Graphical abstract
Keywords: In-silico drug design, SARS-CoV-2, Spike glycoprotein, Peptide vaccine, Computational model
Abstract
The far-reaching effects of the SARS-CoV-2 pandemic have crippled the progress of the world today. With the introduction of newer and newer mutated variants of the virus, it has become necessary to have a vaccine that remains useful against all the mutated strains of SARS-CoV-2. In this regard, peptide vaccines turn out to be a cheap alternative to the traditionally designed vaccines owing to their much quicker and computationally easier, and more robust design procedures. Here, in this article, we hypothesize that there are three possible peptide vaccine regions that can be targeted to prevent the surge of SARS-CoV-2. The candidates that were selected, were surface-exposed and were not sequestered by any neighbouring amino acids. They were also found to be capable of generating both B-cell and T-cell immune responses. Most importantly, none of them contains any spike protein mutation of the currently prevailing variants of SARS-CoV-2. From these findings, we have therefore concluded that these three regions can be used in wet labs for peptide vaccine design against the upcoming strains of SARS-CoV-2.
Introduction
Since the inception of SARS-CoV-2 back in December 2019, according to WHO, there have been 211,730,035 reported cases of it with 4,430,697 deaths as of 23 August 2021 [1]. With the introduction of various mutated strains of this virus that are even more virulent than their predecessors, methodical circulation of vaccines throughout the world has been a silver lining in this regard. mRNA vaccines like Pfizer BioNTech (BNT162b2) and Moderna (mRNA1273); adenovirus vector vaccines such as Oxford-AstraZeneca (Covishield), Sputnik V, Janssen, Convidicea, Sputnik Light; inactivated virus vaccines such as Sinopharm, CoronaVac, Covaxin; subunit vaccines such as EpiVacCorona, ZF2001, Abdala, Soberana 02 have granted emergency use around the world [2], [3]. Recent studies have shown that non-steroidal anti-inflammatory drugs (NSAIDS), particularly indomethacin can be a good option for initial therapeutic proceedings against COVID patients [4]. Besides, the inactivation of the estrogen signaling pathway in lung cells [5] might also help in damping the COVID-19 severity. Studies have also reflected the mechanisms behind natural and vaccine-induced immunity against SARS-COV-2 in humans [6], but the existence of long-term acquired immunity is under scrutiny [7], [8].
Some mutated strains have been classified by the WHO as the Variants of Concern (VOC) on the basis of increased transmissibility, resistance towards vaccines and varied clinical symptoms. One of the deadliest strains of COVID-19 is the B.1.617.2 or Delta variant as it is more contagious (40–60% more transmissible than the B.1.1.7 or Alpha variant) [9]. This strain was first documented in India around October 2020. It can break the vaccine cover quite easily. This strain can remain unaffected by a single dose of the Pfizer BioNTech vaccine [10]. Even the effectiveness of the AstraZeneca ChAdOx1 nCoV-19 vaccine after the first dose was quite low for people affected by the Delta variant, but the effectiveness rose to 74.5% (with a confidence interval of 95%) after the second dose [11]. Other SARS-CoV-2 Variants of Concern are the Beta strain (B.1.351, B.1.351.2, B.1.351.3) and the Gamma strain (P.1, P.1.1, P.1.2).
Some mutated strains of COVID-19 have altered amino acids in their Receptor Binding Domain (RBD) which can increase their binding affinity with the human ACE-2 receptor and hence, making them more infectious. A list of such of such variants and their corresponding alterations in the RBD domain is shown in Table 1 . Thus, the question of long-term immunity against a variety of SARS-CoV-2 strains becomes the most important question that needs to be answered as soon as possible. Effective COVID-19 vaccines with durable specific immunity to encounter the real virus in coming times and an ability to stimulate immune responses from both B-cells and T-cells are on the hunt. In this regard, peptide vaccines can be a good option as they are synthetically designed [12], and can be prepared by analysing the conserved sequences which are potential epitopes. One can also swap target epitopes and amino acids with relative ease.
Table 1.
List of some of commonly observed mutated strains of SARS-CoV-2 and their mutation in the receptor binding domain.
