Abstract
Objective:
To report our experience with Robot Assisted (RA) Autologous Cryopreserved Ovarian Tissue Transplantation (ACOTT) with the utility of a neovascularizing ECM scaffold
Design:
Case series with meta-analytic update
Setting:
Academic
Patients:
7 recipients of RA- ACOTT
Interventions:
Before or shortly after initiating chemotherapy, ovarian tissue was cryopreserved from 7 women, who then underwent RA-ACOTT 9.9±1.8 years (range 7–12) later. Perioperatively, they received transdermal estrogen and low dose aspirin to enhance graft vascularization. Ovarian cortical pieces were thawed and sutured on an ECM scaffold, which was then robotically anastomosed to the bivalved remaining ovary in 6 cases, and retroperitoneally (heterotopic) to the lower abdomen in one
Outcome Measures:
Ovarian function return, number of oocytes/embryos, aneuploidy %, livebirths, neonatal outcomes were recorded. Graft longevity was compared to the mean from the meta-analytic data
Results:
Ovarian function returned 13.9±2.7 weeks (11–16.2) after ACOTT, and oocytes were retrieved in all cases with 12.3±6.9 embryos generated. In contrast to orthotopic, the heterotopic ACOTT demonstrated low embryo quality and 80% aneuploidy rate. While a recipient did not attempt and 2 need a surrogate to conceive, 4/4 delivered 6 healthy children, compared with 115/460 (25%) pregnancy rate from the meta-analytical data (n=79). The mean graft longevity (43.2±23.6/47.4±22.8 months w/wo sensitivity analysis) trended longer (p=0.057/0.015) than the meta-analytical mean (29.4±22.7), even after matching age at cryopreservation.
Conclusions:
In this series, RA- ACOTT resulted in extended graft longevity, with ovarian functions restored in all cases, even when the tissues were cryopreserved after chemotherapy exposure
Funding:
R21HD053112, NIH RO1HD053112
Trial registration:
N/A
Keywords: Fertility preservation, Cryopreservation, ovarian tissue transplantation, robotic surgery, extracellular matrix
INTRODUCTION
Autologous transplantation with cryopreserved ovarian tissue (ACOTT) is a key fertility preservation technique. ACOTT was considered an experimental approach until recently, but it is now recognized as a valid clinical technique by the American Society of Reproductive Medicine and several other specialty societies around the world (1, 2). The first ACOTT with previously cryopreserved tissue was performed in 1999 and reported in 2000 (3). In that orthotopic laparoscopic approach, ovarian cortical pieces were sutured onto a poly-cellulose scaffold (Surgicel, Johnson and Johnson, NJ, USA) and then grafted subperitoneally on the pelvic side wall (3, 4). The procedure resulted in the restoration of ovarian endocrine and ovulatory functions, though the patient did not desire to conceive. Subsequent two decades of progress resulted in an estimated >130 livebirths (5–8).
In a recent meta-analysis performed by our group, we found that the worldwide livebirth rate was 37.7% per woman after ACOTT, with at least 63.9% of the recipients having ovarian endocrine function > 6 months duration. However, the average duration of ovarian function was only 26.9 months, a major limitation of the ACOTT procedure. Based on the cumulative experience from 2000–2017, we found that the mean graft longevity was 26.9 ± 25.6 months when approximately half of the cortex of an ovary was transplanted (8). Because ovarian cortical grafts have to acquire their blood supply through neovasculogenesis which can take up to 10 days (9), a significant proportion of primordial follicles are lost by the time the graft is fully vascularized (7, 9). A xenografting study estimated that nearly two thirds of the ovarian reserve is lost during the initial ischemic period after transplantation (10, 11). As our meta-analysis showed, these ovarian reserve losses are reflected in the limited longevity of ovarian auto-transplants.
With the aim of improving ovarian transplant survival and longevity and to address this Achilles heel in fertility preservation by ovarian tissue cryopreservation, we previously developed and reported a surgical approach where the thawed cortical pieces are first sutured onto a neovascularizing extracellular matrix scaffold (Alloderm) and then grafted to the patient’s remaining ovary with robot-assisted laparoscopy (12). That previous report included the short term follow up of the technique with two cases and only reported on one livebirth and one ongoing pregnancy. Here we report our extended experience with robot-assisted ovarian auto-transplantation with Alloderm on seven cases with additional livebirths in 4 patients, with the hypothesis that our ovarian transplantation approach improves transplantation longevity compared to the historical data. To that end we updated the longevity data from our 2017 meta-analysis (8).
MATERIALS AND METHODS
Patient Selection and Pre-Op Preparation
Seven consecutive women who provided informed consent for an IRB-approved ovarian tissue cryopreservation and transplantation study, and who subsequently underwent ACOTT were included. The cases shared the following characteristics: slow freezing technique, ovarian failure caused by gonadotoxic treatments (chemo and/or radiation therapy), perioperative pharmacological support (transdermal estrogen 4 weeks prior and post-op and baby aspirin 10 days prior), robot assisted ACOTT by single surgeon (K.O.), utility of ECM scaffold (Alloderm® LifeCell Corp, Branchburg, NJ, USA), and a minimum of 1-year follow up. Of the 7 patients, cases 1–5, and 7 had already received some form of chemotherapy (Supplemental Table 1) but the ovarian cryopreservation was performed before they received the brunt of the gonadotoxic regimens, prior to hematopoietic stem cell transplantation. (Supplemental Table 1)
The clinical characteristics of the participants are shown in Supplemental Table 1. Prior to the ovarian transplant, an extensive medical evaluation was performed to determine the suitability for the procedure. Medical clearances were obtained from the patients’ medical- and/or surgical-oncologists, or hematologists, where applicable. For every patient, a sample of the cryopreserved ovarian tissue was thawed and histologically analyzed for primordial follicle density and to rule out the presence of cancer cells (12). There are currently no established guidelines in determining the amount of tissue to thaw for transplantation. Because pre-ovarian tissue cryopreservation ovarian reservation was not reliable due to six patients having already received chemotherapy and one having had a prior unilateral oophorectomy, the follicle density and the patients’ preferences empirically guided us in deciding how much tissue to thaw and transplant. Prior to the transplant, each patient was treated with transdermal estradiol for four weeks and low dose Aspirin for 10 days to aid the graft revascularization as we reported previously (12). Transdermal estradiol was continued post-operatively, until the documentation of ovarian follicle growth.
Ovarian Tissue Cryopreservation, Thawing, and the Surgical Technique
All ovarian tissues were cryopreserved with the slow freezing method and thawed with the rapid thaw approach, as we previously reported (3, 12). All transplants were performed with robotic assistance, after suturing the cortical pieces onto an ECM scaffold (Alloderm) under a surgical microscope (Figure 1). We have previously described our surgical approach in detail (12), including in a video format (13). When an ovary was present, it was bivalved and the graft was anastomosed to that bivalved in situ ovary with stromal side of the cortical pieces and the recipient site juxtaposed. If the ovary was too atrophic, the mesosalpinx was denuded to provide additional vascular surface and then transplant was extended and sutured onto this surface (13). The utility of peri-operative baby aspirin ensured that early microclotting does not occur, and the graft receives extended perfusion before revascularization. In one case, where a total abdominal hysterectomy, pelvic lymph node dissection and bilateral salpingo-oophorectomy were performed for pelvic cancer, the graft was transplanted to the abdominal wall retroperitoneally (13). This patient later underwent a conventional laparoscopic surgery for an omental flap procedure to improve graft vascularization (Video 1).
Figure 1.
