Abstract
Background
COVID-19 pandemic resulted in about 165 million infections and 3.4 million deaths all over the world across 15 months. The most severe clinical presentation of COVID-19 diseases is interstitial pneumonia.
Methods
In this paper we describe clinical outcomes based on radiological features as well as the pattern of haematochemical parameters and IgG/IgM antibodies in 75 patients hospitalized due to COVID-related interstitial pneumonia not requiring intensive care assistance. Each patient underwent routine laboratory tests, including inflammatory markers and coagulation profile at baseline. Computed Tomography (CT) was performed at baseline and after 3 months to assess the persistence of radiological sequelae. A Generalized Linear Model (GLM) was used to test for each patient the association between individual haematochemical parameters at the time of hospital admission and the subsequent radiological features after three months. The presence of IgG antibodies was quantitatively determined in 70 patients at the time of hospital admission and after 3 months. A subgroup of 49 and 21 patients underwent additional dosage of IgG after 6 and 12 months, respectively. IgM serological antibodies were available for 17 patients at baseline and 61 at T3, with additional follow-up for 51 and 20 subjects after 6 and 12 months, respectively.
Results
Only 28 out of 75 patients discharged from the hospital were totally healed after 3 months, while 47 patients (62.7%) still presented radiological sequelae. According to the GLM model, specific haematochemical baseline parameters—such as IL-6, GPT, platelets and eosinophil count—showed a statistically significant association with the presence of radiological sequelae at month 3 highlighting an OR = 0.5, thus meaning that subjects completely healed after 3 months presented half levels of IL-6 at baseline compared to patients with sequelae. In general, IgG serum levels were always higher than IgM at the time of hospitalization (75% at T0; n = 12 out of 16 patients with data available in both visits), after 3 months (72.1%; n = 44 out of 61 pts.), after 6 months (56.8%; 25 out of 44 pts.), and one year after hospitalization (60%; 12 out of 20 pts.). Overall, IgG and IgM serum levels presented a statistically significant decreasing trend from the baseline to month 3, 6 and 12. One patient presented an increase in IgM between baseline and month 3 but negative PCR test for SARS-COV2 on throat swab.
Conclusions
As supported by our findings on 75 patients, COVID-related interstitial pneumonia triggers early IgG levels (higher than IgM) that gradually decrease over 12 months. Mid-term sequelae are still detectable at lung Computed Tomography after 3 months from the hospital admission. Occasionally, it is possible to observe increase of IgM levels in presence of low concentrations of IgG and negative PCR ELISA tests for SARS-COV2 RNA. Baseline levels of IL-6 could be proposed as predictor of radiological mid/long-term sequelae after COVID-related interstitial pneumonia.
Introduction
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is responsible for coronavirus disease-19 (COVID-19), resulted in about 165 million infections and 3.4 million deaths all over the world. Increasing evidence in humans suggest that the virus is responsible for the production of IgA, IgM and IgG neutralizing antibodies against viral nucleocapside (N) and spike (S) proteins after the infection [1]. Interestingly, the vast majority of them are asymptomatic or mild-symptomatic, with no need for medical assistance.
Indeed, the high number of infected individuals suggests that they can contribute to the spread of the novel coronavirus among their communities and should therefore be included in the assessment of infection risk [2]. However, the lifespan of neutralizing antibodies is still matter of debate. Based on literature data, the latter depends on different factors. The length of the infection and severity of the disease are the most relevant from a clinical point of view [3]. Indeed, recent studies [4–6], demonstrated a rapid decline of antibodies post the onset of symptoms (POS), even though some individuals showed high levels of neutralizing antibodies after 60 days POS [7–9]. Moreover, the conversion of Ig still needs to be clarified. Initial studies suggest that the IgG can be produced earlier or simultaneously compared to IgM or IgA [10–12]. However, it has been reported that few days POS some individuals only displayed neutralizing IgM or IgA activity, thus suggesting that the capacity of these latter antibodies could be involved in virus neutralization even in absence of IgG [7, 13]. The production of neutralizing IgA at early stages of the infection could effectively eliminate the virus in the respiratory mucosa, whereas the persistence of both IgA and IgG in the serum over the time can trigger the immune response in mild cases [13].
Notably, the high levels of IgG in severe cases may trigger both the innate and adaptive immunity to efficiently eliminate the novel coronavirus [14, 15]. It has been observed that the kinetics of SARS-CoV-2 IgM antibodies reaches a peak after 14 days POS, while IgG levels persist over time until a rapid decline 5–6 months after the onset of the disease [9, 16, 17]. At the same time, the possibility of asymptomatic re-infection has been reported [18]. There are also increasing evidence of possible predictors of disease severity such as IL-6 [19, 20], which has also been identified as a potential target for pharmaceutical treatments [21–23]. In this paper we describe the kinetics and patterns of IgG/IgM antibodies, clinical outcomes based on radiological features and baseline concentration of specific haematochemical parameters measured in 75 patients hospitalized due to COVID-related interstitial pneumonia (with mild to severe symptoms not requiring Intensive Care Unit assistance) followed for 3, 6 and 12 months.
Methods
We analyzed IgG and IgM neutralizing antibodies in 74 patients with PCR-confirmed positive tests to SARS-COV-2 RNA (Polymerase Chain Reaction performed with device DxC 700 AU Chemistry Analyzer Beckman Coulter, Indianapolis USA) on a total of 75 subjects hospitalized at the Division of Pneumology of San Cesario COVID Hospital (Local Health Authority ASL Lecce) between April and September 2020 with diagnosis of COVID19-related interstitial pneumonia confirmed by computed tomography (CT) presenting mild to severe symptoms. All hospitalized patients were treated according to the currently available international/national guidelines or protocols in the frame of Good Clinical Practice and required oxygen administration (all day long) at the moment of hospital admission, but not Intensive Care Unit assistance.
The presence of IgG anti-nucleocapside (N) antibodies was quantitatively determined (with analyzer DiaSorin Liaison XL, Vercelli, Italy) for 69 patients at the time of hospital admission and in 70 patients after 3 months during the first follow-up visit. A subgroup of 49 patients underwent a second dosage of IgG after six months and 21 of them had a further IgG determination at 12 months. In a subgroup of 17 patients we also determined IgM levels anti-spike protein (S) at T0 (with analyzer Alinity, Abbott Diagnostics, Chicago, USA), but at month 3 (T3) we had IgM data available for 61 patients. IgM levels were also available for 51 and 20 subjects at 6 months (T6) and after one year (T12), respectively. The significance in the changes of IgG and IgM antibodies between the baseline and 3-6-12 months was assessed by repeated measure ANOVA by using F-test to statistically test the equality of means.