| Commonly observed variants | Pango Lineage | Mutations in the receptor binding domain (RBD) in the surface glycoprotein |
|---|---|---|
| Alpha | B.1.1.7 | E484K, S494P, N501Y |
| Beta | B.1.351, B.1.351.2, B.1.351.3 | K417N, E484K, N501Y |
| Gamma | P.1, P.1.1, P.1.2 | K417N, E484K, N501Y |
| Delta | B.1.617.2, AY.1, AY.2, AY.3 | K417N, L452R, T478K |
| Omicron | B.1.1.529 | G339D, S371L, S373P, S375F, K417N, N440K, G446S, S477N, T478K, E484A, Q493R, G496S, Q498R, N501Y, Y505H |
| Lambda | C.37 | L452Q, F490S |
| Mu | B.1.621 | R346K, E484K, N501Y |
| Kappa | B.1.617.1 | L452R, E484Q |
| Eta | B.1.525 | E484K |
| Iota | B.1.526 | L452R, S477N, E484K |
| Epsilon | B.1.427, B.1.429 | L452R |
| Zeta | P.2 | E484K |
| _ | B.1.1.519 | T478K |
| _ | B.1.620 | S477N, E484K |
| _ | B.1.177 | T445C |
| _ | B.1.617.3 | L452R, E484Q |
It is also possible to contemplate tailoring the vaccine to a particular host population and alter it for individual community needs. Moreover, being synthetic in nature, they would be cost-effective in terms of storage and transportation [13]. The latest evolution in solid-phase peptide synthesis (SPPS) using automatic synthesizers and the application of microwave approaches render peptide vaccines as suitable for large-scale production with low costs and high reproducibility [14]. Normally they are water-soluble and more stable under simple storage conditions [15] and their stability can be easily obtained using standard physicochemical characterization methods. Furthermore, as the viral strains are mutating, the peptide vaccines could be quickly tailored to cope with those specific mutating strains by epitope recognition among the targeted human population [13] which would be very difficult to accommodate in traditional vaccine regimes.
This implies the peptide vaccines can respond rapidly as needed to changes in the SARS-CoV-2 virus and can be easily modified to cope with upcoming viral variants. Over the past, peptide vaccines have exhibited tremendous efficiency against multiple types of cancers, ranging from their applications against cancer-causing Human Papillomavirus (HPV) [16] to patients with unresectable Stage III or IV melanoma [17]. In a similar way, many promising peptide-based vaccines in COVID-19 are also in development namely FlowVax COVID-19 vaccine by Flow Pharma, DPX COVID-19 by IVM Inc, Ii-Key Peptide-based Covid-19 vaccine by Generex Biotechnology [18].
In this article, we have described a novel computational approach that takes into account the surface-exposed protein of SARS-CoV-2, that is, spike glycoprotein, and recommends the best possible vaccine candidates for the virus [19]. The method offers two primary advantages in comparison with other competent techniques. Firstly, it is based on an alignment-free mathematical model and hence it avoids the enormous computational cost involved in residue-residue comparison while applying the otherwise frequently-used alignment models for vaccine design. Secondly, it considers only those vaccine targets that remain conserved against mutation, thus ensuring long-term immunity against various strains of the virus.
The spike protein has been chosen as the basis for this design as it is the antigenic component among all structural proteins for SARS-CoV-2 and it is responsible for inducing the host immune response. Vaccines designed from spike protein can induce protective immunity against viral infection [20].
The hypothesis
Defining the problem