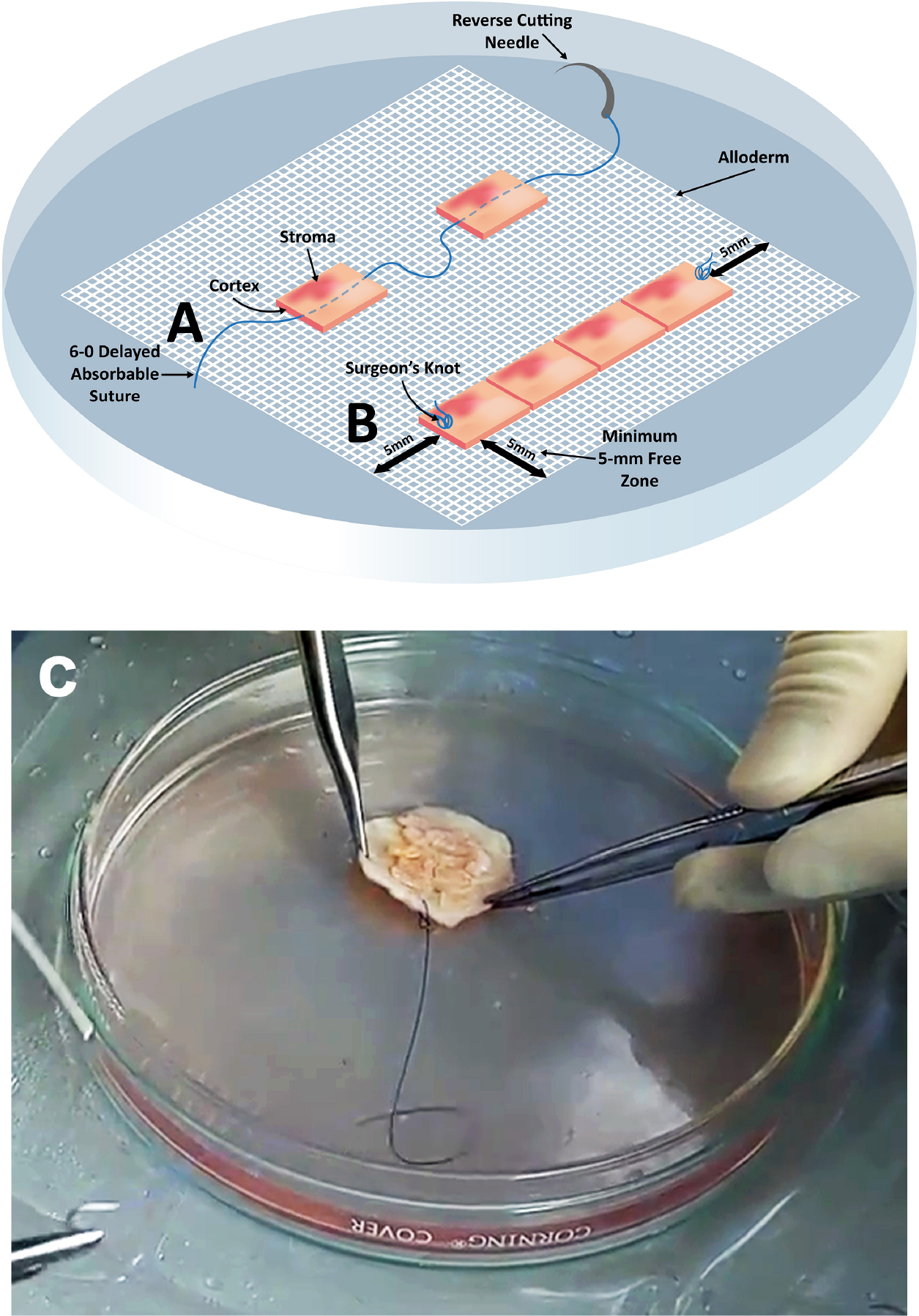
The technique of graft preparation with ECM (Alloderm) scaffold. A) Ovarian cortical pieces are sutured to the “shiny” side of hydrated medium thickness Alloderm, with stromal surface facing up (shiny-to-shiny principle). A 6–0 delayed absorbable suture is first tied 5-mm from the matrix edge and then threaded through the stroma of the first piece, then through the matrix and then back through the stroma of the next piece, avoiding any piercing through the cortex. Depending on the dimensions of the recipient site, numerous pieces can be strung together, and several rows are created on Alloderm in this fashion. A surgeon’s knot is tied again when the row is completed. (B) The rows are created with at least 5-mm clearance from the edges so that this margin can be used to anastomose the graft to the recipient surface. (C) A photograph of a completed ECM graft, ready for transplantation. Medical illustration drawn by K.O.; artwork by G.B.
Post-Ovarian Transplant Follow Up
In each case, we performed a color-doppler ultrasound within 7–10 days of the surgery to document blood flow and retention of the graft. Afterwards, the patients were kept on hormone replacement with continuous transdermal estradiol and cyclical micronized progesterone. Starting 4–6 weeks after the transplantation, the graft was evaluated by ultrasound examination every 2–4 weeks. Once the follicle growth was documented, hormone replacement was discontinued. We then periodically measured serum E2, FSH, LH and progesterone levels and performed pelvic ultrasound exams to monitor ovarian follicle growth. Because all patients preferred to cryopreserve embryos or oocytes in case their grafts prematurely ceased function, and/or they needed to use a gestational carrier due to a past hysterectomy or radiation-induced uterine damage, they underwent ovarian stimulation and oocyte retrievals as was described previously (12). In general, an early-follicula-start antagonist protocol was used with a recombinant FSH preparation, and the trigger was performed singularly with 250 mcg of recombinant hCG (Ovidrel, Merck, New Jersey, USA) or dually by adding 1 mg leuprolide acetate 34–35 ahead. In general, we perform oocyte retrieval sooner after the trigger with ovarian transplant patients compared to similar age non-transplant patients because of the higher risk of premature LH surge with the former. If a premature LH surge was encountered ahead of the trigger, we switched to a long protocol with low dose luteal phase leuprolide acetate suppression. In the case of the heterotopic transplant, no antagonist was needed as our previous experience showed that the heterotopic transplants do not trigger premature LH surge. In such transplants, we administered gonadotropins and hCG near the graft area with the aim of direct delivery to the graft. A more detailed account of the ovarian stimulation approaches in ovarian transplantation can be found in a forthcoming book chapter (14). Three patients consented to pre-implantation genetic testing for aneuploidy (PGT-A), and their embryos were tested via NGS at blastocyst stage. In one of those three, a non-invasive PGT-A approach, through the evaluation of DNA in spent embryo culture media, was also used.
Update of the 2017 Meta-Analysis:
To serve as a historical reference point, we updated our 2017 meta-analysis (8) as of November 2020 from peer-reviewed manuscripts, meeting abstracts, or through personal correspondence with the authors (Supplemental Figure 1). From this updated dataset, we identified the studies that reported graft longevity. From those, we only included studies (“inclusion criteria”) with recipients who had clearly defined ovarian insufficiency, menopause (a minimum of 12-month amenorrhea) or undergone bilateral oophorectomy before ACOTT, and had at least one year of follow up, to match our case-series dataset. Those who had repetitive ovarian transplant procedures were excluded. To summarize the current state of success from all ACOTT worldwide, we also updated the total number of livebirths + ongoing pregnancies (LB+OG) from the same meta-analytical dataset. To calculate the LB+OG pregnancy rate, only studies which specify the total number of transplant recipients (denominator) were included (8, 15–31). While we included the 6 births from this report in the total LB+OG count worldwide, those were not included in the meta-analytical LB+OG rate.
