Abstract
It is well known that cardiovascular disease (CVD) manifests differently in women and men. The underlying causes of these differences over the aging lifespan is less well understood. Sex differences in cardiac and vascular phenotypes are seen in childhood and tend to track along distinct trajectories related to dimorphism in genetic factors as well as response to risk exposures and hormonal changes over the life course. These differences underlie sex- specific variation in cardiovascular events later in life, including myocardial infarction, heart failure, ischemic stroke, and peripheral vascular disease. With respect to cardiac phenotypes, females have intrinsically smaller body-size-adjusted cardiac volumes and then tend to experience greater age-related wall thickening and myocardial stiffening with aging. With respect to vascular phenotypes, sexual dimorphism in both physiology and pathophysiology are also seen, including overt differences in blood pressure trajectories. The majority of sex differences in myocardial and vascular alterations that manifest with aging appear to follow relatively consistent trajectories from the very early to the very later stages of life. This review aims to synthesize recent cardiovascular aging-related research to highlight clinically relevant studies in diverse female and male populations that can inform approaches to improving the diagnosis, management, and prognosis of CVD risks in the aging population at large.
Keywords: Cardiovascular Diseases, Myocardial Biology, Vascular Disease, Women, Sex, Gender
INTRODUCTION
Sex differences in cardiovascular phenotypes are frequently recognized from cross-sectional studies as well as studies that have examined differences in the incidence and longer-term outcomes for a variety of cardiovascular disease (CVD) conditions. Whereas many differences in CVD outcomes arise from factors related to gender, which is based on socially constructed features, we will focus predominantly on differences in both outcomes and pre-clinical characteristics that arise from factors related to sex, which is based on biologically defined traits.1 There is now a large and compelling body of data indicating that biological sex is directly and significantly related to differences in cardiovascular traits. From emerging evidence, we are now beginning to understand that these differences likely emanate from intrinsically distinct phenotypic “starting points” that precede age-related changes, combined with divergent trajectories that appear mediated by female-male differences in the response to various risk exposures. These elements represent the complexity of interactions between sex, risk exposures, and time-dependent pathophysiology, which contribute in aggregate to consistently observed patterns in how women and men tend to manifest subclinical as well as clinical forms of CVD differently.2 Herein, we review several features of the accumulating research on cardiovascular aging, including information relevant to our understanding of age-related cardiac phenotypes, vascular phenotypes, and the role of hormones. The goal of this review is to highlight key aspects of our current understanding of sex differences in myocardial and vascular aging and identify potential future directions.
Sex and the Mechanisms of Cardiovascular Aging
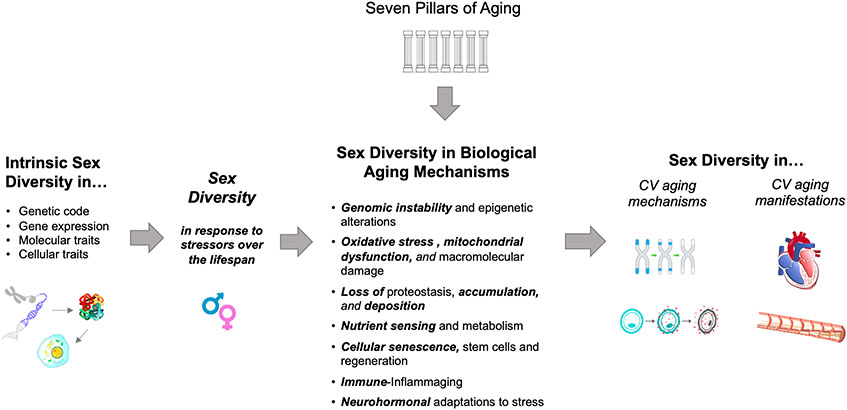
At the outset, it is important to recognize that most insights pertaining to cardiovascular aging arise from what is now a robust foundation of geroscience. Accordingly, the complexity of molecular mechanisms underlying common chronic diseases of aging has been extensively discussed, with multiple conceptual as well as analytical models proposed.3 One seminal and often referenced scheme, known as “The Seven Pillars of Aging”, highlights key interconnected processes that include epigenetics, macromolecular damage, proteostasis, metabolism, stem cells and regeneration, inflammation, and adaptation to stress.4 This framework has been expanded and translated through prior efforts aimed at clarifying the mechanisms that are relevant to aging of the cardiovascular system and the development of common age-related CVD phenotypes.5-7 The same framework can be further adapted for understanding sex-based variation in the mechanisms of cardiovascular aging that contribute sex differences in manifest cardiovascular aging phenotypes (Figure 1). Notwithstanding the relative paucity of data in this nascent field, there are some general themes that have emerged from across experimental, translational, and clinical investigations. The first theme is centered on how intrinsic genetic factors influence sex variation in life course trajectories of cardiovascular phenotypes, based on an abundance of data and that sex differences in traits such as lipid profiles and intima-medial thickness are seen as early as childhood and prepuberty.8-10 The second general theme is a relative excess versus relative deficiency of molecular processes with potential to preserve myocardial integrity in females versus males, respectively (Table 1). In turn, the correlative myocardial phenotypes that tend to dominate with advancing age are concentric versus eccentric left ventricular (LV) phenotypes in women compared to men. The third general theme is a heightened vascular sensitivity to a variety of mechanistic stressors in females that likely contributes to accelerated vascular aging phenotypes seen in women; notably, propensity for severe forms of certain vascular diseases exists earlier in life for males – in whom vascular aging trajectories are present but less pronounced. In the next sections, we will review the reported evidence regarding myocardial and vascular aging phenotypes that tend to exhibit sexual dimorphism in the context of these two themes, while also providing special attention to the aging phenotypes that predominate in women (Figure 2). For additional reference, we provide a summary of the human observational and trial studies that have offered data relevant to all the findings discussed (Table 2).
Figure 1.
Conceptual Overview of Sex-Based Variation in Mechanisms and Manifestations of Cardiovascular Aging
Table 1.
Sex Differences in Cardiovascular Aging Mechanisms and Phenotypic Manifestations
| Cardiovascular Aging Mechanism | Cardiovascular Aging Manifestation | |||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| Cardiac | ↑ Cardiac collagen accumulation* ↑Fibroblast activity ↑Reactive interstitial fibrosis ↓ Myocyte loss (−) autophagy and cellular turnover (−) stem cell regeneration (−) telomere shortening (−) aging miRNA expression ↑ Post-cardiac injury inflammatory transcriptome* ↓ Estradiol protection from ischemia/reperfusion injury* ↓ Estrogenic protection of reactive oxygen species |
↑ Myocyte loss ↑autophagy, necrosis, and apoptosis ↓ stem cell regeneration Different fibroblast secretomes Different valvular interstitial cell expression profiles Higher somatic mutation accumulation rate in DNA Earlier immune aging Decreased mitochondrial lifespan ↓ Connexin expression in atrial myocytes |
↑ Wall thickening in response to risk exposures ↑ MV leaflet thickening, prolapse, calcification risk ↑ Systolic torsion, LV shortening, LVEF ↑ LV end-systolic elastance ↑ Concentric remodeling ↑ Diastolic dysfunction Pre-clinical & clinical HFpEF phenotype |
↑ AV calcification risk ↑ Bradyarrhythmia risk ↑ Eccentric remodeling ↑ Systolic dysfunction Pre-clinical & clinical HFrEF phenotype |
| Vascular | ↑ sensitivity to cardiometabolic risk factors* ↑ Endothelial dysfunction* ↑Oxidative stress ↑Inflammation trajectory ↑ROS production ↑Nitric oxide suppression ↓ Vascular permeability to PDE3 inhibitor*142 ↓ Anti-inflammatory macrophage protection with age* ↑ Salt sensitivity with age* ↓ Estrogenic vasodilation post-menopause*70 ↑ Perivascular fibrosis* ↓ Baroreflex sensitivity ↑ Mineralocorticoid receptor expression51 |
(−) sensitivity to cardiometabolic risk factors ↑ No. endothelial cells Differential immune cell migration marker expression (↑VCAM-1) ↑ Macrophage adrenergic receptor expression ↓ Carotid wall shear stress ↑ Pulse wave velocity ↓ Acetylcholine response |
Accelerated arterial stiffness Accelerated plaque formation in aging Accelerated incident hypertension with age ↑ ↑ Coronary microvascular dysfunction Accelerated renal dysfunction Coronary events shift from erosion-based to rupture-based with aging ↑ Multi-vessel peripheral arterial disease |
Stable progressive aging trajectory Elevated morbidity and mortality due to long-term exposure to risk factors, chronic vascular lipid accumulation Coronary events more likely to be rupture-based from early age Earlier presentation of myocardial infarction ↑ Aortic aneurysm formation ↑ Aortic dissection risk |
Trajectory change associated with advancing age and/or estrogen decline
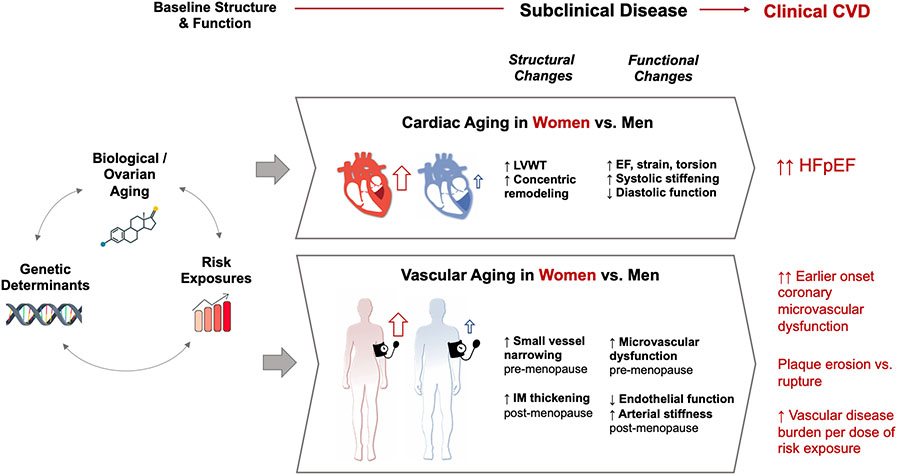
Figure 2.
