Abstract
Salvia ekimiana Celep & Doğan is an endemic species of Turkey. To our knowledge, the number of studies on biological activities and phytochemical profiling of this plant is quite limited. Therefore, this study aimed to analyze its activities and phytochemical content in detail. The qualitative-quantitative compositions were determined via spectrophotometric and chromatographic (LC-MS/MS and HPLC) techniques. 1,1-Diphenyl-2-picrylhydrazyl radical (DPPH•) and 2,2′-Azino-bis 3-ethylbenzothiazoline-6-sulfonic acid (ABTS•+) radical scavenging and ascorbate-iron (III)-catalyzed phospholipid peroxidation experiments were performed to measure antioxidant capacity. Hyaluronidase, collagenase, and elastase enzyme inhibition tests were determined in vitro using a spectrophotometer. Antiproliferative activity was evaluated in human lung cancer (A549) and human breast cancer (MCF7) cells. The murine fibroblast (L929) cell line was used as a normal control cell. While the subextract rich in phenolic compounds was n-butanol extract, rosmarinic acid was defined as the main secondary metabolite. The highest antioxidant activity observed for the n-butanol subextract included the following: DPPH• EC50: 0.08±0.00 mg/mL, TEAC/ABTS: 2.19±0.09 mmol/L Trolox, MDA EC50: 0.42±0.03 mg/mL. The methanolic extract, the ethyl acetate, and n-butanol subextracts displayed significant inhibitory activity on collagenase, while the other subextracts did not show any inhibitory activity on hyaluronidase and elastase. Due to strong interactions with their active sites, molecular docking showed luteolin 7-glucuronide, apigenin 7-glucuronide, and luteolin 5-glucoside had the highest binding affinity with target enzymes. The chloroform subextract showed significant cytotoxicity in all cell lines. These novel results revealed that S. ekimiana has strong antioxidant, collagenase enzyme inhibitory, and cytotoxic potential.
Keywords: Salvia ekimiana; Antioxidant; Enzyme inhibition; Molecular docking, Cytotoxicity; LC-MS/MS
1. Introduction
Turkey has a wide variety of biotic and abiotic diversity. Lamiaceae is the third largest family by taxon number, and the Salvia genus belonging to the Lamiaceae family is a large genus with 107 taxa in Turkey (Celep and Dirmenci, 2017). The Salvia species have been used for medical purposes given their degassing, appetizing, stomach pain relief, colds and cough suppression, antipyretic, pain-relieving, and anti-inflammatory properties. They also possess antiseptic and wound-healing properties (Sezik and Yeşilada, 1999; Akkol et al., 2008). Phytochemical analysis of the Salvia species has shown the presence of phenolic acids, phenolic glycosides, flavonoids, anthocyanins, coumarins, polysaccharides, terpenoids, and essential oils in these plants (Amiri 2007). Anatolian sage extracts and their essential components, di- and triterpenoids, have been extensively studied for their pharmacological properties, especially antibacterial, cytotoxic, antioxidant/antiradical, and anticholinesterase activities (Topcu et al., 2008). The endemism ratio based on the Salvia genus's taxon number in Turkey is 54%, and Salvia ekimiana Celep & Doğan is one of the endemic species. S. ekimiana is limited to Yozgat in Central Anatolia and grows in the open Pinus sylvestris forest and mountain steppes (Celep and Doğan, 2010). To date the antioxidant and acetylcholinesterase (AchE), butyrylcholinesterase (BchE), tyrosinase (TYRO), and lipoxygenase (LOX) enzyme inhibition activities of S. ekimiana have been studied, and rosmarinic acid, protocatechuic acid, hydroxybenzoic acid, caffeic acid, p-coumaric acid, and ferulic acid were detected in methanol extract (Orhan et al., 2012).
Reactive oxygen species (ROS) produced with endogenous or exogenous stimuli are radicals that mutate key genes, causing normal cells to differentiate into cancer cells and damage DNA, proteins, and lipids. High amounts of intracellular ROS are targeted by the antioxidants to hinder cancer cells' growth and proliferation (Sammar et al. 2019)). Notably, biologically and medically important plants modulate a wide range of cell signaling pathways in different cancer cell lines. However, ROS is associated not only with cancer; it is also known that accumulated ROS in the skin can indirectly activate dermal enzymes, such as collagenase and elastase. Thus, elastase and collagenase synthesis accelerates skin aging by causing symptoms such as wrinkles, freckles, pallor, looseness, and leathery appearance (Jiratchayamaethasakul et al.2020). In addition, ROS-mediated degradation of hyaluronic acid is prevented by antioxidants by inhibiting the hyaluronidase enzyme (Esser et al., 2012). In this context, natural products with significant ROS-scavenging activity, due to their pharmacological properties and minimal non-target effects, have been extensively investigated for their potential therapeutic effects (Butt et al., 2017; Farooqi et al., 2018; Lin et al., 2018; Farooqi et al., 2019).
Due to the limited number of studies with S. ekimiana and the absence of characterization of their cytotoxic activity, we evaluated the extracts obtained from the plant for antioxidant, enzyme inhibitory, and cytotoxic activities. Composition analysis was carried out with LC-MS/MS analysis. Antioxidant activities were studied with radical scavenging assays and ascorbate-iron (III)-catalyzed phospholipid peroxidation assays. The effects of the extracts on hyaluronidase, collagenase, and elastase enzyme inhibition were determined in vitro. To understand the possible mechanisms of enzyme inhibition observed, docking analyses were performed on identified compounds. In cytotoxicity experiments, A549, MCF7, and L929 cell lines were used.
2. Materials and Methods
2.1. Plant Material and Reagents
Salvia ekimiana was collected from Yozgat/Turkey on 23.06.2018 to be used in experimental studies (near Aştaş village 39°35’ 19’’N, 035°49’ 43’’ E, 1815 m). An herbarium specimen of the plant is preserved under the code of Budak 3313 in the Herbarium of Bozok University Faculty of Arts and Sciences. All the chemicals and standards were analytical grade and were purchased from Sigma Chemical Company (St. Louis, MO, USA).
2.2. Extraction Procedure
The aerial part of the herbal material (163 g) was macerated with 80% methanol (MeOH) and kept for 24 hours, and the same process was repeated four times. The filtered and combined methanol extracts were concentrated using a rotary evaporator (37–38°C, under reduced pressure). Lyophilized 80% MeOH extract was dispersed in water, and fractionated with n-hexane, chloroform, ethyl acetate, and n-butanol. The obtained subextracts and the remaining water subextract were lyophilized after concentrating on the rotary evaporator.
2.3. Total Phenolic (TFC) and Flavonoid Content (TPC)
The TFC and TPC were determined using well-known methods such as Folin-Ciocalteu and aluminum chloride (AlCl3) colorimetric assays. The results are reported as gallic acid equivalents (GAE) in mg/g plant extract and as catechin (CA) equivalents in mg/g plant extract, respectively (Singleton et al., 1999, Zhishen et al., 1999).
2.4. Qualitative and Quantitative Chromatographic Analysis
2.4.1. Analysis with Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) Systems
AbSciex 3200 MS/MS detector was used for LC-MS/MS analysis. Negative ionization mode was preferred for ionization. Chromatographic separations were made with ODS 150 × 4.6 mm, i.d., 3 μm column using Shimadzu 20A HPLC. The column oven temperature was set to 40°C, and the flow rate was adjusted to 0.5 mL/minute. Mobile phases (A) methanol: water: formic acid (10: 89: 1, v/v/v) and (B) methanol: water: formic acid (89: 10:1, v/v/h). The concentration of B was increased from 10% to 100% in 40 minutes. For mass scanning (EMS), a mass range of 100-1000 amu was chosen.
2.4.2. Analysis with High-Performance Liquid Chromatography (HPLC) Systems
Photodiode Array (PDA) detector combined with Agilent 1100 liquid chromatography system was used to perform quantitative analysis of rosmarinic acid amount in the extracts. A reverse phase C18 analytical column (250 × 4.6 mm i.d., 5 μm particle size) was used as a stationary phase at room temperature. Detection was performed between 200 and 550 nm wavelengths. Three sets of solvent systems, solvents A, B, and C, were used for elution and were pumped at a 1 mL/min flow rate. Solvent A consisted of methanol, water, acetic acid (10,88,2; v/v/v/), Solvent B consisted of methanol, water, acetic acid (90,88,2; v/v/v/), and solvent C consisted of methanol.
2.5. Antioxidant Activity
2.5.1. DPPH• Radical Scavenging Activity
DPPH free radical scavenging activities of 80% methanol extract, subextracts, and standard rosmarinic acid was determined by the Gyamfi et al. (1999) method (Gyamfi et al., 1999). Tris-HCl buffer (50 nM, pH 7.4) and DPPH radical prepared in 0.1 mM methanol were used in the experiment. The extracts' stock solution was prepared at 4 mg/mL concentration for n-hexane subextract and 1 mg/mL for other subextracts and methanol extracts. A series of dilutions were prepared from stock solutions to calculate the EC50 (Half maximal effective concentration) value. After incubating in the dark for 30 minutes at room temperature, the absorbances were read at 517 nm. Estimated EC50 values are given as the mean value of triplicate analyses using Eq. 1.
| (1) |
2.5.2. ABTS•+ Radical Scavenging Activity
The ABTS•+ radical scavenging detection was performed as reported by Re et al. (1999). The ABTS•+ radical (7 mM) and potassium persulfate (2.45 mM) were dissolved in water and kept at room temperature in the dark for 16 hours before the experiment. The extracts and rosmarinic acid stock solutions were diluted in the concentration range of 0.25-1 mg/mL, and 10 μL of these samples were combined with a 990 μL radical solution; a kinetic measurement was performed at 734 nm for 30 minutes.