Statistical Methods:
Data were analyzed by SAS version 9.4. Continuous variables were reported as mean/median with standard deviation and range, whereas categorical variables were presented as frequencies and percentages. Due to the skewed distribution the differences between groups were evaluated using non-parametric Wilcoxon-test. A sensitivity analysis was performed for longevity comparisons, to account for a recipient with underlying POI. Additionally, longevity data were further compared with age-matching.
IRB Approval:
All patients underwent ovarian tissue cryopreservation with informed consent under an Institutional Review Board (IRB)-approved ovarian tissue cryopreservation and transplantation protocol. The results of the transplant procedures were reviewed retrospectively (Yale IRB Protocol ID 2000030279). No IRB-approval was required for the meta-analytical update as the raw data were obtained from publicly available publications.
RESULTS
Patient Population:
On average, patients had a robot-assisted ACOTT approximately 10 years after the cryopreservation (range 7–12). The mean ages at cryopreservation and transplantation were 22.0 ± 5.1 (range 16–32) and 31.9 ± 5.6 (range 26–42), respectively. The indications for ovarian tissue cryopreservation are shown in Supplemental Table 1. In each case, after the initial primordial follicle density assessment and based on the patient or the couple’s goals, an amount of cryopreserved tissue equivalent of 41 – 58% of the entire cortex of an ovary was thawed and transplanted. One woman received ovarian tissue transplantation after acute lymphoblastic leukemia (ALL). The test sample did not show any leukemic or malignant cells by expert histopathological analysis under light microscopy and as no specific markers were available, no further tissue testing was recommended. All but one woman underwent orthotopic ovarian transplantation onto the remaining ovary with or without extension onto the mesosalpinx. In one case, ovarian auto-transplantation was performed retroperitoneally to the lower abdominal wall. In that case, the patient had initially undergone unilateral salpingo-oophorectomy due to an ovarian mass which was later diagnosed as an endometrioid carcinoma on permanent sections. Then the patient was scheduled for a total abdominal hysterectomy with salpingo-oophorectomy of the remaining adnexa. Though the peritoneal washings were positive for adenocarcinoma at the time of this second surgery, her remaining ovary appeared normal and about half of the cortex was harvested and cryopreserved. Ovarian biopsies from that ovary were negative for malignancy. Prior to the ovarian transplant, histological evaluation of the test tissue also did not reveal any malignant cells. However, because of the extensive pelvic scarring due to previous radical surgery and out of abundance of caution for a recurrence in the transplanted tissue, a heterotopic approach was chosen.
Ovarian Transplantation Outcomes:
All transplants resulted in the resumption of estradiol production, follicle growth and ovulation. The mean length of time from the day of transplant to the restoration of ovarian function, as defined by follicle growth and an accompanying rise in serum E2 levels, was 14.8 ± 4.3 weeks (range 11–23 weeks). Of the seven recipients, four attempted pregnancy as of the time of this report and two are in the process of undergoing IVF to accumulate embryos to be used with a gestational carrier. The motive of the remaining recipient was to restore ovarian endocrine function (case-3, Table 1) but her ovarian follicle density was low, likely due to a pre-existing auto-immune polyglandular disease combined with prior chemotherapy exposure. She had inconsistent follow-up and her ovarian function ceased 16 months after the transplantation. However, 50% of her ovarian cortical tissue remains in cryostorage and consequently she has the option to return in the future for a second ovarian transplant to restore her fertility.
Table 1.
Outcomes with Robot-Assisted Ovarian Transplantation
| # | Age at Transplantation (years) | Type of Transplant | Time to Function (weeks) | Longevity (months) | FollicleDensity (/mm2) (mean± SD) | % of Ovary Cortex Grafte d | # of M2 Oocytes Retrieved | Fertilization Rate | # of nonarrested Embryos/PGT- A results if any | Pregnancy Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 35 | Orthotopic | 11 | 49 | 0.39 ± 0.17 | 50% | 11 | 100% | 11 (no PGT-A) | Livebirthx2 (Frozen ETx2) |
| 2 | 29 | Orthotopic | 11.5 | 89 (ongoing) | 1.28 ± 0.75 | 41% | 12 | 92% | 9 (no PGT-A) | Livebirthx2 (Fresh ET, spontaneous) |
| 3 | 33 | Orthotopic | 16 | 18 | 0.43 ± 0.64 | 50% | 1 | N/A | N/A (Oocyte Cryo only) | For endocrine function |
| 4 | 26 | Orthotopic | 11 | 50 (ongoing) | 1.17 ± 0.6 | 48% | 24 | 100% | 4 euploid 4 aneuploid 2 no PGT-A | Livebirth (Fresh ET) |
| 5 | 26 | Orthotopic | 16 | 38.3 (ongoing) | 0.40 ± 0.34 | 58% | 30 | 93% | 5 euploid 5 aneuploid | Livebirth (Frozen ET) |
| 6 | 42 | Heterototopic | 15 | 36.3 (ongoing) | 0.45 ± 0.58 | 46% | 16 | 87.5% | 3 aneuploid* (non-invasive PGT-A) 1 untested | Hysterectomy; GC being arranged |
| 7 | 32 | Orthotopic | 23 | 22 (ongoing) | 2.87 ± 0.88 | 58% | 3 | 100% | 0 | RT Damage: GC being arranged |
N/A: not applicable. PGT-A: Preimplantation Genetic Testing for Aneuploidy. ET: embryo transfer. GC: gestational carrier.
The only euploid result came from an early blastocyst which was submitted in its entirety for PGT-A, after it failed to expand and began degenerating. Hence not included in the table.
Ovarian Stimulation Cycles and IVF Cycle Outcomes:
All patients underwent ovarian stimulation and oocyte retrieval. However, case-3 dropped out after one retrieval for oocyte cryopreservation, and case-7 paused treatment after three retrievals for embryo cryopreservation. These retrievals resulted in 13.9 ± 10.5 (range 1–30) metaphase-II oocytes; 16 ± 9.7 (range 3–30) when excluding case-3 with single cycle of egg freezing. Of those who underwent IVF, the fertilization rate with ICSI was 95.4 ± 5.4% (range 87.5–100%). This yielded 12.3 ± 6.9 (range 2–22) day 3–5 embryos; 14.4 ± 5.2 (range 9 – 22) excluding the case-7 who has not completed her attempts. Of those, 8.8 ± 2.8 (range 4–11) were non-arrested embryos (Table 2). Of all the embryos, 52% were high grade (Grade 1 for cleavage stage or grade A for blastocyst stage), 39% were medium grade (grade 1.5 and 2 for cleavage and B for blastocyst) and only 9% were low grade (≤grade 3 for cleavage stage and C for blastocyst). Of the seven cases, three had a total of 23 embryos analyzed with PGT-A at blastocyst stage. This analysis revealed a mean aneuploidy rate of 56.5% of all embryos tested for all transplant types (50–80%). When broken down based on the ovarian transplantation type, 9 of 18 (50%) embryos from the orthotopic ACOTT and four of five embryos (80%) from the heterotopic ACOTT recipient were aneuploid. Three of the latter were tested using a non-invasive PGT-A approach and the only euploid result came from an early blastocyst which was submitted in its entirety for analysis, after it failed to expand and began degenerating.
Table 2.
