Abstract
Emotion-driven impulse control difficulties are associated with negative psychological outcomes. Extant research suggests that high frequency heart rate variability (HF-HRV) may be indicative of emotion-driven impulse control difficulties and potentially moderated by negative emotion. In the current study, 248 eleven- to 14-year-olds and their parent engaged in a negatively emotionally arousing conflict task at Time 1. Adolescents’ HF-HRV and negative emotional expression and experience were assessed before, during, and/or after the task. Adolescents reported on their levels of emotion-driven impulse control difficulties at Time 1 and one year later. Results revealed that higher levels of HF-HRV reactivity (i.e., higher HF-HRV augmentation) predicted higher levels of emotion-driven impulse control difficulties one year later among adolescents who experienced higher negative emotion. These findings suggest that negative emotional context should be considered when examining HF-HRV reactivity as a risk factor for emotion-driven impulse control difficulties and associated outcomes.
Keywords: heart rate variability, respiratory sinus arrhythmia, impulse control, adolescent, emotion
Emotion-driven impulse control difficulties refer to adolescents acting impulsively (e.g., engaging in risk behaviors) in response to high levels of negative emotion. Emotion-driven impulse control difficulties are a transdiagnostic risk factor for psychopathology and health risk behaviors, such as substance use (Moore et al., 2017; Neumann et al., 2010; Watkins et al., 2015). This is particularly true in adolescence, when emotion reactivity is heightened and when impulse control and emotion regulation capacities are not fully developed (Chambers et al., 2003; Cole et al., 1994). As these psychopathologies/risk behaviors can contribute to premature death and disability and as impulse control and emotion regulation are still developing in adolescents (Whiteford et al., 2013), it is imperative that we understand what factors predict the development of emotion-driven impulse control difficulties during adolescence.
Heart rate variability (HRV) may be one predictor of the development of emotion-driven impulse control difficulties in adolescence. HRV is a measure of the variation in the time between consecutive heart beats (Acharya et al., 2007) and is thought to reflect parasympathetic and sympathetic activity of the autonomic nervous system (Malik et al., 1996). The heart is innervated by sympathetic and parasympathetic nerves, which increase and decrease, respectively, heart rate. These nerves are partially controlled by a group of brain regions (i.e., central autonomic network) that integrate sensory information (e.g., current autonomic functioning) and information about emotional arousal (e.g., appraisal of threat) to adjust autonomic responding (Thayer & Lane, 2000). Thus, HRV is a reflection of regulatory processes that are responding to environmental demands and may be related to levels of emotion-driven impulse control difficulties.
One metric of HRV is high-frequency HRV (HF-HRV). HF-HRV reflects parasympathetic activity on the heart. This is important because the parasympathetic system exerts a much quicker influence on the heart (e.g., .5 seconds) than the sympathetic system (e.g., 5 seconds), meaning that parasympathetic activity enables the heart to immediately respond to environmental demands (Thayer & Lane, 2000). It is therefore useful to specifically use HF-HRV to examine self-regulation on a moment-to-moment basis.
HF-HRV has been measured both at rest and in response to a challenge. At rest, HF-HRV reflects the capacity to regulate arousal (Appelhans & Luecken, 2006). Put another way, higher resting HF-HRV reflects higher capacity to self-regulate. In contrast, lower resting HF-HRV reflects lower capacity to self-regulate. Indeed, a large body of literature has linked higher resting HF-HRV to better emotion regulation and psychosocial functioning in children, adolescents, and adults (Beauchaine, 2015; Cui et al., 2015; Geisler et al., 2010; McLaughlin et al., 2014; Vazquez et al., 2016). One study found that higher resting HF-HRV was associated with reduced emotion-driven impulse control difficulties in young adults (Williams et al., 2015). However, work specifically examining associations between resting HF-HRV and emotion-driven impulse control difficulties in adolescents has been limited.
HF-HRV can also be measured in response to a challenge, which is referred to as HF-HRV reactivity. HF-HRV reactivity reflects the degree to which parasympathetic activity increases or decreases during a challenge state compared with a rest state. An increase in HF-HRV from rest to challenge (i.e., HF-HRV augmentation) indicates that there is increased parasympathetic activity during the challenge state compared with the resting state. This may not be adaptive during a challenge because increased parasympathetic activity would not as readily enable the mobilization of resources (e.g., increased heart rate) needed to face the challenge (Porges, 1995). In contrast, a decrease in HF-HRV from rest to challenge (i.e., HF-HRV suppression) indicates reduced parasympathetic activity during the challenge state compared with the resting state, which may be adaptive because more resources are mobilized to respond to the challenge (Porges, 1995; Sharpley & Gordon, 1999).
Consistent with these findings, most of the extant literature links a decrease in HF-HRV from rest to challenge (i.e., parasympathetic withdrawal) to lower levels of psychopathology symptoms and emotion regulation (e.g., El-Sheikh et al., 2011; Gentzler et al., 2009; Perry et al., 2011; James et al., 2017; Scott & Weems, 2014). This is the case in community and clinical samples. In a cross-sectional study, Gentzler and colleagues (2009) found that greater decreases in HF-HRV in response to negatively emotionally arousing film clips were related to better emotion regulation and reduced depressive symptoms among children and early adolescents. Another study found that children without a history of suicidal ideation exhibited greater decreases in HF-HRV during a parent conflict task compared to children with a history of suicidal ideation (James et al., 2017). Put another way, these studies have shown that increases in HF-HRV from rest to challenge are associated with reduced emotion regulation ability and increased psychopathology symptoms. Importantly, emotion-driven impulse control difficulties are implicated in externalizing and internalizing pathology (Neumann et al., 2010) and are considered a facet of emotion regulation, so it may be the case that decreases in HF-HRV from rest to challenge are also associated with reduced emotion-driven impulse control difficulties (or increases in HF-HRV with increased emotion-driven impulse control difficulties).
Notably, other studies have found the opposite—that increases in HF-HRV from rest to challenge are related to better psychosocial functioning or that decreases in HF-HRV from rest to challenge are related to worse psychosocial functioning (e.g., De Vries-Bouw et al., 2011; Hastings et al., 2008; Vasilev et al., 2009; Beauchaine et al., 2013; Pang and Beauchaine, 2012). Most of these studies have been conducted in clinical samples instead of community samples. In a study on male adolescents with a history of delinquent behavior, De Vries-Bouw and colleagues (2011) found that those with greater decreases in HF-HRV from rest to a public speaking task were more likely to reoffend within a 5-year follow up period. Moreover, Beauchaine and colleagues (2013) similarly found that greater decreases in HF-HRV from rest to a challenging block-building task were associated with worse emotion regulation and prosocial behavior in a sample of children with attention-deficit/hyperactivity disorder (ADHD). Some studies using community samples have found similar findings. One study found that children with greater increases in HF-HRV from rest to a social task had fewer internalizing and externalizing symptoms 6 to 12 months later (Hastings et al., 2008). Thus, it may also be the case that decreases in HF-HRV from rest to a challenge state, particularly in clinical samples, are associated with increased emotion-driven impulse control difficulties or that increases in HF-HRV from rest to challenge state are associated with reduced emotion-driven impulse control difficulties.