Computed Tomography (CT) examination was performed (Brillance TC Philips 16 Slice, model 2011) for all patients along with pneumological visit at baseline and after 3 months while undergoing the first follow up visit in order to assess the persistence of radiological sequelae affecting physiological lung function. Radiological classification was based on the findings officially certified by the radiologists who performed the Computed Tomography. The presence of the following radiological findings at CT examination was indicating mild post-pneumonia features: leucocitaries infiltrates, lymphadenomegaly, lymphadenopathy, generic alterations of lung radiological aspect, pervulosity lymph node, broncovascular texture reinforcement. The following radiological findings at CT examination were classified as indicative of severe post-pneumonia features: infiltrative lesions, areas of parenchymal hyperdensity (framed glass), pleural effusion, pericardial effusion, focal nodular lesions, lesions or thickening and parenchymal consolidation phenomena with an infiltrative / expansive character, micronodular and nodular formations or microformations, pleural thickening or consolidation areas, dense or lamellar peri-lesional striae at the bases, dense or lamellar striae of fibrotic or ectatic type, fibro-sclerotic aspects at the apices, sub-pleural interstitium thickening, pleural thickening, irregularity with frayed appearance, para-fibrotic emphysema, sub-pleural fibrosclerotic aspects, thickening of pericardial sheets, fibro-cicatricial striae, inter or intralobular interstitium thickening, interstitial septa thickening, disventilative diaphragmatic features, intra-scissural effusion, paraseptal and bullous aspects of fibrotic-retracting emphysema, scissural thickening, basal interstitial thickening, peri-broncovascular interstitium thickening, ectasia of bronchial and bronchiolar structures, cicatricial aspects, bullous-cystic formations or microformations.
At the time of hospital admission, each patient underwent routine laboratory tests on blood serum samples to determine the following parameters: RBC (x 10^6/μL), WBC (x 10^3/μL), HGB (g/dL), HCT (%), MCV (fL), MCH (pg), MCHC (g/dL), PLT(x 10^3/μL), RDW-SD (fL), RDW-CV (%), PDW (fL), MPV (fL), P-LCR (%), NEUT%, LINF%, BAS%, EOS%, MONOC%, VES (mm/h), PT (%), INR, PTT (s), FIBRINOGEN (mg/dL), D-DIMER (ng/mL), AZOTEMIA (mg/dL),CREATININ (mg/dL), Egfr, GOT (U/L), GPT (U/L),GGT (U/L),LDH (U/L), CPK (U/L), Bilirubin total/direct/indirect (mg/dl), PCR (mg/dl), VES (mg/dl), IL-6 (pg/mL), FERRITIN (ng/mL), PROCALCITONIN (ng/mL), NT-proBNP (pcg/mL), TROPONIN HS (ng/L), OMOCISTEIN (mcmol/L). We used a Generalized Linear Model (GLM) considering the baseline haematochemical exams as dependent variables and the 3-month clinical outcome (healing or long-term sequelae based on radiological evidence of healing) as independent variable. Only the dependent variables which resulted significantly associated with the independent ones (clinical outcome) were kept and run in the model. The model was implemented with a method of stepwise selection on dependent variables (full model). The selections were analyzed considering the test AIC for each step. Both the correlation coefficient and the VIF (Variation Inflation Factor) were used to verify multicollinearity: the ratio between VIF and R2 is inversely proportional and is equal to 1/1-R2, while multicollinearity is certain at a level of 0.9 of correlation coefficient (or higher), and the VIF values for the included variables should be less than 10. The proposed model is the best performing in terms of AIC (Akaike Information Criterion) as it also guarantees the lowest level of collinearity among the variables. The calculation of VIF (by a value of R2 = 0.5) in our model presented a value of 2.0, far away from the critical VIF value set at 10.0 for showing multicollinearity.
Results
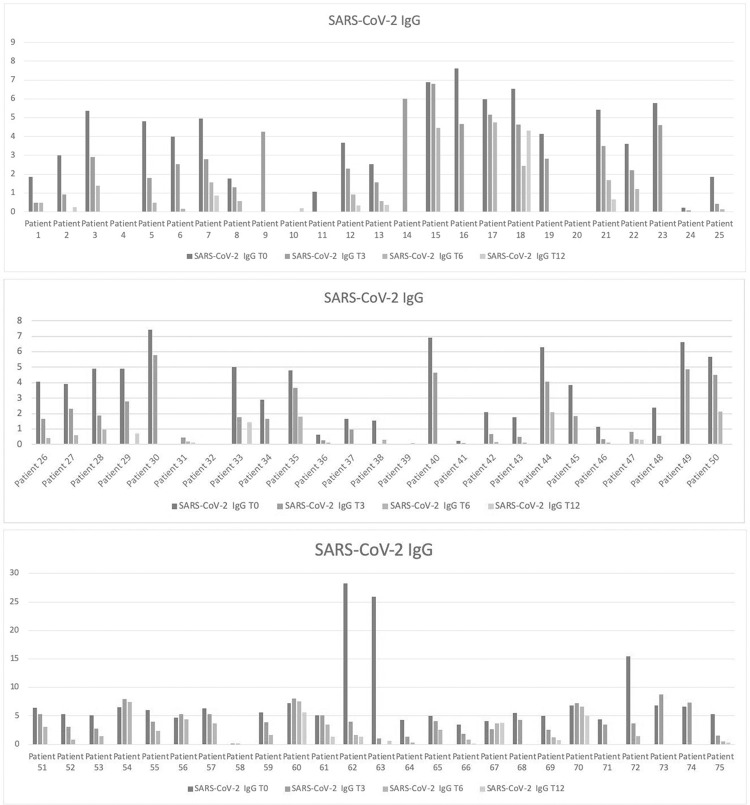
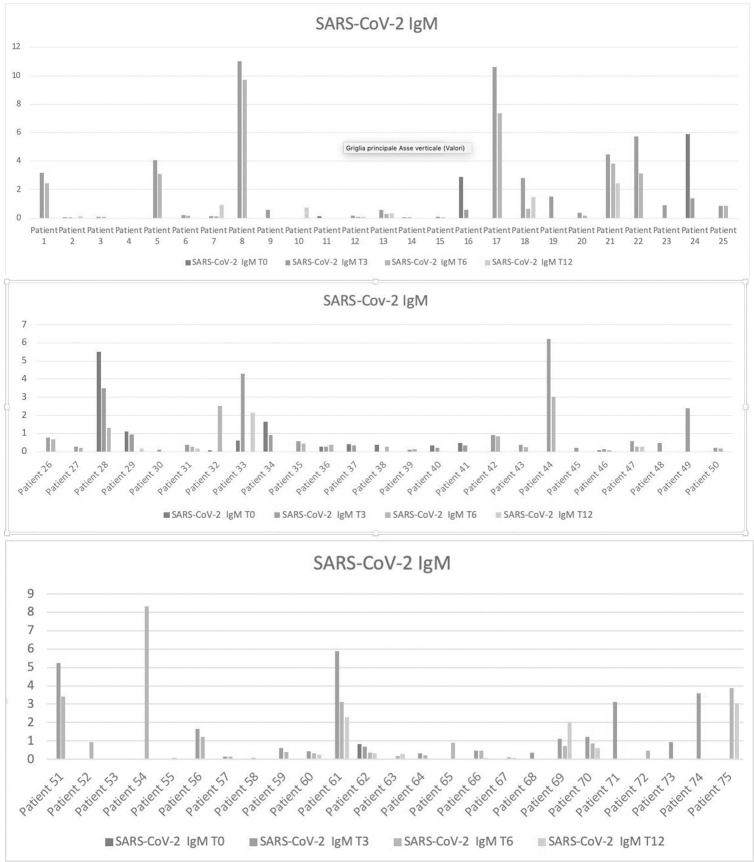
As showed in Table 1 and resumed in Fig 1A–1C, data concerning IgG were available for a total of 69 patients at the baseline (T0), 70 patients after three months (T3), 49 at month 6 (T6) and 21 after twelve months (T12). IgM serological antibodies level were available for 17 patients at baseline, 61 at T3, 51 at T6 and 20 subjects at T12 (Fig 2A–2C).
