Abstract
The maximum curvature of a steerable needle in soft tissue is highly sensitive to needle shaft stiffness, which has motivated use of small diameter needles in the past. However, desired needle payloads constrain minimum shaft diameters, and shearing along the needle shaft can occur at small diameters and high curvatures. We provide a new way to adjust needle shaft stiffness (thereby enhancing maximum curvature, i.e. “steerability”) at diameters selected based on needle payload requirements. We propose helical dovetail laser patterning to increase needle steerability without reducing shaft diameter. Experiments in phantoms and ex vivo animal muscle, brain, liver, and inflated lung tissues demonstrate high steerability in soft tissues. These experiments use needle diameters suitable for various clinical scenarios, and which have been previously limited by steering challenges without helical dovetail patterning. We show that steerable needle targeting remains accurate with established controllers and demonstrate interventional payload delivery (brachytherapy seeds and radiofrequency ablation) through the needle. Helical dovetail patterning decouples steerability from diameter in needle design. It enables diameter to be selected based on clinical requirements rather than being carefully tuned to tissue properties. These results pave the way for new sensors and interventional tools to be integrated into high-curvature steerable needles.
Index Terms—: Medical Robotics, Steerable Needles, Surgical Robotics, Medical Devices
I. Introduction
Bevel tip steerable needles can be used to accurately target desired locations in tissue and travel along curved paths that are useful for avoiding obstacles [1]–[3]. Steerable needles have been proposed for interventions in the liver [4], lungs [5], kidneys [4], prostate [6], and brain [7]. In combination with motion planning, robotic needle steering enables the needle to follow curvilinear, collision-free paths to reach hard-to-access target locations [8]–[10].
Since the advent of bevel tip steerable needles, effort has been devoted to increasing needle maximum curvature (i.e. “steerability”) in tissue by changing needle design (see [11] for a review). Initial research studied the effects of varying tip geometric parameters and insertion speed [12]. Design innovations quickly followed, including incorporating larger tips on smaller shafts and adding a pre-bend or “kink” just behind the needle tip to increase steerability in phantoms [13] and cadaver brain [7]. The effects of parameters (including kinked tip length and angle) on needle performance were subsequently characterized in liver tissue [14]. A flexure hinge was incorporated to obtain the beneficial effects of the kink, but with less tissue damage during axial rotation [15]. A variable-length flexure was proposed in [16], which requires a small-diameter stylet shaft. A pull-wire was incorporated to enable direct control over the flexure angle [17], [18], at the cost of increased mechanical complexity.
The above discussion of needle design innovations considers only bevel tip steerable needles, where insertion combined with axial shaft rotation is used to steer the needle. There are a number of other noteworthy steerable needle designs including (but not limited to) use of a curved stylet in conjunction with an outer cannula proposed in [19] and then later adapted with variations in stiffness and insertion approaches by others [20], [21], a programmable bevel which eliminates the need for axial rotation [22], and shape memory alloy actuation [23], among other innovative designs which are reviewed in [11]. Each of these prior steerable needle designs has its own unique strengths and weaknesses. Here, we restrict our attention solely to bevel tip steerable needles that are controlled using insertion and axial rotation. We focus on bevel tip steerable needles because they have been shown to be effective and are mechanically simple, in that they only require two degrees of freedom to actuate and do not have active components in the needle itself.
To achieve high steerability for bevel tip steerable needles, the stiffness of the needle must be tuned relative to the properties of the tissue into which it is inserted. This is typically achieved by reducing shaft diameter until the needle steers well, and hence very small diameter needles have often been used in the past [13], [16], [24]–[26]. However, there are drawbacks to reducing shaft diameter in order to enhance needle steerability. First, as we show in this paper, with too great a reduction in needle diameter (i.e. to within the ranges previously suggested for high curvature needles in biological tissues) it is possible for the needle to shear through tissue along the shaft (see Fig. 4), potentially causing serious damage to the tissue. Second, the needle has to be adequately sized to perform a desired intervention such as diagnostic tissue collection or the delivery of sensors or therapy. While this can be done in a two-step process with an outer sheath [4], [5], it is also sometimes desirable to deliver the therapy directly through the bevel tip steerable needle itself (e.g. to preclude movement of the sheath within tissue while the secondary tool is being inserted). While therapy delivery has been demonstrated in bevel tip steerable needles [14], the radiofrequency ablation wire used is a special case among interventional payloads, since its diameter can be adjusted to suit the needs of the steerable needle (especially if one is not factoring specific ablation zone size objectives into the design process). To enable integration of other interventional tools or therapies (e.g. brachytherapy seeds, other types of ablators with fixed diameters, etc.) it is desirable to have independent control of needle diameter and steerability in the design process.
