelines, Single-sided deafness, Test battery, Unilateral hearing loss
PURPOSE
When cochlear implants (CIs) were first approved for children, the initial goal was unilateral sound awareness. With multichannel CIs, speech perception became an achievable and expected goal. Outcome measures moved from detection of speech to closed set word recognition, to open-set word recognition, to sentences in quiet, and ultimately sentence perception in noise. With bilateral cochlear implantation becoming standard of care for children in the US with bilateral severe to profound hearing loss, clinicians began to describe outcomes in terms of individual ear word recognition and bilateral performance on speech perception tasks (Uhler et al. 2017). Now children with hearing loss in only one ear are receiving CIs and clinicians are challenged with programming, testing, and evaluating performance in children who have hearing thresholds within the normal range on the contralateral side. This is a considerable challenge as so much of the groundwork for evaluation of CI patients has been laid by working with patients who have bilateral hearing loss. Children who seek cochlear implantation for unilateral hearing loss (UHL) or single-sided deafness (SSD) are seeking implantation not solely for better speech understanding, but in the hopes of achieving binaural hearing. Candidacy considerations, counseling, habilitation, and evaluation postactivation must look beyond simple speech perception and move toward evaluation that encompasses tasks associated with binaural hearing. The aim of this review is to summarize the current literature regarding CI outcomes for children with SSD and provide guidance for candidacy, outcome measures, and mapping of children with SSD + CI. The following recommendations were developed based on published research and experience of clinicians managing children and adults with SSD + CI. While many of these principles may be applicable to children who have asymmetric hearing loss (AHL) wherein there is a mild-to-moderate hearing loss in the better ear, these recommendations focus on children with SSD who have thresholds falling within the normal to near normal range in the better ear (Vincent et al. 2015).
BACKGROUND
UHL is known to occur in approximately 0.6 to 0.7 per 1000 live births in the U.S. (Centers for Disease Control Early Hearing Detection and Intervention [CDC] Database). By school-age, the number of children with UHL is estimated to be 2.5 to 6% (Bess 1998; Ross et al. 2010; Shargorodsky 2010). The impact of UHL includes difficulty understanding speech in noise (Bess & Tharpe 1984; Bess et al. 1986; Sangen et al. 2017; Corbin et al. 2021) and localizing on the horizontal plane (Bess & Tharpe 1984; Bess et al. 1986; Johnstone et al. 2010; Sangen et al. 2017; Corbin et al. 2021), resulting in an increased risk for problems with speech and language (Bess & Tharpe 1984; Fischer & Lieu 2014; Anne et al. 2017; Sangen et al. 2017), cognition (Bess & Tharpe 1984; Ead et al. 2013; Fischer & Lieu 2014), behavior (Bess & Tharpe 1984; Culbertson & Gilbert 1986), and quality of life (QoL) (Umansky et al. 2011; Roland et al. 2016).
Although a hearing aid (HA) may be beneficial for children with mild-to-moderate UHL, it is contra-indicated in those with more significant degrees of UHL, often referred to as SSD (Bagatto et al. 2019). Traditionally, hearing technologies available for school-age children with SSD have included re-routing devices such as contralateral-routing-of-signal (CROS) HAs and bone conduction devices (BCD). Each re-routing device has advantages and disadvantages, although they are typically contraindicated in young children with SSD (McKay et al. 2008; Bagatto et al. 2019). The auditory deprivation associated with SSD causes irreversible changes in the auditory cortex (Kral et al. 2013a, 2013b; Gordon et al. 2015), which re-routing devices would not be expected to prevent as they do not provide hearing to the affected ear.
Cochlear implantation, previously reserved for individuals with bilateral severe to profound sensorineural hearing loss (SNHL), is the only SSD treatment that provides hearing to the affected ear and enables binaural auditory stimulation of the brain. The Food and Drug Administration (FDA) has approved cochlear implantation for adults and children aged 5 years and older with SSD. MED-EL received approval in 2019 (MED-EL Corporation 2019) and Cochlear Americas received approval in 2022 (Cochlear Corporation 2022). Although published outcomes are limited, several positive trends have emerged (Benchetrit et al. 2021). Length of device use has been reported between 6 and 12 hours per day, which compares favorably with bilateral users (Beck et al. 2017; Polonenko et al. 2017; Ganek et al. 2019; Ramos Macías et al. 2019; Deep et al. 2021; Ehrmann-Mueller et al. 2020; Brown et al. 2021). Subjective benefit has been reported in recipients with follow-up ranging from 4 months to 3.5 years (Arndt et al. 2015; Beck et al. 2017; Thomas et al. 2017; Ramos Macías et al. 2019; Brown et al. 2021). Improved speech understanding in the presence of competing noise (Sladen et al. 2017; Thomas et al. 2017; Zeitler et al. 2019; Ehrmann-Mueller et al. 2020; Brown et al. 2021; Park et al. 2021a), tinnitus suppression (Zeitler et al. 2019), and enhancement of localization (Arndt et al. 2015; Távora-Vieira & Rajan 2015; Ehrmann-Mueller et al. 2020; Brown et al. 2021) have also been demonstrated.
CANDIDACY CONSIDERATIONS
Medical Considerations
The etiology of SSD is more often unknown in comparison to children with bilateral SNHL, particularly when it comes to genetic causes (Usami et al. 2017). Although genetic etiologies account for the majority of bilateral SNHL, genetic testing for SSD is not standard unless a specific syndromic etiology is suspected. The reason is lack of cost effectiveness since most genetic variants included in commercial comprehensive hearing loss panels are associated with nonsyndromic bilateral SNHL.
Eighth nerve anatomy is a critical aspect of SSD CI candidacy. Significant hypoplasia or aplasia of the eighth nerve, often referred to as cochlear nerve deficiency (CND), is a contraindication to CI for children with SSD. The incidence of CND has been reported to be as high as 46% in children with SSD who underwent high resolution magnetic resonance imaging (MRI) (Clemmens et al. 2013; Usami et al. 2017; Zhan et al. 2020). High resolution 3D MRI is essential to diagnose CND. An MRI may reveal CND when CT temporal bone imaging demonstrates normal bony landmarks, including diameter of the internal auditory canal (IAC) and cochlear aperture (Casselman & Bensimon 1997; Adunka et al. 2006; Young et al. 2012). Children with SSD due to CND may have auditory brainstem response (ABR) findings consistent with SNHL or unilateral auditory neuropathy spectrum disorder (ANSD). Cochlear implantation is contraindicated in these cases as the electric signal presented to an ear with CND would be significantly degraded. Therefore, the prognosis is poor and likelihood of nonuse is high (Arndt et al. 2015; MacCutcheon et al. 2019; van Wieringen et al. 2019; Zhan et al. 2020; Dewyer et al. 2021; Selleck et al. 2021). In addition, there is risk of negative impact on speech perception, as may occur with traditional amplification of SSD.
At present the SSD + CI literature describes pediatric recipients with anatomy enabling full electrode insertions. Adequate electrode array insertion should be considered during candidacy evaluation for similar reasons as noted above regarding CND.
Children with SSD at risk of progressive loss in their better hearing ear merit special consideration. It is advantageous to implant the SSD ear rather than wait for the contralateral ear to decline. This approach reduces the period of auditory deprivation, which may improve speech perception in the implanted ear, while simultaneously providing binaural hearing. Two common causes of progressive loss in the initially better hearing ear of children with SSD are cochlear malformation and congenital cytomegalovirus (cCMV) infection. Congenital CMV is one of the most common causes of acquired SNHL (Morton & Nance 2006; Grosse et al. 2008), but often undiagnosed in otherwise asymptomatic children unless viral testing is done during the neonatal period. Children with enlarged vestibular aqueduct have bilateral inner ear malformations twice as often as unilateral and are at high risk of progression to CI candidacy during childhood (Hodge et al. 2021).
SSD due to bacterial meningitis requires timely intervention. If profound SNHL occurs, rapidly progressive ossification may obliterate the inner ear and preclude successful implantation. Therefore, early implantation of any ear deafened by bacterial meningitis is necessary.
Age and Length of Deafness
Younger age of implantation is one of the most important factors influencing outcomes in the pediatric CI population (Leigh et al. 2013; Ching et al. 2017). However, the range and degree of benefit associated with age of CI and length of unilateral deafness for children with SSD is not yet fully understood. The current FDA indications for SSD specify that candidates have maximum 10-year duration of deafness and minimum age of 5 years. The lower age limit likely reflects concerns about safety as indications were changed based on adult data and not data obtained specifically from children. Paradoxically, the limit of 10-years of deafness is reflective of the potential negative impact of a lengthy period of auditory deprivation. Considering the importance of neuroplasticity in CI outcomes, it is not unreasonable to assume younger age at implant would be advantageous in SSD + CI outcomes. There is also evidence that brain reorganization in response to SSD may impede binaural central integration after CI perhaps as early as 2 years after onset (Kral & Sharma 2012; Kral et al. 2013a, 2013b). This phenomenon may explain poorer outcomes in some older children with congenital or longstanding SSD (Arndt et al. 2015; Gordon et al. 2015; Rauch et al. 2021). This provides further rationale to implant children with SSD before the arbitrary age of 5 years, especially if they are congenitally deafened. Regarding safety, there is growing evidence demonstrating safety of implantation of children as young as 6 months using modern techniques (Hoff et al. 2019; American Academy of Otolaryngology Head and Neck Surgery 2020).
Audiological Considerations
Children of any age with unilateral, moderate-to-profound SNHL should be referred for an audiological evaluation of CI candidacy. Evaluation procedures are similar to those completed in children with bilateral hearing loss, with the addition of measures meant to evaluate binaural hearing abilities. Specifically, evaluations should consist of case history, threshold assessment, verification of current hearing technology, and validation of current levels of functioning. The goal should be to obtain a full picture of the child’s current level of auditory function and collect baseline data for postactivation testing. Specific recommendations for CI candidacy test batteries and outcomes are described in the “Test Battery” section to follow.
As cochlear implantation in children with SSD is a relatively new treatment option, the professional community relies on evidence obtained in trials with adults with SSD + CI and from children with bilateral hearing loss to guide informed decision-making regarding candidacy. Off-label cochlear implantation has become more routine for children with varying degrees and configurations of hearing loss (Carlson et al. 2018). These procedures have resulted in improved speech perception outcomes for many children (Carlson et al. 2015; Wilson et al. 2016; Meredith et al. 2017; Carlson et al. 2018; Rajan et al. 2018; Park et al. 2019a; Teagle et al. 2019; Varadarajan et al. 2020). For example, Leigh and colleagues (2016) compared phoneme perception abilities between children using HAs and children using CIs and determined that HA users with a 60 dB HL three-frequency pure-tone average (3FPTA; average at 500, 1000, and 2000 Hz) had a 75% likelihood of improved word recognition with a CI, which increased to a 95% likelihood of improved scores with a 3FPTA of 82 dB HL. Aided speech intelligibility index (SII) scores can predict speech understanding with 0.0 implying no available speech information and 1.0 indicating full audibility. Work evaluating outcomes with HAs related to the aided SII suggest that values lower than 0.65 are associated with poor audibility and outcomes (Stiles et al. 2012; McCreery et al. 2013; Tomblin et al. 2015; Leal et al. 2016). McCreery (2014) recommended that children who have an aided SII of less than 0.65 should be considered for cochlear implantation regardless of pure tone thresholds. Even sloping configurations with 3FPTAs as low as 43 dB HL can result in an aided SII of less than 0.65 (Leal et al. 2016). As these degrees and configurations of loss have shown the potential to provide insufficient access to speech for bilateral losses, children meeting these criteria unilaterally should be considered for cochlear implantation as well. The current FDA labeling for SSD includes a four-frequency pure-tone average (4FPTA; dB average at 500, 1000, 2000, and 4000 Hz) of ≥ 90 dB HL for MED-EL devices (MED-EL Corporation 2019) and >80 dB HL for Cochlear devices (Cochlear Corporation, 2022). Given the current evidence suggesting that bilateral guidelines are too restrictive, these SSD criteria may be too restrictive for children as well. Therefore, children with a unilateral 3FPTA ≥ 60 dB HL and/or an aided SII < 0.65 should be evaluated and considered for possible cochlear implantation. Of course, ear and frequency specific data may be challenging to obtain in some infants and very young children. In such cases, frequency-specific ABR testing may be used to estimate hearing thresholds.
