Abstract
Objective
To evaluate the risk of severe COVID-19 in individuals with severe mental disorders, substance use disorders, and common mental disorders in the total adult population of Region Stockholm (N = 1,516,270), and to explore possible underlying mechanisms to the increased risk.
Methods
In this prospective cohort study, we examined the risk of hospitalization and treatment in an intensive care unit (ICU) with COVID-19, and death from COVID-19 for individuals with mental disorders. Associations were step by step adjusted for (1) sociodemographic/economic factors, (2) indicators of virus exposure, (3) somatic conditions, and (4) psychopharmacological treatment.
Results
In model 1 (adjusted for age, sex and living in a care home for elderly people), people with a mental disorder had increased risks for inpatient care (HR = 1.5), ICU care (HR = 1.5), and mortality (HR = 1.4) from COVID-19. There was an increased risk of dying from COVID-19 in all subgroups of mental disorders, particularly in people with a severe mental disorder (HR = 1.9). Different covariates had different effects on the association depending on the outcome and on sex, age, or psychiatric diagnosis of the participants.
Conclusion
People with mental disorders have an increased risk of severe COVID-19, including mortality. The increased risk was partly explained by the examined covariates.
Keywords: COVID-19, Severe mental disorders, Substance-related disorders, Common mental disorders
1. Introduction
Individuals with mental disorders may be more susceptible to SARS-CoV-2 infection and its complications compared to persons without mental disorders [1,2]. Several underlying mechanisms of the increased risk of severe COVID-19 in individuals with mental disorders have been suggested, including (1) lower socioeconomic status, (2) increased exposure to the SARS-CoV-2 virus, (3) higher levels of co-existing medical conditions known to predict severe COVID-19, and (4) side-effects of medications.
More specifically, low socioeconomic status is common in individuals with mental disorders [3]. According to a recent report in Sweden, low socioeconomic status was associated with an increased risk of severe COVID-19 [4].
Individuals with mental disorders may be more exposed than those without mental disorders to SARS-CoV-2 infection due to more frequent health care utilization [5]. Furthermore, these individuals may not have access to resources or knowledge to navigate and apply public health agency advisories and recommendations [6]. In addition, individuals with mental disorders are more likely than others to have unskilled and temporary employments and, as a result, may not have the possibility to work from home [7].
Moreover, having a mental disorder is associated with a range of medical conditions (i.e. obesity, diabetes type II, hypertension, pulmonary diseases, and cardiovascular disease) that have all been found to predict greater complications with a COVID-19 infection [[8], [9], [10], [11], [12]]. Previous studies have shown an increased mortality risk in similar infections as COVID-19 (i.e., influenza and pneumonia), and increased risk of thromboembolism for patients with psychotic and bipolar disorders (i.e., severe mental disorders, SMD) compared to those without an SMD [13,14]. Finally, a few studies suggest that there may be an increased risk of complicated COVID-19 in patients with psychopharmacological treatment (i.e., benzodiazepines and atypical antipsychotics) [15,16].
1.1. Previous studies
Several previous studies have showed higher risks of hospitalization and mortality due to Covid-19 infection in individuals with mental disorders, compared to those without a mental disorder [17,18]. In a study from the United States (N = 7348) researchers found an increased risk of mortality (following a SARS-CoV-2 infection) for patients with schizophrenia (AOR = 3.1) and mood disorders (AOR = 1.5) when adjusting for age, sex, and race [19]. No increased risk was found for patients with anxiety disorders. When further adjusting for several medical conditions, the results were moderately attenuated. In another study from Israel (N = 51,078), researcher found an increased risk for of hospitalization (AOR = 2.2) and mortality (AOR = 3.3) when adjusting for age and sex [20]. These increased risks were moderately attenuated when additionally adjusting for sociodemographic and medical conditions. Finally, a study from Denmark (N = 144,321) found similar risk increases of mortality for patients with schizophrenia (HR = 2.8), bipolar disorder (HR = 2.3) and mood disorders (HR = 1.8), but no increased risk for other psychiatric disorders (Hazard ratios fully adjusted for age, sex, sociodemographic and medical factors) [21].
Regarding substance use disorders, a study from the United States, including a sample with confirmed COVID-19 (N = 11,124) found that patients with substance use disorders had an increased risk of hospitalization (OR = 1.8) and mortality (1.3), when compared to matched controls (matched on demographics, obesity, and diabetes) [22]. When further adjusting the association for other medical conditions, the increased risk of hospitalization was somewhat attenuated (OR = 1.5) and there was no evidence of an increased risk of mortality (OR = 1.0).
In conclusion, patients with schizophrenia have been found to be at an increased risk of severe COVID-19, even after adjusting for sociodemographic factors and medical conditions, whereas the results for other groups of mental disorders are inconclusive. Further, the results from previous studies have generally shown that risks are reduced when adjusting for the included covariates, but there is still limited evidence of what categories of covariates that explain the associations [18].
1.2. The Swedish strategy
During the COVID-19 pandemic, different countries have applied different strategies to combat the spread of the disease. In the first year of the pandemic, Sweden applied a less invasive strategy compared to many other countries, with no general lockdown and open restaurants, kindergartens, schools, and other public spaces [23]. Given the high number of COVID-19 related deaths compared to neighboring countries, this strategy received critique [24]. During the first year of the pandemic in Sweden, Stockholm Region was most severely affected [23]. At a later stage, at the turn of the year 2020/2021, Sweden introduced additional measures to combat the spread such as recommending face masks on public transport, further limits on gatherings, restrictions on open hours for bars/restaurants etc [24]. Given the different strategies to combat the spread of the disease, and the different rates if COVID-19 related deaths between countries, country-specific evaluations of the effect of mental illness on COVID outcomes is of much interest.
1.3. The current study
In a prospective register-based cohort study of the total population in Stockholm Region, covering 1/5th of the Swedish population, we aimed to examine the risk of severe COVID-19 as indicated by hospitalization with COVID-19, intensive care with COVID-19, and death in COVID-19 for individuals diagnosed with a mental disorder between January 1st 2019 - February 29th 2020, compared with individuals with no mental disorder recorded during this time period.