Overview of Female-Predominant Cardiovascular Aging Phenotypes
Table 2.
Major Findings from Observational Studies and Randomized Trials
| Study ID | Sample Size | Setting | Age (Median, Mean, or age range) |
Major Finding Relevant to CV Aging in Women Compared to Men |
|---|---|---|---|---|
| Observational studies | ||||
| 32594163 | 3754 | Patients undergoing coronary angiography | 60 | Concentric remodelling ↑ Eccentric hypertrophy ↓ |
| 16203909 | 2042 | Community-dwelling | 62 | Age-related Ventricular-Vascular Stiffening ↑ |
| 20660804 | 4062 | Framingham heart study | 45 | Age-associated increase in LV wall thickness ↑ |
| 27280886 | 8410 | Asymptomatic participants | 50 | Age-related increase in LV wall thickness, LV mass index, and NT-proBNP ↑ |
| 16567580 | 2618 | Dallas Heart Study | 45 | Left ventricular ejection fractions ↑ |
| 32506162 | 1367 | Patients underwent positron emission tomography (PET) | 63 | Left ventricular ejection fractions (EF) ↑ EF>=65% is associated with MACE in women, but not in men. |
| 23742210 | 5307 | Subjects with normal echocardiography studies. | ~40 | Age-related increase in LV ejection fraction, LV fractional shortening and LV muscle mass index ↑ |
| 23147172 | 1478 | MESA | 65 | Myocardial torsion ↑ |
| 23871886 | 1231 | MESA | 67 | Potentially protective β-adrenergic effect ↓ |
| 19706861 | 136247 | Patients with acute coronary syndrome | 60-69 | Women with STEMI had higher 30-day mortality ↑ |
| 24574260 | 279 | HFpEF patients | 71 | Pronounced diastolic dysfunction to HFpEF ↑ |
| 30007554 | 22681 | Community-based cohorts | 60 | Association of BMI with HFpEF versus HFrEF ↑ |
| 26404197 | 1552 | Patients with nonobstructive CAD | 51 | Coronary microvascular abnormalities ↑ |
| 20579539 | 189 | Women with suspected ischemia undergoing coronary angiogram | 55 | Association of Coronary microvascular reactivity to adenosine with major adverse outcomes ↑ |
| 27511975 | 435 | Stable coronary artery disease or acute coronary syndromes | 62 | Plaque erosion ↑ Cholesterol and calcium content ↓ |
| 32861960 | 790 | Patients with revascularization of the iliofemoral arteries | 68 | Rupture-prone characteristics ↑ |
| 31983726 | 1021 | Patients underwent OCT of culprit lesions | 69 | Thin cap fibroatheroma ↑ |
| 16365194 | 6110 | MESA | 62 | CAC score ↓ |
| 32828763 | 1255 | Patients with suspected CAD | 60 | Calcified plaque volume progression ↑ |
| 30586725 | 28,732 | ARIC Study Community Surveillance | 48 | Annual incidence of AMI hospitalizations ↑ |
| 28329052 | 113,407 | Patients hospitalized for ACS | 68 | STEMI annual incidence ↑ |
| 26302759 | 5606911420 | CHD mortality rates | - | CHD mortality rates ↑ |
| 13679479 | 1860 | Patients with non-diabetic renal disease | 52 | Rate of renal disease progression ↑ |
| 31940010 | 32833 | Community based cohorts | 48 | Blood pressure elevation ↑ |
| 21695075 | 30,372 | Population-based cohorts | - | Blood pressure elevation ↑ |
| 32063062 | 32833 | Community based cohorts | 37 | Cardiometabolic-risk related SBP elevation ↑ |
| 15123572 | 521 | Framingham heart study | 57 | Progression in arterial stiffness ↑ |
| 32130922 | 80415 | Participants in the health examinations | 48-60 | Progression in arterial stiffness ↑ |
| 33486987 | 2026 | Asklepios study | 38 | Progression in arterial stiffness ↑ |
| 10334811 | 89 | Healthy subjects | 49 | Sympathetic activity ↓ Parasympathetic activity ↑ |
| 15687139 | 51 | Healthy adults | 27-28 | Tonic sympathoadrenal activity-related autonomic nervous system ↓ |
| 33587655 | 27 542 | Community based cohorts | - | CVD risk associated with SBP levels ↑ |
| 10919931 | 1028 | Healthy Children | 6-12 | Environmental and genetic factors may have different effects on serum cholesterol in girls and boys. |
| 19389672 | 267 | Healthy pupils | 10 | Sex differences of carotid intima-media thickness exist in pupils |
| Randomized Trials | ||||
|
26551272
NCT01206062 |
9361 | SBP of 130 mm Hg or higher and an increased cardiovascular risk, but without diabetes | 68 | Intensive versus standard blood pressure control resulted in 16% and 28% lower risk of major outcome in women and men respectively |
|
20228401
NCT00000620 |
4733 | SBP of 130 mm Hg with diabetes | 62 | Intensive versus standard blood pressure control did not result in significant benefit in diabetic women and men. |
|
34491661
NCT03015311 |
9624 | Patients with hypertension | 66 | Intensive versus standard blood pressure control resulted in 21% and 30% lower risk of major outcome in women and men respectively |
| 18259011 | 863 | Patients with hypertension | 66 | Women had less hypertrophy regression during long-term antihypertensive treatment. |
| 32946164 NCT02887183 |
794 | Patients with chronic HFrEF | 66 | In women with HFrEF, treatment with S/V was associated with significant NT-proBNP reduction, health status improvement and reverse cardiac remodelling. |
| 31736337 NCT01920711 |
4896 | Patients with heart failure with preserved ejection fraction | 72 | As compared with valsartan, sacubitril-valsartan seemed to reduce the risk of heart failure hospitalization more in women than in men. |
Abbreviations: LV: left ventricular; MACE: Major adverse cardiac events; MESA: Multi-Ethnic Study of Atherosclerosis; STEMI: ST-segment elevation myocardial infarction; HFpEF: heart failure with preserved ejection fraction; BMI: body mass index; CAD: coronary artery disease; CAC: coronary artery calcium; ARIC: Atherosclerosis risk in the community study; AMI: acute myocardial infarction; CHD: coronary heart disease; CVD: cardiovascular disease; ACS: acute coronary syndrome; S/V: sacubitril valsartan.
Sex-Related Differences in Myocardial Aging
Mechanisms.
Myocardial aging encompasses multiple changes occurring over the human lifespan. Extending predominantly from sex-biased in gene expression in addition to sex chromosomal differences and potentially epigenetic factors,11-13 sex-based differences in myocardial aging trajectories include molecular, cellular, and interstitial changes that eventually result in macroscopic differences in size, shape, and function of the heart. Cellular longevity, in general, tends to exhibit intrinsic sex differences with males compared to females having consistently shorter telomeres, less robust mitochondrial function, and higher likelihood of deleterious somatic mutation.14-16 Accordingly, relative differences specific to myocyte aging trajectories are also seen. Relative to the female phenotype, the male myocytes undergo both more cellular loss and reactive hypertrophy due to increased necrosis and apoptosis.17,18 Some of these sex differences may well be mediated in early- to mid-life by estrogen and its derivatives, which have been shown to attenuate injury from ischemia and reperfusion,19-21 mitigate reactive oxygen species damage by modulating mitochondrial activity,22 and reduce cardiac fibroblast activation in part by downregulating collagen and matrix metalloprotease production and modifying the micro-RNA regulated fibrotic response to inflammation.23-26 The direct effects of relative estrogen loss in later life are uncertain. However, in murine models, extracellular matrix composition shifts distinctly between young and old female hearts in comparison to male hearts in terms of collagen composition.23,25 In particular, the ratio of collagen to elastin is significantly increased in females with the shift towards higher collagen production in the myocardial extracellular matrix.24,25
Myocardial Phenotypes.