2.5.3. Ascorbate-iron (III)-catalyzed phospholipid peroxidation
The inhibitory effects of the extracts on ascorbate-iron (III)-catalyzed phospholipid peroxidation were made according to the method of Aruoma et al. (1997). Folch type VII type bovine brain extract was stirred with Phosphate-buffered saline (PBS) (10 mM, pH 7.4), kept in an ultrasonic bath until there was a transparent suspension in the ice bath. 0.2 mL of suspension, 0.5 mL of PBS buffer, 0.1 mL of FeCl3 (1 mM), and 0.1 mL of sample solutions were mixed, and the peroxidation reaction was accelerated by the inclusion of 0.1 mL of ascorbate (1 mM). The mixture was left at 37°C for 60 minutes, and after incubation, first 50 μL of butylated hydroxytoluene (2% w/v), then 1 mL of trichloroacetic acid (2.8% w/v) and 1 mL of 2-thiobarbituric acid (1% w / v in 0.05 M NaOH) was added. The mixture was heated at 100°C for 20 minutes. The reaction thiobarbituric acid (TBA) 2-malondialdehyde (MDA) chromogens were extracted with n-butanol, and the absorbance of the n-butanol phases was read at 532 nm. Percent inhibition was calculated using Eq. 1, and EC50 values were calculated using nonlinear regression curves (Sigma Plot 2001 version 7.0, SPSS Inc., Chicago, IL).
2.6. Enzyme inhibitory activity studies
2.6.1. Hyaluronidase inhibitory activity
Measuring the amount of N-acetyl glucosamine released from sodium hyaluronate forms the basis of the inhibition of hyaluronidase enzyme activity. 50 μL bovine hyaluronidase (7900 units/mL in 0.1 M acetate buffer) was stirred with 50 μl of the extracts dissolved in 5 % dimethyl sulfoxide (DMSO). The control group consisted of 50 μL of 5 % DMSO without extract. After 20 minutes of incubation at 37°C, 50 μL of calcium chloride (12.5 mM) was added the mixture and incubated for another 20 minutes at the same temperature. After adding 250 μL sodium hyaluronate (1.2 mg/mL), it was incubated again for 40 minutes. At the end of incubation, the mixture was processed with 100 μL 0.2 M sodium borate and 50 μL 0.4 M NaOH and then left for 3 minutes in a boiling water bath. After adding 1.5 mL of p-dimethylaminobenzaldehyde to the reaction mixture, the mixture was cooled to room temperature. Tannic acid was used as a standard in the experiment. The mixture was incubated for another 20 minutes at 37°C for 20 minutes for the color formation and the absorbance was measured at 585 nm (Beckmann Dual Spectrometer; Beckman, Fullerton, CA, USA) (Lee et al., 2008; Tumen et al., 2017; Sahasrabudhe and Deodhar, 2010).
2.6.2. Collagenase inhibitory activity
Clostridium histolyticum (ChC) was dissolved in 50 mM Tricine buffer (with 0.4M NaCl and 0.01M CaCl2, pH 7.5) while the extracts and standard were dissolved in DMSO. 2 mM N-[3-(2-Furyl)acryloyl]-Leu-Gly-Pro-Ala (FALGPA) was prepared in the Tricine buffer. Enzyme, buffer, and test sample were added to wells at 25 μL and incubated for 15 minutes. Epigallocatechin gallate (EGCG) was used as a standard in the experiment. A 50 μL of the volume of the prepared substrate was added to the mixture to approximately quantify the optical density (OD) decrease at 340 nm. The following formula was used to calculate the ChC inhibitory activity of each sample studied:
| (2) |
ODcontrol and ODsample represent the optical densities in the sample's absence and presence, respectively (Barrantes et al., 2003; Tumen et al., 2018; Güragac Dereli et al., 2020).
2.6.3. Elastase inhibitory activity
Human neutrophil elastase enzyme (HNE) (17 mU/mL) and samples were mixed in 0.1 M Tris-HCl buffer (pH 7.5) and then incubated at 25°C for 5 minutes. After adding N-Methoxysuccinyl-Ala-Ala-Pro-Val p-nitroanilide (MAAPVN), it was re-incubated for 1 hour at 37°C. EGCG was used as a standard in the experiment. The OD resulting from the formation of p-nitroaniline at 405 nm was read after soybean trypsin inhibitor (1 mg/mL) was added to terminate the reaction. The HNE inhibitory activities were calculated using Eq. 2 (Melzig et al. 2001; Genc et al. 2020).
2.7. Molecular docking
Hyaluronidase (PDB ID: 2PE4) (Chao et al., 2007), elastase (PDB ID: 1BRU) (Nakanishi et al., 2000), and collagenase (PDB ID: 2Y6I) (Eckhard et al., 2011)) enzymes were imported from the protein databank to the UCSF Chimera 1.14rc software. Heteroatoms and water molecules present in enzyme structures were removed. The grid box (25*25*25 Å3) was created from the ligands of the elastase (x: 23.204, y: 47.660, z: 17.090) and collagenase (x: 24.145, y: −2.734, z: 15.883) enzymes on active site coordinates, and the Tyr75 amino acid for the hyaluronidase (x: 40.23, y: −26.31, z: −22.73) enzyme. Protein and ligand structures were prepared and converted into pdbqt file format with the AutoDockTools-1.5.6 program. All compounds were docked with Auto Dock Vina (Trott and Olson, 2010) for hyaluronidase, elastase, and collagenase enzymes. Protein-ligand interactions were analyzed and visualized using the BIOVIA Discovery Studio Visualizer.
2.8. Cytotoxicity studies
2.8.1. Cell culture
A549 (ATCC CCL-185, Human Lung Cancer Cell Series), MCF7 (ATCC CCL-222, Human Breast Cancer Cell Series), and L929 (ATCC CCL-1 Mouse Fibroblast Cell Line) were purchased from American Type Culture Collection (Manassas, VA, USA). A549 and MCF7 were grown in Dulbecco's Modified Eagle's Medium (DMEM) medium containing 1% penicillin/streptomycin and 10% fetal bovine serum (FBS) (Gibco Invitrogen, Grand Island, NY, USA). For the L929 cell line, Eagle's Minimum Essential Medium (EMEM) containing 1% penicillin/streptomycin and 10% horse serum was used as a medium. Cell cultures were kept at 37°C in 5% CO2 and 95% air.
2.8.2. 3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide (MTT) colorimetric cell viability assay
After the MCF7, A549, and L929 cells were propagated in the medium, they were plated in 96-well microplates at 104 cell/well in 100 μL. After 24 hours, the medium in the wells was discarded. The extracts prepared by diluting in the medium (containing 0.05 % DMSO) were added to the plate by taking 100 μL from low concentration to high concentration in the range of 7.8-2000 μg/mL. After 24 hours, 0.5 mg/mL MTT working solution was dispensed to drained 96 well plates. Following incubation for approximately 3 hours, the solutions were removed and DMSO (100 μL) was added. At 540 nm wavelength, the OD of the cells in the plates was measured in an ELISA device (Bio-Rad Laboratories Inc., USA). The mean OD value of control wells was considered as 100% viability. Percent viability was calculated by proportioning the OD of the wells treated with a solvent containing DMSO and samples to the control OD value.
2.9. Statistical Analysis
The Levene test was used to evaluate variance homogeneity. One-way analysis of variance was used for comparisons between more than two groups. The Dunnett T3 test and Tukey's test were used for multiple comparisons. The data were evaluated with SPSS Version 11.0 statistic software package. The significance level was set at p <0.05.
3. Results and Discussion
3.1. Total Phenolic (TFC) and Flavonoid Content (TPC)
TFC and TPC of the extracts were characterized based on the methods described in the experimental section. The highest TFC was found in n-butanol subextract with a value of 617.77 ± 55.66 mgGAE/gextract, and the highest TPC was found in 80% methanol extract with 323.18 ± 7.65 mgCA / gextract value. Results are shown in Table 1. When the TFC and TPC of S. ekimiana were examined, it was noted that S. ekimiana had a richer content than the S. aramiensis and S. virgata species studied (Şeker Karatoprak et al., 2016; Şeker Karatoprak et al., 2020).
Table 1.