We additionally aimed to explore risk differences between three subgroups; SMD, substance use disorders (SUD), and common mental disorders (CMD) compared to those with no mental disorder. Finally, we aimed to explore whether associations were explained by (a) sociodemographic/economic factors, (b) indicators of higher exposure to SARS-CoV-2 (i.e., the amount of outpatient care contacts, home-visits, and work-related exposure), (c) co-existing medical conditions established to increase the risk of more severe COVID-19 (i.e., cardiac disease, diabetes, obesity, chronic kidney disease, chronic obstructive pulmonary disease, and cancer), and (d) psychopharmacological treatment (i.e., atypical antipsychotic drugs and benzodiazepines).
Delineating the risk and exploring the association between a mental disorder and severe COVID-19 infection is crucial in both the acute phase of the current and future pandemics. The results will inform decision makers and care providers on how to organize care and vaccine administration in times of pandemics.
2. Methods
2.1. Study cohort and data
The study was based on the regional health care database in Stockholm Region (the VAL-databases) [25] which includes data on all out- and inpatient care with diagnosis according to WHO's International Classification of Disease [ICD] version 10 (ICD-10) and data from record linkages with socioeconomic and demographic factors from national registers held by Statistics Sweden [26]. The study cohort comprised all residents aged 25 and older in Stockholm Region by March 1st 2020. After excluding 165,881 (10%) with missing data on any of the included variables, 1,516,270 participants remained for analyses. Details about the variables and data sources included in the study are shown in Supplementary Table S1. Ethical approval was obtained by the Swedish Ethical Review Authority (original approval: Dnr 2020–03122, and update: Dnr 2021–00810).
2.2. Exposure
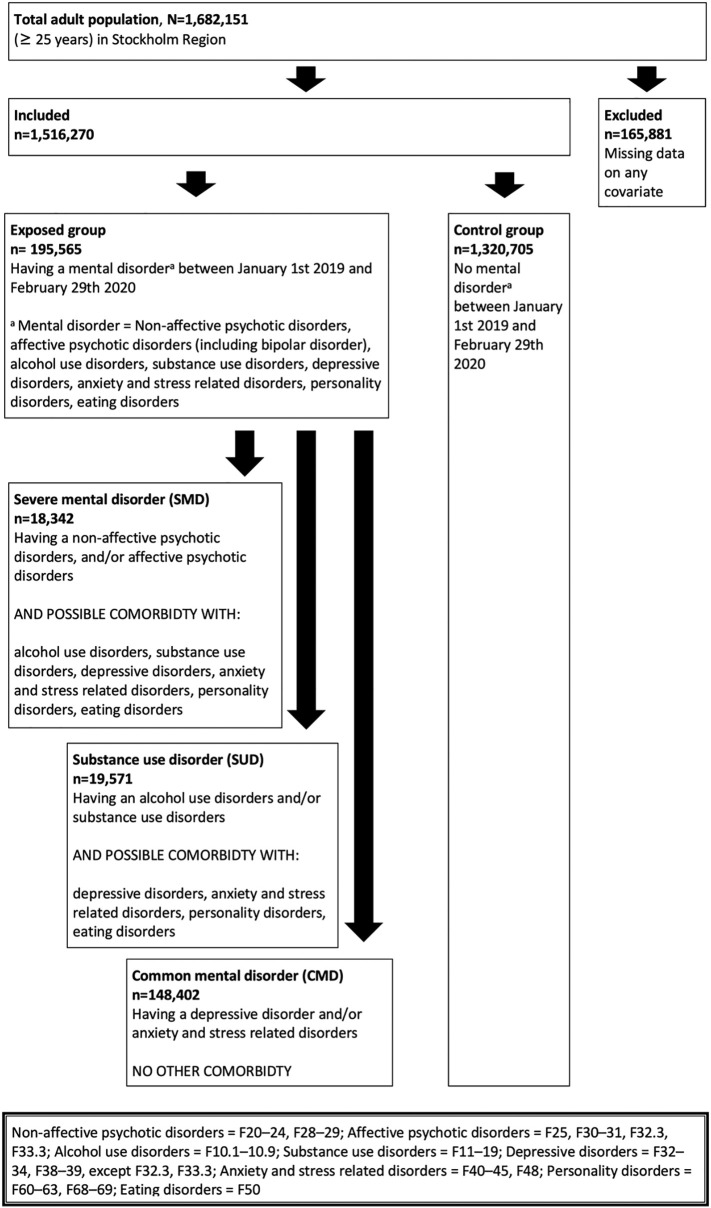
Patients with at least one healthcare contact in in- or outpatient care (including primary care) between January 1st 2019 and February 29th 2020, with any of the following diagnoses, were included in the exposed group: non-affective psychotic disorders (ICD-10: F20–24, F28–29); affective psychotic disorders (F25, F30–31, F32.3, F33.3); alcohol use disorders (F10.1–10.9, but not F10.0 [acute intoxication]); substance use disorders (F11–19); depressive disorders (F32–34, F38–39, except F32.3, F33.3); anxiety and stress related disorders (F40–45, F48); personality disorders (F60–63, F68–69); eating disorders (F50). Diagnoses were additionally subdivided into three subgroups, and participants were assigned a subgroup in a hierarchical order: SMD, SUD and CMD. See Fig. 1 for a flowchart aimed at graphically illustrate the inclusion and exclusion process and the division into subgroups. SMD included individuals with non-affective and affective psychotic disorders including bipolar disorder (and possible comorbidity with other mental disorders) [27]. SUD included individuals with alcohol- and/or substance use disorders (and possible comorbidity with other mental disorders, but not non-affective and affective psychotic disorders). Finally, CMD included individuals with depressive, anxiety- and stress-related disorders (and no other comorbidities) [28]. In addition, we explored the risk of severe COVID-19 in a subgroup of those with SMD, specifically those with SMD and comorbid alcohol and/or substance use disorders (n = 2076).
Fig. 1.
Flow-chart illustrating inclusion, exclusion and categorization into SMD, SUD, and CMD categories.
2.3. Outcomes
The following three COVID-19 outcomes were included: inpatient care with diagnosed COVID-19 (ICD-10 U07.1–07.2), intensive care unit (ICU) care with diagnosed COVID-19, and death due to COVID-19.
