Abstract
Peer rejection powerfully predicts adolescent anxiety. While cognitive differences influence anxious responses to social feedback, little is known about neural contributions. Twelve anxious and 12 age-, gender- and IQ-matched, psychiatrically-healthy adolescents received ‘not interested’ and ‘interested’ feedback from unknown peers during a Chatroom task administered in a neuroimaging scanner. No group differences emerged in subjective ratings to peer feedback, but all participants reported more negative emotion at being rejected (than accepted) by peers to whom they had assigned high desirability ratings. Further highlighting the salience of such feedback, all adolescents, independent of anxiety levels, manifested elevated responses in the amygdala-hippocampal complex bilaterally, during the anticipation of feedback. However, anxious adolescents differed from healthy adolescents in their patterns of persistent amygdala-hippocampal activation following rejection. These data carry interesting implications for using neuroimaging data to inform psychotherapeutic approaches to social anxiety.
Keywords: anxiety, adolescence, peer rejection, amygdala
Adolescence is characterized by a number of changes in the social domain which may be mediated by maturation of brain circuits (Nelson, Leibenluft, McClure, & Pine, 2005; Sebastian, Viding, Williams, & Blakemore, 2010). One notable change is an increase in the emotional salience of peers. Adolescents spend more time interacting with peers than do either young children or adults, and they exert a great deal of energy forming peer networks and soliciting peer approval (Steinberg & Morris, 2001). Although solicitation of peer approval can invite risky behavior, positive experiences with peers are generally psychologically beneficial (Allen, Porter, McFarland, Marsh, & McElhaney, 2005; Gardner & Steinberg, 2005). On the other hand, negative peer experiences often incur marked adverse outcomes on overall well-being (Gazelle & Rudolph, 2004; Hawker & Boulton, 2000; Kingery, Erdley, Marshall, Whitaker, & Reuter, 2010; Steinberg & Morris, 2001; Storch & Ledley, 2005). For a subset of adolescents, intense peer-focused emotional experiences, especially those that involve peer rejection, can induce or exacerbate clinical disorders (Gazelle & Rudolph, 2004; Kingery et al., 2010; La Greca & Harrison, 2005). As early-emerging symptoms of anxiety and depression predict mood and anxiety problems throughout the lifespan (Pine, Cohen, Cohen, & Brook, 1999), identifying differences in responses to peer rejection at multiple levels of analysis in anxious and psychiatrically-healthy adolescents can help elucidate the nature of maladaptive social functioning, and inform targets for therapeutic interventions.
Cognitive factors such as distorted information-processing and ineffective coping strategies may play a role in determining anxious responses to social rejection (Kingery et al., 2010). More particularly, anxious adolescents show greater vigilance than non-anxious adolescents for salient affective stimuli, including social signals (Dalgleish et al., 2003; Waters, Lipp, & Spence, 2004). They also appraise ambiguous social situations more negatively and hold more negative views of their own social competence (Kingery et al., 2010). Finally, anxious youth engage in more problematic forms of coping in the face of difficult peer relationships, selecting passive-avoidant and emotion-focused strategies rather than active self- or problem-directed strategies (Erath, Flanagan, & Bierman, 2007; Sandstrom, 2004). These findings are consistent with cognitive and neuroscience theories of anxiety, which suggest that heightened bottom-up emotional reactivity interacts with difficulties in top-down regulatory control, producing exaggerated responses to negative experiences (Bishop, 2007; Rapee & Heimberg, 1997). An important result of these cognitive patterns is that they can create positive feedback loops (La Greca & Lopez, 1998). For example, being hyper-vigilant for negative social information may ultimately allow the socially anxious adolescent to detect more negative social signals, reinforcing the tendency toward vigilance. Thus, anxious cognitive patterns about feared social situations tend to maintain and even precipitate further peer rejection.
While patterns of anxious social responding have been well characterized at the cognitive and behavioral levels, these patterns have not been clearly mapped onto neural circuits, particularly in adolescents. If behavioral patterns of anxious responding to social rejection are related to differences in cognitive responses, then they may also be reflected in patterns of brain response. Some studies have begun to elucidate the neural signature of response to social rejection (Eisenberger & Lieberman, 2004; Sebastian et al., 2010), but these have mainly focused on psychiatrically-healthy adults (Eisenberger, Gable, & Lieberman, 2007; Eisenberger, Lieberman, & Williams, 2003; Somerville, Heatherton, & Kelley, 2006) and typically-developing adolescents (Gunther Moor, van Leijenhorst, Rombouts, Crone, & Van der Molen; Guyer, McClure-Tone, Shiffrin, Pine, & Nelson, 2009; Masten et al., 2009; Masten, Eisenberger, Pfeifer, & Dapretto, 2010). Only one study has probed the neural signature of social responding in anxious adolescents, focusing on the anticipation of peer evaluations (Guyer et al., 2008). Thus, current predictions on the neural substrates of anxious responding to social rejection draw not only on this single study, but more widely, on results from neuroimaging studies of affective processing in anxious individuals. These data clearly implicate aberrant amygdala function to emotional stimuli, including social stimuli, as a core feature of adult (Freitas-Ferrari et al., 2010; Phelps & LeDoux, 2005) and adolescent anxiety disorders and related phenotypes (Beesdo et al., 2009; Guyer et al., 2008; McClure et al., 2007; Monk, 2008; Pine, Helfinstein, Bar-Haim, Nelson, & Fox, 2009).