Summary Outcomes for Robotic Ovarian Transplantation and IVF
| Variable | Mean ± SD (Range) |
|---|---|
| Age at Cryopreservation | 22 ± 5.1 (16 – 32) |
| Age at Transplantation | 31.9 ± 5.6 (26 – 42) |
| Primordial Follicle Density (mm2) | 1 ± 0.9 (0.39 – 1.28) |
| % Ovary Transplanted | 50.1 ± 6.2 (41 – 58) |
| Time to Function (weeks) | 14.8 ± 4.3 (11 – 23) |
| Longevity (months) | 43.2 ± 23.6 (18 – 89) |
| Number of Oocytes Retrieved† | 16 ± 9.7 (3 – 30) |
| Fertilization %† | 95.4 ± 5.4 (87.5 – 100) |
| Number of embryos/patient with and without case-7 included* | 12.3 ± 6.9 (2 – 22) / 14.4 ± 5.2 (9 – 22) |
| Number of non-arrested embryos/patient with and without case-7 included * | 7.3 ± 4.4 (0 – 11) / 8.8 ± 2.8 (4 – 11) |
| % Aneuploid Embryos of Tested | 56.5% (50–80%) |
Data are from seven recipients unless otherwise stated.
Case-3 not included as transplanted for endocrine function only
Case-7 paused treatment after having 3 retrieval attempts
Pregnancy and Neonatal Outcomes:
Of the four women who attempted pregnancy (cases 1,2,4,5), all conceived at least one child at the time of this report. Two patients, whose first pregnancies were reported previously (12), had 1 additional livebirth each during this study period (cases 1 and 2). In case-1, both pregnancies were from a single frozen embryo transfer. In case-2, the first pregnancy was from a fresh embryo transfer and the second one was spontaneously conceived. The remaining two patients (cases 4 and 5) had one child each at the time of this report. The case-4 conceived with single fresh and the case-5 with single frozen embryo transfer. (Table 3) Case-4 is currently being prepared for a frozen embryo transfer for her attempt for a second child.
Table 3.
Pregnancy Outcomes
| Child # | ACOTT Case # | Mode of Conception | Gestational Age | Complications | Mode of Delivery | Gender & Birthweight | Neonatal complications |
|---|---|---|---|---|---|---|---|
| 1 | 1 (First birth) | Frozen embryo transfer | 39 wks 5 days | None | Vaginal delivery | Female 3,600 gr. | None |
| 2 | 1 (Second birth) | Frozen embryo transfer | 35 wks 4 days | Preeclampsia | Vaginal delivery after labor induction | Male 2,863 gr. | NICU admission for preterm birth, discharged without complications |
| 3 | 2 (First birth) | Fresh embryo transfer | 41 wks 3 days | None | C-section due to failure to progress | Female 3,827 gr. | None |
| 4 | 2 (Second birth) | Spontaneous | 38 wks 4 days | Pregnancyinduced hypertension at 38 weeks | Repeat C section due to previous Csection | Male 3,175 gr. | None |
| 5 | 4 | Fresh embryo transfer | 40 wks 4 days | None | C-section due to failure to progress | Male 3,827 gr. | None |
| 6 | 5 | Frozen embryo transfer | 37 wks 3 days | Pregnancy-induced hypertension at 33 weeks | C-section due to failure to progress | Female 3,629 gr. | None |
NICU: Neonatal Intensive Care Unit
All deliveries except one were at term with a mean gestational age of 38.7 ± 2.2 weeks and there were no significant antepartum, postpartum or neonatal complications. The case-2 underwent labor induction at 35 gestational weeks for mild preeclampsia. The baby was admitted to NICU because of prematurity but was discharged without any complications. The case-5 was induced at 37 weeks due to pregnancy-induced hypertension. Cases 2, 4 and 5 were delivered via C-section due to failure to progress, and the case-2 delivered the second baby via repeat C-section due to the previous uterine surgery. At the time of this report, all children were developing without any health problems.
As a reference point, we updated the LB + OG pregnancy rates from our previous meta-analytical dataset. After the update from peer-reviewed manuscripts, meeting abstracts or through personal correspondence with authors, and including the 6 LBs (6 babies) from our cohort, we identified 518 recipients, who received 631 ACOTTs. Those resulted in 141 LB (147 babies) + 11 OG pregnancies worldwide. To calculate the LB + OG rate, we only included studies which specify the total number of transplant recipients (denominator) (8, 14–31). After the exclusion of 23 recipients who did not intend pregnancy, the LB+OG rate per woman receiving a transplant was 25% (115 LB+OG in 460 recipients). While all four women who attempted pregnancy had 1–2 livebirth each in our series, a statistical comparison was not justified due to the small numbers.
Graft Longevity:
Five of the seven transplants were still functioning at the time of this report. The mean longevity of the transplants was 43.2±23.6 months (18–89). Of the two recipients whose grafts ceased function (cases 1 and 3), one (Case-1) did so after 49 months (Table 1). This patient had received several courses of gonadotoxic chemotherapy before her ovarian tissue was cryopreserved. After her graft stopped functioning, she conceived her second child via a frozen embryo transfer. In case-3, the graft function ceased after 18 months. However, this patient was suffering from a poly-glandular auto-immune disorder and had low pre-transplant follicular density, presumably caused by auto-immune oophoritis and the one course of gonadotoxic CHOP regimen (Supplemental Table 1) she received before ovarian tissue cryopreservation. When case-3, whose premature graft failure was likely due to pre-existing autoimmune POI was excluded, the graft longevity was 47.4±22.8 months (22–89).
To assess whether our robot-assisted ovarian transplantation approach with Alloderm improves ovarian graft longevity, we compared the mean ovarian function length from this case series to the data from the updated meta-analytical database (8, 15, 16, 18, 21, 22, 24–28, 30–56). After the exclusion of 17 cases with repetitive transplants, these updated data showed mean/median (SD) longevity of 29.2/22.9 (22.6) months from 79 transplants worldwide. In comparison, the graft longevity from the 7 recipients was mean/median=43.2/38.3 (23.6) months in our cohort. The two-group comparison of longevity showed a trend for extended longevity with the robot-assisted ovarian transplant technique compared to those previously reported (two-sided p=0.057 from Wilcoxon test). In a sensitivity analysis after excluding case-3 in our cohort, who had pre-existing auto-immune POI (sample sizes of 79 and 6, respectively), graft longevity was 47.4/43.7 (22.8) months and Wilcoxon test yielded p=0.015 for the same comparison.
Age at cryopreservation can affect ovarian transplant longevity. Though we lacked sufficient sample size for more definitive confounder-adjustment, we performed an exploratory comparison with age-matched samples. In the original samples, the mean age at cryopreservation was 22.0 (N=7) in our case series vs. 28.6 in the meta-analytic data (N=79), with similar SDs of 5–6 (Supplemental Table 2). After matching age, both groups had the mean age of 22, and the mean (SD) of longevity was 33.7 (22.1) months in the meta-analytic group (N=28) vs. 43.2 (23.6) months in our case series (N=7), with p=0.21. When we excluded case-3 in our group (who had pre-existing auto-immune POI), the mean (SD) longevity was 47.4 (22.8) in our case series (N=6), where the two-group comparison yielded p=0.07 (Supplemental Table 2). Thus, a robust trend for over 10 months longer longevity was demonstrated in our case series despite reduced statistical power due to lower sample size after age-matching.
Of note, this improvement was observed even though a statistically significantly higher proportion of our patients received chemotherapy prior to ovarian tissue cryopreservation compared to those in the age-matched samples (86%=6/7 in our series vs. 27%=7/26 in the meta-analytic data; p=0.008).
DISCUSSION
Here we report the first extended series of robot-assisted ACOTT with an ECM scaffold (Alloderm). In this cohort, the procedure resulted in the restoration of ovarian function in all recipients. Despite the relatively small numbers and the fact that 6 of 7 recipients had already received some chemotherapy before the tissue cryopreservation, the mean longevity exceeded that of previously reported procedures by at least 10 months (8). The ovarian transplants resulted in embryo generation and/or six livebirths in all recipients who intended pregnancy. Though we suspect that the high success with our approach, relative to the meta-analytical data, is likely due to the improved surgical precision with robotic surgery and potential vascularization re-enhancing features of Alloderm (12), numerous other factors could have also contributed. These may include our unique pharmacological pre-op priming and the fact that all procedures were performed by the same team with extensive experience.
