Abstract
Objective:
While serum bone turnover markers (BTMs) and bone mineral density (BMD) have been confirmed as useable risk assessment tools for postmenopausal osteoporosis, the associations between BTMs and BMD changes are still ambiguous. The aim of this study was to explore the underlying associations between BTMs and BMD changes in postmenopausal women.
Methods:
Between January 2015 and October 2020, 135 postmenopausal women were retrospectively enrolled. They were divided into two groups according to lumbar spine (LS) 1-4 BMD change (1 y T-score minus baseline T-score, Group 1 [n = 36] < 0 and Group 2 [n = 99] ≥ 0). The changes of BTMs (N-terminal middle segment osteocalcin [N-MID], propeptide of type I procollagen [P1NP], and β-C-terminal telopeptide of type I collagen [β-CTX]) and their associations with LS 1-4 BMD change were analyzed. The biochemical indices and clinical parameters related with LS 1-4 BMD change were also evaluated.
Results:
The 1 year N-MID, P1NP, β-CTX and Phosphorus in Group 2 were lower than those in Group 1 (P < 0.05), their changes within 1 year were significantly negatively correlated with LS 1-4 BMD change (R2 = –0.200, P < 0.001; R2 = –0.230, P < 0.001; R2 = –0.186, P < 0.001; R2 = –0.044, P = 0.015; respectively). Except for the Phosphorus change (area under the curve [AUC] = 0.623), the changes of N-MID, P1NP, and β-CTX and their 1 year levels had similar AUC to diagnose the short-term LS 1-4 BMD change (AUC > 0.7 for all, with the AUC of 1 y P1NP being the largest at 0.803). Binary logistic regression analysis showed that the physical activity and drug intervention were the determinant factors for the LS 1-4 BMD change (odds ratio = 6.856, 95% confidence interval: 2.058-22.839, P = 0.002; odds ratio = 5.114, 95% confidence interval: 1.551-16.864, P = 0.007; respectively).
Conclusions:
Declining N-MID, P1NP, β-CTX, and Phosphorus are associated with the short-term increase of LS 1-4 BMD within 1 year. Physical activity and drug intervention are factors significantly influencing the change of LS 1-4 BMD in postmenopausal women.
Keywords: Bone mineral density, Bone turnover marker, Drug intervention, Osteoporosis, Physical activity, Postmenopausal women
Estrogen withdrawal is considered to be an important cause of accelerated bone turnover and subsequent osteoporosis in postmenopausal women.1 Recent studies have reported that the prevalence of osteoporosis among Chinese women over 50 years of age has reached 29.1%, equating to 49 million women.2 Osteoporotic fracture (OF) is an endpoint of postmenopausal osteoporosis (PMOP). By 2050, it is estimated that $25.4 billion will be spent annually on the treatment of OF.3 The COVID-19 pandemic has certainly worsened this reality.4 With the increase in medical costs and disability and mortality rates, tremendous pressure and unprecedented challenges are put on the clinical and public health system. Therefore, it is particularly important to study bone mineral density (BMD) changes in postmenopausal women and take timely intervention measures to minimize the harm of PMOP to human health.
BMD measured by dual-energy X-ray absorptiometry (DXA) is an international method for the clinical evaluation of bone mass. The heritability of BMD ranges from 0.50 to 0.85, which could be used to predict osteoporosis and assess the risk of OF.5,6 Baseline BMD before treatment provides a reference for the occurrence and severity of PMOP, whereas serial BMD measurements offer supporting evidence for monitoring bone mass change and formulating treatment measures. However, according to the 2013 International Osteoporosis Foundation Asia Pacific Audit report, there is less than 1 DXA system per million people in China.7 This means that people who have received a baseline BMD assessment are likely forced to interrupt subsequent evaluations. Meanwhile, due to the small gap in the identification of subsequent BMD changes and OF, the value of serial BMD monitoring within specific populations is met with skepticism.8,9
Serum bone turnover markers (BTMs) are ideal noninvasive biomarkers reflecting the state of bone metabolism. They are relatively stable in body fluids and can be easily detected by existing quantitative techniques. Compared to BMD, BTMs can directly reflect the change in bone metabolism and an individual's response to treatment before bone morphological change.10,11 Some studies have shown that increased levels of certain BTMs are significantly associated with declining lumbar BMD in older women12 and may even indicate a high risk of subsequent OF,13 whereas a decline in BTMs posttreatment reduces the incidence of such adverse events.14
Despite the value of BTMs and BMD in the evaluation of bone health having been well demonstrated in previous investigations, only limited cross-sectional studies have shown the correlations between them. More evidence is needed to clarify the association between the changes of BTMs and BMD relative to an initial baseline assessment in postmenopausal women. Such studies will provide a theoretical basis for clinicians to better evaluate bone mass based on BTMs, especially in situations where BMD results are not available. The aim of this study was to explore the underlying associations between BTMs and BMD in postmenopausal women.
METHODS
Participant
This study was a retrospective study and approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University (No. [2020]150). It included Chinese postmenopausal women who were examined by DXA at our hospital and followed up for approximately 1 year between January 2015 and October 2020. Clinical data were obtained from the outpatient or inpatient Electronic Medical Record of the First Affiliated Hospital of Sun Yat-sen University. The baseline and subsequent follow-up data included: (1) age; (2) height; (3) weight; (4) menopausal duration; (5) drug intervention records; (6) lifestyle; (7) BMD; and (8) serum BTMs and biochemical indices, including 25-hydroxy vitamin D (25[OH]D), N-terminal middle segment osteocalcin (N-MID), propeptide of type I procollagen (P1NP), β-C-terminal telopeptide of type I collagen (β-CTX), uric acid (UA), alkaline phosphatase (ALP), Calcium, Phosphorus, Magnesium, acid phosphatase (ACP), nonprostatic acid phosphatase (NACP), and prostatic acid phosphatase (PACP). Body mass index was also calculated (kg/m2).
The inclusion criteria were: (1) age of ≥50 years; (2) menopausal duration of ≥1 year; and (3) complete clinical data.
The exclusion criteria were: (1) any co-morbidity that could significantly affect bone metabolism, eg, thyroid disease, diabetes, cancer, kidney disease, and ankylosing spondylitis; (2) OF occurrence in the past or during the follow-up period; (3) previous treatment with anti-osteoporosis drugs; (4) previous treatment with hormones, eg, estrogen or glucocorticoid; or (5) a stop or change in treatment and lifestyle during the follow-up period.
A flowchart of the participant selection is showed in Supplemental Digital Content Figure 1.