Consistent with the recognition that sex-biased gene expression is relevant to multi-organ system phenotypes,13 there is clear evidence that females and males exhibit differences in cardiac morphology from early in life. Accordingly, normal values for cardiac chamber dimensions have long been reported in the setting of sex-specific reference limits by cardiac imaging and radiology specialists according to evidence-based guidelines.27 In turn, the patterns of cardiac aging (i.e., age-related cardiac remodeling) also differ by sex. When cross-sectionally compared to men, healthy appearing middle-aged to older-aged adult women have smaller left ventricular (LV) dimensions and lower stroke volumes, even after accounting for body size.28 Longitudinal differences are also seen, with women exhibiting higher age-related relative wall thickening10,27,29,30 and greater age-related systolic stiffening, greater systolic torsion, and greater circumferential shortening of the LV.31-33 Extending from the sex differences in cardiac remodeling seen in apparently healthy aging, females compared to males demonstrate greater concentric remodeling and diastolic dysfunction in response to stressors such as the afterload stress of aortic stenosis.34 These trends are observed in the context of more pronounced local inflammation and greater accumulation of predominantly interstitial myocardial fibrosis in females compared to males.35-37
Myocardial Outcomes.
Sex-specific changes in myocardial aging at least partly account for the frequently observed sex differences observed in phenotypes of heart failure. Older women are more likely than men of any age to develop heart failure with preserved ejection fraction (HFpEF), even after adjusting for differences in age and the potential effects of survival bias in older aged men compared to older aged women. Consistent data from observational studies and clinical trials have demonstrated that the cardiac aging phenotypes that are more common among women include more pronounced concentric remodeling and greater diastolic dysfunction that can predispose elderly women to HFpEF compared to men.38,39 The long-term burden of cardiometabolic risk factors have also been hypothesized to contribute to the progressive myocardial remodeling that eventually leads to cardiac dysfunction.39,40 Women appear more affected by the so-called cardiometabolic type of pre-clinical or clinical heart failure that tends to manifest with advancing age in the setting of hypertension, obesity, type 2 diabetes mellitus, or metabolic syndrome, all of which can also serve to increase risk for HFpEF in particular.40-44 Notably, women are also less likely to exhibit beneficial remodeling and reversal of LV hypertrophy after treatment for hypertension compared to men.45 Further reinforcing evidence of sex-specific susceptibility to cardiac disease phenotypes are emerging clinical trials data on sex-specific responsiveness to cardiac disease targeted therapies such as sacubitril/valsartan among many others.46-48
Sex-Related Differences in Vascular Aging
Mechanisms.
Vascular aging trajectories manifest in parallel with myocardial aging phenotypes, and also arise from sexual dimorphism in genetic factors.49 Amidst the multiple mechanisms implicated in overall vascular aging, several features are noted to be more sex specific.50 At the outset, the vasculature in females compare to males has been found to exhibit greater mineralocorticoid receptor expression51 and lesser baroreflex sensitivity,52,53 which set the stage for what appears to be greater salt sensitivity54 and differential neural-hemodynamic regulation of blood pressure55 with aging. In addition, in the setting of hormone receptor expression throughout the vasculature,56-58 studies have found that younger and premenopausal arteries exhibit limited amounts of endothelial inflammation as well as a robust vasodilatory capacity that is mediated by nitric oxide and serves to limit oxidative damage.59,60 In models and studies of the mid-to-later life vasculature, estrogen decline is associated with endothelial dysfunction dysfunction59 in the setting of increased reactive oxygen species production,22,61,62 oxidative stress22 and nitric oxide suppression.59 In particular, experimental and physiology data suggest that reductions in nitric oxide resulting from elevations in oxidative stress contribute not only to endothelial dysfunction but also to arterial stiffening in this setting.63 Concurrently, there is evidence of a more prominent trajectory in females not only in systemic inflammatory profiles but also inflammation at the cellular level, including reduced anti-inflammatory macrophage activity.64 These age-related changes, combined with baseline sex differences in vascular diameter even when accounting for body size, appear to promote a greater vascular sensitivity to CVD risk exposures and accelerated atherosclerosis in older women compared to age matched men.60,65,66 54,59,67-70
Coronary Vascular Phenotypes.
Patterns of vascular aging differ by sex across the range of vascular beds including the coronary and peripheral vasculature. With respect to coronary disease, women are more vulnerable to endothelial dysfunction and microvascular remodeling and these differences appear to start at a younger age in females than in males. Extensive literature on sex disparities in cardiac disease highlight the increased prevalence of coronary microvascular dysfunction contributing frequently to anginal symptoms in women, who can often present in younger and middle age.71-73 Extending from abnormalities in vascular function, structural vascular alterations including those derived from the coronary atherosclerotic process also differ by sex across the age spectrum. Younger-aged women have lower calcium scores than younger-aged men but women experience a faster increase in calcification score and progression of calcified plaque with advancing age.74,75 In the setting of clinical coronary disease, non-culprit coronary artery plaques in women compared to those in men tend to demonstrate greater plaque stability with smaller lipid arcs, fewer cholesterol crystals, and less lesion calcification. These plaques, however, are also more likely to exhibit plaque erosion, which also results in atherothrombotic events.76,77 Notably, whereas the incidence of thin cap fibroatheroma in women is lower than men before the age of 70, it becomes greater than men after the age of 70.78 Accordingly, coronary events in women shift from being more erosion-based in younger age to being more rupture-based in older age.79,80
Coronary Vascular Outcomes.
Beyond subclinical vascular alterations, which may or may not ultimately present with directly attributable symptoms in many aging adults, the clinically manifest presentations of vascular disease tend to also differ by sex and in ways that are more evident with aging. With respect to coronary disease in particular, women present clinically more often than men with non-obstructive CAD. Non-obstructive CAD, especially when it develops early in life, is much more likely than obstructive CAD to go unrecognized and thus untreated. Thus, after the passage of time, a case of non-obstructive CAD when finally manifest clinically can be found as more diffuse and extensive than a case of obstructive CAD that presents with a similar burden of clinical symptoms. This phenomenon may at least partly contribute to the observed tendency of non-obstructive CAD to promote epicardial and microvascular spasm and conduit vessel stiffening leading to myocardial ischemia. Potentially related to anatomical as well as physiological and pathophysiological sex-specific traits (e.g. females having smaller caliber coronary arteries than men even after accounting for differences in body size), women across the age spectrum are more likely than men to experience angina, ischemia, and acute coronary syndrome in the presence of non-obstructive CAD.81 Treatment advances for non-obstructive CAD lag behind those for obstructive CAD. This may be one reason why management of CAD in men has benefitted more from extensive advances in medical and interventional therapies, with accumulating evidence suggesting that CAD incidence and mortality rates have been plateauing and may even be increasing in young and middle-aged women.82-84
Systemic Vascular Phenotypes.
Similar to findings for the coronary vasculature, systemic vascular sex differences are also observed. Hemodynamic and neurohormonal factors contribute to age-related alterations in systemic vascular function in both sexes. While maintaining cardiac output and tissue perfusion at levels similar to males, females tend to manifest higher resting heart rates and augmented pulsatile load, particularly with aging. These trends reflect sex differences in age-related changes of dependence on sympathetic versus parasympathetic activation responses to hemodynamic stress and a potentially greater relative hemodynamic load experienced by females compared to males with advancing age.85-87 Accordingly, several studies have observed accelerated increase in measures of arterial stiffness in females compared to males, beginning in mid-life and most evident during the postmenopausal period.63,88 With respect to vascular structural changes, males have overall thicker femoral intima-media thickness (IMT) but females demonstrate more pronounced increases in femoral IMT with an increasing number of risk factors, even in the absence of symptoms.89 Differences in blood pressure trajectories are also noted. Studies also indicate that young and middle aged women have lower baseline blood pressure but more rapid blood pressure elevation over the life course, especially in the setting of cardiometabolic disease states such as hypercholesterolemia and diabetes.90-93 A similar pattern has been observed for central arterial stiffness. Although the average arterial stiffness is lower in women, it also increases more rapidly in women even after adjusting for body size and aortic diameter.88,94-96 These vascular differences are clinically reflected in patterns of hypertension prevalence over the life course—prior to age 45, hypertension is more prevalent among men; after age 65, hypertension is more prevalent among women. Collectively, these studies show that although premenopausal women have better overall vascular function than men of similar age, age-related vascular dysfunction progresses at a faster rate in women after controlling for sex-specific basal vascular function.97,98
Systemic Vascular Outcomes.