2.4. Covariates
The covariates were selected on the basis of their documented or likely relationship with mental disorders and severe COVID-19 [4,29,30]. Since several of the covariates may precede or follow from mental disorders, e.g., socioeconomic factors and somatic disorders [29,30], we regard them as explanatory factors without clear-cut differences between confounders and mediators. The covariates were grouped into the following five categories representing (1) basic covariates (sex, living in a care home for elderly people), (2) socioeconomic factors (country of birth, educational attainment, income, living in a socioeconomically vulnerable area, percentage residents in the residential area with COVID-19 requiring inpatient care, household size, crowded housing), (3) indicators of exposure to SARS-CoV-2 (a job exposure measure based on possibility to work from home, [4] number of physical healthcare visits during the study period (outside home and in the home, respectively), (4) somatic disorders (obesity, cardiac disease, cancer, diabetes, chronic pulmonary disease, chronic kidney disease), and (5) psychotropic medication (benzodiazepines, and atypical neuroleptics). All covariates were measured before the study baseline except the number of healthcare contacts, which were measured from baseline and up to five days before end of follow-up (censoring or event), to capture healthcare-related exposure to COVID-19. Details including categorization procedures, data sources, ICD-10 and ATC codes are listed in Supplementary Table S1. All covariates and categories are shown in Table 1 , and their associations to the outcomes are presented in Supplementary Table S2.
Table 1.
The distribution of covariates, divided by mental disorder or not before the study period.
| Mental disorder |
No mental disorder |
Cohen's ha | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Sex (% men) | 68,793 | 35 | 670,330 | 51 | −0.32 |
| Age group | |||||
| 18–49 | 108,285 | 55 | 654,894 | 50 | |
| 50–69 | 60,861 | 31 | 427,259 | 32 | |
| 70+ | 26,419 | 14 | 238,552 | 18 | |
| Living in care home | 3484 | 2 | 12,946 | 1 | |
| Country of birth | |||||
| Sweden | 148,426 | 76 | 947,430 | 72 | |
| Other Nordic countries | 6320 | 3 | 44,959 | 3 | |
| Middle East | 11,260 | 6 | 72,233 | 5 | |
| South Europe | 5807 | 3 | 45,961 | 3 | |
| North and East Africa | 3374 | 2 | 36,078 | 3 | |
| Other OECD countries | 3813 | 2 | 31,627 | 2 | |
| Other countries | 16,565 | 8 | 142,417 | 11 | |
| Education | |||||
| ≤9 yrs | 27,256 | 14 | 162,641 | 12 | |
| 10–12 yrs | 75,832 | 39 | 484,313 | 37 | |
| >12 yrs | 92,477 | 47 | 673,751 | 51 | |
| Income, quintiles | |||||
| Lowest | 48,861 | 25 | 219,304 | 17 | 0.20 |
| 2nd | 45,968 | 24 | 254,411 | 19 | |
| 3rd | 40,088 | 20 | 265,103 | 20 | |
| 4th | 34,351 | 18 | 281,803 | 21 | |
| Highest | 26,297 | 13 | 300,084 | 23 | −0.26 |
| Socioeconomically vulnerable area | 16,882 | 9 | 102,562 | 8 | |
| Proportion with inpatient COVID-19 diagnosis in area, quintiles | |||||
| Lowest | 36,665 | 19 | 260,276 | 20 | |
| 2nd | 37,251 | 19 | 266,258 | 20 | |
| 3rd | 44,022 | 23 | 281,952 | 21 | |
| 4th | 39,296 | 20 | 258,752 | 20 | |
| Highest | 38,331 | 20 | 253,467 | 19 | |
| Household size | |||||
| 1 | 60,853 | 31 | 285,839 | 22 | 0.20 |
| 2 | 56,359 | 29 | 425,768 | 32 | |
| 3 | 31,592 | 16 | 216,016 | 16 | |
| 4 | 28,663 | 15 | 233,734 | 18 | |
| 5 | 11,287 | 6 | 93,989 | 7 | |
| 6+ | 6811 | 3 | 65,359 | 5 | |
| Crowded housing | 26,116 | 13 | 196,543 | 15 | |
| Job exposure | |||||
| No occupation | 71,761 | 37 | 434,540 | 33 | |
| Work from home >50% | 58,954 | 30 | 461,132 | 35 | |
| Work from home <50% | 47,393 | 24 | 331,063 | 25 | |
| Work from home <50%, healthcare | 17,457 | 9 | 93,970 | 7 | |
| Healthcare visits, outside home | |||||
| 0 | 32,818 | 17 | 545,827 | 41 | −0.54 |
| 1–5 | 78,314 | 40 | 532,510 | 40 | |
| >5 | 84,433 | 43 | 242,368 | 18 | 0.55 |
| Healthcare visits, in home | |||||
| 0 | 179,614 | 92 | 1,263,218 | 96 | |
| 1–5 | 8627 | 4 | 32,848 | 2 | |
| >5 | 7324 | 4 | 24,639 | 2 | |
| Somatic disorders | |||||
| Obesity | 13,409 | 7 | 43,065 | 3 | |
| CVD | 9390 | 5 | 57,682 | 4 | |
| Cancer | 10,531 | 5 | 73,819 | 6 | |
| Diabetes | 15,217 | 8 | 87,031 | 7 | |
| COPD | 7248 | 4 | 25,863 | 2 | |
| Kidney | 5081 | 3 | 28,732 | 2 | |
| Psychotropics | |||||
| Benzodiazepines | 50,286 | 26 | 91,000 | 7 | 0.53 |
| Atypical neuroleptics | 17,776 | 9 | 6046 | 0,5 | 0.47 |
Cohen's h = Standardized difference of proportions. Cohen's h > 0.20 indicates small effect size, >0.50 moderate effect size, >0.80 large effect size (Cohen, 1988).
Cohen's h < 0.20 were omitted from the table.