Table 1. Serum levels of IgG and IgM antibodies determined at baseline (T0), month 3 (T3), month 6 (T6) and month 12 (T12).
| SARS-CoV-2 IgG (index value) | SARS-CoV-2 IgM (index value) | |||||||
|---|---|---|---|---|---|---|---|---|
| T0 | T3 | T6 | T12 | T0 | T3 | T6 | T12 | |
| Patient 1 | 1,87 | 0,48 | 0,48 | NA | NA | 3,18 | 2,43 | NA |
| Patient 2 | 2,99 | 0,91 | NA | 0,26 | 0,06 | 0,05 | NA | 0,11 |
| Patient 3 | 5,37 | 2,91 | 1,38 | NA | NA | 0,1 | 0,1 | NA |
| Patient 4 | NA | NA | NA | NA | NA | NA | NA | NA |
| Patient 5 | 4,8 | 1,79 | 0,48 | NA | NA | 4,09 | 3,11 | NA |
| Patient 6 | 4 | 2,53 | 0,16 | NA | NA | 0,21 | 0,16 | NA |
| Patient 7 | 4,95 | 2,79 | 1,58 | 0,85 | NA | 0,11 | 0,12 | 0,93 |
| Patient 8 | 1,77 | 1,29 | 0,58 | NA | NA | 11,02 | 9,73 | NA |
| Patient 9 | NA | 4,24 | NA | NA | NA | 0,59 | NA | NA |
| Patient 10 | NA | NA | NA | 0,2 | NA | NA | NA | 0,72 |
| Patient 11 | 1,07 | NA | NA | NA | 0,11 | NA | NA | NA |
| Patient 12 | 3,66 | 2,29 | 0,93 | 0,34 | NA | 0,18 | 0,1 | 0,09 |
| Patient 13 | 2,53 | 1,58 | 0,58 | 0,38 | NA | 0,56 | 0,29 | 0,32 |
| Patient 14 | NA | 6,01 | NA | NA | 0,04 | 0,04 | NA | NA |
| Patient 15 | 6,88 | 6,79 | 4,46 | NA | NA | 0,07 | 0,06 | NA |
| Patient 16 | 7,62 | 4,66 | NA | NA | 2,89 | 0,59 | NA | NA |
| Patient 17 | 5,98 | 5,15 | 4,75 | NA | NA | 10,59 | 7,36 | NA |
| Patient 18 | 6,53 | 4,64 | 2,43 | 4,31 | NA | 2,82 | 0,65 | 1,46 |
| Patient 19 | 4,14 | 2,82 | NA | NA | NA | 1,51 | NA | NA |
| Patient 20 | 0,03 | 0,01 | 0,01 | NA | NA | 0,37 | 0,18 | NA |
| Patient 21 | 5,42 | 3,5 | 1,67 | 0,66 | NA | 4,46 | 3,83 | 2,45 |
| Patient 22 | 3,61 | 2,22 | 1,22 | NA | NA | 5,75 | 3,15 | NA |
| Patient 23 | 5,78 | 4,6 | NA | NA | NA | 0,91 | NA | NA |
| Patient 24 | 0,22 | 0,07 | NA | NA | 5,91 | 1,38 | NA | NA |
| Patient 25 | 1,87 | 0,44 | 0,13 | NA | NA | 0,88 | 0,87 | NA |
| Patient 26 | 4,05 | 1,66 | 0,4 | NA | NA | 0,77 | 0,66 | NA |
| Patient 27 | 3,93 | 2,3 | 0,58 | NA | NA | 0,26 | 0,22 | NA |
| Patient 28 | 4,9 | 1,87 | 0,96 | NA | 5,52 | 3,5 | 1,3 | NA |
| Patient 29 | 4,89 | 2,78 | NA | 0,71 | 1,1 | 0,96 | NA | 0,18 |
| Patient 30 | 7,42 | 5,79 | NA | NA | NA | 0,11 | NA | NA |
| Patient 31 | NA | 0,46 | 0,21 | 0,11 | NA | 0,36 | 0,27 | 0,17 |
| Patient 32 | 0,01 | NA | 0,01 | NA | 0,06 | NA | 2,53 | NA |
| Patient 33 | 5,03 | 1,77 | NA | 1,42 | 0,61 | 4,31 | NA | 2,16 |
| Patient 34 | 2,89 | 1,66 | NA | NA | 1,64 | 0,91 | NA | NA |
| Patient 35 | 4,81 | 3,66 | 1,79 | NA | NA | 0,56 | 0,44 | NA |
| Patient 36 | 0,64 | 0,27 | 0,14 | NA | 0,28 | 0,27 | 0,36 | NA |
| Patient 37 | 1,66 | 0,95 | NA | NA | 0,42 | 0,34 | NA | NA |
| Patient 38 | 1,54 | NA | 0,3 | NA | 0,36 | NA | 0,27 | NA |
| Patient 39 | 0,05 | 0,06 | 0,08 | NA | NA | 0,09 | 0,14 | NA |
| Patient 40 | 6,91 | 4,65 | NA | NA | 0,34 | 0,2 | 0 | NA |
| Patient 41 | 0,23 | 0,09 | NA | NA | 0,48 | 0,33 | NA | NA |
| Patient 42 | 2,08 | 0,66 | 0,17 | NA | NA | 0,9 | 0,85 | NA |
| Patient 43 | 1,76 | 0,48 | 0,13 | NA | NA | 0,36 | 0,25 | NA |
| Patient 44 | 6,3 | 4,07 | 2,08 | NA | NA | 6,22 | 3,03 | NA |
| Patient 45 | 3,84 | 1,85 | NA | NA | NA | 0,22 | NA | NA |
| Patient 46 | 1,14 | 0,34 | 0,11 | 0,05 | 0,07 | 0,15 | 0,07 | NA |
| Patient 47 | NA | 0,81 | 0,36 | 0,29 | NA | 0,58 | 0,26 | 0,26 |
| Patient 48 | 2,37 | 0,55 | NA | NA | NA | 0,48 | NA | NA |
| Patient 49 | 6,62 | 4,86 | NA | NA | NA | 2,4 | NA | NA |
| Patient 50 | 5,68 | 4,49 | 2,14 | NA | NA | 0,2 | 0,17 | NA |
| Patient 51 | 6,4 | 5,29 | 3,03 | NA | NA | 5,22 | 3,42 | NA |
| Patient 52 | 5,25 | 3,03 | 0,8 | NA | NA | NA | 0,93 | NA |
| Patient 53 | 5,06 | 2,75 | 1,42 | NA | NA | NA | 0,05 | NA |
| Patient 54 | 6,47 | 7,9 | 7,44 | NA | NA | NA | 8,33 | NA |
| Patient 55 | 6,05 | 4,03 | 2,32 | NA | NA | NA | 0,07 | NA |
| Patient 56 | 4,72 | 5,35 | 4,39 | NA | NA | 1,67 | 1,22 | NA |
| Patient 57 | 6,31 | 5,31 | 3,71 | NA | NA | 0,16 | 0,15 | NA |
| Patient 58 | 0,13 | 0,1 | NA | NA | NA | 0,09 | NA | NA |
| Patient 59 | 5,61 | 3,92 | 1,65 | NA | NA | 0,62 | 0,4 | NA |
| Patient 60 | 7,2 | 8,09 | 7,58 | 5,62 | NA | 0,43 | 0,32 | 0,25 |
| Patient 61 | 5,12 | 5,05 | 3,46 | 1,31 | NA | 5,89 | 3,12 | 2,29 |
| Patient 62 | 28,25 | 3,96 | 1,63 | 1,37 | 0,83 | 0,69 | 0,37 | 0,31 |
| Patient 63 | 25,96 | 1 | NA | 0,64 | NA | NA | 0,18 | 0,3 |
| Patient 64 | 4,26 | 1,36 | 0,33 | NA | NA | 0,31 | 0,23 | NA |
| Patient 65 | 4,95 | 4,06 | 2,6 | NA | NA | NA | 0,9 | NA |
| Patient 66 | 3,52 | 1,8 | 0,83 | 0,11 | NA | 0,48 | 0,47 | 0,08 |
| Patient 67 | 4,06 | 2,69 | 3,66 | 3,77 | NA | NA | 0,1 | 0,06 |
| Patient 68 | 5,53 | 4,25 | NA | NA | NA | 0,36 | NA | NA |
| Patient 69 | 4,95 | 2,55 | 1,22 | 0,71 | NA | 1,1 | 0,73 | 2 |
| Patient 70 | 6,79 | 7,28 | 6,63 | 4,96 | NA | 1,23 | 0,88 | 0,61 |
| Patient 71 | 4,42 | 3,48 | NA | NA | NA | 3,12 | NA | NA |
| Patient 72 | 15,43 | 3,69 | 1,4 | NA | NA | NA | 0,48 | NA |
| Patient 73 | 6,83 | 8,79 | NA | NA | NA | 0,94 | NA | NA |
| Patient 74 | 6,67 | 7,33 | NA | NA | NA | 3,6 | NA | NA |
| Patient 75 | 5,3 | 1,5 | 0,5 | 0,33 | NA | NA | 3,89 | 3,06 |
*NA: Not Available
Fig 1.