BMD assessment and grouping
The BMD (g/cm2) and T-score were recorded for all selected participants at the lumbar spine (LS) 1-4, total hip (TH), and femoral neck (FN) using a Lunar iDXA dual-energy X-ray absorptiometer (GE Healthcare, Chicago, IL). Before use, the device was calibrated according to the manufacturer's standard procedures, and all measurements were performed per the manufacturer's recommendations. The coefficients of variation (CVs) of BMD measurements of the LS 1-4, TH, and FN were 0.8%, 0.8%, and 1.4%, respectively. The participants were divided into two groups according to the difference of the T-scores of LS 1-4 (difference = 1 y T-score – baseline T-score): Group 1 with difference < 0 and Group 2 with difference ≥ 0.
Biochemical and immunological analysis
Blood samples were obtained in the morning via the antecubital vein of participants (after fasting for ≥8 h) and were sent to the department laboratory within 3 hours for postprocessing. The analyzers were calibrated daily before the analysis of all serum samples using quality control standards provided by the manufacturers. Quantitative analysis of serum UA, ALP, Calcium, Phosphorus, Magnesium, ACP, NACP, and PACP was carried out using an AU5800 automatic biochemistry analyzer and its corresponding reagents (Beckman Coulter, Pasadena, CA), with intra- and inter-assay CVs ranging from 0.5% to 4.9%. The levels of serum 25(OH)D, N-MID, P1NP, and β-CTX were measured using a Cobas 6000 analyzer series and its corresponding reagents (Roche, Basel, Switzerland), with intra- and inter-assay CVs ranging from 0.6% to 4.3%.
Classification of lifestyles
Smoking behavior, alcohol intake, and physical activity in daily life were included to describe the difference in lifestyle between the two groups. Concerning smoking behavior and alcohol intake, “Never” referred to no smoking or drinking during the follow-up period, whereas “Past and present” referred to smoking or drinking during the follow-up period, even if only once. “Cannot meet daily needs” referred to movement inadequate to meet the needs of daily life (shopping, climbing stairs, or exercising), whereas “Normal activity” referred to adequate movement to meet these needs.
Clinical drug intervention
The choices of drugs used for treatment were made by doctors depending on the person's disease type, age, stage of disease, and adverse drug reaction. The drug types and doses in this study were as follows: (1) calcium carbonate and vitamin D3 Tablets (calcium, 600 mg; vitamin D3, 125IU): one tablet once a day; (2) calcitriol soft capsules (calcitriol, 0.25 μg): one capsule two times a day; (3) alendronate sodium and vitamin D3 tablets (Ale; alendronic acid, 70 mg; vitamin D3, 2800IU): one tablet once a week; and (4) zoledronic acid injection (Zol; zoledronic acid, 5 mg): one bottle once a year. Among them, the former two were classified as basic drugs (Bd) in the treatment of PMOP, whereas the latter two were classified as antiresorptive drugs (inhibiting bone absorption).
Statistical analysis
SPSS software (version 22, IBM, Armonk, New York, NY) was used for data analysis. Continuous variables are presented as mean ± standard deviation. Comparisons between the two groups were made via an independent samples t test. The paired t test was used for the comparison of baseline and 1 year follow-up data within groups. The chi-square test was used to compare the gap in lifestyle and treatment between the two groups. The correlations between clinical parameters and LS 1-4 BMD change of 135 participants were analyzed. Normality was tested with the Shapiro-Wilk normality test and Spearman correlations were used, instead of Pearson, if data were not normal. The correlation coefficient was expressed by the linear regression coefficient (R2). The receiver operating characteristic (ROC) curve was constructed to evaluate the diagnostic effectiveness of the selected variable for BMD change. The area under the curve (AUC) was used as an accuracy index for evaluating the diagnostic performance of the variable. Diagnostic parameters, such as sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic efficiency (DE), were calculated for each diagnostic method.15 The Kappa value was determined to assess the extent of consistency. Kappa values <0.40, 0.41-0.60, 0.61-0.80, and >0.80 were considered as fair, moderate, substantial, and near-perfect agreement, respectively.16 Binary logistic regression was used to evaluate the influence factors of LS 1-4 BMD change. Collinearity diagnostics were examined for potential presence of collinearity between independent variables. P < 0.05 was considered a statistically significant difference.
RESULTS
Participant demographic and clinical characteristics
A total of 135 participants were enrolled, involving 36 women in Group 1 (26.7%) and 99 women in Group 2 (73.3%), and the average follow-up period was 12.3 ± 1.5 months. Table 1 shows the baseline characteristics of the two groups. There were no statistically significant differences in age, body mass index, menopausal duration, and follow-up time between the two groups (P > 0.05). The baseline BTMs and biochemical indices were all within the normal reference ranges and also showed no statistically significant difference (P > 0.05). This means that the variables included in the comparison are comparable. The baseline BMD of LS 1-4, TH, and FN in Group 1 was significantly higher than those in Group 2 (P < 0.05).
TABLE 1.
Baseline characteristics of general clinical data, serum BTMs, and biochemical indices in the two groups
| Variable | Reference Range | Group 1 (n = 36) | Group 2 (n = 99) | t | P |
| Age (y) | - | 65.3 ± 8.5 | 66.9 ± 8.3 | −0.951 | 0.344 |
| BMI (kg/m2) | - | 22.1 ± 2.9 | 22.9 ± 3.7 | −1.189 | 0.237 |
| Menopausal duration (y) | - | 13.2 ± 8.2 | 14.7 ± 8.2 | −0.071 | 0.944 |
| Follow-up time (mo) | - | 12.5 ± 1.8 | 12.2 ± 1.4 | 0.841 | 0.404 |
| LS 1-4 BMD (g/cm2) | - | 0.964 ± 0.184 | 0.884 ± 0.163 | 2.430 | 0.016 |
| TH BMD (g/cm2) | - | 0.825 ± 0.123 | 0.764 ± 0.123 | 2.537 | 0.012 |
| FN BMD (g/cm2) | - | 0.749 ± 0.106 | 0.703 ± 0.114 | 2.132 | 0.035 |
| 25(OH)D (ng/mL) | >25 | 29.9 ± 13.1 | 26.8 ± 8.2 | 1.326 | 0.191 |
| N-MID (ng/mL) | 14-46 | 15.70 ± 6.86 | 17.23 ± 8.03 | −1.013 | 0.313 |
| P1NP (ng/mL) | 0.00-36.40 | 36.22 ± 18.80 | 39.13 ± 21.20 | −0.726 | 0.469 |
| β-CTX (ng/mL) | ≤1.008 | 0.306 ± 0.212 | 0.329 ± 0.232 | −0.533 | 0.595 |
| UA (μmol/L) | 140-360 | 319.9 ± 80.2 | 322.0 ± 68.8 | −0.149 | 0.882 |
| ALP (U/L) | 0-110 | 69.2 ± 14.0 | 74.5 ± 19.9 | –1.470 | 0.144 |
| Calcium (mmol/L) | 2.10-2.60 | 2.31 ± 0.10 | 2.28 ± 0.11 | 1.576 | 0.118 |
| Phosphorus (mmol/L) | 0.97-1.62 | 1.12 ± 0.13 | 1.13 ± 0.15 | −0.287 | 0.774 |
| Magnesium (mmol/L) | 0.70-1.10 | 0.90 ± 0.07 | 0.88 ± 0.08 | 1.669 | 0.097 |
| ACP (U/L) | 0.00-10.00 | 7.9 ± 2.0 | 8.1 ± 1.9 | −0.712 | 0.477 |
| NACP (U/L) | 0.00-6.50 | 4.3 ± 1.0 | 4.4 ± 1.0 | −0.210 | 0.834 |
| PACP (U/L) | 0.00-3.50 | 3.7 ± 0.7 | 3.8 ± 0.9 | −0.333 | 0.739 |
Data are presented as mean ± standard deviation. All P values were calculated with the t test. P value < 0.05 was considered to indicate a statistically significant difference (highlighted in bold). - indicates none.