The main focus of this in silico experiment will be to test whether our computational approach [19] is able to recommend the most suitable peptide vaccine targets against SARS-CoV-2 such that they evade all possible mutations in the frequently observed strains of concern of the virus and are not concealed by any neighbouring amino acids. The factors involved in evaluating this hypothesis include studying the mutation probability and solvent accessibility across the spike protein sequence and checking the epitope potential and autoimmunity of the selected peptide targets. The experiment's outcome will be a list of peptide candidates based on which the vaccines can be prepared and checked for their efficacy in wet labs.
By determining mutation probability as an input parameter in this method, we ensure that the vaccine targets are based on only those regions of the spike protein which have shown negligible tendency to vary, both with time and among the host population.
Mathematical foundation for the selection
The first stage of the method involves a two-stepped model described as follows.
Step 1: Defining a ‘w’ parameter to find conserved and exposed regions
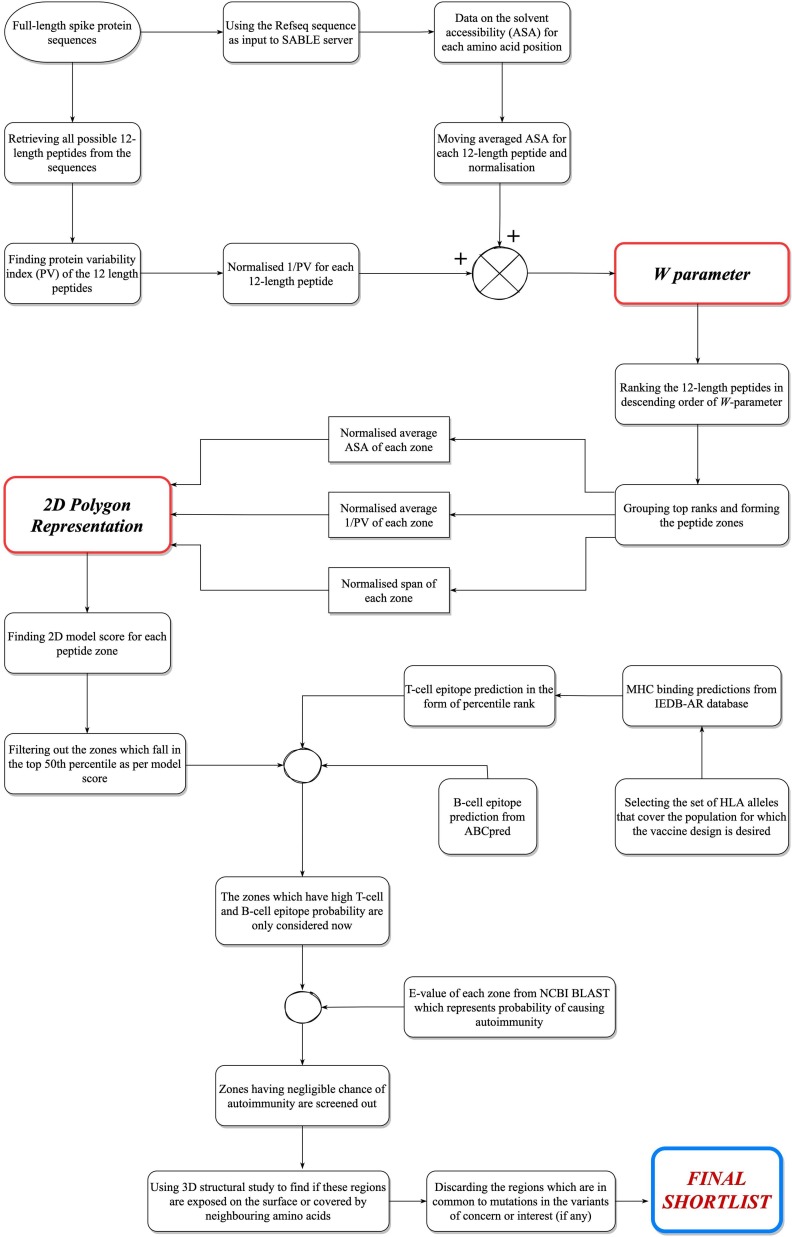
The process begins with calculating a w parameter for every possible 12-length peptide from the spike protein sequence. The w parameter depends on quantified representations of both surface exposure (ASA) and conservativeness (1/PV) for all these peptides. A ranking list for all the 12-length peptides is prepared in descending order of their w values. The top-ranked peptides are then “grouped” as per their position in the sequence, by following certain necessary conditions [19]. This eventually gave 16 grouped “peptide zones”.
Step 2: Using 2D Polygon Representation, a mathematical model, to find peptide groups that are conserved, exposed and spanned across a large area
This step deals with calculating a score for these zones with the help of a mathematical model, namely, “2D Polygon Representation” [19]. The model uses the normalized versions of three different parameters – average ASA, average 1/PV, and the span of each zone. Span gives the area that the peptide region covers on the surface of the protein. Average ASA or average 1/PV are defined as the average of the ASA and 1/PV values respectively of all the 12-length peptides that constitute the grouped peptide zone under consideration. In other words, average ASA and average 1/PV signify the overall surface exposure and the overall conservativeness respectively, of the peptide zone. The normalization of all the three parameters has been done on a common scale to indicate that each of the three parameters was of equal weightage. Fig. 1 shows the diagrammatic representation of the 2D Polygon model. The model represents them as the lengths of three concurrent arms, 120 degrees apart from each other. The area of the triangle formed by the three free ends of the arms is considered as the score generated by the model for that peptide zone. The zones are now ranked in descending order of their respective model scores. As in Fig. 1, OA, OB and OC represent the three arms and the area of ΔABC gives the score. Here |OA| = length of OA arm, gives the normalised average ASA. Similarly, |OB| gives normalised average 1/PV and |OC| gives the normalised length of the peptide region.
Fig. 1.
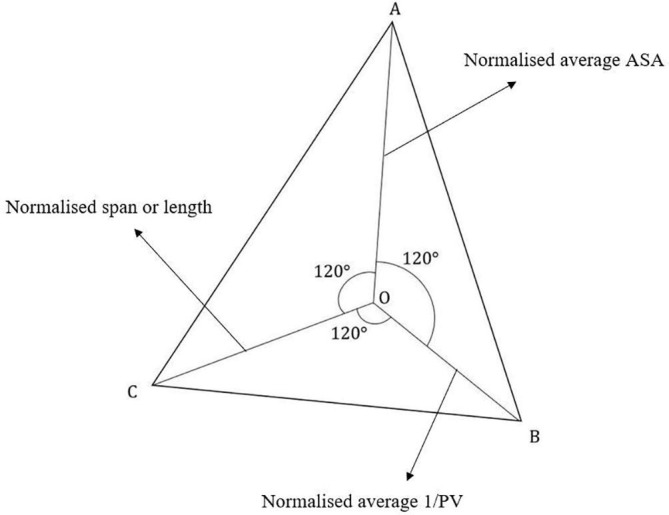
Geometrical overview of the 2D Polygon Representation (The three arms OA, OB and OC represent the three parameters used in the model).