a. Serum IgG antibodies levels in a subgroup of patients from 1 to 25. Serum IgG antibodies levels in a subgroup of patients from 1 to 25 at baseline (T0; blue line), month 3 (T3; orange line), month 6 (T6; gray line and month 12 (T12 Yellow line data available on a smaller subgroup of 7 patients). b. Serum IgG antibodies levels in a subgroup of patients from 26 to 50. Serum IgG antibodies levels in a subgroup of patients from 26 to 50 at baseline (T0; blue line), month 3 (T3; orange line), month 6 (T6; gray line and month 12 (T12 Yellow line data available on a smaller subgroup of 5 patients). c. Serum IgG antibodies levels in a subgroup of patients from 51 to 75. Serum IgG antibodies levels in a subgroup of patients from 51 to 75 at baseline (T0; blue line), month 3 (T3; orange line), month 6 (T6; gray line and month 12 (T12 Yellow line data available on a smaller subgroup of 9 patients).
Fig 2.
a. Serum IgM Antibodies levels in a subgroup of patients from 1 to 25. Serum IgM Antibodies levels in a subgroup of patients from 1 to 25 at baseline (T0; blue line), month 3 (T3; orange line), month 6 (T6; grey line), month 12 (T12; yellow line). b. Serum IgM Antibodies levels in a subgroup of patients from 26 to 50. Serum IgM Antibodies levels in a subgroup of patients from 26 to 50 at baseline (T0; blue line), month 3 (T3; orange line), month 6 (T6; grey line), month 12 (T12; yellow line). c. Serum IgM Antibodies levels in a subgroup of patients from 51 to 75. Serum IgM Antibodies levels in a subgroup of patients from 51 to 75 at baseline (T0; blue line), month 3 (T3; orange line), month 6 (T6; grey line), month 12 (T12; yellow line).
Comparison of IgG and IgM serum levels from 0 to 12 months
We had available both IgG and IgM values at the baseline (T0) only for 16 patients with 12 of them (75%) presenting IgG values higher than IgM. After three months (T3), information about both IgG and IgM levels were available for a total of 61 patients; among them, 44 subjects (72%) had higher IgG values than IgM, 1 person had equal values and the remaining 16 (26%) presented lower values of IgG compared to IgM. At month six (T6), IgG and IgM values were simultaneously available for 44 patients, with 25 of them (56.8%) presenting IgG values higher than IgM, 1 had equal serum levels of both kind of antibodies while the remaining 18 (40.9%) presented lower values of IgG compared to those of IgM. After 12 months (T12), we had available both IgG and IgM serum levels only for 20 patients, with 12 of them (60%) presenting higher IgG values than IgM.
IgG serum levels from 0 to 12 months
IgG values at the baseline (T0) and after three months (T3) were simultaneously available for 66 patients (with 58 of them, namely 87.8%, presenting higher values of IgG at the baseline compared to T3), but for 44 of these subjects we had also available IgG concentrations at T6, that showed a constant decrease in 41 subjects compared to T0. Only for 14 patients, serum levels of IgG were available up to T12 showing always lower values after one year compared to the baseline, although some fluctuations between month 3 and 6 were detectable in 4 patients. Overall, IgG serum levels showed a statistically significant reduction from T0 to T12 (p<0,001), with a lower effect detectable at one year compared to intermediate follow-up visits (T3 and T6) due to a small number of patients observed at T12.
IgM serum levels from 0 to 12 months
IgM values at the baseline (T0) and after three months (T3) were available for 14 patients: 11 of them (78.5%) had higher IgM values at the baseline than at T3 and 1 had equal values. A female patient showed an increase of IgM between baseline and month 3 (higher than IgG levels), despite a double negative result for SARS-COV2 RNA (ELISA test on two consecutive throat swabs). Extended data about serum levels of IgM from T0 up to T3 and T6 were available for 4 patients (showing always a decrease from T0 to T6), while only one of these subjects underwent additional serum determination of IgM also after 12 months (showing a stable decrease across one year). However, a larger number (n = 38) of serum IgM levels were available between T3 and T6 (lacking of T0 determinations), showing a general decreasing trend in 34 patients (89,4%), stable values in 1 case, and an increase in 3 subjects. Twelve of these 38 subjects were followed up for IgM concentrations from T3 up to one year, presenting always constant reductions between month 3 and 12 in all the patient except in two (with some fluctuations between T3 and T6 in four patients). Overall, IgM serum levels showed a statistically significant reduction from T0 to T12 (p<0,001), with a lower effect detectable at one year compared to intermediate follow-up visits (T3 and T6) due to a small number of patients observed at T12.
Radiological features and haematochemical parameters
Table 2 describes the specific findings observed at Computed Tomography at baseline and after 3 months per each of the 75 hospitalized patients.