Sex differences in vascular aging patterns, beginning early in life, offer pathophysiological insights into why women manifest vascular outcomes differently – including presenting greater risk for myocardial infarction and stroke risk beginning at lower blood pressure thresholds and consistently experiencing worse stroke outcomes than men.99,100 Despite these differences seen in observational cohorts, it is worth noting that most published randomized control trials suggest that both sexes derive cardiovascular benefits with intensive control of modifiable risk factors such as blood pressure (Table 2).101,102 Although these trials were not pre-specified to evaluate for sex differences, and further work is needed to investigate potential sex-specific effects, the results to date are reassuring regarding the derivable benefit of treating hypertension across the lifespan. Perhaps more concerning are the sex differences seen in vascular outcomes for which there exist fewer options for early intervention. For instance, although aortic dissection is more prevalent in males than females, associated mortality is significantly higher in females.103 Similarly, although abdominal aortic aneurysm is more common in males than females, females have a significantly greater risk of rupture.104 For lower extremity peripheral arterial disease (PAD), the prevalence is reported to be similar or greater in women compared to men, although women with PAD have a greater extent of multi-vessel atherosclerotic disease identified at revascularization.105,106 Late presentation or under-recognition could account for some proportion of each of these disease disparities. However, the consistent theme of a more vulnerable and less stable clinical vascular phenotype in aging women points to the likelihood of common underlying factors – potentially accumulating and augmenting their effects with advancing age.
Hormonal Effects on Life Course Trajectories
A comprehensive examination of sex differences in cardiovascular aging phenotypes involves not only discerning how females and males differ but also a careful consideration of the sex-specific factors that impact aging trajectories – particularly sex hormones. There remains limited data on how age-related decrease in male sex hormones (e.g. testosterone and its metabolite dihydrotestosterone) may be contributors versus biomarkers of age-related CVD risk in men.107,108 On the other hand, a large body of evidence has emerged regarding the role of ovarian aging – reflected by life course changes in female sex hormones – on age-related CVD risk in women.109,110 Considered a metric biological aging in females, reproductive longevity can be estimated using a variety of measures (e.g. age at first or last reproduction and age of menarche and menopause). Accelerated ovarian aging (i.e. shortened reproductive longevity) has been linked to epigenetic, metabolic, and oxidative stressors and the development of age-related risk factors as well as both subclinical and clinical CVD phenotypes.111-116 Accompanying variations in sex hormones have been associated with especially pronounced alterations in glucose metabolism, lipid homeostasis, and adipose tissue distribution in females.117,118 These findings are concordant with the long-recognized greater risk of cardiovascular outcomes conferred by cardiometabolic risk factors, particularly obesity and diabetes, in women compared to men with or without overt clinical cardiac disease.119-121
The Stages of Reproductive Aging Workshop identified ten sequential stages of relatively distinct female sex hormone profiles as representing key phases of biological versus chronological aging in females, with each stage organized as either before or after the final menstrual period – considered the cardinal ovarian aging event.122 Importantly, across each of these life stages, the age-related decreases in estradiol and increases in follicle-stimulating hormone are known to be progressive, rather than sudden, with variations in longitudinal trajectories that can be used to identify females as having accelerated versus delayed ovarian aging profiles.123 Concordant with the concept that ovarian aging is more a continuous rather than precipitous process, 10 to 20 year longitudinal studies have found that trajectories of anti-mullerian hormone levels – a measure of ovarian reserve – are associated with worsening lipid profiles as well as excess risk for CVD and particularly coronary heart disease.124,125 Therefore, the more pronounced age-related cardiovascular risks seen in postmenopausal compared to premenopausal women and similarly aged men appear less likely due to an abrupt withdrawal of endogenous sex steroids during the ‘menopausal transition’, and more likely related to a progressive accumulation of hormone mediated effects that begin in early adulthood, accelerate in midlife, and culminate in late life. This framework for considering ovarian aging effects of the cardiovascular system is aligned with the observed steadily progressing subclinical myocardial and vascular changes in aging women that begin their course decades prior to the menopausal transition and then, later on, further increase in rate of development.
The longitudinal perspective of ovarian aging is also potentially helpful for considering why most trials of exogenous hormone replacement therapy after menopause have shown no reduction in either subclinical or overt CVD outcomes.126-129 Notwithstanding the effects of cumulative estrogen deficiency, conventional approaches to hormone replacement therapy may represent a mismatch of dosage and timing that may or may not be resolved by future studies.130-132 Therefore, at present, there remains clinical equipoise around hormone therapies.133 Relatedly, there remains a lack of clarity regarding the complex inter-related influences of general somatic aging, ovarian aging, and cardiovascular risk.110,134 While ovarian aging can promote the development of subclinical and then clinical myocardial and vascular disease, cardiovascular risk may also predispose to ovarian aging (e.g. atherosclerosis involving microvasculature of the ovaries),135 and age-related somatic mutations can affect both.136,137
Notably, additional approaches to clarifying hormone-related cardiovascular risk can involve examining the relative effects of sex-determining hormones in gender-affirming hormone therapy within transgender populations. Early data suggest that transgender females experience increased thromboembolic disease, whereas transgender males develop lipid profiles reflecting their gender rather than natal sex potentially without adverse cardiovascular risk.138,139 Further longitudinal and outcomes studies are needed to improve our understanding of the interaction between sex-determining hormones and the aging trajectory.140,141
Conclusion
Over the past two decades, tremendous progress has been made in unveiling and clarifying age-based sex variation in cardiovascular phenotypes and outcomes. Evidence to date indicates that female-male differences in the development and progression of CVD arise from a combination of intrinsic, stochastic, and environmental factors influencing myocardial and vascular aging trajectories. In particular, intrinsic biological between-sex differences manifest as measurable relative dimorphism in cardiac and vascular structure and function, and this dimorphism sets the stage for the frequently observed sex divergent trajectories of response to cardiovascular risk exposures over the life course. The accumulating body of data suggests that sex differences in cardiovascular risk should be accounted for in all clinical trials and in a manner that also accounts for the differing trajectories of risk in women. It is possible that trials that deliberately include younger female populations and address risk factor modifications at an earlier age may reveal previously unforeseen benefits in the management of CVD in women. Together with ongoing work to investigate sex-based variation in cardiovascular risks across the lifespan, these broadened approaches promise to further our understanding of how to better mitigate potentially adverse sequelae of cardiovascular aging in both sexes.
Funding/Support.
This study was funded in part by the National Natural Science Foundation of China (Grant ID. 82103908), the Shandong Provincial Natural Science Foundation (ZR2021QH014), the Doris Duke Charitable Foundation Grant 2020059, the Finnish Foundation for Cardiovascular Research, the Emil Aaltonen Foundation, the Academy of Finland grant no 321351, and National Institutes of Health grants R01p-HL134168, R01-HL131532, R01-HL143227, R01-HL142983, R01-HL146158, K23-HL153888, K99HL157421, and U54-AG065141.
Role of the Funder/Sponsor.
The funding sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or, decision to submit the manuscript for publication.
Footnotes
Disclosures. SC has served as a consultant for Zogenix.