ACP, acid phosphatase; ALP, alkaline phosphatase; β-CTX, β-C-terminal telopeptide of type I collagen; BMD, bone mineral density; 25(OH)D, 25-hydroxy vitamin D; BMI, body mass index; BTMs, bone turnover markers; FN, femoral neck; LS, lumbar spine; NACP, non-prostatic acid phosphatase; N-MID, N-terminal middle segment osteocalcin; PACP, prostatic acid phosphatase; P1NP, propeptide of type I procollagen; TH, total hip; UA, uric acid.
The differences in lifestyle and drug intervention between the two groups
The rates of smoking and drinking in Group 1 (36.1% and 52.8%, respectively) were higher than those in Group 2 (19.2% and 26.3%, respectively), whereas the normal physical activity rate in Group 1 was lower than that in Group 2 (44.4% vs 81.8%), and both of these differences were statistically significant (P < 0.05). Concerning treatments, compared to Group 2, untreated women or those treated with Bds alone accounted for a greater proportion in Group 1 (58.3%), and the number of women treated with antiresorptive drugs with or without Bds in Group 2 was nearly double that in Group 1 (76.8% vs 41.7%). The treatment composition of the participants in the two groups showed a significant difference (P < 0.001; Table 2).
TABLE 2.
Comparison of the lifestyles and drug interventions of the two groups within 1 year follow-up
| Variable | Group 1 (n = 36) | Group 2 (n = 99) | χ2 | P |
| Lifestyle | ||||
| Smoking behavior | ||||
| Nevera | 23 (63.9) | 80 (80.8) | 4.179 | 0.041 |
| Past and presentb | 13 (36.1) | 19 (19.2) | ||
| Alcohol intake | ||||
| Neverc | 17 (47.2) | 73 (73.7) | 8.352 | 0.004 |
| Past and presentd | 19 (52.8) | 26 (26.3) | ||
| Physical activity | ||||
| Cannot meet daily needse | 20 (55.6) | 18 (18.2) | 18.233 | < 0.001 |
| Normal activityf | 16 (44.4) | 81 (81.8) | ||
| Treatment | ||||
| None or basic drugs | 21 (58.3) | 23 (23.2) | 14.805 | < 0.001 |
| Antiresorptive drugs with/without basic drugs | 15 (41.7) | 76 (76.8) |
Data are presented as n (%). All P values were calculated with the chi-square test. P value < 0.05 was considered to indicate a statistically significant difference (highlighted in bold).
referred to no smoking during the follow-up period.
referred to smoking during the follow-up period, even if only once.
referred to no drinking during the follow-up period.
referred to drinking during the follow-up period, even if only once.
referred to movement inadequate to meet the needs of daily life (shopping, climbing stairs, or exercising).
referred to adequate movement to meet the daily needs.
The details of drug intervention between the two groups
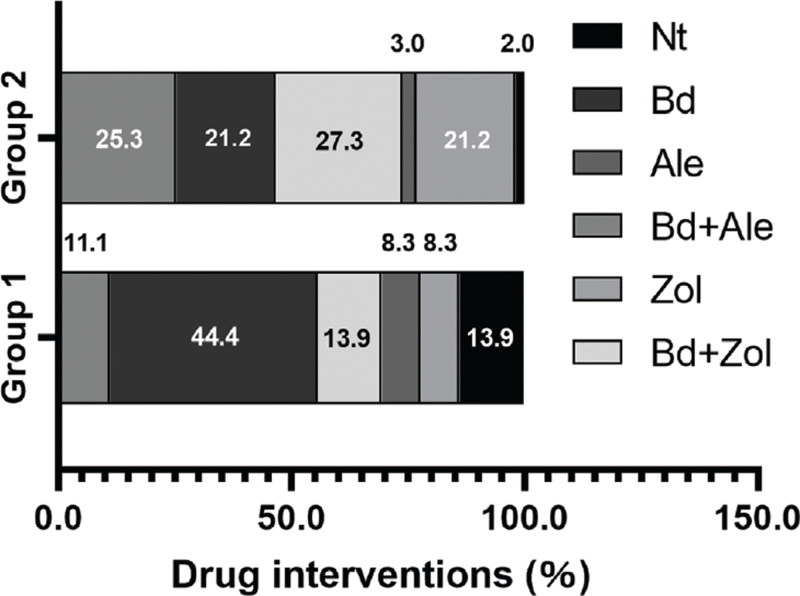
More details of the drug intervention are shown in Fig. 1. Compared to Group 1 (13.9%, 11.1%, and 8.3%, respectively), the usage rates of Bd + Zol, Bd + Ale, and Zol were higher in Group 2 (27.3%, 25.3%, and 21.2%, respectively). However, the rates of no treatment (Nt), Bd use, and Ale use were lower in Group 2 (2.0%, 21.2%, and 3.0%, respectively) than in Group 1 (13.9%, 44.4%, and 8.3%, respectively).
FIG. 1.
The constituent ratios of different drug interventions in the two groups. Nt, no treatment; Bd, basic drugs (calcium carbonate and vitamin D3 tablets + calcitriol soft capsules); Ale, alendronate sodium and vitamin D3 tablets; Bd + Ale, alendronate sodium and vitamin D3 tablets + basic drugs; Zol, zoledronic acid injection; Bd + Zol, zoledronic acid injection + basic drugs.