Total phenol, total flavonoid, and rosmarinic acid amount of S. ekimiana extracts.
| Extracts | Total Phenol (mgGAE/gextract)a |
Total Flavonoids (mgCA/gextract)b |
Amount of Rosmarinic acidc |
|---|---|---|---|
| Se MeOH | 454.77 ± 63.22 | 323.18 ± 7.65 | 96.26± 2.17 |
| Se Hex | 125.88 ± 41.63 | 37.90 ± 3.33 | 5.92 ± 0.93 |
| Se CH | 226.46 ± 27.30 | 126.34 ± 2.49 | n.d |
| Se EtoAc | 470.20 ± 40.27 | 318.11 ± 71.80 | 192.11 ± 0.43 |
| Se BuOH | 617.77 ± 55.66 | 282.68 ± 68.51 | 248.65 ± 4.20 |
| Se Water | 221.21 ± 12.95 | 131.19 ± 7.66 | 3.15 ± 0.01 |
mgGAE/gextract: Total phenols given as gallic acid equivs milligrams of gallic acid per gram (dry weight) of extract.
mgCA/gextract: Total flavonoids given as catechin per gram (dry weight) of extract.
mg/gextract Values are given as means ± standard error, n = 3. Se MeOH; S. ekimiana 80% methanol extract, Se Hex; S. ekimiana n- hexane subextract, Se CH; S. ekimiana chloroform subextract, Se EtOAc; S. ekimiana ethyl acetate subextract, Se BuOH; S. ekimiana n-butanol subextract, Se Water; S. ekimiana water subextract, n.d: not determined.
3.2. Qualitative and Quantitative Chromatographic Analysis
Based on the results of the LC-MS/MS analysis presented in Table 2, compound 1, eluted at 5,6 min, showed a molecular ion peak at m/z 167 [M-H]− which yielded the ions at m/z 152 due to the loss of a methyl group and m/z 123 due to the loss of a CO2. Thus, this compound must have included both a methyl and a carboxylic acid unit. Vanillic acid fragmentation pattern (Li et al., 2016) matched with compound 1, and compound 1 was identified as vanillic acid previously determined in several Salvia species (Liu et al., 2007; Orhan et al., 2012). Compound 2 showed m/z 137 [M-H]− molecular ion peak, fragmented to m/z 109 ion. The fragmentation pattern of compound 2 was similar to protocatechuic acid, which was at 154 amu molecular weight. Compound 2 was 17 amu (most probably an OH) higher than protocatechuic acid. Thus, the compound was identified as protocatechualdehyde previously determined in Salvia species (Zeng et al., 2006). Compound 3 had a molecular ion peak at m/z 179 [M-H]− and the product ions were observed at m/z 161 [M-H−H2O] and m/z 135 [M-H− CO2]. The fragmentation pattern of compound 3 matched with the caffeic acid previously determined in several Salvia species (Akkol et al., 2008; Al-Qudah et al., 2014). Compound 7 eluted at 19.5 min, showed molecular ion peak at m/z 359 [M-H]−, and was fragmented to m/z 191 due to the loss of a caffeoyl moiety (−162 amu). Further fragmentation was determined at m/z 179, m/z 161, and m/z 135, similar to caffeic acid. The fragmentation pattern of compound 7 fully matches with rosmarinic acid (caffeic acid dimer), which was determined as the primary compound of all extracts, except the chloroform subextract. Rosmarinic acid was one of the most abundant characteristic compounds of the Salvia species (Akkol et al., 2008; Chen et al., 2011; Cvetkovikj et al., 2013; Göger et al., 2006). The same molecular ion and the same MS spectrum but a different retention time (11,5) was observed in 80% methanol extract and n-butanol subextract. Compound 4 was identified as a rosmarinic acid isomer. The isomer of rosmarinic acid was previously determined in the Lamiaceae family; methyl ether derivative of rosmarinic acid was also defined as compound 12 (Miron et al., 2011). Compound 8 showed a molecular ion peak at m/z 381 [M-H]− and presented a caffeic acid fragmentation pattern. The 162 Amu between molecular ion peak and m/z 219 enabled us to identify compound 9 as caffeic acid derivative glucoside. Compounds 5 and 6 showed characteristic MS fragmentation of luteolin glucoside and luteolin glucuronide with molecular ion peaks at m/z 447 [M-H]− and m/z 461 [M-H]−, respectively. Due to the loss of glucose and a glucuronide moiety, both compounds gave the same base peak ion at m/z 285 (luteolin) fragmented to m/z 151 and 133, which allowed us to identify these compounds as luteolin glucoside and luteolin glucuronide, respectively. Luteolin (compound 13) was also determined as an aglycon. Luteolin and its derivatives are common in the Salvia species (Şeker Karatoprak et al., 2020; Cvetkovikj et al., 2013; Kontogianni et al., 2013). Compound 9 showed a molecular ion at m/z 431 [M–H]− and a base peak ion at m/z 269 (apigenin). The loss of a glucose moiety meant that compound 9 was apigenin glucoside. Compound 10 showed a molecular ion at m/z 461 [M–H]− and m/z 269 (apigenin) due to the loss of a glucuronide moiety. Thus, compound 10 was identified as apigenin glucuronide. Apigenin and its derivatives are common in the Salvia species, and compound 14 was determined as apigenin in our analysis (Figure 1) (Şeker Karatoprak et al., 2020; Kontogianni et al., 2013; Lu and Foo, 1999).
Table 2.
LC-MS/MS results of the S. ekimiana extracts.
| RT | [M-H] | MS2 | Compound | Extract | |
|---|---|---|---|---|---|
| 1 | 5,6 | 167 | 152, 139,123 | Vanillic acid | Se EtoAc, Se MeOH |
| 2 | 8,5 | 137 | 109 | Protocatechualdehyde | Se EtoAc |
| 3 | 11,4 | 179 | 161,135 | Caffeic acid | Se EtoAc, |
| 4 | 11,5 | 359 | 197, 179, 161 | Rosmarinic acid isomer | Se MeOH, Se BuOH |
| 5 | 18,2 | 447 | 285 | Luteolin glucoside | Se EtoAc, Se BuOH |
| 6 | 18,9 | 461 | 285, 151, 133 | Luteolin glucuronide | Se MeOH, Se BuOH |
| 7 | 19,5 | 359 | 197, 179, 161, 135 | Rosmarinic acid | Se Hex, Se EtoAc, Se MeOH, Se BuOH |
| 8 | 19,6 | 381 | 219, 179, 135 | Caffeic acid derivative glucoside | Se MeOH |
| 9 | 20,5 | 431 | 263, 269 | Apigenin glucoside | Se EtoAc |
| 10 | 21,3 | 445 | 269, 175, 113 | Apigenin glucuronide | Se EtoAc, Se MeOH, Se BuOH |
| 11 | 21,7 | 475 | 337, 299,284, 113 | 300 MW, methoxy flavonoid like diosmetin kaemferide, + glucuronide | Se EtoAc, Se MeOH, Se BuOH |
| 12 | 22,1 | 373 | 355, 311, 197, 179, 135 | Rosmarinic acid methyl ether | Se EtoAc |
| 13 | 26,0 | 285 | 257, 199, 175, 151, 133 | Luteolin | Se CH, Se EtoAc |
| 14 | 28,4 | 269 | 225, 151, 117 | Apigenin | Se EtoAc |
| 15 | 28,9 | 299 | 284, 183, 151 | 300 MW, methoxy flavonoid like diosmetin kaemferide, hispidulin | Se EtoAc |
| 16 | 29,3 | 327 | 309, 291, 229, 211 | Unknown, hydroxy-trimethoxyflavone | Se EtoAc |
| 17 | 32,0 | 299 | 284, 256, 227, 151, 133 | Unknown methoxy flavonoid | Se CH |
| 18 | 34,5 | 283 | 268, 239, 211 | Unknown methoxy flavonoid | Se CH |
| 19 | 36,0 | 339 | 325,311,297 | Unknown methoxy flavonoid | Se CH, Se Hex |
Figure 1.
LC-MS/MS chromatogram of 80% methanol extract of S. ekimiana.
The rosmarinic acid amount in the extracts was determined by comparing the retention time and UV spectra of the standard rosmarinic acid with HPLC. Serial dilutions of rosmarinic acid prepared in the concentration range of 0.012-0.84 mg/mL were used to calculate the quantitative data calibration curve. Rosmarinic acid was the predominant compound of the S. ekimiana, as mentioned by Orhan et al. (2012). While the highest amount of rosmarinic acid was found in the n-butanol subextract (248.65±4.20 mg/gextract) (Figure 2), it was not determined in the chloroform extract (Table 1).
Figure 2.
HPLC chromatogram of S. ekimiana n-butanol subextract.
Se MeOH; S. ekimiana 80% methanol extract, Se Hex; S. ekimiana n-hexane subextract, Se CH; S. ekimiana chloroform subextract, Se EtOAc; S. ekimiana ethyl acetate subextract, Se BuOH; S. ekimiana n-butanol subextract, Se Water; S. ekimiana water subextract
3.3. Antioxidant Activity
The extracts' antioxidant activity was evaluated by DPPH• and ABTS•+ radical scavenging and ascorbate-iron (III)-catalyzed phospholipid peroxidation assays. Results are given in Table 3. ABTS•+ and DPPH• tests are frequently used methods to evaluate the antioxidant potency of natural products, both of which are techniques based on stable colored radical quench (Kedare and Singh, 2011). Eighty percent methanol extract, ethyl acetate, n-butanol, and water subextracts were statistically similar to the standard rosmarinic acid (p<0.01). The EC50 values of the extracts studied were in the range of 0.08-2.09 mg/mL. Orhan et al. (2012) reported that S. ekimiana methanol extract scavenged 57.64% of the DPPH radical at 1000 μg/mL concentration. In our study, 80% methanol extract scavenged 50% of the same radical at a concentration of 140 μg/mL.
Table 3.