The zones in the top 50th percentile as per the 2D model score are to be considered for two further steps – first, to check the epitope potential of the regions and second, to check whether they cause autoimmune threats in human hosts. The epitope potential of the regions was primarily judged by studying the T-cell epitopes given by the MHC-II binding predictions in Immune Epitope Database Analysis Resource (IEDB-AR) [21]. Finally, we checked if the qualified zones pose any significant chance of causing autoimmune threats [19], using NCBI Basic Local Alignment Search Tool (BLAST) server [22].
Analysing the peptide regions obtained
3D visualization of the shortlisted peptide regions using PyMOL
PyMOL, a widely used molecular visualization tool [23], has been used here to observe the location of the selected peptide regions on the spike protein. Previously, it was the two-stepped mathematical approach that used a quantified representation of ASA to determine surface exposure. But now, using PyMOL, the regions are visualised in a 3D structural model to understand whether they are surface accessible or, partially or fully covered by neighbouring amino acids. For this, we have retrieved a 3D structural model having Protein Data Bank ID “6VYB” of the spike protein of SARS-CoV-2 [24]. The model is available in the RCSB Protein Data Bank [25]. The PyMOL interface contains multiple options to modify the way the models or the zones highlighted in the model are visualised.
Checking whether the peptide regions are also capable of producing B-cell immune response
Previously, the analysis was mainly focused on verifying the epitope potential of the regions by determining their T-cell immunogenicity, considering their binding with MHC-II alleles. But in addition to this, it is also necessary to know about their B-cell immunogenicity. We have used the ABCpred server for this purpose [26]. The server takes the full-length amino acid sequence of the spike protein as input. The threshold and the number of amino acids desired in each B-cell epitope can be selected accordingly. Finally, using a recurrent neural network, the server predicts the relevant B-cell epitopes. We have compared the shortlisted peptide regions obtained from our analyses with this list. In the end, we were able to comprehend that the peptide candidates show both T-cell and B-cell immunogenicity.
Analysing and comparing the mutations of the most frequently occurring variants of SARS-CoV-2 with the shortlisted peptide regions
The Centers for Disease Control and Prevention (CDC) presents a list of all the variants of concern and the variants of interest of SARS-CoV-2 with respect to the current scenario [27]. Simultaneously, a list of all synonymous and non-synonymous mutations occurring in the spike protein domain of each of these variants have been given in the CoVariants website [28]. Now, in this regard, every time a new strain of the virus arrives, it is always necessary to check whether the existing or proposed vaccines can work against these mutated variants. So, for an effective peptide vaccine design, it is imperative that the vaccine candidates are selected in a way that they do not contain any region where the mutations in these variants have occurred. For this purpose, we have compared our shortlisted peptide regions with the places of mutation of the variants of concern and interest as well as with that of the recently emerging Omicron variant. Finally, the comparison showed that there was nothing in common between the mutations and the shortlisted peptide regions, thus proving our method’s efficacy.
Data sources
The spike protein sequences for our study have been retrieved from the NCBI database [29]. We have particularly used the sequence YP_009724390.1 as input for the ABCpred server for determining the B-cell epitopes [30]. The SABLE server [31], [32], [33], [34] has been used to obtain the solvent accessibility values for each amino acid position of the spike protein sequence. Using the moving average technique with a window of 12, the quantified expressions of surface exposure (ASA) of the 12-length peptides are then obtained.
Fig. 2 gives a flowchart of the entire protocol of designing vaccine candidates for SARS-CoV-2. Hence, by executing all the aforementioned steps in this approach, we hypothesize that this will give us the regions on the surface glycoprotein of SARS-CoV-2 that should be targeted for designing effective vaccines.
Fig. 2.
Complete overview of our approach to design peptide vaccine targets for COVID 19.
Evaluation of the hypothesis
With the help of the two-stepped mathematical approach (w parameter and 2D Polygon Representation), peptide regions were selected which were surface accessible, conserved and spanned across a broad area [19]. The ones which lacked insufficient T-cell immunogenicity and showed significant chances of auto-immunity were discarded thereafter. Table 2 gives the list of the remaining peptide regions which were shortlisted as possible vaccine candidates.
Table 2.
List of the finally selected peptide vaccine candidates from spike protein of SARS-CoV-2 [19].