The differences in the levels of the BTMs, biochemical indices, and BMD at 1 year follow-up between the two groups
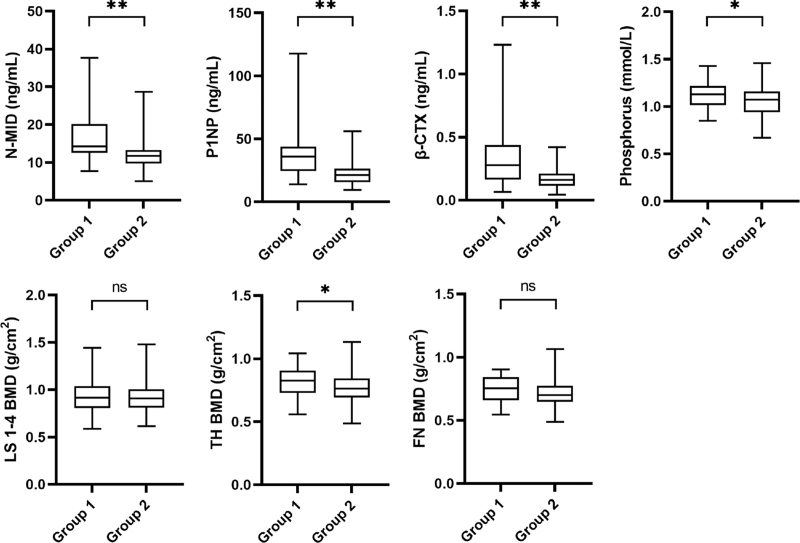
At 1 year follow-up, the levels of N-MID (16.61 ± 6.54 vs 11.98 ± 3.36 ng/mL), P1NP (39.98 ± 23.51 vs 22.49 ± 8.73 ng/mL), β-CTX (0.351 ± 0.261 vs 0.168 ± 0.075 ng/mL), and Phosphorus (1.13 ± 0.15 vs 1.06 ± 0.15 mmol/L) were significantly higher in Group 1 than in Group 2 (P < 0.05). No significant differences were observed in the 25(OH)D (31.0 ± 12.4 vs 30.0 ± 9.3 ng/mL), UA (323.9 ± 75.7 vs 328.2 ± 72.6 μmol/L), ALP (68.7 ± 12.7 vs 65.6 ± 14.1 U/L), Calcium (2.27 ± 0.11 vs 2.25 ± 0.11 mmol/L), Magnesium (0.87 ± 0.08 vs 0.87 ± 0.08 mmol/L), ACP (8.2 ± 1.5 vs 8.0 ± 1.4 U/L), NACP (4.3 ± 0.8 vs 4.2 ± 0.8 U/L), and PACP (3.9 ± 0.8 vs 3.8 ± 0.6 U/L) levels between the two groups (Group 1 vs Group 2; P > 0.05). As for LS 1-4 (0.929 ± 0.169 vs 0.921 ± 0.166 g/cm2) and FN (0.748 ± 0.095 vs 0.710 ± 0.105 g/cm2) BMD, no differences were evidenced for Group 1 in comparison with Group 2 (P > 0.05). However, the TH BMD in Group 1 was still higher than that in Group 2 (0.822 ± 0.117 vs 0.775 ± 0.120 g/cm2; P < 0.05; Fig. 2).
FIG. 2.
Comparison of the differences in N-MID, P1NP, β-CTX, Phosphorus, and BMD at 1 year follow-up between the two groups. All P values were calculated with the t test. ns, not significant. ∗P < 0.05. ∗∗P < 0.01. β-CTX, β-C-terminal telopeptide of type I collagen; BMD, bone mineral density; FN, femoral neck; LS, lumbar spine; N-MID, N-terminal middle segment osteocalcin; P1NP, propeptide of type I procollagen; TH, total hip.
The differences in BMD at baseline and 1 year follow-up
There were significant differences in LS 1-4 BMD in both Group 1 and Group 2 (P < 0.001). For TH BMD, a statistically significant difference was only seen in Group 2 (P < 0.01). The difference was nonsignificant in FN BMD in either Group 1 or Group 2 (P > 0.05). The results showed that the BMD in different sites had almost the same change trend, but different change degrees (Supplemental Digital Content Table 1).
The correlations between BTMs, lifestyle, treatment, and LS 1-4 BMD change
Table 3 shows the correlations between N-MID, P1NP, β-CTX, Phosphorus, lifestyle, treatment, and the change in LS 1-4 BMD. The changes of BTMs were calculated as the variable value at 1 year follow-up minus the variable value at baseline. The results showed that the levels of N-MID, P1NP, and β-CTX at 1 year follow-up were negatively correlated with the change of LS 1-4 BMD (R2 = −0.069, P = 0.002; R2 = −0.167, P < 0.001; R2 = −0.116, P < 0.001, respectively). What's more, the changes in N-MID, P1NP, β-CTX, and Phosphorus were negatively correlated with the change in LS 1-4 BMD (R2 = −0.200, P < 0.001; R2 = −0.230, P < 0.001; R2 = −0.186, P < 0.001; R2 = −0.044, P = 0.015, respectively), which indicated that an increase in N-MID, P1NP, β-CTX, or Phosphorus was associated with a decrease in LS 1-4 BMD within the follow-up.
TABLE 3.
Correlation analysis of the correlations between BTMs, lifestyle, treatment, and LS 1-4 BMD changea
| LS 1-4 BMD change | ||
| Variable | R 2 | P |
| 1 y N-NID | −0.069 | 0.002 |
| N-NID change | −0.200 | < 0.001 |
| 1 y P1NP | −0.167 | < 0.001 |
| P1NP change | −0.230 | < 0.001 |
| 1 y β-CTX | −0.116 | < 0.001 |
| β-CTX change | −0.186 | < 0.001 |
| 1 y Phosphorus | −0.002 | 0.626 |
| Phosphorus change | −0.044 | 0.015 |
| Smoking behavior | −0.019 | 0.112 |
| Alcohol intake | −0.039 | 0.021 |
| Physical activity | 0.090 | < 0.001 |
| Treatment | 0.134 | < 0.001 |
All P values were calculated with the Pearson or Spearman correlation analysis. P value < 0.05 was considered to indicate a statistically significant difference (highlighted in bold).
BMD, bone mineral density; BTMs, bone turnover markers; β -CTX, β -C-terminal telopeptide of type I collagen; LS, lumbar spine; N-NID, N-terminal middle segment osteocalcin; P1NP, propeptide of type I procollagen; R2, correlation coefficient.
change was calculated as the variable value at year follow-up minus the variable value at baseline.
For lifestyle and treatment, physical activity and drug intervention were observed to have significant positive correlations with the change of LS 1-4 BMD (R2 = 0.090, P < 0.001; R2 = 0.134, P < 0.001, respectively), while alcohol intake was negatively correlated with LS 1-4 BMD change (R2 = −0.039, P = 0.021). No correlation between smoking behavior and LS 1-4 BMD change was observed (R2 = −0.019, P = 0.112).