There are two sides to the estrogen priming prior and during ovarian transplantation. One may be concerned that E2 may lower serum FSH levels and hence negatively affect revascularization as a rodent study suggested that gonadotropins may aid in that process (57). However, our preoperative transdermal estrogen treatment (Climara 0.1 mg/wk) is given for a brief period of time (about 4 weeks) and does not significantly lower FSH levels in these patients with established ovarian failure and hence our patients may benefit from both. The estrogenization may also enhance reproductive tissue quality including those of the recipient areas, in addition to providing neoangiogenic effects. Furthermore, recent data in rodents show that estrogen action reduces primordial follicle activation (58). and hence ERT may prevent ischemia-triggered primordial follicle activation and depletion, until full vascularization is achieved.
While encouraging, our report also identified areas that need further investigation. First, in the only case of a heterotopic ovarian transplant in this series, we consistently encountered poor embryo development. After 11 months of attempting IVF, we could not obtain a blastocyst. In the hopes of improving oocyte quality and following the encouraging preliminary results of a primate study (59), we performed a laparoscopic omental flap procedure on this patient to improve graft vascularization (video 1). After the omental flap procedure, we were able to cryopreserve a 7-cell day-3 embryo and two blastocysts from this patient, though the latter were aneuploid. Further studies will be needed to determine if a routine vascular omental flap is beneficial to improve heterotopic ovarian transplant outcomes. In our prior experience with heterotopic ovarian transplants, we also encountered poor embryo development, though those grafts were implanted subcutaneously, either in the forearm (60) or the lower abdomen (61). Due to medical risks with invasive surgery, we recently performed a subcutaneous heterotopic ovarian autotransplantation under local anesthesia (see the third procedure at this link https://youtu.be/CqMl_WXtCmM) (13). In that case as well, repeated retrievals only resulted in poor-quality oocytes and embryos. In the current robotic technique, the tissues are placed retroperitoneally below the rectus abdominus muscle, a deeper location than that of subcutaneous transplants. Livebirths have been reported with a similar heterotopic ovarian transplantation technique (54, 55) and it is plausible that the currently cryopreserved embryos will result in a conception when transferred to a gestational carrier in the patient reported here.
For the robot-assisted heterotopic transplant case reported here, there could be other reasons for the poor response, including the relatively advanced age of the patient at the time of the cryopreservation and the potential negative impact of pelvic cancer on oocyte quality. Though the ovary where the cryopreserved tissues originated from was found to be free of cancer by gross and microscopic examination, peritoneal washings were positive for adenocarcinoma. Consequently, it is possible that the tumor cells induced inflammatory changes that had a detrimental effect upon the germ cell pool (62). It is also possible that the blood flow, vascularization patterns and paracrine/endocrine factors may not be optimal for oocyte development in heterotopic sites. Future studies will determine whether heterotopic ovarian transplants should have room in fertility restoration or whether they should be reserved to restore ovarian endocrine function only.
One advantage of heterotopic ovarian transplantation is easy access to the transplanted tissue for monitoring and removal, should there be a risk of disease recurrence. In case-6, we are regularly monitoring the graft volume and anatomy for any signs of cancer recurrence, and the graft may be easily removed once the pregnancy goal is achieved. However, in instances where ovarian tissue was cryopreserved in the presence of a malignant tumor in the same ovary, removal of the graft soon after the achievement of fertility is recommended (54). In fact, in the first report of livebirth after abdominal wall ovarian transplantation, the graft was removed during the C-section delivery, and it was found to contain local recurrence of a granulosa cell tumor (54).
In this manuscript, we reported the live delivery of a child from a woman who received ovarian tissue transplantation after ALL (Supplemental Table 1). Concerns have been expressed in auto-transplanting cryopreserved ovarian tissue from women with history of acute leukemias, as these tissues may harbor leukemic cells (63). However, ovarian cryopreservation is typically performed (as was the case with our patient) after the initial consolidation treatment, when the patient is in remission and about to receive gonadotoxic preconditioning chemotherapy for hematopoietic stem cell transplantation. During the remission, there are no circulating leukemic cells (31, 64). In fact, a quantitative PCR analysis showed that there is no or negligible degree of malignant cell contamination in ovarian tissues of acute leukemia patients who were in remission at the time of the harvesting; the xenografting of these tissues did not transmit the disease to immunodeficient mice. (64). In a case reported by Shapira et al (31) in a leukemia survivor, there was no recurrence during the 28-month follow up and the patient had two children from the transplant. Sonmezer et al (63) reported an ALL survivor who underwent multiple IVF cycles after ovarian transplantation. The recipient conceived, and the graft was removed during the cesarean section 25 months following the transplantation. Histopathological examination revealed no leukemic cells in the excised graft and as there were no specific cancer markers available, no further testing was recommended by the pathologist. However, when there are specific markers available for a given leukemia (for example Philadelphia chromosome in CML etc.), a PCR or FISH analysis can be performed with such markers (31). Some proposed xenografting experiments but such experiments are not only costly, but their clinical usefulness has not been established (64). In our case, the patient delivered a baby after the orthotopic ovarian tissue transplantation and the patient is currently disease free for nearly four years following the transplantation. While xenografting studies and a limited number of clinical observations suggest that performing ovarian tissue cryopreservation in acute leukemia patients during remission is safe, larger clinical studies will be needed to confirm this conclusion.
Though based on small number of cases, we observed seemingly high aneuploidy rates from embryos in women whose ovarian tissues were cryopreserved at a relatively young age. We performed PGT-A on the embryos of 16, 18 and 32-year-old recipients. Of the 21 embryos tested, a mean of 56.5% were aneuploid (50–80%). The aneuploidy rate was 80% for the heterotopic transplant case and 50% for the orthotopic transplant patients. A recent large study indicated that the aneuploidy rates may show a biphasic age pattern, highest in extremes of reproductive life span (67). In that study, the rate of aneuploidy by PGT-A was 44.4% in embryos from women with a mean age of 22 years. Consequently, the aneuploidy rate for the two recipients in our series whose tissues were cryopreserved at ages 16 and 18, do not seem to be increased.
However, an 80% aneuploidy rate appears to be higher than expected for the recipient (Case-6) who underwent ovarian tissue cryopreservation at the age of 32. The study by Franasiak et al reported 31% aneuploidy rate for a woman aged 32 years (65). Previous studies suggest that the ovarian milieu can affect meiotic integrity and oocyte quality (66, 67). Thus, it is possible that the heterotopic environment impairs oocyte development and quality. We prefer performing PGT-A in ovarian transplant patients, especially for those planning on utilizing a gestational carrier, to increase the likelihood of implantation and to reduce the number of attempts. Though the suitability of PGT-A for improving IVF outcomes has been questioned in young women (70), because of the high incidence of aneuploidy in ovarian auto-transplant recipients, it may be a strategy that is worth further exploration.