| Starting position of the candidate in the sequence | Ending position of the candidate in the sequence | Peptide stretch |
|---|---|---|
| 1021 | 1035 | SANLAATKMSECVLG |
| 527 | 541 | PKKSTNLVKNKCVNF |
| 459 | 470 | SNLKPFERDIST |
| 987 | 1001 | VEAEVQIDRLITGRL |
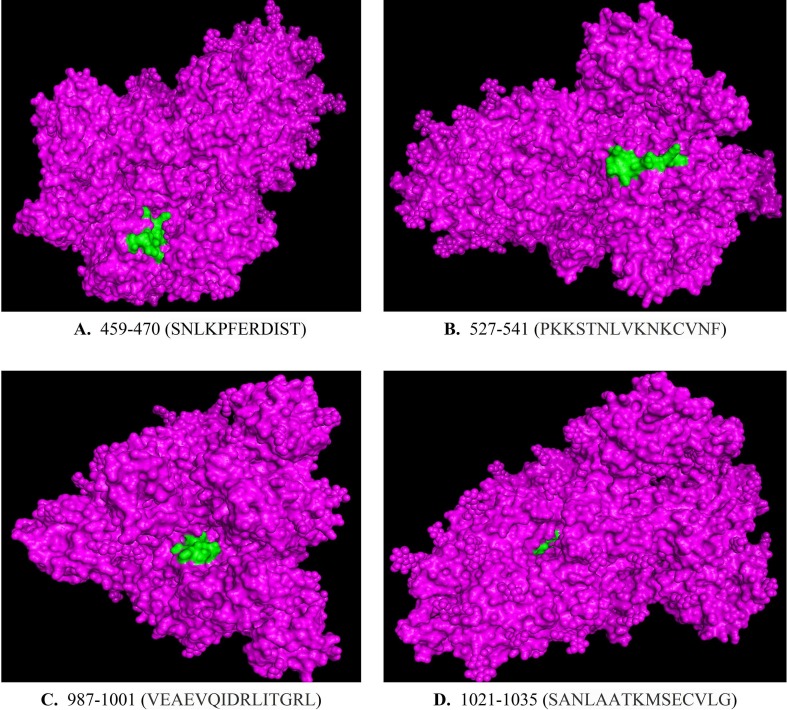
Now, using PyMOL, we have visualised these 4 peptide regions. The 3D model “6VYB” was first loaded in the software before highlighting these peptide regions. Fig. 3 (A)–(D) show an overview of the vaccine targets on the 3D simulation in PyMOL.
Fig. 3.
A brief overview of the geometrical location of the four target regions (A) 459–470, (B) 527–541, (C) 987–1001 and (D) 1021–1035, on the 3D model of the trimeric structure of spike protein. The target regions have been highlighted in green. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
As in Fig. 3(A)–(D), the regions 527–541, 459–470 and 987–1001 are very well surface-exposed. On the contrary, the region 1021–1035 is almost fully covered by its neighbouring amino acids, and hence, not accessible even after having a high normalized average ASA. A possible explanation for this is that the spike protein has a trimeric structure, that is, it consists of three chains A, B and C. Our analyses are limited only to the protein sequence of a single chain. Because the region 1021–1035 falls in the place where the three chains converge together, it gets enclosed within the junction area of the chains and as a whole, it is not well accessible. Ultimately, we are left with three peptide candidates: 527–541, 459–470 and 987–1001, which can be tested further. Region 1021–1035, meanwhile, has to be discarded.
Using the ABCpred server, we have then checked the B-cell immunogenicity of the three regions tentatively selected. We have used a threshold of 0.65 for a stricter condition of predicting the B-cell epitopes. We looked for all possible 16-length B-cell epitopes from the server using that threshold, as listed in Table S1 of the Supplementary file. The corresponding score given beside each entry in Table S1 in the file represents the probability of such a peptide being a B-cell epitope. A higher score means a greater probability of that happening. Now, on comparing the three regions 527–541, 459–470 and 987–1001 with this list, we found that all of them were contained partially or fully with the matching epitopes. This shows that these regions not only can cause a T-cell immune response but can also generate a B-cell response. Table 3 gives a list of the best matching B-cell epitope with the best possible score corresponding to the three peptide regions.
Table 3.
Comparison of the three selected regions with the best matching B-cell epitope having the best possible score with threshold = 0.65.
| Peptide regions | B-cell epitope | Start position | End position | Score |
|---|---|---|---|---|
| PKKSTNLVKNKCVNF | CGPKKSTNLVKNKCVN | 525 | 540 | 0.86 |
| VEAEVQIDRLITGRL | EAEVQIDRLITGRLQS | 988 | 1003 | 0.73 |
| SNLKPFERDIST | RKSNLKPFERDISTEI | 457 | 472 | 0.65 |
In the end, we retrieved the information about the mutations in the variants of concern and variants of interest of SARS-CoV-2 (as of August 18, 2021). In Table 4 , for each variant of concern and each variant of interest, we have compared the three regions with the spike protein mutations associated with the variant. For every case, we found that there were no matches. We further compared the regions with the mutations occurring in the spike protein of the currently surging Omicron variant of SARS-CoV-2 [28], and observed that for this case as well, there was no coherence between the mutations and the peptide targets.
Table 4.
