Abstract
COVID-19 has caused a global pandemic and millions of deaths. It is imperative to develop effective countermeasures against the causative viral agent, SARS-CoV-2 and its many variants. Vaccines and therapeutic antibodies are the most effective approaches for preventing and treating COVID-19, respectively. SARS-CoV-2 enters host cells through the activities of the virus-surface spike (S) protein. Accordingly, the S protein is a prime target for vaccines and therapeutic antibodies. Dealing with particles with dimensions on the scale of nanometers, nanotechnology has emerged as a critical tool for rapidly designing and developing safe, effective, and urgently needed vaccines and therapeutics to control the COVID-19 pandemic. For example, nanotechnology was key to the fast-track approval of two mRNA vaccines for their wide use in human populations. In this review article, we first explore the roles of nanotechnology in battling COVID-19, including protein nanoparticles (for presentation of protein vaccines), lipid nanoparticles (for formulation with mRNAs), and nanobodies (as unique therapeutic antibodies). We then summarize the currently available COVID-19 vaccines and therapeutics based on nanotechnology.
Introduction
Nanotechnology is defined as the design, characterization, development, and employment of particles with dimensions on the scale of nanometers (1–100 nm).1,2 This technology has yielded nano-structures with unique properties, such as improved bioavailability, targeted drug delivery, and enhanced target-binding affinity.3 The development of nanotechnology has provided a cutting-edge platform for treating and preventing infectious diseases, including COVID-19 (caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2)), Zika, dengue, influenza, Ebola, AIDS (caused by HIV-1), cancer caused by human papillomavirus, diseases associated with respiratory syncytial virus, and herpes simplex viral infections.3–8 One of the most notable and important applications of nanotechnology is to the design and development of effective vaccines and therapeutics to control viral diseases.
COVID-19, a recently emerged respiratory infectious disease, was first reported in 2019 and has caused devastating damages to global health and economy.9 The causative agent SARS-CoV-2 is a novel beta-coronavirus.9 Two other beta-coronaviruses, SARS-CoV-1 and Middle East respiratory syndrome coronavirus (MERS-CoV), caused outbreaks in 2002–2003 and have been infecting humans since 2012, respectively.10–18 SARS-CoV-2 spreads in human populations much more widely than both SARS-CoV-1 and MERS-CoV. Currently, the numbers of COVID-19 cases and fatalities continues to grow. Rapid development of a variety of effective and safe vaccines and therapeutic agents is a key strategy for stopping this global pandemic.
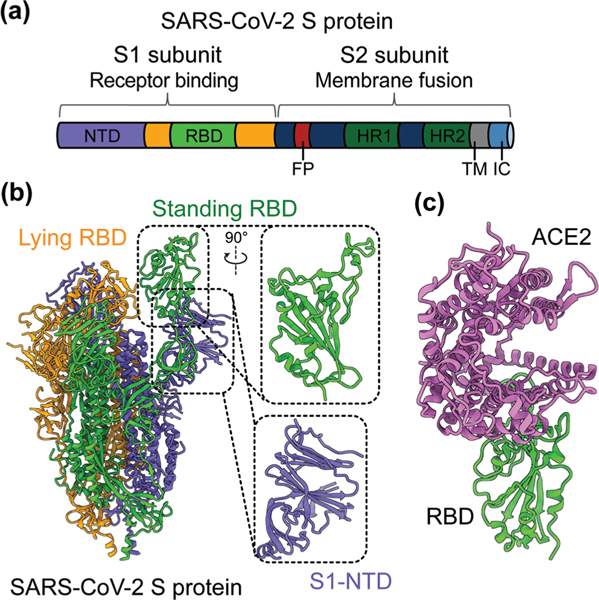
SARS-CoV-2 is a single-stranded and positive-sense RNA virus. The viral genome encodes four major structural proteins, including spike (S), envelope (E), nucleocapsid (N), and membrane (M), in addition to sixteen non-structural proteins (nsp1–16) and several accessory proteins (3a, 6, 7a, 7b, 8, and 10).19,20 The S protein plays an essential role in viral entry and is a critical determinant of viral infection and pathogenesis. Accordingly, it is an important target for vaccines and therapeutics.20–25 The S protein contains a receptor-binding S1 subunit and a membrane-fusion S2 subunit. The S1 subunit contains a N-terminal domain (NTD) and a C-terminal domain (CTD), also known as the receptor-binding domain (RBD) (Fig. 1a and b).21,26,27 The RBDs of both SARS-CoV-2 and SARS-CoV-1 recognize angiotensin-converting enzyme 2 (ACE2) as their cellular receptor for viral attachment to host cells.26,28–30 SARS-CoV-2 RBD binds to human ACE2 more tightly than SARS-CoV-1 RBD does (Fig. 1c).26,27 ACE2 binding by the RBD may trigger a conformational change in the S protein (i.e., transition of the S protein from its pre-fusion conformation to post-fusion conformation). The conformational change of the S protein leads to fusion of viral and cell membranes and SARS-CoV-2 entry into host cells.23,31–33 The RBD in the trimeric S protein of SARS-CoV-2 can exist in two states, “up” and “down”, but the binding of ACE2 to the RBD only occurs when the RBD in the “up” position (Fig. 1b and c).32,34 The strong ACE2 binding and potential hidden state of the RBD present challenges to the development of potent vaccines and therapeutics targeting SARS-CoV-2.
Fig. 1.
Structure of SARS-CoV-2 S protein. (a) Schematics of SARS-CoV-2 spike (S) protein domain organization. NTD, N-terminal domain; RBD, receptor-binding domain; FP, fusion peptide; HR1 and HR2, heptad region 1 and 2; TM: transmembrane domain; IC: intracellular tail. (b) Cryo-EM structure of SARS-CoV-2 S protein trimer (PDB 6VYB) with closed up views of the S1-NTD and RBD. Three copies of the S protein in the trimer are colored in green, orange and purple, respectively. (c) Crystal structure of SARS-CoV-2 RBD in complex with the host cell receptor angiotensin-converting enzyme 2 (ACE2) (PDB 6 M0J). SARS-CoV-2 RBD is colored in green and ACE2 is in orchid.
In response to the rapid human-to-human spread of SARS-CoV-2 and the devastating pandemic, several nanotechnology-based tools, including protein nanoparticles (PNPs), lipid nanoparticles (LNPs), and nanobodies (Nbs), have been used to combat COVID-19 at a speed never before seen for other infectious diseases. In the remainder of this review, we will describe the roles of these nanotechnology-based tools in combating COVID-19, and will also summarize the currently available PNPs, NLPs, and Nbs targeting COVID-19.
Role of nanotechnology in the development of COVID-19 vaccines and therapeutic antibodies
Protein nanoparticles as a tool for delivery of SARS-CoV-2 vaccines
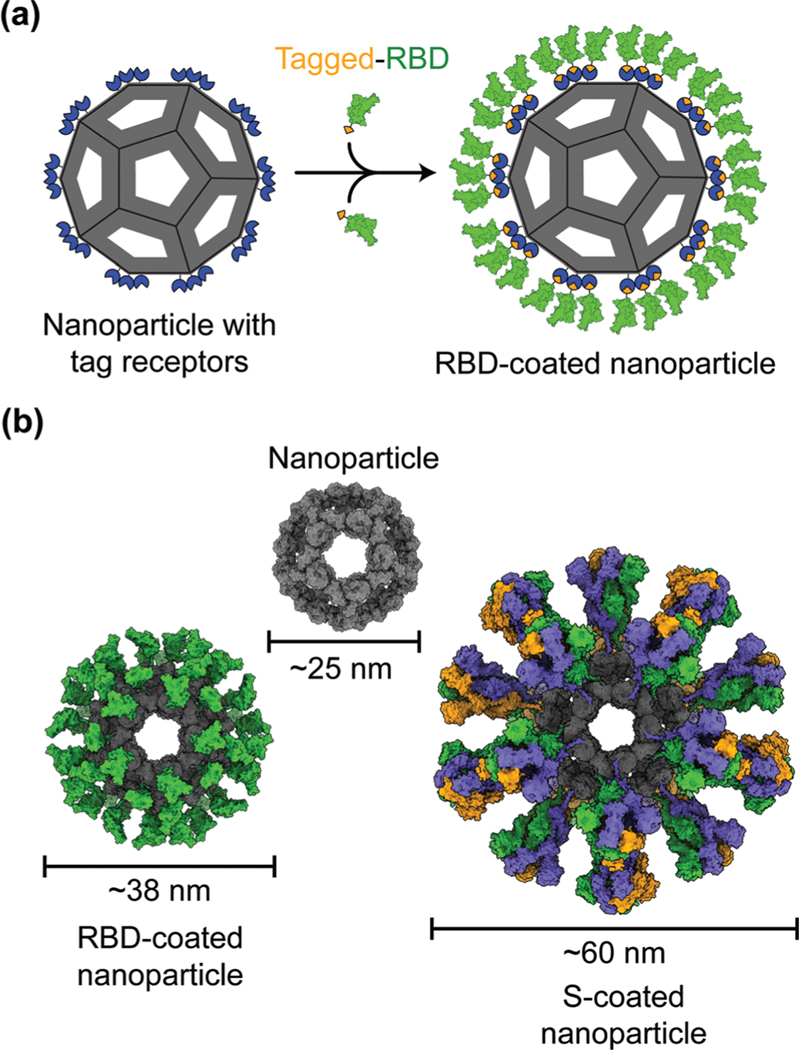
Protein nanoparticles are effective and safe delivery vehicles for protein-based vaccines. Nanoparticle-forming proteins are unusual in that they can spontaneously assemble into large multimers at nanometer sizes (such as 24-meric ferritin and 60-meric lumazine synthase). These virus particle-like multimers can then display many copies of target antigens (i.e., SARS-CoV-2 RBD or S protein) on their surface (Fig. 2).35–37 These viral antigens may be attached to nanoparticles via covalent or noncovalent interactions. Alternatively, nanoparticle proteins and viral antigens can be encoded in a single peptide chain that self assembles into antigen-presenting nanoparticles.38 In general, nanoparticles mimic the structures of virus particles and present antigens with high densities and in repetitive patterns; these features are what the mammalian immune systems have evolved to recognize.2 Hence, nanoparticle-based vaccines enhance antigen uptake by antigen-presenting cells (APCs) and stimulate the immune system to produce strong humoral (antibody) and cellular immune responses specific to the target antigens, conferring protection against SARS-CoV-2 infection.35,37,39
Fig. 2.
Models of protein nanoparticle-based SARS-CoV-2 vaccines. (a) 2D diagram of a receptor-binding domain (RBD)-coated protein nanoparticle vaccine. The RBD (green) is captured by the nanoparticle (gray) through the interactions between the SpyTag (orange) on the RBD and SpyCatcher (blue) on the nanoparticle. (b) 3D models of the nanoparticle coated by 60 copies of SARS-CoV-2 RBD or 20 copies of SARS-CoV-2 spike (S) trimer.
Despite having the size and structure similar to virus particles, nanoparticles lack viral genetic material that could drive infection or replication. Hence in contrast to live attenuated virus vaccines, which may recover virulence, nanoparticle-based vaccines are relatively safe.40 Also, in contrast to inactivated virus vaccines whose antigens may have been destructed due to the inactivation procedures, nanoparticle-based vaccines present antigens in their original forms.41 Moreover, unlike recombinant protein subunit vaccines, which generally have low immunogenicity, nanoparticles have immunostimulatory functions that induce antigen-specific immune responses even in the absence of an adjuvant.42–44 The above being said, the inclusion of adjuvants in the vaccine components can further improve the immunogenicity and efficacy of nanoparticle-based vaccines. Indeed, antibodies induced by nanoparticle-based COVID-19 vaccines demonstrate significantly higher neutralization titers than those induced by recombinant proteins.37 Accordingly, nanoparticle-based COVID-19 vaccines are able to potently neutralize SARS-CoV-2 infection and protect immunized animals against SARS-CoV-2 challenge.37,45
Lipid nanoparticles as a tool for delivery of SARS-CoV-2 mRNA vaccines
mRNA vaccines represent a new approach to protecting humans against viral infections. mRNAs can be rapidly synthesized at large scales. However, naked mRNAs are unstable and can be easily degraded under various physical, chemical, and enzymatic conditions. To improve mRNA vaccines’ stability, several strategies have been developed, including chemical modification of mRNAs, conjugation of mRNAs to other molecules, and encapsulation of mRNAs in lipid nanoparticles (LNPs).46–48 Among these approaches, LNPs demonstrate strong potentials in stabilizing mRNA vaccines and protecting them from degradation, ensuring effective and targeted delivery of mRNA vaccines.49
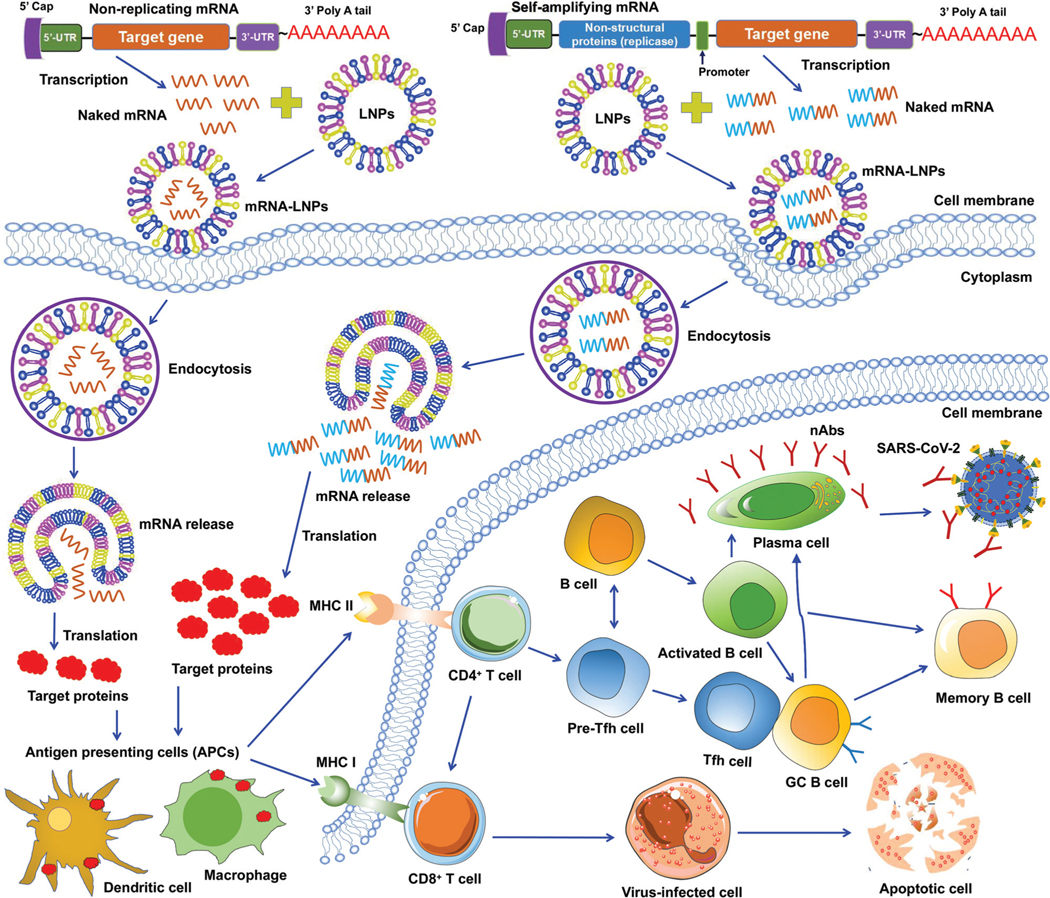
To date, two types of mRNA vaccines have been reported against infectious diseases: non-replicating mRNAs and self-amplifying mRNAs. Non-replicating mRNAs contain the coding antigen sequence, 5′- and 3′-untranslated regions (UTRs), 5′-cap structure, and 3′-poly(A) tail for amplification.46,50 Self-amplifying mRNAs, also called replicons, are generated by replacing the structural proteins of an RNA virus (often an alphavirus or a flavivirus) with viral RNA-dependent RNA polymerases and antigen-encoding mRNAs.51,52 Both types of mRNAs can be delivered through LNPs. Like protein-based vaccines, non-replicating mRNA vaccines are safer because they do not contain any infectious viral components. If self-amplifying mRNAs are used, it is necessary to detect immune responses specific to both target proteins and RNA polymerases since the latter may induce an independent immune response. Both types of mRNA vaccines have been developed against SARS-CoV-2 infection (Fig. 3).47,53–55
Fig. 3.