The performances of the BTMs in diagnosing the change of LS 1-4 BMD
Receiver operating characteristic curves of N-MID, P1NP, β-CTX, their changes, and Phosphorus change were shown in Supplemental Digital Content Figure 2. Among these seven markers, 1 year N-MID, P1NP, β-CTX and their changes had AUC values larger than 0.7. 1 year P1NP had the highest AUC (0.803, 95% confidence interval [CI]: 0.726-0.866) for the diagnosis of LS 1-4 BMD change. The AUC, cut-off value, sensitivity, specificity, positive predictive value, negative predictive value, Kappa value, and diagnostic efficiency for seven markers are depicted in Table 4.
TABLE 4.
Performance characteristics of the single biomarker for diagnosing LS 1-4 BMD change
| Variable | AUC (95% CI) | P | Cut-off value | Sensitivity (%, 95% CI) | Specificity (%, 95% CI) | PPV (%, 95% CI) | NPV (%, 95% CI) | Kappa value (95% CI) | DE (%, 95% CI) |
| 1 y N-MID (ng/mL) | 0.751 (0.669-0.821) | < 0.001 | 13.24 | 72.7 (62.9-81.2) | 72.2 (54.8-85.8) | 87.8 (78.7-94.0) | 49.1 (35.1-63.2) | 0.391 (0.235-0.556) | 72.6 (65.1-80.1) |
| N-MID change (ng/mL) | 0.764 (0.684-0.833) | < 0.001 | −5.65 | 43.4 (33.5-53.8) | 97.2 (85.5-99.9) | 97.7 (88.0-99.9) | 38.5 (28.4-49.2) | 0.274 (0.179-0.379) | 57.8 (49.5-66.1) |
| 1 y P1NP (ng/mL) | 0.803 (0.726-0.866) | < 0.001 | 33.66 | 89.9 (82.2-95.0) | 58.3 (40.8-74.5) | 85.6 (77.3-91.7) | 67.7 (48.6-83.3) | 0.505 (0.323-0.671) | 81.5 (75.0-88.1) |
| P1NP change (ng/mL) | 0.768 (0.688-0.837) | < 0.001 | −9.12 | 57.6 (47.2-67.5) | 91.7 (77.5-98.2) | 95.0 (86.1-99.0) | 44.0 (32.5-55.9) | 0.366 (0.233-0.498) | 66.7 (58.8-74.7) |
| 1 y β-CTX (ng/mL) | 0.753 (0.671-0.823) | < 0.001 | 0.238 | 86.9 (78.6-92.8) | 61.1 (43.5-76.9) | 86.0 (77.6-92.1) | 62.9 (44.9-78.5) | 0.484 (0.295-0.639) | 80.0 (73.3-86.7) |
| β-CTX change (ng/mL) | 0.753 (0.672-0.823) | < 0.001 | −0.111 | 51.5 (41.3-61.7) | 86.1 (70.5-95.3) | 91.1 (80.4-97.0) | 39.2 (28.4-50.9) | 0.273 (0.149-0.399) | 60.7 (52.5-68.9) |
| Phosphorus change (mmol/L) | 0.623 (0.536-0.705) | 0.029 | −0.04 | 60.6 (50.3-70.3) | 63.9 (46.2-79.2) | 82.2 (71.5-90.2) | 37.1 (25.2-50.3) | 0.199 (0.038-0.351) | 61.5 (53.3-69.7) |
Data are presented as value, value (95% CI) or % (95% CI). P value < 0.05 was considered to indicate a statistically significant difference (highlighted in bold).
AUC, area under the curve; BMD, bone mineral density; β-CTX, β-C-terminal telopeptide of type I collagen; CI, confidence interval; DE, diagnostic efficiency; N-NID, N-terminal middle segment osteocalcin; NPV, negative predictive value; PPV, positive predictive value; P1NP, propeptide of type I procollagen.
Binary logistic regression analysis of factors affecting LS 1-4 BMD change
The variables with statistical significance (P < 0.05), as verified by the independent sample t test (or chi-square test) and the correlation analysis, were identified as candidate-independent variables for constructing the binary logistic regression model. The practical significance and collinearity problems of candidate independent variables were also taken into consideration. The results show that the maximum value of each variance inflation factor is less than 10, showing that the collinearity between variables is acceptable. The independent variables like BTMs and their changes, Phosphorus change, alcohol intake, physical activity, and treatment were included in the final model (Table 5). Physical activity and treatment were found to be strong determinant factors for LS 1-4 BMD change (P < 0.05). Women with more physical activities were found to be 6.856 times more likely to have a positive change of LS 1-4 BMD than women with less activity (odds ratio = 6.856, 95% CI: 2.058-22.839). Meanwhile, women who were treated with antiresorptive drugs were 5.114 times more likely to have increased LS 1-4 BMD than those who were untreated or treated with Bds (odds ratio = 5.114, 95% CI: 1.551-16.864). Other variables were not the defining factors for the change of LS 1-4 BMD (P > 0.05).
TABLE 5.
Binary logistic regression analysis of the factors influencing LS 1-4 BMD change
| Variable | β | SE | Wald | OR (95% CI) | P |
| 1 y N-MID | −0.108 | 0.108 | 0.989 | 0.898 (0.726-1.110) | 0.320 |
| N-NID change | −0.064 | 0.106 | 0.360 | 0.938 (0.762-1.156) | 0.548 |
| 1 y P1NP | −0.053 | 0.041 | 1.613 | 0.949 (0.875-1.029) | 0.204 |
| P1NP change | −0.002 | 0.035 | 0.004 | 0.998 (0.933-1.068) | 0.952 |
| 1 y β-CTX | −2.662 | 3.779 | 0.496 | 0.070 (0.000-114.913) | 0.481 |
| β-CTX change | −1.524 | 2.520 | 0.366 | 0.218 (0.002-30.394) | 0.545 |
| Phosphorus change | −0.210 | 1.894 | 0.012 | 0.811 (0.020-33.181) | 0.912 |
| Alcohol intake | −0.719 | 0.591 | 1.478 | 0.487 (0.153-1.553) | 0.224 |
| Physical activity | 1.925 | 0.614 | 9.834 | 6.856 (2.058-22.839) | 0.002 |
| Treatment | 1.632 | 0.609 | 7.185 | 5.114 (1.551-16.864) | 0.007 |
Data are presented as value, or value (95% CI). P value < 0.05 was considered to indicate a statistically significant difference (highlighted in bold).
BMD, bone mineral density; β, regression coefficient; β-CTX, β-C-terminal telopeptide of type I collagen; CI, confidence interval; LS, lumbar spine; N-NID, N-terminal middle segment osteocalcin; OR, odds ratio; P1NP, propeptide of type I procollagen; SE, standard error.