Antioxidant activity of S. ekimiana extracts.
| Extracts | DPPHA EC50 (mg/mL) |
TEACB (mmol/L Trolox) |
TBAC EC50 (mg/mL) |
|---|---|---|---|
| Se MeOH | 0.14±0.01* | 1.72±0.21d (1 mg/mL) 1.18±0.18b (0.5 mg/mL) |
0.62±0.03 bc |
| Se Hex | 2.09±0.69 | 0.53±0.01a (1 mg/mL) 0.33±0.03a (0.5 mg/mL) |
2.80±0.19 d |
| Se CH | 0.56±0.10 | 1.18±0.13b (1 mg/mL) 1.01±0.21b (0.5 mg/mL) |
0.85±0.11 e |
| Se EtoAc | 0.12±0.04* | 1.66±0.10d (1 mg/mL) 1.18±0.13b (0.5 mg/mL) |
0.72±0.06c |
| Se BuOH | 0.08±0.00* | 2.19±0.09e (1 mg/mL) 1.41±0.17b (0.5 mg/mL) |
0.42±0.03ab |
| Se Water | 0.24±0.01* | 1.37±0.07b (1 mg/mL) 1.23±0.15b (0.5 mg/mL) |
2.45±0.13f |
| RA | 0.004±0.00 | 2.31±0.06e (0.5 mg/mL) 0.97±0.04b (0.25 mg/mL) |
0.17±0.01a |
DPPH radical scavenging
TEAC is defined as the concentration of Trolox (mmol/L) having the ABTS•+ radical scavenging
Inhibition of malondialdehyde formation. Values (mg/mL) expressed as mean ± standard errors (n = 3), statistical analyses by Tukey comparison test. Bars with the same lower case letters (a-f) are not significantly (p > 0.05) different
p< 0.01. Se MeOH; S. ekimiana 80% methanol extract, Se Hex; S. ekimiana n-hexane subextract, Se CH; S. ekimiana chloroform subextract, Se EtOAc; S. ekimiana ethyl acetate subextract, Se BuOH; S. ekimiana n-butanol subextract, Se Water; S. ekimiana water subextract, RA: rosmarinic acid.
Hydrophilic and lipophilic antioxidants such as flavonoids, hydroxycinnamic acids, carotenoids can be measured with the ABTS•+ radical scavenging method. However, a full and accurate evaluation of the loss of ABTS•+ must be recorded continuously for the entire reaction time. Hence, herein, the kinetic measurement was measured for 30 minutes (Dong et al., 2015). In the experiment, n-butanol subextract showed the same activity at the concentration of 1 mg/mL with the 0.5 mg/mL concentrated rosmarinic acid (p> 0.05). All extracts, except n-hexane subextract at 1 mg/mL, had statistically analogous activity to 0.25 mg/mL concentrated rosmarinic acid (p> 0.05). The n-hexane subextract had the lowest activity.
Phospholipids rich in polyunsaturated fatty acids are biologically substantial molecules and are susceptible to degradation from hydroxyl radicals. Accordingly, it is vital to find new natural substances that prevent oxidative damage. The inhibition of the formation of TBA-reactive compounds at physiological pH is measured based on their degradation in bovine-brain-derived phospholipid liposomes via Ascorbate-Fe (III) -catalyzed hydroxyl radical. The oxidative treatment with ascorbate-iron (III) resulted in increased MDA levels due to the enhancement of the lipid peroxidation in the control group. It was determined that the extracts decreased MDA formation compared to the control, and the extracts had a protective effect against phospholipid peroxidation. According to the experiment results, the n-butanol subextract was statistically significant with rosmarinic acid with an EC50 value of 0.42±0.03 mg/mL (p>0.05). The Salvia species have been shown to possess antioxidant activity with high polyphenol content (Orhan et al., 2012; Koşar et al., 2011; Gantner et al., 2018; Boukhary et al., 2016), and to our knowledge, the effects of S. ekimiana on the ABTS radical and lipid peroxidation have been revealed for the first time in this study. Multiple studies have corroborated that various Salvia species possess remarkable antioxidant activity, secondary to mediation of various cellular mechanisms. DPPH, ABTS, FRAP, and superoxide anion scavenging activities, and β-carotene bleaching properties of Salvia fruticosa, Salvia clandestina, Salvia officinalis, Salvia sclarea, Salvia pomifera, Salvia tomentosa, S. aethiopis, S. candidissima, S. limbata, S. microstegia, S. nemorosa, S. pachystachys, S. verticillata, and S. virgata were investigated. According to the results, the antioxidant capacity has been ascribed to phenolic compounds (Tosun et al., 2009; Erdoğan et al., 2014; Boukhary et al., 2016; Vergine et al., 2019).
3.4. Enzyme inhibitory activity studies
Matrix metalloproteinases (MMPs) are zinc-containing extracellular proteinases divided into five subgroups according to substrate types: gelatinase, collagenase, stromelysin, membrane-type MMPs (MT-MMPs), to name a few. Collagenase, which is responsible for the breakdown of collagen, is also responsible for the remodeling of the extracellular matrix (ECM). Elastase is primarily responsible for the breakdown of elastin in the ECM. Since elastin and collagen are responsible for skin elasticity and structural integrity, unwanted wrinkles, scars, and aging skin occur when its depleted (Pientaweeratch et al., 2016; Demeule et al., 2000). Our results established that none of the extracts had significant inhibitory activity on the elastase enzyme at 100 μg/mL concentration compared to EGCG, showing the most potent inhibitory activity on elastase (Figure 3).
Figure 3.
Effects of the MeOH extract, subextracts of MeOH extract from S. ekimiana on elastase enzyme inhibitory activity. The results are given as the mean ± S.D. * p<0.05; ** p<0.01; *** p<0.001. Se MeOH; S. ekimiana 80% methanol extract, Se Hex; S. ekimiana n- hexane subextract, Se CH; S. ekimiana chloroform subextract, Se EtOAc; S. ekimiana ethyl acetate subextract, Se BuOH; S. ekimiana n-butanol subextract, Se Water; S. ekimiana water subextract.
The methanol extract showed an inhibitory value of 36.41% and 26.5% for both collagenase and hyaluronidase, respectively. In the collagenase activity assay, EGCG exhibited the most robust inhibitory activity at 59.34%. Among the subextracts, EtOAc and BuOH extracts showed 29.44% and 33.12% inhibition of collagenase (Figure 4). In the hyaluronidase activity assay, tannic acid exhibited the strongest inhibition at 69.03%. However, none of the subextracts showed any inhibitory activity in this assay (Figure 5). Notably, the activity of S. ekimiana on these enzymes has been evaluated herein for the first time. In a previous study with S. plebeia leaf methanolic extract and fractions, and ethyl acetate and butanol fractions were reported to inhibit elastase activity by 65.2 ± 1.30% and 31.7 ± 1.23%, respectively, at 330 μg/mL (Chang et al. 2017). In one of our previous studies, we evaluated the antioxidant activity and collagenase and elastase enzyme inhibition of S. aramiensis. We determined >50% inhibition of the methanol extract prepared from the aerial part on both enzymes (Şeker Karatoprak et al., 2020). Ippoushi et al. (2000) reported the hyaluronidase inhibitory activity of Salvia officinalis leaf ethanol extract as 15% (Ippoushi et al., 2000).
Figure 4.
Effects of the MeOH extract, subextracts of MeOH extract from S. ekimiana on collagenase enzyme inhibitory activity. The results are given as the mean ± S.D. *p<0.05; **p<0.01; *** p<0.001. Se MeOH; S. ekimiana 80% methanol extract, Se Hex; S. ekimiana n- hexane subextract, Se CH; S. ekimiana chloroform subextract, Se EtOAc; S. ekimiana ethyl acetate subextract, Se BuOH; S. ekimiana n-butanol subextract, Se Water; S. ekimiana water subextract.
Figure 5.
Effects of the MeOH extract, subextracts of MeOH extract from S. ekimiana on hyaluronidase enzyme inhibitory activity. The results are given as the mean ± S.D. * p<0.05; ** p<0.01; ***p<0.001. Se MeOH; S. ekimiana 80% methanol extract, Se Hex; S. ekimiana n- hexane subextract, Se CH; S. ekimiana chloroform subextract, Se EtOAc; S. ekimiana ethyl acetate subextract, Se BuOH; S. ekimiana n-butanol subextract, Se Water; S. ekimiana water subextract.
The inhibitory effect of S. ekimiana likely involves several mechanisms. The Zn ion, which is in the active site of collagenase and plays a crucial role, facilitates the interaction with the inhibitor (Mc Donald et al., 1996). Phenolic compounds, such as catechin and EGCG, have been recognized as collagenase inhibitors by inactivating the Zn+2 ion for catalytic activity due to their metal chelation properties (Ghimeray et al., 2015; Thring et al., 2009). S. ekimiana exhibited enzyme inhibitory activity by chelating Zn through its rich phenolic compounds (Jung et al., 2014; Thring et al., 2009; Mc Donald et al., 1996). Moreover, the hydroxyl groups of polyphenolic compounds can interact with the backbone or other functional group side chain of collagenase. In addition, the benzene ring's hydrophobic interaction in the structure of the polyphenolic compound and the collagenase can lead to a dysfunctional enzyme due to conformational changes (Malesev and Kuntic, 2007). Therefore, these results shed light on the high collagenase activity observed in S. ekimiana extracts due to their high number of polyphenolic compounds.