Experience With Alternative Technology
Re-routing technology such as CROS and BCD are typically avoided in young children with SSD for several reasons (McKay et al. 2008; Bagatto et al. 2019). First, to avoid having a poor signal on the deafened side transmitted to the better-hearing ear, users must be mature enough to manipulate their device and/or environment. Also, to avoid occlusion of the better-hearing ear with a CROS device, children need to have large enough ear canals to accommodate the device. Most importantly these technologies do not promote binaural hearing as stimulation is provided only to one auditory pathway. Delaying intervention that stimulates both auditory pathways can result in auditory deprivation in the ear with SSD, which has been associated with irreversible changes in the auditory cortex (Kral et al. 2013a, 2013b; Gordon et al. 2015). The current FDA labeling indicates a 30-day trial with a CROS or BCD prior to cochlear implantation. The authors of the current article do not believe this should be required for pediatric SSD patients seeking CI. When the goal is binaural hearing, there is no need to trial a device that does not stimulate both auditory pathways. If the goal is to manage and mitigate the impact of SSD, and there is hesitancy around implantation, a trial period with a re-routing device can be considered.
A trial with traditional amplification is not recommended if an aided SII is less than 0.65 despite the current FDA criteria specification of unilateral aided open-set word recognition of 5% or poorer. Unilateral word recognition in children with CIs averages above 70% (Teagle et al. 2019), revealing a large gap in what current guidelines consider acceptable for children with UHL and what children are capable of with a CI. Furthermore, children with SSD do not typically present for consideration of cochlear implantation because the family would like for them to better discriminate single words in their poorer-hearing ear. That is, children with SSD present with difficulties in learning, localization, hearing in noise, and QoL necessitating further consideration of the whole child beyond the audiogram or single-word recognition when determining candidacy. Specific recommendations for preoperative and postactivation measurements of these important variables are included in the “Test Battery” section of this document.
Counseling
A logical first step in counseling families of children with UHL is to identify the needs and goals of the family. Here, clinicians may probe the family on their family dynamics, their feelings around surgery, and their understanding of their child’s needs. Services need to be family-focused, allowing for modifications based on unique family-identified concerns, priorities, goals, and desires (Moeller et al. 2013).
Counseling families of children with SSD requires a lengthy discussion on the developmental risks associated with SSD and the associated consequences for speech understanding in noise and sound localization. Families need this information to fully understand the long-term consequences of SSD and evaluate the possible treatment options. This conversation may emphasize the importance of bilateral input for the process of separating speech and noise and finding sounds in space. Families should be presented with all options to manage SSD, including re-routing devices to appreciate how each technology may or may not help meet the goals they have for their child. Families must also understand that studies demonstrate auditory neural plasticity is greatest during the first few years of life (Sharma et al. 2002). Polonenko and colleagues (2017) used electroencephalography to measure cortical responses to sound among a small group of children with left SSD since infancy who received an implant prior to 3.6 years of age. Results showed rapid improvement of cortical responsivity after a few months of device use suggesting that when children with SSD are implanted at young ages, they may be able to make use of neural plasticity.
Generally speaking, research suggests that children with SSD should expect benefit from a CI for speech perception in noise and quiet as well as with localization (Benchetrit et al. 2021; Brown et al. 2021), but reasonable expectations should be established prior to surgery (Deep et al. 2021; Rauch et al. 2021). Families who choose cochlear implantation need to understand the high degree of variability in patient performance and the inability to accurately predict outcomes. In addition to age and duration of SSD, variation in SSD + CI outcomes is likely influenced by a host of variables including, but not limited to, neuroanatomy, etiology of hearing loss, electrode insertion length, duration and consistency of device use, and type and duration of behavioral auditory skill building therapy post-CI. In addition, the time needed to adjust to electrical hearing and subsequently achieve benefits for speech understanding in noise and localization may vary across individuals. Families and older children in particular should understand that CI outcomes are best with device use during all waking hours (Easwar et al. 2018; Park et al. 2019b; Gagnon et al. 2020). Early work has been suggestive of teen and pre-teenaged children having difficulty adjusting to device use, potentially due to social pressures and the scrutiny a visible device brings to a previously invisible disability (Thomas et al. 2017). Families should also be prepared for the listening therapy recommended following implantation (Evans & Dillon 2019). Most importantly, families should understand that while safe and effective, SSD + CI in children is still a relatively recent development, and long-term evidence regarding speech, language, cognitive, and behavioral outcomes among children with SSD + CI is still evolving.
Parents of children with SSD and additional disabilities that put them at high risk for language delay independent of hearing loss, such as autism spectrum disorder and/or Down syndrome, warrant special counseling considerations. For these families, discussion about potential advantages of improved hearing regardless of spoken language status is important. In addition, counseling regarding the need for long-term, consistent CI use in the absence of measurable benefit is important for parents and professionals working with these children as auditory skills may be challenging to measure and/or slower to emerge than in typically developing children.
Candidacy Determination
Once current level of audiological function, medical status, imaging, case history, and QoL has been obtained, the child’s case should be discussed with the CI team. A multidisciplinary team (typically audiologist, surgeon, and speech and language pathologist at a minimum) can help synthesize information to determine whether the child is expected to have improved outcomes with a CI as opposed to a traditional HA, re-routing device, or no intervention. CI team staffing can also be particularly helpful in making sure candidates are well-selected and that realistic expectations have been established before moving forward. Implant teams may provide summaries of the team discussion as a report that outlines the criteria for which candidacy was based. Supplemental reports including speech and language evaluations and parental and teacher feedback may also be included. This report may help support insurance approval and should be substantiated by literature.
TEST BATTERY
Threshold Assessment and Immittance Measures
Preoperative assessments should include immittance measures, and pure tone air and bone conduction testing using warble tone stimuli. The test battery should consist of age-appropriate behavioral assessment and cross-check measures (Jerger & Hayes 1976; American Academy of Audiology 2020). It is recommended that octave thresholds be measured between 125 and 8000 Hz, and inter-octaves 3000 and 6000 Hz can be included in at least the better-hearing ear. It is important to find any early signs of noise-induced or progressive hearing loss in the better-hearing ear and measure low frequencies (e.g., 125 Hz) for consideration of interaural pitch matching and evaluation of hearing preservation.
Reliable behavioral testing can be difficult to obtain with very young children. In these cases, objective assessments of auditory function are the gold standard and should be completed following the Joint Commission on Infant Hearing Early Hearing Detection and Intervention Guidelines (The Joint Committee on Infant Hearing 2019), particularly when an infant does not pass their newborn hearing screening. Testing should be conducted as outlined in the Clinical Guidance Document on Assessment of Hearing in Infants and Young Children (American Academy of Audiology 2020). Additionally, consideration for effective masking levels should be used to isolate the ear with hearing loss for accurate assessment (Lightfoot et al. 2010; Lau & Small 2020) and to avoid under-estimating the degree of loss as this may further delay intervention including possible provision of a CI.
Postactivation, unaided hearing assessment of both ears should be continued at regular intervals. There are no available data that estimate the prevalence of progression to bilateral hearing loss among children with CIs and SSD. Studies that have investigated the incidence of progression in children with UHL of varying degrees have estimated that between 10 and 17% of children with UHL may progress to a bilateral hearing loss (Uwiera et al. 2009; Haffey et al. 2013; Fitzpatrick et al. 2017). Unaided hearing of the implanted ear should be monitored as well (Dillon et al. 2020) and electric-acoustic stimulation should be considered for children with postoperative residual hearing (Skarzynski & Lorens 2010; Wolfe et al. 2017; Park et al. 2019a).
As with all CI recipients, routine validation of audibility from the CI is required postactivation (Academy Task Force on Guidelines for Cochlear Implants 2019). Sound field testing using warble tones is used with traditional recipients to ensure audibility of conversational speech. Isolating the implanted side in the sound field is complicated in children with SSD because the stimulus will reach the CI processor and the better hearing ear simultaneously. Masking is typically used in audiometric testing to avoid crossover from the ear contralateral to the test ear. Electric hearing does not create crossover to the non-test ear, and there are no formulas for calculating effective masking for this test configuration. As the goal is to occlude the better hearing ear enough to prevent its contribution to testing, the plug-and-muff method is used for sound field detection in adult studies of CI recipients with SSD (Roland et al. 2011; Firszt et al. 2012; Friedmann et al. 2016; Galvin et al. 2019). The ear plugs should be small enough to ensure an appropriate fit, and the muffs should be an appropriate pediatric size (Park et al. 2021b). The plug-and-muff technique does not provide complete attenuation of the ear with normal hearing thresholds (Berger et al. 2003; Galvin et al. 2019). Clinicians should keep in mind that the estimated attenuation from this configuration is limited to the noise reduction rating (NRR) of the device with the highest attenuation value +5 dB (US Department of Labor 1993), which may not be enough for children with normal thresholds. To ensure that the thresholds obtained in the sound field are attributable to the detection from the speech processor rather than the occluded better hearing ear, thresholds should be screened with the plug-and-muff alone at 10 to 15 dB above the assumed CI thresholds. If there is no response, occlusion was sufficient for accurate CI detection threshold. If there is detection, the provider should be aware that the assumed CI detection threshold may be a response from the occluded contralateral ear.
Verification of Hearing Technology
Preoperative speech perception testing is an essential component of the candidacy evaluation to assess functional access to sound and establish a baseline for monitoring progress with the CI. The authors recommend that candidacy assessment can be completed in the child’s everyday listening situation whether that may be with a CROS, BCD, or no assistive technology. It may be necessary to obtain ear-specific word recognition scores through use of a traditional HA for purposes of insurance authorization. It is important that the HA or re-routing device used for the assessment is appropriate for the child’s hearing loss and is programmed to DSL v5.0 prescriptive targets (Bagatto et al. 2005; Seewald et al. 2005) using probe microphone measures. The reader is directed to the Clinical Practice Guidelines for Pediatric Amplification from the American Academy of Audiology (2013) for further guidance. For traditional HAs, the clinician should make note of the SII for 65 dB speech inputs. Aided SIIs of 0.65 or below should be considered to reflect poor potential for adequate audibility with a HA (Stiles et al. 2012; McCreery et al. 2013; Tomblin et al. 2015; Leal et al. 2016).
Postactivation, the function of the CI speech processor should be verified through obtaining sound field detection as described above, confirmation of microphone function, and listening checks via direct audio input (DAI) and/or streaming.
Word Recognition Testing
Preoperative speech perception testing can be administered once the HA or re-routing device has been properly verified. This testing should be completed using recorded speech stimuli (Roeser & Clark 2008; Uhler et al. 2016) calibrated and presented at 60 dBA from a single loudspeaker at 0° azimuth approximately 1 m from the center of the listener’s head as outlined by the Pediatric Minimum Speech Test Battery (PMSTB; Uhler et al. 2017). For pediatric patients, selection of developmentally appropriate test stimuli is of great importance. The PMSTB was developed to provide a standardized, hierarchical protocol to assist pediatric audiologists in test selection for evaluating speech perception in children with hearing loss (Uhler et al. 2017). The PMSTB provides guidance not only on test selection, but also administration parameters, with an emphasis on administering the most appropriate test and conditions given a child’s language age, auditory skills, and response abilities, while avoiding potential ceiling or floor effects. The authors recommend the use of a modified version of the PMSTB as children with thresholds in the normal hearing range in one ear are likely able to repeat more challenging word lists at a younger age than children with prelingual bilateral SNHL. Table 1 lists the recommended hierarchy.