Overall, research on HF-HRV reactivity from rest to challenge and psychosocial outcomes is mixed, with slightly more research indicating that decreases in HF-HRV from rest to challenge are associated with decreased negative psychosocial outcomes. It may be that associations between HF-HRV reactivity and outcomes depend on how arousing the task is. Specifically, greater decreases in HF-HRV from rest to challenge may be adaptive in situations of high emotional arousal because greater parasympathetic withdrawal is needed to enable action (e.g., Gentzler et al., 2009; James et al., 2017). This would indicate that increases in HF-HRV from rest to challenge are not adaptive and may be related to negative psychosocial outcomes. On the contrary, increases in HF-HRV from rest to challenge may be adaptive in situations of low emotional arousal, because in these situations, it might be important to maintain high HF-HRV and engagement of the parasympathetic nervous system (e.g., Hastings et al., 2008). It is possible in this situation (i.e., low negative emotion) that decreases in HF-HRV from rest to challenge may be related to negative psychosocial outcomes. Therefore, it may be that the association between HF-HRV reactivity from rest to challenge and outcomes is moderated by how arousing the task is. Given that participants in a sample will have varying responses to a challenging task, one way to examine emotional arousal to a task would be to test an individual’s appraisal of the task and how emotionally arousing it is as a moderator (Thayer et al., 2012). To date, however, the potential moderating role of negative emotion on this relation has not been examined. Thus, the present study aimed to fill this gap in the literature by examining adolescents’ experienced and expressed negative emotional responses to a challenging task as a moderator of the association between HF-HRV changes from rest to challenge (i.e., HF-HRV reactivity) and a psychosocial outcome (i.e., emotion-driven impulse control difficulties). We specifically focused on emotion-driven impulse control difficulties because it is a transdiagnostic risk factor for psychopathology (Neumann et al., 2010), undergoes rapid development in adolescence (Chambers et al., 2003; Cole et al., 1994) and has not to our knowledge been specifically examined in previous studies.
The specific challenge employed in this study is a parent-adolescent conflict interaction task. This task has been widely used to elicit arousal and has been shown to produce a psychophysiological response in adolescents (i.e., increases in heart rate and blood pressure) (Chaplin et al., 2012; Poon et al., 2016). However, the task does lead to a range in emotion reactivity with some youth being more aroused emotionally than others (Turpyn et al., 2015).
Current Study
The current study examined the relations between resting HF-HRV (e.g., HF-HRV measured at rest) and HF-HRV reactivity (e.g., HF-HRV changes from rest to a challenge—a parent-adolescent conflict interaction task) in 11 to 14-year-olds and adolescents’ self-reported emotion-driven impulse control difficulties 1 year later at ages 12 to 15 years. We also examined adolescents’ experienced and expressed negative emotion during the parent-adolescent conflict interaction task as moderators of the relation between HF-HRV reactivity and future emotion-driven impulse control difficulties. Our hypotheses were as follows:
Adolescents’ lower resting HF-HRV will be associated with higher emotion-driven impulse control difficulties 1 year later.
Adolescents’ expressed negative emotion and experienced negative emotion will moderate the relation between HF-HRV reactivity and emotion-driven impulse control difficulties 1 year later. We predicted that higher HF-HRV reactivity (increases in HF-HRV from rest to conflict) will be associated with higher emotion-driven impulse control difficulties at higher levels of expressed and experienced negative emotion. In addition, higher HF-HRV reactivity will be associated with lower emotion-driven impulse control difficulties at lower levels of expressed and experienced negative emotion.
Method
Subjects
Subjects were 248 early adolescents at the start of the study (M = 12.61, Range of age: 11 to 14, SD = .64; 127 boys, 51%). The sample of subjects was representative of their communities, with 67.30% White, 10.50% Black, 3.60% Asian, .40% Pacific Islander, .40% American Indian, 4.83% Biracial, and 2.80% other. In this sample, 10.10% did not provide information about their race. In addition, 12.10% were Hispanic and 1.20% were Hispanic and non-White. Subjects were also predominantly from families with incomes greater than $100,000 (n = 155, 62.5%; 14.1% between $75,000–100,000; 5.6% between $60,000–74,999; 1.6 % between $45,000–59,999; 12.8% <$45,000, 2 did not report income). Subjects were recruited from the local community and were required to be between the ages of 12-14 years at the start of the study (there was one 11 year old who was about to turn 12 that was included), have an IQ ≥ 80, and adequate English proficiency to complete study questionnaires. Exclusion criterion were prenatal drug exposure, and diagnosis of autism spectrum disorder or psychotic disorder for the adolescent.
Procedure
Adolescents completed a challenging, negatively emotionally arousing parent-adolescent interaction task with their primary caregiver at baseline and completed self-report questionnaires at baseline and at 1 year follow-up. The parent-adolescent interaction task included a conflict discussion and a substance use discussion. The order of the two discussions was counterbalanced. Following the discussion tasks, adolescents quietly completed questionnaires and then interviews. For the purposes of this study, we only examined the parent-adolescent conflict discussion given our interest in examining reactivity within a conflict context. All participants gave their informed consent prior to their inclusion in the study. Study procedures were approved by the University’s Institutional Review Board. Data collection took place from 2012 to 2019.
Parent-Adolescent Interaction Task (PAIT)
Resting Task.
Adolescents and their primary caregiver completed the Issues Checklist (Prinz et al., 1979), a list of common family conflicts (e.g., fighting with sibling, cleaning room) that are rated on a scale of 1 to 5, based on how much anger they induced in the past month. The highest mutually rated topic of conflict was selected for the discussion. Next, adolescents and their primary caregiver had a 25-minute adaptation period during which they listened to two 5-minute relaxation tapes and sat quietly for the remainder of the time.
Conflict Task.
Adolescents and their primary caregiver discussed the selected topic of conflict for 10 minutes. The adolescent and their parent were told to discuss the topic and try to reach a resolution to the conflict. If the adolescent and their parent ended the discussion prior to the 10 minutes, they were instructed to continue for the full 10 minutes.
Experienced negative emotion was measured following the conflict task. Emotion expression throughout the task was later coded. Using Biopac MP150 (Biopac Systems, Santa Barbara, CA), electrocardiography (ECG) was used to measure HRV for 5 minutes before the task (15 minutes into relaxation), for 10 minutes during the conflict task and then again for 10 minutes after the task. Our current analyses focus on the resting and conflict task HRV collection. A sampling frequency of 1,000 Hz was employed. Disposable pre-gelled Ag/AgCl electrodes were placed on the adolescent according to Lead II Placement (right and left chest, where clavicle meets the arm, and left abdomen, fifth intercostal space at midaxillary line).
Measures
Self-Reported Emotion-Driven Impulse Control Difficulties
The impulse control difficulties subscale of the Difficulties in Emotion Regulation Scale (Gratz & Roemer, 2004) was used to assess emotion-driven impulse control difficulties at all timepoints. Adolescents rated the extent to which they experience the specified statements on a scale from 1 (almost never) to 5 (almost always). Example items are as follows: “When I’m upset, I have difficulty controlling my behaviors” and “When I’m upset, I feel out of control.” This six-item subscale has been used in several studies using community samples (Dixon et al., 2017). Internal consistency in the current sample was alpha of .83 for Time 1 and .81 for Time 2.