REFERENCES
- 1.Seeland U, Nemcsik J, Lønnebakken MT, Kublickiene K, Schluchter H, Park C, Pucci G, Mozos I and Bruno RM. Sex and Gender Aspects in Vascular Ageing - Focus on Epidemiology, Pathophysiology, and Outcomes. Heart Lung Circ. 2021;30:1637–1646. [DOI] [PubMed] [Google Scholar]
- 2.Hemal K, Pagidipati NJ, Coles A, Dolor RJ, Mark DB, Pellikka PA, Hoffmann U, Litwin SE, Daubert MA, Shah SH, Ariani K, Bullock-Palmer RP, Martinez B, Lee KL and Douglas PS. Sex Differences in Demographics, Risk Factors, Presentation, and Noninvasive Testing in Stable Outpatients With Suspected Coronary Artery Disease: Insights From the PROMISE Trial. JACC Cardiovasc Imaging. 2016;9:337–46. PMC4982809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mc Auley MT, Guimera AM, Hodgson D, McDonald N, Mooney KM, Morgan AE and Proctor CJ. Modelling the molecular mechanisms of aging. Biosci Rep. 2017;37.PMC5322748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA, Richardson A, Schadt EE, Wyss-Coray T and Sierra F. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–13.PMC4852871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marian AJ, Bhatnagar A, Bolli R and Belmonte JCI. Introduction to Cardiovascular Aging Compendium. Circulation Research. 2018;123:737–739. [DOI] [PubMed] [Google Scholar]
- 6.Ungvari Z, Tarantini S, Donato AJ, Galvan V and Csiszar A. Mechanisms of Vascular Aging. Circulation Research. 2018;123:849–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakayama H, Nishida K and Otsu K. Macromolecular Degradation Systems and Cardiovascular Aging. Circulation Research. 2016;118:1577–1592. [DOI] [PubMed] [Google Scholar]
- 8.Guillaume M, Lapidus L and Lambert A. Differences in associations of familial and nutritional factors with serum lipids between boys and girls: the Luxembourg Child Study. Am J Clin Nutr. 2000;72:384–8. [DOI] [PubMed] [Google Scholar]
- 9.Bohm B, Hartmann K, Buck M and Oberhoffer R. Sex differences of carotid intima-media thickness in healthy children and adolescents. Atherosclerosis. 2009;206:458–63. [DOI] [PubMed] [Google Scholar]
- 10.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ and Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation. 2005;112:2254–62. [DOI] [PubMed] [Google Scholar]
- 11.Hartman RJG, Huisman SE and den Ruijter HM. Sex differences in cardiovascular epigenetics—a systematic review. Biology of Sex Differences. 2018;9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ingleby FC, Flis I and Morrow EH. Sex-biased gene expression and sexual conflict throughout development. Cold Spring Harb Perspect Biol. 2014;7:a017632.PMC4292171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naqvi S, Godfrey AK, Hughes JF, Goodheart ML, Mitchell RN and Page DC. Conservation, acquisition, and functional impact of sex-biased gene expression in mammals. Science. 2019;365.PMC6896219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrett EL and Richardson DS. Sex differences in telomeres and lifespan. Aging cell. 2011;10:913–921. [DOI] [PubMed] [Google Scholar]
- 15.Podolskiy DI, Lobanov AV, Kryukov GV and Gladyshev VN. Analysis of cancer genomes reveals basic features of human aging and its role in cancer development. Nature communications. 2016;7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viña J, Sastre J, Pallardó F and Borrás C. Mitochondrial theory of aging: importance to explain why females live longer than males. Antioxidants and Redox Signaling. 2003;5:549–556. [DOI] [PubMed] [Google Scholar]
- 17.Olivetti G, Melissari M, Capasso J and Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circulation research. 1991;68:1560–1568. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X-P, Vatner SF, Shen Y-T, Rossi F, Tian Y, Peppas A, Resuello RR, Natividad FF and Vatner DE. Increased apoptosis and myocyte enlargement with decreased cardiac mass; distinctive features of the aging male, but not female, monkey heart. Journal of molecular and cellular cardiology. 2007;43:487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menazza S, Sun J, Appachi S, Chambliss KL, Kim SH, Aponte A, Khan S, Katzenellenbogen JA, Katzenellenbogen BS and Shaul PW. Non-nuclear estrogen receptor alpha activation in endothelium reduces cardiac ischemia-reperfusion injury in mice. Journal of molecular and cellular cardiology. 2017;107:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rocca C, Femminò S, Aquila G, Granieri MC, De Francesco EM, Pasqua T, Rigiracciolo DC, Fortini F, Cerra MC and Maggiolini M. Notch1 mediates preconditioning protection induced by GPER in normotensive and hypertensive female rat hearts. Frontiers in physiology. 2018;9:521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shao Q, Fallica J, Casin KM, Murphy E, Steenbergen C and Kohr MJ. Characterization of the sex-dependent myocardial S-nitrosothiol proteome. American Journal of Physiology-Heart and Circulatory Physiology. 2016;310:H505–H515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lagranha CJ, Deschamps A, Aponte A, Steenbergen C and Murphy E. Sex differences in the phosphorylation of mitochondrial proteins result in reduced production of reactive oxygen species and cardioprotection in females. Circulation research. 2010;106:1681–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Achkar A, Saliba Y and Fares N. Differential Gender-Dependent Patterns of Cardiac Fibrosis and Fibroblast Phenotypes in Aging Mice. Oxidative medicine and cellular longevity. 2020;2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dworatzek E, Baczko I and Kararigas G. Effects of aging on cardiac extracellular matrix in men and women. PROTEOMICS–Clinical Applications. 2016;10:84–91. [DOI] [PubMed] [Google Scholar]
- 25.Dworatzek E, Mahmoodzadeh S, Schriever C, Kusumoto K, Kramer L, Santos G, Fliegner D, Leung Y-K, Ho S-M and Zimmermann W-H. Sex-specific regulation of collagen I and III expression by 17β-Estradiol in cardiac fibroblasts: role of estrogen receptors. Cardiovascular research. 2019;115:315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Queirós AM, Eschen C, Fliegner D, Kararigas G, Dworatzek E, Westphal C, Ruderisch HS and Regitz-Zagrosek V. Sex-and estrogen-dependent regulation of a miRNA network in the healthy and hypertrophied heart. International journal of cardiology. 2013;169:331–338. [DOI] [PubMed] [Google Scholar]
- 27.Bairey Merz CN, Nelson MD, Cheng S and Wei J. Sex differences and the left ventricle: morphology matters. Eur Heart J Cardiovasc Imaging. 2020;21:991–993.PMC7440960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oneglia A, Nelson MD and Merz CNB. Sex Differences in Cardiovascular Aging and Heart Failure. Current Heart Failure Reports. 2020;17:409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng S, Xanthakis V, Sullivan LM, Lieb W, Massaro J, Aragam J, Benjamin EJ and Vasan RS. Correlates of echocardiographic indices of cardiac remodeling over the adult life course: longitudinal observations from the Framingham Heart Study. Circulation. 2010;122:570–8.PMC2942081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen C, Sung KT, Shih SC, Liu CC, Kuo JY, Hou CJ, Hung CL and Yeh HI. Age, Gender and Load-Related Influences on Left Ventricular Geometric Remodeling, Systolic Mid-Wall Function, and NT-ProBNP in Asymptomatic Asian Population. PLoS One. 2016;11:e0156467.PMC4900638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung AK, Das SR, Leonard D, Peshock RM, Kazi F, Abdullah SM, Canham RM, Levine BD and Drazner MH. Women have higher left ventricular ejection fractions than men independent of differences in left ventricular volume: the Dallas Heart Study. Circulation. 2006;113:1597–604. [DOI] [PubMed] [Google Scholar]
- 32.Gebhard C, Stahli BE, Gebhard CE, Tasnady H, Zihler D, Wischnewsky MB, Jenni R and Tanner FC. Age- and gender-dependent left ventricular remodeling. Echocardiography. 2013;30:1143–50. [DOI] [PubMed] [Google Scholar]
- 33.Yoneyama K, Gjesdal O, Choi EY, Wu CO, Hundley WG, Gomes AS, Liu CY, McClelland RL, Bluemke DA and Lima JA. Age, sex, and hypertension-related remodeling influences left ventricular torsion assessed by tagged cardiac magnetic resonance in asymptomatic individuals: the multi-ethnic study of atherosclerosis. Circulation. 2012;126:2481–90.PMC3558706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tastet L, Kwiecinski J, Pibarot P, Capoulade R, Everett Russell J, Newby David E, Shen M, Guzzetti E, Arsenault M, Bédard É, Larose É, Beaudoin J, Dweck M and Clavel M-A. Sex-Related Differences in the Extent of Myocardial Fibrosis in Patients With Aortic Valve Stenosis. JACC: Cardiovascular Imaging. 2020;13:699–711. [DOI] [PubMed] [Google Scholar]
- 35.Liu CY, Liu YC, Wu C, Armstrong A, Volpe GJ, van der Geest RJ, Liu Y, Hundley WG, Gomes AS, Liu S, Nacif M, Bluemke DA and Lima JAC. Evaluation of age-related interstitial myocardial fibrosis with cardiac magnetic resonance contrast-enhanced T1 mapping: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2013;62:1280–1287.PMC3807823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalez A, Schelbert EB, Diez J and Butler J. Myocardial Interstitial Fibrosis in Heart Failure: Biological and Translational Perspectives. J Am Coll Cardiol. 2018;71:1696–1706. [DOI] [PubMed] [Google Scholar]