Working models of lipid nanoparticle-based SARS-CoV-2 mRNA vaccines. Non-replicating and self-amplifying mRNAs are encapsulated with lipid nanoparticles (LNPs) and delivered into cells. Non-replicating mRNAs only encode target proteins whereas self-amplifying mRNAs encode both target proteins and viral non-structural proteins that are required for mRNA amplification. Target proteins translated from these mRNAs are recognized by antigen-presenting cells (APCs), including dendritic cell and macrophage, followed by activation of T (CD4+ and CD8+) and B cells that kill virus-infected cells or generate specific neutralizing antibodies (nAbs) that combat SARS-CoV-2 infection. GC B cell, germinal center B cell. 5’- and 3’-UTR, 5’- and 3’-untranslated regions. MHC I and II, major histocompatibility complex class I and class II molecules. nAbs, neutralizing antibodies.
Unlike DNA vaccines, mRNA vaccines do not need to cross nuclear membranes or integrate into cellular genomes, making them easier to be delivered.56 After LNP-mediated mRNAs are delivered into cells, they are internalized via endocytosis, released into the cytoplasm, and then translated to target proteins. Different from non-replicating mRNAs, the self-amplifying mRNAs can produce multiple copies of subgenomic mRNAs that express a higher level of target proteins.52,57 In either case, the target proteins are subsequently recognized by APCs (e.g., dendritic cells and macrophages), which subsequently activate T cells and B cells. Activated T cells will generate CD8+ and CD4+ T-cell responses to kill virus-infected cells and also help trigger B-cell responses. Some activated B cells differentiate into plasma cells to produce virus-neutralizing antibodies, whereas other activated B cells differentiate into germinal center B (GC B) cells, which may interact with T follicular helper cells (Tfh) cells or become memory B cells (Fig. 3).
A potential side effect of mRNA vaccines is that mRNAs can be recognized by pattern-recognition receptors (e.g., toll-like receptors: TLR3, TLR7, or TLR8) in endosomes. These receptors activate innate immune receptors, such as retinoic acid-inducible gene I (RIG-1), protein kinase RNA-activated (PKR), or melanoma differentiation-associated antigen 5 (MDA5), which in turn upregulate the secretion of proinflammatory cytokines and chemokines.56,58 To overcome this potential side effect, naturally-occurring modified nucleotides (e.g., pseudouridine-5′-triphosphate) can be introduced during mRNA synthesis to inhibit activation of these innate immune receptors.46,47,58
Nanobodies as unique SARS-CoV-2 therapeutics
Nanobodies are single-domain antibodies derived from camelid heavy-chain-only antibodies.59,60 They are connected to nanotechnology due to their nano-scale size (about 4 nm by 2.5 nm). With a molecular weight of ∼12–14 kDa, nanobodies are among the smallest naturally occurring antibodies. Because of their small size, they can bind to hidden areas of antigens that are normally unreachable to conventional antibodies.59,60 Because of their elongated CDR3 loops, they are capable of binding to antigens with high affinities. Nanobodies can be expressed at high yield in a variety of expression systems (e.g., bacteria, yeasts and mammalian cells), allowing cheap and large-scale productions of nanobodies.59–63 They are stable under extreme conditions (e.g., extreme temperatures and extreme pH), making them easy to be stored and transported.62,63 Together, these features make nanobodies ideal prophylactic and therapeutic agents. They would be particularly suited to meet the urgent and large demands for battling the COVID-19 global pandemic.
Monomeric nanobodies do not contain the Fc portion; consequently, they are unable to enter the Fc receptor-dependent pathway and generally lack cytotoxic activity arising from activation of the complement system and immune cells.64,65 Monomeric nanobodies generally have a short half-life because their size is below the renal filtration capacity, but this can be overcome by constructing Fc-tagged dimeric nanobodies or linking nanobodies to albumin to increase their size (Fig. 3).60,61,66 It is worth noting that the molecular weights of Fc-tagged dimeric nanobodies and albumin-linked nanobodies (both ∼75–80 kDa) are still much smaller than those of conventional antibodies (∼160 kDa), maintaining their small size advantage over conventional antibodies.61,66 Moreover, when Fc is fused with nanobodies, the amino acids of the Fc portion can be mutated to alleviate potential Fc-associated side effects.
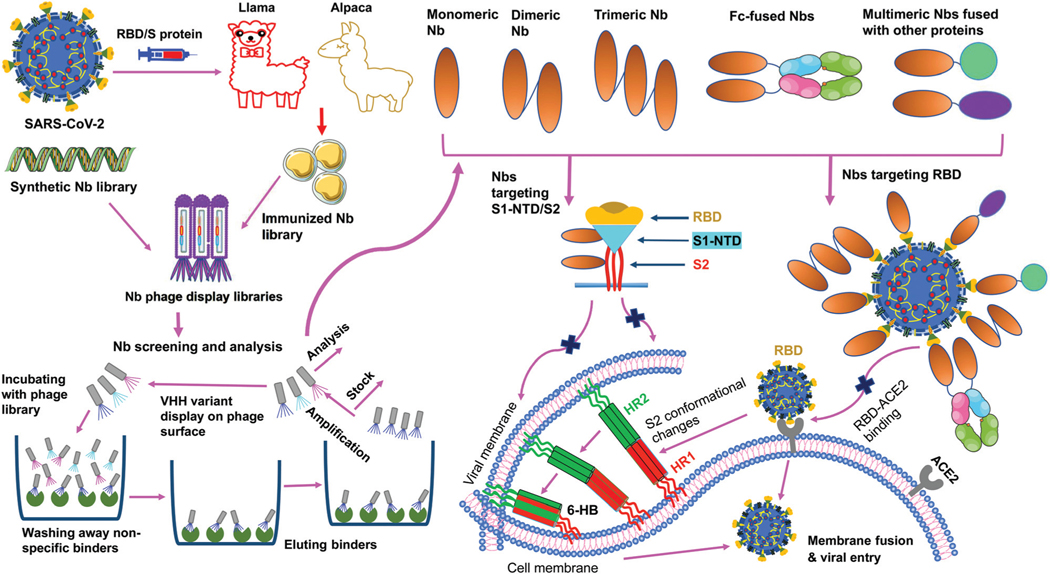
SARS-CoV-2 nanobodies can be developed by immunizing camelids (llama or alpaca) with target antigens.67,68 Subsequently, immunized nanobody libraries can be established and screened. Alternatively, naïve nanobody libraries may be directly screened, skipping the immunization step.63,69,70 However, for nanobodies screened from naïve nanobody libraries, in vitro affinity maturation is usually needed to improve the nanobody’s affinity for target antigens. Theoretically, neutralizing nanobodies function by binding to their target antigens (such as the RBD, S1, or S2 of SARS-CoV-2 S protein). Binding of nanobodies to the RBD may prevent the RBD from binding its receptor ACE2, thus blocking viral attachment and viral entry. Binding of nanobodies to the S1 or S2 may prevent the S protein from transitioning to its post-fusion conformation, thereby blocking membrane fusion and viral entry. The generation and potential mechanisms of SARS-CoV-2-targeting nanobodies are described in Fig. 4.
Fig. 4.
Generation and function of SARS-CoV-2-specific nanobodies. Nanobodies (Nbs) are generated by immunization of animals or screened from synthetic Nb phage display libraries. After a series of processes (incubation, wash, elution, amplification, and analysis), specific monomeric Nbs are screened; based on the identified monomeric Nbs, multimeric (dimeric, trimeric, Fc or other protein-fused) Nbs can be constructed. Nbs that target SARS-CoV-2 receptor-binding domain (RBD) in the spike (S) protein S1 subunit prevent the RBD-angiotensin-converting enzyme 2 (ACE2) receptor binding, whereas Nbs that target N-terminal domain (NTD) in the S1 subunit (S1-NTD) or S2 subunit may inhibit the conformational changes of the S protein or S2-mediated membrane fusion, thereby preventing subsequent steps in viral infection. HR1 and HR2, heptad repeat 1 and 2; 6-HB, 6-helix bundle.
Currently developed COVID-19 vaccines based on protein nanoparticles
Nanoparticle-based SARS-CoV-2 vaccines in preclinical development.
A number of nanoparticle-based SARS-CoV-2 vaccines are in preclinical development (Table 1). Most of these vaccines use viral S protein or its RBD fragment as target antigens. They have been shown to elicit neutralizing antibodies against SARS-CoV-2 infection in vitro and some of them may protect vaccinated animals against SARS-CoV-2 infection.
Table 1.
Representative SARS-CoV-2 vaccines based on protein nanoparticles and lipid nanoparticles
| Vaccine name | Target | Animals/ humans | Ab responses and neutralizing Abs | Cellular and other responses | Protection and/or potential side effects | Ref. |
|---|---|---|---|---|---|---|
|
| ||||||
| Nanoparticle-based SARS-CoV-2 vaccines | ||||||
| RBD-NP | RBD | Mice NHPs | Induced SARS-CoV-2 S-specific Abs, with neutralizing Ab titers being 10-fold higher than a stabilized S protein against SARS-CoV-2 WT infection; neutralized SARS-CoV-2 B.1.1.7 variant | Elicited SARS-CoV-2 RBD-specific CD4+ T cell responses | Protected NHPs against SARS-CoV-2 infection | 72 and 73 |
| VLP-RBD | RBD | Mice | Induced long-term RBD-specific Abs with neutralizing activity against live SARS-CoV-2 and pseudotyped SARS-CoV-2 WT and B.1.1.7, B.1.351, and P.1 variants, SARS-CoV-1, and SARS-related bat CoVs | N/A | Protected BALB/c mice against mouse-adapted SARS-CoV-2 challenge, with reduced clinical signs and pathological changes | 37 |
| RBD-SpyVLP RBD-mi3 | RBD | Mice Pigs | Induced SARS-CoV-2 RBDspecific Abs and neutralizing Abs against pseudotyped and/or live SARS-CoV-2 infection | Elicited SARS-CoV-2 RBD-specific B-cell responses and weak T-cell responses | N/A | 74 and 75 |
| RBD-ferritin S2GΔHR2 | RBD S | Mice NHPs | Induced SARS-CoV-2 S/RBDspecific Abs and more potent neutralizing Abs than RBD alone or S2P protein against pseudotyped SARS-CoV-2 infection; elicited neutralizing Abs against pseudotyped SARS-CoV-2 B.1.1.7, B.1.351, and B.1.617 variants | Elicited SARS-CoV-2 S-specific T-cell immunity (S2GΔHR2), and high GC B cell responses | Protected NHPs from SARS-CoV-2 infection, with reduced virus replication and pathological damage | 35, 39 and 71 |
| S1-VLP | S1 | Mice | Induced SARS-CoV-2 S/RBDspecific Abs and neutralizing Abs against SARS-CoV-2 WT and B.1.1.7 variant | N/A | N/A | 76 |
| SpFN | S | Hamsters | Induced SARS-CoV-2 S/RBDspecific Abs and neutralizing Abs against pseudotyped SARS-CoV-2 WA1, B.1.1.7, and B.1.351 variants | N/A | Protected hamsters from SARS-CoV-2 (B.1.1.7 or B.1.351 variant) infection, with reduced virus replication and decreased weight loss, virus titers, and lung pathology | 45 |
| S-LuS | S | Mice | Induced higher SARS-CoV-2 S-specific Abs and pseudovirus neutralizing Abs than those by S2P protein | N/A | N/A | 77 |
| S-Fer SΔC-Fer | S S | Mice | Induced higher SARS-CoV-2 S/ RBD-specific Abs and pseudovirus neutralizing Abs than RBD monomer or S trimer protein | N/A | N/A | 36 |
| NVX-CoV2373 | S | Humans | Induced SARS-CoV-2 S-specific IgG Abs and neutralizing Abs against infection of live SARS-CoV-2 | Elicited SARS-CoV-2 S-specific CD4+ T-cell responses | Protected vaccinated people against SARS-CoV-2 infection, showing efficacy against B.1.1.7 and B.1.351 variants; no serious adverse effects were identified, except for mild fever | 38, 78–80 |
| Lipid nanoparticle-based SARS-CoV-2 mRNA vaccines | ||||||
| RBD-hFc | RBD | Mice | Induced SARS-CoV-2 S-specific IgG Abs and neutralizing Abs against pseudotyped SARS-CoV-2 infection | Elicited SARS-CoV-2 S-specific Th1 cell responses | Protected 70% of vaccinated hACE2-Tg mice from SARS-CoV-2 infection | 85 and 86 |
| mRNA-RBD | RBD | Mice | Induced SARS-CoV-2 RBD- specific IgG Abs and neutralizing Abs against pseudotyped and live SARS-CoV-2 infection | Elicited SARS-CoV-2 RBD-specific T-cell responses | Protected hACE2-Tg mice from SARS-CoV-2 infection | 83 |
| RBD-LNP | RBD | Mice | Induced SARS-CoV-2 and S1-LNP S1 SARS-CoV-1 RBD-specific IgG Abs and neutralizing Abs against pseudotyped and live SARS-CoV-2 infection | Elicited SARS-CoV-2 RBD-specific T-cell, Tfh, GC B, and plasma cell responses | N/A | 47 |
| RBD Full-length Δfurin | RBD S | Mice | Induced SARS-CoV-2 S/RBDspecific IgG Abs and neutralizing Abs against pseudotyped and live SARS-CoV-2 infection | Elicited SARS-CoV-2 RBD-specific Tfh, GC B, T-cell, B-cell, LLPC and MBC responses | N/A | 81 and 82 |
| saRNA | S | Mice | Induced SARS-CoV-2 S-specific IgG Abs and neutralizing Abs against pseudotyped and live SARS-CoV-2 infection | Elicited SARS-CoV-2 S-specific T-cell responses | N/A | 89 |
| LION/ repRNA-CoV2S | S | Mice NHPs | Induced SARS-CoV-2 S-specific IgG Abs and neutralizing Abs against pseudotyped and/or live SARS-CoV-2 infection | Elicited SARS-CoV-2 S1/RBD-specific T-cell responses (in spleen, lung (for mice) and PBMCs (for NHPs) | N/A | 53 |
| CV07050101 | S | NHPs | Induced SARS-CoV-2 S-specific IgG Abs without neutralizing activity | Elicited SARS-CoV-2 S-specific T-cell responses | N/A | 87 |
| LUNAR-COV19 | S | Mice | Induced SARS-CoV-2 S-specific IgG and IgM Abs, S1/RBDspecific IgG Abs, and neutralizing Abs against live SARS-CoV-2 infection | Elicited SARS-CoV-2 S-specific CD8+ T and CD4+ T/Th1-dominant T-cell responses | Protected hACE2-Tg mice from SARS-CoV-2 infection | 90 |
| mRNA-LNP | S | NHPs | Induced SARS-CoV-2 S1/S2/ RBD-specific Abs and neutralizing Abs against pseudotyped and live SARS-CoV-2 infection | Elicited SARS-CoV-2 S-specific memory B-cell, GC B cell, Tfh, and T-cell responses | Protected infant NHPs from SARS-CoV-2 infection | 94 |
| MRT5500 | S | Mice Hamsters NHPs | Induced SARS-CoV-2 S-specific IgG Abs and neutralizing Abs against pseudotyped or live SARS-CoV-2 infection | Elicited SARS-CoV-2 S-specific T-cell responses in mice and NHPs | Protected hamsters from SARS-CoV-2 infection without weight loss and lung pathology | 92 |
| mRNA-1273 | S | Mice NHPs Humans | Induced SARS-CoV-2 S-specific IgG Abs and neutralizing Abs against pseudotyped (wild-type and D614 mutant) and live SARS-CoV-2 infection | Elicited SARS-CoV-2 S-specific CD4+ or CD8+ T-cell responses | Protected immunized mice and NHPs from SARS-CoV-2 infection without causing disease enhancement effect; had protective efficacy in preventing COVID-19 disease in human; elicited local and systemic adverse effects (e.g., fatigue, chills, headache, myalgia, pain), allergic reactions, etc. | 54, 88, 91, 97–100 and 138 |
| BNT162b1 BNT162b2 | RBD S | Mice NHPs Humans | Induced dose-dependent, and SARS-CoV-2 RBD/S-specific Abs and neutralizing Abs against pseudotyped and live SARS-CoV-2 infection | Elicited SARS-CoV-2 RBD-specific CD4+ and CD8+ T-cell responses | Protected immunized mice and NHPs from SARS-CoV-2 infection; had protective efficacy in preventing COVID-19 disease in humans, with local and systemic reactions (fatigue, headache, myalgia, local pain, etc.), allergic reactions, and rare severe adverse events (for BNT162b2) | 55, 84, 93, 101–103, 131 and 140 |
| Lipid nanoparticles displaying SARS-CoV-2 therapeutic agents | ||||||
| hsACE2 | hACE2 | Mice | Secreted hACE2 in blood circulation and expressed hACE2 in bronchoalveolar lavage fluid; hACE2 bound SARS-CoV-2 RBD and neutralized pseudotyped SARS-CoV-2 infection | N/A | N/A | 96 |
Abs, antibodies; ACE2, angiotensin-converting enzyme 2; BAL, bronchoalveolar-lavage; COVID-19, Coronavirus disease 2019; GC B, germinal center B; hACE2-Tg, human ACE2-transgenic; HR2, heptad repeat region 2; LLPCs, long-lived plasma cells; LuS, lumazine synthase; MBCs, memory B cells; NHPs, non-human primates; RBD, receptor-binding domain; S, spike; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; S2P, S protein with two proline mutations in the S2 subunit; Tfh, T follicular helper cells; WT, wild-type.