One way in which amygdala activity may differ in anxious individuals is in the duration of activity after onset of amygdala engagement. In adults, a pattern of prolonged emotional responding to negatively valenced stimuli has been found in mood-dysregulated individuals (Siegle, Steinhauer, Thase, Stenger, & Carter, 2002). This inability to ‘recover’ from a negative emotional experience may arise from dysfunctions in the inhibition of amygdala activation, or possibly even active up-regulation of amygdala responsiveness (Davidson, 2002; Jackson et al., 2003). Although not studied extensively, anxious youth may be even more susceptible than adults to regulatory distortions, especially during social events of extreme salience, because of the delayed structural and functional maturation of regions like the ventral prefrontal cortex, which exerts regulatory control on amygdala activity (Gogtay et al., 2004; Monk, 2008).
In the present study, we assessed amygdala activation patterns during a peer feedback task in a group of anxious and non-anxious adolescents using functional neuroimaging. We focused on brain activation before and after receipt of ‘reject’ feedback from unknown peers. Based on previous studies of anxious youth and neuroimaging data in anxious adults, we predicted that the current sample of anxious adolescents would display a pattern of persistent amygdala activity after receiving rejecting feedback, guided by the hypothesis that emotional amygdala reactivity to a feared social event is highly prominent in anxious adolescents. To assess specificity of these patterns to reject feedback only, we also investigated amygdala responses to the receipt of ‘accept’ feedback from peers. A more exploratory analysis also considered additional cortical regions of the brain given previous findings of perturbed prefrontal activity during emotional processing in anxious adolescents (Monk, 2008) and involvement of other cortical regions such as the anterior cingulate cortex during social processing in typically-developing adolescents (Masten et al., 2009; Masten et al., 2010).
Methods
Participants
Participants were 12 medication-free adolescents with a current DSM-IV anxiety diagnosis and 12 psychiatrically healthy adolescents. All patients were recruited when they sought treatment for anxiety related to social situations while healthy adolescents were recruited through local schools and newspaper advertisements. Participants from each group were matched on age, gender, IQ and SES (Table 1).
Table 1:
Demographics, diagnoses and self-reported affective ratings across participants
| Anxious patients (n = 12) |
Healthy controls (n = 12) |
|
|---|---|---|
| Demographics | ||
| Mean age | 11.88 (2.48) | 12.23 (2.44) |
| Number of females (% of sample) | 8 (66.7%) | 8 (66.7%) |
| SES | 8.30 (.82) | 7.82 (1.33) |
| Full scale IQ | 115.50 (14.71) | 119.17 (7.96) |
| Anxiety diagnosis | ||
| Generalized Anxiety Disorder | 7 | - |
| Social Phobia | 7 | - |
| Separation Anxiety | 4 | - |
| Specific Phobia | 5 | - |
| Affective ratings | ||
| To reject feedback | 47.54 (20.64) | 55.95(15.12) |
| From high desired individuals | 42.12 (17.06) | 50.38 (16.63) |
| From low desired individuals | 52.64 (26.64) | 61.94 (17.89) |
| To accept feedback | 51.15 (17.33) | 58.38 (14.36) |
| From high desired individuals | 58.89 (20.71) | 67.08 (15.72) |
| From low desired individuals | 43.31 (20.95) | 47.61 (19.87) |
The Kiddie Schedule for Affective Disorders – Present and Lifetime version (K-SADS-PL Kaufman, Birmaher, Brent, Ryan, & Rao, 2000) determined patient diagnoses and confirmed healthy status of comparison subjects. Patient diagnoses included generalized anxiety disorder (n=7), social phobia (SP; n=7), and separation anxiety disorder (n=4). Four subjects were diagnosed with two anxiety disorders while two subjects received all three diagnoses. Secondary current diagnoses in patients included major depressive disorder (MDD; n=1), attention-deficit/hyperactivity disorder (ADHD; n=1) and specific phobia (n=5). Patients who did not meet criteria for current SP (n=5) reported clinically significant social concerns, such as fear of social interaction or social performance. Other inclusion criteria for patients comprised: clinically significant anxiety on the Pediatric Anxiety Rating Scale (PARS; score >=10); significant impairment on the Child Global Assessment Schedule (CGAS: Shaffer et al., 1983; score <60); and persistent anxiety during 3 weeks of supportive therapy. Exclusion criteria were: obsessive compulsive disorder, Tourette’s syndrome, post-traumatic stress disorder, oppositional defiant disorder, or conduct disorder; exposure to trauma; suicidal ideation; lifetime history of mania, psychosis, or pervasive developmental disorder (PDD); IQ<70; contraindications for magnetic resonance imaging (e.g. pacemaker, pregnancy, braces), and use of any psychoactive substance. Healthy adolescents were free of current psychiatric disorder and lifetime history of psychosis, PDD, major affective disorder, CD, ADHD, and anorexia.