DISCUSSION
The imbalance of bone metabolism caused by increased activity of osteoclasts is known as the pathophysiologic basis of PMOP.1 As reliable indicators of bone health, however, the relationship between BTMs and BMD changes still lacks the support of strong evidence, which leads to incomplete understanding of PMOP and subsequent treatment-related decision making. The present study determined the associations between BTMs and BMD changes and found that declining N-MID, P1NP, β-CTX, and Phosphorus are significantly associated with a short-term positive change in LS 1-4 BMD over 1 year. We also studied the differences in smoking, drinking, and physical activity among women with different BMD changes (Table 2), and confirmed that appropriate physical activity is beneficial to the maintenance of and positive change in BMD (Table 5).17,18 Smoking and drinking have been also reported to be associated with the impairment of bone remodeling, and were known risk factors for the decrease of BMD.19,20 However, in our present study, smoking had an insignificant association with LS 1-4 BMD change, and the statistical differences of the logistic regression analysis of alcohol intake was not significant either. We speculate that this contradiction may be caused by the small sample size and short follow-up period.
Timely drug intervention is an important measure to block the continuous decline of BMD in postmenopausal women. Calcium, vitamin D, and calcitriol are considered Bds for this treatment. However, a significant change in BMD is rarely observed in patients receiving only Bd treatment, and the total effective rate is relatively low.21,22,23 Bisphosphonates can improve BMD by inhibiting the activity of osteoclasts. They have been on the market for a long time and have reliable effects.23,24 For patients newly diagnosed with PMOP, antiresorptive drugs are recommended before bone-forming drugs, according to guidelines.25 Therefore, bisphosphonate drugs have been widely used in China. In the comparison of drug interventions between the two groups in our study, most participants receiving no treatment or treatment with Bds alone showed a decline in BMD. The effect of an antiresorptive polypill alone seems weaker than that combined with Bds (Fig. 1). This indicates the doses and types insufficient to prevent and treat PMOP using Bds alone. More interesting is that the participants with higher baseline LS 1-4 BMD were more susceptible to suffering a decline in the following year, which was manifested in an overall decline in BMD of the LS L1-4, TH, and FN. Those women with lower baseline BMD often experienced an increase in BMD within 1 year. This phenomenon may be attributed to low awareness, untimely medical measures taken, and unhealthy life habits in the populations with high initial BMD. Although the result is not convincing enough to change current treatment strategy, it emphasizes the importance of periodic risk assessment and possible medical interventions to take in advance for this population, because this population is more likely to suffer a decline in BMD within 1 year after initial diagnosis.11,26
Vitamin D plays an important role in promoting calcium absorption, maintaining calcium homeostasis, inhibiting osteoclast formation, and promoting bone mineralization.27 Recent studies have shown that vitamin D can significantly downregulate the cellular response to TNF-α and IL-6,28 which have been proved to be closely related to the development of PMOP.29 25(OH)D is the main form of vitamin D stored in the body and can be measured to reflect its overall level. Al-Daghri et al30 pointed out that the correlation between 25(OH)D and sex steroid indices may be an important mechanism affecting BMD in postmenopausal women. In this study, although there was no significant difference in the baseline and 1-year 25(OH)D levels between the two groups, the 1-year 25(OH)D level in Group 2 was significantly higher than the baseline level, confirming that 25(OH)D may play a positive role in the improvement of BMD.
N-MID is the hydrolytic fragment of osteocalcin secreted by osteoblasts and can be used to reflect the process of bone formation. Since it can also be released from the bone matrix during bone resorption, its accuracy as a marker of bone formation remains controversial.26,31 In the present study, the 1 year N-MID in Group 2 was lower than that in Group 1 (Fig. 2), and lower than the baseline level in Group 2 as well (Table 1), indicating that the decline in bone turnover accelerates the deposition of the bone matrix, accompanied by a subsequent positive change in BMD.
P1NP and β-CTX are the markers recommended by the International Osteoporosis Foundation and the International Federation of Clinical Chemistry and Laboratory Medicine to reflect bone formation and resorption.32 The level of bone turnover and bone loss in postmenopausal women is higher than that in premenopausal women, and this can be reflected by the rise in P1NP and β-CTX.33 Whether the risk of PMOP and subsequent OF can be predicted by P1NP or β-CTX is still debatable. By comparing the relationship between P1NP, β-CTX, and BMD in postmenopausal women, Azizieh et al34 found that P1NP and the P1NP/β-CTX ratio were significantly correlated with BMD in the hip and spine, whereas β-CTX has nothing to do with BMD. Qu et al pointed out that β-CTX in a fracture group of older women was significantly higher than that in a nonfracture group. They believed that a high level of β-CTX is better than P1NP in predicting the risk of OF.26 Previous studies by Garnero et al13 and Wright et al35 emphasized the potential value of β-CTX in reflecting changes in bone mass. The sample sizes and study populations used in different studies may contribute to the differences in the results. In our study, both of 1 year P1NP, 1 year β-CTX, and their changes were found to have negative correlations with LS 1-4 BMD change (Table 3), and could be identified as biomarkers to diagnose this tendency.
UA is the end product of purine metabolism. Due to its antioxidant properties, UA is thought to cause gout while maintaining bone mass by inhibiting osteoclastic resorption and promoting osteoblast differentiation.36 In older Japanese men, higher serum UA concentrations are associated with a lower risk of vertebral fracture measured by morphology.37 In postmenopausal women, however, the relationship between UA and BMD is equivocal.38,39 No association between UA and BMD changes was found in our follow-up participants, and more convincing research is needed to clarify this relationship.
The changes in enzymes related to bone metabolism were also discussed in this study. ALP is a group of glycoproteases that can hydrolyze phosphates under alkaline conditions. Only approximately half of ALP in healthy adult blood comes from bone, which shows a lack of specificity. Clinically, ALP is often used to reflect bone metabolism activity and monitor drug response after treatment.18,21,24,26 In our study, the 1 year ALP in Group 2 declined, which reflected a good response of the body to drugs to a certain extent. Compared to bone, the prostate is the primary source of ACP and PACP (the level of NACP is equal to ACP minus PACP). An abnormal increase in those enzymes is often used as an auxiliary diagnostic marker for prostate cancer or bone diseases. There was no significant change in the baseline and 1 year levels of these enzymes in the present study, which indicates that the change in LS 1-4 BMD was independent of their changes.
Calcium, Phosphorus, and Magnesium are essential elements for the human body and play an important role in maintaining bone homeostasis. The key parts of calcium and phosphorus metabolism are controlled by the parathyroid hormone (PTH)–1,25 dihydroxyvitamin D (1,25[OH]2D)–fibroblast growth factor-23 (FGF23) axis.40 Magnesium deficiency can affect PTH and 1,25(OH)2D, destroy the calcium balance in the body, and lead to hypocalcemia.41 The close relationship between them makes them important objects of research into the pathological mechanism of osteoporosis. Dietary magnesium intake is considered to predict short-term bone resorption over a period of 2 years.35 Compared to that in healthy women, a lower serum magnesium concentration can be found in postmenopausal patients with osteoporosis.42 A decline in vertebral BMD in rodent models supports the conclusion that bone metabolic disorders are caused by magnesium deficiency.43 Unlike Magnesium studies, some research has suggested that there are no differences in Calcium and Phosphorus levels between people with different BMD levels;26,30 it is speculated that this may be because levels of mineral salts tend to be normal in patients with primary osteoporosis.26 In our study, there were some differences in Calcium, Phosphorus, and Magnesium levels across the times and groups. Among them, only Phosphorus change was found to have a negative correlation with LS 1-4 BMD change. The AUC of it was less than 0.7 and its diagnostic value needs more studies to verify in the future.