Protein nanoparticle vaccines displaying SARS-CoV-2 RBD.
Compared to recombinant SARS-CoV-2 RBD by itself or the full-length S protein, protein nanoparticle vaccines displaying SARS-CoV-2 RBD demonstrate good immunogenicity and/or protective effects in various animal models. First, 24-meric ferritin nanoparticles displaying 24 copies of SARS-CoV-2 RBD induced antibodies that potently neutralized pseudoviruses; they also protected non-human primates (NHPs) from SARS-CoV-2 infection, reducing virus replication and pathogenesis.35,71 Here, the ferritin and SARS-CoV-2 RBD were expressed in the same polypeptide chain. Second, a computationally designed 120-meric nanoparticle (named I53–50) displaying 60 copies of SARS-CoV-2 RBD (RBD-NP) induced 10 times more neutralizing antibodies in mice than the stabilized SARS-CoV-2 full-length S protein; it also protected NHPs from SARS-CoV-2 infection.72,73 Here, the nanoparticle protein and the RBD were also expressed in the same polypeptide chain. Third, a 60-meric lumazine synthase nanoparticle displaying 120 copies of SARS-CoV-2 RBD induced >5 times more neutralizing antibodies in mice than RBD by itself; it also conferred nearly complete protection to mice challenged by SARS-CoV-2 (mouse-adapted strain).37 The induced neutralizing antibodies lasted long time in mice. Moreover, the lumazine synthase nanoparticle vaccine was also effective against SARS-CoV-2 variants (B.1.1.7, B.1.351, and P.1), SARS-CoV-1, and SARS-related bat coronavirus. In this nanoparticle vaccine, Fc-tagged dimeric RBD is connected to protein A-tagged lumazine synthase through noncovalent Fc/protein A binding.37 Fourth, 60-meric nanoparticles (SpyCatcher-mi3 or SpyCatcher003-mi3) displaying SARS-CoV-2 RBD induced neutralizing antibodies in mice and pigs.74,75 These vaccines are temperature-resistant and maintains immunogenicity after lyophilization. Here, the RBD is connected to the nanoparticle protein through covalent SpyTag : SpyCatcher binding. Overall, all of these nanoparticle-RBD vaccines prove the concept that target vaccines presented on the surface of nanoparticle scaffolds are significantly better recognized by mammalian immune systems than vaccines by themselves.
Nanoparticle vaccines displaying SARS-CoV-2 S protein or S1 fragment.
Several nanoparticle vaccines presenting SARS-CoV-2 S protein or S1 subunit have been developed and tested in preclinical studies.36,39,45,76 The 60-mer trimeric lumazine synthase nanoparticle was used again to present the S protein, but the connection between lumazine synthase and the S protein was through the covalent SpyTag : SpyCatcher binding.77 The 24-meric ferritin nanoparticle was also used to present several truncated versions of the S protein, and the connection between ferritin and the S protein was through the covalent SpyTag : SpyCatcher binding.77 These nanoparticle vaccines elicited neutralizing antibodies against pseudotyped SARS-CoV-2 and its variants (B.1.1.7, B.1.351, P.1, and B.1.617); they also demonstrated longer retention and stronger presentation on follicular dendritic cell dendrites, and induced higher germinal center B cell responses in immunized mice.39,77 Some of these nanoparticle vaccines protected hamsters against SARS-CoV-2 infection, with reduced disease and virus replication, as well as decreased weight loss, virus titers, and lung pathology.45 Bacteriophage AP205 nanoparticles presenting SARS-CoV-2 S1 resulted in strong neutralizing antibodies in immunized mice.76
Nanoparticle-based SARS-CoV-2 vaccines under clinical trials.
The only nanoparticle vaccine currently being tested in clinical trials is a recombinant SARS-CoV-2 S protein that is expressed in insect cells and self assembles into nanoparticles without any scaffold protein (NVX-CoV2373; Novavax).38 A phase 1/2 trial showed that this vaccine, which is formulated with Matrix-M1 adjuvant, elicited specific neutralizing antibodies against SARS-CoV-2. No serious adverse events were reported except for mild fever. A phase 2 trial revealed good immunogenicity and tolerance of this vaccine in younger and older adults. Phase 2a–b and Phase 3 trials revealed its efficacy against SARS-CoV-2 variants (B.1.351 or B.1.1.7).78–80
Currently developed SARS-CoV-2 mRNA vaccines based on lipid nanoparticles
LNP-based SARS-CoV-2 mRNAs are among the most advanced COVID-19 vaccines under development. Vaccines of this class have been developed preclinically and clinically. Several of them have been approved for human use to prevent SARS-CoV-2 infection (Table 1).
SARS-CoV-2 mRNA vaccines or therapeutics under preclinical development.
More than 10 LNP-based mRNA vaccines have been developed preclinically (Table 1). They encode SARS-CoV-2 S protein or its fragments, such as RBD and S1. These vaccines demonstrate good immunogenicity in animal models, including mice, hamsters, and NHPs. In addition, mRNAs can encode therapeutic agents (e.g., recombinant ACE2) to directly inhibit SARS-CoV-2.
mRNA vaccines encoding SARS-CoV-2 RBD or S1 fragment.
LNP-encapsulated non-replicating mRNA vaccines encoding SARS-CoV-2 RBD have been developed and shown protective efficacy in animal models against SARS-CoV-2 infection.47,81–84 An mRNA encoding the human Fc-tagged RBD (RBD-hFc) and formulated with LNPs elicited robust neutralizing IgG antibodies and Th1-based cellular immune responses in mice, partially protecting hACE2-transgenic (hACE2-Tg) mice from SARS-CoV-2 infection.85,86 mRNAs encoding the RBD without Fc (mRNA-RBD) also showed strong immunogenicity and elicited potent immune responses. Notably, a single-dose injection of an mRNA-RBD vaccine elicited sufficient neutralizing antibodies to protect hACE2-Tg mice from SARS-CoV-2 infection; passive transfer of sera of mice immunized with this mRNA inhibited SARS-CoV-2 infection of the same mouse model.83 An mRNA vaccine (named BNT162b1) encoding a trimerized SARS-CoV-2 RBD and formulated with LNPs also induced T-cell responses and neutralizing antibodies in mice and NHPs, protecting immunized NHPs from SARS-CoV-2 infection.84 It has been shown that SARS-CoV-2 RBD-specific Tfh, GC B, and memory B cell (MBC) responses were generated after mice were immunized with LNP-formulated mRNA-RBD vaccines.81,82 Compared to LNP-formulated RBD-mRNA vaccines, LNP-formulated mRNA-S1 vaccine induced significantly lower-titer neutralizing antibodies against SARS-CoV-2,47 suggesting that the RBD should play an more important role than other parts of the S1 fragment in the development of LNP-based SARS-CoV-2 mRNA vaccines.
mRNA vaccines encoding SARS-CoV-2 S protein.
In addition to SARS-CoV-2 RBD, S protein is also a critical target for the development of mRNA vaccines. mRNA vaccines encoding SARS-CoV-2 S protein (mRNA-S) have been extensively studied, and two types of mRNA-S vaccines (self-amplifying and nonreplicating) have been developed and tested for immunogenicity and protective efficacy in animal models.53,54,87,88
Several self-amplifying mRNA-S vaccines are being developed preclinically. These include an LNP-formulated, self-amplifying mRNA-S vaccine encoding alphavirus replicase which induced T-cell responses and neutralizing antibodies in mice.89 In addition, a replicon mRNA-S vaccine (LION/repRNA-CoV2S), which was derived from alphavirus and formulated with lipid inorganic nanoparticles (LIONs), also elicited specific neutralizing antibody responses in mice and NHPs.53 Furthermore, a self-replicating mRNA-S vaccine encoding alphavirus replicon (LUNAR-COV19) induced T-cell responses and neutralizing antibodies that protected hACE2-Tg mice from SARS-CoV-2.90
Non-replicating mRNAs encoding SARS-CoV-2 S protein have also been extensively studied. Several of them have demonstrated protective effective in animal models challenged by SARS-CoV-2. An LNP-formulated mRNA-S vaccine induced neutralizing antibody and specific T-cell, long-lived plasma cell (LLPC), and MBC responses.81,82 mRNA-1273, BNT162b2, and MRT5500 are three LNP-encapsulated mRNA-S vaccines encoding a stabilized prefusion SARS-CoV-2 S protein. In animal models (mice, hamsters, and NHPs), these mRNA-S vaccines induced neutralizing antibodies and specific T-cell responses, protecting immunized animal models against SARS-CoV-2 infection.84,88,91–93 Importantly, these mRNA-S vaccines did not cause vaccine-associated enhancement of disease after SARS-CoV-2 challenge.54,84,91 Other mRNA-S vaccines also elicited T-cell responses and neutralizing IgG antibodies specific to the RBD, S1, and S2 fragments; these immune responses lasted for 22 weeks and protected infant NHPs from SARS-CoV-2 infection.94
mRNA vaccines encoding SARS-CoV-2 therapeutics.
In addition to expressing SARS-CoV-2-specific proteins as vaccine targets, mRNAs can also express SARS-CoV-2 therapeutics to directly inhibit SARS-CoV-2 entry. As the cellular receptor for SARS-CoV-2, ACE2 plays an essential role in SARS-CoV-2 entry.23,95 mRNAs encoding ACE2 receptor (mRNA-ACE2) produce recombinant ACE2 that directly binds to SARS-CoV-2 and blocks the virus from binding to cell-surface ACE2.96 It has been shown that after injection of mRNA-ACE2 into mice, secreted ACE2 reached a peak level in the blood circulation 6 hours and was detectable in bronchoalveolar lavage fluid 24 and 48 hours.96
SARS-CoV-2 mRNA vaccines under clinical trials.
At least three SARS-CoV-2 mRNA vaccines have shown good efficacy in human clinical trials (Table 1). mRNA-1273 vaccine is from Moderna, and encodes a stabilized pre-fusion SARS-CoV-2 S protein (hence mRNA-S vaccine). BNT162b1 and BNT162b2 vaccines are from Pfizer-BioNTech, and they encode a trimerized SARS-CoV-2 RBD (hence mRNA-RBD vaccine) and a stabilized pre-fusion SARS-CoV-2 S protein (hence mRNA-S vaccine), respectively. All three vaccines are formulated with LNPs.
In a Phase I clinical trial among people 18–55 years old, the mRNA-1273 vaccine elicited specific T-cell responses and IgG antibodies in a dose-dependent manner, neutralizing both pseudotyped and live SARS-CoV-2 infection.97 In another Phase I trial among older adults (56–70 or ≥71 years old), this vaccine also induced dose-dependent antibody responses with neutralizing activity, as well as CD4+ T-cell responses.98 The vaccine largely caused mild or moderate local and systemic adverse effects, including fatigue, chills, headache, myalgia, and pain at the injection site; only three participants at the highest injection dosage reported severe adverse effects.97,98 In addition, the vaccine induced durable antibodies that were maintained for 119 days after the first immunization but declined over time; these antibodies were able to neutralize both pseudotyped and authentic SARS-CoV-2 infections.99 In a Phase III trial, this mRNA vaccine was 94.1% effective in preventing COVID-19 illness in all participants, including those who were ≥65 years of age or had a SARS-CoV-2 infection at the time of vaccination.100
In a phase I clinical trial, BNT162b1 and BNT162b2 mRNA vaccines elicited similar dose-dependent SARS-CoV-2 neutralizing antibodies in adults 18–55 or 65–85 years old.101 Compared to BNT162b1, BNT162b2 was associated with fewer and milder systemic reactions. In phase I/II trials, BNT162b1 induced RBD-specific antibody responses, neutralizing antibodies, and T-cell responses in healthy adults aged 18–55 years, with mild to moderate local and systemic reactions.102,103 BNT162b2 exhibited 95% efficacy in prevention of COVID-19 in a phase III clinical trial, with mild side effects including pain at the injection site, fatigue, and headache.55 In a phase IV clinical trial, most of the participants developed at least one side effect, such as injection site pain, fatigue, headache, muscle pain, or chills.104
SARS-CoV-2 mRNA vaccines approved for use in humans.
On December 11, 2019, the U.S. Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) for the BNT162b2 vaccine to be used to prevent COVID-19 in humans. The vaccine is injected intramuscularly in two doses (30 μg per 0.3 ml each), with the boost (second) dose delivered 3 weeks after the first. An interim recommendation was issued the following day for the use of this vaccine in persons at or over 16 years of age.105 On August 23, 2021, the FDA issued a full regulatory approval for the BNT162b2 vaccine to be used in individuals ≥16 years of age.106–108 Currently, the BNT162b2 vaccine has been authorized by the FDA for children aged 12–15 years (at the same dosages as older people) and aged 5–11 years (at a reduced dosage of 10 μg per 0.2 ml each) using the same injection route and interval.109,110 On December 18, 2020, the FDA issued another EUA for mRNA-1273 to be intramuscularly used in two doses (100 μg per 0.5 ml each) separated by 4 weeks. Currently, the vaccine is used for adults ≥18 years of age and children or adolescents aged 18 years or younger.111,112 To date, over half of the U.S. populations have been vaccinated with one of these two mRNA vaccines.