TABLE 1.
Recommended lists by age and ability
| Age | Word Recognition | Sentence Recognition |
|---|---|---|
| 2–3 years | ESP | None |
| 3–5 years | MLNT/LNT | BKB-SIN* |
| 5–6 years | CNC words | Baby Bio BKB-SIN |
| 6 years and older | CNC words | Baby Bio; BKB-SIN; AZBio Sentences† |
•≤25% administer an easier test.
•25–79% repeat at follow-up.
•≥80% at 2 visits move to next level of words and/or sentences‡
The BKB-SIN is generally not recommended until age 5 years, but has been used in children with SSD + CI who are as young as 3.5-years old (Park et al. 2021a).
Many sentences on the standard AZBio are not appropriate for children and should be used with caution.
If child struggles in more difficult condition, return to easier condition to show consistent performance from last visit (i.e., repeat LNT after CNC words).
CI, cochlear implants; SSD, single-sided deafness.
Adapted from the Pediatric Minimum Speech Test Battery (PMSTB; Uhler et al. 2017).
The age at which a child can participate in word and sentence recognition may vary, but should be attempted as early as possible, starting with a closed-set ESP low-verbal and then expanding to open-set word recognition as soon as possible. Single-word speech recognition testing should be completed preoperatively and at regular postactivation intervals in the sound field in the following conditions: better hearing ear alone, aided in the affected ear alone (HA if appropriate preoperatively and CI postactivation), and bilaterally. The same word recognition test should be used for all three conditions. This allows scores from the bilateral condition to be compared to the normal-hearing ear alone to check for potential binaural interference from the HA/CI. In a candidacy evaluation, this comparison highlights the difference between what the child is capable of perceiving with the normal-hearing ear and what they can perceive in the impaired ear.
For preoperative aided or earphone word recognition testing, the better hearing ear must be masked to isolate the aided ear and avoid crossover. Speech-shaped noise presented via insert earphone at 40 dB HL is recommended (Martin et al. 1998). This test configuration is the only currently available way to isolate an acoustically amplified ear and prevent crossover. Further research is necessary to find the most valid and reliable methods of isolated word recognition testing for acoustically amplified speech in this unique group.
Postactivation word recognition should be tested at regular intervals as per recommended clinical guidelines for CI care (Academy Task Force on Guidelines for Cochlear Implants 2019). Testing speech recognition in the sound field while isolating the CI is challenging for children with SSD + CI. Although adult studies have found success using masking to isolate the CI ear (Bernstein et al. 2017; Buss et al. 2018), children are more sensitive to masking than adults (Johnstone & Litovsky 2006; Corbin et al. 2016; Leibold & Buss 2019). As mentioned previously, masking formulas were created to prevent crossover from the test ear and have not been adapted for sound field testing contralateral to electric input. Additionally, children with SSD have been shown to exhibit neural changes that may impact selective attention and audition (Propst et al. 2010; Tibbetts et al. 2011; Schmithorst et al. 2014; Zhang et al. 2018; Vanderauwera et al. 2020). These biological differences may make it more difficult for children with SSD to attend to speech with a contralateral masker. Practically speaking, it may not be realistic to expect a child to be comfortable with equipment required for the plug-and-muff technique for the length of time required to complete isolated speech recognition, and the attenuation will likely be insufficient (Galvin et al. 2019). DAI from a sound source to the speech processor has been shown to be an effective way to isolate a CI ear in studies involving teens and adults (Roland et al. 2011; Friedmann et al. 2016; Sevier et al. 2019), but these devices are often proprietary and have internal calibration methods to control the stimulus level. Deep et al. (2021) successfully used commercially available DAI equipment with children for isolated ear speech recognition. These methods bring variability, but the plug-and-muff and masking methods have biological unpredictability that the DAI method does not. For routine monitoring of speech recognition in the CI ear alone, the recommendation is to use DAI at a volume level reported by the user to be comfortable (Park et al. 2021b).
Spatial Hearing Assessment
For children with asymmetrical hearing loss, real-world hearing difficulties and subsequent benefits of cochlear implantation are not well-captured via standard audiometric assessment measures. Although it is important to measure ear-specific benefits of cochlear implantation, including auditory detection and word recognition as described above, for children with SSD, it may be arguably more important to assess binaural hearing skills. In doing so, the clinician can better estimate performance in the child’s preoperative everyday listening modality and query potential binaural advantages gained postactivation through bilateral stimulation provided by the CI. Both speech-in-noise (SIN) and localization testing have emerged as key measures to objectively assess ‘binaural benefit’ of cochlear implantation in individuals with SSD + CI.
SPEECH-IN-NOISE TESTING
Spatially separated SIN assessment is sensitive to monaural versus binaural hearing and is an excellent measure for quantifying binaural advantages. Binaural hearing capabilities have been studied in adult SSD + CI recipients, and researchers generally agree that the addition of the CI allows the listener to benefit from the head shadow effect (Arndt et al. 2015; Bernstein et al. 2017; Dirks et al. 2019; Sullivan et al. 2020). Studies have also provided evidence that some adult SSD + CI recipients have improved speech in noise performance secondary to binaural redundancy and binaural squelch (Vermeire & Van de Heyning 2009; Mertens et al. 2015). Pediatric SSD + CI patients have shown improvement in SIN tasks as well (Sladen et al. 2017; Thomas et al. 2017; Zeitler et al. 2019; Benchetrit et al. 2021; Ehrmann-Mueller et al. 2020; Park et al. 2021a).
Careful consideration must be given to target-masker spatial configuration during SIN testing. Testing solely with both target and masker signals co-located at 0-degrees azimuth will not fully assess the consequences of monaural listening or benefits of CI use. Separating the target and masker locations allows the clinician to assess a recipient’s use of interaural difference cues and can be completed in most 2-loudspeaker clinical audiometry booths as outlined by King and colleagues (King et al. 2021; Selleck et al. 2021).
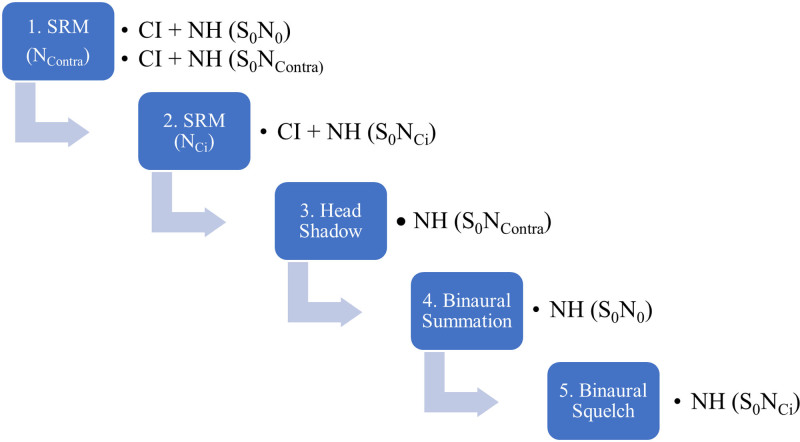
The authors propose the following three target-to-masker spatial configurations for SIN testing: (1) target and masker co-located in front (S0N0); (2) target to the front, masker 90° to the affected ear (S0Nci); and (3) target to front, masker 90° to the normal-hearing ear (S0Ncontra). These configurations are frequently used in the adult and pediatric studies involving the effectiveness of CI in SSD (Buechner et al. 2010; Firszt et al. 2012; Friedmann et al. 2016; Buss et al. 2018; Galvin et al. 2019; Deep et al. 2021; Park et al. 2021a). This testing paradigm and reasoning has been well described by Gartrell et al. (2014), but in brief, it allows clinicians to estimate effects of binaural summation, head shadow, binaural squelch, and SRM in two listening conditions by using three target/masker spatial conditions as outlined in Table 2. Current evidence suggests that after 1 year of device use, pediatric SSD + CI recipients may experience benefit in all three conditions and SRM in both spatially separated conditions (Park et al. 2021a).
TABLE 2.
Calculating Spatial Speech Recognition Scores using SNR-50 outcomes (paraphrased from Gartrell et al. 2014)
| Spatial Measure | Calculation Listing Modality (Target/Masker) |
|---|---|
| Binaural summation | NH (S0N0) – CI/HA + NH (S0N0) |
| Head shadow | NH (S0NContra) – CI/HA + NH (S0NContra) |
| Binaural squelch | NH (S0NCi) – CI/HA + NH (S0NCi) |
| SRM (NCi) | CI/HA + NH (S0N0) – CI/HA + NH (S0NCi) |
| SRM (NContra) | CI/HA + NH (S0N0) – CI/HA + NH (S0NContra) |
NH = listening with normal-hearing ear alone (device removed).
CI/HA + NH = listening with CI/HA or HA and normal-hearing ear together (device on).
S0 = speech signal located at 0° azimuth.
N0 = Noise/masking signal located at 0° azimuth.
NContra = Noise/masking signal directed toward the normal-hearing ear.
NCi = Noise/masking signal directed toward the deaf ear; SRM = spatial release from masking.
It is recommended that the SIN battery can be completed preoperatively as part of the candidacy testing as well as at regular postactivation intervals. Very young children may not be able to participate in SIN testing, although it should be incorporated into the test schedule when appropriate along with subjective parental questionnaires. Given the well-known effects of development on SIN abilities (e.g., Corbin et al. 2016; Griffin et al. 2019; Leibold & Buss 2019; Corbin et al. 2021), the clinician is encouraged to measure SIN performance with and without the CI in all three conditions within the same visit. For example, comparing pre-op-aided SIN performance to performance measured with the CI when the child is a year older may be an invalid comparison since SIN abilities will improve due to development. Comparing aided to unaided scores obtained within the same visit will provide evidence of the amount of overall SIN benefit due to the CI. Once unaided SIN scores stabilize, repeat testing in the unaided condition is no longer as critical. When a child reaches this plateau will vary depending on test selection and listening conditions, but likely will occur in their teenage years, when SIN testing has been shown to yield adult-like scores (Etymōtic Research 2005; Corbin et al. 2016; Holder et al. 2016; Griffin et al. 2019). SRM likely can be calculated and compared to preoperative values as current research in this population suggests that SIN scores improve with age but SRM may not (Park et al. 2021a). The pediatric audiologist may need to adapt the protocol for the very young listener for whom listening in six conditions could be fatiguing and ultimately yield unreliable data. In this scenario, the audiologist should feel justified in prioritizing the proposed conditions to ensure reliable data are collected in the most valuable conditions first for the patient with SSD + CI (see Fig. 1). However, data should be collected in all six conditions as soon as possible. As this battery could be fatiguing, spatial hearing measures should be prioritized in the SSD + CI population.
Fig. 1.
Binaural hearing measures presented as a test hierarchy and prioritized by order of relevance for the pediatric patient with SSD + CI. CI, cochlear implants; SSD, single-sided deafness.