Task procedures
An ecologically-valid neuroimaging paradigm was developed to examine in vivo neural responses to negative and positive social feedback (Guyer et al., 2008; Guyer et al., 2009). Study participants attended two visits. At the first visit, participants received clinical assessments and information about the study. They were told that they would participate in a collaborative nationwide study across several institutions to examine internet communication across teenagers. Participants were led to believe that they would chat online with another participant from a collaborating institution following their scan session. At the second visit, participants completed the paradigm during functional magnetic resonance imaging (fMRI) acquisition. In actuality, there were no other institutions or participants involved, nor did social interactions occur. All study procedures were approved by the institutional review board at the National Institute of Mental Health (NIMH). All participants and parents/legal guardians provided written informed consent prior to participation. Participants were informed that they would receive misinformation at some point during the course of their testing at NIMH and were debriefed extensively at the conclusion of this study. No adverse reactions to the misinformation were noted.
The paradigm comprised two runs. Data from run 1 concerned neural substrates of self-evaluative processes regarding anticipated social interactions and are already published (Guyer et al., 2008). Data from run 2 formed the basis of the current hypotheses. This run comprised 40 trials presented across 8 minutes (Figure 1). Each trial consisted of three task phases. Participants first viewed a photograph of an unknown peer, whom they believed to be a participant from another institution, for a period of either 3000, 4000, or 5000 msec. Subsequently, the words ‘Not Interested’ or ‘Interested’ (indicating the peer’s desire to talk to the subject) appeared below the photograph to provide peer feedback. After 100 msec, subjects were prompted to rate how they felt on a scale from 0 to 100, where lower ratings indicated greater negative affect. The duration of this rating period was 6900 msec. Two event types were created: initial presentation of the photograph formed the ‘pre-feedback’ event and presentation of the feedback plus the rating response formed the ‘post-feedback’ event. Each event was further divided into ‘Not interested’ or ‘Interested’ feedback events to assess ‘reject’ and ‘accept’ feedback conditions respectively. Task stimuli were drawn from a teen facial expression dataset developed within our laboratory, which contains digital head shots of 20 male and 20 female actors posing happy expressions. These actors varied in age (11-17 years), race, and ethnicity. All reject and accept feedback was randomly assigned (half to each gender). Data in the present study were only from participants who indicated that they believed they would interact with one of the depicted individuals after the scan (80% of the recruited sample). The task was programmed by using E-Prime version 1.1 by Psychological Software Tools (Pittsburgh, PA).
Figure 1:
Schematic of Phase 3 of the Chatroom Task, with reject and accept feedback conditions
fMRI data acquisition and preprocessing
Scanning occurred in a General Electric (Waukesha, WI) Signa 3 tesla magnet. A hand-held two-button response box recorded behavioral ratings (NIMH engineering core, Bethesda, MD). Task stimuli were projected onto a screen at the foot of the scanner bed and viewed with a mirror mounted on the head coil. Head movement was constrained by foam padding. Functional scans were preceded by a localizer and a manual shim procedure. For functional image acquisition, each brain volume contained 29 contiguous 3.3 mm axial slices acquired parallel to the AC/PC line using a single shot gradient echo with T2* weighting with a repetition time (TR) of 2300 ms and echo time (TE) of 23 ms. Voxel dimension was 3.3 x 3.75 x 3.75 mm. A 64 x 64 matrix and field of view (FOV) of 24 cm were used. A high resolution anatomical image was also acquired per subject using a T1-weighted standardized magnetization prepared spoiled gradient recalled echo sequence to aid with spatial normalization using the following parameters: 124 1 mm axial slices, TR of 8100 ms, TE of 32 ms, flip angle of 15°, NEX = 1, 256 x 256 matrix, bandwidth = 31.2 KHz, and FOV of 24 cm.
Standard preprocessing of echo-planar imaging (EPI) data was conducted using the Analysis of Functional and Neural Images (AFNI) software version 2.56b (http://afni.nimh.nih.gov/afni/download/). This included slice time correction; motion correction, including removal of individuals from analyses who moved >3.5 mm in any plane; spatial smoothing with a 6 mm full-width half-maximum Gaussian smoothing kernel; removal of large signal deviations > 2.5 SD from the mean using an AFNI de-spiking algorithm applied on a voxelwise basis; a bandpass filtering algorithm to smooth cyclical fluctuations in signal (either >.011 or <.15 s) that were not temporally indicative of a hemodynamic response; and normalization of blood oxygen level-dependent (BOLD) signal intensity to percentage signal change using each subject’s voxel-wise time series mean as a baseline. Movement artifact was mitigated by using motion correction parameters in the statistical model as nuisance covariates along with a covariate for mean intensity and linear drift. All images were aligned to AC-PC plane and then spatially normalized into Talairach space using algorithms contained in AFNI. Finally, all images were re-sliced using a tri-linear function supplied by AFNI to a 1x1x1 mm resolution.
Data analyses
As subjects initial responses to the social stimuli (e.g., prior to receiving feedback) may moderate affective ratings (Guyer et al., 2008), we asked subjects to first rate their desire to chat with individuals depicted in all photographs. We then performed a median split on these responses to generate high and low desirability groups of stimuli for each participant. Affect ratings following reject and accept feedback were analyzed using a 2x2x2 analysis of variance (ANOVA) with Group (anxious, healthy) as the between-subjects factor and Feedback (reject, accept) and Desirability (high vs. low) as the within-subjects factors.