The BNT162b2 and mRNA-1273 vaccines are generally effective in inducing immune responses in individuals, particularly healthy people.113–115 It takes about two weeks after the second vaccination for immune responses to reach high levels.116 Vaccine-induced T cells, particularly memory T cells, were detectable 100 days after the second dose of the BNT162b2 vaccine.117 Overall, the immune responses can last up to 6 months.118 Reduced antibody or cellular immune responses were observed in elderly people, organ transplant patients, cancer patients, and patients with other immunosuppressed conditions.115,119–130 Between the two mRNA vaccines, mRNA-1273 led to better outcomes than BNT162b2 against COVID-19-related hospitalizations.107 Compared with an authorized viral vector vaccine (Ad26·COV2 from Johnson & Johnson), both of the mRNA vaccines induced more potent immune responses such as higher levels of S and RBD-specific antibodies.107
While overall both mRNA vaccines are safe to use in humans, some side effects have been identified. After the first or second dose of either of the two mRNA vaccines, regular local and systemic side reactions have been reported. These side effects include injection-site pain, muscle pain, joint pain, headache, fever, fatigue, malaise, chills, nausea, myalgia, and swelling.55,131–135 The side effects also include cardiovascular adverse events, such as tachycardia, flushing, hypertension, hypotension, and peripheral coldness. Allergic reactions were reported after receiving either of the two mRNA vaccines.136–138 Moreover, people >75 years old may also develop acute myocardial infarction, cardiac arrest, and circulatory collapse.139 For the BNT162b2 vaccine, some rare serious cases were reported in adults or elderly people, including Miller Fisher syndrome (a nerve disease related to Guillain-Barré syndrome), intracerebral hemorrhage due to vasculitis, and multisystem inflammation and organ dysfunction.140–142 Also for BNT162b2, myocarditis were reported in children, particularly in boys, after the second dose of the vaccine.143 It is believed that local or systemic adverse reactions are associated with increased SARS-CoV-2-specific IgG antibody responses.144
To further boost protection against COVID-19, the FDA has authorized a third dose of the two mRNA vaccines for individuals with immunocompromised diseases. The CDC recommended timing for the third dose for individuals aged 18 years or order is six months after the second dose. The third dose of BNT162b2 induced a moderate increase of S-specific antibody and/or cellular immune responses or neutralizing antibodies in some kidney transplant recipients and leukemia patients, which improved protection against SARS-CoV-2 infection.145–149 However, some organ (such as kidney) transplant recipients receiving immunosuppressive drugs did not develop effective S or RBD-specific antibody responses or neutralizing antibodies.150,151 Patients with solid tumors receiving chemotherapy produced moderately increased neutralizing antibodies, but not T-cell responses.152 The aforementioned side effects after the third dose of BNT162b2 were similar to, or better than, the second dose in immunocompromised individuals, the elderly, and other individuals.153,154
Currently developed nanobodies as anti-SARS-CoV-2 therapeutics
SARS-CoV-2 RBD is a key target for the development of potent nanobodies. Several SARS-CoV-2-specific nanobodies have been developed preclinically. Almost all of them target the RBD (Table 2).
Table 2.
Representative SARS-CoV-2-targeting therapeutic nanobodies
| Nanobody name | Target | Binding affinity and mechanism | Structure | Neutralizing activity | Protection and/or pharmacokinetics (PK) | Ref. |
|---|---|---|---|---|---|---|
|
| ||||||
| 1E2 4D8 2F2 3F11 5F8 |
RBD | Bound to RBD (EC50 0.996–35.52 nM), blocking the RBD-ACE2 binding | No | Neutralized pseudotyped (IC50 0.9–69 ng ml−1) and live (IC50 0.13–0.51 μg ml−1) SARS-CoV-2 infection | N/A | 156 |
| Ty1 Ty1-Fc |
RBD | Bound to RBD (Kd 5–10 nM), preventing the RBD-ACE2 binding | Yes, cryo-EM structure (for Ty1) | Neutralized pseudotyped (IC50 0.77 μg ml−1 for Ty1; IC50 0.012 μg ml−1 for Ty1- Fc) SARS-CoV-2 infection | N/A | 67 |
| Sb23 | RBD | Bound to RBD, competing its binding with ACE2 | Yes, crystal structure | Neutralized pseudotyped (IC50 0.6 μg ml−1) SARS-CoV-2 infection | N/A | 70 |
| H11-D4 H11-H4 H11-D4-Fc H11-H4-Fc |
RBD | Bound to RBD (Kd 39 and 12 nM for H11-D4 and h11-H4), blocking the RBD-ACE2 interaction | Yes, crystal and cryo-EM structures (for H11-D4 and H11-H4) | Neutralized live (IC50 4–6 nM for H11-H4-Fc; 18 nM for H11-D4-Fc) SARS-CoV-2 infection | N/A | 155 |
| Nb20 Nb21 Nb89 Nb203 Nb213 |
RBD | Nbs 20 and 21 bound to RBD (Kd < 1 pM), overlapping with the ACE2 binding site | Yes, crystal structure (for Nb20) | Neutralized pseudotyped (IC50 0.045–0.133 nM) and live (IC50 0.022–0.154 nM) SARS-CoV-2 infection, with multimeric Nbs improving neutralizing activity against infection of pseudotyped (IC50 1.3–4.1 pM) and live SARS-CoV-2 | N/A | 68 |
| Nb3 Nb6 Nb11 mNb6 Nb3-tri Nb6-tri Nb11-tri mNb6-tri PiN-21 |
RBD | Bound to RBD by inhibiting the RBD-ACE2 interaction (via blocking the binding site or locking the RBD into an inactive conformation) | Yes, crystal (for mNb6) and cryo- EM (for Nb6, Nb11, and mNb6) structures | Neutralized pseudotyped (IC50 2.0–3.9 μM) SARS-CoV-2 infection, with trimeric or matured Nbs enhancing neutralizing activity against infection of pseudotyped (IC50 1.2–400 nM for tri-Nbs; 0.12 nM for mNb6-tri) and live (IC50 0.16–140 nM for tri-Nbs; 0.054 nM for mNb6-tri) SARS-CoV-2 | Prophylactically and therapeutically prevented hamsters from SARS-CoV-2 infection; inhalation treatment inhibited weight loss, with decreased lung viral titers and lung pathology | 69 and 159 |
| C1 H3 C5 C1-trimer H3-trimer C5-trimer C5-Fc |
RBD | Bound to WT or variant RBD (Kd 0.0003–2.523 nM) by overlapping with the ACE2 epitope (for H3 and C5) or a different epitope (for C1) | Yes, crystal (for C1, H3, and C5) and cryo-EM (for C5) structures | Neutralized (IC50 0.025–8.2 nM) infection of SARS-CoV-2 variants (B.1.1.7 or B.1.351) (for C1-trimer, C5-trimer, or H3-trimer) | C5-trimer or C5-Fc therapeutically prevented hamsters from SARS-CoV-2 infection | 158 |
| Nanosota-I Nanosota-IC Nanosota-IC-Fc |
RBD | Bound to RBD (Kd 15.7 pg for Nanosota-1C-Fc), blocking the RBD-ACE2 binding | Yes, crystal structure (for Nanosota-1C) | Nanosota-IC-Fc inhibited infection of pseudotyped and/or live (ND50 0.16 μg ml−1) SARS-CoV-2 and variant (D614G) | Nanosota-IC-Fc prophylactically and therapeutically inhibited hamsters and hACE2-Tg mice from SARS-CoV-2 infection; >10-day half-life and high tissue bioavailability | 63 |
| K-874A | S1 (RBD-NTD) | Bound to S1 region (Kd 1.4 nM) between RBD and NTD of S protein | Cryo-EM structure | Blocked viral membrane fusion, neutralizing SARS-CoV-2 B.1.1.7 variant | Therapeutically protected hamsters from SARS-CoV-2 infection | 157 |
ACE2, angiotensin-converting enzyme 2; Cryo-EM: cryo-electron microscopy; EC50, half maximal effective concentration; hACE2-Tg, human ACE2-transgenic; IC50, half maximal inhibitory concentration; ND50, 50% neutralization dose; Kd, equilibrium dissociation constant; Nbs, nanobodies; RBD, receptor-binding domain; WT, wild-type.
A number of nanobodies have been discovered from the camelid (e.g., llama and alpaca) immune system. In one approach, camelid animals were immunized with recombinant SARS-CoV-2 S1 or RBD protein, immunized nanobody libraries were established from the camelid immune cells, and these libraries were subsequently screened using proteomic strategy or phage display methods. Nanobodies that have been discovered using this approach include Nb20, Nb21, Nb89, Nb34, Nb95, and Ty1.67,68 In another approach, naïve nanobody libraries were established from naïve camelid immune cells and subsequently screened. Nanobodies screened from the naive library generally have low affinity for the target antigen and need to go through in vitro affinity maturation procedures. Nanobodies that have been discovered using this approach include Nb3, Nb6, Nb11, Sb23, 1E2, 4D8, H11-H4, H11-D4, and Nanosota-1C.63,69,70,155,156 For example, affinity-matured Nb6 and Nanosota-1 were both generated by mutating several amino acids at the antigen-binding sites and demonstrated much higher RBD-binding affinity and potent SARS-CoV-2 neutralizing potency than the respective nanobodies before the affinity maturation.63,69 Most nanobodies discovered from either of the above approach so far target SARS-CoV-2 RBD. Some of these nanobodies (such as Nb6, Nb11, Nb20, Nb21, Sb23, and Nanosota-1) recognize the RBD epitopes that overlap with the hACE2 binding site, whereas some other nanobodies (such as Nb34, Nb95, and K-874A) recognize the RBD epitopes that do not overlap with the ACE2 binding site.63,68–70,157 The former nanobodies directly block viral attachment to ACE2, whereas the latter may block the conformational changes of the S protein and hence inhibit the viral membrane fusion process.63,67–69,156,157
Multivalent (dimeric or trimeric) nanobodies have been constructed to improve the nanobodies’ target binding affinity, neutralizing potency, and in vivo half-life. One of the approaches is to link monomeric nanobodies linearly. These nanobodies include Nb3-tri, Nb6-tri, Nb11-tri, Nb203, Nb213, Nb20–20, Nb21–21, Nb21–Nb34, C1-trimer, H3-trimer, and C5-trimer. Compared to monomeric nanobodies, the linearly linked dimeric or trimeric nanobodies all neutralized pseudotyped SARS-CoV-2 infection more potently.63,68,69,158 In particular, compared to monomeric Nb6, its linearly linked dimer and trimer bound to SARS-CoV-2 RBD 750- and >20 000 times more strongly, respectively, and they neutralized pseudotyped SARS-CoV-2 infection 10 and 2000 times more potently respectively.69 These multivalent nanobodies maintain the high solubility, yield, and thermostability of monomeric nanobodies. Another approach to building multivalent nanobodies is to add a multivalent tag to the nanobodies. Dimeric Fc tags have been added to nanobodies, including Nanosota-1C-Fc 1E2-Fc, 4D8-Fc, 2F2-Fc, 3F11-Fc, 5F8-Fc, Ty1-Fc, and C5-Fc. Compared to monomeric nanobodies, the Fc-tagged dimeric nanobodies demonstrated binding to the RBD more tightly and neutralize SARS-CoV-2 infection more potently.63,67,156,158 A common misconception about building multivalent nanobodies is that multivalency makes nanobodies lose their advantages of being small. However, compared to conventional antibodies whose molecular weight is ∼160 kDa, Fc-tagged dimeric nanobodies are still much smaller with a molecular weight of ∼75–80 kDa. These multivalent nanobodies can still access cryptic epitopes on their targets because their antigen-binding sites are identical to monomeric nanobodies. Moreover, multivalent nanobodies maintain their good physical and chemical stabilities and production yields.63 It has been shown that some of the multivalent nanobodies even maintain their RBD-binding affinity and neutralizing activity after aerosolization, lyophilization, or heat treatment.69 Importantly, multivalent nanobodies have extended in vivo half-life because their molecular weight exceeds the molecular weight cutoff for kidney clearance (which is ∼60 kDa).63 Overall, multivalent nanobodies possess many advantages over monomeric nanobodies as antiviral therapeutics.
Currently, only a few anti-SARS-CoV-2 nanobodies have been tested and shown to be efficacious in animal models. For example, single-dose injection of hamsters with Fc-fused Nanosota-1C, K-874A, C5-trimer, and C5-Fc protected the animals from SARS-CoV-2 infection both prophylactically and/or therapeutically.63,157,158 Inhalable treatment of hamsters with PiN-21 nanobody prevented SARS-CoV-2 infection with reduced weight loss and decreased viral titers and lung pathology.159 Fc-fused Nanosota-1C also protected hACE2-Tg mice from SARS-CoV-2 infection with reduced viral titers and/or absence of lung pathology in the lung.63 To date, however, no SARS-CoV-2-specific nanobody has been evaluated for efficacy in humans.
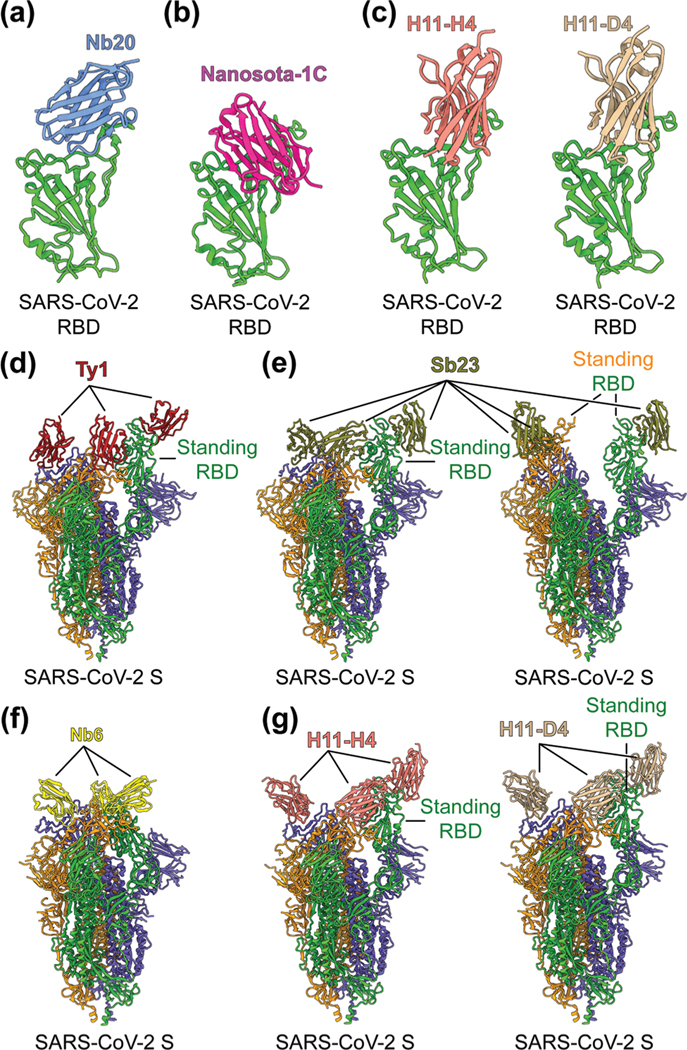
To identify the neutralizing epitopes of nanobodies, crystal structures of nanobody/RBD complexes and cryo-EM structures of nanobody/S complexes have been determined for the following nanobodies: Nb11, mNb6, C1, H3, C5, K-874A, Nb20, Nanosota-1C, H11-H4, H11-D4, Ty1, Sb23, and Nb6 (Fig. 5a–g).63,67–70,155,157,158 Whereas ACE2 only binds to SARS-CoV-2 RBD in the “up” position of the S protein, nanobodies can bind to SARS-CoV-2 RBD in the “up” or “down” conformation of the S protein due to their small size (Fig. 5d–g).67,70 Structural analysis of nanobodies in complex with the RBD or S protein is important for understanding the neutralizing mechanisms of the nanobodies and also provides a structural framework for optimizing the target-binding affinity and therapeutic potency of the nanobodies.
Fig. 5.