Two open-set SIN tests are referenced in the PMSTB and are recommended for testing as noted in Table 1: the BKB-SIN (Bench et al. 1979; Etymōtic Research 2005) and the Pediatric AZBio Sentence Test (Spahr et al. 2014). The BKB-SIN consists of 18 list pairs, with list pairs 1 to 8 including more challenging signal-to-noise ratios (SNRs). Clinicians may find these list pairs are more appropriate for use with children with SSD + CI, who may exhibit ceiling effects in some conditions when tested with less challenging lists. It is important to note that the test result (SNR-50) becomes more reliable as the examiner administers more lists per condition. This is especially relevant for young children as the confidence interval is quite large when administering just 1 or 2 lists per condition (see Table 5 of the BKB-SIN user manual; Etymōtic Research 2005). The Pediatric AzBio Sentence Test, commonly referred to as the “BabyBio,” has become a frequently used speech perception test for evaluation of CI benefit in children (Spahr et al. 2014). Unlike the BKB-SIN, the entire sentence list is administered at a fixed SNR. The PMSTB recommends a +5 dB SNR; however, the clinician may find a +5 dB SNR to be too favorable for many children with SSD. Finding an appropriate SNR that avoids a ceiling or floor effect will take additional time but is a critical step. Once established, testing in all conditions should be completed at the same SNR. Confidence intervals should be consulted to determine if changes in performance are statistically significant (see Table 1 of Spahr et al. 2014).
Certainly, there are many more speech perception tests that could be utilized to assess SIN benefit, each having its own benefits and limitations. Most SIN stimuli recommended in the PMSTB are sentence-level stimuli. Sentence-level stimuli may better represent a real-world listening task than a monosyllabic word recognition task, but it is important to consider the potential limitations. First, factors such as working memory, language, and cognition have the potential to confound test results (Osman & Sullivan 2014; McCreery et al. 2017; MacCutcheon et al. 2019). Second, test administration for sentence-level stimuli can often be more time intensive than word-level stimuli. This is especially relevant for children with SSD + CI, who require testing across multiple listening conditions. Additionally, there are a limited number of lists among commercially available tests and presenting a minimum of six of them each year throughout childhood carries concerns about list learning. The Words In Noise (WIN) Test could prove to be an efficient alternative, although there are just eight commercially available lists (Wilson et al. 2007a; Wilson & McArdle 2007). The WIN test carries normative data for children 6 to 12 years (Wilson et al. 2010). Target stimuli are monosyllable words from the NU No. 6 (Tillman & Carhart 1966) presented in multi-talker babble and scored as an SNR-50. The WIN has been found to be a more sensitive tool for measuring speech recognition in noise than the BKB-SIN (Wilson et al. 2007b). The standard AzBio is also an option for older children; however, the content of some lists may be inappropriate even for teens. Development of a single efficient, sensitive, and appropriate test for children with SSD + CI is needed.
Considerations for the Very Young Child
Because of the lack of commercially available SIN tests for children younger than 5 years of age (Schafer 2010), clinicians may need to rely on subjective questionnaires to probe hearing in noise. Alternative test stimuli created for research purposes may hold promise such as The Children’s Realistic Index of Speech Perception (CRISP, Litovsky 2005) and Phrases in Noise Test (PINT, Schafer et al. 2012), but further work is needed before routine clinical use can be adopted. There is a great need for more efficient, reliable, and sensitive SIN tests with plentiful lists that can be used in the pediatric population with SSD, especially for the very young child. This should remain an area of continued investigation in the field.
Localization Testing
With regard to sound localization in children and adults with SSD + CI, studies have reported that the majority who regularly use their CI have improved localization (Arndt et al. 2015; Távora-Vieira & Rajan 2015; Benchetrit et al. 2021; Ehrmann-Mueller et al. 2020). This was true despite some children with SSD + CI having longer duration of deafness and older age at implant. Studies of localization in adults generally agree that patients with SSD + CI are able to access interaural level differences (ILD) to localize sound, but they do not use interaural timing differences (ITD) for localization (Dorman et al. 2015; Dirks et al. 2019). Similar studies of the pediatric population are lacking, so the cues that children with SSD + CI use for localization, especially those with congenital SSD, is not well understood. Clinical measurement of localization is not generally feasible due to space and time constraints. Clinicians may need to rely on subjective questionnaires to estimate localization performance.
Subjective Questionnaires
Subjective questionnaires completed by the parent, teacher, speech-language pathologist, or child can provide valuable information about current levels of functioning and the potential impact of SSD. There are questionnaires designed to capture a child’s auditory skill development, progress with speech and language, listening behaviors/abilities in quiet versus noise, spatial hearing, perceived listening effort and fatigue, academics, and QoL. The Consensus Practice Parameter (Bagatto et al. 2019) and the Phonak Compendium on UHL (Smith & Drexler 2018) outline several options by age and abilities evaluated. Clinicians should use questionnaires preoperatively as part of the candidacy evaluation and postactivation to monitor progress. The questionnaires chosen will be subject to the age and behaviors targeted.
Auditory Skills Development
Subjective outcome evaluation tools in pediatric audiology are plentiful. Bagatto et al. (2011) performed a critical review of 12 outcome measures designed to evaluate auditory-related behaviors in children birth to 6 years of age who use amplification. Two questionnaires were rated highly in the critical review: LittlEARS Auditory Questionnaires (Tsiakpini et al. 2004) and the Parents’ Evaluation of Aural/Oral Performance of Children (PEACH) Rating Scale (Ching & Hill 2007). The LittlEARS may not be sensitive enough to difficulties reported by parents of children with SSD as the targeted behaviors are not specific to binaural hearing. The authors recommend the use of the PEACH Rating Scale for infants and young children as the items may be more sensitive to the effects of SSD (Sangen et al. 2019).
Quality of Life
QoL measures have been identified as important tools for measuring the benefits of cochlear implantation (Lin & Niparko 2006). To assess hearing-related QoL in the pediatric population, the HEAR-QL (Umansky et al. 2011) has been found to be a sensitive tool in children with SSD (Umansky et al. 2011; Griffin et al. 2019). The HEAR-QL-26 is a 26-item questionnaire designed for children aged 7 to 12 years and assesses QoL in three subscales: perceived difficulty hearing in certain environments/situations (Environments), impact of hearing loss on social/sports activities (Activities), and impact of hearing loss on the child’s feelings (Feelings). The HEAR-QL-28 is a 28-item questionnaire designed for adolescents aged 13 to 18 years and assesses QoL in four subscales: Family and Friends, Activities, School and Feelings. A preschool version of the HEAR-QL is still in development.
Hearing in Everyday Listening Environments
The Speech, Spatial, and Qualities of Hearing (SSQ) questionnaire (Gatehouse & Noble 2004) provides a means to obtain information about how an individual perceives these functions of hearing in their everyday listening environments. The SSQ targets hearing functions that rely on an intact binaural auditory system and is an appropriate tool to evaluate the efficacy of interventions for individuals with SSD. The SSQ has been widely used in adult (Galvin et al. 2019) and pediatric (Hassepass et al. 2012; Arndt et al. 2015; Beck et al. 2017; Thomas et al. 2017; Ramos Macías et al. 2019; Rauch et al. 2021) outcome studies of CI for SSD. Three versions of the SSQ have been adapted specifically for the pediatric population: SSQ-Parents, SSQ-Child, and SSQ-Teacher of the Deaf (Galvin & Noble 2013). The literature thus far has reported improvement in self-perceived benefit among pediatric SSD + CI patients between the pre and postactivation test intervals using the SSQ (Arndt et al. 2015; Beck et al. 2017; Thomas et al. 2017; Ramos Macías et al. 2019).
Academic Performance
Academic performance is an important metric for longitudinal research in this population as researchers are still learning about the impact of cochlear implantation on academic performance in children with SSD. The Screening Instrument for Targeting Educational Risk (SIFTER; Anderson 1989) is a commonly used tool both in the clinical and research settings. It has been found to be sensitive in its ability to detect differences between children with UHL and normal hearing (Dancer et al. 1995; Bess 1998). The child’s teacher compares how the child with hearing loss performs in the classroom compared to their peers on five domains: Academics, Attention, Communication, Class Participation, and School Behavior.
Listening Effort
Children with hearing loss exert disproportionately more effort to hear compared to peers with normal hearing, and thus are significantly more fatigued (Hornsby et al. 2017). Children with SSD experience listening fatigue similarly to children with bilateral hearing loss (Bess et al. 2020). The listening effort pragmatic subscale of the SSQ-Child has been successfully used to measure perceived changes in listening effort in children with SSD + CI (Lopez et al. 2021). At present, researchers at Vanderbilt University are developing a new scale to measure listening-related fatigue in children with hearing loss and address the paucity of self-report measures for listening effort. Like the SSQ, there are three versions: a child, a parent, and a teacher scale. Ranging from 8 to 12 items, the instruments use a 5-point Likert scale. This scale is in its final stages of development (Bess et al. 2020), with plans to be made freely available to clinicians. This questionnaire may prove to be a useful instrument to quantify potentially unmeasured benefits of CI for individuals with SSD.
Localization
The loss of binaural hearing significantly impacts localization (Humes et al. 1980; Johnstone et al. 2010), but most clinics are not able to assess this skill. The spatial hearing subtest of the SSQ has many questions targeting localization. The Auditory Behavior in Everyday Life (ABEL) from Purdy and colleagues (2002) also has questions to capture spatial hearing abilities.
Tinnitus
Measuring tinnitus severity has been a routine component of SSD + CI test batteries since the earliest studies published in 2008 (Van de Heyning et al. 2008). There are currently no pediatric measures to evaluate subjective tinnitus, despite the notable prevalence in 40% of children with UHL (Piotrowska et al. 2015). The Tinnitus Handicap Inventory (THI; Newman et al. 1996) is a self-report measure originally designed for use in adults but has been previously used in research studies on children as young as 8 years old (Smith et al. 2019). The THI has 25 questions that gauge the patient’s response to their tinnitus and thus the extent to which intervention is required. It has been used in adult outcome studies of SSD + CI (Gartrell et al. 2014; Dillon et al. 2017). The Tinnitus Functional Index (TFI; Meikle et al. 2012), also designed for use in adults but used with children as young as 8 years old (Smith et al., 2019), is a 25-question self-report scale with eight subscales: Intrusive, Sense of Control, Cognitive, Sleep, Auditory, Relaxation, QoL, and Emotional. The greater specificity in answer choices as well as the targeted subscales could make the TFI a preferred measure choice over the THI. The TFI has been suggested as the standard for evaluations of subjective experience of tinnitus in adults with SSD (Van de Heyning et al. 2016). With both measures, clinicians may need to use the questionnaires in an interview format with simplified language that is developmentally appropriate for the child. The development of a validated, tinnitus questionnaire for use specifically in children is greatly needed.
DEVICE PROGRAMMING
Device programming methods are similar to those recommended for children with bilateral hearing loss, with the ultimate goal of using electric stimulation to provide audibility for the development of speech recognition (Academy Task Force on Guidelines for Cochlear Implants 2019). As researchers learn more about how children with SSD use their CIs and how different programming strategies and methods affect outcomes, these recommendations will likely change. For example, there is emerging literature suggesting that programming based on approximate cochlear place frequency may impact outcomes and acceptance among individuals with SSD + CI (Dillon et al. 2019; Dorman et al. 2019).
There are a few general recommendations to consider when programming children with SSD + CI. First, children with SSD have experience with sound from birth and may be more comfortable with descriptive terminology surrounding loudness and pitch perception, especially as they have a typically hearing ear for comparison. Second, they are sensitive to sound around them during programming. When setting levels, it is important to occlude the normal-hearing ear to minimize distractions, and limit their ability to attend to cues (such as mouse or keyboard clicks) that may cause false positive responses (Buss et al. 2018). Electrically evoked stapedial reflex threshold (eSRT) measurements are a very useful tool for setting upper stimulation levels, especially in early days of implant use (Shapiro & Bradham 2012). Third, due to their experience with sound, children with SSD + CI may be able to tolerate higher stimulation levels at activation than children with bilateral hearing loss. Hence, they may not need as many progressive programs to adapt to CI use as typical pediatric recipients. Finally, it may be helpful to remove the plug or muff while the microphone is active to ensure equal loudness between ears. This practice has been advocated in the pediatric bimodal literature (Davidson et al. 2015), but it is unclear whether this is helpful for pediatric SSD recipients.