Fig. 4:
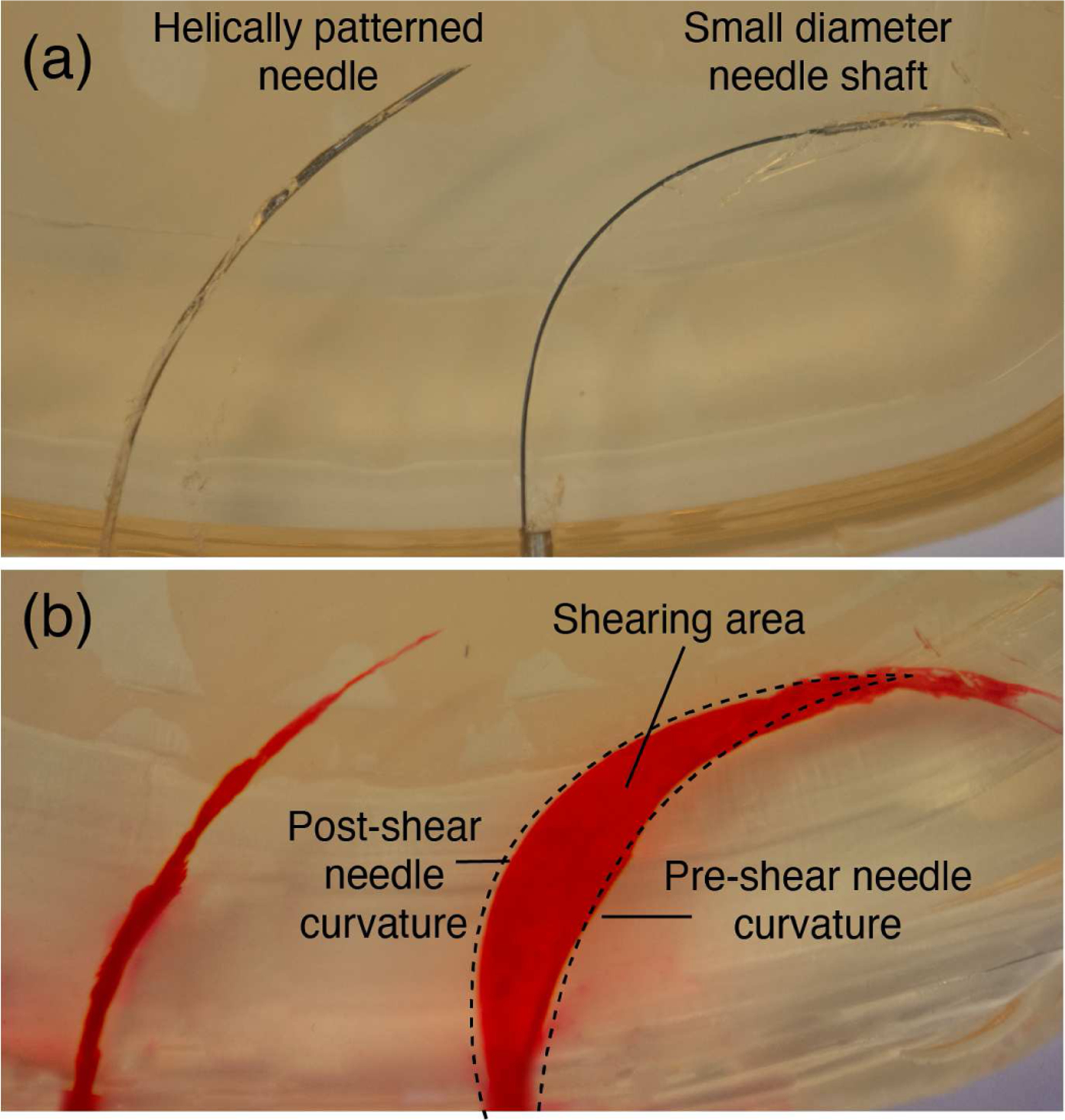
Helically patterned needle inserted in tissue vs. a needle with a small diameter shaft (0.36 mm OD, 0.24 mm ID) with a large bent tip. (a) shows the patterned needle (left) and the small shaft needle (right) inserted in the gelatin. (b) shows the tissue damage caused by each insertion. Red dye was injected into the path created by each needle after the needle was removed from the gelatin.
The purpose of this paper is to propose helical dovetail laser patterning to achieve this by adjusting needle shaft stiffness through an approach other than adjusting needle diameter. The helical dovetail is a pattern, similar to a repeating series of interlocking puzzle pieces, that winds helically around the needle shaft (see Fig. 1). It was previously suggested for use in catheters, with the goal of reducing bending stiffness while maintaining good axial and torsional stiffness [27]. We apply this pattern to a steerable needle for the first time in this paper. We incorporate this helical dovetail just proximal to a flexure hinge of the type proposed in [15], with design enhancements to prevent unintended flexing in the wrong direction and angled surfaces that contact one another at full articulation in the correct direction. To demonstrate that the helical dovetail enhances steerability, we perform insertions in artificial tissue phantoms, as well as ex vivo porcine muscle, lung tissue, bovine liver, and ovine brain. We experimentally show that needles of the same tube dimensions with patterning perform with higher steerability in every attempted tissue type over needles that don’t have patterning. We also show that this needle is capable of delivering a variety of interventional therapies including brachytherapy seeds and thermal ablation probes, which would be too large to deliver through existing small-diameter, high-curvature needles.
Fig. 1:
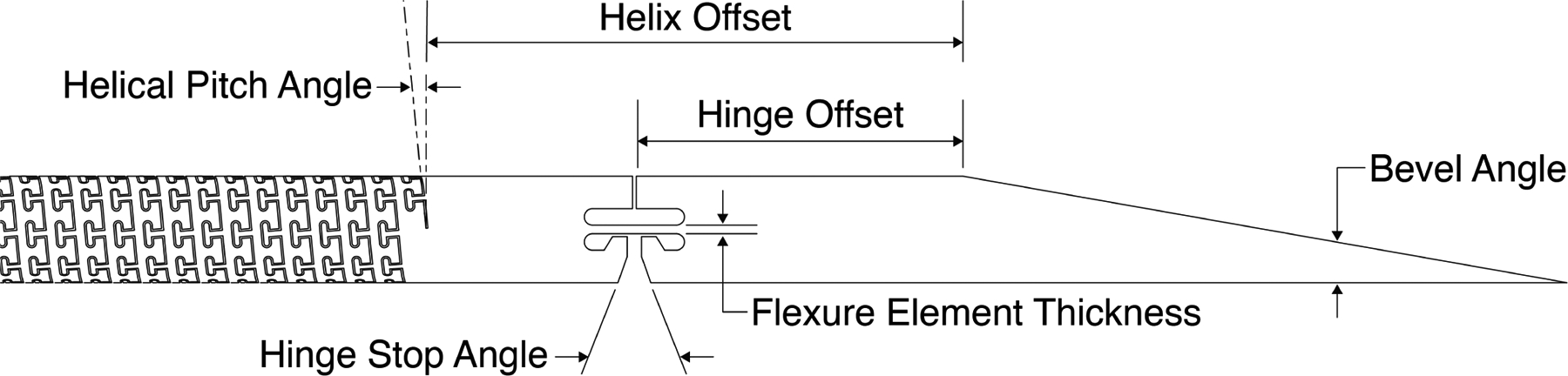
Laser cut needle design with helical dovetail patterning on the shaft. Geometric parameters of the design include the bevel tip angle, the hinge offset, the hinge stop angle, the flexure element thickness, the helix offset, and the helical pitch angle.
II. Needle Design
The helical dovetail needle concept we propose is shown schematically in Fig. 1, and a photograph of a prototype is shown in Fig. 2. The helical dovetail pattern and hinge are laser cut into the shaft of the needle.
Fig. 2:
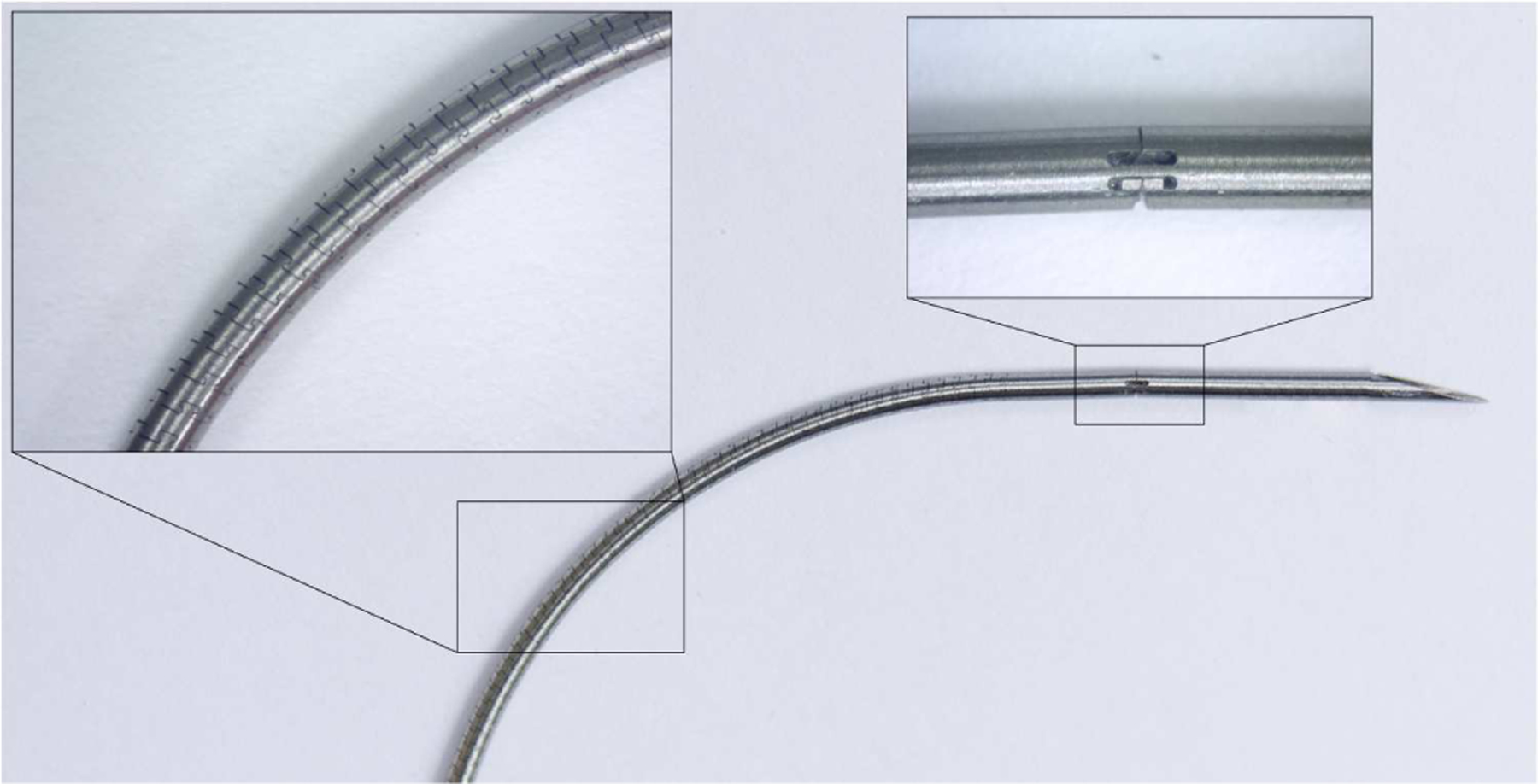
A photograph of a curved, helically patterned needle. The insets show the helical patterning and the hinge design under a microscope.
To visually illustrate the helical dovetail pattern’s ability to modify shaft stiffness, Fig. 3 shows how a helical dovetail patterned needle bends under its own weight, compared to the same needle with no patterning. The needles shown are the same as those used in the experiments in Sections IV, V, and VI, with parameters for the helical patterning and hinge as shown in Table I.
Fig. 3:
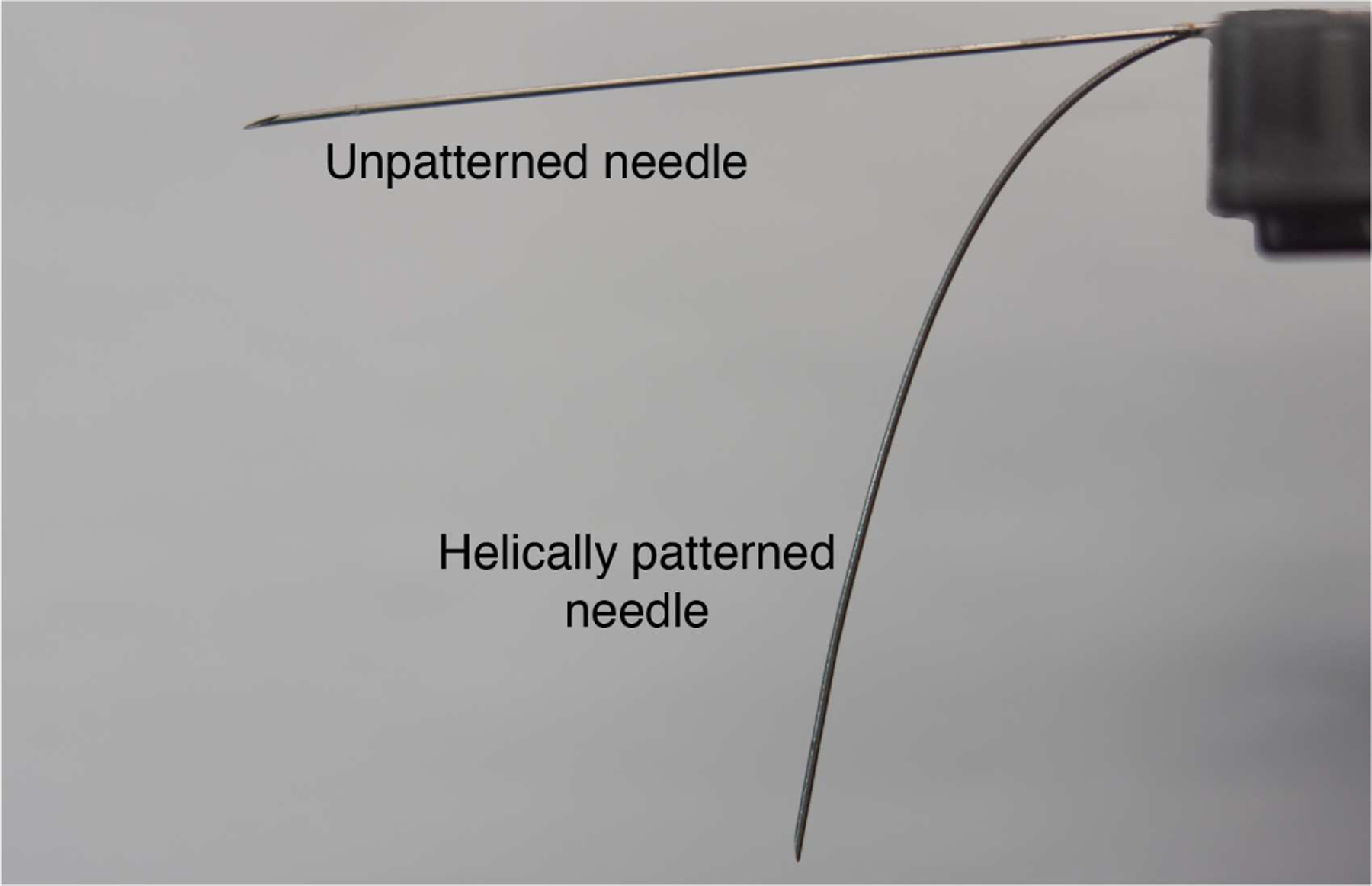
Illustration of the stiffness difference between two needles of the same size with and without helical patterning. The two needles are cantilevered and bend under self-weight.
Table I:
Geometric needle parameters for the bevel tip needle with helical dovetail shaft patterning used in the experiments in this paper.
| Helical Pitch Angle | 10deg |
| Nominal Laser Kerf | 22μm |
| Helix Offset | 15mm |
| Hinge Offset | 10mm |
| Bevel Angle | 15deg |
| Flexure Element Thickness | 0.1mm |
| Hinge Stop Angle | 18deg |
The prototypes in this paper are made from superelastic Nitinol tubing (Euroflex GmbH) that was laser cut by MDI LLC. (Medical Device Imagineering, Inc., Somerset, NJ) using a fiber laser (Rofin Inc.). We incorporate a flexure hinge in the needles in this paper as proposed in [15]. The motivation for such a hinge is to obtain the enhanced steerability associated with previous kinked tip designs while reducing tissue damage during axial rotation. Prior flexure tip designs have been fabricated either by gluing small Nitinol wires side-by-side [15], or by cutting a square notch in the shaft [28], [29]. The flexure design we propose here is laser cut into the shaft and incorporates two additional beneficial features. First, for safety purposes, we include a hard stop to prevent the hinge from bending in the wrong direction (i.e. toward the bevel, rather than away), which could damage prior designs - especially the notched designs. Note that such bending would not happen under intended use of the needle, but might accidentally occur as the needle was being handled prior to insertion in a real-world operating room. Second, a stop angle for desired hinge bending is defined by two angled surfaces. These provide a more stable surface contact when the hinge closes, rather than the point contact seen in prior designs.
Note that this helical dovetail needle design provides many parameters for future optimization studies (see Table I). In this paper, our purpose is not to suggest that we have optimized all relevant parameters, but rather to propose the new design concept and illustrate its value in (1) enhancing needle steerability at a fixed diameter, and (2) enabling integration of interventional payloads. But first, we illustrate a negative phenomenon that can occur with small-diameter, high-curvature steerable needles under certain circumstances, which motivates that smaller is not necessarily better, regardless of whether an interventional tool is being integrated or not.