Structures of SARS-CoV-2 RBD or S protein trimer in complex with neutralizing nanobodies. (a–c) Crystal structures of SARS-CoV-2 receptor-binding domain (RBD) in complex with (a) neutralizing nanobodies Nb20 (PDB 7JVB), (b) Nanosota-1C (PDB 7KM5), (c) H11-H4 (PDB 6ZBP) or H11-D4 (PDB 6YZ5). SARS-CoV-2 RBD is colored in green and the four neutralizing nanobodies are colored in blue, magenta, salmon and tan, respectively. (d–g) Cryo-EM structures of SARS-CoV-2 spike (S) trimer in complex with neutralizing nanobodies (d) Ty1 with one RBD in the standing position (PDB 6ZXN), (e) Sb23 with one (PDB 7A25) and two (PDB 7A29) RBDs in the standing position, (f) Nb6 with all RBDs in the lying-down position (PDB 7KKK), (g) H11-H4 (PDB 6ZHD) and H11-D4 (PDB 6Z43) with one RBD in the standing position, respectively. Three copies of the S protein in the trimer are colored in green, orange and purple, respectively. Neutralizing nanobodies are colored in red (Ty1), olive (Sb23), yellow (Nb6), salmon (H11-H4), and tan (H11-D4), respectively.
Potential challenges nanotechnology-based COVID-19 vaccines and therapeutics
SARS-CoV-2 mutates at fast paces, particularly in the RBD region of the S protein. Multiple mutant virus strains, including Omicron variant, have been identified all over the world, and they almost all harbor mutations in the RBD.160–164 Although the currently available COVID-19 mRNA-S vaccines are effective against original virus strain and some mutant variants, many vaccine-induced antibodies are less effective against mutant strains with the K417N/T, L452R, E484K, N501Y, K417N-E484K-N501Y, or T478K mutations in the RBD.72,165–169 Hence there is an urgent need for developing universal vaccines, particularly those that can induce protective T-cell responses against newly-emerged and future SARS-CoV-2 variant strains. In contrast to the fast-mutating RBD, the S2 region is more conserved among different SARS-CoV-2 strains and among SARS-CoV-2, SARS-CoV-1, and SARS-related beta-coronaviruses. Thus, the S2 region may serve as an important target for the development of pan-beta-coronavirus vaccines. Other conserved structural proteins such as N or M may also serve as targets for developing SARS-CoV-2 vaccines with broad efficacy. However, it remains to be seen whether vaccines targeting the S2 region of the S protein, N protein, and M protein can induce potent neutralizing or protective immune responses. If the answer is yes, both protein nanoparticle-based protein vaccines and lipid nanoparticle-based mRNA vaccines can incorporate these more conserved antigens as immunogens.
Like vaccines, nanobody therapy also faces a similar challenge in neutralizing variant SARS-CoV-2 strains. Three potential strategies can be used. First, nanobodies can target different epitopes on SARS-CoV-2 S protein because mutations in multiple epitopes are less likely to occur than single-site mutations. To this end, multimeric nanobodies can be constructed by combining two or more nanobodies recognizing different epitopes on the same SARS-CoV-2 S protein. Alternatively, cocktails of nanobodies targeting different epitopes on the S protein may be used in therapies. Second, nanobodies can be developed to target conserved and essential epitopes on the S protein. Because of the high sequence conservation of the S2 region among SARS-CoV-2, SARS-CoV-1, and SARS-related coronaviruses, it is possible to develop broadly neutralizing nanobodies that target the S2 subunit. One advantage of nanobodies over conventional antibodies is that nanobodies can access cryptic epitopes that are essential for the function of the S protein. Third, nanobodies can be quickly adapted to new virus strains through in vitro affinity maturation using phage display. Overall, new nanobody therapies have the flexibility to be adapted to new SARS-CoV-2 variants.
While conventional antibodies have been developed for decades and technologies for developing them are much mature, the development of nanobodies as human therapeutics is still in its infancy. Nanobodies are generally produced by immunizing large camelid animals such as camels and llama, which can be difficult to access and also require very restricted regulatory procedures. Fortunately, recent development of naïve nanobody libraries makes it possible to rapidly screen target nanobodies quickly and conveniently. Nanobodies are generally safe to use as they have low immunogenicity and toxicity in humans.170–172 Nanobodies, such as Caplacizumab-yhdp (Cablivi), have been approved as therapeutic agents to treat human diseases, including thrombotic thrombocytopenic purpura (aTTP),173,174 confirming the safety of nanobodies.
Conclusion and future perspectives
Since the COVID-19 pandemic began, extensive global efforts and increased funds have been devoted to the development of effective vaccines and therapeutics to battle this pandemic. Using nanotechnology as a tool, protein nanoparticle-based and lipid nanoparticle-formulated mRNA vaccines have been developed rapidly and shown to be effective in animals. In particular, two mRNA vaccines have demonstrated both safety and high efficacy in preventing SARS-CoV-2 infection in humans. Future efforts will be needed to expand the spectrum of these vaccines against divergent virus variants. Notably, lipid nanoparticles have contributed substantially to the battle against COVID-19 by formulating with mRNA vaccines and delivering them into immune cells. It is worth noting that lipid nanoparticles can also deliver DNA to immune cells to treat nonviral diseases.175–177 Hence lipid nanoparticle-formulated DNA vaccines may be developed to battle COVID-19, and the result can be compared with the data from traditional approaches for DNA delivery.178–181 While several SARS-CoV-2-specific conventional antibodies have proceeded into human clinical trials,22,182,183 SARS-CoV-2-specific nanobodies are still being developed at the preclinical stage, and only a few of them have been tested for efficacy in animal models. We hope that some of the potent and cost-effective SARS-CoV-2-neutralizing nanobodies will move forward to human clinical trials where their safety and therapeutic efficacy can be tested in humans. Overall, nanotechnology has been and will continue to be one of the major scientific weapons in the global battle against the COVID-19 pandemic.
Acknowledgements
This study was supported by NIH grants (R01AI139092, R01AI137472, and R01AI157975).
Footnotes
Conflicts of interest
The University of Minnesota has filed a patent on the lumazine synthase nanoparticle-based SARS-CoV-2 RBD vaccine with F. L and L. D. as two of the inventors and another patent on the Nanosota-1 candidate drugs with F. L. as one of the inventors. Other authors have declared that no competing interests exist.
References
- 1.Blecher K, Nasir A. and Friedman A, Virulence, 2011, 2, 395–401. [DOI] [PubMed] [Google Scholar]
- 2.Singh L, Kruger HG, Maguire GEM, Govender T. and Parboosing R, Ther. Adv. Infect. Dis, 2017, 4, 105–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akilesh MS and Wadhwani A, Curr. Drug Res. Rev, 2021, 13, 120–129. [DOI] [PubMed] [Google Scholar]
- 4.Milane L. and Amiji M, Drug Delivery Transl. Res, 2021, 11, 1309–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffmann MAG, Bar-On Y, Yang Z, Gristick HB, Gnanapragasam PNP, Vielmetter J, Nussenzweig MC and Bjorkman PJ, Proc. Natl. Acad. Sci. U. S. A, 2020, 117, 18719–18728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakravarty M. and Vora A, Drug Delivery Transl. Res, 2021, 11, 748–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee YT, Ko EJ, Kim KH, Hwang HS, Lee Y, Kwon YM, Kim MC, Lee YN, Jung YJ and Kang SM, J. Biomed. Nanotechnol, 2017, 13, 84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rupar MJ, Golusinski P, Golusinski W. and Masternak MM, Rep. Pract. Oncol. Radiother, 2019, 24, 544–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF and Shi ZL, Nature, 2020, 579, 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du L, He Y, Zhou Y, Liu S, Zheng BJ and Jiang S, Nat. Rev. Microbiol, 2009, 7, 226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD and Fouchier RA, N. Engl. J. Med, 2012, 367, 1814–1820. [DOI] [PubMed] [Google Scholar]
- 12.Li F, Annu. Rev. Virol, 2016, 3, 237–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du L, Zhao G, Kou Z, Ma C, Sun S, Poon VK, Lu L, Wang L, Debnath AK, Zheng BJ, Zhou Y. and Jiang S, J. Virol, 2013, 87, 9939–9942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du L, Tai W, Zhou Y. and Jiang S, Expert Rev. Vaccines, 2016, 15, 1123–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui J, Li F. and Shi ZL, Nat. Rev. Microbiol, 2019, 17, 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Y, Jiang S. and Du L, Expert Rev. Vaccines, 2018, 17, 677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang N, Shang J, Li C, Zhou K. and Du L, Expert Rev. Vaccines, 2020, 19, 817–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du L, Yang Y, Zhou Y, Lu L, Li F. and Jiang S, Expert Opin. Ther. Targets, 2017, 21, 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang N, Shang J, Jiang S. and Du L, Front. Microbiol, 2020, 11, 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du L, Yang Y. and Zhang X, Cell. Mol. Immunol, 2021, 18, 2293–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y. and Du L, Signal Transduction Targeted Ther., 2021, 6, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang S, Zhang X, Yang Y, Hotez PJ and Du L, Nat. Biomed. Eng, 2020, 4, 1134–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang S, Zhang X. and Du L, Expert Opin. Ther. Targets, 2021, 25, 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang S, Hillyer C. and Du L, Trends Immunol., 2020, 41, 355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tai W, Zhang X, He Y, Jiang S. and Du L, Antiviral Res., 2020, 179, 104820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, Geng Q, Auerbach A. and Li F, Nature, 2020, 581, 221–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, Zhang Q, Shi X, Wang Q, Zhang L. and Wang X, Nature, 2020, 581, 215–220. [DOI] [PubMed] [Google Scholar]
- 28.Wan Y, Shang J, Graham R, Baric RS and Li F, J. Virol, 2020, 94, e00127–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li F, Li W, Farzan M. and Harrison SC, Science, 2005, 309, 1864–1868. [DOI] [PubMed] [Google Scholar]
- 30.Li F, Virol J., 2015, 89, 1954–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia S, Liu M, Wang C, Xu W, Lan Q, Feng S, Qi F, Bao L, Du L, Liu S, Qin C, Sun F, Shi Z, Zhu Y, Jiang S. and Lu L, Cell Res., 2020, 30, 343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A. and Li F, Proc. Natl. Acad. Sci. U. S. A, 2020, 117, 11727–11734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang S, Du L. and Shi Z, Emerging Microbes Infect., 2020, 9, 275–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT and Veesler D, Cell, 2020, 181, 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He L, Lin X, Wang Y, Abraham C, Sou C, Ngo T, Zhang Y, Wilson IA and Zhu J, Sci. Adv, 2021, 7, eabf1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Powell AE, Zhang K, Sanyal M, Tang S, Weidenbacher PA, Li S, Pham TD, Pak JE, Chiu W. and Kim PS, ACS Cent. Sci, 2021, 7, 183–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geng Q, Tai W, Baxter VK, Shi J, Wan Y, Zhang X, Montgomery SA, Taft-Benz SA, Anderson EJ, Knight AC, Dinnon KH 3rd, Leist SR, Baric RS, Shang J, Hong SW, Drelich A, Tseng CK, Jenkins M,Heise M, Du L. and Li F, PLoS Pathog., 2021, 17, e1009897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keech C, Albert G, Cho I, Robertson A, Reed P, Neal S, Plested JS, Zhu M, Cloney-Clark S, Zhou H, Smith G, Patel N, Frieman MB, Haupt RE, Logue J, McGrath M, Weston S, Piedra PA, Desai C, Callahan K, Lewis M, Price-Abbott P, Formica N,Shinde V, Fries L, Lickliter JD, Griffin P, Wilkinson B. and Glenn GM, N. Engl. J. Med, 2020, 383, 2320–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang YN, Paynter J, Sou C, Fourfouris T, Wang Y, Abraham C, Ngo T, Zhang Y, He L. and Zhu J, Sci. Adv, 2021, 7, eabj3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pati R, Shevtsov M. and Sonawane A, Front. Immunol, 2018, 9, 2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shinde V, Cho I, Plested JS, Agrawal S, Fiske J, Cai R, Zhou H, Pham X, Zhu M, Cloney-Clark S, Wang N, Zhou B, Lewis M, Price-Abbott P, Patel N, Massare MJ, Smith G, Keech C, Fries L. and Glenn GM, Lancet Infect. Dis, 2021, 22, 73–84. [DOI] [PubMed] [Google Scholar]
- 42.Smith DM, Simon JK and Baker JR Jr., Nat. Rev. Immunol, 2013, 13, 592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al-Halifa S, Gauthier L, Arpin D, Bourgault S. and Archambault D, Front. Immunol, 2019, 10, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang N, Li C, Jiang S. and Du L, Vaccines, 2020, 8, 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wuertz KM, Barkei EK, Chen WH, Martinez EJ, Lakhal-Naouar I, Jagodzinski LL, Paquin-Proulx D, Gromowski GD, Swafford I, Ganesh A, Dong M, Zeng X, Thomas PV, Sankhala RS, Hajduczki A, Peterson CE, Kuklis C, Soman S, Wieczorek L, Zemil M, Anderson A, Darden J, Hernandez H, Grove H, Dussupt V, Hack H, de la Barrera R, Zarling S, Wood JF, Froude JW, Gagne M, Henry AR, Mokhtari EB, Mudvari P, Krebs SJ, Pekosz AS, Currier JR, Kar S, Porto M, Winn A, Radzyminski K, Lewis MG, Vasan S, Suthar M, Polonis VR, Matyas GR, Boritz EA, Douek DC, Seder RA, Daye SP, Rao M, Peel SA, Joyce MG, Bolton DL, Michael NL and Modjarrad K, npj Vaccines, 2021, 6, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tai W, Zhang X, Yang Y, Zhu J. and Du L, Transl. Res, 2021, S1931–5244(21)00280–2. [Google Scholar]