Table 2. Specific findings observed at Computed Tomography at baseline (T0) and after 3 months (T3) per each of the 75 hospitalized patients.
| Infiltrative lesions or parenchymal hyperdensity with framed glass aspect | Lymphadenomegalies, Lymphadenopathies or nodularities | Pleural or pericardial thickness/effusion | Lung plot thickness/ectasia or fibrotic aspects | Alterations, lesions or thickening of parenchimal or pleural base | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T3 | T0 | T3 | T0 | T3 | T0 | T3 | T0 | T3 | |
| PATIENT 1 | 0 | NA | 0 | 1 | 0 | 0 | 0 | |||
| PATIENT 2 | 1 | NA | 0 | NA | 0 | NA | NA | NA | 1 | NA |
| PATIENT 3 | 2 | 0 | NA | 0 | NA | 0 | 2 | 2 | 2 | 2 |
| PATIENT 4 | 1 | 2 | 1 | 1 | 0 | 1 | 2 | NA | 1 | 1 |
| PATIENT 5 | NA | NA | 1 | NA | 0 | NA | 0 | NA | NA | NA |
| PATIENT 6 | 1 | 2 | 1 | 1 | 2 | 1 | 2 | 2 | 1 | 1 |
| PATIENT 7 | NA | 0 | NA | 0 | 0 | 1 | 1 | NA | 1 | 1 |
| PATIENT 8 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 |
| PATIENT 9 | 1 | NA | 1 | 0 | 0 | 0 | NA | NA | NA | NA |
| PATIENT 10 | 0 | NA | 1 | NA | 0 | NA | NA | NA | 1 | NA |
| PATIENT 11 | 1 | NA | 1 | NA | 1 | NA | NA | NA | 1 | NA |
| PATIENT 12 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | NA | 2 |
| PATIENT 13 | 2 | 0 | 1 | 1 | 0 | 0 | 2 | NA | NA | NA |
| PATIENT 14 | 2 | 2 | 1 | 0 | NA | 0 | NA | NA | 1 | 1 |
| PATIENT 15 | 2 | NA | 1 | 1 | 0 | 0 | 2 | 2 | 1 | 2 |
| PATIENT 16 | 1 | NA | 1 | NA | 0 | NA | 2 | NA | NA | NA |
| PATIENT 17 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | NA | 1 |
| PATIENT 18 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | NA |
| PATIENT 19 | 1 | 2 | 1 | 1 | 0 | 1 | 1 | NA | 1 | 1 |
| PATIENT 20 | 1 | 2 | 1 | 1 | 0 | 0 | 1 | 2 | 1 | 2 |
| PATIENT 21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NA | |
| PATIENT 22 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | NA | 1 |
| PATIENT 23 | NA | NA | 1 | 1 | 0 | 0 | 1 | 1 | NA | NA |
| PATIENT 24 | 0 | NA | 0 | NA | 0 | NA | 0 | NA | 0 | NA |
| PATIENT 25 | 0 | NA | 1 | NA | 0 | NA | 0 | NA | NA | NA |
| PATIENT 26 | 0 | 0 | 1 | 1 | NA | 0 | 0 | 0 | NA | NA |
| PATIENT 27 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NA | NA |
| PATIENT 28 | 0 | NA | 0 | NA | 0 | NA | 1 | NA | NA | NA |
| PATIENT 29 | 0 | NA | 1 | NA | 0 | NA | NA | NA | 1 | NA |
| PATIENT 30 | 1 | 1 | 1 | 1 | 1 | 0 | NA | 1 | 1 | 1 |
| PATIENT 31 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| PATIENT 32 | 0 | NA | 0 | NA | 0 | NA | 0 | NA | 1 | NA |
| PATIENT 33 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| PATIENT 34 | 1 | NA | 0 | NA | 0 | NA | 0 | NA | NA | NA |
| PATIENT 35 | 1 | 2 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 |
| PATIENT 36 | 1 | NA | 1 | NA | 0 | NA | NA | NA | 1 | NA |
| PATIENT 37 | 0 | NA | 1 | 1 | 0 | NA | 0 | NA | NA | NA |
| PATIENT 38 | 0 | NA | 0 | NA | 0 | NA | 0 | NA | 1 | NA |
| PATIENT 39 | 1 | 1 | 1 | 1 | 0 | 0 | NA | NA | 1 | NA |
| PATIENT 40 | 0 | NA | 1 | NA | 0 | NA | NA | NA | 1 | NA |
| PATIENT 41 | 0 | NA | 1 | NA | 1 | NA | 1 | NA | 1 | NA |
| PATIENT 42 | 0 | NA | 0 | NA | 0 | NA | 0 | NA | NA | NA |
| PATIENT 43 | 1 | 1 | 0 | 1 | 0 | 0 | NA | NA | 1 | 1 |
| PATIENT 44 | 1 | NA | 1 | NA | 0 | NA | NA | NA | NA | NA |
| PATIENT 45 | NA | NA | 1 | NA | 1 | NA | 1 | NA | 1 | NA |
| PATIENT 46 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 |
| PATIENT 47 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | NA | 0 | 1 |
| PATIENT 48 | 0 | NA | 0 | NA | 0 | NA | 0 | NA | 1 | NA |
| PATIENT 49 | NA | NA | 1 | 1 | 0 | 0 | 1 | 2 | 1 | 1 |
| PATIENT 50 | 2 | 2 | 0 | 0 | 0 | 0 | 2 | 2 | NA | NA |
| PATIENT 51 | 1 | 1 | NA | 0 | NA | 0 | 0 | 0 | NA | NA |
| PATIENT 52 | 1 | 1 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 1 |
| PATIENT 53 | 2 | 2 | 1 | 1 | 0 | 0 | NA | NA | NA | NA |
| PATIENT 54 | 2 | 1 | 1 | 1 | 0 | 0 | 1 | 2 | 1 | 1 |
| PATIENT 55 | 2 | 2 | 1 | 1 | 0 | 0 | NA | 1 | 1 | 1 |
| PATIENT 56 | 2 | 0 | 1 | 1 | 1 | 1 | NA | NA | NA | NA |
| PATIENT 57 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| PATIENT 58 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NA | NA |
| PATIENT 59 | 2 | 2 | 0 | 0 | 0 | 0 | NA | NA | NA | NA |
| PATIENT 60 | 1 | 2 | 1 | 1 | 0 | 0 | 1 | 2 | NA | 1 |
| PATIENT 61 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 1 |
| PATIENT 62 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | NA | NA |
| PATIENT 63 | 2 | 2 | NA | NA | 0 | 0 | 2 | 1 | 1 | 1 |
| PATIENT 64 | 1 | 0 | 1 | 1 | 0 | 0 | NA | NA | NA | NA |
| PATIENT 65 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NA | NA |
| PATIENT 66 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NA | NA |
| PATIENT 67 | 1 | 0 | NA | 0 | NA | 0 | NA | 0 | NA | NA |
| PATIENT 68 | 2 | 2 | 1 | 1 | NA | 0 | NA | 2 | 2 | 2 |
| PATIENT 69 | 2 | 2 | 1 | 1 | 0 | 0 | 2 | 2 | NA | NA |
| PATIENT 70 | 2 | 0 | 0 | NA | NA | NA | NA | NA | ||
| PATIENT 71 | 2 | 1 | 1 | 1 | 0 | 0 | 2 | NA | NA | NA |
| PATIENT 72 | 1 | NA | 1 | NA | 0 | NA | NA | NA | NA | |
| PATIENT 73 | 2 | 0 | 1 | 0 | NA | NA | NA | 2 | NA | |
| PATIENT 74 | 2 | 2 | 1 | 1 | 1 | 1 | 2 | NA | 2 | 1 |
| PATIENT 75 | 1 | 0 | 0 | 0 | 2 | 2 | 2 | 0 | 1 | 1 |
*NA: Not Available; 0: No radiological findings (healing); 1: Mild radiological findings; 2: Severe radiological findings.