III. Illustration of Shearing at Small Diameters
In this section, we demonstrate experimentally that shearing can occur at small needle shaft diameters and high curvatures. This example further motivates a shift away from using shaft diameter for tuning steerable needle properties to tissue requirements. The shearing effect occurs along the needle shaft, when the shaft slices through tissue due to lateral forces imparted to the tissue after the needle’s tip has passed.
To illustrate this, consider a needle with a 0.36 mm OD and 0.24 mm ID, to which is affixed a larger, kinked tip made of stainless steel (1.0 mm OD). This needle design and these dimensions are similar to those previously used in e.g. [13], [24], among others. We inserted this needle into 10% by weight Knox gelatin (Kraft Foods Global Inc., IL), which is a commonly used phantom tissue in needle steering research [24]. The results are shown in the right-hand images in Fig. 4 (a) and (b), where the sheared cavity is highlighted with red dye. By increasing the diameter of the needle and applying the helical dovetail pattern (i.e. using the needle described by Table I), we can maintain high curvature in this particular phantom tissue, while eliminating the shearing effect, as shown in the left-hand images in the figure.
IV. The Effect Of Patterning on Needle Steerability In Tissue
We performed a series of needle insertions into various tissue types to demonstrate that helical dovetail patterning enhances needle steerability. These experiments demonstrate that needles of fixed diameters, useful for interventional tool integration (see Section VI), achieve high steerability with patterning when they would otherwise not steer well in these tissues. The steerable needles were the same tube dimensions as those mentioned in previous sections, with geometric parameters of the helical patterning and flexure hinge described in Table I. We compared four different needles: helically patterned with a hinge, helically patterned without a hinge, no patterning with a hinge, and no patterning or hinge. All needle designs had a beveled tip. We performed insertions using a steerable needle robot [30], and integrated a 6-DOF magnetic tracking coil (Northern Digital Inc., Canada) into the tip of the needle, which was used to measure radius of curvature. We integrated the sensor distal to the flexure hinge (where a hinge exists) to position it as close to the needle tip as possible.
To evaluate the needle curvature in tissues of varying stiffness, we used 3 different phantom materials and 3 different ex vivo animal tissues. For phantom tissues, we used 10% by weight Knox Gelatin (used as a phantom tissue in [15]), 6.1% by weight Knox Gelatin (used as a stand-in for brain tissue in [13], [31]), and polyvinyl chloride (PVC) mixed at an 80% plastic/20% softener ratio (used as a stand-in for prostate tissue in [2], [32], [33]). We also evaluated steerability in three ex vivo animal tissues: porcine loin (used, for example, in [15]), bovine liver (used, for example in [25]), and ovine brain. For each tissue type, we performed five insertions and measured the radius of curvature. The results are shown in Table II. These insertions were conducted at a velocity of 5 mm/s to an insertion depth of 75 mm for all samples except the ovine brain, where we inserted to 50 mm due to the small size of the organ.
Table II:
Average radius of curvature (in mm) values for the helically patterned needles with and without a flexure hinge, and the unpatterned needles with an identical flexure hinge and without a hinge in phantom and ex vivo tissues. All tube dimensions were identical.
| 10% Gelatin | 60.1 | 69.5 | 1290.9 | 410.6 |
| 6.1% Gelatin | 71.9 | 77.2 | 1209.3 | 548.4 |
| PVC | 41.4 | 77.1 | 116.6 | 366.1 |
| Bovine Liver | 35.5 | 54.8 | 150.6 | 1112.7 |
| Porcine Loin | 61.0 | 90.0 | 171.2 | 792.0 |
| Ovine Brain | 61.8 | 69.8 | 342.0 | 661.7 |
Despite the wide range of tissue stiffness in these experiments, the helically patterned needles consistently demonstrated higher steerability (i.e. lower radius of curvature) compared to needles without the helical patterning. However, the point here is not to build the highest curvature needles ever produced, but rather that these curvatures were achieved in prototypes with diameters that otherwise would not steer well in these tissues, as shown in the two columns marked ”unpatterned” in Table II. The table shows the results of insertions of needles that are identical in other ways (diameter, hinge parameters, etc.), where the only difference is the presence or absence of helical dovetail patterning and a hinge. Note that the needles exhibited very low steerability without the helical dovetail patterning. The helically patterned needle with a hinge outperforms all the other designs, although even without a hinge we see an improvement in curvature over the needles without patterning. These results demonstrate the ability to substantially improve the steerability of a needle of fixed diameter.
V. Closed-Loop Control in Inflated Porcine Lung
In this section, we describe targeting experiments in ex vivo porcine lung tissue. The purpose is to demonstrate that the helical dovetail needles can be steered to desired target locations with an established closed-loop controller [34].