2.5. Statistical analyses
The associations between exposure and outcomes were analyzed using Cox proportional hazard regressions with age as the underlying timescale. The use of attained age as the underlying time scale is the most adequate choice in epidemiological cohorts when age is strongly associated to the outcome [31]. When using age as the underlying time scale, age is inherently controlled for in the analyses [32]. Participants were followed from March 1st 2020 to either the date on an outcome event, moving from the Stockholm Region, death due to any cause, or January 14th 2021, whichever occurred first. The proportional hazard assumption was tested using Schoenfeld residuals and no major violations were found. Cox-regression models were explored with a cumulative number of covariates, by the categories of covariates described above to investigate their explanatory effect on the estimates.
We performed two sensitivity analyses. First, we excluded all participants who lived in a care home for elderly people during the study period, because risk factors for the outcomes in these facilities may differ from other settings [4]. Second, patients with less severe COVID-19 may have been diagnosed with COVID-19 in inpatient care because they were in the hospital for other reasons (e.g., mental inpatient care). To check for a possible effect of misclassification, we repeated the analysis of association between our exposure and the outcome of inpatient care for COVID-19 only in the following clinics: infection, lung disorder, geriatric, internal medicine, and emergency clinics.
2.6. Availability of data and materials
Due to Swedish legal restrictions and the current ethical approval for the study, data are not publicly available to share.
3. Results
From March 1st 2020 to January 14th 2021, 11,897 (78 out of 10,000) of the cohort had been treated in inpatient care and 1282 (8 out of 10,000) in ICU care with COVID-19; and 2996 (20 out of 10,000) had died from COVID-19. Some of the covariates that were associated with the COVID-19 outcomes were more common among people with a mental disorder, such as low income (25% vs. 17%), high number (>5) of healthcare visits (43% vs. 18%), medication with benzodiazepines (26% vs. 7%) and atypical neuroleptics (9% vs. 0.5%) (See Table 1). On the other hand, male sex, which is associated with inpatient care and mortality from COVID-19, were less prevalent in the group of people with mental disorders (35% vs. 51%). Differences between groups on the distribution of covariates were more substantial when specifically comparing SMD and SUD with no mental disorders (see Supplementary Table S3).
People with a mental disorder had increased risks, as indicated by HRs, for inpatient and ICU care with COVID-19, and mortality from COVID-19 (Table 2 ). In models with adjustment for sex and living in care homes for elderly, the HRs (and 95% CIs) were 1.5 (1.5–1.6) for inpatient care, 1.5 (1.3–1.8) for ICU care, and 1.4 (1.2–1.5) for mortality from COVID-19. Adjusting for other covariates had limited attenuating effects on the association. This was most notable for the covariates job exposure and healthcare contacts for the outcome inpatient care, and psychotropic medication for the outcome ICU care. However, people with mental disorders had marked increased risks for all three COVID-19 outcomes in the fully adjusted models.
Table 2.
Associations between having a psychiatric disorder and inpatient and ICU care with covid-19 and mortality from covid-19 in the full study population and stratified by sex and age group.
| Inpatient care | ICU | Mortality | |||||||
|---|---|---|---|---|---|---|---|---|---|
| a) Main analysis | 11,897 cases | 1281 cases | 2996 cases | ||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | ||||
| Model 1 | 1.5 | 1.5 | 1.6 | 1.5 | 1.3 | 1.8 | 1.4 | 1.2 | 1.5 |
| Model 2 | 1.5 | 1.4 | 1.6 | 1.5 | 1.3 | 1.7 | 1.3 | 1.2 | 1.5 |
| Model 3 | 1.4 | 1.3 | 1.5 | 1.6 | 1.3 | 1.8 | 1.3 | 1.2 | 1.5 |
| Model 4 | 1.4 | 1.3 | 1.4 | 1.5 | 1.3 | 1.8 | 1.3 | 1.2 | 1.4 |
| Model 5 | 1.3 | 1.3 | 1.4 | 1.4 | 1.1 | 1.6 | 1.2 | 1.1 | 1.4 |
| b) Stratified by sex | |||||||||
| Women | 5223 cases | 363 cases | 1380 cases | ||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | ||||
| Model 1 | 1.5 | 1.4 | 1.6 | 1.6 | 1.3 | 2.1 | 1.3 | 1.1 | 1.5 |
| Model 2 | 1.5 | 1.4 | 1.6 | 1.6 | 1.2 | 2.0 | 1.3 | 1.1 | 1.4 |
| Model 3 | 1.4 | 1.3 | 1.5 | 1.5 | 1.1 | 1.9 | 1.3 | 1.1 | 1.5 |
| Model 4 | 1.3 | 1.2 | 1.4 | 1.4 | 1.1 | 1.9 | 1.2 | 1.1 | 1.4 |
| Model 5 | 1.3 | 1.2 | 1.4 | 1.3 | 1.0 | 1.8 | 1.2 | 1.0 | 1.4 |
| Men | 6678 cases | 918 cases | 1616 cases | ||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | ||||
| Model 1 | 1.5 | 1.4 | 1.6 | 1.5 | 1.2 | 1.8 | 1.5 | 1.3 | 1.7 |
| Model 2 | 1.5 | 1.4 | 1.6 | 1.4 | 1.2 | 1.7 | 1.4 | 1.2 | 1.6 |
| Model 3 | 1.4 | 1.3 | 1.5 | 1.5 | 1.3 | 1.9 | 1.4 | 1.2 | 1.6 |
| Model 4 | 1.4 | 1.3 | 1.5 | 1.5 | 1.2 | 1.8 | 1.4 | 1.2 | 1.6 |
| Model 5 | 1.3 | 1.2 | 1.5 | 1.3 | 1.1 | 1.7 | 1.3 | 1.1 | 1.5 |
| c) Stratified by age group | |||||||||
| 25–69 years | 6137 cases | 925 cases | 318 cases | ||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | ||||
| Model 1 | 1.5 | 1.4 | 1.6 | 1.6 | 1.4 | 1.9 | 2.0 | 1.5 | 2.6 |
| Model 2 | 1.4 | 1.4 | 1.6 | 1.6 | 1.3 | 1.9 | 1.7 | 1.3 | 2.2 |
| Model 3 | 1.4 | 1.3 | 1.5 | 1.7 | 1.4 | 2.0 | 1.6 | 1.2 | 2.2 |
| Model 4 | 1.3 | 1.3 | 1.4 | 1.6 | 1.3 | 1.9 | 1.5 | 1.1 | 2.0 |
| Model 5 | 1.3 | 1.2 | 1.4 | 1.4 | 1.1 | 1.7 | 1.4 | 1.0 | 1.9 |
| 70+ years | 5760 cases | 356 cases | 2678 cases | ||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | ||||
| Model 1 | 1.6 | 1.5 | 1.7 | 1.3 | 0.9 | 1.8 | 1.3 | 1.2 | 1.4 |
| Model 2 | 1.5 | 1.4 | 1.7 | 1.2 | 0.9 | 1.7 | 1.3 | 1.1 | 1.4 |
| Model 3 | 1.3 | 1.2 | 1.4 | 1.2 | 0.8 | 1.7 | 1.3 | 1.1 | 1.4 |
| Model 4 | 1.3 | 1.2 | 1.4 | 1.2 | 0.8 | 1.7 | 1.2 | 1.1 | 1.4 |
| Model 5 | 1.3 | 1.2 | 1.4 | 1.2 | 0.8 | 1.7 | 1.2 | 1.1 | 1.3 |
Statistical models were performed with an increasing number of covariates. Adjustments in Model 1: sex, living in care home; Model 2: model 1 plus country of birth, vulnerable area, covid-19 inpatients in area, education, income, household size, crowded housing; Model 3: model 2 plus job exposure, healthcare contacts; Model 4: model 3 plus somatic disorders; Model 5: model 4 plus psychotropics.