Comparison of the three peptide vaccine candidates with the mutations present in the most significant variants of SARS-CoV-2. The table shows that the regions are all free from any of the listed mutations.
| As of August 18, 2021 | ||||
|---|---|---|---|---|
| For variants of concern | Variant | Spike Protein mutations | Peptide Sequences to be matched | Remark |
| Alpha (B.1.1.7) Originated in: United Kingdom |
69del, 70del, 144del, E484K, S494P, N501Y, A570D, D614G, P681H, T716I, S982A, D1118H, K1191N | PKKSTNLVKNKCVNF (527-541) | No matches found | |
| SNLKPFERDIST (459-470) | ||||
| VEAEVQIDRLITGRL (987-1001) | ||||
| Beta (B.1.351, B.1.351.2, B.1.351.3) Originated in: South Africa |
D80A, D215G, 241del, 242del, 243del, K417N, E484K, N501Y, D614G, A701V | PKKSTNLVKNKCVNF (527-541) | No matches found | |
| SNLKPFERDIST (459-470) | ||||
| VEAEVQIDRLITGRL (987-1001) | ||||
| Delta (B.1.617.2, AY.1, AY.2, AY.3) Originated in: India |
T19R, V70F, T95I, G142D, E156-, F157-, R158G, A222V, W258L, K417N, L452R, T478K, D614G, P681R, D950N | PKKSTNLVKNKCVNF (527-541) | No matches found | |
| SNLKPFERDIST (459-470) | ||||
| VEAEVQIDRLITGRL (987-1001) | ||||
| Gamma (P.1, P.1.1, P.1.2) Originated in: Japan, Brazil |
L18F, T20N, P26S, D138Y, R190S, K417T, E484K, N501Y, D614G, H655Y, T1027I | PKKSTNLVKNKCVNF (527-541) | No matches found | |
| SNLKPFERDIST (459-470) | ||||
| VEAEVQIDRLITGRL (987-1001) | ||||
| For variants of interest | B.1.427 Originated in: United States (California) |
L452R, D614G | PKKSTNLVKNKCVNF (527-541) | No matches found |
| SNLKPFERDIST (459-470) | ||||
| VEAEVQIDRLITGRL (987-1001) | ||||
| B.1.429 Originated in: United States (California) |
S13I, W152C, L452R, D614G | PKKSTNLVKNKCVNF (527-541) | No matches found | |
| SNLKPFERDIST (459-470) | ||||
| VEAEVQIDRLITGRL (987-1001) | ||||
| Eta (B.1.525) Originated in: United Kingdom, Nigeria |
A67V, 69del, 70del, 144del, E484K, D614G, Q677H, F888L | PKKSTNLVKNKCVNF (527-541) | No matches found | |
| SNLKPFERDIST (459-470) | ||||
| VEAEVQIDRLITGRL (987-1001) | ||||
| Iota (B.1.526) Originated in: United States (New York) |
L5F, D80G, T95I, Y144-, F157S, D253G, L452R, S477N, E484K, D614G, A701V, T859N, D950H, Q957R | PKKSTNLVKNKCVNF (527-541) | No matches found | |
| SNLKPFERDIST (459-470) | ||||
| VEAEVQIDRLITGRL (987-1001) | ||||
| Kappa (B.1.617.1) Originated in: India |
T95I, G142D, E154K, L452R, E484Q, D614G, P681R, Q1071H | PKKSTNLVKNKCVNF (527-541) | No matches found | |
| SNLKPFERDIST (459-470) | ||||
| VEAEVQIDRLITGRL (987-1001) | ||||
| B.1.617.3 Originated in: India |
T19R, G142D, L452R, E484Q, D614G, P681R, D950N | PKKSTNLVKNKCVNF (527-541) | No matches found | |
| SNLKPFERDIST (459-470) | ||||
| VEAEVQIDRLITGRL (987-1001) | ||||
Therefore, we can comment that the 3 regions, PKKSTNLVKNKCVNF (527–541), SNLKPFERDIST (459–470) and VEAEVQIDRLITGRL (987–1001) have the following characteristics as listed below:
3.1 They are surface-accessible, conserved and occupy a broad area on the spike protein surface.
3.2 They show both B-cell and T-cell immunogenicity.
3.3 They have negligible chances of causing any autoimmune threats in a human host.
3.4 Among the significant variants of SARS-CoV-2 in the current standpoint, there is no such mutation that falls within our proposed peptide vaccine candidates.
Taking all the above characteristics into account, we can therefore suggest that these peptide regions are suitable for vaccine design against SARS-CoV-2. A special significance of the region SNLKPFERDIST (459–470) is that it is a highly surface-accessible and a highly conserved peptide region falling under the receptor-binding domain (RBD) of the spike protein, that is, the region where it binds with the ACE2 receptor which ranges from 318 to 513 amino acid residues [35]. Moreover, the RBD region is a critical target for neutralizing antibodies and some vaccines [36]. Now as we are able to find the region, SNLKPFERDIST (459–470) which completely lies within this RBD domain, the peptide vaccine designed using this amino acid stretch can indeed be a suitable candidate for a potential therapeutic. Additionally, as this peptide region is highly conserved throughout the various strains, we can hypothesize that it plays a critical role in attaching with the human ACE2 receptor directly or indirectly.
However, these peptides when used alone, generally show low immunogenicity. The targets can be, thus, coupled with innate immune agonists in the form of adjuvants to improve the immunogenic response and ensure a long-term immunity in the host body [37]. Furthermore, in vitro and in vivo evaluation of such a vaccine on animal models through wet lab tests is also needed to verify its efficacy before performing its trial on the infected patients.