We first determined the radius of curvature of the needle in inflated lung tissue. Due to the many airways and other obstacles present in lung tissue, we scanned the lung using the CT scanner shown in Fig. 5 (The Xoran xCAT, Xoran Technologies, USA), and performed segmentation according to [35]. We then selected a region where the needle could be inserted without rotation and with no collisions, and inserted along this path. Using magnetic tracker measurements collected during insertion, we measured the radius of curvature in inflated lung as 100 mm. Note that the best prior radius of curvature result we have achieved in deflated lung tissue was 255 mm [5], [9]. Inflation reduces lung density, making needles steer with worse curvature in inflated lung. These results underscore the value of helical dovetail patterning in very soft tissues, such as inflated lung.
Fig. 5:
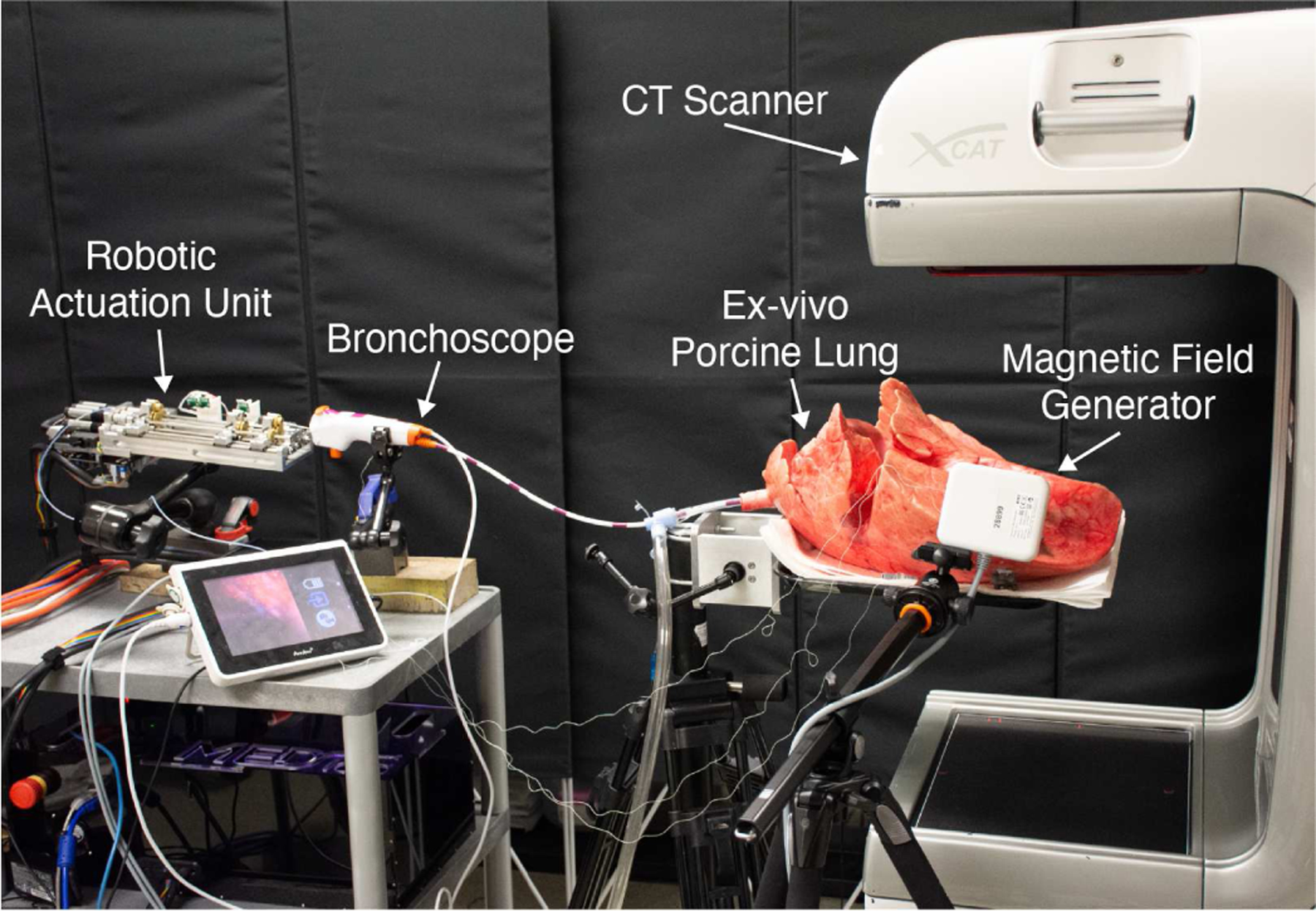
A photograph of the experimental setup for our ex vivo porcine lung targeting experiments.
We performed a set of targeting experiments in ex vivo porcine lung, as follows. We used the actuation unit described in [30], integrated with the overall system concept in [5], in which the needle is delivered through a bronchoscope. After inflating the lung via an endotracheal tube, we captured a preoperative CT scan using a portable CT scanner (Xoran Technologies, USA) and performed lung segmentation as described in [35]. We then manually selected target points in the peripheral lung and steered our needle to each. The experimental setup was as shown in Fig. 5. Final tip error in magnetic tracker space was as shown in Table III. The mean targeting error for all 14 runs was 1.92 mm. The excellent targeting error is consistent with prior results in the literature [7], [25], [34]. This illustrates that the helical patterning has not interfered with closed loop control.
Table III:
Final targeting error for each of the insertions under closed-loop control in inflated ex vivo porcine lung tissue.
| 1 | 2.8 |
| 2 | 1.8 |
| 3 | 0.6 |
| 4 | 4.2 |
| 5 | 2.4 |
| 6 | 1.5 |
| 7 | 1.5 |
| 8 | 2.0 |
| 9 | 1.2 |
| 10 | 0.9 |
| 11 | 1.5 |
| 12 | 1.3 |
| 13 | 2.5 |
| 14 | 2.8 |
VI. Integration of Interventional Payloads
To demonstrate the advantage of decoupling needle stiffness (and hence steerability) from shaft diameter, we provide two examples of delivering interventional tools with the helically patterned steerable needle: brachytherapy and radiofrequency (RF) ablation. Brachytherapy seeds are small implants that locally deliver radiation to tumors. Since radiation is localized around the seed, accurate placement is vital for the success of the overall procedure. Furthermore, the seeds have a pre-defined cylindrical shape with a fixed diameter, which places constraints on needle diameter. Fig. 6 shows several needle trajectories in gelatin, in which we delivered small cylinders representing brachytherapy seeds into phantom tissue. One standard clinical size for brachytherapy seeds is 0.8 mm in diameter and 4.5 mm long [36]. To replicate this, we cut segments of this length and diameter from nitinol wire, and deployed them through our needle.
Fig. 6:
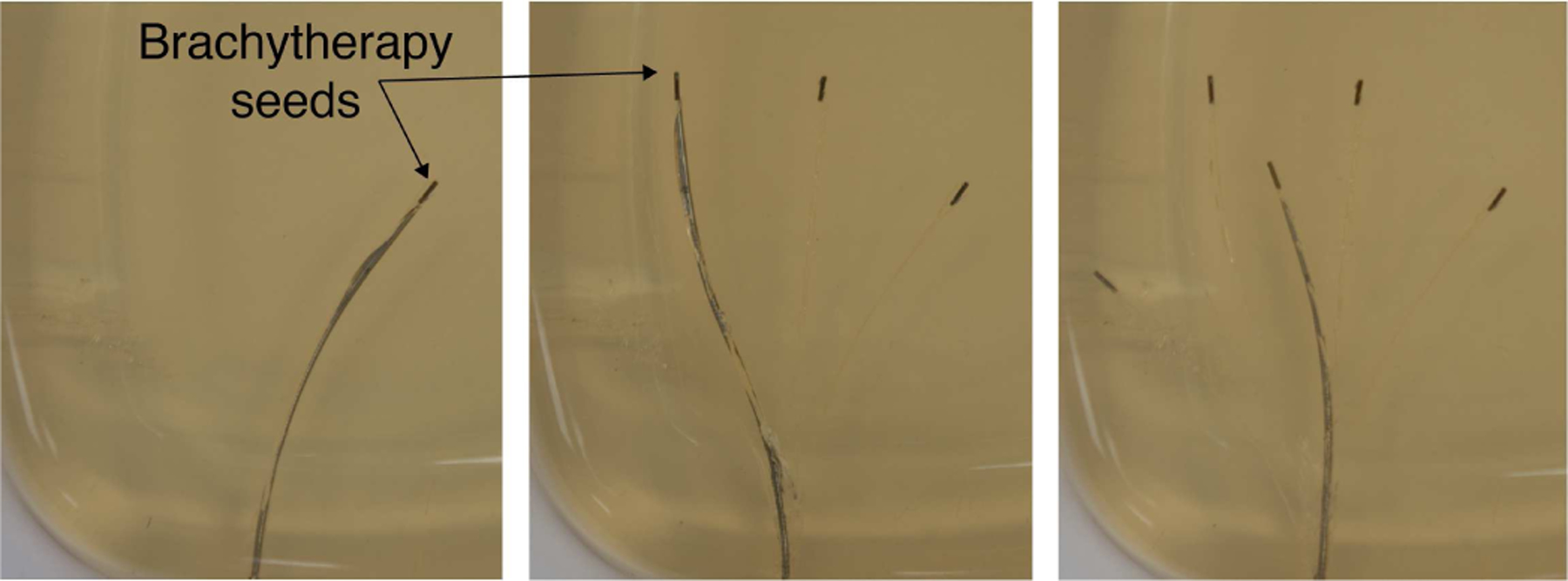
Helically patterned needle steering through Knox gelatin. We deployed five cylinders the size of brachytherapy seeds (4.5 mm × 0.8 mm) to different locations.
We also successfully integrated an RF ablation probe into our steerable needle, as shown in Fig. 7. Here, we used a 2MHz RF generator (Basco India, Tamilnadu State, India) with a 20 gauge nitinol wire to thermally ablate chicken breast. Note that ablation probes exist in a range of sizes (12 – 24 gauge). Larger probe sizes produce larger ablation zones [37], which can be desirable based on the clinical application. Our purpose here was not to optimize the ablation zone, but simply to demonstrate that a probe with a clinically realistic diameter can be integrated with our needle and used to deliver RF energy to tissue. Note that in the future, helical dovetail patterning will enable the ablation zone to be considered in the design process, rather than having the wire diameter (and hence ablation zone volume) constrained to be small in order to optimize needle steerability.
Fig. 7:
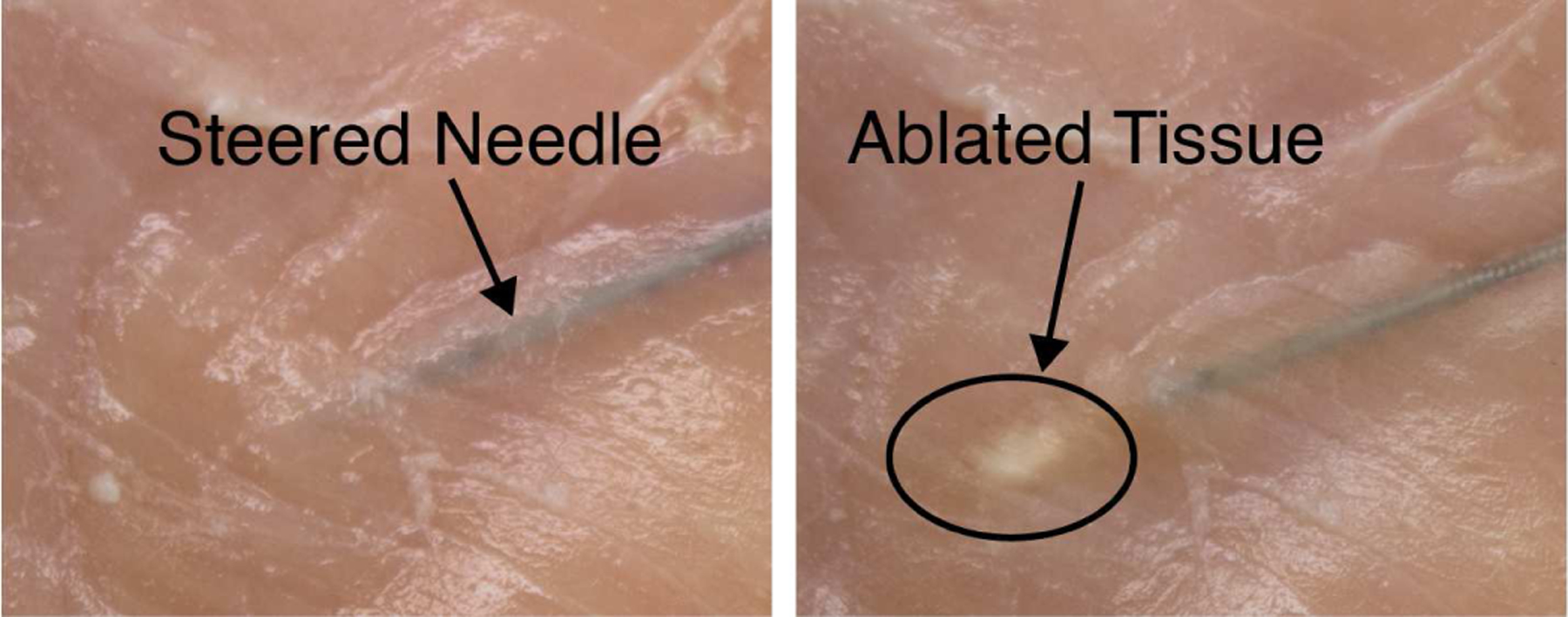
The needle was inserted into chicken breast and an ablation tool (20 gauge wire) was deployed through it. We successfully locally ablated the tissue around the tool through the needle.
VII. Conclusion
This paper describes a new way to decouple needle steerability from needle shaft diameter. This newfound flexibility expands the options available in the design process, which is particularly useful when one is interested in integrating interventional or diagnostic and therapeutic interventions into a steerable needle. We showed that helical dovetail patterning can make needles that would otherwise steer minimally in a given soft tissue steer with high curvature. We proved that this is true in a variety of phantoms and ex vivo tissues, and is particularly useful in lung tissue, demonstrating much higher steerability in inflated lung tissue than has ever previously been demonstrated - even in deflated lung. We also showed that helical dovetail patterning does not interfere with accurate robotic control and demonstrated steering to desired targets in inflated ex vivo porcine lung tissue.
In future work, the design parameters of the helical dovetail needle can be optimized for specific tissues, diameters, and/or payloads. Future work will also involve ex vivo and in vivo experiments in a variety of tissue types with paths that steer around anatomical obstacles (e.g., blood vessels, airways, sensitive tissues). The results in this paper serve to pave the way for interventional tool integration in steerable needles designed for diverse applications throughout the human body.
Acknowledgments
This material is based upon work supported in part by the National Institutes of Health under NIBIB training grant T32EB021937. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the NIH.
Biographies