HF-HRV Resting and Reactivity
ECG data were imported into Biopac AcqKnowledge (Version 4.2) where it was processed. After R peaks were identified, the data were visually inspected for artifacts and manually corrected by trained research assistants. Data with more than 5% of artifacts were removed from the data set (approximately 3%). Next, R peak time series were imported into R. The R package RHRV Version 4.2.2 (García Martínez et al., 2017) was used to perform spectral-based analyses to calculate HF-HRV.
Fast Fourier transformation was used to extract values in the 0.23-0.5 frequency band (an age-appropriate respiratory frequency band for this adolescent sample) (Shader et al., 2018; Cui, et al., 2019). Both the resting task and the conflict task were split into 1-minute epochs; thus, HF-HRV values were obtained for every 1-minute epoch. These values were natural log transformed and averaged to create an average HF-HRV value for the resting task and an average HF-HRV value for the conflict task. Resting HRV was measured as average HF-HRV during the resting task. HF-HRV reactivity was measured as the residualized change score from average HF-HRV during the resting task and average HF-HRV during the conflict task; a higher HF-HRV reactivity score indicates an increase in HF-HRV from rest to challenge (i.e., increased HF-HRV augmentation).
Experienced Negative Emotion
Adolescents were asked about their subjective emotion experience after the parent-adolescent interaction task. Specifically, adolescents were asked to rate on a scale from 0 (not at all) to 10 (more than ever) how frustrated or irritated they felt in the moment (during and immediately after task).
Expressed Negative Emotion
Coded negative emotion was used to measure adolescents’ expressed negative emotion expression. Using a validated coding system (Chaplin, 2010), trained coders rated adolescents’ expressions of negative emotion during the conflict task from videorecordings. Coders watched the 10-minute conflict task and then provided ratings of negative emotion on a scale from 1 (none) to 5 (high) based on facial, vocal, gestural, and postural cues indicative of sadness, fear, anger, contempt, and aggression (e.g., downturned mouth, narrowed eyes). Coders underwent a 6-hour training session and had bimonthly coding meetings to discuss coding questions. To assess for interrater reliability, 38 tapes were double coded. Interrater reliability for negative emotion was deemed adequate (intraclass correlation coefficient [ICC] = .77).
Missing Data
Of the 248 adolescents at Time 1, 26 adolescents did not complete Time 2 (10% of original sample). Most attrition was due to scheduling difficulties. In addition to attrition, 18 participants did not have HF-HRV data due to technical malfunctions, seven participants did not have HF-HRV data due to excessive artifacts in the data, three participants did not have expressed negative emotion data due to problems with videorecording equipment, and three participants did not have experienced emotion data due to data collection problems. In total, 57 participants (23% of total sample) had some sort of missing data. Adolescents with missing data did not differ from adolescents with complete data on gender, race, age, or any study variable at Time 1 (i.e., emotion-driven impulse control difficulties, expressed negative emotion, experienced negative emotion at Time 1) (ps > .05). All missing data were imputed. This means that we did not exclude any participants and had a final sample of 248.
Data Analysis
All analyses were performed in R. Preliminary analyses examined descriptive statistics and zero-order correlations among key study variables (i.e., resting HF-HRV, conflict HF-HRV, HF-HRV reactivity, experienced negative emotion, expressed negative emotion, sex, age at Time 1, and emotion-driven impulse control difficulties at Time 1 and Time 2). Next, data were examined for assumptions of the general linear model. To retain the original sample size, the R package “mice” (van Buuren & Groothuis-Oudshoorn, 2010) was used to create multiply imputed data sets (number of imputed data sets = 10, number of iterations = 50, method = predictive mean matching).
To test Hypothesis 1, a linear regression was used to examine resting HF-HRV at Time 1 as a predictor of emotion-driven impulse control difficulties at Time 2, controlling for Time 1 emotion-driven impulse control difficulties, sex, and Time 1 age.
To test Hypothesis 2, two-step hierarchical linear regressions were used to examine Time 1 expressed negative emotion and Time 1 experienced negative emotion as moderators of Time 1 HF-HRV reactivity predicting Time 2 emotion-driven impulse control difficulties, controlling for Time 1 emotion-driven impulse control difficulties, sex, and Time 1 age. A total of two models were tested. All continuous predictor variables were mean-centered prior to inclusion in the models. For experienced negative emotion, the Step 1 model included Time 1 age, sex, Time 1 emotion-driven impulse control difficulties, Time 1 experienced negative emotion, and Time 1 HF-HRV reactivity. The Step 2 model additionally included the interaction between experienced negative emotion and HF-HRV reactivity at Time 1. For expressed negative emotion, the Step 1 model included Time 1 age, sex, Time 1 emotion-driven impulse control difficulties, Time 1 expressed negative emotion, and Time 1 HF-HRV reactivity. The Step 2 model additionally included the interaction between expressed negative emotion and HF-HRV reactivity at Time 1. Using Preacher and colleagues’ (2006) simple slopes macro, interactions were probed by testing the conditional effects of HF-HRV reactivity at different levels of the moderator (i.e., at the mean, one and two standard deviations below the mean, and one and two standard deviations above the mean; Preacher et al., 2006).
Results
Data met assumptions of the general linear model (e.g., normally distributed residuals, homoskedasticity). Skewness and kurtosis for all variables were adequate. Descriptive statistics and zero-order bivariate correlations among study variables are reported in Table 1. Resting HF-HRV was positively correlated with conflict HF-HRV (r = .81 p < .001) and negatively correlated with expressed negative emotion (r = −.14, p < .05). Moreover, conflict HF-HRV was positively related to HF-HRV reactivity (i.e., greater HF-HRV augmentation; r = .59, p < .01). In addition, expressed negative emotion and experienced negative emotion were positively correlated (r = .22, p < .01). Expressed negative emotion and experienced negative emotion were also related to increased emotion-driven impulse control difficulties at Time 1 (ps < .05).
Table 1.
Correlations and Descriptive Statistics
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1. Age | 1.00 | ||||||||
| 2. Sex | −.14* | 1.00 | |||||||
| 3. Resting HF-HRV | −.07 | .06 | 1.00 | ||||||
| 4. Conflict HF-HRV | −.04 | .07 | .81*** | 1.00 | |||||
| 5. HF HRV Reactivity | −.01 | .08 | .00 | .59** | 1.00 | ||||
| 6. Experienced Emotion | .05 | −.15* | −.04 | −.05 | .06 | 1.00 | |||
| 7. Expressed Emotion | .02 | −.08 | −.14* | −.10 | −.01 | .22** | 1.00 | ||
| 8. Time 1 EIC | .12 | −.07 | −.01 | .04 | .05 | .25** | .16* | 1.00 | |
| 9. Time 2 EIC | −.01 | −.10 | .02 | .07 | .08 | .15* | .05 | .50** | 1.00 |
| Mean | 12.61 | - | 5.28 | 4.84 | 0 | 1.91 | 1.77 | 11.82 | 10.59 |
| SD | .64 | - | .89 | .87 | .52 | 2.41 | .89 | 4.74 | 4.19 |
| Range | 11-14 | - | 2.63-7.64 | 2.27-7.54 | −2.0-1.60 | 0-10 | 1-5 | 6-30 | 6-28 |
Note. EIC = Emotion-Driven Impulse Control Difficulties; HF-HRV = High Frequency Heart Rate Variability. Correlates, means, and standard deviations calculated using non-multiple imputed dataset.
p <.05
p <.01
p<.001
Hypothesis 1: Resting HRV and Emotion-Driven Impulse Control Difficulties
Resting HF-HRV was not a significant predictor of emotion-driven impulse control difficulties at Time 2, covarying for emotion-driven impulse control difficulties, sex, and age at Time 1 (ps > .05; see Table 2).