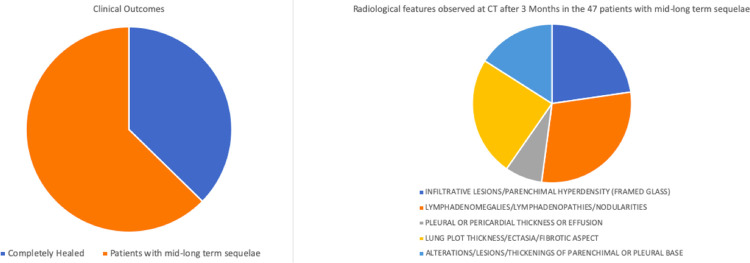
As showed in Fig 3, only 28 of the 75 hospitalized patients (37,3% of the total) were completely healed at month 3 as demonstrated by radiological investigations. Radiological features detected by Computed Tomography after 3 months in the remaining 47 patients (62.7%) are showed in Fig 3 and included the following (each patient could have presented more than one feature): infiltrative lesions or parenchimal hyperdensity with framed glass aspect (detectable in 27 patients; 36% of CT exams), lymphadenomegalies/lymphadenopathies or nodularities (35 patients; 46.6% of CT exams), pleural or pericardial thickness or effusion (9 patients; 12% of CT exams), lung plot thickness/ectasia or fibrotic aspects (29 patients; 38,6% of CT exams), alterations/lesions or thickening of parenchimal or pleural base (observed in 19 patients; 25.3% of CT exams).
Fig 3. Clinical outcomes.
Clinical outcomes (28 patients completely healed + 47 patients showing persistence of radiological sequelae) after 3 months from the hospital admission due to interstitial pneumonia (mild to moderate symptoms) and classification of the radiological sequelae observed in case of disease persistence.
Table 3 shows the average values, standard deviation and maximum/minimum values of all the haematochemical parameters dosed in the 75 hospitalized patients. Explorative descriptive analyses have been performed to test the consistency of the baseline characteristics of our hospitalized patients with the results of the evidence published by Kabak et al. [24] confirming that the number of neutrophils and leucocytes were below the normal minimum average count, and also the neutrophil to lymphocyte ratio was suboptimal.
Table 3. Average values (with min/max and std deviation) of haematochemical parameters dosed at the time of hospital admission in the 75 hospitalized patients.
| Parameter | Average | Standard Deviation | Mínimum Value | Máximum Value |
|---|---|---|---|---|
| WBC (x 10^3/L) | 6,314932 | 2,196404 | 3,47 | 19,08 |
| RBC (x 10^6/L) | 4,777808 | 0,478314 | 3,36 | 5,97 |
| HGB (g/dL) | 13,40274 | 1,485576 | 9,1 | 16,1 |
| HCT (%) | 41,89726 | 3,91081 | 30,4 | 48,5 |
| MCV (fL) | 88,08904 | 7,458693 | 65 | 99,6 |
| MCH (pg) | 28,1726 | 2,795048 | 19,5 | 32,8 |
| MCHC (g/dL) | 31,94795 | 1,147474 | 29,8 | 34,6 |
| PLT(x10^3/æL) | 240,4384 | 64,56607 | 88 | 434 |
| RDW-SD (fL) | 45,47083 | 4,963002 | 34,6 | 58 |
| RDW-CV (%) | 14,2875 | 1,599466 | 11,6 | 20 |
| PDW (fL) | 13,64 | 2,643088 | 9,2 | 24,7 |
| MPV (fL) | 11,00571 | 1,071622 | 8,9 | 15 |
| P-LCR (%) | 33,54638 | 8,43673 | 16,5 | 62 |
| NEUT% | 55,56575 | 7,821697 | 36,6 | 71,2 |
| LINF% | 33,28356 | 7,029798 | 18,7 | 51,1 |
| MONO% | 7,908219 | 1,758926 | 4,4 | 13,9 |
| EOSI% | 2,561644 | 1,365714 | 0,5 | 7,3 |
| BASO% | 0,653425 | 0,285327 | 0,2 | 1,4 |
| NEUTROF (x 10^3/æL) | 3,561096 | 1,620583 | 1,33 | 13,53 |
| LINFOC (x 10^3/æL) | 2,064658 | 0,664099 | 0,96 | 4,14 |
| MONOC(x 10^3/L) | 0,485694 | 0,150762 | 0,21 | 1,13 |
| EOSIN (x 10^3/L) | 0,156027 | 0,087793 | 0,03 | 0,58 |
| BASOF(x 10^3/L) | 0,041389 | 0,022661 | 0,01 | 0,12 |
| VES (mm/h) | 12,81159 | 13,34748 | 2 | 88 |
| PT (%) | 97,33472 | 9,403343 | 73,6 | 123 |
| INR | 1,021111 | 0,059138 | 0,88 | 1,15 |
| PTT (s) | 32,16364 | 3,287257 | 22 | 41 |
| FIBRINOGEN (mg/dL) | 255,9559 | 58,15585 | 129 | 451 |
| D-DIMER (ng/mL) | 673,4264 | 1052,08 | 91 | 8188 |
| AZOTEMIA (mg/dL) | 37,34 | 10,23432 | 20 | 64 |
| CREATININ (mg/dL) | 0,831857 | 0,158711 | 0,59 | 1,25 |
| GOT (U/L) | 24,79452 | 22,7852 | 12 | 207 |
| GPT (U/L) | 25,39706 | 17,26885 | 9 | 103 |
| GGT (U/L) | 30,23611 | 40,59643 | 7 | 337 |
| LDH (U/L) | 209,3065 | 73,01978 | 146 | 706 |
| CPK (U/L) | 145,3944 | 453,4975 | 15 | 3890 |
| BILIRUBIN TOT (mg/dL) | 0,65125 | 0,309566 | 0,32 | 1,95 |
| BILIRUBIN DIRECT (mg/dL) | 0,122639 | 0,059623 | 0,04 | 0,33 |
| BILIRUBINA INDIRECT (mg/dL) | 0,528889 | 0,267274 | 0,27 | 1,72 |
| PCR (mg/dL) | 0,253014 | 0,321683 | 0,01 | 1,63 |
| IL-6 (pg/mL) | 42,34648 | 69,52357 | 1,5 | 408 |
According to the model, despite the small effect depicted, for the parameters showing a statistically significant association with the presence of radiological sequelae at month 3 –namely IL-6 or GPT, platelets and eosinophil count–it was possible to highlight an OR = 0.5 (Table 4), thus meaning that subjects completely healed after 3 months presented half levels of IL-6 at the time of hospitalization (as well as GPT, platelets and eosinophils) compared to patients with mid/long term sequelae.