Another clinically relevant observation from this report is that despite the chemotherapy exposure prior to ovarian cryopreservation, four transplant recipients cryopreserved numerous good quality embryos and had 1–2 livebirths. Of those, three had received alkylating agent regimens. While some questioned the feasibility of tissue cryopreservation after gonadotoxic chemotherapy exposure (69, 70), our data show that sufficient primordial follicle reserve survives in young women to enable successful outcomes after ovarian transplants. This could be due to the presence of large ovarian reserve in young women, as well as the higher ability of the primordial follicle oocytes to repair chemotherapy induced DNA damage (71,72,73,74,75). Hence, ovarian cryopreservation should not be routinely withheld from young females who have already begun receiving gonadotoxic chemotherapy. However, in such patients, primordial follicle density should be assessed to determine the feasibility prior to ovarian auto-transplantation.
While this case series reports on novel advances in transplantation techniques, it is based on a relatively small number of recipients at a single center. In addition, age at cryopreservation and whether the ovary was exposed to previous gonadotoxic chemotherapy can affect the graft longevity. While we attempted an exploratory analysis with age-matched data, this reduced the sample size and statistical power. Of note that, after age-matching, we still found a trend for extended longevity of 10–14 months with our approach, despite the fact that a statistically significantly larger proportion of our patients had already received gonadotoxic chemotherapy prior to tissue cryopreservation compared to the age-matched meta-analytical controls. Thus, the trend for extended longevity with our approach based on historical controls justifies larger prospective studies to further test the ACOTT technique reported herewith. However, ovarian transplantation is still a relatively rare procedure due to its currently low utilization, and thus, such prospective studies may not yet be feasible. Despite more than two decades of progress, the number of reported LB+OG pregnancies is 158 worldwide, based on our update as of November 2020, including the livebirths we reported here. In the meantime, our series represents a meaningful patient population relative to the entirety of published reports on ovarian transplantation success. However, given that ACOTT is no longer considered experimental by the ASRM (1), we expect its more widespread utility. In that context, we believe that this report will provide guidance to those who are planning to establish an ovarian transplantation program in their centers and will stimulate further research to improve ACOTT success.
Supplementary Material
Supplemental figure 1. Inclusion/exclusion flow diagram for the update of 2017 Meta-analysis
Supplemental table 1. Medical diagnosis, treatment, and indications for ovarian tissue cryopreservation
Supplemental table 2. Graft longevity comparison between the meta-analytic controls and the case series.
Video 1. Omental flap procedure to improve graft vascularization.
ACKNOWLEDGMENT
We thank Richard Hochberg, PhD for his critical reading of our manuscript. We also thank Pei Hui MD, PhD of Department of Pathology at Yale University School of Medicine for histological screening of ovarian samples in transplant patients. We also would like to thank Farr Nezhat, MD for his guidance in the omental flap procedure shown in Video 1.
FUNDING
KO’s work on ECM models was supported by NIH grant R21HD053112. HB is partly supported by the NIH through Grant UL1 TR001860.
Footnotes
CONFLICT OF INTEREST
The authors have nothing to disclose.
Orthotopic ovarian tissue transplantation with robotic surgery and a neovascularizing human extracellular matrix scaffold results in high success based on longevity, endocrine function, follicle development, embryo development and livebirths.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.ASRM. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril 2019;112:1022–33. [DOI] [PubMed] [Google Scholar]
- 2.Oktay K, Harvey BE, Partridge AH, Quinn GP, Reinecke J, Taylor HS et al. Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol 2018;36:1994–2001. [DOI] [PubMed] [Google Scholar]
- 3.Oktay K, Karlikaya G. Ovarian function after transplantation of frozen, banked autologous ovarian tissue. N Engl J Med 2000;342:1919. [DOI] [PubMed] [Google Scholar]
- 4.Oktay K, Aydin BA, Karlikaya G. A technique for laparoscopic transplantation of frozen-banked ovarian tissue. Fertil Steril 2001;75:1212–6. [DOI] [PubMed] [Google Scholar]
- 5.Christianson MS, Oktay K. Advances in fertility-preservation surgery: navigating new frontiers. Fertil Steril 2019;112:438–45. [DOI] [PubMed] [Google Scholar]
- 6.Lotz L, Dittrich R, Hoffmann I, Beckmann MW. Ovarian Tissue Transplantation: Experience From Germany and Worldwide Efficacy. Clin Med insights Reprod Health 2019;13:1179558119867357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marin L, Bedoschi G, Kawahara T, Oktay KH. History, Evolution and Current State of Ovarian Tissue Auto-Transplantation with Cryopreserved Tissue: a Successful Translational Research Journey from 1999 to 2020. Reprod Sci 2020;27:955–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pacheco F, Oktay K. Current Success and Efficiency of Autologous Ovarian Transplantation: A Meta-Analysis. Reprod Sci 2017;24:1111–20. [DOI] [PubMed] [Google Scholar]
- 9.Soleimani R, Heytens E, Oktay K. Enhancement of neoangiogenesis and follicle survival by sphingosine-1-phosphate in human ovarian tissue xenotransplants. PLoS One 2011;6:e19475–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baird DT, Webb R, Campbell BK, Harkness LM, Gosden RG. Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at −196 C. Endocrinology 1999;140:462–71. [DOI] [PubMed] [Google Scholar]
- 11.Gosden RG, Baird DT, Wade JC, Webb R. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at −196 degrees C. Hum Reprod. 1994;9:597–603. [DOI] [PubMed] [Google Scholar]
- 12.Oktay K, Bedoschi G, Pacheco F, Turan V, Emirdar V. First pregnancies, live birth, and in vitro fertilization outcomes after transplantation of frozen-banked ovarian tissue with a human extracellular matrix scaffold using robot-assisted minimally invasive surgery. Am J Obstet Gynecol 2016;214:94.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oktay K, Taylan E, Kawahara T, Cillo GM. Robot-assisted orthotopic and heterotopic ovarian tissue transplantation techniques: surgical advances since our first success in 2000. Fertil Steril 2019;111:604–6. [DOI] [PubMed] [Google Scholar]
- 14.Oktay K, Babayev S. Oocyte Retrieval and IVF Approach in Patients with Transplanted Ovarian Tissue. In: Oktay K, ed. Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation: Elsevier, Cambridge, MA, USA, 2022. [Google Scholar]
- 15.Fabbri R, Seracchioli R, Vicenti R, Paradisi R, Rossi S, De Meis L et al. Successful achievement after heterotopic transplantations of long-term stored ovarian tissue in Hodgkin’s lymphoma survivor. Gynecol Endocrinol 2019;35:470–2. [DOI] [PubMed] [Google Scholar]