3.5. Molecular docking analysis
Molecular docking studies are frequently used in drug design and lead compound development stages (Ferreira et al., 2015). With molecular docking studies, the interaction energy of small molecular weight compounds with macromolecules such as target protein, DNA, RNA, and hydrophobic interactions and hydrogen bonds at the atomic level can be calculated (De Ruyck et al., 2016). From this point on, the possible interaction energies of the compounds detected in the LC-MS/MS analysis of S. ekimiana extracts with the enzyme's hyaluronidase (PDB ID: 2PE4), elastase (PDB ID: 1BRU), and collagenase (PDB ID: 2Y6I) were measured. The epigallocatechin gallate compound used as the standard substance in enzyme experiments for elastase and collagenase was docked and generated interaction energy of −7.6 kcal/mol, −7.7 kcal/mol for elastase and collagenase, respectively. The tannic acid compound used as a standard substance was molecular docking for the hyaluronidase enzyme and formed −9.4 kcal/mol interaction energy. The molecular docking protein-ligand interaction energy results performed with AutoDock Vina for all three enzymes are shown in Table 4.
Table 4.
AutoDock Vina molecular docking interaction energies (kcal/mol) of compounds with hyaluronidase, elastase, and collagenase enzymes detected by LC-MS/MS analysis of S. ekimiana extracts.
| Compounds from S. ekimiana extracts | Hyaluronidase(PDB ID: 2PE4) | Elastase(PDB ID: 1BRU) | Collagenase(PDB ID: 2Y6I) |
|---|---|---|---|
| 5-Hydroxy-3′,4′,7-trimethoxyflavone | −7.3 | −6.6 | −6.3 |
| 5-Hydroxy-7,8,4′-trimethoxyflavone | −6.9 | −5.9 | −6.3 |
| Apigenin 4′-glucoside | −8.1 | −7.5 | −7.4 |
| Apigenin 7-O-beta-D-glucoside | −8.5 | −8.0 | −7.4 |
| Apigenin-7-O-glucoside | −9.1 | −7.8 | −7.4 |
| Apigenin | −7.5 | −7.0 | −6.7 |
| Apigenin 4′-glucoside | −7.9 | −7.4 | −7.4 |
| Apigenin 7′-glucoside | −8.5 | −7.9 | −7.2 |
| Apigenin 7-glucuronide | −9.5 | −8.1 | −7.7 |
| Caffeic acid | −6.5 | −5.6 | −5.9 |
| DiosMetin | −9.0 | −8.0 | −7.6 |
| Hispidulin | −7.4 | −6.7 | −6.5 |
| Luteolin | −7.9 | −7.0 | −6.6 |
| Luteolin 3′-glucoside | −8.8 | −7.5 | −7.2 |
| Luteolin 4′-glucoside | −8.0 | −7.5 | −7.1 |
| Luteolin 5′-glucoside | −9.0 | −7.8 | −7.9 |
| Luteolin 7′-glucoside | −9.3 | −7.9 | −7.5 |
| Luteolin 3′-o-glucuronide | −9.2 | −8.0 | −7.5 |
| Luteolin 7′-o-glucuronide | −9.6 | −8.1 | −7.8 |
| Physcion | −7.0 | −6.1 | −6.4 |
| Protocatechualdehyde | −5.7 | −5.0 | −5.1 |
| Questin | −7.4 | −7.1 | −6.6 |
| Rosmarinic acid methyl ester | −7.9 | −6.3 | −6.6 |
| Rosmarinic acid | −8.2 | −6.5 | −6.9 |
| Vanilic acid | −5.7 | −5.3 | −5.1 |
| Epigallocatechin gallate* | - | −7.6 | −7.7 |
| Tannic acid* | −9.4 | - | - |
Compound used as reference in enzyme assays.
Table 4 confirms that almost all of the compounds are able to form high interactions with hyaluronidase. The 2D and 3D interactions of luteolin 7′-o-glucuronide compound (−9.6 kcal/mol), which creates the highest interaction with hyaluronidase, as shown in Figure 6. The active site of the hyaluronidase enzyme was formed by taking the Tyr75 amino acid as the center. The interaction of the ligands with Tyr75 is vital for the inhibition of this enzyme (Chao et al., 2007). The luteolin 7′-o-glucuronide compound formed a 2.36 Å length hydrogen bond with Tyr75, while the other H bonds formed with two Tyr210 (2.91 Å and 2.45 Å) and one Tyr286 (2.05 Å). It also produced pi-cation with Asp129 and Glu131, pi-pi stacked interactions with Tyr202 and Trp321.
Figure 6.
Hydrogen bonds surface area of 3D protein-ligand interaction and 2D schematic interaction diagram of luteolin 7-glucuronide compound at hyaluronidase enzyme active site (PDB ID: 2PE4).
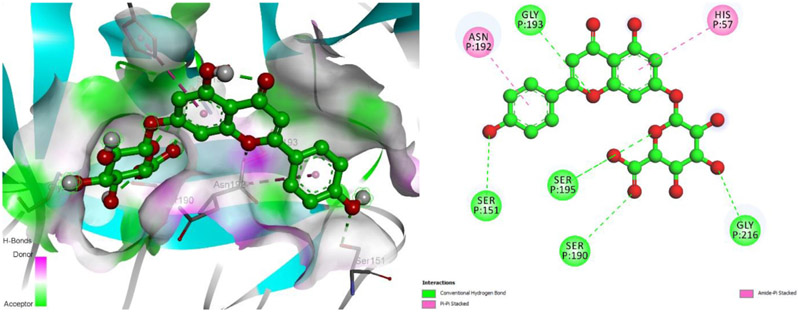
The compounds detected from the S. ekimiana extracts produced interaction energies ranging from −5.0 kcal/mol to −8.1 kcal/mol at the elastase enzyme's active site. The 2D and 3D protein-ligand interactions of the apigenin 7-glucuronide compound, which has the lowest interaction energy are shown in Figure 7. Five hydrogen bonds with apigenin 7-glucuronide, Gly193 (2.15 Å), Ser151 (1.85 Å), Ser195 (2.75 Å), Ser190, and Gly216 (2.15 Å) amino acids, and Pi-Pi stacked/amide-Pi stacked interactions with Asn192 and His57, also created hydrophobic interactions with Asn192.
Figure 7.
3D protein-ligand interaction with hydrogen bonds surface map and 2D schematic interaction diagram of apigenin 7-glucuronide compound at elastase enzyme active site (PDB ID: 1BRU).
The interaction of the collagenase enzyme and the compounds contained in S. ekimiana extracts were analyzed by molecular docking study. While epigallocatechin gallate used as reference compound generated −7.7 kcal/mol interaction energy, luteolin 5′-glucoside generated −7.9 kcal/mol. Therefore, it was determined to study the interactions of luteolin 5′-glucoside with the collagenase enzyme. As shown in Figure 8, luteolin 5′-glucoside generated hydrogen bonds with Glu559, Glu524, Leu550 (2.60 Å), Thr551 (3.30 Å) and Tyr496 (3.8 Å), unfavorable donor-donor interaction with Gly493 (3.01 Å), Pi-cation/Pi-anion interaction with Zn2+ and Glu555, Pi-Pi stacked with Trp539 and His527, Van der Waals interactions with His523. According to the molecular docking analysis of the components of the S. ekimiana extracts with hyaluronidase, elastase, and collagenase enzymes, it was inferred that some of the components form interactions with all three target enzymes. Luteolin 7-glucuronide, apigenin 7-glucuronide, and luteolin 5-glucoside selected compounds formed 4 to 5 strong hydrogen bonds with target enzymes. Based on LC-MS/MS and HPLC chromatograms, the low peak intensity of luteolin 7-glucuronide, apigenin 7-glucuronide, and luteolin 5-glucoside indicated that the amount of these compounds in the extracts was low. Therefore, the extracts' enzyme inhibition activity appeared low compared to the standards used.
Figure 8.
3D and 2D protein-ligand interactions at the collagenase active site of the luteolin 5-glucoside compound (PDB ID: 2Y6I).
3.6. Cytotoxicity studies
The extracts' toxicity was evaluated with the MTT assay in A549, MCF7, and L929 cell lines. The results are expressed as a percentage (%), and the cell control group viability is considered 100%, and other groups are calculated. Results are shown in Table 5. The IC50 values of the extracts are presented in Table 6. Eighty percent methanol extract in the concentration range of 7.8-500 μg/mL failed to inhibiting A549 cell viability significantly compared to the control. Cell viability decreased in the 80% methanol extract group at 1000 and 2000 μg/mL concentrations (p <0.001). Compared to the control, the n-hexane subextract did not significantly affect viability at concentrations in the range 7.8-1000 μg/mL. While the chloroform subextract reduced the viability in the concentration range of 7.8-2000 μg/mL, and this reduction was significant at p <0.001 at concentrations between 250 and 2000 μg/mL. In the concentration range of 7.8-125 μg/mL, ethyl acetate subextract did not significantly affect viability, but at concentrations ranging from 500 to 2000 μg/mL, viability was significantly reduced compared to the control group (p <0.001). n-Butanol subextract had a significant effect on cell viability in the concentration range between 250 and 2000 μg/mL (p <0.001). The water subextract only affected cell viability at a 2000 μg/mL (p <0.001). When the IC50 values were examined, it was determined that the chloroform subextract was quite effective compared to the other extracts with a value of 49.46 μg/mL. In the MCF7 cell line, the efficiency of the 80% methanol extract, n-hexane, ethyl acetate, and water subextracts were more effective than in the A549 cell. In contrast, the n-butanol subextract strongly increased cell death at 1000 and 2000 μg/mL compared to the A549 cell line, but this effect was not seen at lower concentrations. The chloroform subextract exhibited approximately 95% inhibition of proliferation in the cell line, even at a concentration of 125 μg/mL. Similar to the effect in A549 cell, chloroform subextract showed a strong effect in MCF7 cell line with an IC50 value of 46.16 μg/mL.