- 47.Tai W, Zhang X, Drelich A, Shi J, Hsu JC, Luchsinger L, Hillyer CD, Tseng CK, Jiang S. and Du L, Cell Res., 2020, 30, 932–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wadhwa A, Aljabbari A, Lokras A, Foged C. and Thakur A, Pharmaceutics, 2020, 12, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim J, Eygeris Y, Gupta M. and Sahay G, Adv. Drug Delivery Rev, 2021, 170, 83–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jackson NAC, Kester KE, Casimiro D, Gurunathan S. and DeRosa F, npj Vaccines, 2020, 5, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ballesteros-Briones MC, Silva-Pilipich N, Herrador-Canete G, Vanrell L. and Smerdou C, Curr. Opin. Virol, 2020, 44, 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bloom K, van den Berg F. and Arbuthnot P, Gene Ther., 2021, 28, 117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Erasmus JH, Khandhar AP, O’Connor MA, Walls AC, Hemann EA, Murapa P, Archer J, Leventhal S, Fuller JT, Lewis TB, Draves KE, Randall S, Guerriero KA, Duthie MS, Carter D, Reed SG, Hawman DW, Feldmann H, Gale M Jr., Veesler D, Berglund P. and Fuller DH, Sci. Transl. Med, 2020, 12, eabc9396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Corbett KS, Flynn B, Foulds KE, Francica JR, Boyoglu-Barnum S, Werner AP, Flach B, O’Connell S, Bock KW, Minai M, Nagata BM, Andersen H, Martinez DR, Noe AT, Douek N, Donaldson MM, Nji NN, Alvarado GS, Edwards DK, Flebbe DR, Lamb E, Doria-Rose NA, Lin BC, Louder MK, O’Dell S, Schmidt SD, Phung E, Chang LA, Yap C, Todd JM, Pessaint L, Van Ry A, Browne S, Greenhouse J, Putman-Taylor T, Strasbaugh A, Campbell TA, Cook A, Dodson A, Steingrebe K, Shi W, Zhang Y, Abiona OM, Wang L, Pegu A, Yang ES, Leung K, Zhou T, Teng IT, Widge A, Gordon I, Novik L, Gillespie RA, Loomis RJ, Moliva JI, Stewart-Jones G, Himansu S, Kong WP, Nason MC, Morabito KM, Ruckwardt TJ, Ledgerwood JE, Gaudinski MR, Kwong PD, Mascola JR, Carfi A, Lewis MG, Baric RS, McDermott A, Moore IN,Sullivan NJ, Roederer M, Seder RA and Graham BS, N. Engl. J. Med, 2020, 383, 1544–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Perez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr., Hammitt LL, Tureci O, Nell H, Schaefer A, Unal S, Tresnan DB, Mather S, Dormitzer PR, Sahin U, Jansen KU, Gruber WC and Group CCT, N. Engl. J. Med, 2020, 383, 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reichmuth AM, Oberli MA, Jaklenec A, Langer R. and Blankschtein D, Ther. Delivery, 2016, 7, 319–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blakney AK, Ip S. and Geall AJ, Vaccines, 2021, 9, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Linares-Fernandez S, Lacroix C, Exposito JY and Verrier B, Trends Mol. Med, 2020, 26, 311–323. [DOI] [PubMed] [Google Scholar]
- 59.Hoey RJ, Eom H. and Horn JR, Exp. Biol. Med, 2019, 244, 1568–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muyldermans S, FEBS J, 2021, 288, 2084–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao G, He L, Sun S, Qiu H, Tai W, Chen J, Li J, Chen Y, Guo Y, Wang Y, Shang J, Ji K, Fan R, Du E, Jiang S, Li F, Du L. and Zhou Y, J. Virol, 2018, 92, e00837–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.He L, Tai W, Li J, Chen Y, Gao Y, Li J, Sun S, Zhou Y, Du L. and Zhao G, Viruses, 2019, 11, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ye G, Gallant J, Zheng J, Massey C, Shi K, Tai W, Odle A, Vickers M, Shang J, Wan Y, Du L, Aihara H, Perlman S, LeBeau A. and Li F, eLife, 2021, 10, e64815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wan Y, Shang J, Sun S, Tai W, Chen J, Geng Q, He L, Chen Y, Wu J, Shi Z, Zhou Y, Du L. and Li F, J. Virol, 2020, 94, e02015–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peyron I, Kizlik-Masson C, Dubois MD, Atsou S, Ferriere S, Denis CV, Lenting PJ, Casari C. and Christophe OD, Res. Pract. Thromb. Haemostasis, 2020, 4, 1087–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xenaki KT, Dorrestijn B, Muns JA, Adamzek K, Doulkeridou S, Houthoff H, Oliveira S. and van PM Bergen En Henegouwen, Theranostics, 2021, 11, 5525–5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hanke L, Vidakovics Perez L, Sheward DJ, Das H, Schulte T, Moliner-Morro A, Corcoran M, Achour A, Karlsson Hedestam GB, Hallberg BM, Murrell B. and McInerney GM, Nat. Commun, 2020, 11, 4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xiang Y, Nambulli S, Xiao Z, Liu H, Sang Z, Duprex WP, Schneidman-Duhovny D, Zhang C. and Shi Y, Science, 2020, 370, 1479–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schoof M, Faust B, Saunders RA, Sangwan S, Rezelj V, Hoppe N, Boone M, Billesbolle CB, Puchades C, Azumaya CM, Kratochvil HT, Zimanyi M, Deshpande I, Liang J, Dickinson S, Nguyen HC, Chio CM, Merz GE, Thompson MC, Diwanji D, Schaefer K, Anand AA, Dobzinski N, Zha BS, Simoneau CR, Leon K, White KM, Chio US, Gupta M, Jin M, Li F, Liu Y, Zhang K, Bulkley D, Sun M, Smith AM, Rizo AN, Moss F, Brilot AF, Pourmal S, Trenker R, Pospiech T, Gupta S, Barsi-Rhyne B, Belyy V, Barile-Hill AW, Nock S, Liu Y, Krogan NJ, Ralston CY, Swaney DL, Garcia-Sastre A,Ott M, Vignuzzi M, Consortium QSB, Walter P. and Manglik A, Science, 2020, 370, 1473–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Custodio TF, Das H, Sheward DJ, Hanke L, Pazicky S, Pieprzyk J, Sorgenfrei M, Schroer MA, Gruzinov AY, Jeffries CM, Graewert MA, Svergun DI, Dobrev N, Remans K, Seeger MA, McInerney GM, Murrell B, Hallberg BM and Low C, Nat. Commun, 2020, 11, 5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li H, Guo L, Zheng H, Li J, Zhao X, Li J, Liang Y, Yang F, Zhao Y, Yang J, Xue M, Zuo Y, Zhou J, Chen Y, Yang Z, Li Y, Jin W, Shi H, He Z, Li Q. and Liu L, Bioconjugate Chem., 2021, 32, 1034–1046. [DOI] [PubMed] [Google Scholar]
- 72.Arunachalam PS, Walls AC, Golden N, Atyeo C, Fischinger S, Li C, Aye P, Navarro MJ, Lai L, Edara VV, Roltgen K, Rogers K, Shirreff L, Ferrell DE, Wrenn S, Pettie D, Kraft JC, Miranda MC, Kepl E, Sydeman C, Brunette N, Murphy M, Fiala B, Carter L, White AG, Trisal M, Hsieh CL, Russell-Lodrigue K, Monjure C, Dufour J, Spencer S, Doyle-Meyers L, Bohm RP, Maness NJ, Roy C, Plante JA, Plante KS, Zhu A, Gorman MJ, Shin S, Shen X, Fontenot J, Gupta S, O’Hagan DT, Van Der Most R, Rappuoli R, Coffman RL, Novack D, McLellan JS, Subramaniam S, Montefiori D, Boyd SD, Flynn JL, Alter G, Villinger F, Kleanthous H, Rappaport J, Suthar MS, King NP, Veesler D. and Pulendran B, Nature, 2021, 594, 253–258. [DOI] [PubMed] [Google Scholar]
- 73.Walls AC, Fiala B, Schafer A, Wrenn S, Pham MN, Murphy M, Tse LV, Shehata L, O’Connor MA, Chen C, Navarro MJ, Miranda MC, Pettie D, Ravichandran R, Kraft JC, Ogohara C, Palser A, Chalk S, Lee EC, Guerriero K, Kepl E, Chow CM, Sydeman C, Hodge EA, Brown B, Fuller JT, Dinnon KH 3rd, Gralinski LE, Leist SR, Gully KL, Lewis TB, Guttman M, Chu HY, Lee KK, Fuller DH, Baric RS, Kellam P, Carter L, Pepper M, Sheahan TP, Veesler D. and King NP, Cell, 2020, 183, 1367–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kang YF, Sun C, Zhuang Z, Yuan RY, Zheng Q, Li JP, Zhou PP, Chen XC, Liu Z, Zhang X, Yu XH, Kong XW, Zhu QY, Zhong Q, Xu M, Zhong NS, Zeng YX, Feng GK, Ke C, Zhao JC and Zeng MS, ACS Nano, 2021, 15, 2738–2752. [DOI] [PubMed] [Google Scholar]
- 75.Tan TK, Rijal P, Rahikainen R, Keeble AH, Schimanski L, Hussain S, Harvey R, Hayes JWP, Edwards JC, McLean RK, Martini V, Pedrera M, Thakur N, Conceicao C, Dietrich I, Shelton H, Ludi A, Wilsden G, Browning C, Zagrajek AK, Bialy D, Bhat S, Stevenson-Leggett P, Hollinghurst P, Tully M, Moffat K, Chiu C, Waters R, Gray A, Azhar M, Mioulet V, Newman J, Asfor AS, Burman A, Crossley S, Hammond JA, Tchilian E, Charleston B, Bailey D, Tuthill TJ, Graham SP, Duyvesteyn HME, Malinauskas T, Huo J, Tree JA, Buttigieg KR, Owens RJ, Carroll MW, Daniels RS, McCauley JW, Stuart DI, Huang KA, Howarth M. and Townsend R, Nat. Commun, 2021, 12, 542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van Oosten L, Altenburg JJ, Fougeroux C, Geertsema C, van den End F, Evers WAC, Westphal AH, Lindhoud S, van den Berg W, Swarts DC, Deurhof L, Suhrbier A, Le TT, Torres Morales S, Myeni SK, Kikkert M, Sander AF, de Jongh WA, Dagil R, Nielsen MA, Salanti A, Sogaard M, Keijzer TMP, Weijers D, Eppink MHM, Wijffels RH, van Oers MM, Martens DE and Pijlman GP, mBio, 2021, 12, e0181321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang B, Chao CW, Tsybovsky Y, Abiona OM, Hutchinson GB, Moliva JI, Olia AS, Pegu A, Phung E, Stewart-Jones GBE, Verardi R, Wang L, Wang S, Werner A, Yang ES, Yap C, Zhou T, Mascola JR, Sullivan NJ, Graham BS, Corbett KS and Kwong PD, Sci. Rep, 2020, 10, 18149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Formica N, Mallory R, Albert G, Robinson M, Plested JS, Cho I, Robertson A, Dubovsky F, Glenn GM and nCo VSG, PLoS Med., 2021, 18, e1003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Heath PT, Galiza EP, Baxter DN, Boffito M, Browne D, Burns F, Chadwick DR, Clark R, Cosgrove C, Galloway J, Goodman AL, Heer A, Higham A, Iyengar S, Jamal A, Jeanes C, Kalra PA, Kyriakidou C, McAuley DF, Meyrick A, Minassian AM, Minton J, Moore P, Munsoor I, Nicholls H, Osanlou O, Packham J, Pretswell CH, San Francisco Ramos A, Saralaya D, Sheridan RP, Smith R, Soiza RL, Swift PA, Thomson EC, Turner J, Viljoen ME, Albert G, Cho I, Dubovsky F, Glenn G, Rivers J, Robertson A, Smith K, Toback S. and nCo VSG, N. Engl. J. Med, 2021, 385, 1172–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shinde V, Bhikha S, Hoosain Z, Archary M, Bhorat Q, Fairlie L, Lalloo U, Masilela MSL, Moodley D, Hanley S, Fouche L, Louw C, Tameris M, Singh N, Goga A, Dheda K, Grobbelaar C, Kruger G, CarrimGaney N, Baillie V, de Oliveira T, Lombard Koen A, Lombaard JJ, Mngqibisa R, Bhorat AE, Benade G, Lalloo N, Pitsi A, Vollgraaff PL, Luabeya A, Esmail A, Petrick FG, Oommen-Jose A, Foulkes S, Ahmed K, Thombrayil A, Fries L, Cloney-Clark S, Zhu M, Bennett C, Albert G, Faust E, Plested JS, Robertson A, Neal S, Cho I, Glenn GM, Dubovsky F, Madhi SA and nCo VSG, N. Engl. J. Med, 2021, 384, 1899–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Laczkó D, Hogan MJ, Toulmin SA, Hicks P, Lederer K, Gaudette BT, Castaño D, Amanat F, Muramatsu H, Oguin TH 3rd, Ojha A, Zhang L, Mu Z, Parks R, Manzoni TB, Roper B, Strohmeier S, Tombácz I, Arwood L, Nachbagauer R, Karikó K, Greenhouse J, Pessaint L, Porto M, Putman-Taylor T, Strasbaugh A, Campbell TA, Lin PJC, Tam YK, Sempowski GD, Farzan M, Choe H, Saunders KO, Haynes BF, Andersen H, Eisenlohr LC, Weissman D, Krammer F, Bates P, Allman D, Locci M. and Pardi N, Immunity, 2020, 53, 724–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lederer K, Castano D, Gomez Atria D, Oguin TH 3rd, Wang S, Manzoni TB, Muramatsu H, Hogan MJ, Amanat F, Cherubin P, Lundgreen KA, Tam YK, Fan SHY, Eisenlohr LC, Maillard I, Weissman D, Bates P, Krammer F, Sempowski GD, Pardi N. and Locci M, Immunity, 2020, 53, 1281–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang Q, Ji K, Tian S, Wang F, Huang B, Tong Z, Tan S, Hao J, Wang Q, Tan W, Gao GF and Yan J, Nat. Commun, 2021, 12, 776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vogel AB, Kanevsky I, Che Y, Swanson KA, Muik A, Vormehr M, Kranz LM, Walzer KC, Hein S, Güler A, Loschko J, Maddur MS, Ota-Setlik A, Tompkins K, Cole J, Lui BG, Ziegenhals T, Plaschke A, Eisel D, Dany SC, Fesser S, Erbar S, Bates F, Schneider D, Jesionek B, Sänger B, Wallisch AK, Feuchter Y, Junginger H, Krumm SA, Heinen AP, AdamsQuack P, Schlereth J, Schille S, Kröner C, de la Caridad Güimil Garcia, Hiller T, Fischer L, Sellers RS, Choudhary S, Gonzalez O, Vascotto F, Gutman MR, Fontenot JA, Hall-Ursone S, Brasky K, Griffor MC, Han S, Su AAH, Lees JA, Nedoma NL, Mashalidis EH, Sahasrabudhe PV, Tan CY, Pavliakova D, Singh G, Fontes-Garfias C, Pride M, Scully IL, Ciolino T, Obregon J, Gazi M, Carrion R Jr., Alfson KJ, Kalina WV, Kaushal D, Shi PY, Klamp T, Rosenbaum C, Kuhn AN, Türeci Ö, Dormitzer PR, Jansen KU and Sahin U, Nature, 2021, 592, 283–289. [DOI] [PubMed] [Google Scholar]
- 85.Elia U, Ramishetti S, Rosenfeld R, Dammes N, Bar-Haim E, Naidu GS, Makdasi E, Yahalom-Ronen Y, Tamir H, Paran N, Cohen O. and Peer D, ACS Nano, 2021, 15, 9627–9637. [DOI] [PubMed] [Google Scholar]
- 86.Elia U, Rotem S, Bar-Haim E, Ramishetti S, Naidu GS, Gur D, Aftalion M, Israeli M, Bercovich-Kinori A, Alcalay R, Makdasi E, Chitlaru T, Rosenfeld R, Israely T, Melamed S, Abutbul Ionita I, Danino D, Peer D. and Cohen O, Nano Lett., 2021, 21, 4774–4779. [DOI] [PubMed] [Google Scholar]