Discussion
The findings described here takes a step ahead from our previous work [19] to propose target regions on the spike protein that can be utilised for vaccine design against the most impactful strains of SARS-CoV-2 prevailing right now. Moreover, from the structural analysis of the spike protein explained in this article, we have found that one particular peptide region, mentioned in the previous work, remains sequestered by its neighbouring amino acids, for which it has not been recommended for use.
The three remaining peptide candidates which we highly recommend for vaccine production are conserved that is, they have not been affected by any sort of mutations in these variants. Simultaneously, these three regions are also well exposed on the surface of the spike protein, have negligible chance of causing autoimmune disorders, and are capable of producing adequate B-cell and T-cell epitopes in the host.
However, our approach is limited to an in silico study. Therefore, through different phases of human trials, it is yet to be seen whether vaccines prepared using these targets can sustain a prolonged immunity in the human host. Safety is another area of concern when it comes to choosing the proper adjuvants for this design.
Nevertheless, the protocol suggests that the peptide vaccines prepared using these regions have a high possibility of preventing outbreaks if used in response to the introduction of a new strain of the virus as well as work efficiently against the existing strains. They can be universally used as a proactive safeguard against SARS-CoV-2 by mitigating the risk of its re-emergence in humans. With these peptides, or by adopting the approach communicated in this article, perhaps we can also prevent a “SARS-CoV-3” in the future, that is, this technique can also be followed up to prevent future pandemics.
It is now up to the wet lab scientists to use these peptide stretches and combine them with suitable adjuvants to design effective peptide vaccine formulations. We envision that with this procedure, the recurring outbreaks of the COVID 19 strains can be reduced while minimising the massive economic losses as well.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Ethical approval
Not required.
CRediT authorship contribution statement
Subhamoy Biswas: Methodology, Formal analysis, Software, Visualization, Resources, Data curation, Writing – original draft. Sumanta Dey: Visualization, Resources, Formal analysis, Validation, Writing – original draft. Shreyans Chatterjee: Resources, Formal analysis, Writing – original draft. Ashesh Nandy: Supervision, Project administration, Validation, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank Professor Subhash C. Basak (Department of Chemistry and Biochemistry, University of Minnesota Duluth, Minnesota, US) for his constant support during the preparation of the work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mehy.2022.110810.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.World Health Organization. WHO Coronavirus (COVID 19) Dashboard. [accessed on 2021 August 23] https://covid19.who.int/.
- 2.Wikipedia contributors. COVID-19 vaccine. Wikipedia, The Free Encyclopaedia. [accessed on 2021 August 12] https://en.wikipedia.org/wiki/Covid-19_vaccine.
- 3.World Health Organization. WHO lists additional COVID-19 vaccine for emergency use and issues interim policy recommendations. 2021 May 7 [accessed on 2021 August 12] https://www.who.int/news/item/07-05-2021-who-lists-additional-covid-19-vaccine-for-emergency-use-and-issues-interim-policy-recommendations.
- 4.Oh K.K., Adnan M., Cho D.H. Network pharmacology approach to decipher signaling pathways associated with target proteins of NSAIDs against COVID-19. Sci Rep. 2021;11:9606. doi: 10.1038/s41598-021-88313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oh KK, Adnan M, Cho DH. Drug-repurposing against COVID-19 by targeting a key signaling pathway: An in silico study, Medical Hypotheses, Volume 155, 2021, 110656, ISSN 0306-9877, doi: 10.1016/j.mehy.2021.110656. [DOI] [PMC free article] [PubMed]
- 6.Sadarangani M., Marchant A., Kollmann T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat Rev Immunol. 2021;21(8):475–484. doi: 10.1038/s41577-021-00578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dan J.M., Mateus J., Kato Y.u., Hastie K.M., Yu E.D., Faliti C.E., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529) doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaebler C., Wang Z., Lorenzi J.C.C., Muecksch F., Finkin S., Tokuyama M., et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591(7851):639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagen A. How Dangerous Is the Delta Variant (B.1.617.2)? [blog]. American Society for Microbiology. 2021 July 30 [accessed on 2021 August 12]. https://asm.org/Articles/2021/July/How-Dangerous-is-the-Delta-Variant-B-1-617-2.
- 10.Planas D., Veyer D., Baidaliuk A., Staropoli I., Guivel-Benhassine F., Rajah M.M., et al. Reduced sensitivity of SARS-CoV-2 variant delta to antibody neutralization. Nature. 2021;596(7871):276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 11.Lopez Bernal J., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med. 2021;385(7):585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatterjee S, Dey S, Nandy A, Basak SC. A Computational Search for Peptide Vaccines Using Novel Mathematical Descriptors of Sequences of Emerging Pathogens. Topics in Medicinal Chemistry. Springer, Berlin, Heidelberg, 2020. doi: 10.1007/7355_2020_108.