Table 5.
Cytotoxic effects of S. ekimiana extracts.
| A549 cell line (% Viability) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Extracts Concentrations (μg/mL) | |||||||||
| 7.8 | 15.6 | 31.25 | 62.5 | 125 | 250 | 500 | 1000 | 2000 | |
| SeMeOH | 106.31±14.48 | 103.90±11.62 | 91.04±7.44 | 81.85±9.26 | 80.16±6.29 | 83.37±6.59 | 78.76±5.29 | 18.92±0.92*** | 25.66±3.96*** |
| Se Hex | 104.18±4.95 | 104.34±6.68 | 103.09±10.85 | 102.69±12.43 | 104.42±8.26 | 102.65±4.76 | 82.37±8.75 | 71.97±4.38 | 64.18±8.2** |
| Se CH | 60.56±6.65** | 61.49±8.30** | 59.36±4.32** | 26.06±5.93*** | 28.43±4.9*** | 16.63±4.74*** | 15.50±0.62*** | 17.11±1.11*** | 11.77±2.73*** |
| Se EtoAc | 125.29±7.34 | 120.28±11.75 | 113.38±14.98 | 109.08±13.54 | 105.84±8.20 | 58.02±2.16** | 32.19±5.64*** | 34.73±3.25*** | 30.07±3.11*** |
| SeBuOH | 117.34±10.95 | 102.12±6.06 | 113.50±11.09 | 93.35±8.94 | 74.26±7.65 | 33.72±5.05*** | 17.48±1.88*** | 18.81±2.43*** | 20.08±2.96*** |
| Se Water | 114.42±4.77 | 112.15±10.98 | 112.92±9.25 | 108.45±8.95 | 107.17±9.03 | 96.57±10.73 | 95.97±5.31 | 88.80±3.91 | 20.60±0.94*** |
| MCF 7 cell line (% Viability) | |||||||||
| Extracts Concentrations (μg/mL) | |||||||||
| 7.8 | 15.6 | 31.25 | 62.5 | 125 | 250 | 500 | 1000 | 2000 | |
| Se MeOH | 95.05±4.65 | 90.58±2.98 | 87.16±5.04 | 88.65±5.88 | 82.29±4.79 | 57.71±6.08** | 41.62±2.71*** | 3.62±0.65*** | 4.21±0.34*** |
| Se Hex | 109.18±6.75 | 93.96±8.32 | 97.90±12.43 | 81.19±11.67 | 55.92±6.16** | 35.93±5.16*** | 13.83±3.9*** | 3.24±0.50*** | 2.69±0.14*** |
| Se CH | 80.94±8.50 | 74.36±9.11* | 52.20±8.23** | 26.05±3.71*** | 5.37±0.75*** | 2.33±0.08*** | 2.51±0.24*** | 2.76±0.19*** | 2.77±0.06*** |
| Se EtoAc | 114.41±8.72 | 109.22±13.22 | 109.64±7.5 | 76.34±5.21 | 65.06±8.86** | 34.52±4.61*** | 3.88±0.46*** | 5.40±1.83*** | 8.01±1.63*** |
| Se BuOH | 102.48±12.01 | 105.83±10.57 | 104.58±4.43 | 102.93±16.50 | 81.71±7.78 | 55.63±1.55** | 25.12±2.22*** | 4.69±1.35*** | 5.32±0.46*** |
| Se Water | 129.26±10.65 | 129.87±12.74 | 122.70±8.53 | 121.78±6.17 | 112.43±6.54 | 111.12±6.88 | 82.33±9.14 | 31.22±6.05*** | 4.62±0.30*** |
| L929 cell line (% Viability) | |||||||||
| Extracts Concentrations (μg/mL) | |||||||||
| 7.8 | 15.6 | 31.25 | 62.5 | 125 | 250 | 500 | 1000 | 2000 | |
| SeMeOH | 102.33±8.67 | 100.28±4.97 | 84.30±13.17 | 84.89±8.63 | 70.79±10.22 | 55.22±8.14** | 46.10±10.64*** | 18.42±2.95*** | 16.50±0.87*** |
| Se Hex | 98.71±15.70 | 93.61±11.98 | 86.58±10.30 | 83.92±18.41 | 86.62±11.04 | 88.99±7.62 | 77.41±10.37 | 68.09±9.15** | 53.38±9.50** |
| Se CH | 76.40±2.98 | 64.86±4.49** | 44.95±3.81*** | 32.88±2.84*** | 16.29±1.25*** | 13.35±0.59*** | 20.44±2.17*** | 16.44±0.26*** | 14.56±0.91*** |
| Se EtoAc | 117.13±13.54 | 90.79±15.95 | 72.66±6.47 | 56.23±4.70** | 51.04±5.24** | 40.31±3.91*** | 29.20±3.71*** | 26.04±1.72*** | 19.68±0.97*** |
| SeBuOH | 107.94±5.04 | 99.72±5.88 | 94.27±6.23 | 87.41±3.44 | 96.34±6.28 | 99.24±11.50 | 99.34±9.54 | 31.50±8.31*** | 24.12±4.70*** |
| Se Water | 105.05±17.01 | 112.86±9.33 | 98.03±14.09 | 73.10±12.53 | 83.46±13.62 | 74.48±14.36 | 67.65±8.75** | 66.21±9.38** | 31.30±1.16*** |
Values (μg/mL) given as mean ± standard errors (n = 3), statistical analyses by Dunnett T3 comparison test.
p<0.05
p<0.01
p<0.001.
Se MeOH; S. ekimiana 80% methanol extract, Se Hex; S. ekimiana n- hexane subextract, Se CH; S. ekimiana chloroform subextract, Se EtOAc; S. ekimiana ethyl acetate subextract, Se BuOH; S. ekimiana n-butanol subextract, Se Water; S. ekimiana water subextract.
Table 6.
IC50 values of extracts in cell lines.
| IC50 (μg/mL) | |||
|---|---|---|---|
| Extracts | A549 | MCF 7 | L929 |
| SeMeOH | 632.93±10.52 | 351.35±51.87 | 227.84±40.56 |
| Se Hex | >2000 | 121.80±2.67 | >2000 |
| Se CH | 49.46±5.26 | 46.16±4.00 | 31.82±4.18 |
| Se EtoAc | 268.95±34.09 | 153.12±10.31 | 128.52±17.46 |
| SeBuOH | 145.95±5.56 | 226.02±67.41 | 730.58±45.12 |
| Se Water | >1000 | >1000 | >1000 |
Values (μg/mL) given as mean ± standard errors (n = 3).
The L929 mouse fibroblast cell line was included in our study as it meets international standards (ISO 10993 part 5, 1999; ISO 7405, 1997) as a reference for cytotoxicity assays (Karamustafa et al., 2006). Generally, in the L929 cell line, the extracts had analogous effects. n-Butanol subextract did not induce toxicity in the L929 cell line below 500 μg/mL, and chloroform subextract has the highest cytotoxic effect, significantly attenuating viability at all tested concentrations. n-Butanol subextract has higher IC50 (730.58 μg/mL) value in L929 cell line in contrast to cancer cells. Based on the results, the antiproliferative efficacy of chloroform extract is thought to be promising. Anatolian Salvia species are rich in Di and triterpenoids, flavonoids, and phenolic compounds (Topçu et al., 2017). It was determined that extracts rich in phenolic composition have lower cytotoxic activity than chloroform subextract. The strong activity of the chloroform subextract as well is probably due to the presence of diterpenes. Topcu et al. (2008) studied the cytotoxic activity of 16 Salvia species grown in Anatolia and reported that the compounds responsible for the effect are abietane diterpenoids. Considering that great efforts are made to evaluate the antiproliferative effect on new natural extracts obtained from plants and that the researches are increasing day by day (Mahomoodally et al., 2019; Della Valle et al.2020; Yagi et al. 2020), it is obvious that the data obtained from the study will contribute to this field.