Primary fMRI analyses explored event-related response amplitudes to four event types: post-reject, pre-reject, post-accept and pre-accept. Because desirability had no effect on amygdala response to feedback, we collapsed event types across this variable. This maximized the number of trials in each condition. Statistical models with a gamma variate basis function were first convolved with the hemodynamic response function of each subject in AFNI. The basis function was set to the onset time of each event-type. These regression analyses produced beta coefficients for each event-type for each subject. Comparing coefficients for given event types generated subject-level contrast values. Our key contrast of interest assessed changes in the response to reject feedback, generated by post-reject minus pre-reject. Group analyses were performed on this contrast in the whole brain by submitting individual contrast values of subjects in each group to a between-subjects t-test (two-tailed). Results were evaluated using a two-pass approach to determine statistical significance. On the first pass, we used a whole-brain p<0.005 two-tailed t test uncorrected for multiple comparisons throughout the brain. If activation clusters included either side of the amygdala, our a priori region of interest, (ROI), on this first pass we then used a spatial extent threshold generated from Monte Carlo simulations to separately control for multiple comparisons. Within the amygdalae, suprathreshold cluster size had to exceed 194 voxels, corresponding to an ROI-corrected value of p<.05.
Significant effects in the amygdala ROIs that emerged from group analysis were followed up by secondary analyses performed in SPSS to clarify the pattern of these effects. We used the 3dmaskave AFNI program to compute average activation values of all voxels within the functionally-defined ROI mask for each participant. Threshold parameters for the mask were based on the results from the primary ROI analyses, using t=2.03, p<.05 and minimum cluster size 194. Mean activation values for functional clusters were extracted per participant for the following contrasts: post-reject minus baseline and pre-reject minus baseline. Amygdala response data for changes in reject were analyzed in a 2x2 repeated measures ANOVA with Group (anxious, healthy) as the between-subjects factor and Time (pre, post) as the within-subjects factor. Significant interactions were decomposed with post-hoc analyses. Additional analyses evaluated associations between extracted activation values and behavioral ratings of affective responses to feedback.
To assess the specificity of Group and Time main and interaction effects to reject feedback, mean activation values for these same functional clusters were extracted per participant for accept feedback, by computing ‘post-accept minus baseline’ and ‘pre-accept minus baseline’ contrasts. ANOVA analyses were then repeated for the accept feedback data with Group and Time as between- and within-group factors respectively. A final set of exploratory analyses examined cortical regions previously implicated in anxious adolescents or during social information-processing in typically-developing adolescents.
Results
Self-reported affective ratings
Affective ratings to reject and accept peer feedback are depicted in Table 1. There was a main effect of desirability [F(1,22) = 6.25, p < .05], that was further modified by a feedback-by-desirability interaction [F(1,22) = 14.60, p < .001]. Being rejected by a high desirable individual was associated with more negative emotion than being accepted by a high desirable individual [t(23) = 3.62, p < .05]. In contrast affective ratings following rejection and acceptance for low desirable individuals did not differ [t(23) = −1.63, p = n.s.]. However there were no significant effects of group [F(1,22) = 2.29, p = n.s.] or feedback [F(1,22) = .25, p = n.s.] on affective ratings.
Group differences in changes in brain activation to reject feedback
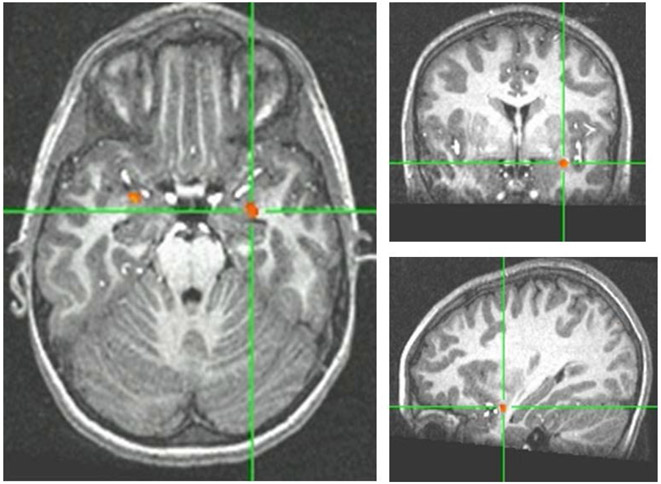
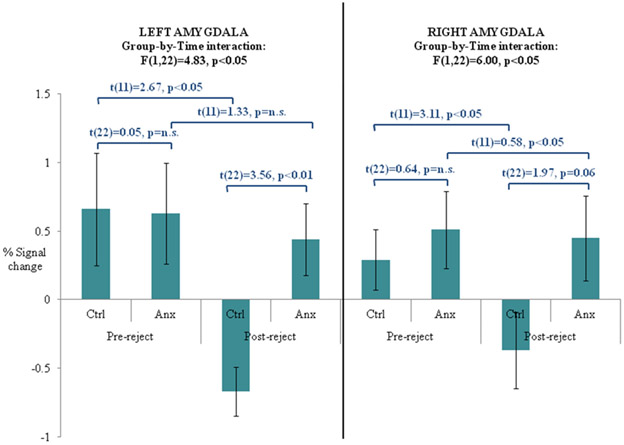
Group differences in the changes in response to reject feedback contrast (post-reject minus pre-reject collapsed across desirability) were revealed in eleven regions that survived our first pass whole brain uncorrected analysis of p<.005 (Table 2). Two of these were located bilaterally within the amygdalae-hippocampal complex (Figure 2a). The direction of results indicated greater responses among anxious adolescents in the post-reject condition after subtracting pre-reject activity. Both of these regions survived the minimum cluster size controlling for multiple comparisons within the amygdalae ROI. Accordingly, activation values for each individual subject were extracted and analyzed using a repeated-measures ANOVA. As expected, comparable results characterized each region. A significant effect of time in both left (F(1,22)=8.61, p<0.01) and right (F(1,22)=9.04, p<0.01) regions in this amygdalae-hippocampal complex indicated changes in activity across all subjects after reject feedback. These effects were further qualified by a significant group-by-time interaction in both regions (Figure 2b), where changes in regional activity from pre- to post-reject differed across groups. Post-hoc t tests indicated that while healthy subjects showed significant deactivation from pre-reject to post-reject in both left and right regions of the amygdalae-hippocampal complex, anxious patients showed similar levels of activity across time in both regions. Moreover, significant group differences only emerged during post-reject feedback. These sets of results were unchanged after co-varying for mean affective ratings to reject feedback. No significant correlations emerged between changes in activity in these amygdalae-hippocampal regions from pre-reject to post-reject feedback and affective ratings.