Table 4. Results of the General Linear Model (stepwise full model) used to test for each patient the association between haematochemical examinations performed at the baseline (dependent variables) and the subsequent radiological features at month 3 (independent variable).
| Parameter | DF | Estimate | Standard | T vale | Pr(T) | OR |
|---|---|---|---|---|---|---|
| Error | ||||||
| Intercept | 1 | -1,35266 | 0,735325 | -1,84 | 0,0761 | |
| PLT(x 10^3/æL) | 1 | 0,002572 | 0,001031 | 2,49 | 0,0186 | 0,500643 |
| RDW-SD (fL) | 1 | 0,031326 | 0,01323 | 2,37 | 0,0248 | 0,507831 |
| EOSI% | 1 | -0,09729 | 0,050906 | -1,91 | 0,0659 | 0,475697 |
| GPT (U/L) | 1 | 0,022311 | 0,005839 | 3,82 | 0,0006 | 0,505578 |
| Direct BILIRUBIN | 1 | -2,28613 | 1,324487 | -1,73 | 0,095 | 0,092278 |
| IL-6 (pg/mL) | 1 | -0,00222 | 0,000832 | -2,67 | 0,0123 | 0,499444 |
| Raíz MSE | 0,39906 | |||||
| DependentMean | 0,58333 | |||||
| R 2 | 0,4722 | |||||
| R-SqAdjust | 0,363 | |||||
| AIC | -21,9257 | |||||
| AICC | -16,5923 | |||||
| BIC | -73,9257 | |||||
| C(p) | . | |||||
| PRESS | 6,75673 | |||||
| SBC | -48,841 | |||||
| ASE | 0,12829 |
*The model kept and run only the parameters showing statistical significance: PLT, RDW-SD, EOSI, GPT, Direct Bilirubin, and IL-6.
Discussion
Our study involved 75 patients hospitalized in Southern Italy due to symptomatic COVID-19 infection during the months of April and May 2021 (with follow up at 3, 6 and 12 months), which required prolonged oxygen therapy (administered 24 hours per day) along with antinflammatory and antiviral treatments available at the time of the study. On a total of 75 hospitalized patients, only 28 were completely healed after 3 months as documented by negative Computed Tomography (first follow up pneumological visit). The remaining 47 patients showed persistence of radiological sequelae at month 3 that were significantly associated with higher levels of IL-6 measured at the time of hospital admissions, namely two-folds compared to individuals who did not present any radiological alteration at month 3.
These findings seem to be consistent with other literature data and suggest that, among the inflammatory markers, IL-6 could be empirically proposed in medical practice as a possible predictor of mid/long-term unfavorable radiological outcomes. This latter data comes from the use of a Generalized Linear Model (GLM) which considered the baseline haematochemical exams as dependent variables and the 3-month clinical outcome (healing or long-term sequelae based on radiological evidence) as independent variable. Our findings are consistent with those of Herold et al, who found that the levels of IL-6 predict respiratory failure in hospitalized symptomatic COVID-19 patients. Moreover, some authors have already proposed anti-IL-6 as target for the treatment of severe COVID-19 patients [21–23]. We have also performed an explorative descriptive analysis on our haematochemical parameters at the time of hospital admission and we found that the findings of another study carried out by Kabak et al. [24] were confirmed for all the parameters (neutrophil and leucocytes were below the normal minimum average count, and also the neutrophil to lymphocyte ratio was suboptimal) except for lymphocyte and platelet; however it was not possible for us to repeat the comparison with a control group as performed in the study of Kabak et al. [24].
Our data show that COVID-19 patients with moderate to severe symptoms and radiologically confirmed diagnosis of interstitial pneumonia present high levels of IgG, which decline in the subsequent 3–12 months. However, it is known that in addition to specific antibodies response, SARS-COV2 infection is able to trigger additional immunologic response through the activation of T cells (CD4+ T helper lymphocyte memory) potentially conferring to infected subjects a longer protection against the coronavirus that goes beyond the detectable levels of antibodies [25].
Generally speaking, IgG serum levels were always higher than IgM at the moment of hospitalization (75% at T0; n = 12 out of 16 subjects with both data available), after 3 months (72.1%; n = 44 out of 61), after 6 months (56.8%; 25 out of 44), and at one year after hospitalization (60%; 12 out of 20). Interestingly, 18 patients (40.9%) showed IgM levels higher than IgG at month six (T6), and 8 patients (40%) presented the same pattern of IgM being higher than IgG at one year from hospital admission. Overall, IgG serum levels presented a statistically significant decreasing trend from the baseline to month 3 (T3; data available for 66 patients), month 6 (T6; 44 patients with data available for both visits) and month 12 (T12; 14 patients followed up at one year), although the concentration of this kind of antibodies was likely to present variations with small increase between T0 and T3 or T6 in a limited number of patients (n = 6 patients). In all the 14 patients with IgG complete serological data available from T0 to T12 we observed a reduction of IgG after twelve months compared to the baseline. The persistence of specific antibodies IgG after 3 months that was confirmed by our findings is consistent with results of Wajnberg et al. who indicated that this kind of immune response can persist at least for 5 months [8].
IgM serum concentrations showed a statistically significant decreasing trend over the time: IgM were higher at T0 in 78.5% vs. T3 of the 14 patients with both available data; in 89.4% of the 38 subjects tested from T3 to T6; in 83.3% of the 12 serum tests performed from T3 up to T6 and T12. Two patients showed higher levels of IgM at T3 compared to T0, while additional three and two patients increased their IgM from T3 to T6 and from T3 up to T12, respectively. Finally, our findings show the possibility of sporadic increase of IgM after 3 months from the hospitalization, in the frame of negative SARS-COV2 diagnostic tests (PCR ELISA tests performed on throat swabs), as observed in one female patient.
Conclusions
Based on our findings on 75 patients, COVID-related interstitial pneumonia presenting mild or moderate clinical features (not requiring ICU but only ordinary hospitalization with administration of oxygen as well as pharmacological treatments) might result in mid-term sequelae still detectable at lung Computed Tomography after 3 months from the initial hospital admission. Baseline levels of IL-6 could be proposed as predictor of mid/long term sequelae detectable at imaging at least after 3 months. Individuals infected by SARS-COV2 who develop interstitial pneumonia show early levels of IgG–that are usually higher than IgM–significantly decreasing but still present after 3 and 6 months. Occasionally, it is possible to detect again increases in IgM levels in presence of low levels of IgG and negative PCR ELISA tests for SARS-COV2 RNA.
Supporting information
(XLSX)
Acknowledgments
The authors are grateful to the nurses Daniela Fiordiso and Rosaria Tommasi (San Cesario Hospital, Local Health Authority, ASL Lecce) who collaborated to data collection.
Data Availability
DOI to access the dataset from figshare: https://doi.org/10.6084/m9.figshare.17009063.v1 However, data are also available upon request to the corresponding author at the following email address: piscitelli@unescochairnapoli.it or to the Local Helath Authority ASL Lecce repol@ausl.le.it
Funding Statement
The authors received no specific funding for this work.