Table 2.
Resting HRV Predicting Emotion-Driven Impulse Control Difficulties 1 Year Later
| b | SE | R 2 | |
|---|---|---|---|
| Time 1 Age | −.32 | .38 | - |
| Time 1 Sex | −.51 | .48 | - |
| Time 1 EIC | .46*** | .05 | - |
| Time 1 Resting HF-HRV | .09 | .27 | .27 |
Note. EIC = Emotion-Driven Impulse Control Difficulties; HF_HRV = High Frequency Heart Rate Variability. Regressions performed on multiple imputed dataset.
p <.05
p <.01
p<.001
HF-HRV Reactivity and Emotion-Driven Impulse Control Difficulties
Hypothesis 2a: HF-HRV Reactivity Interacting with Experienced Negative Emotion to Predict Emotion-Driven Impulse Control Difficulties One Year Later
Experienced negative emotion significantly moderated the relation between HF-HRV reactivity and emotion-driven impulse control difficulties 1 year later (b = .42, SE = .20, p < .05; see Table 3). Follow-ups indicated that higher HF-HRV reactivity (i.e., greater augmentation) predicted higher emotion-driven impulse control difficulties at Time 2 when experienced negative emotion was two standard deviations above the mean (p > .05), but not two standard deviations below the mean (p > .05) or at the mean (p > .05; Figure 1). Regions of significance analyses showed that the relation between HF-HRV reactivity and emotion-driven impulse control difficulties was significant at levels of experienced negative emotion above 2.75. In sum, higher HF-HRV reactivity (i.e., greater augmentation) to the challenge predicted higher emotion-driven impulse control difficulties 1 year later when experienced negative emotion was higher, which is consistent with our hypothesis.
Table 3.
HRV Reactivity and Experienced Negative Emotion Predicting Emotion-Driven Impulse Control Difficulties 1 Year Later
| b | SE | R2 | ΔR2 | |
|---|---|---|---|---|
| Step 1 | ||||
| Time 1 Age | −.34 | .45 | - | - |
| Time 1 Sex | −.57 | .49 | - | - |
| Time 1 EIC | .46*** | .06 | - | - |
| HF-HRV Reactivity | .38 | .51 | - | - |
| Experienced Emotion | .06 | .11 | .29 | - |
| Step 2 | ||||
| HF-HRV Reactivity x Experienced Emotion |
.42* | .20 | .30 | .01* |
Note. EIC = Emotion-Driven Impulse Control Difficulties; HF-HRV = High Frequency Heart Rate Variability. Regressions performed on multiple imputed dataset.
p <.05
p <.01
p<.001
Figure 1.
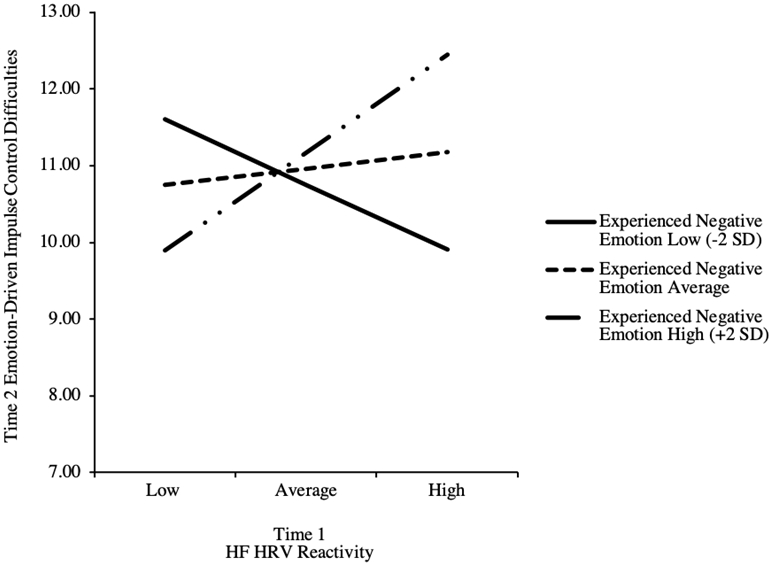
Simple Slopes of HF-HRV Reactivity x Experienced Negative Emotion Interaction
There were no significant main effects of HF-HRV reactivity or experienced negative emotion predicting emotion-driven impulse control difficulties at Time 2 (see Table 3). In terms of covariates, higher emotion-driven impulse control difficulties at Time 1 was associated with higher emotion-driven impulse control difficulties at Time 2 and there were no significant main effects of age or sex (see Table 3).
Hypothesis 2b: HF-HRV Reactivity Interacting with Expressed Negative Emotion to Predict Emotion-Driven Impulse Control Difficulties One Year Later
Expressed negative emotion did not significantly moderate the relation between HF-HRV reactivity and emotion-driven impulse control difficulties 1 year later (see Table 4). There were no significant main effects of HF-HRV reactivity or expressed negative emotion in predicting emotion-driven impulse control difficulties at Time 2 (see Table 4). In terms of covariates, higher emotion-driven impulse control difficulties at Time 1 was associated with higher emotion-driven impulse control difficulties at Time 2 and there were no significant main effects of sex and age (see Table 4).
Table 4.
HRV Reactivity and Expressed Negative Emotion Predicting Emotion-Driven Impulse Control Difficulties 1 Year Later
| b | SE | R 2 | ΔR2 | |
|---|---|---|---|---|
| Step 1 | ||||
| Time 1 Age | −.39 | .40 | - | - |
| Time 1 Sex | −.55 | .51 | - | - |
| Time 1 EIC | .48*** | .06 | - | - |
| HF-HRV Reactivity | .30 | .50 | - | - |
| Expressed Emotion | −.23 | .29 | .29 | - |
| Step 2 | ||||
| HF-HRV Reactivity x Expressed Emotion |
−.11 | .60 | .29 | .00 |
Note. EIC= Emotion-Driven Impulse Control Difficulties; HF-HRV = High Frequency Heart Rate Variability. Regressions performed on multiple imputed dataset.
p <.05
p <.01
p<.001
Discussion
The purpose of this study was to examine resting HF-HRV and HF-HRV reactivity to a negatively emotionally arousing parent-adolescent conflict task in early adolescence as predictors of changes in emotion-driven impulse control difficulties over 1 year. We also examined adolescents’ negative emotion experience and negative emotion expression during the conflict as moderators of the relation between adolescents’ HF-HRV reactivity to the conflict and their later emotion-driven impulse control difficulties. We obtained three major findings in this study. First, resting HRV was not associated with emotion-driven impulse control difficulties 1 year later in adolescence. Second, HF-HRV reactivity to conflict interacted with adolescents’ negative emotion experience to predict emotion-driven impulse control difficulties over 1 year. Specifically, among adolescents who reported higher levels (i.e., moderate levels) of experienced negative emotion, higher HF-HRV reactivity (i.e., greater augmentation) to conflict predicted higher emotion-driven impulse control difficulties. Third, expressed negative emotion during the conflict did not moderate the relation between HF-HRV reactivity and emotion-driven impulse control difficulties for adolescents. These findings and their clinical implications are elaborated below.