The results were quite similar between women and men with a tendency towards higher HRs for COVID-19 mortality in men with a mental disorder than for their female counterparts (Table 2). In stratified analyses with people aged 25–69 and people aged 70 or older, the results differed more markedly between age groups. Most notably, in the younger age group, people with a mental disorder had a higher HR for mortality from COVID-19, compared with their counterparts in the older age group (2.0 [1.5–2.6] and 1.3 [1.2–1.4], respectively formodel 1). Adjusting for the additional covariates had a stronger attenuating effect on the association in the younger age group, and the HRs were quite similar for the two age groups in the fully adjusted models (1.4 [1.0–1.9] and 1.2 [1.1–1.3], respectively), indicating that other risk factors explained a greater part of the higher risk of COVID-19 mortality in people with mental disorders in the younger age group. However, adjustment for the COVID-19 exposure indicators (model 3) had a notable attenuating impact on the association between mental disorder and inpatient care with COVID-19 in the older age group (Table 2).
Among the diagnostic groups of mental disorders (SMD, SUD and CMD), individuals with CMD had the lowest risk increase for all the outcomes as compared with controls (Table 3 ). Individuals with SMD had a clearly increased risk for mortality from COVID-19 (1.9 [1.5–2.4]), where those with SMD and comorbid alcohol/substance use disorder showed particularly high risk increases compared to controls (5.1 [2.6–9.8]). Adjustments for the covariates attenuated the associations with inpatient care and mortality from COVID-19 in people with SMD and SUD, while adjustments seemed to have less impact for people with only CMD. However, statistical power was low in some of these analyses which limits the conclusions to be drawn.
Table 3.
Associations between having a psychiatric disorder and inpatient and ICU care with covid-19 and mortality from covid-19, by subgroup of psychiatric disorders.
| Inpatient care |
SMD |
SUD |
CMD |
SMD+ |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10,201 cases |
10,259 cases |
11,562 cases |
9997 cases |
|||||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |||||
| Model 1 | 1.8 | 1.6 | 2.1 | 1.7 | 1.5 | 1.9 | 1.5 | 1.4 | 1.5 | 3.0 | 2.2 | 4.2 |
| Model 2 | 1.7 | 1.5 | 1.9 | 1.6 | 1.4 | 1.8 | 1.4 | 1.4 | 1.5 | 2.8 | 2.0 | 3.9 |
| Model 3 | 1.5 | 1.3 | 1.7 | 1.4 | 1.2 | 1.6 | 1.4 | 1.3 | 1.4 | 2.4 | 1.7 | 3.4 |
| Model 4 | 1.5 | 1.3 | 1.7 | 1.3 | 1.1 | 1.5 | 1.3 | 1.3 | 1.4 | 2.3 | 1.6 | 3.2 |
| Model 5 | 1.4 | 1.2 | 1.7 | 1.3 | 1.1 | 1.4 | 1.3 | 1.2 | 1.4 | 2.3 | 1.6 | 3.2 |
| ICU |
SMD |
SUD |
CMD |
SMD+ |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1116 cases |
1135 cases |
1245 cases |
1089 cases |
|||||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |||||
| Model 1 | 1.8 | 1.3 | 2.7 | 1.8 | 1.3 | 2.5 | 1.4 | 1.2 | 1.7 | 4,8 | 2.4 | 9.7 |
| Model 2 | 1.7 | 1.1 | 2.5 | 1.8 | 1.3 | 2.5 | 1.4 | 1.1 | 1.7 | 4.6 | 2.3 | 9.3 |
| Model 3 | 1.8 | 1.2 | 2.6 | 1.8 | 1.3 | 2.5 | 1.5 | 1.2 | 1.8 | 4.9 | 2.4 | 10.0 |
| Model 4 | 1.7 | 1.2 | 2.6 | 1.8 | 1.3 | 2.4 | 1.4 | 1.2 | 1.7 | 4.9 | 2.4 | 10.0 |
| Model 5 | 1.5 | 0.9 | 2.4 | 1.7 | 1.2 | 2.4 | 1.3 | 1.1 | 1.6 | 4.3 | 1.9 | 9.6 |
| Mortality |
SMD |
SUD |
CMD |
SMD+ |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2564 cases |
2560 cases |
2881 cases |
2492 cases |
|||||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |||||
| Model 1 | 1.9 | 1.5 | 2.4 | 1.6 | 1.3 | 2.0 | 1.2 | 1.1 | 1.4 | 5.1 | 2.6 | 9.8 |
| Model 2 | 1.7 | 1.4 | 2.1 | 1.5 | 1.2 | 1.9 | 1.2 | 1.1 | 1.4 | 4.4 | 2.3 | 8.5 |
| Model 3 | 1.7 | 1.3 | 2.1 | 1.4 | 1.1 | 1.8 | 1.2 | 1.1 | 1.4 | 4.7 | 2.4 | 9.0 |
| Model 4 | 1.7 | 1.4 | 2.2 | 1.3 | 1.0 | 1.6 | 1.2 | 1.1 | 1.3 | 3.3 | 1.7 | 6.4 |
| Model 5 | 1.5 | 1.2 | 1.9 | 1.3 | 1.0 | 1.6 | 1.2 | 1.0 | 1.3 | 2.9 | 1.5 | 5.7 |
Severe mental disorders (SMD), substance use disorders (SUD), common mental disorders (CMD), and Severe mental disorders with comorbid alcohol/substance use (SMD+). Statistical models were performed with an increasing number of covariates. Adjustments in Model 1: sex, living in care home; Model 2: model 1 plus country of birth, vulnerable area, covid-19 inpatients in area, education, income, household size, crowded housing; Model 3: model 2 plus job exposure, healthcare contacts; Model 4: model 3 plus somatic disorders; Model 5: model 4 plus psychotropics.