- 37.Paulus WJ and Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–71. [DOI] [PubMed] [Google Scholar]
- 38.Beale AL, Meyer P, Marwick TH, Lam CSP and Kaye DM. Sex Differences in Cardiovascular Pathophysiology: Why Women Are Overrepresented in Heart Failure With Preserved Ejection Fraction. Circulation. 2018;138:198–205. [DOI] [PubMed] [Google Scholar]
- 39.Gori M, Lam CS, Gupta DK, Santos AB, Cheng S, Shah AM, Claggett B, Zile MR, Kraigher-Krainer E, Pieske B, Voors AA, Packer M, Bransford T, Lefkowitz M, McMurray JJ, Solomon SD and Investigators P. Sex-specific cardiovascular structure and function in heart failure with preserved ejection fraction. Eur J Heart Fail. 2014;16:535–42. [DOI] [PubMed] [Google Scholar]
- 40.Gerdts E and Regitz-Zagrosek V. Sex differences in cardiometabolic disorders. Nat Med. 2019;25:1657–1666. [DOI] [PubMed] [Google Scholar]
- 41.Ho JE, Lyass A, Lee DS, Vasan RS, Kannel WB, Larson MG and Levy D. Predictors of new-onset heart failure: differences in preserved versus reduced ejection fraction. Circ Heart Fail. 2013;6:279–86.PMC3705220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savji N, Meijers WC, Bartz TM, Bhambhani V, Cushman M, Nayor M, Kizer JR, Sarma A, Blaha MJ, Gansevoort RT, Gardin JM, Hillege HL, Ji F, Kop WJ, Lau ES, Lee DS, Sadreyev R, van Gilst WH, Wang TJ, Zanni MV, Vasan RS, Allen NB, Psaty BM, van der Harst P, Levy D, Larson M, Shah SJ, de Boer RA, Gottdiener JS and Ho JE. The Association of Obesity and Cardiometabolic Traits With Incident HFpEF and HFrEF. JACC Heart Fail. 2018;6:701–709.PMC6076337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy E, Amanakis G, Fillmore N, Parks RJ and Sun J. Sex Differences in Metabolic Cardiomyopathy. Cardiovasc Res. 2017;113:370–377.PMC5852638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schulze PC, Drosatos K and Goldberg IJ. Lipid Use and Misuse by the Heart. Circ Res. 2016;118:1736–51.PMC5340419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerdts E, Okin PM, de Simone G, Cramariuc D, Wachtell K, Boman K and Devereux RB. Gender differences in left ventricular structure and function during antihypertensive treatment: the Losartan Intervention for Endpoint Reduction in Hypertension Study. Hypertension. 2008;51:1109–14. [DOI] [PubMed] [Google Scholar]
- 46.Ibrahim NE, Piña IL, Camacho A, Bapat D, Felker GM, Maisel AS, Butler J, Prescott MF, Abbas CA, Solomon SD and Januzzi JL Jr. Sex-based differences in biomarkers, health status, and reverse cardiac remodelling in patients with heart failure with reduced ejection fraction treated with sacubitril/valsartan. Eur J Heart Fail. 2020;22:2018–2025.PMC7756516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McMurray JJV, Jackson AM, Lam CSP, Redfield MM, Anand IS, Ge J, Lefkowitz MP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Rizkala AR, Sabarwal SV, Shah AM, Shah SJ, Shi VC, van Veldhuisen DJ, Zannad F, Zile MR, Cikes M, Goncalvesova E, Katova T, Kosztin A, Lelonek M, Sweitzer N, Vardeny O, Claggett B, Jhund PS and Solomon SD. Effects of Sacubitril-Valsartan Versus Valsartan in Women Compared With Men With Heart Failure and Preserved Ejection Fraction: Insights From PARAGON-HF. Circulation. 2020;141:338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tamargo J, Rosano G, Walther T, Duarte J, Niessner A, Kaski J, Ceconi C, Drexel H, Kjeldsen K, Savarese G, Torp-Pedersen C, Atar D, Lewis B and Agewall S. Gender differences in the effects of cardiovascular drugs. European Heart Journal - Cardiovascular Pharmacotherapy. 2017;3:163–182. [DOI] [PubMed] [Google Scholar]
- 49.Kauko A, Aittokallio J, Vaura F, Ji H, Ebinger JE, Niiranen T and Cheng S. Sex Differences in Genetic Risk for Hypertension. Hypertension. 2021;78:1153–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dela Justina V, Miguez JSG, Priviero F, Sullivan JC, Giachini FR and Webb RC. Sex Differences in Molecular Mechanisms of Cardiovascular Aging. Frontiers in Aging. 2021;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DuPont JJ, Kim SK, Kenney RM and Jaffe IZ. Sex differences in the time course and mechanisms of vascular and cardiac aging in mice: role of the smooth muscle cell mineralocorticoid receptor. American Journal of Physiology-Heart and Circulatory Physiology. 2021;320:H169–H180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huikuri HV, Pikkuja¨ msa¨ SM, Airaksinen KJ, Ika¨ heimo MJ, Rantala AO, Kauma H, Lilja M and Kesa¨ niemi YA. Sex-related differences in autonomic modulation of heart rate in middle-aged subjects. Circulation. 1996;94:122–125. [DOI] [PubMed] [Google Scholar]
- 53.Lacolley P, Regnault V, Segers P and Laurent S. Vascular smooth muscle cells and arterial stiffening: relevance in development, aging, and disease. Physiological reviews. 2017;97:1555–1617. [DOI] [PubMed] [Google Scholar]
- 54.Tominaga T, Suzuki H, Ogata Y, Matsukawa S and Saruta T. The role of sex hormones and sodium intake in postmenopausal hypertension. Journal of human hypertension. 1991;5:495–500. [PubMed] [Google Scholar]
- 55.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH and Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance: implications for human blood pressure regulation. Hypertension. 2009;53:571–6.PMC3733790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vitale C, Fini M, Speziale G and Chierchia S. Gender differences in the cardiovascular effects of sex hormones. Fundam Clin Pharmacol. 2010;24:675–85. [DOI] [PubMed] [Google Scholar]
- 57.Crandall CJ and Barrett-Connor E. Endogenous sex steroid levels and cardiovascular disease in relation to the menopause: a systematic review. Endocrinol Metab Clin North Am. 2013;42:227–53. [DOI] [PubMed] [Google Scholar]
- 58.Connelly PJ, Casey H, Montezano AC, Touyz RM and Delles C. Sex steroids receptors, hypertension, and vascular ageing. J Hum Hypertens. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moreau KL, Stauffer BL, Kohrt WM and Seals DR. Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. The Journal of Clinical Endocrinology & Metabolism. 2013;98:4507–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moreau KL. Modulatory influence of sex hormones on vascular aging. Am J Physiol Heart Circ Physiol. 2019;316:H522–h526.PMC6957359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu H, Yanamandala M, Lee TC and Kim JK. Mitochondrial p38β and manganese superoxide dismutase interaction mediated by estrogen in cardiomyocytes. PloS one. 2014;9:e85272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun X and Feinberg MW. Vascular endothelial senescence: pathobiological insights, emerging long noncoding RNA targets, challenges and therapeutic opportunities. Frontiers in Physiology. 2021;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.DuPont JJ, Kenney RM, Patel AR and Jaffe IZ. Sex differences in mechanisms of arterial stiffness. British journal of pharmacology. 2019;176:4208–4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sowers MR, Jannausch M, Randolph JF, McConnell D, Little R, Lasley B, Pasternak R, Sutton-Tyrrell K and Matthews KA. Androgens are associated with hemostatic and inflammatory factors among women at the mid-life. The Journal of Clinical Endocrinology & Metabolism. 2005;90:6064–6071. [DOI] [PubMed] [Google Scholar]
- 65.Moreau KL, Babcock MC and Hildreth KL. Sex differences in vascular aging in response to testosterone. Biol Sex Differ. 2020;11:18.PMC7161199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stanhewicz AE, Wenner MM and Stachenfeld NS. Sex differences in endothelial function important to vascular health and overall cardiovascular disease risk across the lifespan. Am J Physiol Heart Circ Physiol. 2018;315:H1569–H1588.PMC6734083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coutinho T, Yam Y, Chow BJ, Dwivedi G and Inácio J. Sex differences in associations of arterial compliance with coronary artery plaque and calcification burden. Journal of the American Heart Association. 2017;6:e006079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rees M and Stevenson J. Primary prevention of coronary heart disease in women. Menopause international. 2008;14:40–45. [DOI] [PubMed] [Google Scholar]
- 69.AlGhatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P, Scuteri A, Najjar SS, Ferrucci L and Lakatta EG. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension. 2013;62:934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reis SE, Gloth ST, Blumenthal RS, Resar JR, Zacur HA, Gerstenblith G and Brinker JA. Ethinyl estradiol acutely attenuates abnormal coronary vasomotor responses to acetylcholine in postmenopausal women. Circulation. 1994;89:52–60. [DOI] [PubMed] [Google Scholar]