There are considerations for processor settings and streaming. The authors recommend use of an ear level processor as research has suggested that microphone placement is important for spatial hearing (Jones et al. 2016). It is reasonable to expect that the placement of the microphone on/in the pinna is more likely to mimic how the typically-hearing ear is collecting sound than an off-the-ear (OTE) device where the microphone is positioned at the magnet site. OTE device placement may further affect binaural processing by impacting interaural timing and level cues, particularly if the device is in a more posterior location. It is also important to enable the device’s streaming capabilities to support direct connect or wireless streaming to allow for isolation of the CI for testing and listening therapy. Research had not yet established a clear understanding of the role of microphone and input processing strategies, but it is important to consider as research continues to evolve.
HABILITATION
The amount and type of listening therapy necessary for children with SSD + CI has not been assessed directly. However, auditory-based therapy has historically been a hallmark of pediatric hearing intervention. Greaver et al. describe and recommend auditory therapy in the CI ear alone based on a hierarchy of auditory skills and a focus on development of binaural integration with bilateral listening (Greaver et al. 2017). Other pediatric studies mention that similar methods have been employed with participants (Rauch et al. 2021; Park et al. 2021a), although most do not mention whether subjects participated in therapy or not. Given the importance of auditory habilitation in pediatric CI users in general, it is prudent to recommend developmentally appropriate listening therapy for children with SSD + CI. This therapy should use direct connect techniques to isolate the CI ear (Dillon et al. 2017; Evans & Dillon 2019) and incorporate methods to encourage development of binaural skills such as spatial hearing (Greaver et al. 2017).
SUMMARY OF GUIDELINES FOR ASSESSMENT AND MANAGEMENT OF COCHLEAR IMPLANTION IN CHILDREN WITH SINGLE-SIDED DEAFNESS
Cochlear implantation to address SSD in an ear with cochlear nerve deficiency is contraindicated. Accurate diagnosis of nerve deficiency is important because it is present in almost half of children with SSD. Therefore, high resolution 3D MRI of the internal auditory canals is recommended rather than computer tomography alone.
Cochlear implantation should be considered a priority for children at risk of hearing loss progression in the better hearing ear. Children with SSD due to bacterial meningitis should be implanted promptly.
Younger age at implantation is expected to be advantageous in children with SSD. Children with longer lengths of deafness may experience fewer benefits and should be counseled as such. The impact of age and length of deafness is not yet fully understood in this population.
A CI evaluation is recommended for children with a unilateral three frequency pure tone average (3FPTA) of >60 dB HL and/or an aided SII < 0.65 because these children are unlikely to receive adequate benefit from traditional amplification.
Trials with re-routing devices are not recommended for children seeking binaural hearing as these devices are not able to provide the brain with bilateral input and the trial could delay a time-sensitive procedure.
Counseling for families considering SSD + CI should include information about developmental disadvantages of SSD such as the inability to develop spatial hearing in the absence of bilateral input, resultant difficulty with localizing sound and hearing in noise, and listening fatigue. Counseling should stress the importance of neuroplasticity and thus the potential advantage for a younger age at implantation to improve outcomes. Counseling should include a discussion of the importance of postimplant listening therapy, full-time use, reasonable expectations, and audiologic follow-up.
Candidacy test batteries should include age-appropriate behavioral assessment and cross-check, spatial hearing assessment in the child’s everyday listening condition, and relevant subjective questionnaires. Recorded aided word recognition testing with contralateral masking following the hierarchy recommended in Table 1 should be completed preoperatively if the child uses traditional amplification and/or if required by insurance.
Postactivation test batteries completed at regular intervals should include regular assessment of unaided hearing, validation of audibility from the CI, isolated single-word recognition using DAI, spatial hearing assessment with and without the CI, and relevant subjective questionnaires.
Evaluation of audibility in the sound field should be completed while using a plug-and-muff technique and screening in the plug-and-muff alone condition (with the processor off) to evaluate the possibility that thresholds are reflective of the occluded better hearing ear.
Spatial hearing assessments should be prioritized in children with SSD. This can be accomplished using SIN testing with three target-to-masker configurations including speech and masker collocated in front, speech in front with maker to the affected ear, and speech in front with masker to the better hearing ear.
Device programming considerations include plugging of the contralateral ear during mapping, use of eSRT, and considerations for rapid adaptation.
Auditory listening therapy based on a hierarchy of auditory skills and development of binaural integration is strongly recommended.
Clinicians who work with children who have SSD + CI should recognize that this is a rapidly evolving field and should keep abreast of the literature and current research outcomes.
CONCLUSION
Cochlear implantation is an effective intervention for children with SSD. As implantation becomes more common and research continues to emerge, clinicians are likely to gain more insight into best practices for candidacy, evaluation, programming, and therapy. This report outlines the current state of knowledge and provides a framework for clinically feasible assessment and intervention in children with SSD + CI (see Table, Supplemental Digital Content 1, http://links.lww.com/EANDH/A998, which summarizes these recommendations). It is important that clinicians stay up to date on the rapidly emerging literature for this population. At present, the pediatric literature is limited to an extent that precludes guideline development via a systematic review. The guidelines presented here were developed based on the available literature and clinician experience. As research in this evolving field becomes more prevalent, they should be revisited with a systematic review.
ACKNOWLEDGMENTS
NY: Advisory Board, Advanced Bionics; Surgical Advisory Board, MED-EL Corporation, USA. Research grant support to the University from MED-EL Corporation, USA (to L. R. P.).
This article was written by the American Cochlear Implant Alliance Task Force, which included authors Lisa Park, Amanda Griffin, Douglas Sladen, Sara Neumann, and Nancy Young. All authors contributed equally to this work, drafted sections, commented and edited throughout the process, and discussed implications.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and text of this article on the journal’s Web site (www.ear-hearing.com).
The authors have no conflicts of interest to disclose.
REFERENCES
- Academy Task Force on Guidelines for Cochlear Implants. Clinical Practice Guidelines: Cochlear Implants, (2019). American Academy of Audiology. [DOI] [PubMed] [Google Scholar]
- Adunka O. F., Roush P. A., Teagle H. F., Brown C. J., Zdanski C. J., Jewells V., Buchman C. A. Internal auditory canal morphology in children with cochlear nerve deficiency. Otol Neurotol, (2006). 27, 793–801. [DOI] [PubMed] [Google Scholar]
- American Academy of Audiology. Clinical guidance document: Assessment of hearing in infants and young children, (2020). Available at: https://www.audiology.org/sites/default/files/publications/resources/ClinGuidDoc_Assess_Hear_Infants_Children_1.23.20.pdf.
- American Academy of Audiology Task Force on Pediatric Amplification. Clinical Practice Guidelines: Pediatric Amplification, (2013). Reston, VA: American Academy of Audiology. Available at: https://www.audiology.org/sites/default/files/publications/PediatricAmplificationGuidelines.pdf. [Google Scholar]
- American Academy of Otolaryngology Head and Neck Surgery. Position Statement: Pediatric Cochlear Implants. (2020). Available at: https://www.entnet.org/content/position-statement-pediatric-cochlear-implants.
- Anderson K. SIFTER: Screening Instrument For Targeting Educational Risk in children identified by hearing screening or who have known hearing loss: User’s manual, (1989). Educational Audiology Association. [Google Scholar]
- Anne S., Lieu J. E. C., Cohen M. S. Speech and language consequences of unilateral hearing loss: A systematic review. Otolaryngol Head Neck Surg, (2017). 157, 572–579. [DOI] [PubMed] [Google Scholar]
- Arndt S., Prosse S., Laszig R., Wesarg T., Aschendorff A., Hassepass F. Cochlear implantation in children with single-sided deafness: Does aetiology and duration of deafness matter? Audiol Neurootol, (2015). 20(Suppl 1), 21–30. [DOI] [PubMed] [Google Scholar]
- Bagatto M., DesGeorges J., King A., Kitterick P., Laurnagaray D., Lewis D., Roush P., Sladen D. P., Tharpe A. M. Consensus practice parameter: Audiological assessment and management of unilateral hearing loss in children. Int J Audiol, (2019). 0, 1–11. [DOI] [PubMed] [Google Scholar]
- Bagatto M., Moodie S., Scollie S., Seewald R., Moodie S., Pumford J., Liu K. P. Clinical protocols for hearing instrument fitting in the Desired Sensation Level method. Trends Amplif, (2005). 9, 199–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagatto M. P., Moodie S. T., Malandrino A. C., Richert F. M., Clench D. A., Scollie S. D. The University of Western Ontario Pediatric Audiological Monitoring Protocol (UWO PedAMP). Trends Amplif, (2011). 15, 57–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck R. L., Aschendorff A., Hassepaß F., Wesarg T., Kröger S., Jakob T. F., Arndt S. Cochlear implantation in children with congenital unilateral deafness: A case series. Otol Neurotol, (2017). 38, e570–e576. [DOI] [PubMed] [Google Scholar]
- Bench J., Kowal A., Bamford J. The BKB (Bamford-Kowal-Bench) sentence lists for partially-hearing children. Br J Audiol, (1979). 13, 108–112. [DOI] [PubMed] [Google Scholar]
- Benchetrit L., Ronner E. A., Anne S., Cohen M. S. Cochlear implantation in children with single-sided deafness: A systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg, (2021). 147, 58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. H., Kieper R. W., Gauger D. Hearing protection: Surpassing the limits to attenuation imposed by the bone-conduction pathways. J Acoust Soc Am, (2003). 114(4 Pt 1), 1955–1967. [DOI] [PubMed] [Google Scholar]
- Bernstein J. G. W., Schuchman G. I., Rivera A. L. Head shadow and binaural squelch for unilaterally deaf cochlear implantees. Otol Neurotol, (2017). 38, e195–e202. [DOI] [PubMed] [Google Scholar]
- Bess F. H., Dodd-Murphy J., Parker R. A. Children with minimal sensorineural hearing loss: Prevalence, educational performance, and functional status. Ear Hear, (1998). 19, 339–354. [DOI] [PubMed] [Google Scholar]
- Bess F. H., Davis H., Camarata S., Hornsby B. W. Y. Listening-related fatigue in children with unilateral hearing loss. Lang Speech Hear Serv Sch, (2020). 51, 84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bess F. H., Tharpe A. M. Unilateral hearing impairment in children. Pediatrics, (1984). 74, 206–216. [PubMed] [Google Scholar]
- Bess F.H., Tharpe A.M., Gibler A.M. Auditory performance of children with unilateral sensorineural hearing loss. Ear Hear, (1986). 7, 20–26. [DOI] [PubMed] [Google Scholar]
- Brown K.D., Dillon M.T., Park L.R. Benefits of cochlear implantation in childhood unilateral hearing loss (CUHL Trial). [published online ahead of print Sept 20, 2021]. Laryngoscope. (2021). doi:10.1002/lary.29853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buechner A., Brendel M., Lesinski-Schiedat A., Wenzel G., Frohne-Buechner C., Jaeger B., Lenarz T. Cochlear implantation in unilateral deaf subjects associated with ipsilateral tinnitus. Otol Neurotol, (2010). 31, 1381–1385. [DOI] [PubMed] [Google Scholar]
- Buss E., Dillon M. T., Rooth M. A., King E. R., Deres E. J., Buchman C. A., Pillsbury H. C., Brown K. D. Effects of cochlear implantation on binaural hearing in adults with unilateral hearing loss. Trends Hear, (2018). 22, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M. L., Sladen D. P., Gurgel R. K., Tombers N. M., Lohse C. M., Driscoll C. L. Survey of the American Neurotology Society on cochlear implantation: Part 1, candidacy assessment and expanding indications. Otol Neurotol, (2018). 39, e12–e19. [DOI] [PubMed] [Google Scholar]
- Carlson M. L., Sladen D. P., Haynes D. S., Driscoll C. L., DeJong M. D., Erickson H. C., Sunderhaus L. W., Hedley-Williams A., Rosenzweig E. A., Davis T. J., Gifford R. H. Evidence for the expansion of pediatric cochlear implant candidacy. Otol Neurotol, (2015). 36, 43–50. [DOI] [PubMed] [Google Scholar]
- Casselman J. W., Bensimon J. L. Imaging of the inner ear. Radiologe, (1997). 37, 954–963. [DOI] [PubMed] [Google Scholar]
- Ching T. Y. C., Dillon H., Button L., Seeto M., Van Buynder P., Marnane V., Cupples L., Leigh G. Age at intervention for permanent hearing loss and 5-year language outcomes. Pediatrics, (2017). 140, e20164274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching T. Y., Hill M. The Parents’ Evaluation of Aural/Oral Performance of Children (PEACH) scale: Normative data. J Am Acad Audiol, (2007). 18, 220–235. [DOI] [PubMed] [Google Scholar]
- Clemmens C. S., Guidi J., Caroff A., Cohn S. J., Brant J. A., Laury A. M., Bilaniuk L. T., Germiller J. A. Unilateral cochlear nerve deficiency in children. Otolaryngol Head Neck Surg, (2013). 149, 318–325. [DOI] [PubMed] [Google Scholar]
- Cochlear Corporation. Cochlear Nucleus CI512 cochlear implant [package insert]. (2022). U.S. Food and Drug Administration website. https://www.accessdata.fda.gov/cdrh_docs/pdf/P970051S205D.pdf. Revised December 2021. Retrieved January 2022.