- 16.Silber SJ, DeRosa M, Goldsmith S, Fan Y, Castleman L, Melnick J. Cryopreservation and transplantation of ovarian tissue: results from one center in the USA. J Assist Reprod Genet 2018;35:2205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen AK, Macklon KT, Fedder J, Ernst E, Humaidan P, Andersen CY. 86 successful births and 9 ongoing pregnancies worldwide in women transplanted with frozen-thawed ovarian tissue: focus on birth and perinatal outcome in 40 of these children. J Assist Reprod Genet 2017;34:325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bystrova O, Lapina E, Kalugina A, Lisyanskaya A, Tapilskaya N, Manikhas G. Heterotopic transplantation of cryopreserved ovarian tissue in cancer patients: a case series. Gynecol Endocrinol 2019;35:1043–9. [DOI] [PubMed] [Google Scholar]
- 19.Diaz-Garcia C, Domingo J, Garcia-Velasco JA, Herraiz S, Mirabet V, Iniesta I et al. Oocyte vitrification versus ovarian cortex transplantation in fertility preservation for adult women undergoing gonadotoxic treatments: a prospective cohort study. Fertil Steril 2018;109:478–85 e2. [DOI] [PubMed] [Google Scholar]
- 20.von Wolff M, Andersen CY, Woodruff TK, Nawroth F. FertiPROTEKT, Oncofertility Consortium and the Danish Fertility-Preservation Networks - What Can We Learn From Their Experiences? Clin Med Insights Reprod Health 2019;13:1179558119845865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoekman EJ, Louwe LA, Rooijers M, van der Westerlaken LAJ, Klijn NF, Pilgram GSK et al. Ovarian tissue cryopreservation: Low usage rates and high live-birth rate after transplantation. Acta Obstet Gynecol Scand 2020;99:213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imbert R, Moffa F, Tsepelidis S, Simon P, Delbaere A, Devreker F et al. Safety and usefulness of cryopreservation of ovarian tissue to preserve fertility: a 12-year retrospective analysis. Hum Reprod 2014;29:1931–40. [DOI] [PubMed] [Google Scholar]
- 23.Dueholm Hjorth IM, Kristensen SG, Dueholm M, Humaidan P. Reproductive outcomes after in vitro fertilization treatment in a cohort of Danish women transplanted with cryopreserved ovarian tissue. Fertil Steril 2020;114:379–87. [DOI] [PubMed] [Google Scholar]
- 24.Kim SS. Assessment of long term endocrine function after transplantation of frozen-thawed human ovarian tissue to the heterotopic site: 10 year longitudinal follow-up study. J Assist Reprod Genet 2012;29:489–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poirot C, Fortin A, Dhedin N, Brice P, Socie G, Lacorte JM et al. Post-transplant outcome of ovarian tissue cryopreserved after chemotherapy in hematologic malignancies. Haematologica 2019;104:e360–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poirot C, Fortin A, Lacorte JM, Akakpo JP, Genestie C, Vernant JP et al. Impact of cancer chemotherapy before ovarian cortex cryopreservation on ovarian tissue transplantation. Hum Reprod 2019;34:1083–94. [DOI] [PubMed] [Google Scholar]
- 27.Tanbo T, Greggains G, Storeng R, Busund B, Langebrekke A, Fedorcsak P. Autotransplantation of cryopreserved ovarian tissue after treatment for malignant disease - the first Norwegian results. Acta Obstet Gynecol Scand 2015;94:937–41. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Wallberg KA, Tanbo T, Tinkanen H, Thurin-Kjellberg A, Nedstrand E, Kitlinski ML et al. Ovarian tissue cryopreservation and transplantation among alternatives for fertility preservation in the Nordic countries - compilation of 20 years of multicenter experience. Acta Obstet Gynecol Scand 2016;95:1015–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruan X, Cheng J, Korell M, Du J, Kong W, Lu D et al. Ovarian tissue cryopreservation and transplantation prevents iatrogenic premature ovarian insufficiency: first 10 cases in China. Climacteric 2020;23:574–80. [DOI] [PubMed] [Google Scholar]
- 30.Meirow D, Ra’anani H, Shapira M, Brenghausen M, Derech Chaim S, Aviel-Ronen S et al. Transplantations of frozen-thawed ovarian tissue demonstrate high reproductive performance and the need to revise restrictive criteria. Fertil Steril 2016;106:467–74. [DOI] [PubMed] [Google Scholar]
- 31.Shapira M, Raanani H, Barshack I, Amariglio N, Derech-Haim S, Marciano MN et al. First delivery in a leukemia survivor after transplantation of cryopreserved ovarian tissue, evaluated for leukemia cells contamination. Fertil Steril 2018;109:48–53. [DOI] [PubMed] [Google Scholar]
- 32.Donnez J, Squifflet J, Jadoul P, Demylle D, Cheron AC, Van Langendonckt A et al. Pregnancy and live birth after autotransplantation of frozen-thawed ovarian tissue in a patient with metastatic disease undergoing chemotherapy and hematopoietic stem cell transplantation. Fertil Steril 2011;95:1787 e1–4. [DOI] [PubMed] [Google Scholar]
- 33.Donnez J, Jadoul P, Pirard C, Hutchings G, Demylle D, Squifflet J et al. Live birth after transplantation of frozen-thawed ovarian tissue after bilateral oophorectomy for benign disease. Fertil Steril 2012;98:720–5. [DOI] [PubMed] [Google Scholar]
- 34.Donnez J, Dolmans M-M. Ovarian cortex transplantation: 60 reported live births brings the success and worldwide expansion of the technique towards routine clinical practice. J Assist Reprod Genet 2015;32:1167–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fabbri R, Pasquinelli G, Magnani V, Macciocca M, Vicenti R, Parazza I et al. Autotransplantation of cryopreserved ovarian tissue in oncological patients: recovery of ovarian function. Future Oncol 2014;10:549–61. [DOI] [PubMed] [Google Scholar]
- 36.Jensen AK, Kristensen SG, Macklon KT, Jeppesen JV, Fedder J, Ernst E et al. Outcomes of transplantations of cryopreserved ovarian tissue to 41 women in Denmark. Hum Reprod 2015;30:2838–45. [DOI] [PubMed] [Google Scholar]
- 37.Van der Ven H, Liebenthron J, Beckmann M, Toth B, Korell M, Krussel J et al. Ninety-five orthotopic transplantations in 74 women of ovarian tissue after cytotoxic treatment in a fertility preservation network: tissue activity, pregnancy and delivery rates. Hum Reprod 2016;31:2031–41. [DOI] [PubMed] [Google Scholar]
- 38.Revelli A, Marchino G, Dolfin E, Molinari E, Delle Piane L, Salvagno F et al. Live birth after orthotopic grafting of autologous cryopreserved ovarian tissue and spontaneous conception in Italy. Fertil Steril 2013;99:227–30. [DOI] [PubMed] [Google Scholar]
- 39.Lorenzo F, Villamayor M, Viola J, Tiveron M, Young E. Second children born after autotransplantation of cryopreserved ovarian tissue in a young patient previously treated with chemotherapy for Askin’s diseasethe successful of fertility preservation program. Fertil Steril 2016;106.26456229 [Google Scholar]
- 40.Isachenko V, Morgenstern B, Todorov P, Isachenko E, Mallmann P, Hanstein B et al. Long-term (24h) cooling of ovarian fragments in the presence of permeable cryoprotectants prior to freezing: Two unsuccesful IVF-cycles and spontaneous pregnancy with baby born after re-transplantation. Cryobiology 2020;93:115–20. [DOI] [PubMed] [Google Scholar]
- 41.Tammiste T, Kask K, Padrik P, Idla K, Rosenstein K, Jatsenko T et al. A case report and follow-up of the first live birth after heterotopic transplantation of cryopreserved ovarian tissue in Eastern Europe. BMC Womens Health 2019;19:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Radwan P, Abramik A, Wilczynski J, Radwan M. Successful autotransplantation of cryopreserved ovarian tissue with recovery of the ovarian function. Ginekol Pol 2016;87:235–40. [DOI] [PubMed] [Google Scholar]
- 43.Radford JA, Lieberman BA, Brison DR, Smith ARB, Critchlow JD, Russell SA et al. Orthotopic reimplantation of cryopreserved ovarian cortical strips after high-dose chemotherapy for Hodgkin’s lymphoma. Lancet 2001;357:1172–5. [DOI] [PubMed] [Google Scholar]
- 44.Burmeister L, Kovacs GT, Osianlis T. First Australian pregnancy after ovarian tissue cryopreservation and subsequent autotransplantation. Med J Aust 2013;198:158–9. [DOI] [PubMed] [Google Scholar]