- 71.Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO and Lerman A. Prevalence of Coronary Microvascular Dysfunction Among Patients With Chest Pain and Nonobstructive Coronary Artery Disease. JACC Cardiovasc Interv. 2015;8:1445–1453. [DOI] [PubMed] [Google Scholar]
- 72.Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G and Bairey Merz CN. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825–32.PMC2898523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shaw LJ, Bugiardini R and Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol. 2009;54:1561–75.PMC2789479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McClelland RL, Chung H, Detrano R, Post W and Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2006;113:30–7. [DOI] [PubMed] [Google Scholar]
- 75.Lee SE, Sung JM, Andreini D, Al-Mallah MH, Budoff MJ, Cademartiri F, Chinnaiyan K, Choi JH, Chun EJ, Conte E, Gottlieb I, Hadamitzky M, Kim YJ, Lee BK, Leipsic JA, Maffei E, Marques H, de Araujo Goncalves P, Pontone G, Shin S, Stone PH, Samady H, Virmani R, Narula J, Berman DS, Shaw LJ, Bax JJ, Lin FY, Min JK and Chang HJ. Sex Differences in Compositional Plaque Volume Progression in Patients With Coronary Artery Disease. JACC Cardiovasc Imaging. 2020;13:2386–2396. [DOI] [PubMed] [Google Scholar]
- 76.Kataoka Y, Puri R, Hammadah M, Duggal B, Uno K, Kapadia SR, Tuzcu EM, Nissen SE, King P and Nicholls SJ. Sex Differences in Nonculprit Coronary Plaque Microstructures on Frequency-Domain Optical Coherence Tomography in Acute Coronary Syndromes and Stable Coronary Artery Disease. Circ Cardiovasc Imaging. 2016;9. [DOI] [PubMed] [Google Scholar]
- 77.de Bakker M, Timmerman N, van Koeverden ID, de Kleijn DPV, de Borst GJ, Pasterkamp G, Boersma E and den Ruijter HM. The age- and sex-specific composition of atherosclerotic plaques in vascular surgery patients. Atherosclerosis. 2020;310:1–10. [DOI] [PubMed] [Google Scholar]
- 78.Sato T, Minami Y, Asakura K, Katamine M, Kato A, Katsura A, Muramatsu Y, Kakizaki R, Nemoto T, Hashimoto T, Fujiyoshi K, Kameda R, Meguro K, Shimohama T, Ako J. Age- and Gender-Related Differences in Coronary Lesion Plaque Composition on Optical Coherence Tomography. Circ J. 2020. Feb 25;84(3):463–470. doi: 10.1253/circj.CJ-19-0859. Epub 2020 Jan 25. PMID: 31983726. [DOI] [PubMed] [Google Scholar]
- 79.Yahagi K, Davis HR, Arbustini E and Virmani R. Sex differences in coronary artery disease: pathological observations. Atherosclerosis. 2015;239:260–267. [DOI] [PubMed] [Google Scholar]
- 80.Chakrabarti S, Morton JS and Davidge ST. Mechanisms of estrogen effects on the endothelium: an overview. Canadian Journal of Cardiology. 2014;30:705–712. [DOI] [PubMed] [Google Scholar]
- 81.Pepine CJ, Ferdinand KC, Shaw LJ, Light-McGroary KA, Shah RU, Gulati M, Duvernoy C, Walsh MN, Bairey Merz CN and Committee ACiW. Emergence of Nonobstructive Coronary Artery Disease: A Woman's Problem and Need for Change in Definition on Angiography. J Am Coll Cardiol. 2015;66:1918–33.PMC4618799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arora S, Stouffer GA, Kucharska-Newton AM, Qamar A, Vaduganathan M, Pandey A, Porterfield D, Blankstein R, Rosamond WD, Bhatt DL and Caughey MC. Twenty Year Trends and Sex Differences in Young Adults Hospitalized With Acute Myocardial Infarction. Circulation. 2019;139:1047–1056.PMC6380926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gabet A, Danchin N, Juilliere Y and Olie V. Acute coronary syndrome in women: rising hospitalizations in middle-aged French women, 2004-14. Eur Heart J. 2017;38:1060–1065. [DOI] [PubMed] [Google Scholar]
- 84.Wilmot KA, O'Flaherty M, Capewell S, Ford ES and Vaccarino V. Coronary Heart Disease Mortality Declines in the United States From 1979 Through 2011: Evidence for Stagnation in Young Adults, Especially Women. Circulation. 2015;132:997–1002.PMC4828724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hart EC, Joyner MJ, Wallin BG and Charkoudian N. Sex, ageing and resting blood pressure: gaining insights from the integrated balance of neural and haemodynamic factors. J Physiol. 2012;590:2069–79.PMC3447151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Joyner MJ, Barnes JN, Hart EC, Wallin BG and Charkoudian N. Neural control of the circulation: how sex and age differences interact in humans. Compr Physiol. 2015;5:193–215.PMC4459710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Joyner MJ, Wallin BG and Charkoudian N. Sex differences and blood pressure regulation in humans. Exp Physiol. 2016;101:349–55. [DOI] [PubMed] [Google Scholar]
- 88.Lu Y, Pechlaner R, Cai J, Yuan H, Huang Z, Yang G, Wang J, Chen Z, Kiechl S and Xu Q. Trajectories of Age-Related Arterial Stiffness in Chinese Men and Women. J Am Coll Cardiol. 2020;75:870–880. [DOI] [PubMed] [Google Scholar]
- 89.Paul TK, Chen W, Srinivasan SR, Toprak A, He J and Berenson GS. Gender divergence on the impact of multiple cardiovascular risk factors on the femoral artery intima-media thickness in asymptomatic young adults: the Bogalusa Heart Study. Am J Med Sci. 2012;343:40–5.PMC3179814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ji H, Kim A, Ebinger JE, Niiranen TJ, Claggett BL, Bairey Merz CN and Cheng S. Sex Differences in Blood Pressure Trajectories Over the Life Course. JAMA Cardiol. 2020;5:19–26.PMC6990675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wills AK, Lawlor DA, Matthews FE, Sayer AA, Bakra E, Ben-Shlomo Y, Benzeval M, Brunner E, Cooper R, Kivimaki M, Kuh D, Muniz-Terrera G and Hardy R. Life course trajectories of systolic blood pressure using longitudinal data from eight UK cohorts. PLoS Med. 2011;8:e1000440.PMC3114857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shen W, Zhang T, Li S, Zhang H, Xi B, Shen H, Fernandez C, Bazzano L, He J and Chen W. Race and Sex Differences of Long-Term Blood Pressure Profiles From Childhood and Adult Hypertension: The Bogalusa Heart Study. Hypertension. 2017;70:66–74.PMC5711390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ji H, Kim A, Ebinger JE, Niiranen TJ, Claggett BL, Merz CNB and Cheng S. Cardiometabolic Risk-Related Blood Pressure Trajectories Differ by Sex. Hypertension. 2020;75:e6–e9.PMC7286096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS and Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–45. [DOI] [PubMed] [Google Scholar]
- 95.Mitchell GF, Gudnason V, Launer LJ, Aspelund T and Harris TB. Hemodynamics of increased pulse pressure in older women in the community-based Age, Gene/Environment Susceptibility-Reykjavik Study. Hypertension. 2008;51:1123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Avolio AP, Kuznetsova T, Heyndrickx GR, Kerkhof PLM and Li JK. Arterial Flow, Pulse Pressure and Pulse Wave Velocity in Men and Women at Various Ages. Adv Exp Med Biol. 2018;1065:153–168. [DOI] [PubMed] [Google Scholar]
- 97.Barnett SR, Morin RJ, Kiely DK, Gagnon M, Azhar G, Knight EL, Nelson JC and Lipsitz LA. Effects of age and gender on autonomic control of blood pressure dynamics. Hypertension. 1999;33:1195–200. [DOI] [PubMed] [Google Scholar]
- 98.Christou DD, Jones PP, Jordan J, Diedrich A, Robertson D and Seals DR. Women have lower tonic autonomic support of arterial blood pressure and less effective baroreflex buffering than men. Circulation. 2005;111:494–8. [DOI] [PubMed] [Google Scholar]
- 99.Ji H, Niiranen TJ, Rader F, Henglin M, Kim A, Ebinger JE, Claggett B, Merz CNB and Cheng S. Sex Differences in Blood Pressure Associations With Cardiovascular Outcomes. Circulation. 2021;143:761–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A and Lisabeth L. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–26.PMC2665267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Group SR, Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr., Fine LJ, Cutler JA, Cushman WC, Cheung AK and Ambrosius WT. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–16.PMC4689591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Group AS, Cushman WC, Evans GW, Byington RP, Goff DC Jr., Grimm RH Jr., Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC and Ismail-Beigi F. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–85.PMC4123215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nienaber CA, Fattori R, Mehta RH, Richartz BM, Evangelista A, Petzsch M, Cooper JV, Januzzi JL, Ince H, Sechtem U, Bossone E, Fang J, Smith DE, Isselbacher EM, Pape LA and Eagle KA. Gender-related differences in acute aortic dissection. Circulation. 2004;109:3014–21. [DOI] [PubMed] [Google Scholar]
- 104.Boese AC, Chang L, Yin K-J, Chen YE, Lee J-P and Hamblin MH. Sex differences in abdominal aortic aneurysms. American Journal of Physiology-Heart and Circulatory Physiology. 2018;314:H1137–H1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jackson EA, Munir K, Schreiber T, Rubin JR, Cuff R, Gallagher KA, Henke PK, Gurm HS and Grossman PM. Impact of sex on morbidity and mortality rates after lower extremity interventions for peripheral arterial disease: observations from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium. J Am Coll Cardiol. 2014;63:2525–2530. [DOI] [PubMed] [Google Scholar]