In the sensitivity analyses, excluding people living in care home (N = 16,430) or analyzing only COVID-19 in selected clinics as inpatient cases (9393 cases) yielded similar results as the main analyses (Supplementary Table S4).
4. Discussion
In line with previous studies, we found that being affected with a mental disorder during a COVID-19 outbreak predisposes to inpatient care or intensive care unit (ICU) care with COVID-19, and death due to COVID-19.
Comparing the risk increases in our study to the estimations in previous studies is challenging, as the studies to some extent differ in regard to population, outcome, covariates and analysis. With that said, in comparison to previous studies exploring increased risk of severe COVID-19 outcomes in patients with schizophrenia and bipolar disorder, [[19], [20], [21]] the risk increases for patients with SMD in our study was smaller. For example, the fully adjusted HRs in the Danish study [21] was 2.8 and 2.3 for schizophrenia and bipolar disorder respectively, compared to our result of the SMD group (including both schizophrenia and bipolar disorder) where the age, sex and living in care home corrected HR (model 1) was 1.9 and the fully adjusted HR 1.5. Given the differences between studies, and other differences in healthcare and, COVID-19 strategies between countries, it is hard to conclude why these risk increases differ.
An interesting result in the current study, was that individuals with CMDs and no other comorbid mental disorder had an increased risk of severe COVID-19 including death. This was unpredicted, as the group also comprised individuals with a diagnosis recorded within primary care, and as previous studies did not find any increased risk for anxiety disorders [19] or other disorders than schizophrenia, bipolar disorder, and major depression [21]. However, the increased risk for the CMD groups was moderate (HR = 1.2–1.5 between outcomes) and may to some extent be driven by individuals with severe depression. Future studies of the CMD group may explore these increased risks in further analyses.
During the study period (March 1st 2020 to January 14th 2021) Sweden applied a less strict approach to combat the spread of the disease. However, the present study does not show any evidence of a worse trajectory for those with mental disorders in comparison to the results found in other countries. Irrespective, individuals with mental disorders, especially those with very severe illness (i.e., SMD and comorbid alcohol/substance use disorders) clearly have increased risk for severe COVID-19, even when adjusting for a range of covariates. This result is especially important as it seems that those with SMD also may be under-vaccinated for COVID-19 [33].
The Cox models showed that different categories of covariates had different effects on the association depending on the outcome and on sex, age or psychiatric diagnosis of the participants.
Regarding the examination of covariates, we found that sociodemographic/ economic factors seemed to partly explain, the increased risk of death due to COVID-19 for the younger age group and those with SMDs (all ages). It is worth noting that the association between mental disorders and socioeconomic variables have been found to differ between regions, which may limit the generalizability of this result. For example, in a meta-analysis on the association between socioeconomic factors and major depression, a stronger association was found in North American studies compared to European studies [34]. Exposure to SARS-CoV-2 may contribute to the increased risk of being admitted to inpatient care, especially in women, the elderly (70+) and those with SMD and SUD. The variables (or levels of a variable) that increased hospitalization risk were number of home visits, having no employment or having employment where it was difficult to work from home. However, we cannot conclude that this increased risk of hospitalization is solely explained by increased exposure to SARS-CoV-2 during home visits when it may also be due to reversed causality, i.e., the frailty of the patient. Somatic comorbidities seemed to explain part of the increased risk of death due to COVID-19 for the younger age group, for those with SUD, and those with SMD and a comorbid alcohol/substance use disorder. Interestingly, somatic comorbidities did not appear to explain the increased risk of death due to COVID-19 for the whole SMD group, although it is well known that this population has an increased risk of somatic conditions such as, but not limited to, heart disease, obesity and COPD [9]. A possible explanation to this unexpected result is that individuals with SMDs may be under-diagnosed regarding somatic conditions [35]. It is also possible that the previous adjustment variables, such as the socioeconomic factors, capture some of the differences in somatic conditions. Medication with benzodiazepines and atypical neuroleptics seemed to explain some of the variance in the increased risk of admission to ICU care in people with mental disorders, especially in the younger age group. These medications also seemed to explain some of the variance in risk of ICU admission and death among people with SMD. Future studies may further explore this association to assess if there is any causal effect of psychotropic mediation on severe COVID-19 outcomes.
A main result of the current study is that the higher risk for severe COVID-19 infection to a large extent seems to be explained by factors other than those we examined in this study. Recently published studies have highlighted the concept of frailty as an important factor in explaining the risk of severe COVID-19 [36]. This usage of frailty refers to a condition characterized by reduced strength, endurance and physiological function that increases the individual's vulnerability to serious illness or death. Such frailty increases naturally with age, but can also be found in younger people, and is possibly a better predictor than age per se for severe COVID-19 [36]. Notably, frailty has been shown in several studies to be common in individuals with mental disorders [37,38]. In people with SMD, a high risk remained even after adjustments, indicating an additional frailty in individuals with SMD. This is in line with previous studies showing that frailty among those with SMD may give rise to an increased vulnerability to similar infections as COVID-19 (i.e., influenza and pneumonia) [14]. Researchers have suggested that patients with serious mental disorders, particularly schizophrenia, may not receive adequate care, which may explain some of the exacerbated mortality [1]. Further, patients and relatives may not identify early signs of COVID-19, and patients may not receive appropriate assessment and treatment due to stigma and discrimination associated with serious mental illness [39].