It is well known that bone loss in healthy postmenopausal women progresses slowly and steadily with aging.44 The clinical value of repeated BMD measurements in the short term is highly debated. However, it should be noted that the repeated screening in the present study was meaningful. In our study, only the change in LS 1-4 BMD was significant in participants in Group 1, whereas both the BMD changes in LS 1-4 and TH were significant in Group 2. Aggressive anti-osteoporosis therapy may have accelerated the BMD changes within a short period. There is more controversy over the value of repeated BMD measurements to predict the risk of fragile fractures. As evidenced by the Berry et al 2014 publication in JAMA, repeating a BMD test in 4 years provided little additional value beyond baseline BMD when assessing fracture risk.45 This inefficiency was even found in bone health assessments after thyroid-stimulating suppression therapy in postmenopausal women with differentiated thyroid carcinoma.46 Even though evidence is insufficient, clinicians should select potential populations suitable for repeated BMD assessments carefully in order to avoid unnecessary radiation exposure and possible additional expense.
This study has its own limitations. First, the phenomenon of “regression to the mean” is a potential confounder for all studies with repeated measurements. Based on the limited data available, whether the change of clinical parameters is a real effect or a statistical regression to the mean needs more research. Second, the time interval of follow-up was 1 year in this study, and data on the BTMs and BMD changes within the year were unavailable. Third, although the study was conducted in a single center and the same measuring equipment was used, the variation of repeated measurements may still exist. Furthermore, observational studies do not allow interventions with specific therapeutic drugs, so the effects of bone-forming drugs and estrogen replacement therapy on BTMs and BMD changes were not discussed in this study. Notably, the change in BMD, not OF, was the main point of our study, so we excluded participants with OF during the study to avoid the significant impact of OF on BTMs levels.31 A multicenter, prospective, randomized controlled study will help to further clarify the underlying link between BTMs and BMD changes in postmenopausal women. Given the above concerns, the results of the present study should be interpreted with caution.
CONCLUSIONS
To the best of our knowledge, this is one of the few real-world studies concerning the associations between BTMs and BMD changes in postmenopausal women. Our results highlight the potential roles of the decreased BTMs, especially the changes of N-MID, P1NP, β-CTX, and Phosphorus, as diagnostic markers of short-term LS 1-4 BMD change over 1 year. This study also confirms that appropriate physical activity and drug intervention are powerful protective factors for positive changes in LS1-4 BMD. These findings will help clinicians to gain insight into the change in BMD based on limited clinical data, and to initiate timely interventions.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgments
The authors are thankful to all of the women who participated in this study.
Footnotes
S. Z., X. M., and Z. W. contributed equally to this work.
Funding/support: This study was funded by the National Natural Science Foundation of China (31570976) and the Science and Technology Program of Guangzhou (201604020148).
Financial disclosure/conflicts of interest: None reported.
Supplemental digital content is available for this article.
REFERENCES
- 1.Emmanuelle NE, Marie-Cécile V, Florence T, et al. Critical role of estrogens on bone homeostasis in both male and female: from physiology to medical implications. Int J Mol Sci 2021; 22:1568–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng X, Zhao K, Zha X, et al. China Health Big Data (China Biobank) Project Investigators. Opportunistic screening using low-dose CT and the prevalence of osteoporosis in China: a nationwide, multicenter study. J Bone Miner Res 2021; 36:427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Si L, Winzenberg TM, Jiang Q, Chen M, Palmer AJ. Projection of osteoporosis-related fractures and costs in China: 2010-2050. Osteoporos Int 2015; 26:1929–1937. [DOI] [PubMed] [Google Scholar]
- 4.Fuggle NR, Singer A, Gill C, et al. How has COVID-19 affected the treatment of osteoporosis? An IOF-NOF-ESCEO global survey. Osteoporos Int 2021; 32:611–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ralston SH, Uitterlinden AG. Genetics of osteoporosis. Endocr Rev 2010; 31:629–662. [DOI] [PubMed] [Google Scholar]
- 6.Siris ES, Adler R, Bilezikian J, et al. The clinical diagnosis of osteoporosis: a position statement from the national bone health alliance working group. Osteoporos Int 2014; 25:1439–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mithal A, Ebeling P, Kyer CS. The Asia-Pacific Regional Audit: epidemiology, costs & burden of osteoporosis in 2013. Available at: https://www.iofbonehealth.org/sites/default/files/media/PDFs/Regional%20Audits/2013-Asia_Pacific_Audit_0_0.pdf. Accessed 2013. [Google Scholar]
- 8.Kline GA, Morin SN, Feldman S, Lix LM, Leslie WD. Diminishing value from multiple serial bone densitometry in women receiving anti-resorptive medication for osteoporosis. J Clin Endocrinol Metab 2021; 106:2718–2725. [DOI] [PubMed] [Google Scholar]
- 9.Crandall CJ, Larson J, Wright NC, et al. Serial bone density measurement and incident fracture risk discrimination in postmenopausal women. JAMA Intern Med 2020; 180:1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eastell R, Pigott T, Gossiel F, Naylor KE, Walsh JS, Peel NFA. Diagnosis of endocrine disease: bone turnover markers: are they clinically useful? Eur J Endocrinol 2018; 178:R19–R31. [DOI] [PubMed] [Google Scholar]
- 11.Lane NE, Saag K, O’Neill TJ, et al. Real-world bone turnover marker use: impact on treatment decisions and fracture. Osteoporos Int 2021; 32:831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu Z, Zhou H, Wang Y, Yao X. Associations between bone turnover markers and bone mineral density in older adults. J Orthop Surg (Hong Kong) 2021; 29:1–7. [DOI] [PubMed] [Google Scholar]
- 13.Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res 2000; 15:1526–1536. [DOI] [PubMed] [Google Scholar]
- 14.Bauer DC. Clinical use of bone turnover markers. JAMA 2019; 322:569–570. [DOI] [PubMed] [Google Scholar]
- 15.González-Sapienza G, Lorenzo C, Nieto A. Improved immunodiagnosis of cystic hydatid disease by using a synthetic peptide with higher diagnostic value than that of its parent protein, Echinococcus granulosus antigen B. J Clin Microbiol 2000; 38:3979–3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33:159–174. [PubMed] [Google Scholar]