- Corbin N. E., Bonino A. Y., Buss E., Leibold L. J. Development of open-set word recognition in children: Speech-shaped noise and two-talker speech maskers. Ear Hear, (2016). 37, 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin N. E., Buss E., Leibold L. J. Spatial hearing and functional auditory skills in children with unilateral hearing loss. J Speech Lang Hear Res, (2021). 64, 4495–4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson J. L., Gilbert L. E. Children with unilateral sensorineural hearing loss: Cognitive, academic, and social development. Ear Hear, (1986). 7, 38–42. [DOI] [PubMed] [Google Scholar]
- Dancer J., Burl N.T., Waters S. Effects of unilateral hearing loss on teacher responses to the SIFTER. Am Ann Deaf, (1995). 140, 291–294. [DOI] [PubMed] [Google Scholar]
- Davidson L. S., Firszt J. B., Brenner C., Cadieux J. H. Evaluation of hearing aid frequency response fittings in pediatric and young adult bimodal recipients. J Am Acad Audiol, (2015). 26, 393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deep N. L., Gordon S. A., Shapiro W. H., Waltzman S. B., Roland J. T., Jr, Friedmann D. R. Cochlear implantation in children with single-sided deafness. Laryngoscope, (2021). 131, E271–E277. [DOI] [PubMed] [Google Scholar]
- Dewyer N.A., Smith S., Herrmann B., Reinshagen K. B., Lee D. J. Pediatric single-sided deafness: A review of prevalence, radiologic findings, and cochlear implant candidacy [published online ahead of print May 26, 2021]. Ann Otol Rhinol Laryngol. (2021). doi: 10.1177/00034894211019519. [DOI] [PubMed] [Google Scholar]
- Dillon M. T., Buss E., Anderson M. L., King E. R., Deres E. J., Buchman C. A., Brown K. D., Pillsbury H. C. Cochlear implantation in cases of unilateral hearing loss: initial localization abilities. Ear Hear, (2017). 38, 611–619. [DOI] [PubMed] [Google Scholar]
- Dillon M. T., Buss E., O’Connell B. P., Rooth M. A., King E. R., Bucker A. L., Deres E. J., McCarthy S. A., Pillsbury H. C., Brown K. D. Low-frequency hearing preservation with long electrode arrays: Inclusion of unaided hearing threshold assessment in the postoperative test battery. Am J Audiol, (2020). 29, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon M. T., Buss E., Rooth M. A., King E. R., Pillsbury H. C., Brown K. D. Low-frequency pitch perception in cochlear implant recipients with normal hearing in the contralateral ear. J Speech Lang Hear Res, (2019). 62, 2860–2871. [DOI] [PubMed] [Google Scholar]
- Dirks C., Nelson P. B., Sladen D. P., Oxenham A. J. Mechanisms of localization and speech perception with colocated and spatially separated noise and speech maskers under single-sided deafness with a cochlear implant. Ear Hear, (2019). 40, 1293–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman M. F., Cook Natale S., Baxter L., Zeitler D. M., Carlson M. L., Noble J. H. Cochlear place of stimulation is one determinant of cochlear implant sound quality. Audiol Neurootol, (2019). 24, 264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman M. F., Zeitler D., Cook S. J., Loiselle L., Yost W. A., Wanna G. B., Gifford R. H. Interaural level difference cues determine sound source localization by single-sided deaf patients fit with a cochlear implant. Audiol Neurotol, (2015). 20, 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ead B., Hale S., DeAlwis D., Lieu J. E. Pilot study of cognition in children with unilateral hearing loss. Int J Pediatr Otorhinolaryngol, (2013). 77, 1856–1860. [DOI] [PubMed] [Google Scholar]
- Easwar V., Sanfilippo J., Papsin B., Gordon K. Impact of consistency in daily device use on speech perception abilities in children with cochlear implants: Datalogging evidence. J Am Acad Audiol, (2018). 29, 835–846. [DOI] [PubMed] [Google Scholar]
- Ehrmann-Mueller D., Kurz A., Kuehn H., Rak K., Mlynski R., Hagen R., Shehata-Dieler W. Usefulness of cochlear implantation in children with single sided deafness. Int J Pediatr Otorhinolaryngol, (2020). 130, 109808. [DOI] [PubMed] [Google Scholar]
- Etymōtic Research BKB-SIN test. (2005). Speech-in-Noise Test Version 1.03. www.etymotic.com.
- Evans M., Dillon M. The assessment and aural rehabilitation tool for cochlear implant recipients with unilateral hearing loss. Perspect ASHA Spec Interes Groups, (2019). 4, 962–970. [Google Scholar]
- Firszt J.B., Holden L.K., Reeder R.M., Waltzman S. B., Arndt S. Auditory abilities after cochlear implantation in adults with unilateral deafness: A pilot study. Otol Neurotol, (2012). 33, 1339–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer C., Lieu J. Unilateral hearing loss is associated with a negative effect on language scores in adolescents. Int J Pediatr Otorhinolaryngol, (2014). 78, 1611–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick E. M., Al-Essa R. S., Whittingham J., Fitzpatrick J. Characteristics of children with unilateral hearing loss. Int J Audiol, (2017). 56, 819–828. [DOI] [PubMed] [Google Scholar]
- Friedmann D. R., Ahmed O. H., McMenomey S. O., Shapiro W. H., Waltzman S. B., Roland J. T., Jr. Single-sided deafness cochlear implantation: Candidacy, evaluation, and outcomes in children and adults. Otol Neurotol, (2016). 37, e154–e160. [DOI] [PubMed] [Google Scholar]
- Gagnon E. B., Eskridge H., Brown K. D. Pediatric cochlear implant wear time and early language development. Cochlear Implants Int, (2020). 21, 92–97. [DOI] [PubMed] [Google Scholar]
- Galvin J. J., 3rd, Fu Q. J., Wilkinson E. P., Mills D., Hagan S. C., Lupo J. E., Padilla M., Shannon R. V. Benefits of cochlear implantation for single-sided deafness: Data From the House Clinic-University of Southern California-University of California, Los Angeles Clinical Trial. Ear Hear, (2019). 40, 766–781. [DOI] [PubMed] [Google Scholar]
- Galvin K. L., Noble W. Adaptation of the speech, spatial, and qualities of hearing scale for use with children, parents, and teachers. Cochlear Implants Int, (2013). 14, 135–141. [DOI] [PubMed] [Google Scholar]
- Ganek H. V., Cushing S.L., Papsin B.C., Gordon K. A. Cochlear implant use remains consistent over time in children with single-sided deafness. Ear Hear, (2019). 41, 678–685. [DOI] [PubMed] [Google Scholar]
- Gartrell B.C., Jones H.G., Kan A., Gordon K. A. Investigating long-term effects of cochlear implantation in single-sided deafness. Otol Neurotol, (2014). 35, 1525–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatehouse S., Noble W. The Speech, Spatial and Qualities of Hearing Scale (SSQ). Int J Audiol, (2004). 43, 85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon K., Henkin Y., Kral A. Asymmetric hearing during development: The aural preference syndrome and treatment options. Pediatrics, (2015). 136, 141–153. [DOI] [PubMed] [Google Scholar]
- Greaver L., Eskridge H., Teagle H. F. B. Considerations for pediatric cochlear implant recipients with unilateral or asymmetric hearing loss: Assessment, device fitting, and habilitation. Am J Audiol, (2017). 26, 91–98. [DOI] [PubMed] [Google Scholar]
- Griffin A. M., Poissant S. F., Freyman R. L. Speech-in-noise and quality-of-life measures in school-aged children with normal hearing and with unilateral hearing loss. Ear Hear, (2019). 40, 887–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse S. D., Ross D. S., Dollard S. C. Congenital cytomegalovirus (CMV) infection as a cause of permanent bilateral hearing loss: A quantitative assessment. J Clin Virol, (2008). 41, 57–62. [DOI] [PubMed] [Google Scholar]
- Haffey T., Fowler N., Anne S. Evaluation of unilateral sensorineural hearing loss in the pediatric patient. Int J Pediatr Otorhinolaryngol, (2013). 77, 955–958. [DOI] [PubMed] [Google Scholar]
- Hassepass F., Achendorff A., Wesarag T., Kröger S., Laszig R., Beck R. L., Schild C., Arndt S. Unilateral deafness in children: Audiologic and subjective assessment of hearing ability after cochlear implantation. Otol Neurotol, (2012). 34, 53–60. [DOI] [PubMed] [Google Scholar]
- Van de Heyning P., Távora-Vieira D., Mertens G., et al. Towards a unified testing framework for single-sided deafness studies: A consensus paper. Audiol Neurotol, (2016). 21, 391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Heyning P., Vermeire K., Diebl M., Nopp P., Anderson I., De Ridder D. Incapacitating unilateral tinnitus in single-sided deafness treated by cochlear implantation. Ann Otol Rhinol Laryngol, (2008). 117, 645–652. [DOI] [PubMed] [Google Scholar]
- Hodge S. E., Thompson N. J., Park L. R., Brown K. D. Enlarged vestibular aqueduct: Hearing progression and cochlear implant candidacy in pediatric patients. Otol Neurotol, (2021). 42, 203–206. [DOI] [PubMed] [Google Scholar]
- Hoff S., Ryan M., Thomas D., Tournis E., Kenny H., Hajduk J., Young N. M. Safety and effectiveness of cochlear implantation of young children, including those with complicating conditions. Otol Neurotol, (2019). 40, 454–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder J. T., Sheffield S. W., Gifford R. H. Speech understanding in children with normal hearing: sound field normative data for babybio, BKB-SIN, and QuickSIN. Otol Neurotol, (2016). 37, e50–e55. [DOI] [PubMed] [Google Scholar]
- Hornsby B. W. Y., Gustafson S. J., Lancaster H., Cho S. J., Camarata S., Bess F. H. Subjective fatigue in children with hearing loss assessed using self- and parent-proxy report. Am J Audiol, (2017). 26(3S), 393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes L. E., Allen S. K., Bess F. H. Horizontal sound localization skills of unilaterally hearing-impaired children. Audiology, (1980). 19, 508–518. [DOI] [PubMed] [Google Scholar]
- Jerger J. F., Hayes D. The cross-check principle in pediatric audiometry. Arch Otolaryngol, (1976). 102, 614–620. [DOI] [PubMed] [Google Scholar]
- Johnstone P. M., Litovsky R. Y. Effect of masker type and age on speech intelligibility and spatial release from masking in children and adults. J Acoust Soc Am, (2006). 120, 2177–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone P. M., Nábĕlek A. K., Robertson V. S. Sound localization acuity in children with unilateral hearing loss who wear a hearing aid in the impaired ear. J Am Acad Audiol, (2010). 21, 522–534. [DOI] [PubMed] [Google Scholar]