- 87.van Doremalen N, Fischer RJ, Schulz JE, Holbrook MG, Smith BJ, Lovaglio J, Petsch B. and Munster VJ, Viruses, 2021, 13, 1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Corbett KS, Edwards DK, Leist SR, Abiona OM, Boyoglu-Barnum S, Gillespie RA, Himansu S, Schäfer A, Ziwawo CT, DiPiazza AT, Dinnon KH, Elbashir SM, Shaw CA, Woods A, Fritch EJ, Martinez DR, Bock KW, Minai M, Nagata BM, Hutchinson GB, Wu K, Henry C, Bahl K, Garcia-Dominguez D, Ma L, Renzi I, Kong WP, Schmidt SD, Wang L, Zhang Y, Phung E, Chang LA, Loomis RJ, Altaras NE, Narayanan E, Metkar M, Presnyak V, Liu C, Louder MK, Shi W, Leung K, Yang ES, West A, Gully KL, Stevens LJ, Wang N, Wrapp D, Doria-Rose NA, Stewart-Jones G, Bennett H, Alvarado GS, Nason MC, Ruckwardt TJ, McLellan JS, Denison MR, Chappell JD, Moore IN, Morabito KM, Mascola JR, Baric RS, Carfi A. and Graham BS, Nature, 2020, 586, 567–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McKay PF, Hu K, Blakney AK, Samnuan K, Brown JC, Penn R, Zhou J, Bouton CR, Rogers P, Polra K, Lin PJC, Barbosa C, Tam YK, Barclay WS and Shattock RJ, Nat. Commun, 2020, 11, 3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de Alwis R, Gan ES, Chen S, Leong YS, Tan HC, Zhang SL, Yau C, Low JGH, Kalimuddin S, Matsuda D, Allen EC, Hartman P, Park KJ, Alayyoubi M, Bhaskaran H, Dukanovic A, Bao Y, Clemente B, Vega J, Roberts S, Gonzalez JA, Sablad M, Yelin R, Taylor W, Tachikawa K, Parker S, Karmali P, Davis J, Sullivan BM, Sullivan SM, Hughes SG, Chivukula P. and Ooi EE, Mol. Ther, 2021, 29, 1970–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.DiPiazza AT, Leist SR, Abiona OM, Moliva JI, Werner A, Minai M, Nagata BM, Bock KW, Phung E, Schafer A, Dinnon KH 3rd, Chang LA, Loomis RJ, Boyoglu-Barnum S, Alvarado GS, Sullivan NJ, Edwards DK, Morabito KM, Mascola JR, Carfi A, Corbett KS, Moore IN, Baric RS, Graham BS and Ruckwardt TJ, Immunity, 2021, 54, 1869–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kalnin KV, Plitnik T, Kishko M, Zhang J, Zhang D, Beauvais A, Anosova NG, Tibbitts T, DiNapoli J, Ulinski G, Piepenhagen P, Cummings SM, Bangari DS, Ryan S, Huang PD, Huleatt J, Vincent D, Fries K, Karve S, Goldman R, Gopani H, Dias A, Tran K, Zacharia M, Gu X, Boeglin L, Abysalh J, Vargas J, Beaulieu A, Shah M, Jeannotte T, Gillis K, Chivukula S, Swearingen R, Landolfi V, Fu TM, DeRosa F. and Casimiro D, npj Vaccines, 2021, 6, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ji RR, Qu Y, Zhu H, Yang Y, Vogel AB, Sahin U, Qin C. and Hui A, Vaccines, 2021, 9, 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garrido C, Curtis AD 2nd, Dennis M, Pathak SH, Gao H, Montefiori D, Tomai M, Fox CB, Kozlowski PA, Scobey T, Munt JE, Mallory ML, Saha PT, Hudgens MG, Lindesmith LC, Baric RS, Abiona OM, Graham B, Corbett KS, Edwards D, Carfi A, Fouda G, Van Rompay KKA, De Paris K. and Permar SR, Sci. Immunol, 2021, 6, eabj3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tai W, He L, Zhang X, Pu J, Voronin D, Jiang S, Zhou Y. and Du L, Cell. Mol. Immunol, 2020, 17, 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim J, Mukherjee A, Nelson D, Jozic A. and Sahay G, Preprint bioRxiv, 2020, DOI: 10.1101/2020.07.24.205583. [DOI] [Google Scholar]
- 97.Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, McCullough MP, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, McDermott A, Flach B, Doria-Rose NA, Corbett KS, Morabito KM, O’Dell S, Schmidt SD, Swanson PA 2nd, Padilla M, Mascola JR, Neuzil KM, Bennett H, Sun W, Peters E, Makowski M, Albert J, Cross K, Buchanan W, Pikaart-Tautges R, Ledgerwood JE, Graham BS and Beigel JH, N. Engl. J. Med, 2020, 383, 1920–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Anderson EJ, Rouphael NG, Widge AT, Jackson LA, Roberts PC, Makhene M, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, McDermott AB, Flach B, Lin BC, Doria-Rose NA, O’Dell S, Schmidt SD, Corbett KS, Swanson PA II, Padilla M, Neuzil KM, Bennett H, Leav B, Makowski M, Albert J, Cross K, Edara VV, Floyd K, Suthar MS, Martinez DR, Baric R, Buchanan W, Luke CJ, Phadke VK, Rostad CA, Ledgerwood JE, Graham BS, Beigel JH and mRNA-1273 Study Group, N. Engl. J. Med, 2020, 383, 2427–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Widge AT, Rouphael NG, Jackson LA, Anderson EJ, Roberts PC, Makhene M, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, McDermott AB, Flach B, Lin BC, Doria-Rose NA, O’Dell S, Schmidt SD, Neuzil KM, Bennett H, Leav B, Makowski M, Albert J, Cross K, Edara VV, Floyd K, Suthar MS, Buchanan W, Luke CJ, Ledgerwood JE, Mascola JR, Graham BS, Beigel JH and mRNA-1273 Study Group, N. Engl. J. Med, 2021, 384, 80–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T. and Group CS, N. Engl. J. Med, 2021, 384, 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Walsh EE, Frenck RW Jr., Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R, Swanson KA, Li P, Koury K, Kalina W, Cooper D, Fontes-Garfias C, Shi PY, Tureci O, Tompkins KR, Lyke KE, Raabe V, Dormitzer PR, Jansen KU, Sahin U. and Gruber WC, N. Engl. J. Med, 2020, 383, 2439–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, Baum A, Pascal K, Quandt J, Maurus D, Brachtendorf S, Lörks V, Sikorski J, Hilker R, Becker D, Eller AK, Grützner J, Boesler C, Rosenbaum C, Kühnle MC, Luxemburger U, Kemmer-Brück A, Langer D, Bexon M, Bolte S, Karikó K, Palanche T, Fischer B, Schultz A, Shi PY, Fontes-Garfias C, Perez JL, Swanson KA, Loschko J, Scully IL, Cutler M, Kalina W, Kyratsous CA, Cooper D, Dormitzer PR, Jansen KU and Türeci Ö, Nature, 2020, 586, 594–599. [DOI] [PubMed] [Google Scholar]
- 103.Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Raabe V, Bailey R, Swanson KA, Li P, Koury K, Kalina W, Cooper D, Fontes-Garfias C, Shi PY, Tureci O, Tompkins KR, Walsh EE, Frenck R, Falsey AR, Dormitzer PR, Gruber WC, Sahin U. and Jansen KU, Nature, 2020, 586, 589–593. [DOI] [PubMed] [Google Scholar]
- 104.Riad A, Hockova B, Kantorova L, Slavik R, Spurna L, Stebel A, Havrilak M. and Klugar M, Pharmaceuticals, 2021, 14, 873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, McClung N, Campos-Outcalt D, Morgan RL, Mbaeyi S, Romero JR, Talbot HK, Lee GM, Bell BP and Dooling K, MMWR Morb. Mortal. Wkly. Rep, 2020, 69, 1922–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dooling K, Gargano JW, Moulia D, Wallace M, Rosenblum HG, Blain AE, Hadler SC, Plumb ID, Moline H, Gerstein J, Collins JP, Godfrey M, Campos-Outcalt D, Morgan RL, Brooks O, Talbot HK, Lee GM, Daley MF and Oliver SE, MMWR Morb. Mortal. Wkly. Rep, 2021, 70, 1344–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Self WH, Tenforde MW, Rhoads JP, Gaglani M, Ginde AA, Douin DJ, Olson SM, Talbot HK, Casey JD, Mohr NM, Zepeski A, McNeal T, Ghamande S, Gibbs KW, Files DC, Hager DN, Shehu A, Prekker ME, Erickson HL, Gong MN, Mohamed A, Henning DJ, Steingrub JS, Peltan ID, Brown SM, Martin ET, Monto AS, Khan A, Hough CL, Busse LW, Ten Lohuis CC, Duggal A, Wilson JG, Gordon AJ, Qadir N, Chang SY, Mallow C, Rivas C, Babcock HM, Kwon JH, Exline MC, Halasa N, Chappell JD, Lauring AS, Grijalva CG, Rice TW, Jones ID, Stubblefield WB, Baughman A, Womack KN, Lindsell CJ, Hart KW, Zhu Y, Mills L, Lester SN, Stumpf MM, Naioti EA, Kobayashi M, Verani JR, Thornburg NJ, Patel MM and Network IVY, MMWR Morb. Mortal. Wkly. Rep, 2021, 70, 1337–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Parums DV, Med. Sci. Monit, 2021, 27, e934625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wallace M, Woodworth KR, Gargano JW, Scobie HM, Blain AE, Moulia D, Chamberland M, Reisman N, Hadler SC, MacNeil JR, Campos-Outcalt D, Morgan RL, Daley MF, Romero JR, Talbot HK, Lee GM, Bell BP and Oliver SE, MMWR Morb. Mortal. Wkly. Rep, 2021, 70, 749–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Woodworth KR, Moulia D, Collins JP, Hadler SC, Jones JM, Reddy SC, Chamberland M, Campos-Outcalt D, Morgan RL, Brooks O, Talbot HK, Lee GM, Bell BP, Daley MF, Mbaeyi S, Dooling K. and Oliver SE, MMWR Morb. Mortal. Wkly. Rep, 2021, 70, 1579–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, McClung N, Campos-Outcalt D, Morgan RL, Mbaeyi S, Romero JR, Talbot HK, Lee GM, Bell BP and Dooling K, MMWR Morb. Mortal. Wkly. Rep, 2021, 69, 1653–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wodi AP, Ault K, Hunter P, McNally V, Szilagyi PGand Bernstein H, MMWR Morb. Mortal. Wkly. Rep, 2021, 70, 189–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Glatman-Freedman A, Hershkovitz Y, Kaufman Z, Dichtiar R, Keinan-Boker L. and Bromberg M, Emerging Infect. Dis, 2021, 27, 2919–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Montoya JG, Adams AE, Bonetti V, Deng S, Link NA, Pertsch S, Olson K, Li M, Dillon EC and Frosch DL, Microbiol. Spectrum, 2021, e0116221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Coppeta L, Somma G, Ferrari C, Mazza A, Rizza S, Trabucco Aurilio M, Perrone S, Magrini A. and Pietroiusti A, Vaccines, 2021, 9, 947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gil-Manso S, Carbonell D, Lopez-Fernandez L, Miguens I, Alonso R, Buno I, Munoz P, Ochando J, Pion M. and Correa-Rocha R, Front. Immunol, 2021, 12, 726960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rossi C, Lanuti P, Cicalini I, De Bellis D, Pierdomenico L, Del Boccio P, Zucchelli M, Natale L, Sinjari B, Catitti G, Vespa S, Simeone P, Bologna G, Bucci I, Falasca K, Vecchiet J, Stuppia L, De Laurenzi V. and Pieragostino D, Vaccines, 2021, 9, 1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thomas SJ, Moreira ED Jr., Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Perez Marc G, Polack FP, Zerbini C, Bailey R, Swanson KA, Xu X, Roychoudhury S, Koury K, Bouguermouh S, Kalina WV, Cooper D, Frenck RW Jr., Hammitt LL, Tureci O, Nell H, Schaefer A, Unal S, Yang Q, Liberator P, Tresnan DB, Mather S, Dormitzer PR, Sahin U, Gruber WC, Jansen KU and C. C. T. Group, N. Engl. J. Med, 2021, 385, 1761–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yahav D, Rozen-Zvi B, Mashraki T, Atamna A, Ben-Zvi H, Bar-Haim E. and Rahamimov R, BMJ Open, 2021, 11, e055611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ammitzboll C, Bartels LE, Bogh Andersen J, Risbol Vils S, Elbaek Mistegard C, Dahl Johannsen A, From Hermansen ML, Kragh Thomsen M, Erikstrup C, Hauge EM and Troldborg A, ACR Open Rheumatol., 2021, 3, 622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gavriatopoulou M, Terpos E, Ntanasis-Stathopoulos I, Briasoulis A, Gumeni S, Malandrakis P, Fotiou D, Migkou M, Theodorakakou F, Eleutherakis-Papaiakovou E, Kanellias N, Kastritis E, Trougakos IP and Dimopoulos MA, Blood Adv., 2021, 5, 4398–4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rabinowich L, Grupper A, Baruch R, Ben-Yehoyada M, Halperin T, Turner D, Katchman E, Levi S, Houri I, Lubezky N, Shibolet O. and Katchman H, J. Hepatol, 2021, 75, 435–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ferri C, Ursini F, Gragnani L, Raimondo V, Giuggioli D, Foti R, Caminiti M, Olivo D, Cuomo G, Visentini M, Cacciapaglia F, Pellegrini R, Pigatto E, Urraro T, Naclerio C, Tavoni A, Puccetti L, Varcasia G, Cavazzana I, L’Andolina M, Ruscitti P, Vadacca M, Gigliotti P, La Gualana F, Cozzi F, Spinella A, Visalli E, Dal Bosco Y, Amato G, Masini F, Pagano Mariano G, Brittelli R, Aiello V, Caminiti R, Scorpiniti D, Rechichi G, Ferrari T, Monti M, Elia G, Franceschini F, Meliconi R, Casato M, Iannone F, Giacomelli R, Fallahi P, Santini SA, Zignego AL and Antonelli A, J. Autoimmun, 2021, 125, 102744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Davidov Y, Tsaraf K, Cohen-Ezra O, Likhter M, Ben Yakov G, Levy I, Levin EG, Lustig Y, Mor O, Rahav G. and Ben Ari Z, Liver Transpl., 2021, DOI: 10.1002/lt.26366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cucchiari D, Egri N, Bodro M, Herrera S, Del Risco-Zevallos J, Casals-Urquiza J, Cofan F, Moreno A, Rovira J, Banon-Maneus E, Ramirez-Bajo MJ, Ventura-Aguiar P, Perez-Olmos A, Garcia-Pascual M, Pascal M, Vilella A, Trilla A, Rios J, Palou E, Juan M, Bayes B. and Diekmann F, Am. J. Transplant, 2021, 21, 2727–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Herrera S, Colmenero J, Pascal M, Escobedo M, Castel MA, Sole-Gonzalez E, Palou E, Egri N, Ruiz P, Mosquera M, Moreno A, Juan M, Vilella A, Soriano A, Farrero M. and Bodro M, Am. J. Transplant, 2021, 21, 3971–3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Naranbhai V, Pernat CA, Gavralidis A, St Denis KJ, Lam EC, Spring LM, Isakoff SJ, Farmer JR, Zubiri L, Hobbs GS, How J, Brunner AM, Fathi AT, Peterson JL, Sakhi M, Hambelton G, Denault EN, Mortensen LJ, Perriello LA, Bruno MN, Bertaux BY, Lawless AR, Jackson MA, Niehoff E, Barabell C, Nambu CN, Nakajima E, Reinicke T, Bowes C, Berrios-Mairena CJ, Ofoman O, Kirkpatrick GE, Thierauf JC, Reynolds K, Willers H, Beltran WG, Dighe AS, Saff R, Blumenthal K, Sullivan RJ, Chen YB, Kim A, Bardia A, Balazs AB, Iafrate AJ and Gainor JF, J. Clin. Oncol, 2021, JCO2101891, DOI: 10.1200/JCO.21.01891. [DOI] [Google Scholar]