Contrary to our hypothesis, resting HF-HRV did not prospectively predict emotion-driven impulse control difficulties. A large body of literature has linked high resting HF-HRV to good psychosocial outcomes and low resting HF-HRV to poor psychosocial outcomes, such as emotion regulation, in adolescents and adults (Beauchaine, 2015; Cui et al., 2015; Geisler et al., 2010; McLaughlin et al., 2014; Vazquez et al., 2016). One study specifically linked higher resting HF-HRV with lower emotion-driven impulse control difficulties in adults (Williams et al., 2015). Of note, however, some studies have found no relation between resting HF-HRV and psychosocial outcomes, including other facets of emotion regulation (e.g., Williams et al., 2015). The correlation between resting HF-HRV and emotion-driven impulse control difficulties at Time 1 was also nonsignificant, suggesting that this null finding may not have just been a function of our prospective design. One possibility is that there may not be as strong a relation between resting HF-HRV and emotion-driven impulse control difficulties as with other outcomes such as psychopathology. This is especially the case because we found a negative association between resting HF-HRV and expressed negative emotion during the conflict task, which is consistent with the literature and suggests that the measurement of resting HF-HRV was adequate. Similarly, there may also not be as strong a relation between resting HF-HRV and emotion-driven impulse control difficulties among adolescents as among adults. HF-HRV is age-dependent and there is research to indicate that HF-HRV changes through the life span (e.g., Reardon & Malik, 1996). Perhaps it is the case that resting HF-HRV is more predictive of emotion-driven impulse control difficulties among adults than young adolescents. Further research on this matter is warranted.
The major finding in this article is that HF-HRV reactivity to a challenge interacted with experienced negative emotion to prospectively predict increases in emotion-driven impulse control difficulties 1 year later. Specifically, as hypothesized, higher HF-HRV reactivity (i.e., HF-HRV augmentation) to the parent-adolescent conflict task predicted higher emotion-driven impulse control difficulties among adolescents who had higher levels (i.e., moderate levels) of experienced negative emotion during the conflict task. As noted earlier, HF-HRV would normally be expected to decrease in response to higher negative emotion as the body decreases activation of the parasympathetic system to manage the negative emotion. And, importantly, greater decreases in HF-HRV from rest to challenge may be particularly adaptive in situations in which the person experiences high negative emotion because those individuals find the task arousing and benefit from HF-HRV decreases (parasympathetic withdrawal). For adolescents, higher HF-HRV (parasympathetic) response to a challenge that is experienced negatively would not be adaptive and thus may lead to less adaptive future regulatory ability and greater emotion-driven impulse control difficulties. Over the long term, this may make it difficult for the adolescent to manage life challenges and subsequently engage in dysregulated, impulsive behavior as a way to cope with these stressors.
These findings are consistent with recent research indicating that negative emotion interacting with HF-HRV reactivity in predicting psychosocial outcomes is likely. Similar to the current study, James et al. (2017) had children complete a parent-child conflict task and found that children without a history of suicidal ideation had greater decreases in HF-HRV reactivity compared to children with a history of suicidal ideation. Importantly, all children on average had significantly increased experienced negative emotion in the conflict task (compared with a “fun” discussion task), suggesting that the task was negatively emotionally arousing. In addition, other studies that have demonstrated increases in HF-HRV as being linked to worse psychosocial outcomes have employed negatively emotionally arousing (e.g., film clips; Gentzler et al., 2009; Beauchaine et al., 2007) and cognitively demanding tasks (El-Sheikh, Hinnant, & Erath, 2011) that have been found to elicit psychophysiological responses in other studies (e.g., Aguado et al., 2016; Westermann et al., 1996; Dickerson & Kemeny, 2004).
Therefore, similar to the current study, it appears that individuals who had increased HF-HRV reactivity (i.e., greater augmentation) during tasks that are likely to be negatively emotionally arousing also had increased psychosocial impairment. On the contrary, one study that employed a low emotionally arousing task (i.e., nonconflict social task) found that greater decreases in HF-HRV were associated with more psychosocial symptoms (Hastings et al., 2008). This too is consistent with the theory that reductions in HF-HRV are helpful when experiencing high, not low negative emotion—when engagement of the parasympathetic nervous system would be optimal to maintain social engagement (Porges, 2001). Taken together, the current study as well as most extant research demonstrates that higher emotion tasks induce decreases in HF-HRV reactivity that are associated with reduced psychopathology.
Some studies are inconsistent with this theory that decreases in HF-HRV are associated with improved psychosocial outcomes in the context of higher negative emotion. For example, De Vries-Bouw et al. (2011) had adolescents with a history of delinquent behavior complete a public speaking task, as shown in previous studies to elicit a stress response (e.g., Hostinar et al., 2014). Results revealed that adolescents with greater decreases in HF-HRV (which also corresponded to an increase in experienced negative emotion) were associated with increased risk of offending over a 5-year follow-up period. This may suggest that decreases in HF-HRV are associated with negative psychosocial outcomes in the context of higher negative emotion. Other studies have shown a similar pattern of findings among children diagnosed with externalizing psychopathology (ADHD; for example, Beauchaine et al., 2013). It is possible that relations between HF-HRV reactivity and psychosocial functioning are different in clinical samples compared with community samples. In other words, findings from clinical samples may not be generalizable to a community sample as in the current study. It is imperative that more research is done to replicate the current findings and better understand how emotional context interacts with HF-HRV reactivity to predict psychosocial outcomes—in community and clinical samples of adolescents.
In addition, it is important to note that in the current study higher than average levels of experienced negative emotion (i.e., one to two standard deviations from mean) represented moderate levels of negative emotion. That is, moderate levels of experienced negative emotion interacted with HF-HRV reactivity to predict increased emotion-driven impulse control difficulties. This suggests that reductions in HF-HRV are adaptive even at moderate levels of experienced negative emotion. The implication of this is that at moderate levels of negative emotion, it may be preferable to disengage parasympathetic resources that better enable social engagement and cognitive processing. Future research should examine the level of negative emotion at which HF-HRV reactivity is predictive of poor psychosocial outcomes.
Interestingly, HF-HRV reactivity and emotion-driven impulse control difficulties were not related among adolescents with low experienced negative emotion. We had initially predicted that HF-HRV increases from rest to challenge would be associated with lower emotion-driven impulse control difficulties among those with low negative emotion because these youth would be maintaining parasympathetic engagement appropriate to the emotional arousal of the situation. It is possible that these effects are not very strong and that we did not have the power in our study to detect these effects.