References
- 1.Kowitdamrong E, Puthanakit T, Jantarabenjakul W, Prompetchara E, Suchartlikitwong P, Putcharoen O, et al. Antibody responses to SARS-CoV-2 in patients with differing severities of coronavirus disease 2019. PLoS One. 2020. Oct 9;15(10):e0240502. doi: 10.1371/journal.pone.0240502 ; PMCID: PMC7546485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stefanelli P, Bella A, Fedele G, Pancheri S, Leone P, Vacca P, et al. Prevalence of SARS-CoV-2 IgG antibodies in an area of northeastern Italy with a high incidence of COVID-19 cases: a population-based study. ClinMicrobiolInfect. 2021. Apr;27(4):633.e1–633.e7. doi: 10.1016/j.cmi.2020.11.013 Epub 2020 Nov 28. ; PMCID: PMC7695553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orner EP, Rodgers MA, Hock K, Tang MS, Taylor R, Gardiner M, et al. Comparison of SARS-CoV-2 IgM and IgG seroconversion profiles among hospitalized patients in two US cities. Diagn Microbiol Infect Dis. 2021. Apr;99(4):115300. doi: 10.1016/j.diagmicrobio.2020.115300 Epub 2020 Dec 24. ; PMCID: PMC7759125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ibarrondo FJ, Fulcher JA, Goodman-Meza D, Elliott J, Hofmann C, Hausner MA, et al. Rapid Decay of Anti-SARS-CoV-2 Antibodies in Persons with Mild Covid-19. N Engl J Med. 2020. Sep 10;383(11):1085–1087. doi: 10.1056/NEJMc2025179 Epub 2020 Jul 21. Erratum in: N Engl J Med. 2020 Jul 23;: ; PMCID: PMC7397184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward H. et al. Declining prevalence of antibody positivity to SARS-CoV-2: a community study of 365,000 adults.http://medrxiv.org/lookup/doi/10.1101/2020.10.26.20219725. 2020. [Google Scholar]
- 6.Edridge AWD, Kaczorowska J, Hoste ACR, Bakker M, Klein M, Loens K, et al. Seasonal coronavirus protective immunity is short-lasting. Nat Med. 2020. Nov;26(11):1691–1693. doi: 10.1038/s41591-020-1083-1 Epub 2020 Sep 14. . [DOI] [PubMed] [Google Scholar]
- 7.Seow J, Graham C, Merrick B, Acors S, Pickering S, Steel KJA, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol. 2020. Dec;5(12):1598–1607. doi: 10.1038/s41564-020-00813-8 Epub 2020 Oct 26. ; PMCID: PMC7610833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wajnberg A, Amanat F, Firpo A, Altman DR, Bailey MJ, Mansour M, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020. Dec 4;370(6521):1227–1230. doi: 10.1126/science.abd7728 Epub 2020 Oct 28. ; PMCID: PMC7810037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maine GN, Lao KM, Krishnan SM, Afolayan-Oloye O, Fatemi S, Kumar S, et al. Longitudinal characterization of the IgM and IgG humoral response in symptomatic COVID-19 patients using the Abbott Architect. J ClinVirol. 2020. Dec;133:104663. doi: 10.1016/j.jcv.2020.104663 Epub 2020 Oct 27. ; PMCID: PMC7590643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long QX, Liu BZ, Deng HJ, Wu GC, Deng K, Chen YK, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020. Jun;26(6):845–848. doi: 10.1038/s41591-020-0897-1 Epub 2020 Apr 29. . [DOI] [PubMed] [Google Scholar]
- 11.Lee YL, Liao CH, Liu PY, Cheng CY, Chung MY, Liu CE, et al. Dynamics of anti-SARS-Cov-2 IgM and IgG antibodies among COVID-19 patients. J Infect. 2020. Aug;81(2):e55–e58. doi: 10.1016/j.jinf.2020.04.019 Epub 2020 Apr 23. ; PMCID: PMC7177139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020. May;20(5):565–574. doi: 10.1016/S1473-3099(20)30196-1 Epub 2020 Mar 23. ; PMCID: PMC7158907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sterlin D, Mathian A, Miyara M, Mohr A, Anna F, Claër L, et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med. 2021. Jan 20;13(577):eabd2223. doi: 10.1126/scitranslmed.abd2223 Epub 2020 Dec 7. ; PMCID: PMC7857408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carsetti R, Zaffina S, Piano Mortari E, Terreri S, Corrente F, Capponi C, et al. Different Innate and Adaptive Immune Responses to SARS-CoV-2 Infection of Asymptomatic, Mild, and Severe Cases. Front Immunol. 2020. Dec 16;11:610300. doi: 10.3389/fimmu.2020.610300 ; PMCID: PMC7772470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakraborty S, Gonzalez J, Edwards K, Mallajosyula V, Buzzanco AS, Sherwood R, et al. Proinflammatory IgG Fc structures in patients with severe COVID-19. Nat Immunol. 2021. Jan;22(1):67–73. doi: 10.1038/s41590-020-00828-7 Epub 2020 Nov 9. ; PMCID: PMC8130642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun B, Feng Y, Mo X, Zheng P, Wang Q, Li P, et al. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg Microbes Infect. 2020. Dec;9(1):940–948. doi: 10.1080/22221751.2020.1762515 ; PMCID: PMC7273175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou W, Xu X, Chang Z, Wang H, Zhong X, Tong X, et al. The dynamic changes of serum IgM and IgG against SARS-CoV-2 in patients with COVID-19. J Med Virol. 2021. Feb;93(2):924–933. doi: 10.1002/jmv.26353 Epub 2020 Sep 28. ; PMCID: PMC7404900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta V, Bhoyar RC, Jain A, Srivastava S, Upadhayay R, Imran M, et al. Asymptomatic reinfection in two healthcare workers from India with genetically distinct SARS-CoV-2. Clin Infect Dis. 2020. Sep 23:ciaa1451. doi: 10.1093/cid/ciaa1451 Epub ahead of print. ; PMCID: PMC7543380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han H, Ma Q, Li C, Liu R, Zhao L, Wang W, et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020. Dec;9(1):1123–1130. doi: 10.1080/22221751.2020.1770129 ; PMCID: PMC7473317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herold T, Jurinovic V, Arnreich C, Hellmuth JC, von Bergwelt-Baildon M, Klein M, et al. Level of IL-6 predicts respiratory failure in hospitalized symptomatic COVID-19 patients. MedRxiv. 2020. Jan 1. [Google Scholar]
- 21.Zhao M. Cytokine storm and immunomodulatory therapy in COVID-19: Role of chloroquine and anti-IL-6 monoclonal antibodies. Int J Antimicrob Agents. 2020. Jun;55(6):105982. doi: 10.1016/j.ijantimicag.2020.105982 Epub 2020 Apr 16. ; PMCID: PMC7161506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atal S, Fatima Z. IL-6 Inhibitors in the Treatment of Serious COVID-19: A Promising Therapy? Pharmaceut Med. 2020. Aug;34(4):223–231. doi: 10.1007/s40290-020-00342-z ; PMCID: PMC7292936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crisafulli S, Isgrò V, La Corte L, Atzeni F, Trifirò G. Potential Role of Anti-interleukin (IL)-6 Drugs in the Treatment of COVID-19: Rationale, Clinical Evidence and Risks. BioDrugs. 2020. Aug;34(4):415–422. doi: 10.1007/s40259-020-00430-1 ; PMCID: PMC7299248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kabak M, Çil B, Hocanlı I. Relationship between leukocyte, neutrophil, lymphocyte, plateletcounts, and neutrophil to lymphocyte ratio and polymerase chain reaction positivity. Int Immunopharmacol. 2021. Apr;93:107390. doi: 10.1016/j.intimp.2021.107390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng Y, Mentzer AJ, Liu G, Yao X, Yin Z, Dong D, et al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol. 2020. Nov;21(11):1336–1345. doi: 10.1038/s41590-020-0782-6 Epub 2020 Sep 4. ; PMCID: PMC7611020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
DOI to access the dataset from figshare: https://doi.org/10.6084/m9.figshare.17009063.v1 However, data are also available upon request to the corresponding author at the following email address: piscitelli@unescochairnapoli.it or to the Local Helath Authority ASL Lecce repol@ausl.le.it