4. Conclusions
This report is the foremost detailed study on the polyphenolic determination of S. ekimiana extracts and their biological activities consisting of antioxidant, enzyme inhibition, and cytotoxicity. S. ekimiana inhibited collagenase activity establishing its efficacy in wound healing. The results indicate that the phenolic compounds found in S. ekimiana may be responsible for this activity. The more robust activity of the methanol extract compared to the other subextracts indicates synergistic interference of the compounds found in this crude extract. Our novel study shows that S. ekimiana has a potential antiproliferative effect on cancer cell lines and may also be effective in aging-related diseases in which oxidative stress plays an important role. We observed that the S. ekimiana n-butanol subextract had potent radical scavenging capacity and inhibited lipid peroxidation, while chloroform subextract displayed a strong effect on human lung and breast cancer cell lines. Future studies will further characterize the main active ingredients in these extracts and determine their pharmaceutical for a host of human ailments.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors
Abbreviations
- ABTS•+
2,2′-Azino-bis 3-ethylbenzothiazoline-6-sulfonic acid
- AchE
Acetylcholinesterase
- BchE
Butyrylcholinesterase
- CA
Catechin
- DMEM
Dulbecco's Modified Eagle's Medium DPPH•1,1-Diphenyl-2-picrylhydrazyl radical
- EMEM
Eagle's Minimum Essential Medium
- GAE
Gallic acid equivalents
- HPLC
High Performance Liquid Chromatography
- LC-MS/MS
Liquid Chromatography-Tandem Mass Spectrometry
- LOX
Lipoxygenase
- MDA
Malondialdehyde
- MTT
3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide
- PBS
Phospate buffered Saline
- PDA
Photodiode Array
- RA
Rosmarinic Acid
- TBA
Thiobarbituric Acid
- TEAC
Trolox Equivalent Antioxidant Capacity
- TFC
Total Phenolic Content
- TPC
Total Flavonoid Content
- TYRO
Tyrosinase
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Akkol EK, Göger F, Koşar M, Başer KHC, 2008. Phenolic composition and biological activities of Salvia halophila and Salvia virgata from Turkey. Food Chem 108, 942–949. [DOI] [PubMed] [Google Scholar]
- Al-Qudah MA, Al-Jaber HI, Abu Zarga MH, Abu Orabi ST, 2014. Flavonoid and phenolic compounds from Salvia palaestina L. growing wild in Jordan and their antioxidant activities. Phytochemistry 99, 115–120. [DOI] [PubMed] [Google Scholar]
- Amiri H, 2007. Quantitative and qualitative changes of essential oil of Salvia bracteata Bank et Sol. in different growth stages. DARU J. Pharm. Sci 15 (2), 79–82. [Google Scholar]
- Aruoma GI, Spencer JPE, Warren D, Jenner P, Butler J, Halliwell B, 1997. Characterization of food antioxidants, illustrated using commercial garlic and ginger preparations. Food Chem 60, 149–156. [Google Scholar]
- Barrantes E, Guinea M, 2003. Inhibition of collagenase and metalloproteinases by aloins and aloe gel. Life Sci 72 (7), 843–850. [DOI] [PubMed] [Google Scholar]
- Boukhary R, Raafat K, Ghoneim AI, Aboul-Ela M, El-Lakany A, 2016. Anti-inflammatory and antioxidant activities of Salvia fruticosa: An HPLC determination of phenolic contents. Evid. Based Complementary Altern. Med 7178105. doi: 10.1155/2016/7178105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt G, Attar R, Tabassum S, Aras A, Qadir MI, Ozbey U, Alaaeddine N, Ozcelik B, Farooqi AA, 2017. Regulation of signal transduction cascades by pterostilbenes in different cancers: is it a death knell for oncogenic pathways. Cell. Mol. Biol 63 (12), 5–10. [DOI] [PubMed] [Google Scholar]
- Celep F, Dirmenci T, 2017. Systematic and biogeographic overview of Lamiaceae in Turkey. Natural Volatiles & Essential Oils 4 (4), 14–27. [Google Scholar]
- Celep F, Doğan M, 2010. Salvia ekimiana (Lamiaceae), a new species from Turkey. Ann. Bot. Fenn 47, 63–66. [Google Scholar]
- Chang YJ, Lee DU, Nam DY, Cho SM, Hong S, Nam JH, Kim WK, 2017. Inhibitory effect of Salvia plebeia leaf extract on ultraviolet-induced photoaging-associated ion channels and enzymes. Exp. Ther. Med 13 (2), 567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao KL, Muthukumar L, Herzberg O, 2007. Structure of human hyaluronidase-1, a hyaluronan hydrolyzing enzyme involved in tumor growth and angiogenesis. Biochemistry 46 (23), 6911–6920. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhang Q, Wang X, Yang J, Wang Q, 2011. Qualitative analysis and simultaneous quantification of phenolic compounds in the aerial parts of Salvia miltiorrhiza by HPLC-DAD and ESI/MSn. Phytochem. Anal 22 (3), 247–257. [DOI] [PubMed] [Google Scholar]
- Cvetkovikj I, Stefkov G, Acevska J, Stanoeva JP, Karapandzova M, Stefova M, Dimitrovska A, Kulevanova S, 2013. Polyphenolic characterization and chromatographic methods for fast assessment of culinary Salvia species from South East Europe. J. Chromatogr A 1282 (0), 38–45. [DOI] [PubMed] [Google Scholar]
- Della Valle A, Dimmito MP, Zengin G, Pieretti S, Mollica A, Locatelli M, Cichelli A, Novellino E, Ak G, Yerlikaya S, Baloglu MC, Celik Altunoglu Y, Stefanucci A, 2020. Exploring the nutraceutical potential of dried pepper Capsicum annuum L. on market from Altino in Abruzzo region. Antioxidants (Basel, Switzerland) 9 (5), 400. 10.3390/antiox9050400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeule M, Brossard M, Page M, Gingras D, Beliveau R, 2000. Matrix metalloproteinase inhibition by green tea catechins. Biochim. Biophys. Acta 1478, 51–60. [DOI] [PubMed] [Google Scholar]
- De Ruyck J, Brysbaert G, Blossey R, Lensink MF, 2016. Molecular docking as a popular tool in drug design, an in silico travel. Adv. Appl. Bioinform. Chem 28 (9), 1–11. 10.2147/AABC.S105289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong JW, Cai L, Xing Y, Yu J, Ding ZT, 2015. Re-evaluation of ABTS•+ assay for total antioxidant capacity of natural products. Nat. Prod. Com 10 (12), 2169–2172. [PubMed] [Google Scholar]
- Eckhard U, Schönauer E, Nüss D, Brandstetter H, 2011. Structure of collagenase G reveals a chew-and-digest mechanism of bacterial collagenolysis. Nat. Struct. Mol. Biol 18 (10), 1109–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdoğan SS, Karik Ü, Başer KHC, 2014. The determination of antioxidant activity of some Sage populations of in the Marmara Region. Turkish Journal of Agricultural and Natural Sciences 2, 1877–1882. [Google Scholar]
- Esser PR, Wölfle U, Dürr C, von Loewenich FD, Schempp CM, Freudenberg MA, Jakob T, Martin SF, 2012. Contact sensitizers induce skin inflammation via ROS production and hyaluronic acid degradation. PloS one 7 (7), e41340. 10.1371/journal.pone.0041340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi AA, Ahmad A, 2019. Gaze through the clinical lens: molecular and clinical advancements of botanicals. Future Med. Chem 11 (2), 75–77. [DOI] [PubMed] [Google Scholar]
- Farooqi AA, Jabeen S, Attar R, Yaylim I, Xu B, 2018. Quercetin-mediated regulation of signal transduction cascades and microRNAs: natural weapon against cancer. J. Cell. Biochem 119 (12), 9664–9674. [DOI] [PubMed] [Google Scholar]
- Ferreira LG, Dos Santos RN, Oliva G, Andricopulo AD, 2015. Molecular docking and structure-based drug design strategies. Molecules 20 (7), 13384–13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantner M, Brodowska M, Górska-Horczyczak E, Wojtasik-Kalinowska I, Najda A, Pogorzelska E, Godziszewska J, 2018. Antioxidant effect of sage (Salvia officinalis L.) extract on Turkey meatballs packed in cold modified atmosphere. Cyta J. Food 16 (1), 628–636. [Google Scholar]
- Genc Y, Guragac Dereli FT, Saracoglu I, Kupeli Akkol E, 2020. The inhibitory effects of isolated constituents from Plantago major subsp. major L. on collagenase, elastase and hyaluronidase enzymes: Potential wound healer. Saudi Pharm. J 28, 101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghimeray AK, Jung US, Lee HY, Kim YH, Ryu EK, Chang MS, 2015. In vitro antioxidant, collagenase inhibition, and in vivo anti-wrinkle effects of combined formulation containing Punica granatum, Ginkgo biloba, Ficus carica, and Morus alba fruits extract. Clin. Cosmet. Investig. Dermatol 8, 389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göger F, 2006. Salvia virgata Jacq. ve Salvia halophila Hedge’nin antioksidan etkilerinin ve bileşimlerinin belirlenmesi. Anadolu University, Eskişehir, Turkey. Master thesis. [Google Scholar]
- Güragac Dereli FT, Genc Y, Saracoglu I, Küpeli Akkol E, 2020. Enzyme inhibitory assessment of isolated constituents from Plantago holosteum. Scop. Z. Naturforsch C 75 (3-4), 121–128. [DOI] [PubMed] [Google Scholar]