Finally, counter to our hypothesis, the relation between higher HF-HRV reactivity (i.e., HF-HRV augmentation) and emotion-driven impulse control difficulties was not moderated by expressed negative emotion. In other words, there was no significant association between HF-HRV reactivity and emotion-driven impulse control difficulties at any level of expressed negative emotion. We hypothesized that higher HF-HRV reactivity (i.e., HF-HRV augmentation) would predict more emotion-driven impulse control difficulties among adolescents who found the task to be negatively emotionally arousing, as evidenced by higher expressed negative emotion. One possibility for this null finding is that negative emotion expression may be varied across this sample of adolescents, making it more difficult to detect an effect (i.e., increased noise). For example, it is possible that there were adolescents with low expressed negative emotion who were actually feeling high negative emotion but were just suppressing/not showing their negative emotions. Other adolescents, however, may have been showing negative emotion that reflects their true emotion. Future research should explore the link between HF-HRV reactivity and emotional expression specifically to better understand the physiological reactivity seen in different expression profiles.
It is worth underscoring the fact that these results are longitudinal. That is, adolescents at 11 to 14 years of age with greater increases in HF-HRV from rest to challenge had higher emotion-driven impulse control difficulties at 12 to 15 years of age if they had higher experienced negative emotion in response to the conflict task. This indicates that HF-HRV reactivity in combination with negative emotion reactivity to a task predicted psychosocial impairment over 1 year in adolescence. One possibility for these results is that this profile of negative emotion reactivity may become more problematic as adolescents mature and are faced with more negatively emotionally arousing challenges (e.g., puberty, interpersonal concerns). Over time, an inability to adaptively manage these challenges (e.g., high recruitment of parasympathetic resources) may lead to increased negative emotion and problems with effective emotion regulation, which in turn increases the likelihood of experiencing more challenges (e.g., interpersonal conflict due to negative emotion; Selby et al., 2008). Relatedly, an inability to adaptively manage challenges early in adolescence may impair an adolescent’s ability to learn emotion regulation strategies that are typically learned during the adolescent developmental period (Gullone et al., 2010). The result of this is that an adolescent cannot downregulate strong emotions and may react to feeling strong emotions through behaving rashly or impulsively (e.g., use substances) as a way to cope.
Overall, these findings suggest that the relation between HF-HRV reactivity and emotion-driven impulse control difficulties (and perhaps other self-regulatory abilities) is dependent on the emotional context (e.g., whether it is experienced as negative). This can explain why the literature on HF-HRV reactivity to challenge and psychosocial impairment is somewhat mixed, with most studies linking increases in HF-HRV from rest to challenge as worse for emotional functioning (e.g., Gentzler et al., 2009) and a couple of studies finding increases in HF-HRV from rest to challenge as better for emotional functioning (e.g., Hastings et al., 2008). In this case, experienced, but not expressed, negative emotion modulated the relation between HF-HRV reactivity and changes in emotion-driven impulse control difficulties 1 year later.
Clinical Implications
The findings in this article have implications for the prevention and treatment programs that target emotion-driven impulse control difficulties to prevent psychopathology and risk behaviors. This is important because emotion-driven impulse control difficulties are seen in several forms of psychopathology (Moore et al., 2017; Neumann et al., 2010; Watkins et al., 2015) for both adolescents and adults. Given that this study found that higher HF-HRV reactivity (i.e., HF-HRV augmentation) in the context of high experienced negative emotion can predict emotion-driven impulse control difficulties, it may be worthwhile to target physiological reactivity during challenges as a way to reduce escalations in emotion-driven impulse control difficulties. This may be done by increasing physiological flexibility through interventions involving biofeedback and exercise, for example (Sherlin et al., 2009; von Haaren et al., 2016). Similarly, HF-HRV reactivity can serve as a biomarker to identify at-risk youth who would benefit from early interventions. As more studies replicate the findings shown here, we can move toward integrating HF-HRV reactivity into protocols meant to screen youth at risk of psychopathology and related symptoms. Importantly, it is critical to note that among adolescents with clinically significant psychopathology, especially externalizing psychopathology, higher HF-HRV augmentation during high experienced negative emotion may not predict psychopathology, and thus, the aforementioned interventions may not be appropriate for them. More research employing clinical samples is needed to fully understand the clinical implications of HF-HRV reactivity in negative emotion contexts.
Limitations and Future Directions
Although this study has several strengths, there are some limitations that should be addressed in subsequent studies. First, HF-HRV was not collected at each timepoint; therefore, we were unable to test bidirectional relations between resting HF-HRV, HF-HRV reactivity, and emotion-driven impulse control difficulties. It is possible that emotion-driven impulse control difficulties predict resting HF-HRV and HF-HRV reactivity over time, although the current study is unable to examine this. With that said, there is limited evidence to support this: One longitudinal study on depression showed that HF-HRV prospectively predicted depression, but that depression did not prospectively predict HF-HRV (Jandackova et al., 2016). Second, although our parent-adolescent conflict task elicited levels of negative emotion consistent with other laboratory tasks (e.g., James et al., 2017), the limited range of negative emotion is not ideal. The relatively low levels of negative emotion we obtained reduces power to fully detect how negative emotion may interact with HF-HRV reactivity to predict psychosocial outcomes. Future studies should make efforts to increase the range of negative emotion elicited by the parent-adolescent conflict task. Third, this sample is primarily White and middle to upper middle income, which limits our ability to generalize these findings to adolescents of other races/ethnicities or income groups. This is particularly important when examining adolescent-parent interactions, given evidence suggesting that non-White adolescents interact with their parents differently than their White peers (Phinney et al., 2005). Although beyond the scope of the current study, future studies should examine dynamic changes in HF-HRV reactivity throughout and following the task (i.e., recovery). This is critical to investigate because what may be adaptive at the beginning of a challenge may not be adaptive as the challenge continues and then after the challenge is over. Thus, it is important to investigate how dynamic changes in HF-HRV interact with contextual factors to predict emotion-driven impulse control difficulties.
Conclusion
In sum, we found that higher HF-HRV reactivity (i.e., HF-HRV augmentation) predicted greater increases in emotion-driven impulse control difficulties one year later among adolescents with higher experienced negative emotion. That is, higher HF-HRV reactivity (i.e., HF-HRV augmentation) was not adaptive for adolescents with higher levels of experienced negative emotion. It is possible that over time these patterns of reactivity may make it difficult for adolescents to manage life challenges and subsequently engage in impulsive behavior as a way to cope with these challenges. These findings suggest that emotional context should be considered when examining relations between HF-HRV reactivity and psychosocial outcomes. Moreover, if findings are replicated, HF-HRV reactivity can be used as a biomarker to identify vulnerable adolescents in need of intervention to prevent escalations in emotion-driven impulse control difficulties and other outcomes.