Margaret Rox (S’17) received the B.S. degree in mechanical engineering with honors from Lip-scomb University, Nashville, TN, USA, in 2016. She is currently working toward the Ph.D. degree in mechanical engineering at Vanderbilt University, Nashville, TN, USA.
Since 2016, she has been a research assistant with the Medical Engineering and Discovery Laboratory at Vanderbilt University. She is involved in the research of medical robots, continuum robots, and endoscopic robot design and control.

Maxwell Emerson was born in Torrance, CA in 1991. He received the B.S. degree in biological sciences from the University of California Irvine, Irvine, CA, USA, in 2013, and M.S. degree in biomedical engineering at Yale University, New Haven, CT, USA, in 2015.
He is currently pursuing a Ph.D. in mechanical engineering at Vanderbilt University in Nashville, TN, USA. His research interests include modeling and control of continuum robots, steerable needles, medical device engineering, machine learning, probabilistic control and estimation.

Tayfun Efe Ertop (S’20) received the B.S. (with a minor in mechatronics) and M.S. degrees in mechanical engineering from Middle East Technical University, Ankara, Turkey, in 2014 and 2017, respectively.
From 2014 to 2015, he was a R&D Engineer in BAMA Technology (Ankara, Turkey). He worked as a Teaching Assistant with the Mechanical Engineering Department at Middle East Technical University between 2015 and 2017. He is currently working towards the Ph.D. degree in Mechanical Engineering at Vanderbilt University, Nashville, TN, USA. His research interests include medical robotics, continuum robots, and control systems.