- Jones H. G., Kan A., Litovsky R. Y. The effect of microphone placement on interaural level differences and sound localization across the horizontal plane in bilateral cochlear implant users. Ear Hear, (2016). 37, e341–e345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King K., Dillon M. T., O’Connell B. P., Brown K. D., Park L. R. Spatial release from masking in bimodal and bilateral pediatric cochlear implant recipients. Am J Audiol, (2021). 30, 67–75. [DOI] [PubMed] [Google Scholar]
- Kral A., Heid S., Hubka P., Tillein J. Unilateral hearing during development: Hemispheric specificity in plastic reorganizations. Front Syst Neurosci, (2013a). 7, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral A., Hubka P., Heid S., Tillein J. Single-sided deafness leads to unilateral aural preference within an early sensitive period. Brain, (2013b). 136(Pt 1), 180–193. [DOI] [PubMed] [Google Scholar]
- Kral A., Sharma A. Developmental neuroplasticity after cochlear implantation. Trends Neurosci, (2012). 35, 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau R., Small S. A. Effective masking levels for bone conduction auditory brainstem response stimuli in infants and adults with normal hearing. Ear Hear, (2020). 42, 443–455. [DOI] [PubMed] [Google Scholar]
- Leal C., Marriage J., Vickers D. Evaluating recommended audiometric changes to candidacy using the speech intelligibility index. Cochlear Implants Int, (2016). 17(Suppl 1), 8–12. [DOI] [PubMed] [Google Scholar]
- Leibold L. J., Buss E. Masked Speech Recognition in School-Age Children. Front Psychol, (2019). 10, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh J., Dettman S., Dowell R., Briggs R. Communication development in children who receive a cochlear implant by 12 months of age. Otol Neurotol, (2013). 34, 443–450. [DOI] [PubMed] [Google Scholar]
- Leigh J. R., Dettman S. J., Dowell R. C. Evidence-based guidelines for recommending cochlear implantation for young children: Audiological criteria and optimizing age at implantation. Int J Audiol, (2016). 55(Suppl 2), S9–S18. [DOI] [PubMed] [Google Scholar]
- Lightfoot G., Cairns A., Stevens J. Noise levels required to mask stimuli used in auditory brainstem response testing. Int J Audiol, (2010). 49, 794–798. [DOI] [PubMed] [Google Scholar]
- Lin F. R., Niparko J. K. Measuring health-related quality of life after pediatric cochlear implantation: A systematic review. Int J Pediatr Otorhinolaryngol, (2006). 70, 1695–1706. [DOI] [PubMed] [Google Scholar]
- Litovsky R. Y. Speech intelligibility and spatial release from masking in young children. J Acoust Soc Am, (2005). 117, 3091–3099. [DOI] [PubMed] [Google Scholar]
- Lopez E. M., Dillon M. T., Park L. R., Rooth M. A., Richter M. E., Thompson N. J., O’Connell B. P., Pillsbury H. C., Brown K. D. Influence of cochlear implant use on perceived listening effort in adult and pediatric cases of unilateral and asymmetric hearing loss. Otol Neurotol, (2021). 42, e1234–e1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCutcheon D., Pausch F., Füllgrabe C., Eccles R., van der Linde J., Panebianco C., Fels J., Ljung R. The contribution of individual differences in memory span and language ability to spatial release from masking in young children. J Speech Lang Hear Res, (2019). 62, 3741–3751. [DOI] [PubMed] [Google Scholar]
- Martin F. N., Champlin C. A., Chambers J. A. Seventh survey of audiometric practices in the United States. J Am Acad Audiol, (1998). 9, 95–104. [PubMed] [Google Scholar]
- McCreery R. The right time to go from hearing aid to cochlear implant. Hear J, (2014). 67, 30. [Google Scholar]
- McCreery R. W., Bentler R. A., Roush P. A. Characteristics of hearing aid fittings in infants and young children. Ear Hear, (2013). 34, 701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreery R. W., Spratford M., Kirby B., Brennan M. Individual differences in language and working memory affect children’s speech recognition in noise. Int J Audiol, (2017). 56, 306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay S., Gravel J. S., Tharpe A. M. Amplification considerations for children with minimal or mild bilateral hearing loss and unilateral hearing loss. Trends Amplif, (2008). 12, 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MED-EL Corporation. Mi1250 SYNCHRONY 2 Cochlear Implant [package insert]. (2019). U.S. Food and Drug Administration website. https://www.accessdata.fda.gov/cdrh_docs/pdf/P000025S104C.pdf. Revised July 2018. Retrieved January 2022.
- Meikle M. B., Henry J. A., Griest S. E., Stewart B. J., Abrams H. B., McArdle R., Myers P. J., Newman C. W., Sandridge S., Turk D. C., Folmer R. L., Frederick E. J., House J. W., Jacobson G. P., Kinney S. E., Martin W. H., Nagler S. M., Reich G. E., Searchfield G., Sweetow R., et al. The tinnitus functional index: Development of a new clinical measure for chronic, intrusive tinnitus. Ear Hear, (2012). 33, 153–176. [DOI] [PubMed] [Google Scholar]
- Meredith M. A., Rubinstein J. T., Sie K. C. Y., Norton S. J. Cochlear implantation in children with postlingual progressive steeply sloping high-frequency hearing loss. J Am Acad Audiol, (2017). 28, 913–919. [DOI] [PubMed] [Google Scholar]
- Mertens G., Kleine Punte A., De Bodt M., Van de Heyning P. Binaural auditory outcomes in patients with postlingual profound unilateral hearing loss: 3 years after cochlear implantation. Audiol Neurootol, (2015). 20(Suppl 1), 67–72. [DOI] [PubMed] [Google Scholar]
- Moeller M. P., Carr G., Seaver L., Stredler-Brown A., Holzinger D. Best practices in family-centered early intervention for children who are deaf or hard of hearing: An international consensus statement. J Deaf Stud Deaf Educ, (2013). 18, 429–445. [DOI] [PubMed] [Google Scholar]
- Morton C.C., Nance W.E. Newborn hearing screening — A silent revolution. N Engl J Med, (2006). 354, 2151–2164. [DOI] [PubMed] [Google Scholar]
- Newman C. W., Jacobson G. P., Spitzer J. B. Development of the tinnitus handicap inventory. Arch Otolaryngol Head Neck Surg, (1996). 122, 143–148. [DOI] [PubMed] [Google Scholar]
- Osman H., Sullivan J. R. Children’s auditory working memory performance in degraded listening conditions. J Speech Lang Hear Res, (2014). 57, 1503–1511. [DOI] [PubMed] [Google Scholar]
- Park L. R., Dillon M. T., Buss E., O’Connell B. P., Brown K. D. Spatial release from masking in pediatric cochlear implant recipients with single-sided deafness. Am J Audiol, (2021a). 30, 443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L. R., Preston E., Noxon A. S., Dillon M. T. Comparison of test methods to assess the implanted ear alone for pediatric cochlear implant recipients with single-sided deafness. Cochlear Implants Int, (2021b). 22, 283–290. [DOI] [PubMed] [Google Scholar]
- Park L. R., Teagle H. F. B., Gagnon E., Woodard J., Brown K.D. Electric-acoustic stimulation outcomes in children. Ear Hear, (2019a). 40, 849–857. [DOI] [PubMed] [Google Scholar]
- Park L.R., Gagnon E.B., Thompson E., Brown K. D. Age at full-time use predicts language outcomes better than age of surgery in children who use cochlear implants. Am J Audiol, (2019b). 28, 986–992. [DOI] [PubMed] [Google Scholar]
- Piotrowska A., Raj-Koziak D., Lorens A., Skarżyński H. Tinnitus reported by children aged 7 and 12 years. Int J Pediatr Otorhinolaryngol, (2015). 79, 1346–1350. [DOI] [PubMed] [Google Scholar]
- Polonenko M. J., Gordon K. A., Cushing S. L., Papsin B. C. Cortical organization restored by cochlear implantation in young children with single sided deafness. Sci Rep, (2017). 7, 16900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Propst E. J., Greinwald J. H., Schmithorst V. Neuroanatomic differences in children with unilateral sensorineural hearing loss detected using functional magnetic resonance imaging. Arch Otolaryngol Head Neck Surg, (2010). 136, 22–26. [DOI] [PubMed] [Google Scholar]
- Purdy S. C., Farrington D. R., Moran C. A., Chard L. L., Hodgson S. A. A parental questionnaire to evaluate children’s Auditory Behavior in Everyday Life (ABEL). Am J Audiol, (2002). 11, 72–82. [DOI] [PubMed] [Google Scholar]
- Rajan G., Tavora-Vieira D., Baumgartner W. D., Godey B., Müller J., O’Driscoll M., Skarzynski H., Skarzynski P., Usami S. I., Adunka O., Agrawal S., Bruce I., De Bodt M., Caversaccio M., Pilsbury H., Gavilán J., Hagen R., Hagr A., Kameswaran M., Karltorp E., et al. Hearing preservation cochlear implantation in children: The HEARRING Group consensus and practice guide. Cochlear Implants Int, (2018). 19, 1–13. [DOI] [PubMed] [Google Scholar]
- Ramos Macías Á., Borkoski-Barreiro S. A., Falcón González J. C., de Miguel Martínez I., Ramos de Miguel Á. Single-sided deafness and cochlear implantation in congenital and acquired hearing loss in children. Clin Otolaryngol, (2019). 44, 138–143. [DOI] [PubMed] [Google Scholar]
- Rauch A. K., Arndt S., Aschendorff A., Beck R., Speck I., Ketterer M. C., Jakob T. F., Hassepass F. Long-term results of cochlear implantation in children with congenital single-sided deafness. Eur Arch Otorhinolaryngol, (2021). 278, 3245–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeser R., Clark J. Live voice speech recognition audiometry - Stop the madness. Audiol Today, (2008). 20, 32–33. [Google Scholar]
- Roland J., Shapiro W., Waltzman S. Cochlear implantation as a treatment option for single-sided deafness: Speech perception benefit. Audiol Neurotol, (2011). 16, 8–9. [Google Scholar]
- Roland L., Fischer C., Tran K., et al. Quality of life in children who are hearing impaired: Systematic review and meta analysis. Am Acad Otorlarngology, (2016). 155, 208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross D. S., Visser S. N., Holstrum W. J., Qin T., Kenneson A. Highly variable population-based prevalence rates of unilateral hearing loss after the application of common case definitions. Ear Hear, (2010). 31, 126–133. [DOI] [PubMed] [Google Scholar]