Table 2:
Regions surviving threshold criteria for significant between-group differences in the Changes in response to Reject contrast
| Area of Activation | Brodmann Area |
Direction | Talairach Coordinates |
Cluster size |
Max t- value |
|---|---|---|---|---|---|
| Frontal / Parietal | |||||
| Left precentral gyrus | 43 | Anx > Ctrl | −51, −1, 11 | 1086 | 4.90 |
| Right precentral gyrus | 43 | Anx > Ctrl | 50, −16, 17 | 101 | 3.92 |
| Right precentral gyrus | 43 | Anx > Ctrl | 55, −9, 15 | 33 | 3.57 |
| Right precentral gyrus | 4 | Anx > Ctrl | 60, −12, 29 | 28 | 3.32 |
| Left cuneus | 19 | Anx > Ctrl | −15, −90, 26 | 173 | 3.85 |
| Occipital | |||||
| Right lingual gyrus | 19 | Anx > Ctrl | 8, −63, −2 | 46 | 3.35 |
| Left claustrum | Anx > Ctrl | −31, −21, 7 | 708 | 4.55 | |
| Subcortical / Limbic | |||||
| Right insula | 13 | Anx > Ctrl | 39, −25, 16 | 291 | 4.26 |
| Right claustrum | Anx > Ctrl | 34, −16, 10 | 121 | 3.56 | |
| Left amygdala | Anx > Ctrl | −30, −1, −15 | 33 | 3.77 | |
| Right amygdala | Anx > Ctrl | 13, −8, −18 | 29 | 3.67 |
Figure 2:
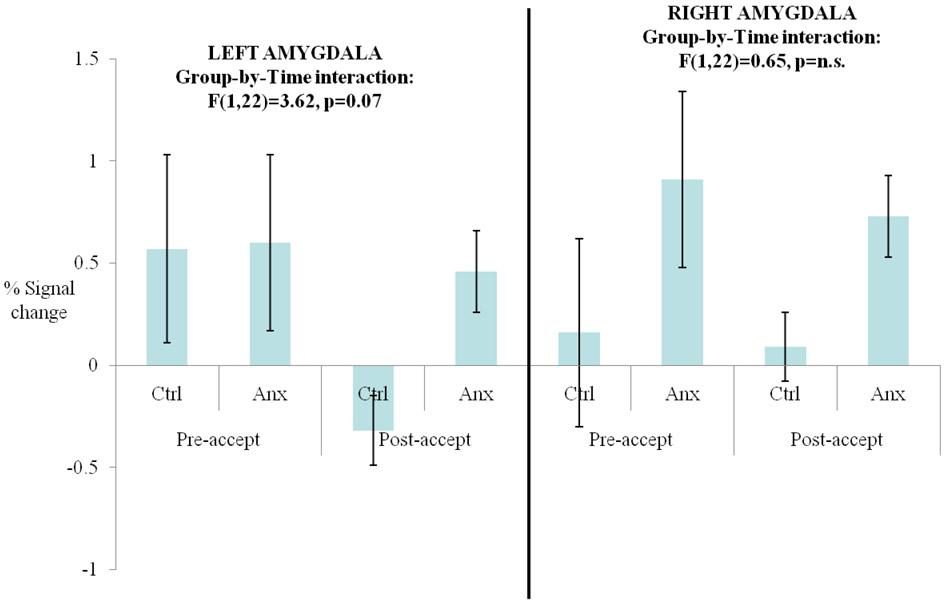
(a) Between-group differences in left and right amygdala activity in the changes in reject contrast; (b) Changes in bilateral amygdala responses from pre-reject to post-reject feedback in anxious and psychiatrically-healthy adolescents; (c) Changes in bilateral amygdala responses from pre-accept to post-accept feedback in anxious and psychiatrically-healthy adolescents.