Inbar Fried received the B.A. and M.S. degrees in computer science from Tufts University, Medford, MA, USA, in 2014 and 2015, respectively. He is currently pursuing a MD degree from the University of North Carolina School of Medicine, and the Ph.D. degree in computer science from the University of North Carolina, Chapel Hill, NC, USA.
From 2015 to 2017 he was a research programmer with the Department of Biomedical Informatics, Harvard Medical School, Boston, MA, USA. His research interests include computational approaches to clinical care, and motion planning for surgical robots. He is currently with the Computational Robotics Research Group.

Mengyu Fu (S’18) received the B.S. degree in automation in 2015 and the M.S. degree in control science and engineering in 2017 from Harbin Institute of Technology, Harbin, China. She is currently pursuing the Ph.D. degree in Computer Science at University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Since 2017, she has been a research assistant with Computational Robotics Research Group, Chapel Hill, NC, USA. Her research interests include general motion planning algorithms and motion planning for medical applications.

Janine Hoelscher (S’18) received the B.S. degree in computer science in 2014 and the M.S. degree in computer science with a minor in psychology in 2017 from Technical University of Darmstadt, Darmstadt, Germany. She is currently pursuing the Ph.D. degree in computer science at University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Since 2018, she has been a research assistant with the Computational Robotics Research Group, in Chapel Hill, NC, USA. Her research interests include motion planning and machine learning for robotics applications.
Ms. Hoelscher received a full scholarship for both her undergraduate and graduate studies by the German Academic Scholarship Foundation.

Alan Kuntz (S’15-M’20) received the B.S. degree in computer science with Honors from the University of New Mexico, Albuquerque, NM, USA, in 2014, the M.S. degree in computer science from the University of North Carolina at Chapel Hill, Chapel Hill, NC, USA, in 2016, and the Ph.D. degree in computer science from the University of North Carolina at Chapel Hill in 2019.
In 2020, he joined the faculty of the School of Computing and the Robotics Center at the University of Utah, Salt Lake City, UT, USA as an Assistant Professor, where he leads the artificial intelligence and robotics in medicine lab. Prior to joining the University of Utah, he was a postdoctoral scholar at the Vanderbilt Institute for Surgery and Engineering and the Department of Mechanical Engineering at Vanderbilt University, Nashville, TN, USA. His research focuses on healthcare applications of artificial intelligence, design optimization, and robot motion planning.

Josephine Granna received the Dipl.-Ing. in 2015 and the Dr.-Ing. in 2019 in mechanical engineering from Leibniz Universitat Hannover, Hanover, Germany.
From 2015 to 2019, she was a Research Assistant with the Laboratory for Continuum Robotics in the Department of Mechanical Engineering at Leibniz Universitat Hannover. Since November 2019, she has been a Postdoctoral Researcher at the Vanderbilt Institute for Surgery and Engineering and the Department of Mechanical Engineering at Vanderbilt University, Nashville, USA. Her research interests include robotic surgery, continuum robotics, optimization, and coverage planning.

Jason E. Mitchell received the B.S. degree in mechanical engineering from Tennessee Technological University, Cookeville, TN, USA, in 1999, and the M.S. and Ph.D. degrees in mechanical engineering from Vanderbilt University, Nashville, TN, USA, in 2002 and 2014 respectively.
He spent several years in industry as a mechanical design and process engineer. He is currently a Research Assistant Professor in the mechanical engineering department at Vanderbilt University, Nashville, TN. His research interests include analysis and design of high precision medical devices and prosthetics.
Dr. Mitchell has 14 patents and more than 40 articles.