- Gyamfi MA, Yonamine M, Aniya Y, 1999. Free-radical scavenging action of medicinal herbs from Ghana: Thonningia sanguinea on experimentally induced liver injuries. Gen. Pharmacol 32, 661–667. [DOI] [PubMed] [Google Scholar]
- Ippoushi K, Yamaguchi Y, Itou H, Azuma K, Higashi H, 2000. Evaluation of inhibitory effects of vegetables and herbs on hyaluronidase and identification of rosmarinic acid as a hyaluronidase inhibitor in Lemon Balm (Melissa officinalis L.). Food Sci. Technol. Res 6, 74–77. [Google Scholar]
- Jiratchayamaethasakul C, Ding Y, Hwang O, Im ST, Jang Y, Myung SW, Lee JM, Kim HS, Ko SC, Lee SH, 2020. 2020. In vitro screening of elastase, collagenase, hyaluronidase, and tyrosinase inhibitory and antioxidant activities of 22 halophyte plant extracts for novel cosmeceuticals. Fish Aquatic Sci 23 (6). 10.1186/s41240-020-00149-8. [DOI] [Google Scholar]
- Jung HY, Shin JC, Park SM, 2014. Pinus densiflora extract protects human skin fibroblasts against UVB-induced photoaging by inhibiting the expression of MMPs and increasing type I procollagen expression. Toxicol. Rep 1, 658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamustafa F, Çelebi N, Değim Z, Yılmaz Ş, 2006. Evaluation of the viability of L-929 cells in the presence of alendronate and absorption enhancers. FABAD J. Pharm. Sci 31, 1–5. [Google Scholar]
- Kedare SB, Singh RP, 2011. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol 48 (4), 412–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontogianni VG, Tomic G, Nikolic I, Nerantzaki AA, Sayyad N, Stosic-Grujicic S, Stojanovic I, Gerothanassis IP, Tzakos AG, 2013. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem 136 (1), 120–129. [DOI] [PubMed] [Google Scholar]
- Koşar M, Göger F, Başer KHC, 2011. In vitro antioxidant properties and phenolic composition of Salvia halophila Hedge from Turkey. Food Chem 129, 374–379. [DOI] [PubMed] [Google Scholar]
- Lee TH, Huang NK, Lai TC, Yang ATY, Wang GJ, 2008. Anemonin, from Clematis crassifolia, potent and selective inducible nitric oxide synthase inhibitor. J. Ethnopharmacol 116 (3), 518–527. [DOI] [PubMed] [Google Scholar]
- Li S, Lin Z, Jiang H, Tong L, Wang H, Chen S, 2016. Rapid identification and assignation of the active ingredients in Fufang banbianlian injection using HPLC-DAD-ESI-IT-TOF-MS. J. Chromatogr. Sci 54 (7), 1225–1237. [DOI] [PubMed] [Google Scholar]
- Lin X, Ozbey U, Sabitaliyevich UY, Attar R, Ozcelik B, Zhang Y, Guo M, Liu M, Alhewairini SS, Farooqi AA, 2018. Maslinic acid as an effective anticancer agent. Cell. Mol. Biol 64 (10), 87–91. [PubMed] [Google Scholar]
- Lu Y, Foo LY, 1999. Rosmarinic acid derivatives from Salvia officinalis. Phytochemistry 51 (1), 91–94. [Google Scholar]
- Mahomoodally MF, Picot-Allain CM, Hosenally M, Ugurlu A, Mollica A, Stefanucci A, Llorent-Martínez EJ, Baloglu MC, Zengin G, 2019. Multi-targeted potential of Pittosporum senacia Putt.: HPLC-ESI-MSn analysis, in silico docking, DNA protection, antimicrobial, enzyme inhibition, anti-cancer and apoptotic activity. Comput. Biol. Chem 83, 107114. 10.1016/j.compbiolchem.2019.107114. [DOI] [PubMed] [Google Scholar]
- Malesev D, Kuntic V, 2007. investigation of metal-flavonoid chelates and the determination of flavonoids via metalflavonoid complexing reactions. J. Serb. Chem. Soc 72, 921–939. [Google Scholar]
- Mc Donald M, Mila I, Scalbert A, 1996. Precipitaion of metal ions by plant polyphenols: optimal conditions and origin of precipitation. J. Agric. Food Chem 44, 599–606. [Google Scholar]
- Melzig MF, Loser B, Ciesielski S, 2001. Inhibition of neutrophil elastase activity by phenolic compounds from plants. Pharmazie 56 (12), 967–970. [PubMed] [Google Scholar]
- Miron TL, Plaza M, Bahrim G, Ibanez E, Herrero M, 2011. Chemical composition of bioactive pressurized extracts of Romanian aromatic plants.J. Chromatogr A 1218 (30), 4918–4927. [DOI] [PubMed] [Google Scholar]
- Nakanishi I, Kinoshita T, Sato A, Tada T, 2000. Structure of porcine pancreatic elastase complexed with FR901277, a novel macrocyclic inhibitor of elastases, at 1.6 Å resolution. Biopolymers 53 (5), 434–445. [DOI] [PubMed] [Google Scholar]
- Orhan IE, Sezer Senol F, Ozturk N, Akaydin G, Sener B, 2012. Profiling of in vitro neurobiological effects and phenolic acids of selected endemic Salvia species. Food Chem 132, 1360–1367. [DOI] [PubMed] [Google Scholar]
- Pientaweeratch S, Panapisal v., Tansirikongkol A, 2016. Antioxidant, anti-collagenase and anti-elastase activities of Phyllanthus emblica, Manilkara zapota and silymarin: an in vitro comparative study for anti-aging applications. Pharm. Biol, 54 (9), 1865–1872. [DOI] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannalaa A, Yanga M, Rice-Evans C, 1999. Antioxidant activity applying an improved ABTS radical cation decolorisation assay. Free Rad. Biol. Med 26, 1231–1237. [DOI] [PubMed] [Google Scholar]
- Sahasrabudhe A, Deodhar M, 2010. Anti-hyaluronidase, anti-elastase activity of Garcinia indica. Int. J. Bot 6, 299–303. [Google Scholar]
- Sammar M, AbuFarich B, Rayan I, Falah M, Rayan A, 2019. Correlation between cytotoxicity in cancer cells and free radicalscavenging activity: In vitro evaluation of 57 medicinal and edible plant extracts. Oncol. Lett 18, 6563–6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Şeker Karatoprak G, İlgün S, Koşar M, 2016. Antioxidant properties and phenolic composition of Salvia virgata Jacq. Turk J. Pharm. Sci 13, 201–212. [Google Scholar]
- Şeker Karatoprak G, Yücel Ç, Göger F, Sobarzo-Sánchez E, Küpeli Akkol E, 2020. Potential antioxidant and enzyme inhibitory effects of nanoliposomal formulation prepared from Salvia aramiensis Rech. f. extract. Antioxidants (Basel) 1; 9 (4). pii: E293. doi: 10.3390/antiox9040293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sezik E, Yesilada E, 1999. Ucucu Yag Tasıyan Turk Halk Ilacları (Turkish folk medicine containing volatile oils). In: Kirimer N, & Mat A (Eds.), Essential Oils—In Honour of Prof. Dr. K. Hüsnü Can Baser On his 50th Birthday (pp. 98–131), Eskisehir: Anadolu University. [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventos RM, 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-ciocalteu reagent. Methods Enzymol 299, 152–178. [Google Scholar]
- Thring TS, Hili P, Naughton DP, 2009. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med 9, 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topcu G, Turkmen Z, Schilling JK, Kingston DGI, Pezzuto JM, Ulubelen A, 2008. Cytotoxic activity of some Anatolian Salvia extracts and isolated abietane diterpenoids. Pharm. Biol 46 (3), 180–184. [Google Scholar]
- Topçu G, Yücer R, Şenol H, 2017. Bioactive constituents of Anatolian Salvia species. In: Georgiev V, & Pavlov A (Eds.), Salvia Biotechnology (pp.31–132). Cham: Springer [Google Scholar]
- Tosun M, Ercisli S, Sengul M, Ozer H, Polat T, Ozturk E, 2009. Antioxidant properties and total phenolic content of eight Salviaspecies from Turkey. Biol. Res 42, 175–181. [PubMed] [Google Scholar]
- Trott O, Olson AJ, 2010. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput Chem 31 (2), 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumen I, Guragac FT, Keles H, Reunanen M, Kupeli-Akkol E, 2017. Characterization and wound repair potential of essential oil Eucalyptus globulus Labill. Fresenius Environ. Bull 26 (11), 6390–6399. [Google Scholar]
- Tumen I, Kupeli-Akkol E, Pranovich A, Reunanen M, Yaman B, 2018. Chemical composition and wound healing activity of inflorescences, leaves, wood, and bark of Marsdenia erecta R. Br. (Apocynaceae). Fresenius Environ. Bull 27 (8), 5590–5598. [Google Scholar]
- Vergine M, Nicolì F, Negro C, Luvisi A, Nutricati E, Accogli RA, Sabella E, Miceli A, 2019. Phytochemical profiles and antioxidant activity of Salvia species from Southern Italy. Rec. Nat. Prod 3, 205–215. [Google Scholar]
- Yagi S, Mohammed ABA, Tzanova T, Schohn H, Abdelgadir H, Stefanucci A, Mollica A, Zengin G, 2020. Chemical profile, antiproliferative, antioxidant, and enzyme inhibition activities and docking studies of Cymbopogon schoenanthus (L.) Spreng. and Cymbopogon nervatus (Hochst.) Chiov. from Sudan. J Food Biochem 44, e13107. 10.1111/jfbc.13107. [DOI] [PubMed] [Google Scholar]
- Zeng G, Xiao H, Liu J, Liang X, 2006. Identification of phenolic constituents in Radix Salvia miltiorrhizae by liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom 20 (3), 499–506. [DOI] [PubMed] [Google Scholar]
- Zhishen J, Mengcheng T, Jianming W, 1999. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64, 555–559. [Google Scholar]