Analyses on activation of these regions from pre-accept to post-accept feedback revealed no significant main or interaction effects of group or time (Figure 2c). However, patterns of anxious and non-anxious groups’ responding in the left amygdala-hippocampal complex were similar to activation patterns following reject feedback.
Finally, we did not find group differences in prefrontal cortex activity during receipt of reject feedback relative to pre-feedback. Although we found significant activation in the cingulate cortex relative to baseline, this did not vary significantly as a function of response-type (accept/reject) or group (anxious/healthy) during receipt of feedback relative to baseline.
Discussion
In the present study, we compared anxious and non-anxious adolescents’ responses to peer rejection, an emotional stimulus that is salient for adolescents, and especially so for anxious adolescents. While a number of prior studies have examined the effects of social rejection on the brain and other physiological systems (Eisenberger & Lieberman, 2004; Eisenberger et al., 2003; Gunther Moor, Crone, & van der Molen; Gunther Moor, van Leijenhorst et al.; Guyer et al., 2009; Masten et al., 2009; Masten et al., 2010; Somerville et al., 2006; Stroud et al., 2009), these studies have generally focused on adults or adolescents without psychopathology and on brain regions such as the anterior cingulate cortex rather than subcortical regions such as the amygdala, which is clearly a focal point for the neural basis of anxiety disorders (Davidson, 2002; Freitas-Ferrari et al.; Monk, 2008 2010; Pine et al., 2009).
Our data clearly demonstrate a pattern of aberrant activity in the amygdala-hippocampal complex in response to peer rejection in anxious adolescents. The primary result from this study shows that while activation in the amygdala-hippocampus complex occurs in both healthy and anxious youth prior to feedback, this activity persists in anxious, but not psychiatrically-healthy individuals after they receive reject feedback from peers. This result is consistent with a previous report from our group on a sample that overlaps with the current sample where we reported amygdala hyperactivation in anxious youth while they contemplated feedback from a peer whom the participant had previously rejected (assigned low desirability ratings to) (Guyer et al., 2008). In both studies, anxious youth are not simply characterized by global hyperactivation in these amygdala regions, but rather by more subtle perturbations in patterns of response that varies with the particular stimulus features, psychological processes, and time-related parameters created through this social context. In the first study (Guyer et al., 2008) what distinguished pathological responding from normal responding was a possible internally-directed focus on retaliatory, anticipatory rejection. In contrast, here, the data suggest that activation in the amygdala-hippocampal complex occurring prior to feedback might persist to a greater degree among anxious than healthy adolescents, in response to actual negative outcomes such as peer rejection. Similar patterns of persistence have been reported in adults with clinical anxiety and depression using nonsocial paradigms (Nitschke et al., 2009; Siegle et al., 2002). However we should note that while we believe a pattern of sustained activity is the most likely explanation for these results, it is also possible that these results reflect a pattern of dual subcortical activation in the anxious group, but not the control group. This activation could occur before feedback and occur again after feedback, however, the degree of jittering between the two phases (pre- and post-feedback) of these trials make it difficult to clearly separate these response patterns in the present dataset.
These findings extend important aspects of our understanding of both adaptive and maladaptive responses in adolescents to social feedback. First, prior studies of affective-processing generally find that the period preceding receipt of a negative event is the most salient for anxious individuals, and where amygdala activity usually deviates from controls (Nitschke et al, 2009). In contrast, here, we found similar levels of amygdala-hippocampal activity just before receiving feedback in both anxious and healthy individuals, reflecting presumably the universal salience of peer feedback for all adolescents compared to other naturally-occurring emotional events. This suggestion is also supported by the absence of group differences in affective ratings to rejection and acceptance in the present data.
Our data instead suggest that the fundamental difference between pathological and normal responding may lie in the ability of anxious adolescents to down-regulate activity in these subcortical regions after a period of activation. The pattern of prolonged activation in affective circuitry following engagement has been implicated as a potential contributor to mood and anxiety disorders in adults (Davidson, 2002; Siegle et al., 2002) and could reflect relatively weak input to subcortical regions such as the amygdala from inhibitory structures such as the ventral prefrontal cortex (Quirk & Beer, 2006). This pattern may be more relevant among adolescents in general, for whom there may be a normative functional imbalance between frontal and subcortical responses, and exaggerated in anxious adolescents in particular, given their cognitive distortions on affective-social signals (Guyer et al., 2008; Monk, 2008). Alternatively, it could reflect strategic failures to engage appropriate top-down inhibitory mechanisms which are physiologically sound. While our findings of prolonged amygdala-hippocampal activation generally occurred following negative outcomes, suggesting particular difficulties deploying inhibitory responses in a negative environment, there was some indication of similar findings during the receipt of peer acceptance. But these effects did not reach significance and will require clarification in future studies. A final finding is that although we found significant elevation of cingulate cortex responses during peer feedback, consistent with prior studies of typically-developing adolescents (Masten et al., 2009; Masten et al., 2010), no group differences were noted, again possibly highlighting the universal salience of peer feedback for adolescents.