Michael Lester was born in Huntsville, AL in 1987. He graduated with the B.S. degree in physics from the University of Alabama at Birmingham, Birmingham, AL, USA, in 2010, and the M.D. degree from Harvard Medical School, Boston, MA, USA, in 2014.
He completed a residency in Internal Medicine at Vanderbilt University Medical Center, Nashville, TN, USA, in 2017 and served as Chief Resident in 2017–2018. He remains at Vanderbilt today, where he is currently a fellow in Pulmonary and Critical Care Medicine. His research interests include translational interventional pulmonary devices and novel imaging techniques.

Fabien Maldonado received the M.D. degree from the School of Medicine at the University of Burgundy, Dijon, France, in 2002. He moved to the U.S. to eventually complete a fellowship in Pulmonary and Critical Care Medicine at Mayo Clinic, Rochester, MN, USA in 2008. He then completed an Interventional Pulmonary fellowship at the Universit’e de la M’editerran’ee, Marseille, France, from 2009 to 2010.
He is a Professor of Medicine, Thoracic Surgery and Mechanical Engineering at Vanderbilt University, which he joined in July 2015, after serving 9 years as a faculty at Mayo Clinic in the division of Pulmonary and Critical Care Medicine. He helped start an Interventional Pulmonology fellowship program at Mayo Clinic as the co-program director, and currently serves as the program director for the interventional pulmonology fellowship at Vanderbilt university. He is a member of the Board of Directors of the American Association of Bronchology and Interventional Pulmonology and serves on several steering committees for the American College of Chest Physicians and the American Thoracic Society. His main research interests include clinical trials in interventional pulmonology, computed-tomography based quantitative imaging for lung cancer and robotic bronchoscopy in collaboration with the Vanderbilt institute for Surgery and Engineering, with funding from the Department of Defense and the National Institutes of Health.

Erin A. Gillaspie received her B.S. in Microbiology and Cell Science at the University of Florida, Gainesville, FL, USA, in 2004 and her M.D. at the University of Miami Miller School of Medicine, Miami, FL, USA, in 2008.
From 2008 to 2013 she completed her general surgery residency at Bassett Medical Center in Cooperstown, NY, USA, and from 2013 to 2016 she completed her fellowship in cardiothoracic surgery at the Mayo Clinic in Rochester, MN, USA. Since 2016 she has been an Assistant Professor of Thoracic Surgery at Vanderbilt University Medical Center, Nashville, TN, USA. During her first two years on staff, she started a thoracic surgery robotics program and completed her M.P.H. with a focus on Epidemiology in 2018 from Vanderbilt University, Nashville, TN. She is the author of more than 50 articles, book chapters and has presented her research around the world. Her research interests include the development of novel technologies for the performance of minimally invasive surgery and the multidisciplinary management of lung cancer.
Dr. Gillaspie has been inducted as a Fellow in the American College of Surgeons. She was also the recipient of the Association for Academic Surgery Young Investigator Award, as well as the WTS-Intuitive Robotics Fellowship and the OT Clagett Traveling Fellowship.

Jason A. Akulian was born in Oakland, CA, USA, in 1976. He received the B.S. degree in biochemistry from the University of California, Riverside, USA, in 1998, MPH degree in epidemiology and biostatistics from Loma Linda University, Loma Linda, CA, USA, in 2001 and MD degree from St. Georges University, Grenada, West Indies in 2005.
From 2005 to 2009, he was an Internal Medicine Resident then Chief Resident at the University of Connecticut, Farmington, CT. He went on to complete training with his fellowships in Pulmonary/Critical Care and Interventional Pulmonology at The Johns Hopkins University School of Medicine, Baltimore, MD in 2013. Since 2013, he has been an Assistant Professor of Medicine with the University of North Carolina at Chapel Hill. He is also the Director of Interventional Pulmonology and the Carolina Center for Pleural Disease.
He is author of 3 book chapters and more than 60 peer-reviewed articles. His research interests include advanced diagnostic and therapeutic bronchoscopy, malignant pleural disease, and tumor-immune interaction in patients with advanced stage lung cancer.

Ron Alterovitz (M’09) received the B.S. degree with Honors from the California Institute of Technology (Caltech), Pasadena, CA in 2001 and a Ph.D. degree in Industrial Engineering and Operations Research from the University of California, Berkeley, CA in 2006.
In 2009, he joined the faculty of the Department of Computer Science at the University of North Carolina at Chapel Hill, NC, where he is currently a Professor and leads the Computational Robotics Research Group. His research focuses on developing novel algorithms for robots to learn and plan their motions, with a focus on enabling robots to perform new, less invasive medical procedures and to assist people in their homes.
Prof. Alterovitz has co-authored a book on Motion Planning in Medicine, was co-awarded a patent for a medical device, and has received multiple best paper awards at robotics and computer-assisted medicine conferences. He is the recipient of an NIH Ruth L. Kirschstein National Research Service Award, two UNC Computer Science Department Excellence in Teaching Awards, an NSF Early Career Development (CAREER) Award, and the Presidential Early Career Award for Scientists and Engineers (PECASE), the highest honor bestowed by the United States Government on science and engineering professionals in the early stages of their independent research careers.