References
- Acharya UR, Krishnan SM, Spaan JA, & Suri JS (Eds.). (2007). Advances in cardiac signal processing. Springer. [Google Scholar]
- Aguado L, Fernández-Cahill M, Román FJ, Blanco I, & de Echegaray J (2016). Evaluative and psychophysiological responses to short film clips of different emotional content. Journal of Psychophysiology, 32(1), 1–19. 10.1027/0269-8803/a000180 [DOI] [Google Scholar]
- Appelhans BM, & Luecken LJ (2006). Heart rate variability as an index of regulated emotional responding. Review of General Psychology, 10(3), 229–240. 10.1037/1089-2680.10.3.229 [DOI] [Google Scholar]
- Beauchaine TP, Gatzke-Kopp L, Neuhaus E, Chipman J, Reid MJ, & Webster-Stratton C (2013). Sympathetic- and parasympathetic-linked cardiac function and prediction of externalizing behavior, emotion regulation, and prosocial behavior among preschoolers treated for ADHD. Journal of Consulting and Clinical Psychology, 81(3), 481–493. 10.1037/a0032302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP (2015). Respiratory sinus arrhythmia: a transdiagnostic biomarker of emotion dysregulation and psychopathology. Current Opinion in Psychology, 3, 43–47. 10.1016/j.copsyc.2015.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, & Potenza MN (2003). Developmental neurocircuitry of motivation in adolescence: A critical period of addiction vulnerability. American Journal of Psychiatry, 160(6), 1041–1052. 10.1176/appi.ajp.160.6.1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin TM (2010). Parent–adolescent interaction task (PAIT) coding system. Yale University School of Medicine. Unpublished manual. [Google Scholar]
- Chaplin TM, Sinha R, Simmons JA, Healy SM, Mayes LC, Hommer RE, & Crowley MJ (2012). Parent–adolescent conflict interactions and adolescent alcohol use. Addictive Behaviors, 37(5), 605–612. 10.1016/j.addbeh.2012.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole PM, Michel MK, & Teti LOD (1994). The development of emotion regulation and dysregulation: a clinical perspective. Monographs Of The Society For Research In Child Development, 59(2–3), 73–102. 10.1111/j.1540-5834.1994.tb01278.x [DOI] [PubMed] [Google Scholar]
- Cui L, Morris AS, Harrist AW, Larzelere RE, Criss MM, & Houltberg BJ (2015). Adolescent RSA responses during an anger discussion task: Relations to emotion regulation and adjustment. Emotion, 15(3), 360–372. 10.1037/emo0000040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Zhang X, Houltberg BJ, Criss MM, & Morris AS (2019). RSA reactivity in response to viewing bullying film and adolescent social adjustment. Developmental Psychobiology, 61(4), 592–604. 10.1002/dev.21835 [DOI] [PubMed] [Google Scholar]
- De Vries-Bouw M, Popma A, Vermeiren R, Doreleijers TAH, Van De Ven PM, & Jansen LMC (2011). The predictive value of low heart rate and heart rate variability during stress for reoffending in delinquent male adolescents: Low ANS reactivity and reoffending in adolescents. Psychophysiology, 48(11), 1597–1604. 10.1111/j.1469-8986.2011.01233.x [DOI] [PubMed] [Google Scholar]
- Dickerson SS, & Kemeny ME (2004). Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130(3), 355–391. 10.1037/0033-2909.130.3.355 [DOI] [PubMed] [Google Scholar]
- Dixon LJ, Tull MT, Lee AA, Kimbrel NA, & Gratz KL (2017). The role of emotion-driven impulse control difficulties in the relation between social anxiety and aggression. Journal of Clinical Psychology, 73(6), 722–732. 10.1002/jclp.22372 [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Hinnant JB, & Erath S (2011). Developmental trajectories of delinquency symptoms in childhood: The role of marital conflict and autonomic nervous system activity. Journal of Abnormal Psychology, 120(1), 16–32. 10.1037/a0020626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García Martínez CA, Otero Quintana A, Vila XA, Lado Touriño MJ, Rodríguez-Liñares L, Rodríguez Presedo JM, & Méndez Penín AJ (2017). Heart Rate Variability Analysis with the R Package RHRV. Springer International Publishing. 10.1007/978-3-319-65355-6 [DOI] [Google Scholar]
- Geisler FCM, Vennewald N, Kubiak T, & Weber H (2010). The impact of heart rate variability on subjective well-being is mediated by emotion regulation. Personality and Individual Differences, 49(7), 723–728. 10.1016/j.paid.2010.06.015 [DOI] [Google Scholar]
- Gentzler AL, Santucci AK, Kovacs M, & Fox NA (2009). Respiratory sinus arrhythmia reactivity predicts emotion regulation and depressive symptoms in at-risk and control children. Biological Psychology, 82(2), 156–163. 10.1016/j.biopsycho.2009.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz KL, & Roemer L (2004). Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the difficulties in emotion regulation scale. Journal of Psychopathology and Behavioral Assessment, 26(1), 41–54. 10.1023/B:JOBA.0000007455.08539.94 [DOI] [Google Scholar]
- Gullone E, Hughes EK, King NJ, & Tonge B (2010). The normative development of emotion regulation strategy use in children and adolescents: a 2-year follow-up study. Journal of Child Psychology and Psychiatry, 51(5), 567–574. 10.1111/j.1469-7610.2009.02183.x [DOI] [PubMed] [Google Scholar]
- Hastings PD, Nuselovici JN, Utendale WT, Coutya J, McShane KE, & Sullivan C (2008). Applying the polyvagal theory to children’s emotion regulation: Social context, socialization, and adjustment. Biological Psychology, 79(3), 299–306. 10.1016/j.biopsycho.2008.07.005 [DOI] [PubMed] [Google Scholar]
- Hinnant JB, & Mona ES (2013). Codevelopment of externalizing and internalizing symptoms in middle to late childhood: Sex, baseline respiratory sinus arrhythmia, and respiratory sinus arrhythmia reactivity as predictors. Development and Psychopathology, 25(2), 419–436. 10.1017/S0954579412001150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, McQuillan MT, Mirous HJ, Grant KE, & Adam EK (2014). Cortisol responses to a group public speaking task for adolescents: variations by age, gender, and race. Psychoneuroendocrinology, 50, 155–166. 10.1016/j.psyneuen.2014.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James KM, Woody ML, Feurer C, Kudinova AY, & Gibb BE (2017). Disrupted physiological reactivity among children with a history of suicidal ideation: Moderation by parental expressed emotion-criticism. Biological Psychology, 130, 22–29. 10.1016/j.biopsycho.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandackova VK, Britton A, Malik M, & Steptoe A (2016). Heart rate variability and depressive symptoms: a cross-lagged analysis over a 10-year period in the Whitehall II study. Psychological Medicine, 46(10), 2121–2131. 10.1017/S003329171600060X [DOI] [PubMed] [Google Scholar]
- Malik M, Bigger JT, John Camm A, Kleiger RE, Malliani A, Moss AJ, & Schwartz PJ (1996). Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. European Heart Journal, 17(3), 354–381. 10.1093/oxfordjournals.eurheartj.a014868 [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Alves S, & Sheridan MA (2014). Vagal regulation and internalizing psychopathology among adolescents exposed to childhood adversity. Developmental Psychobiology, 56(5), 1036–1051. 10.1002/dev.21187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KE, Tull MT, & Gratz KL (2017). Borderline personality disorder symptoms and criminal justice system involvement: The roles of emotion-driven difficulties controlling impulsive behaviors and physical aggression. Comprehensive Psychiatry, 76, 26–35. 10.1016/j.comppsych.2017.03.008 [DOI] [PubMed] [Google Scholar]