While our findings are intriguing, they are subject to several limitations. First, as with most clinical neuroimaging studies of adolescents, we relied on small sample sizes with only 12 participants in each group. Moreover, the patient group reflected diagnostic heterogeneity. Importantly, however, all patients reported clinically elevated social concerns, whereas healthy adolescents did not. Therefore, as these data are among the first to report perturbed amygdala-hippocampal responses to social rejection in clinical populations, they should be interpreted with caution. Second, we did not find group differences in the broader neural networks that interface with subcortical structures such as the prefrontal cortex implicated in previous studies of emotional processing in adolescent patients and healthy comparisons (Guyer et al., 2008; Monk et al., 2006). Thus, while we assume such amygdala differences are occurring because of different activity patterns within a wider network of regions, such as the prefrontal cortex, this possibility remains speculative.
Third, group differences in BOLD activity were found in spite of comparable affective ratings between anxious and non-anxious adolescents. Conversely, where we found differences in affective ratings to negative feedback as a function of the desirability of a partner, we did not observe parallel neural dissociations in the amygdala-hippocampus complex across this variable. Such patterns of brain activation differences without corresponding behavioral differences (and vice versa) are not uncommon in functional neuroimaging research (McClure et al., 2007), and may indicate that there are many factors that influence both self-reported and physiological responding, leading to attenuated covariation. Alternatively, null results may be explained by reduced power associated with fewer trial replicas in each feedback by desirability condition, with only 10 trials probing rejection by ‘high desirable’ individuals and 10 trials probing rejection by ‘low desirable’ individuals. Future studies should include more trials of each of these critical conditions to clarify these results.
In spite of these weaknesses, the results of this study make an important contribution to our understanding of how aspects of social functioning may differ neurally between adolescents with and without clinically-elevated social concerns. More particularly, pathological responses to social stressors, such as rejection may be expressed in anxiety-related brain systems, which in turn influence cognitive appraisals. In contrast to most previous studies that have utilized paradigms requiring participants to engage in highly-structured but somewhat artificial social-affective tasks to probe amygdala responding, the present paradigm was designed to mimic emotionally- and socially-salient events that adolescents are likely to encounter in their daily lives. Because of this increased ecological validity, these findings are likely to be very pertinent for the management of real world adolescent anxiety. To provide a clear direction for therapeutic approaches, additional research is still needed to dissociate different explanations for these pathological neural processes and elucidate their effects on social cognitions.
Acknowledgements
The authors would like to thank Michelle Goldwin and Nina Shiffrin for data processing assistance; Harvey Iwamoto for programming and computer support; and Jennifer Cameron, Ken Towbin, and Alan Zametkin for medical oversight. We thank the families who participated.
Funding
This work was supported by the NIMH intramural research program and Career Development Awards to A.E.G. (K99 MH080076; R00 MH080076) from the NIMH.
Footnotes
Declaration of interests
The authors report no conflicts of interest.
References
- Allen JP, Porter MR, McFarland FC, Marsh P, & McElhaney KB (2005). The two faces of adolescents' success with peers: adolescent popularity, social adaptation, and deviant behavior. Child Development, 76(3), 747–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo K, Lau JY, McClure-Tone EB, Guyer AE, Monk CS, Nelson EE, et al. (2009). Common and specific amygdala engagement perturbations in depressed versus anxious adolescents. Archives of General Psychiatry, 66(3), 275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ (2007). Neurocognitive mechanisms of anxiety: an integrative account. Trends in Cognitive Science, 11(7), 307–316. [DOI] [PubMed] [Google Scholar]
- Dalgleish T, Taghavi R, Neshat-Doost H, Moradi A, Canterbury R, & Yule W (2003). Patterns of processing bias for emotional information across clinical disorders: a comparison of attention, memory, and prospective cognition in children and adolescents with depression, generalized anxiety, and posttraumatic stress disorder. Journal of Clinical Child and Adolescent Psychology, 32(1), 10–21. [DOI] [PubMed] [Google Scholar]
- Davidson RJ (2002). Anxiety and affective style: role of prefrontal cortex and amygdala. Biological Psychiatry, 51(1), 68–80. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Gable SL, & Lieberman MD (2007). Functional magnetic resonance imaging responses relate to differences in real-world social experience. Emotion, 7(4), 745–754. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, & Lieberman MD (2004). Why rejection hurts: a common neural alarm system for physical and social pain. Trends in Cognitive Science, 8(7), 294–300. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, & Williams KD (2003). Does rejection hurt? An FMRI study of social exclusion. Science, 302(5643), 290–292. [DOI] [PubMed] [Google Scholar]
- Erath SA, Flanagan KS, & Bierman KL (2007). Social anxiety and peer relations in early adolescence: behavioral and cognitive factors. Journal of Abnormal Child Psychology, 35(3), 405–416. [DOI] [PubMed] [Google Scholar]
- Freitas-Ferrari MC, Hallak JE, Trzesniak C, Filho AS, Machado-de-Sousa JP, Chagas MH, et al. Neuroimaging in social anxiety disorder: a systematic review of the literature. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 34(4), 565–580. [DOI] [PubMed] [Google Scholar]
- Gardner M, & Steinberg L (2005). Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: an experimental study. Developmental Psychology, 41(4), 625–635. [DOI] [PubMed] [Google Scholar]