Robert J. Webster III (S’97-M’08-SM’14) received the B.S. degree in electrical engineering from Clemson University, Clemson, SC, USA, in 2002, and the M.S. and Ph.D. degrees in mechanical engineering from Johns Hopkins University, Baltimore, MD, USA, in 2004 and 2007, respectively.
In 2008, he joined the Faculty of Vanderbilt University, Nashville, TN, USA, where he is currently a Richard A. Schroeder Professor of Mechanical Engineering, and also a professor of electrical engineering, otolaryngology, neurological surgery, urologic surgery, and medicine (interventional pulmonology). He directs the Medical Engineering and Discovery Laboratory, and co-founded Vanderbilt Institute for Surgery and Engineering, which brings together physicians and engineers to solve challenging clinical problems. He co-founded and serves as President of Virtuoso Surgical, Inc., and EndoTheia, Inc., Nashville, TN, USA.
He has received the IEEE RAS Early Career Award, the NSF CAREER Award, the RSS Early Career Spotlight Award, the IEEE Volz Award, and the Vanderbilt Engineering Award for Excellence in Teaching. He has served as Chair of the SPIE Image-Guided Procedures, Robotic Interventions, and Modeling Conference, Associate Editor of IEEE Transactions on Robotics, and currently serves as Associate Editor for the International Journal of Robotics Research and as a charter member of the NIH Imaging Guided Interventions and Surgery study section. His research interests include surgical robotics, image-guided surgery, and continuum robotics.
References
- [1].Abolhassani N, Patel R, and Moallem M, “Needle insertion into soft tissue: A survey,” Medical Engineering & Physics, vol. 29, no. 4, pp. 413–431, 2007. [DOI] [PubMed] [Google Scholar]
- [2].Reed KB, Majewicz A, Kallem V, Alterovitz R, Goldberg K, Cowan NJ, and Okamura AM, “Robot-assisted needle steering,” IEEE robotics & Automation Magazine, vol. 18, no. 4, pp. 35–46, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Webster RJ III, Kim JS, Cowan NJ, Chirikjian GS, and Okamura AM, “Nonholonomic modeling of needle steering,” The International Journal of Robotics Research, vol. 25, no. 5–6, pp. 509–525, 2006. [Google Scholar]
- [4].Majewicz A, Marra SP, Van Vledder MG, Lin M, Choti MA, Song DY, and Okamura AM, “Behavior of tip-steerable needles in ex vivo and in vivo tissue,” IEEE Transactions on Biomedical Engineering, vol. 59, no. 10, pp. 2705–2715, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Swaney PJ, Mahoney AW, Hartley BI, Remirez AA, Lamers E, Feins RH, Alterovitz R, and Webster RJ III, “Toward transoral peripheral lung access: Combining continuum robots and steerable needles,” Journal of Medical Robotics Research, vol. 02, no. 01, p. 1750001, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Alterovitz R, Goldberg K, and Okamura A, “Planning for steerable bevel-tip needle insertion through 2d soft tissue with obstacles,” in Proceedings of the 2005 IEEE international conference on robotics and automation. IEEE, 2005, pp. 1640–1645. [Google Scholar]
- [7].Minhas D, Engh JA, and Riviere CN, “Testing of neurosurgical needle steering via duty-cycled spinning in brain tissue in vitro,” in Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2009, pp. 258–261. [DOI] [PubMed] [Google Scholar]
- [8].Patil S and Alterovitz R, “Interactive motion planning for steerable needles in 3D environments with obstacles,” in IEEE International Conference on Biomedical Robotics and Biomechatronics, 2010, pp. 893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kuntz A, Torres LG, Feins RH, Webster RJ, and Alterovitz R, “Motion planning for a three-stage multilumen transoral lung access system,” IEEE International Conference on Intelligent Robots and Systems, vol. 2015-Decem, pp. 3255–3261, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pinzi M, Galvan S, and Rodriguez y Baena F, “The adaptive hermite fractal tree (AHFT): a novel surgical 3D path planning approach with curvature and heading constraints,” International Journal of Computer Assisted Radiology and Surgery, vol. 14, no. 4, pp. 659–670, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].van de Berg NJ, van Gerwen DJ, Dankelman J, and van den Dobbelsteen JJ, “Design choices in needle steeringa review,” IEEE/ASME Transactions on Mechatronics, vol. 20, no. 5, pp. 2172–2183, 2015. [Google Scholar]
- [12].Webster RJ III, Memisevic J, and Okamura AM, “Design Considerations for Robotic Needle Steering,” in IEEE International Conference on Robotics and Automation, 2005, pp. 3588–3594. [Google Scholar]
- [13].Engh JA, Podnar G, Kondziolka D, and Riviere CN, “Toward effective needle steering in brain tissue,” in Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2006, pp. 559–562. [DOI] [PubMed] [Google Scholar]
- [14].Adebar TK, Greer JD, Laeseke PF, Hwang GL, and Okamura AM, “Methods for improving the curvature of steerable needles in biological tissue,” IEEE Transactions on Biomedical Engineering, vol. 63, no. 6, pp. 1167–1177, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Swaney PJ, Burgner J, Gilbert HB, and Webster RJ III, “A flexure-based steerable needle: high curvature with reduced tissue damage,” IEEE Transactions on Biomedical Engineering, vol. 60, no. 4, pp. 906–909, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bui VK, Park S, Park J-O, and Ko SY, “A novel curvature-controllable steerable needle for percutaneous intervention,” Journal of Engineering in Medicine, vol. 230, no. 8, pp. 727–738, 2016. [DOI] [PubMed] [Google Scholar]
- [17].van de Berg NJ, Dankelman J, and van den Dobbelsteen JJ, “Design of an actively controlled steerable needle with tendon actuation and FBG-based shape sensing,” Medical Engineering and Physics, vol. 37, no. 6, pp. 617–622, 2015. [DOI] [PubMed] [Google Scholar]
- [18].Gerboni G, Greer JD, Laeseke PF, Hwang GL, and Okamura AM, “Highly articulated robotic needle achieves distributed ablation of liver tissue,” IEEE Robotics and Automation Letters, vol. 2, no. 3, pp. 1367–1374, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Okazawa S, Ebrahimi R, Chuang J, Salcudean S, and Rohling R, “Hand-held steerable needle device,” IEEE/ASME Transactions on Mechatronics, vol. 10, no. 3, pp. 285–296, 2005. [Google Scholar]
- [20].Yang F, Babaiasl M, and Swensen JP, “Fracture-Directed Steerable Needles,” Journal of Medical Robotics Research, vol. 4, no. 1, p. 1842002, 2018. [Google Scholar]
- [21].Gilbert HB, Neimat J, and Webster RJ III, “Concentric Tube Robots as Steerable Needles : Achieving Follow-the-Leader Deployment,” IEEE Transactions on Robotics, pp. 1–13, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Frasson L, Ferroni F, Ko SY, Dogangil G, and Rodriguez y Baena F, “Experimental evaluation of a novel steerable probe with a programmable bevel tip inspired by nature,” Journal of Robotic Surgery, vol. 6, no. 3, pp. 189–197, 2012. [DOI] [PubMed] [Google Scholar]
- [23].Ryu SC, Quek ZF, Renaud P, Black RJ, Daniel BL, and Cutkosky MR, “An optical actuation system and curvature sensor for a MR-compatible active needle,” in 2012 IEEE International Conference on Robotics and Automation, May 2012, pp. 1589–1594, iSSN: 1050–4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lehocky CA and Riviere CN, “Needle insertion with duty-cycled rotation into multiple media,” in Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2012, pp. 916–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Adebar TK, Fletcher AE, and Okamura AM, “3-D Ultrasound-Guided Robotic Needle Steering in Biological Tissue,” IEEE Transactions on Biomedical Engineering, vol. 61, no. 12, pp. 2899–2910, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wedlick TR and Okamura AM, “Characterization of pre-curved needles for steering in tissue,” in International Conference of the IEEE Engineering in Medicine and Biology Society, 2009, pp. 1200–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Majercak DC and Olsen D, “Highly flexible tubular device with high initial torque response for medical use,” U.S. Patent US8 357 140B2. [Google Scholar]
- [28].Swaney PJ, York P, Gilbert HB, Webster RJ, Mahoney AW, and Wellborn P, “Surgical device tip with arc length varying curvature,” US Patent US20 160 346 513A1, Dec., 2016, library Catalog: Google Patents.
- [29].Amack S, “Design of a Compact, Workflow-Oriented Robot for Transbronchial Lung Biopsy,” Master’s thesis, Vanderbilt University, Nashville, TN, 2019. [Google Scholar]
- [30].Amack S, Rox MF, Emerson M, Webster RJ III, Alterovitz R, Kuntz A, Mitchell J, Ertop TE, Gafford J, Maldonado F, and Akulian J, “Design and control of a compact modular robot for transbronchial lung biopsy,” in SPIE 10951, Medical Imaging 2019: Image-Guided Procedures, Robotic Interventions, and Modeling, 2019, p. 109510I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ritter R, Quate E, Gillies G, Grady M, Howard M, and Broaddus W, “Measurement of friction on straight catheters in in vitro brain and phantom material,” IEEE Transactions on Biomedical Engineering, vol. 45, no. 4, pp. 476–485, 1998. [DOI] [PubMed] [Google Scholar]
- [32].Li W, Belmont B, and Shih A, “Design and manufacture of polyvinyl chloride (PVC) tissue mimicking material for needle insertion,” Procedia Manufacturing, vol. 1, pp. 866–878, 2015. [Google Scholar]
- [33].Gerboni G, Greer JD, Laeseke PF, Hwang GL, and Okamura AM, “Highly articulated robotic needle achieves distributed ablation of liver tissue,” IEEE Robotics and Automation Letters, vol. 2, no. 3, pp. 1367–1374, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rucker DC, Das J, Gilbert HB, Swaney PJ, Miga MI, Sarkar N, and Webster RJ III, “Sliding mode control of steerable needles,” IEEE Transactions on Robotics, vol. 29, no. 5, pp. 1289–1299, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fu M, Kuntz A, Webster RJ III, and Alterovitz R, “Safe Motion Planning for Steerable Needles Using Cost Maps Automatically Extracted from Pulmonary Images,” IEEE International Conference on Intelligent Robots and Systems, pp. 4942–4949, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Aronowitz JN and Rivard MJ, “The phylogeny of permanent prostate brachytherapy,” Journal of Contemporary Brachytherapy, vol. 5, no. 2, pp. 89–92, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Nahum Goldberg S, Scott Gazelle G, Dawson SL, Rittman WJ, Mueller PR, and Rosenthal DI, “Tissue ablation with radiofrequency: Effect of probe size, gauge, duration, and temperature on lesion volume,” Academic Radiology, vol. 2, no. 5, pp. 399–404, May 1995. [DOI] [PubMed] [Google Scholar]