4.1. Limitations
The VAL databases provide full coverage of diagnosed mental disorders in both primary and secondary care in the region. The linked registers provide reliable information on several potential explanatory factors with minimal selection but have their limitations. First, we had no information on other potentially important factors such as health behaviors or adherence to restrictions to mitigate disease transmission. Nor did we have any information on COVID-19 infections that did not require healthcare, which prevents us from distinguishing between the risk of being infected and the risk of developing more severe symptoms. The lack of information on risk of COVID-19 infection means limited possibilities to draw conclusions on whether the increased risk of severe COVID-19 for individuals with mental disorders is due to increased infection rates or a frailty of the population (or a combination of both). Although we evaluated some indicators of exposure to the virus, these indicators only comprise a small fraction of possible factors that may increase infection rates. Second, we included several medical conditions that have been explored as confounders in similar studies [[19], [20], [21], [22]]. However, there are other medical conditions such as dementia and other neurological conditions, HIV and other immunocompromised conditions, cerebrovascular disease, pregnancy, and smoking that also have been shown to be associated to COVID-19, which was not included and constitute a limitation to the current study. Third, diagnoses of obesity are heavily underreported (unpublished observation), and consequently, the confounding effect of obesity on the association between mental illness and severe COVID-19 may be biased. More broadly, validation studies of other medical conditions in Swedish registers have generally shown a high agreement between recorded diagnoses and established diagnostic criteria, for example for cardiovascular diseases, autoimmune/immune-mediated diseases, and psychiatric diseases [40]. Fourth, we included only mental disorders diagnosed before the study period to minimize reverse causality, and psychiatric status may have changed over time. Finally, this study is based on the Stockholm Region in Sweden and cannot without caution be generalized to other settings and countries, especially considering differences in the impacts of the disease spread and nations' responses but also in access to healthcare which is subsidized in Sweden.
Disclosures
None.
CRediT authorship contribution statement
A. Sörberg Wallin: Conceptualization, Methodology, Software, Formal analysis, Writing – original draft. A. Ohlis: Methodology, Writing – review & editing. C. Dalman: Conceptualization, Methodology, Resources, Writing – review & editing. J. Ahlen: Conceptualization, Methodology, Writing – original draft, Visualization, Supervision, Project administration.
Acknowledgments
The study was funded by the Center for Epidemiology and Community medicine, within the ALF-agreement, Health Care Services Stockholm County.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.genhosppsych.2022.02.004.
Appendix A. Supplementary data
Supplementary material
Data availability
The authors do not have permission to share data.
References
- 1.Kozloff N., Mulsant B.H., Stergiopoulos V., Voineskos A.N. The COVID-19 global pandemic: implications for people with schizophrenia and related disorders. Schizophr Bull. 2020;46(4):752–757. doi: 10.1093/schbul/sbaa051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cullen W., Gulati G., Kelly B.D. Mental health in the COVID-19 pandemic. QJM. 2020;113(5):311–312. doi: 10.1093/qjmed/hcaa110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kivimäki M., Batty G.D., Pentti J., et al. Association between socioeconomic status and the development of mental and physical health conditions in adulthood: a multi-cohort study. Lancet Public Health. 2020;5(3):e140–e149. doi: 10.1016/S2468-2667(19)30248-8. [DOI] [PubMed] [Google Scholar]
- 4.Bartelink V., Tynelius P., Walander A., et al. 2020. Socioekonomiska Faktorer Och Covid-19 i Stockholms Län. [Google Scholar]
- 5.Sporinova B., Manns B., Tonelli M., et al. Association of mental health disorders with health care utilization and costs among adults with chronic disease. JAMA Netw Open. 2019;2(8) doi: 10.1001/jamanetworkopen.2019.9910. e199910-e199910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shinn A.K., Viron M. Perspectives on the COVID-19 pandemic and individuals with serious mental illness. J Clin Psychiatry. 2020;81(3):0. doi: 10.4088/JCP.20com13412. [DOI] [PubMed] [Google Scholar]
- 7.Stuart H. Mental illness and employment discrimination. Curr Opin Psychiatry. 2006;19(5) doi: 10.1097/01.yco.0000238482.27270.5d. [DOI] [PubMed] [Google Scholar]
- 8.Casey D.E. Metabolic issues and cardiovascular disease in patients with psychiatric disorders. Am J Med. 2005;118(Suppl):15S–22S. doi: 10.1016/j.amjmed.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 9.De Hert M., Correll C.U., Bobes J., et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52–77. doi: 10.1002/j.2051-5545.2011.tb00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novak P., Sanmartin M.X., Ali M.M., Chen J. Health conditions associated with severe illness from COVID-19 among individuals with serious mental illness. Psychiatr Serv. 2020 doi: 10.1176/appi.ps.202000300. Published online. appips202000300. [DOI] [PubMed] [Google Scholar]
- 11.Roy-Byrne P.P., Davidson K.W., Kessler R.C., et al. Anxiety disorders and comorbid medical illness. Gen Hosp Psychiatry. 2008;30(3):208–225. doi: 10.1016/j.genhosppsych.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Gold S.M., Köhler-Forsberg O., Moss-Morris R., et al. Comorbid depression in medical diseases. Nat Rev Dis Primers. 2020;6(1):69. doi: 10.1038/s41572-020-0200-2. [DOI] [PubMed] [Google Scholar]
- 13.Mongan D., Cannon M., Cotter D.R. COVID-19, hypercoagulation and what it could mean for patients with psychotic disorders. Brain Behav Immun. 2020;88:9–10. doi: 10.1016/j.bbi.2020.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olfson M., Gerhard T., Huang C., Crystal S., Stroup T.S. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiat. 2015;72(12):1172–1181. doi: 10.1001/jamapsychiatry.2015.1737. [DOI] [PubMed] [Google Scholar]