- 45.Callejo J, Salvador C, Gonzalez-Nunez S, Almeida L, Rodriguez L, Marques L et al. Live birth in a woman without ovaries after autograft of frozen-thawed ovarian tissue combined with growth factors. J Ovarian Res 2013;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanchez-Serrano M, Crespo J, Mirabet V, Cobo AC, Escriba MJ, Simon C et al. Twins born after transplantation of ovarian cortical tissue and oocyte vitrification. Fertil Steril 2010;93:268 e11–3. [DOI] [PubMed] [Google Scholar]
- 47.Roux C, Amiot C, Agnani G, Aubard Y, Rohrlich PS, Piver P. Live birth after ovarian tissue autograft in a patient with sickle cell disease treated by allogeneic bone marrow transplantation. Fertil Steril 2010;93:2413 e15–9. [DOI] [PubMed] [Google Scholar]
- 48.Povoa A, Xavier P, Calejo L, Soares S, Sousa M, Silva J et al. First transplantation of cryopreserved ovarian tissue in Portugal, stored for 10 years: an unexpected indication. Reprod Biomed Online 2016;32:334–6. [DOI] [PubMed] [Google Scholar]
- 49.Revel A, Laufer N, Ben Meir A, Lebovich M, Mitrani E. Micro-organ ovarian transplantation enables pregnancy: a case report. Hum Reprod 2011;26:1097–103. [DOI] [PubMed] [Google Scholar]
- 50.Milenkovic M, Brannstrom M, Diaz-Garcia C, Lundin K, Selleskog U, Soderlund B et al. Spontaneous twin pregnancy with live births after cryopreservation and re-implantation of ovarian tissue. Gynecol Surg 2017;14:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Macklon KT, Jensen AK, Loft A, Ernst E, Andersen CY. Treatment history and outcome of 24 deliveries worldwide after autotransplantation of cryopreserved ovarian tissue, including two new Danish deliveries years after autotransplantation. J Assist Reprod Genet 2014;31:1557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gellert SE, Pors SE, Kristensen SG, Bay-Bjorn AM, Ernst E, Yding Andersen C. Transplantation of frozen-thawed ovarian tissue: an update on worldwide activity published in peer-reviewed papers and on the Danish cohort. J Assist Reprod Genet 2018;35:561–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poirot C, Abirached F, Prades M, Coussieu C, Bernaudin F, Piver P. Induction of puberty by autograft of cryopreserved ovarian tissue. Lancet 2012;379. [DOI] [PubMed] [Google Scholar]
- 54.Stern CJ, Gook D, Hale LG, Agresta F, Oldham J, Rozen G et al. First reported clinical pregnancy following heterotopic grafting of cryopreserved ovarian tissue in a woman after a bilateral oophorectomy. Hum Reprod 2013;28:2996–9. [DOI] [PubMed] [Google Scholar]
- 55.Stern CJ, Gook D, Hale LG, Agresta F, Oldham J, Rozen G et al. Delivery of twins following heterotopic grafting of frozen-thawed ovarian tissue. Hum Reprod 2014;29:1828. [DOI] [PubMed] [Google Scholar]
- 56.Sonmezer M, Ozkavukcu S, Sukur YE, Kankaya D, Arslan O. First pregnancy and live birth in Turkey following frozen-thawed ovarian tissue transplantation in a patient with acute lymphoblastic leukemia who underwent cord blood transplantation. J Assist Reprod Genet 2020;37:2033–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y, Chang Q, Sun J, Dang L, Ma W, Hei C et al. Effects of HMG on revascularization and follicular survival in heterotopic autotransplants of mouse ovarian tissue. Reprod Biomed Online 2012;24:646–53. [DOI] [PubMed] [Google Scholar]
- 58.Chakravarthi VP, Ghosh S, Roby KF, Wolfe MW, Rumi MAK. A Gatekeeping Role of ESR2 to Maintain the Primordial Follicle Reserve. Endocrinology 2020;161. [DOI] [PubMed] [Google Scholar]
- 59.Zelinski MB, Ting A, Bishop C, Lawson M, Liang L, Hobbs T et al. Vitrified macaque ovarian cortical tissue transplanted to heterotopic sites produces fertilizable oocytes. Fertil Steril 2018;110:1. [Google Scholar]
- 60.Oktay K, Economos K, Kan M, Rucinski J, Veeck L, Rosenwaks Z. Endocrine function and oocyte retrieval after autologous transplantation of ovarian cortical strips to the forearm. JAMA 2001;286:1490–3. [DOI] [PubMed] [Google Scholar]
- 61.Oktay K, Türkçüoğlu I, Rodriguez-Wallberg KA. Four spontaneous pregnancies and three live births following subcutaneous transplantation of frozen banked ovarian tissue: what is the explanation? Fertil Steril 2011;95:804.e7–10. [DOI] [PubMed] [Google Scholar]
- 62.Fortin CS, Leader A, Mahutte N, Hamilton S, Léveillé MC, Villeneuve M et al. Gene expression analysis of follicular cells revealed inflammation as a potential IVF failure cause. J Assist Reprod Genet 2019;36:1195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.ASRM. Ovarian tissue cryopreservation: a committee opinion. Fertil Steril 2014;101:1237–43. [DOI] [PubMed] [Google Scholar]
- 64.Greve T, Clasen-Linde E, Andersen MT, Andersen MK, Sørensen SD, Rosendahl M et al. Cryopreserved ovarian cortex from patients with leukemia in complete remission contains no apparent viable malignant cells. Blood 2012;120:4311–6. [DOI] [PubMed] [Google Scholar]
- 65.Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR et al. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril 2014;101:656–63.e1. [DOI] [PubMed] [Google Scholar]
- 66.Oktem O, Oktay K. Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer 2007;110:2222–9. [DOI] [PubMed] [Google Scholar]
- 67.Spears N, Lopes F, Stefansdottir A, Rossi V, De Felici M, Anderson RA et al. Ovarian damage from chemotherapy and current approaches to its protection. Hum Reprod Update 2019;25:673–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.ASRM. The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Fertil Steril 2018;109:429–36. [DOI] [PubMed] [Google Scholar]
- 69.Anderson RA, Mitchell RT, Kelsey TW, Spears N, Telfer EE, Wallace WH. Cancer treatment and gonadal function: experimental and established strategies for fertility preservation in children and young adults. Lancet Diabetes Endocrinol 2015;3:556–67. [DOI] [PubMed] [Google Scholar]
- 70.Wallace WH, Anderson RA, Irvine DS. Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Oncol 2005;6:209–18. [DOI] [PubMed] [Google Scholar]
- 71.Soleimani R, Heytens E, Darzynkiewicz Z, Oktay K. Mechanisms of chemotherapy-induced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging 2011;3:782–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oktay KH, Bedoschi G, Goldfarb SB, Taylan E, Titus S, Palomaki GE, et al. Increased chemotherapy-induced ovarian reserve loss in women with germline BRCA mutations due to oocyte deoxyribonucleic acid double-strand break repair deficiency. Fertil Steril 2020;113, 1251–60.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Titus S, Szymanska KJ, Musul B, Turan V, Taylan E, Garcia-Milian R, et al. Individual-oocyte transcriptomic analysis shows that genotoxic chemotherapy depletes human primordial follicle reserve in vivo by triggering proapoptotic pathways without growth activation. Sci Rep 2021;11:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, et al. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci Transl Med 2013;5:172ra21: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Turan V, Oktay K. BRCA-related ATM-mediated DNA double-strand break repair and ovarian aging. Hum Reprod Update 2020;26:43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1. Inclusion/exclusion flow diagram for the update of 2017 Meta-analysis
Supplemental table 1. Medical diagnosis, treatment, and indications for ovarian tissue cryopreservation
Supplemental table 2. Graft longevity comparison between the meta-analytic controls and the case series.
Video 1. Omental flap procedure to improve graft vascularization.