- Neumann A, van Lier PAC, Gratz KL, & Koot HM (2010). Multidimensional assessment of emotion regulation difficulties in adolescents using the difficulties in emotion regulation scale. Assessment, 17(1), 138–149. 10.1177/1073191109349579 [DOI] [PubMed] [Google Scholar]
- Pang KC, & Beauchaine TP (2013). Longitudinal patterns of autonomic nervous system responding to emotion evocation among children with conduct problems and/or depression. Developmental Psychobiology, 55(7), 698–706. 10.1002/dev.21065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry NB, Calkins SD, Nelson JA, Leerkes EM, & Marcovitch S (2012). Mothers' responses to children's negative emotions and child emotion regulation: The moderating role of vagal suppression. Developmental Psychobiology, 54(5), 503–513. 10.1002/dev.20608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phinney JS, Kim-Jo T, Osorio S, & Vilhjalmsdottir P (2005). Autonomy and relatedness in adolescent-parent disagreements: Ethnic and developmental factors. Journal of Adolescent Research, 20(1), 8–39. 10.1177/0743558404271237 [DOI] [Google Scholar]
- Porges SW (1995). Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology, 32(4), 301–318. 10.1111/j.1469-8986.1995.tb01213.x [DOI] [PubMed] [Google Scholar]
- Porges SW (2001). The polyvagal theory: phylogenetic substrates of a social nervous system. International Journal of Psychophysiology, 42(2), 123–146. 10.1016/s0167-8760(01)00162-3 [DOI] [PubMed] [Google Scholar]
- Poon JA, Turpyn CC, Hansen A, Jacangelo J, & Chaplin TM (2016). Adolescent substance use & psychopathology: Interactive effects of cortisol reactivity and emotion regulation. Cognitive Therapy and Research, 40(3), 368–380. 10.1007/s10608-015-9729-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, & Bauer DJ (2006). Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics, 31, 437–448. 10.3102/10769986031004437 [DOI] [Google Scholar]
- Prinz RJ, Foster S, Kent RN, & O’Leary KD (1979). Multivariate assessment of conflict in distressed and nondistressed mother-adolescent dyads. Journal of Applied Behavior Analysis, 12(4), 691–700. 10.1901/jaba.1979.12-691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon M, & Malik M (1996). Changes in Heart Rate Variability with Age. Pacing and Clinical Electrophysiology, 19(11), 1863–1866. 10.1111/j.1540-8159.1996.tb03241.x [DOI] [PubMed] [Google Scholar]
- Scott BG, & Weems CF (2014). Resting vagal tone and vagal response to stress: associations with anxiety, aggression, and perceived anxiety control among youths. Psychophysiology, 51(8), 718–727. 10.1111/psyp.12218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby EA, Anestis MD, & Joiner TE (2008). Understanding the relationship between emotional and behavioral dysregulation: Emotional cascades. Behaviour Research and Therapy, 46(5), 593–611. 10.1016/j.brat.2008.02.002 [DOI] [PubMed] [Google Scholar]
- Shader TM, Gatzke-Kopp LM, Crowell SE, Reid MJ, Thayer JF, Vasey MW, … & Beauchaine TP (2018). Quantifying respiratory sinus arrhythmia: Effects of misspecifying breathing frequencies across development. Development and Psychopathology, 30(1), 351–366. 10.1017/S0954579417000669 [DOI] [PubMed] [Google Scholar]
- Sharpley CF, & Gordon JE (1999). Differences between ECG and pulse when measuring heart rate and reactivity under two physical and two psychological stressors. Journal of Behavioral Medicine, 22(3), 285–298. 10.1023/A:1018724608328 [DOI] [PubMed] [Google Scholar]
- Sherlin L, Gevirtz R, Wyckoff S, & Muench F (2009). Effects of respiratory sinus arrhythmia biofeedback versus passive biofeedback control. International Journal of Stress Management, 16(3), 233–248. 10.1037/a0016047 [DOI] [Google Scholar]
- Thayer JF, & Lane RD (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders, 61(3), 201–216. 10.1016/s0165-0327(00)00338-4 [DOI] [PubMed] [Google Scholar]
- Thayer JF, Åhs F, Fredrikson M, Sollers JJ, & Wager TD (2012). A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neuroscience & Biobehavioral Reviews, 36(2), 747–756. 10.1016/j.neubiorev.2011.11.009 [DOI] [PubMed] [Google Scholar]
- Turpyn CC, Chaplin TM, Cook EC, & Martelli AM (2015). A person-centered approach to adolescent emotion regulation: Associations with psychopathology and parenting. Journal of Experimental Child Psychology, 136, 1–16. 10.1016/j.jecp.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Buuren S, & Groothuis-Oudshoorn K (2010). mice: Multivariate imputation by chained equations in R. Journal of Statistical Software, 45(3). 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- Vasilev CA, Crowell SE, Beauchaine TP, Mead HK, & Gatzke-Kopp LM (2009). Correspondence between physiological and self-report measures of emotion dysregulation: A longitudinal investigation of youth with and without psychopathology. Journal of Child Psychology and Psychiatry, 50(11), 1357–1364. 10.1111/j.1469-7610.2009.02172.x [DOI] [PubMed] [Google Scholar]
- Vazquez L, Blood JD, Wu J, Chaplin TM, Hommer RE, Rutherford HJV, … Crowley MJ (2016). High frequency heart-rate variability predicts adolescent depressive symptoms, particularly anhedonia, across one year. Journal of Affective Disorders, 196, 243–247. 10.1016/j.jad.2016.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Haaren B, Ottenbacher J, Muenz J, Neumann R, Boes K, & Ebner-Priemer U (2016). Does a 20-week aerobic exercise training programme increase our capabilities to buffer real-life stressors? A randomized, controlled trial using ambulatory assessment. European Journal of Applied Physiology, 116(2), 383–394. 10.1007/s00421-015-3284-8 [DOI] [PubMed] [Google Scholar]
- Watkins LE, Franz MR, DiLillo D, Gratz KL, & Messman-Moore TL (2015). Does drinking to cope explain links between emotion-driven impulse control difficulties and hazardous drinking? A longitudinal test. Psychology of Addictive Behaviors, 29(4), 875–884. 10.1037/adb0000127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann R, Spies K, Stahl G, & Hesse FW (1996). Relative effectiveness and validity of mood induction procedures: A meta-analysis. European Journal of Social Psychology, 26(4), 557–580. [DOI] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, … Vos T (2013). Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. The Lancet, 382(9904), 1575–1586. 10.1016/S0140-6736(13)61611-6 [DOI] [PubMed] [Google Scholar]
- Williams DP, Cash C, Rankin C, Bernardi A, Koenig J, & Thayer JF (2015). Resting heart rate variability predicts self-reported difficulties in emotion regulation: a focus on different facets of emotion regulation. Frontiers in Psychology, 6, 261. 10.3389/fpsyg.2015.00261 [DOI] [PMC free article] [PubMed] [Google Scholar]