- Sangen A., Dierckx A., Boudewyns A., Dhooge I., Offeciers E., Wouters J., Desloovere C., van Wieringen A. Longitudinal linguistic outcomes of toddlers with congenital single-sided deafness-Six with and twelve without cochlear implant and nineteen normal hearing peers. Clin Otolaryngol, (2019). 44, 671–676. [DOI] [PubMed] [Google Scholar]
- Sangen A., Royackers L., Desloovere C., Wouters J., van Wieringen A. Single-sided deafness affects language and auditory development – A case–control study. Clin Otolaryngol, (2017). 42, 979–987. [DOI] [PubMed] [Google Scholar]
- Schafer E. Speech perception in noise measures for children: A critical review and case studies. J Educ Audiol, (2010). 16, 4–15. [Google Scholar]
- Schafer E. C., Beeler S., Ramos H., Morais M., Monzingo J., Algier K. Developmental effects and spatial hearing in young children with normal-hearing sensitivity. Ear Hear, (2012). 33, e32–e43. [DOI] [PubMed] [Google Scholar]
- Schmithorst V. J., Plante E., Holland S. Unilateral deafness in children affects development of multi-modal modulation and default mode networks. Front Hum Neurosci, (2014). 8, 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seewald R., Moodie S., Scollie S., Bagatto M. The DSL method for pediatric hearing instrument fitting: Historical perspective and current issues. Trends Amplif, (2005). 9, 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selleck A. M., Brown K. D., Park L. R. Cochlear implantation for unilateral hearing loss. Otolaryngol Clin North Am, (2021). 54, 1193–1203. [DOI] [PubMed] [Google Scholar]
- Sevier J. D., Choi S., Hughes M. L. Use of direct-connect for remote speech-perception testing in cochlear implants. Ear Hear, (2019). 40, 1162–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro W. H., Bradham T. S. Cochlear implant programming. Otolaryngol Clin North Am, (2012). 45, 111–127. [DOI] [PubMed] [Google Scholar]
- Shargorodsky J., Curhan S.G., Curhan G.C., Eavey R. Change in prevalence of hearing loss in US adolescents. JAMA, (2010). 304, 772–778. [DOI] [PubMed] [Google Scholar]
- Sharma A., Dorman M.F., Spahr A.J. A sensitive period for the development of the central auditory system in children with cochlear implants: Implications for age of implantation. Ear Hear, (2002). 23, 532–539. [DOI] [PubMed] [Google Scholar]
- Skarzynski H., Lorens A. Electric acoustic stimulation in children. Adv Otorhinolaryngol, (2010). 67, 135–143. [DOI] [PubMed] [Google Scholar]
- Sladen D. P., Frisch C. D., Carlson M. L., Driscoll C. L., Torres J. H., Zeitler D. M. Cochlear implantation for single-sided deafness: A multicenter study. Laryngoscope, (2017). 127, 223–228. [DOI] [PubMed] [Google Scholar]
- Smith C., Drexler J. Phonak Compendium: A Review of Unilateral Hearing Loss in Children, (2018). Warrenville, IL: Phonak. [Google Scholar]
- Smith H., Fackrell K., Kennedy V., Barry J., Partridge L., Hoare D. J. A scoping review to catalogue tinnitus problems in children. Int J Pediatr Otorhinolaryngol, (2019). 122, 141–151. [DOI] [PubMed] [Google Scholar]
- Spahr A. J., Dorman M. F., Litvak L. M., Cook S. J., Loiselle L. M., DeJong M. D., Hedley-Williams A., Sunderhaus L. S., Hayes C. A., Gifford R. H. Development and validation of the pediatric AzBio sentence lists. Ear Hear, (2014). 35, 418–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles D. J., Bentler R. A., McGregor K. K. The Speech Intelligibility Index and the pure-tone average as predictors of lexical ability in children fit with hearing AIDS. J Speech Lang Hear Res, (2012). 55, 764–778. [DOI] [PubMed] [Google Scholar]
- Sullivan C. B., Al-Qurayshi Z., Zhu V., Liu A., Dunn C., Gantz B. J., Hansen M. R. Long-term audiologic outcomes after cochlear implantation for single-sided deafness. Laryngoscope, (2020). 130, 1805–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Távora-Vieira D., Rajan G. P. Cochlear implantation in children with congenital and noncongenital unilateral deafness. Otol Neurotol, (2015). 36, 1457–1458. [DOI] [PubMed] [Google Scholar]
- Teagle H. F. B., Park L. R., Brown K. D., Zdanski C., Pillsbury H. C. Pediatric cochlear implantation: A quarter century in review. Cochlear Implants Int, (2019). 20, 288–298. [DOI] [PubMed] [Google Scholar]
- The Joint Committee on Infant Hearing. Year 2019 position statement: Principles and guidelines for early hearing detection and intervention programs. J Early Hear Detect Interv, (2019). 42, 1–44. [Google Scholar]
- Thomas J. P., Neumann K., Dazert S., Voelter C. Cochlear implantation in children with congenital single-sided deafness. Otol Neurotol, (2017). 38, 496–503. [DOI] [PubMed] [Google Scholar]
- Tibbetts K., Ead B., Umansky A., Coalson R., Schlaggar B. L., Firszt J. B., Lieu J. E. C. Inter-regional brain interactions in children with unilateral hearing loss. Otolaryngol Head Neck Surg, (2011). 144, 602–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillman T.W., Carhart R. An expanded test for speech discrimination utilizing CNC monosyllabic words: Northwestern University Auditory Test No. 6, (1966). Brooks Air Force Base. [DOI] [PubMed] [Google Scholar]
- Tomblin J. B., Harrison M., Ambrose S. E., Walker E. A., Oleson J. J., Moeller M. P. Language outcomes in young children with mild to severe hearing loss. Ear Hear, (2015). 36(Suppl 1), 76S–91S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiakpini L., Weichbold V., Kuehn-Inacker H. LittlEARS Auditory Questionnaire, (2004). Innsbruck, Austria: MED-EL. [Google Scholar]
- Uhler K., Biever A., Gifford R. H. Method of speech stimulus presentation impacts pediatric speech recognition: monitored live voice versus recorded speech. Otol Neurotol, (2016). 37, e70–e74. [DOI] [PubMed] [Google Scholar]
- Uhler K., Warner-Czyz A., Gifford R., Working Group P. Pediatric minimum speech test battery. J Am Acad Audiol, (2017). 28, 232–247. [DOI] [PubMed] [Google Scholar]
- Umansky A. M., Jeffe D. B., Lieu J. E. The HEAR-QL: Quality of life questionnaire for children with hearing loss. J Am Acad Audiol, (2011). 22, 644–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Labor. PPE Selection - Hearing Protection. (1993). https://www.osha.gov/SLTC/etools/shipyard/ship_breaking/ppe/general_ppe/hearing_protection.html. Accessed October 3, 2020.
- Usami S. I., Kitoh R., Moteki H., Nishio S. Y., Kitano T., Kobayashi M., Shinagawa J., Yokota Y., Sugiyama K., Watanabe K. Etiology of single-sided deafness and asymmetrical hearing loss. Acta Otolaryngol, (2017). 137(sup565), S2–S7. [DOI] [PubMed] [Google Scholar]
- Uwiera T. C., DeAlarcon A., Meinzen-Derr J., Cohen A. P., Rasmussen B., Shott G., Greinwald J. Hearing loss progression and contralateral involvement in children with unilateral sensorineural hearing loss. Ann Otol Rhinol Laryngol, (2009). 118, 781–785. [PubMed] [Google Scholar]
- Vanderauwera J., Hellemans E., Verhaert N. Research insights on neural effects of auditory deprivation and restoration in unilateral hearing loss: A systematic review. J Clin Med, (2020). 9, E812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadarajan V. V., Sydlowski S. A., Li M. M., Anne S., Adunka O. F. Evolving criteria for adult and pediatric cochlear implantation. Ear Nose Throat J, (2020). 100, 31–37. [DOI] [PubMed] [Google Scholar]
- Vermeire K., Van de Heyning P. Binaural hearing after cochlear implantation in subjects with unilateral sensorineural deafness and tinnitus. Audiol Neurootol, (2009). 14, 163–171. [DOI] [PubMed] [Google Scholar]
- Vincent C., Arndt S., Firszt J. B., Fraysse B., Kitterick P. T., Papsin B. C., Snik A., Van de Heyning P., Deguine O., Marx M. Identification and evaluation of cochlear implant candidates with asymmetrical hearing loss. Audiol Neurootol, (2015). 20(Suppl 1), 87–89. [DOI] [PubMed] [Google Scholar]
- van Wieringen A., Boudewyns A., Sangen A., Wouters J., Desloovere C. Unilateral congenital hearing loss in children: Challenges and potentials. Hear Res, (2019). 372, 29–41. [DOI] [PubMed] [Google Scholar]
- Wilson K., Ambler M., Hanvey K., Jenkins M., Jiang D., Maggs J., Tzifa K. Cochlear implant assessment and candidacy for children with partial hearing. Cochlear Implants Int, (2016). 17(Suppl 1), 66–69. [DOI] [PubMed] [Google Scholar]
- Wilson R. H., Carnell C. S., Cleghorn A. L. The Words-in-Noise (WIN) test with multitalker babble and speech-spectrum noise maskers. J Am Acad Audiol, (2007a). 18, 522–529. [DOI] [PubMed] [Google Scholar]
- Wilson R. H., Farmer N. M., Gandhi A., Shelburne E., Weaver J. Normative data for the Words-in-Noise Test for 6- to 12-year-old children. J Speech Lang Hear Res, (2010). 53, 1111–1121. [DOI] [PubMed] [Google Scholar]
- Wilson R. H., McArdle R. Intra- and inter-session test, retest reliability of the Words-in-Noise (WIN) test. J Am Acad Audiol, (2007). 18, 813–825. [DOI] [PubMed] [Google Scholar]
- Wilson R. H., McArdle R. A., Smith S. L. An evaluation of the BKB-SIN, HINT, QuickSIN, and WIN materials on listeners with normal hearing and listeners with hearing loss. J Speech Lang Hear Res, (2007b). 50, 844–856. [DOI] [PubMed] [Google Scholar]
- Wolfe J., Neumann S., Schafer E., Marsh M., Wood M., Baker R. S. Potential benefits of an integrated electric-acoustic sound processor with children: A preliminary report. J Am Acad Audiol, (2017). 28, 127–140. [DOI] [PubMed] [Google Scholar]
- Young N. M., Kim F. M., Ryan M. E., Tournis E., Yaras S. Pediatric cochlear implantation of children with eighth nerve deficiency. Int J Pediatr Otorhinolaryngol, (2012). 76, 1442–1448. [DOI] [PubMed] [Google Scholar]
- Zeitler D. M., Sladen D. P., DeJong M. D., Torres J. H., Dorman M. F., Carlson M. L. Cochlear implantation for single-sided deafness in children and adolescents. Int J Pediatr Otorhinolaryngol, (2019). 118, 128–133. [DOI] [PubMed] [Google Scholar]
- Zhan K. Y., Findlen U. M., Allen D. Z., Shannon M. K., Mattingly J. K., Adunka O. F. Therapeutic challenges and clinical characteristics of single-sided deafness in children. Int J Pediatr Otorhinolaryngol, (2020). 135, 110116. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Mao Z., Feng S., Liu X., Lan L., Zhang J., Yu X. Altered functional networks in long-term unilateral hearing loss: A connectome analysis. Brain Behav, (2018). 8, e00912. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.