- 15.Govind R., Fonseca de Freitas D., Pritchard M., Hayes R.D., JH MacCabe. Clozapine treatment and risk of COVID-19 infection: retrospective cohort study. Br J Psychiatry. 2020:1–7. doi: 10.1192/bjp.2020.151. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.May M., Slitzky M., Rostama B., Barlow D., Houseknecht K.L. Antipsychotic-induced immune dysfunction: a consideration for COVID-19 risk. Brain Behav Immun Heal. 2020;6 doi: 10.1016/j.bbih.2020.100097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fond G., Nemani K., Etchecopar-Etchart D., et al. Association between mental health disorders and mortality among patients with COVID-19 in 7 countries: a systematic review and meta-analysis. JAMA Psychiat. 2021 doi: 10.1001/jamapsychiatry.2021.2274. Published online July 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vai B., Mazza M.G., Delli Colli C., et al. Mental disorders and risk of COVID-19-related mortality, hospitalisation, and intensive care unit admission: a systematic review and meta-analysis. Lancet Psychiatry. 2021;8(9):797–812. doi: 10.1016/S2215-0366(21)00232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nemani K., Li C., Olfson M., et al. Association of psychiatric disorders with mortality among patients with COVID-19. JAMA Psychiat. 2021;78(4):380–386. doi: 10.1001/jamapsychiatry.2020.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tzur Bitan D., Krieger I., Kridin K., et al. COVID-19 prevalence and mortality among schizophrenia patients: a large-scale retrospective cohort study. Schizophr Bull. 2021;47(5):1211–1217. doi: 10.1093/schbul/sbab012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barcella C.A., Polcwiartek C., Mohr G.H., et al. Severe mental illness is associated with increased mortality and severe course of COVID-19. Acta Psychiatr Scand. 2021;144(1):82–91. doi: 10.1111/acps.13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baillargeon J., Polychronopoulou E., Kuo Y.-F., Raji M.A. The impact of substance use disorder on COVID-19 outcomes. Psychiatr Serv. 2020;72(5):578–581. doi: 10.1176/appi.ps.202000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ludvigsson J.F. The first eight months of Sweden’s COVID-19 strategy and the key actions and actors that were involved. Acta Paediatr. 2020;109(12):2459–2471. doi: 10.1111/apa.15582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Claeson M., Hanson S. COVID-19 and the Swedish enigma. Lancet (Lond, Engl) 2021;397(10271):259–261. doi: 10.1016/s0140-6736(20)32750-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stockholm Region . 2014. VAL Databaserna: Datalager För Uppföljning Av Vårdhändelser i SLL. [Google Scholar]
- 26.Statistics Sweden About statistics Sweden. 2021. https://www.scb.se/en/About-us/ Published.
- 27.Hardoon S., Hayes J.F., Blackburn R., et al. Recording of severe mental illness in United Kingdom primary care, 2000–2010. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0082365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Björkenstam E., Helgesson M., Norredam M., Sijbrandij M., de Montgomery C.J., Mittendorfer-Rutz E. Common mental disorders among young refugees in Sweden: the role of education and duration of residency. J Affect Disord. 2020;270:189–197. doi: 10.1016/j.jad.2020.02.039. [DOI] [PubMed] [Google Scholar]
- 29.Dohrenwend B.P., Levav I., Shrout P.E., et al. Socioeconomic status and psychiatric disorders: the causation-selection issue. Science. 1992;255(5047):946–952. doi: 10.1126/science.1546291. [DOI] [PubMed] [Google Scholar]
- 30.Firth J., Siddiqi N., Koyanagi A., et al. The lancet psychiatry commission: a blueprint for protecting physical health in people with mental illness. Lancet Psychiatry. 2019;6(8):675–712. doi: 10.1016/s2215-0366(19)30132-4. [DOI] [PubMed] [Google Scholar]
- 31.Thiébaut A.C.M., Bénichou J. Choice of time-scale in Cox’s model analysis of epidemiologic cohort data: a simulation study. Stat Med. 2004;23(24):3803–3820. doi: 10.1002/sim.2098. [DOI] [PubMed] [Google Scholar]
- 32.Griffin B.A., Anderson G.L., Shih R.A., Whitsel E.A. Use of alternative time scales in cox proportional hazard models: implications for time-varying environmental exposures. Stat Med. 2012;31(27):3320–3327. doi: 10.1002/sim.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tzur Bitan D. Patients with schizophrenia are under-vaccinated for COVID-19: a report from Israel. World Psychiatry. 2021;20(2):300–301. doi: 10.1002/wps.20874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lorant V., Deliège D., Eaton W., Robert A., Philippot P., Ansseau M. Socioeconomic inequalities in depression: a Meta-analysis. Am J Epidemiol. 2003;157(2):98–112. doi: 10.1093/aje/kwf182. [DOI] [PubMed] [Google Scholar]
- 35.Laursen T.M., Munk-Olsen T., Gasse C. Chronic somatic comorbidity and excess mortality due to natural causes in persons with schizophrenia or bipolar affective disorder. PLoS One. 2011;6(9):e24597. doi: 10.1371/journal.pone.0024597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hewitt J., Carter B., Vilches-Moraga A., et al. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health. 2020;5(8):e444–e451. doi: 10.1016/S2468-2667(20)30146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andrew M.K., Rockwood K. Psychiatric illness in relation to frailty in community-dwelling elderly people without dementia: a report from the Canadian study of health and aging. Can J Aging. 2007;26(1):33–38. doi: 10.3138/8774-758w-702q-2531. [DOI] [PubMed] [Google Scholar]
- 38.Dent E., Hoogendijk E.O. Psychosocial factors modify the association of frailty with adverse outcomes: a prospective study of hospitalised older people. BMC Geriatr. 2014;14(1):108. doi: 10.1186/1471-2318-14-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fonseca L., Diniz E., Mendonça G., Malinowski F., Mari J., Gadelha A. Schizophrenia and COVID-19: risks and recommendations. Rev Bras Psiquiatr. 2020;42(3):236–238. doi: 10.1590/1516-4446-2020-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ludvigsson J.F., Andersson E., Ekbom A., et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11(1):450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Due to Swedish legal restrictions and the current ethical approval for the study, data are not publicly available to share.
The authors do not have permission to share data.