- Gazelle H, & Rudolph KD (2004). Moving toward and away from the world: social approach and avoidance trajectories in anxious solitary youth. Child Development, 75(3), 829–849. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences, 101(21), 8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther Moor B, Crone EA, & van der Molen MW The heartbrake of social rejection: heart rate deceleration in response to unexpected peer rejection. Psychological Science, 21(9), 1326–1333. [DOI] [PubMed] [Google Scholar]
- Gunther Moor B, van Leijenhorst L, Rombouts SA, Crone EA, & Van der Molen MW Do you like me? Neural correlates of social evaluation and developmental trajectories. Social Neuroscience, 1–22. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Lau JY, McClure-Tone EB, Parrish J, Shiffrin ND, Reynolds RC, et al. (2008). Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Archives of General Psychiatry, 65(11), 1303–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, McClure-Tone EB, Shiffrin ND, Pine DS, & Nelson EE (2009). Probing the neural correlates of anticipated peer evaluation in adolescence. Child Development, 80(4), 1000–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawker DS, & Boulton MJ (2000). Twenty years' research on peer victimization and psychosocial maladjustment: a meta-analytic review of cross-sectional studies. Journal of Child Psychology and Psychiatry, 41(4), 441–455. [PubMed] [Google Scholar]
- Jackson DC, Mueller CJ, Dolski I, Dalton KM, Nitschke JB, Urry HL, et al. (2003). Now you feel it, now you don't: frontal brain electrical asymmetry and individual differences in emotion regulation. Psychological Science, 14(6), 612–617. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent DA, Ryan ND, & Rao U (2000). K-Sads-Pl. Journal of the American Academy of Child and Adolescent Psychiatry, 39(10), 1208. [DOI] [PubMed] [Google Scholar]
- Kingery JN, Erdley CA, Marshall KC, Whitaker KG, & Reuter TR (2010). Peer experiences of anxious and socially withdrawn youth: an integrative review of the developmental and clinical literature. Clinical Child and Family Psychology Review, 13(1), 91–128. [DOI] [PubMed] [Google Scholar]
- La Greca AM, & Harrison HM (2005). Adolescent peer relations, friendships, and romantic relationships: do they predict social anxiety and depression? Journal of Clinical Child and Adolescent Psychology, 34(1), 49–61. [DOI] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, et al. (2009). Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Social, Cognitive and Affective Neuroscience, 4(2), 143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Pfeifer JH, & Dapretto M Witnessing peer rejection during early adolescence: Neural correlates of empathy for experiences of social exclusion. Social Neuroscience, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJ, et al. (2007). Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Archives of General Psychiatry, 64(1), 97–106. [DOI] [PubMed] [Google Scholar]
- Monk CS (2008). The development of emotion-related neural circuitry in health and psychopathology. Developmental Psychopathology, 20(4), 1231–1250. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, & Pine DS (2005). The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine, 35(2), 163–174. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Oathes DJ, Johnstone T, Whalen PJ, Davidson RJ, et al. (2009). Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. American Journal of Psychiatry, 166(3), 302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, & LeDoux JE (2005). Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron, 48(2), 175–187. [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen E, Cohen P, & Brook J (1999). Adolescent depressive symptoms as predictors of adult depression: moodiness or mood disorder? American Journal of Psychiatry, 156(1), 133–135. [DOI] [PubMed] [Google Scholar]
- Pine DS, Helfinstein SM, Bar-Haim Y, Nelson E, & Fox NA (2009). Challenges in developing novel treatments for childhood disorders: lessons from research on anxiety. Neuropsychopharmacology, 34(1), 213–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, & Beer JS (2006). Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Current Opinion in Neurobiology, 16(6), 723–727. [DOI] [PubMed] [Google Scholar]
- Rapee RM, & Heimberg RG (1997). A cognitive-behavioral model of anxiety in social phobia. Behaviour Research and Therapy, 35(8), 741–756. [DOI] [PubMed] [Google Scholar]
- Sandstrom MJ (2004). Pitfalls of the peer world: how children cope with common rejection experiences. Journal of Abnormal Child Psychology, 32(1), 67–81. [DOI] [PubMed] [Google Scholar]
- Sebastian C, Viding E, Williams KD, & Blakemore SJ Social brain development and the affective consequences of ostracism in adolescence. Brain and Cognition, 72(1), 134–145. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, et al. (1983). A children's global assessment scale (CGAS). Archives of General Psychiatry, 40(11), 1228–1231. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, & Carter CS (2002). Can't shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry, 51(9), 693–707. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, & Kelley WM (2006). Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nature Neuroscience, 9(8), 1007–1008. [DOI] [PubMed] [Google Scholar]
- Steinberg L, & Morris AS (2001). Adolescent development. Annual Reviews of Psychology, 52, 83–110. [DOI] [PubMed] [Google Scholar]
- Storch EA, & Ledley DR (2005). Peer victimization and psychosocial adjustment in children: current knowledge and future directions. Clin Pediatr (Phila), 44(1), 29–38. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, et al. (2009). Stress response and the adolescent transition: performance versus peer rejection stressors. Development and Psychopathology, 21(1), 47–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AM, Lipp OV, & Spence SH (2004). Attentional bias toward fear-related stimuli: an investigation with nonselected children and adults and children with anxiety disorders. Journal of Experimental Child Psychology, 89(4), 320–337. [DOI] [PubMed] [Google Scholar]