- 106.McCoach CE, Armstrong EJ, Singh S, Javed U, Anderson D, Yeo KK, Westin GG, Hedayati N, Amsterdam EA and Laird JR. Gender-related variation in the clinical presentation and outcomes of critical limb ischemia. Vasc Med. 2013;18:19–26.PMC3786085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yeap BB. Sex steroids and cardiovascular disease. Asian J Androl. 2014;16:239–47.PMC3955333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yeap BB. Testosterone and cardiovascular disease risk. Curr Opin Endocrinol Diabetes Obes. 2015;22:193–202. [DOI] [PubMed] [Google Scholar]
- 109.Khoudary SRE, Aggarwal B, Beckie TM, Hodis HN, Johnson AE, Langer RD, Limacher MC, Manson JE, Stefanick ML and Allison MA. Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement From the American Heart Association. Circulation. 2020;142:e506–e532. [DOI] [PubMed] [Google Scholar]
- 110.Quinn MM and Cedars MI. Cardiovascular health and ovarian aging. Fertil Steril. 2018;110:790–793. [DOI] [PubMed] [Google Scholar]
- 111.Atsma F, Bartelink ML, Grobbee DE and van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause. 2006;13:265–79. [DOI] [PubMed] [Google Scholar]
- 112.Zhu D, Chung HF, Dobson AJ, Pandeya N, Giles GG, Bruinsma F, Brunner EJ, Kuh D, Hardy R, Avis NE, Gold EB, Derby CA, Matthews KA, Cade JE, Greenwood DC, Demakakos P, Brown DE, Sievert LL, Anderson D, Hayashi K, Lee JS, Mizunuma H, Tillin T, Simonsen MK, Adami HO, Weiderpass E and Mishra GD. Age at natural menopause and risk of incident cardiovascular disease: a pooled analysis of individual patient data. Lancet Public Health. 2019;4:e553–e564.PMC7118366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Honigberg MC, Zekavat SM, Aragam K, Finneran P, Klarin D, Bhatt DL, Januzzi JL Jr., Scott NS and Natarajan P. Association of Premature Natural and Surgical Menopause With Incident Cardiovascular Disease. JAMA. 2019;322:2411–2421.PMC7231649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Muka T, Oliver-Williams C, Kunutsor S, Laven JS, Fauser BC, Chowdhury R, Kavousi M and Franco OH. Association of Age at Onset of Menopause and Time Since Onset of Menopause With Cardiovascular Outcomes, Intermediate Vascular Traits, and All-Cause Mortality: A Systematic Review and Meta-analysis. JAMA Cardiol. 2016;1:767–776. [DOI] [PubMed] [Google Scholar]
- 115.Schuster V, Eggersmann TK, Eifert S, Ueberfuhr P, Zugenmaier B, Kolben TM, Thaler CJ, Kublickiene K, Rieger A, Reichart B, Hagl C, Pichlmaier MA and Guethoff S. Ascending Aortic Disease is Associated with Earlier Menopause and Shorter Reproductive Life Span. J Womens Health (Larchmt). 2016;25:912–9. [DOI] [PubMed] [Google Scholar]
- 116.Chou EL, Pettinger M, Haring B, Allison MA, Mell MW, Hlatky MA, Wactawski-Wende J, Wild RA, Shadyab AH, Wallace RB, Snetselaar LG, Madsen TE, Eagleton MJ, Conrad MF and Liu S. Association of Premature Menopause With Risk of Abdominal Aortic Aneurysm in the Women's Health Initiative. Ann Surg. 2020. [DOI] [PubMed] [Google Scholar]
- 117.Ventura-Clapier R, Dworatzek E, Seeland U, Kararigas G, Arnal JF, Brunelleschi S, Carpenter TC, Erdmann J, Franconi F, Giannetta E, Glezerman M, Hofmann SM, Junien C, Katai M, Kublickiene K, Konig IR, Majdic G, Malorni W, Mieth C, Miller VM, Reynolds RM, Shimokawa H, Tannenbaum C, D'Ursi AM and Regitz-Zagrosek V. Sex in basic research: concepts in the cardiovascular field. Cardiovasc Res. 2017;113:711–724. [DOI] [PubMed] [Google Scholar]
- 118.Mauvais-Jarvis F Sex differences in metabolic homeostasis, diabetes, and obesity. Biol Sex Differ. 2015;6:14.PMC4559072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wilson PW, D'Agostino RB, Sullivan L, Parise H and Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162:1867–72. [DOI] [PubMed] [Google Scholar]
- 120.Garcia M, Mulvagh SL, Merz CN, Buring JE and Manson JE. Cardiovascular Disease in Women: Clinical Perspectives. Circ Res. 2016;118:1273–93.PMC4834856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Berger JS, Elliott L, Gallup D, Roe M, Granger CB, Armstrong PW, Simes RJ, White HD, Van de Werf F, Topol EJ, Hochman JS, Newby LK, Harrington RA, Califf RM, Becker RC and Douglas PS. Sex differences in mortality following acute coronary syndromes. JAMA. 2009;302:874–82.PMC2778841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM and de Villiers TJ. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97:1159–68.PMC3319184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tepper PG, Randolph JF Jr., McConnell DS, Crawford SL, El Khoudary SR, Joffe H, Gold EB, Zheng H, Bromberger JT and Sutton-Tyrrell K. Trajectory clustering of estradiol and follicle-stimulating hormone during the menopausal transition among women in the Study of Women's Health across the Nation (SWAN). J Clin Endocrinol Metab. 2012;97:2872–80.PMC3410268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.de Kat AC, Verschuren WM, Eijkemans MJ, Broekmans FJ and van der Schouw YT. Anti-Müllerian Hormone Trajectories Are Associated With Cardiovascular Disease in Women: Results From the Doetinchem Cohort Study. Circulation. 2017;135:556–565. [DOI] [PubMed] [Google Scholar]
- 125.Tehrani FR, Erfani H, Cheraghi L, Tohidi M and Azizi F. Lipid profiles and ovarian reserve status: a longitudinal study. Hum Reprod. 2014;29:2522–9. [DOI] [PubMed] [Google Scholar]
- 126.Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, Hsia J, Hulley S, Herd A, Khan S, Newby LK, Waters D, Vittinghoff E, Wenger N and Group HR. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA. 2002;288:49–57. [DOI] [PubMed] [Google Scholar]
- 127.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B and Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–13. [DOI] [PubMed] [Google Scholar]
- 128.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J and Writing Group for the Women's Health Initiative I. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. [DOI] [PubMed] [Google Scholar]
- 129.Marjoribanks J, Farquhar C, Roberts H, Lethaby A and Lee J. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2017;1:CD004143.PMC6465148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL and Stefanick ML. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–77. [DOI] [PubMed] [Google Scholar]
- 131.Giordano S, Hage FG, Xing D, Chen YF, Allon S, Chen C and Oparil S. Estrogen and Cardiovascular Disease: Is Timing Everything? Am J Med Sci. 2015;350:27–35.PMC4490077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hodis HN, Mack WJ, Henderson VW, Shoupe D, Budoff MJ, Hwang-Levine J, Li Y, Feng M, Dustin L, Kono N, Stanczyk FZ, Selzer RH, Azen SP and Group ER. Vascular Effects of Early versus Late Postmenopausal Treatment with Estradiol. N Engl J Med. 2016;374:1221–31.PMC4921205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728–753. [DOI] [PubMed] [Google Scholar]
- 134.Bittner V. Menopause, age, and cardiovascular risk: a complex relationship. J Am Coll Cardiol. 2009;54:2374–5. [DOI] [PubMed] [Google Scholar]
- 135.Kok HS, van Asselt KM, van der Schouw YT, van der Tweel I, Peeters PH, Wilson PW, Pearson PL and Grobbee DE. Heart disease risk determines menopausal age rather than the reverse. J Am Coll Cardiol. 2006;47:1976–83. [DOI] [PubMed] [Google Scholar]
- 136.Heimlich JB and Bick AG. Somatic Mutations in Cardiovascular Disease. Circulation Research. 2022;130:149–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schneider A, Saccon TD, Garcia DN, Zanini BM, Isola JVV, Hense JD, Alvarado-Rincón JA, Cavalcante MB, Mason JB, Stout MB, Bartke A and Masternak MM. The Interconnections Between Somatic and Ovarian Aging in Murine Models. The journals of gerontology Series A, Biological sciences and medical sciences. 2021;76:1579–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nota NM, Wiepjes CM, Blok CJMd, Gooren LJG, Kreukels BPC and Heijer Md. Occurrence of Acute Cardiovascular Events in Transgender Individuals Receiving Hormone Therapy. Circulation. 2019;139:1461–1462. [DOI] [PubMed] [Google Scholar]
- 139.Cross-sex Hormones and Acute Cardiovascular Events in Transgender Persons. Annals of Internal Medicine. 2018;169:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Streed CG Jr., Harfouch O, Marvel F, Blumenthal RS, Martin SS and Mukherjee M. Cardiovascular Disease Among Transgender Adults Receiving Hormone Therapy: A Narrative Review. Ann Intern Med. 2017;167:256–267. [DOI] [PubMed] [Google Scholar]
- 141.Connelly PJ, Marie Freel E, Perry C, Ewan J, Touyz RM, Currie G and Delles C. Gender-Affirming Hormone Therapy, Vascular Health and Cardiovascular Disease in Transgender Adults. Hypertension. 2019;74:1266–1274.PMC6887638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang J, Bingaman S and Huxley VH. Intrinsic sex-specific differences in microvascular endothelial cell phosphodiesterases. American Journal of Physiology-Heart and Circulatory Physiology. 2010;298:H1146–H1154. [DOI] [PMC free article] [PubMed] [Google Scholar]