- 13.Nandy A., Dey S., Roy P., Basak S.C. Epidemics and peptide vaccine response: a brief review. Curr Top Med Chem. 2018;18(26):2202–2208. doi: 10.2174/1568026618666181112144745. [DOI] [PubMed] [Google Scholar]
- 14.Skwarczynski M., Toth I. Peptide-based synthetic vaccines. Chem Sci. 2016;7(2):842–854. doi: 10.1039/c5sc03892h. PMID: 28791117; PMCID: PMC5529997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W., Joshi M., Singhania S., Ramsey K., Murthy A. Peptide vaccine: progress and challenges. Vaccines. 2014;2(3):515–536. doi: 10.3390/vaccines2030515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dey S., De A., Nandy A. Rational design of peptide vaccines against multiple types of human papillomavirus. Cancer Inf. 2016;15s1:CIN.S39071. doi: 10.4137/CIN.S39071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Identifier NCT00094653, MDX-010 Antibody, MDX-1379 Melanoma Vaccine, or MDX-010/MDX-1379 Combination Treatment for Patients With Unresectable or Metastatic Melanoma; 2011 June 23 [cited 2022 Jan 06]. Available from: https://clinicaltrials.gov/ct2/show/NCT00094653.
- 18.Di Natale C., La Manna S., De Benedictis I., Brandi P., Marasco D. Perspectives in peptide-based vaccination strategies for syndrome coronavirus 2 pandemic. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.578382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biswas S., Manna S., Nandy A., Basak S.C. New computational approach for peptide vaccine design against SARS-COV-2. Int J Pept Res Ther. 2021;27(4):2257–2273. doi: 10.1007/s10989-021-10251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Y., Yang C., Xu X.-F., Xu W., Liu S.-W. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020;41(9):1141–1149. doi: 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vita R, Mahajan S, Overton JA, Dhanda SK, Martini S, Cantrell JR, Wheeler DK, Sette A, Peters B. The immune epitope database (IEDB): 2018 update. Nucleic Acids Res., 2018. doi: 10.1093/nar/gky1006. [DOI] [PMC free article] [PubMed]
- 22.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 23.PyMOL. Version 2.5 [software]. Schrodinger, LLC. 2010. [accessed on 2021 August 08] https://pymol.org/2/.
- 24.Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARSCoV-2 spike glycoprotein. Cell. 2020;181(2):281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berman H.M., Westbrook J., Feng Z., Gilliland G., Bhat T.N., Weissig H., et al. The protein data bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saha S., Raghava G.P.S. Prediction of continuous B-cell epitopes in an antigen using recurrent neural network. Proteins. 2006;65(1):40–48. doi: 10.1002/prot.21078. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention (CDC). SARS-CoV-2 Variants Classifications and Definitions. [accessed on 2021 August 18] https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html.
- 28.Hodcroft EB. 2021. CoVariants: SARS-CoV-2 Mutations and Variants of Interest. https://covariants.org/.
- 29.National Center for Biotechnology Information (NCBI)[Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; [1988] – [cited 2021 August 14]. Available from: https://www.ncbi.nlm.nih.gov/.
- 30.Wu F., Zhao S.u., Yu B., Chen Y.-M., Wang W., Song Z.-G., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adamczak R., Porollo A., Meller J. Accurate prediction of solvent accessibility using neural networks based regression. Proteins Struct Funct Bioinf. 2004;56(4):753–767. doi: 10.1002/prot.20176. [DOI] [PubMed] [Google Scholar]
- 32.Adamczak R., Porollo A., Meller J. Combining prediction of secondary structure and solvent accessibility in proteins. Proteins Struct Funct Bioinf. 2005;59(3):467–475. doi: 10.1002/prot.20441. [DOI] [PubMed] [Google Scholar]
- 33.Wagner M., Adamczak R., Porollo A., Meller J. Linear regression models for solvent accessibility prediction in proteins. J Comput Biol. 2005;12(3):355–369. doi: 10.1089/cmb.2005.12.355. [DOI] [PubMed] [Google Scholar]
- 34.Porollo A, Adamczak R, Wagner M, Meller J. Maximum Feasibility Approach for Consensus Classifiers: Applications to Protein Structure Prediction, CIRAS 2003 (conference proceedings).
- 35.Di Paola L., Hadi-Alijanvand H., Song X., Hu G., Giuliani A. The discovery of a putative allosteric site in the SARS-CoV-2 spike protein using an integrated structural/dynamic approach. J Proteome Res. 2020;19(11):4576–4586. doi: 10.1021/acs.jproteome.0c00273. [DOI] [PubMed] [Google Scholar]
- 36.Min L., Sun Q. Antibodies and vaccines target RBD of SARS-CoV-2. Front Mol Biosci. 2021;8:247. doi: 10.3389/fmolb.2021.671633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Long Y., Sun J., Liu T., Tang F., Zhang X., Qin Q., et al. CoVac501, a self-adjuvanting peptide vaccine conjugated with TLR7 agonists, against SARS-CoV-2 induces protective immunity. bioRxiv. 2021 doi: 10.1101/2021.04.10.439275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.