- 128.Buttiron Webber T, Provinciali N, Musso M, Ugolini M, Boitano M, Clavarezza M, D’Amico M, Defferrari C, Gozza A, Briata IM, Magnani M, Paciolla F, Menghini N, Marcenaro E, De Palma R, Sacchi N, Innocenti L, Siri G, D’Ecclesiis O, Cevasco I, Gandini S. and DeCensi A, Eur. J. Cancer, 2021, 159, 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Melin J, Svensson MK, Albinsson B, Winqvist O. and Pauksens K, BMC Immunol., 2021, 22, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Herishanu Y, Avivi I, Aharon A, Shefer G, Levi S, Bronstein Y, Morales M, Ziv T, Shorer Arbel Y, Scarfo L, Joffe E, Perry C. and Ghia P, Blood, 2021, 137, 3165–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Amanzio M, Mitsikostas DD, Giovannelli F, Bartoli M, Cipriani GE and Brown WA, Lancet Reg. Health Eur, 2021, 100253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Riad A, Pokorna A, Klugarova J, Antalova N, Kantorova L, Koscik M. and Klugar M, Pharmaceuticals, 2021, 14, 1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Esquivel-Valerio JA, Skinner-Taylor CM, Moreno-Arquieta IA, Cardenas-de la Garza JA, Garcia-Arellano G, Gonzalez-Garcia PL, Almaraz-Juarez FDR and Galarza-Delgado DA, Rheumatol. Int, 2021, 41, 2105–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Klugar M, Riad A, Mekhemar M, Conrad J, Buchbender M, Howaldt HP and Attia S, Biology, 2021, 10, 752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Briggs FBS, Mateen FJ, Schmidt H, Currie KM, Siefers HM, Crouthamel S, Bebo BF, Fiol J, Racke MK, O’Connor KC, Kolaczkowski LG, Klein P, Loud S. and McBurney RN, Neurol. Neuroimmunol. Neuroinflamm, 2021, 9, e1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kaplan B, Farzan S, Coscia G, Rosenthal DW, McInerney A, Jongco AM, Ponda P. and Bonagura VR, Ann. Allergy, Asthma, Immunol, 2021, S1081–1206(21)01172–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Klimek L, Novak N, Hamelmann E, Werfel T, Wagenmann M, Taube C, Bauer A, Merk H, Rabe U, Jung K, Schlenter W, Ring J, Chaker A, Wehrmann W, Becker S, Mulleneisen N, Nemat K, Czech W, Wrede H, Brehler R, Fuchs T, Jakob T, Ankermann T, Schmidt SM, Gerstlauer M, Vogelberg C, Zuberbier T, Hartmann K. and Worm M, Allergo J. Int, 2021, 30, 51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Team Food CC-R and Drug A, MMWR Morb. Mortal. Wkly. Rep, 2021, 70, 125–129.33507892 [Google Scholar]
- 139.Jeet Kaur R, Dutta S, Charan J, Bhardwaj P, Tandon A, Yadav D, Islam S. and Haque M, Int. J. Gen. Med, 2021, 14, 3909–3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nishiguchi Y, Matsuyama H, Maeda K, Shindo A. and Tomimoto H, BMC Neurol., 2021, 21, 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Takeyama R, Fukuda K, Kouzaki Y, Koga T, Hayashi S, Ohtani H. and Inoue T, Acta Neurochir., 2021, DOI: 10.1007/s00701-021-05038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kahn B, Apostolidis SA, Bhatt V, Greenplate AR, Kallish S, LaCava A, Lucas A, Meyer NJ, Negoianu D, Ogdie AR, Shashaty MGS, Takach PA, Zuroff L, Wherry EJ and Anesi GL, Crit. Care Explor, 2021, 3, e0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dionne A, Sperotto F, Chamberlain S, Baker AL, Powell AJ, Prakash A, Castellanos DA, Saleeb SF, de Ferranti SD, Newburger JW and Friedman KG, JAMA Cardiol., 2021, 6, 1446–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rechavi Y, Shashar M, Lellouche J, Yana M, Yakubovich D. and Sharon N, Vaccines, 2021, 9, 977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Massa F, Cremoni M, Gerard A, Grabsi H, Rogier L, Blois M, Couzin C, Hassen NB, Rouleau M, Barbosa S, Martinuzzi E, Fayada J, Bernard G, Favre G, Hofman P, Esnault VLM, Czerkinsky C, Seitz-Polski B, Glaichenhaus N. and Sicard A, EBioMedicine, 2021, 73, 103679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Barda N, Dagan N, Cohen C, Hernan MA, Lipsitch M, Kohane IS, Reis BY and Balicer RD, Lancet, 2021, 398, 2093–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Marlet J, Gatault P, Maakaroun Z, Longuet H, Stefic K, Handala L, Eymieux S, Gyan E, Dartigeas C. and Gaudy-Graffin C, Vaccines, 2021, 9, 1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schrezenmeier E, Rincon-Arevalo H, Stefanski AL, Potekhin A, Staub-Hohenbleicher H, Choi M, Bachmann F, Pross V, Hammett C, Schrezenmeier H, Ludwig C, Jahrsdorfer B, Lino A, Eckardt KU, Kotsch K, Doerner T, Budde K, Sattler A. and Halleck F, J. Am. Soc. Nephrol, 2021, 32, 3027–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Saciuk Y, Kertes J, Shamir Stein N. and Ekka Zohar A, J. Infect. Dis, 2021, 225, 30–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chavarot N, Morel A, Leruez-Ville M, Vilain E, Divard G, Burger C, Serris A, Sberro-Soussan R, Martinez F, Amrouche L, Bererhi L, Lanternier F, Legendre C, Zuber J, Anglicheau D. and Scemla A, Am. J. Transplant, 2021, 21, 4043–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Karaba AH, Zhu X, Liang T, Wang KH, Rittenhouse AG, Akinde O, Eby Y, Ruff JE, Blankson JN, Abedon AT, Alejo JL, Cox AL, Bailey JR, Thompson EA, Klein SL, Warren DS, Garonzik-Wang JM, Boyarsky BJ, Sitaras I, Pekosz A, Segev DL, Tobian AAR and Werbel WA, Preprint medRxiv, 2021, DOI: 10.1101/2021.08.11.21261914. [DOI] [Google Scholar]
- 152.Shroff RT, Chalasani P, Wei R, Pennington D, Quirk G, Schoenle MV, Peyton KL, Uhrlaub JL, Ripperger TJ, Jergovic M, Dalgai S, Wolf A, Whitmer R, Hammad H, Carrier A, Scott AJ, Nikolich-Zugich J, Worobey M, Sprissler R, Dake M, LaFleur BJ and Bhattacharya D, Nat. Med, 2021, 27, 2002–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shapiro Ben David S, Shamir-Stein N, Baruch Gez S, Lerner U, Rahamim-Cohen D. and Ekka Zohar A, Clin. Immunol, 2021, 232, 108860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hause AM, Baggs J, Gee J, Marquez P, Myers TR, Shimabukuro TT and Shay DK, MMWR Morb. Mortal. Wkly. Rep, 2021, 70, 1379–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Huo J, Le Bas A, Ruza RR, Duyvesteyn HME, Mikolajek H, Malinauskas T, Tan TK, Rijal P, Dumoux M, Ward PN, Ren J, Zhou D, Harrison PJ, Weckener M, Clare DK, Vogirala VK, Radecke J, Moynie L, Zhao Y, Gilbert-Jaramillo J, Knight ML, Tree JA, Buttigieg KR, Coombes N, Elmore MJ, Carroll MW, Carrique L, Shah PNM, James W, Townsend AR, Stuart DI, Owens RJ and Naismith JH, Nat. Struct. Mol. Biol, 2020, 27, 846–854. [DOI] [PubMed] [Google Scholar]
- 156.Chi X, Liu X, Wang C, Zhang X, Li X, Hou J, Ren L, Jin Q, Wang J. and Yang W, Nat. Commun, 2020, 11, 4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Haga K, Takai-Todaka R, Matsumura Y, Song C, Takano T, Tojo T, Nagami A, Ishida Y, Masaki H, Tsuchiya M, Ebisudani T, Sugimoto S, Sato T, Yasuda H, Fukunaga K, Sawada A, Nemoto N, Murata K, Morimoto T. and Katayama K, PLoS Pathog., 2021, 17, e1009542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Huo J, Mikolajek H, Le Bas A, Clark JJ, Sharma P, Kipar A, Dormon J, Norman C, Weckener M, Clare DK, Harrison PJ, Tree JA, Buttigieg KR, Salguero FJ, Watson R, Knott D, Carnell O, Ngabo D, Elmore MJ, Fotheringham S, Harding A, Moynie L, Ward PN, Dumoux M, Prince T, Hall Y, Hiscox JA, Owen A, James W, Carroll MW, Stewart JP, Naismith JH and Owens RJ, Nat. Commun, 2021, 12, 5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nambulli S, Xiang Y, Tilston-Lunel NL, Rennick LJ, Sang Z, Klimstra WB, Reed DS, Crossland NA, Shi Y. and Duprex WP, Sci. Adv, 2021, 7, eabh0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mlcochova P, Kemp SA, Dhar MS, Papa G, Meng B, Ferreira IATM, Datir R, Collier DA, Albecka A, Singh S, Pandey R, Brown J, Zhou J, Goonawardane N, Mishra S, Whittaker C, Mellan T, Marwal R, Datta M, Sengupta S, Ponnusamy K, Radhakrishnan VS, Abdullahi A, Charles O, Chattopadhyay P, Devi P, Caputo D, Peacock T, Wattal C, Goel N, Satwik A, Vaishya R, Agarwal M, Indian SARS-CoV-2 Genomics Consortium (INSACOG), Genotype to Phenotype Japan (G2P-Japan) Consortium, CITIID-NIHR BioResource COVID-19 Collaboration, Mavousian A, Lee JH, Bassi J, Silacci-Fegni C, Saliba C, Pinto D, Irie T, Yoshida I, Hamilton WL, Sato K, Bhatt S, Flaxman S, James LC, Corti D, Piccoli L, Barclay WS, Rakshit P, Agrawal A. and Gupta RK, Nature, 2021, 599, 114–119.34488225 [Google Scholar]
- 161.French G, Hulse M, Nguyen D, Sobotka K, Webster K, Corman J, Aboagye-Nyame B, Dion M, Johnson M, Zalinger B. and Ewing M, MMWR Morb. Mortal. Wkly. Rep, 2021, 70, 1613–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Venkatraja B, Srilakshminarayana G. and Krishna Kumar B, Am. J. Trop. Med. Hyg, 2021, tpmd210812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Allen H, Vusirikala A, Flannagan J, Twohig KA, Zaidi A, Chudasama D, Lamagni T. and UK C-G, Lancet Reg. Health Eur, 2021, 100252, DOI: 10.1016/j.lanepe.2021.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Melloul M, Chouati T, Hemlali M, Alaoui Amine S, Touil N, Elannaz H, Ennibi K, Youbi M, Merabet M, Bellefquih AM, Nourlil J, Maaroufi A. and El Fahime E, Microbiol. Resour. Announce, 2021, 10, e0072721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang Z, Schmidt F, Weisblum Y, Muecksch F, Barnes CO, Finkin S, Schaefer-Babajew D, Cipolla M, Gaebler C, Lieberman JA, Oliveira TY, Yang Z, Abernathy ME, Huey-Tubman KE, Hurley A, Turroja M, West KA, Gordon K, Millard KG, Ramos V, Da Silva J, Xu J, Colbert RA, Patel R, Dizon J, Unson-O’Brien C, Shimeliovich I, Gazumyan A, Caskey M, Bjorkman PJ, Casellas R, Hatziioannou T, Bieniasz PD and Nussenzweig MC, Nature, 2021, 592, 616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Garcia-Beltran WF, Lam EC, St Denis K, Nitido AD, Garcia ZH, Hauser BM, Feldman J, Pavlovic MN, Gregory DJ, Poznansky MC, Sigal A, Schmidt AG, Iafrate AJ, Naranbhai V. and Balazs AB, Cell, 2021, 184, 2372–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kuzmina A, Khalaila Y, Voloshin O, Keren-Naus A, Boehm-Cohen L, Raviv Y, Shemer-Avni Y, Rosenberg E. and Taube R, Cell Host Microbe, 2021, 29, 522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Collier DA, De Marco A, Ferreira IATM, Meng B, Datir RP, Walls AC, Kemp SA, Bassi J, Pinto D, Silacci-Fregni C, Bianchi S, Tortorici MA, Bowen J, Culap K, Jaconi S, Cameroni E, Snell G, Pizzuto MS, Pellanda AF, Garzoni C, Riva A, CITIID-NIHR BioResource COVID-19 Collaboration, Elmer A, Kingston N, Graves B, McCoy LE, Smith KGC, Bradley JR, Temperton N, Ceron-Gutierrez L, Barcenas-Morales G, COVID-19 Genomics UK (COG-UK) Consortium, Harvey W, Virgin HW, Lanzavecchia A, Piccoli L, Doffinger R, Wills M, Veesler D, Corti D. and Gupta RK, Nature, 2021, 593, 136–141.33706364 [Google Scholar]
- 169.Zhou D, Dejnirattisai W, Supasa P, Liu C, Mentzer AJ, Ginn HM, Zhao Y, Duyvesteyn HME, Tuekprakhon A, Nutalai R, Wang B, Paesen GC, Lopez-Camacho C, Slon-Campos J, Hallis B, Coombes N, Bewley K, Charlton S, Walter TS, Skelly D, Lumley SF, Dold C, Levin R, Dong T, Pollard AJ, Knight JC, Crook D, Lambe T, Clutterbuck E, Bibi S, Flaxman A, Bittaye M, Belij-Rammerstorfer S, Gilbert S, James W, Carroll MW, Klenerman P, Barnes E, Dunachie SJ, Fry EE, Mongkolsapaya J, Ren J, Stuart DI and Screaton GR, Cell, 2021, 184, 2348–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ackaert C, Smiejkowska N, Xavier C, Sterckx YGJ, Denies S, Stijlemans B, Elkrim Y, Devoogdt N, Caveliers V, Lahoutte T, Muyldermans S, Breckpot K. and Keyaerts M, Front. Immunol, 2021, 12, 632687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Broadbent L, Parke HG, Ferguson LJ, Millar A, Shields MD, Detalle L. and Power UF, Antimicrob. Agents Chemother, 2020, 64, e02034–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Svecova D, Lubell MW, Casset-Semanaz F, Mackenzie H, Grenningloh R. and Krueger JG, J. Am. Acad. Dermatol, 2019, 81, 196–203. [DOI] [PubMed] [Google Scholar]
- 173.Morrison C, Nat. Rev. Drug Discovery, 2019, 18, 485–487. [DOI] [PubMed] [Google Scholar]
- 174.Duggan S, Drugs, 2018, 78, 1639–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kimura S, Khalil IA, Elewa YHA and Harashima H, J. Controlled Release, 2021, 330, 753–764. [DOI] [PubMed] [Google Scholar]
- 176.Francis JE, Skakic I, Dekiwadia C, Shukla R, Taki AC, Walduck A. and Smooker PM, Vaccines, 2020, 8, 551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Maeta M, Miura N, Tanaka H, Nakamura T, Kawanishi R, Nishikawa Y, Asano K, Tanaka M, Tamagawa S, Nakai Y, Tange K, Yoshioka H, Harashima H. and Akita H, Mol. Pharm, 2020, 17, 1237–1247. [DOI] [PubMed] [Google Scholar]
- 178.Gambino F Jr., Tai W, Voronin D, Zhang Y, Zhang X, Shi J, Wang X, Wang N, Du L. and Qiao L, Cell Rep., 2021, 35, 109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Huang D, Huang Y. and Li Z, Methods Mol. Biol, 2020, 2050, 101–112. [DOI] [PubMed] [Google Scholar]
- 180.Shah MA, Ali Z, Ahmad R, Qadri I, Fatima K. and He N, J. Nanosci. Nanotechnol, 2015, 15, 41–53. [DOI] [PubMed] [Google Scholar]
- 181.Borggren M, Nielsen J, Bragstad K, Karlsson I, Krog JS, Williams JA and Fomsgaard A, Hum. Vaccines Immunother, 2015, 11, 1983–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, Musser BJ, Soo Y, Rofail D, Im J, Perry C, Pan C, Hosain R, Mahmood A, Davis JD, Turner KC, Hooper AT, Hamilton JD, Baum A, Kyratsous CA, Kim Y, Cook A, Kampman W, Kohli A, Sachdeva Y, Graber X, Kowal B, DiCioccio T, Stahl N, Lipsich L, Braunstein N, Herman G, Yancopoulos GD and Trial I, N. Engl. J. Med, 2021, 384, 238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J, Huhn G, Cardona J, Mocherla B, Stosor V, Shawa I, Adams AC, Van Naarden J, Custer KL, Shen L, Durante M, Oakley G, Schade AE, Sabo J, Patel DR, Klekotka P, Skovronsky DM and Investigators B, N. Engl. J. Med, 2021, 384, 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]