- 17.Pinheiro MB, Oliveira J, Bauman A, Fairhall N, Kwok W, Sherrington C. Evidence on physical activity and osteoporosis prevention for people aged 65+ years: a systematic review to inform the WHO guidelines on physical activity and sedentary behaviour. Int J Behav Nutr Phys Act 2020; 17:150–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marini S, Barone G, Masini A, et al. The effect of physical activity on bone biomarkers in people with osteoporosis: a systematic review. Front Endocrinol 2020; 11:585689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu Y, Di YP, Chang M, et al. Cigarette smoke-associated inflammation impairs bone remodeling through NFκB activation. J Transl Med 2021; 19:163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedersen KB, Osborn ML, Robertson AC, et al. Chronic ethanol feeding in mice decreases expression of genes for major structural bone proteins in a Nox4-independent manner. J Pharmacol Exp Ther 2020; 373:337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng WB, Dai Y, Hu J, et al. Effects of bisphosphonates on osteoporosis induced by duchenne muscular dystrophy: a prospective study. Endocr Pract 2020; 26:1477–1485. [DOI] [PubMed] [Google Scholar]
- 22.Makan AM, Hout HV, Onder G, et al. Pharmacological management of osteoporosis in nursing home residents: the Shelter study. Maturitas 2021; 143:184–189. [DOI] [PubMed] [Google Scholar]
- 23.Kong L, Zuo K, Ma L. Clinical effect of zoledronic acid in the treatment of senile osteoporosis. Pak J Med Sci 2020; 36:1703–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo BL, Li FQ. The safety observation of zoledronic acid in the treatment of senile osteoporosis. Chin J Osteoporos 2014; 20:820–823. [Google Scholar]
- 25.Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an endocrine society∗ clinical practice guideline. J Clin Endocrinol Metab 2019; 104:1595–1622. [DOI] [PubMed] [Google Scholar]
- 26.Qu XL, Zheng B, Chen TY, Cao ZR, Qu B, Jiang T. Bone turnover markers and bone mineral density to predict osteoporotic fractures in older women: a retrospective comparative study. Orthop Surg 2020; 12:116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allard L, Demoncheaux N, Machuca-Gayet I, et al. Biphasic effects of vitamin D and FGF23 on human osteoclast biology. Calcif Tissue Int 2015; 97:69–79. [DOI] [PubMed] [Google Scholar]
- 28.Lee SU, Na KT, Lee YM, Park JH, Joo SY. Low vitamin D levels in post-menopausal women are associated with complex regional pain syndrome type I in surgically treated distal radius fractures. J Orthop Surg Res 2020; 15:328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azizieh F, Raghupathy R, Shehab D, Al-Jarallah K, Gupta R. Cytokine profiles in osteoporosis suggest a proresorptive bias. Menopause 2017; 24:1057–1064. [DOI] [PubMed] [Google Scholar]
- 30.Al-Daghri NM, Yakout SM, Ansari MGA, Hussain SD, Wani KA, Sabico S. Vitamin D metabolites and sex steroid indices in postmenopausal women with and without low bone mass. Metabolites 2021; 11:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Højsager FD, Rand MS, Pedersen SB, Nissen N, Jørgensen NR. Fracture-induced changes in biomarkers CTX, P1NP, OC, and BAP-a systematic review. Osteoporos Int 2019; 30:2381–2389. [DOI] [PubMed] [Google Scholar]
- 32.Vasikaran S, Eastell R, Bruyère O, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int 2011; 22:391–420. [DOI] [PubMed] [Google Scholar]
- 33.Gossiel F, Altaher H, Reid DM, et al. Bone turnover markers after the menopause: T-score approach. Bone 2018; 111:44–48. [DOI] [PubMed] [Google Scholar]
- 34.Azizieh FY, Shehab D, Al-Jarallah K, Mojiminiyi O, Gupta R, Raghupathy R. Circulatory pattern of cytokines, adipokines and bone markers in postmenopausal women with low BMD. J Inflamm Res 2019; 12:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright HH, Kruger MC, Schutte WD, Wentzel-Viljoen E, Kruger IM, Kruger HS. Magnesium intake predicts bone turnover in postmenopausal black South African women. Nutrients 2019; 11:2519–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaushal N, Vohora D, Jalali RK, Jha S. Review of the literature examining the association of serum uric acid with osteoporosis and mechanistic insights into its effect on bone metabolism. Endocr Metab Immune Disord Drug Targets 2019; 19:259–273. [DOI] [PubMed] [Google Scholar]
- 37.Iki M, Yura A, Fujita Y, et al. Relationships between serum uric acid concentrations, uric acid lowering medications, and vertebral fracture in community-dwelling elderly Japanese men: Fujiwara-kyo osteoporosis risk in men (FORMEN) cohort study. Bone 2020; 139:115519. [DOI] [PubMed] [Google Scholar]
- 38.Xiong A, Yao Q, He J, Fu W, Yu J, Zhang Z. No causal effect of serum urate on bone-related outcomes among a population of postmenopausal women and elderly men of Chinese Han ethnicity-a Mendelian randomization study. Osteoporos Int 2016; 27:1031–1039. [DOI] [PubMed] [Google Scholar]
- 39.Yan DD, Wang J, Hou XH, et al. Association of serum uric acid levels with osteoporosis and bone turnover markers in a Chinese population. Acta Pharmacol Sin 2018; 39:626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun M, Wu X, Yu Y, et al. Disorders of calcium and phosphorus metabolism and the proteomics/metabolomics-based research. Front Cell Dev Biol 2020; 8:576110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castiglioni S, Cazzaniga A, Albisetti W, Maier JA. Magnesium and osteoporosis: current state of knowledge and future research directions. Nutrients 2013; 5:3022–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang J, Yu D, Ji J, Wang N, Yu S, Yu B. The association between the concentration of serum magnesium and postmenopausal osteoporosis. Front Med 2020; 7:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Belluci MM, de-Molon RS, Rossa C, Jr, et al. Severe magnesium deficiency compromises systemic bone mineral density and aggravates inflammatory bone resorption. J Nutr Biochem 2020; 77:108301. [DOI] [PubMed] [Google Scholar]
- 44.Tai V, Leung W, Grey A, et al. Calcium intake and bone mineral density: systematic review and meta-analysis. BMJ 2015; 351:h4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berry SD, Samelson EJ, Pencina MJ, et al. Repeat bone mineral density screening and prediction of hip and major osteoporotic fracture. JAMA 2013; 310:1256–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chung CW, Choi HS, Kong SH, et al. Measurements of bone health after thyroid-stimulating suppression therapy in postmenopausal women with differentiated thyroid carcinoma: bone mineral density versus the trabecular bone score. J Clin Med 2021; 10:1964–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.