Abstract
Background:
In patients undergoing resection of intrahepatic cholangiocarcinoma (ICC), hypervascularity during the arterial phase of contrast-enhanced computed tomography (CT) is associated with better prognosis than hypovascularity. However, the prognostic implications of arterial enhancement pattern in patients with unresectable ICC are unknown. We assessed the prognostic implications of arterial enhancement pattern in patients with resectable and unresectable ICC.
Methods:
Consecutive patients who underwent surgery or gemcitabine-plus-cisplatin chemotherapy for ICC during 2003–2015 and CT with dynamic enhancement for diagnosis were included. After review by 2 radiologists, tumors were categorized according to the percentage of the tumor exhibiting arterial enhancement as hypervascular (>50% of tumor exhibiting enhancement), peripherally enhancing(10%−50%), and hypovascular (<10%). In each cohort (surgical and medical), overall survival (OS) curves were generated using the Kaplan-Meier method, and differences between curves were evaluated with Cox analysis.
Results:
The study included 56 patients treated surgically and 89 patients with unresectable ICC. Mean (SD) tumor density in the hypervascular, peripherally enhancing, and hypovascular groups was 119.3 (45.2) Hounsfield units (HU), 72.1 (15.9) HU, and 59.9 (14.4) HU, respectively, in the surgical cohort and 93.6 (17.5) HU, 66.6 (16.2) HU, and 48.7 (14.3) HU, respectively, in the medical cohort. In both cohorts, the 5-year OS rate was significantly higher in the hypervascular group than in the hypovascular group (surgical, 67.6% vs 22.5%, P=.038; medical, 15.4% vs 0%, P=.030). In both cohorts, a Cox proportional hazards model analysis showed that hypervascularity was significantly associated with better OS.
Conclusion:
Hypervascularity during the arterial CT phase is a positive prognostic biomarker in patients undergoing ICC resection and patients with unresectable ICC.
Keywords: Intrahepatic cholangiocarcinoma, cholangiocarcinoma, arterial enhancement, computed tomography, liver resection, chemotherapy
PRÉCIS
Hypervascularity during the arterial CT phase is a positive prognostic biomarker in both patients with resectable and unresectable intrahepatic cholangiocarcinoma.
INTRODUCTION
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver malignancy after hepatocellular carcinoma1 and affects 5000 to 8000 people per year in the United States,2 with a rising incidence over the past decades.1,3,4 Three macroscopic types of ICC have been described: mass-forming, periductal infiltrating, and intraductal growing.1,3
Surgical resection is the only curative treatment strategy for ICC, and 5-year overall survival (OS) rates after surgery range from 22% to 36%.4–6 However, most patients have disease deemed unresectable at initial presentation and undergo systemic chemotherapy3,4 with gemcitabine and cisplatin, the suggested first-line medical treatment.7,8
Histologically, ICC often demonstrates features of well-differentiated to moderately differentiated adenocarcinoma with accompanying fibrous stroma.9,10 The presence of fibrous stroma in ICC has been associated with decreased survival 11,12 because of its relationship with hypovascularity in the arterial phase of contrast-enhanced cross-sectional imaging.11,13,14
This suggests that hypervascularity of ICC in the arterial phase could be used as a surrogate or “biomarker” for favorable tumor biology. Indeed, some investigators have demonstrated that hypervascularity of ICC in the arterial phase is associated with better survival after resection13,15–21 and less lymph node involvement.22 However, the prognostic implications of arterial enhancement patterns in patients with unresectable ICC remain unknown. The aim of this study was to determine whether arterial hypervascularity predicts survival in patients with unresectable ICC and to validate arterial hypervascularity as a prognostic factor in patients undergoing ICC resection.
METHODS
Patient selection
The Institutional Review Board of The University of Texas MD Anderson Cancer Center approved this study protocol (PA17–0640_MOD003) and waived the requirement for informed consent. Consecutive patients who underwent treatment of mass-forming ICC during 2003–2015 and underwent contrast-enhanced computed tomography (CT) with dynamic enhancement at the time of diagnosis were included in the study. To identify patients who underwent resection of ICC, a prospectively maintained Hepatobiliary Surgery database was reviewed. To identify unresectable patients who underwent medical therapy, a prospectively maintained database of the Department of Gastrointestinal Medical Oncology was reviewed. Only patients who received gemcitabine and cisplatin as first-line chemotherapy were included. Patients were deemed to have unresectable disease when they had distant metastases or extensive local extension. Histopathologic diagnosis of ICC was made with percutaneous or surgical biopsy, and immunohistological evaluation was additionally performed if necessary. T category and N category were classified according to the AJCC Cancer Stating Manual, eighth edition.
Imaging studies and assessment of enhancement in the arterial phase
Contrast-enhanced CT was performed with multidetector row CT, with 4, 16, or 64 slices (Light-Speed; GE Health care, Piscataway, NJ), using a slice thickness of 5mm and reconstruction at 2.5mm. Images were acquired with either a triphasic liver protocol or biphasic technique. For the triphasic liver protocol, images of the abdomen were obtained at approximately 35 s and 70 s after the start of ioversol injection at a rate of 5mL/s, in addition to unenhanced images. Excretory-phase images were acquired approximately 5–6 min after ioversol injection. For the biphasic technique, images of the thorax were obtained approximately 25 to 30 s after the start of ioversol injection at a rate of 2 to 3 mL/s, and images of the abdomen/pelvis were obtained approximately 70 s after ioversol injection. Images of the thorax covered the superior aspect of the abdomen and allowed visualization of the intrahepatic tumor.
The pattern of arterial enhancement on CT images was reviewed by radiologists who specialize in liver imaging (H.C.K., V.C.) and were blinded to clinical data. The radiologists qualitatively assessed the proportion of the tumor that was hypervascular compared to non-tumorous liver on the same slice. Tumors with arterial enhancement involving greater than 50% of the tumor were categorized as “hypervascular” (Figure 1A); tumors with enhancement involving 10% to 50% of the tumor along the periphery were categorized as “peripherally enhancing (Figure 1B); and diffusely non enhancing or hypoenhancing tumors were categorized as “hypovascular” (Figure 1C).21–23 Only patients for whom consensus agreement was reached were included in the study.
Fig. 1.
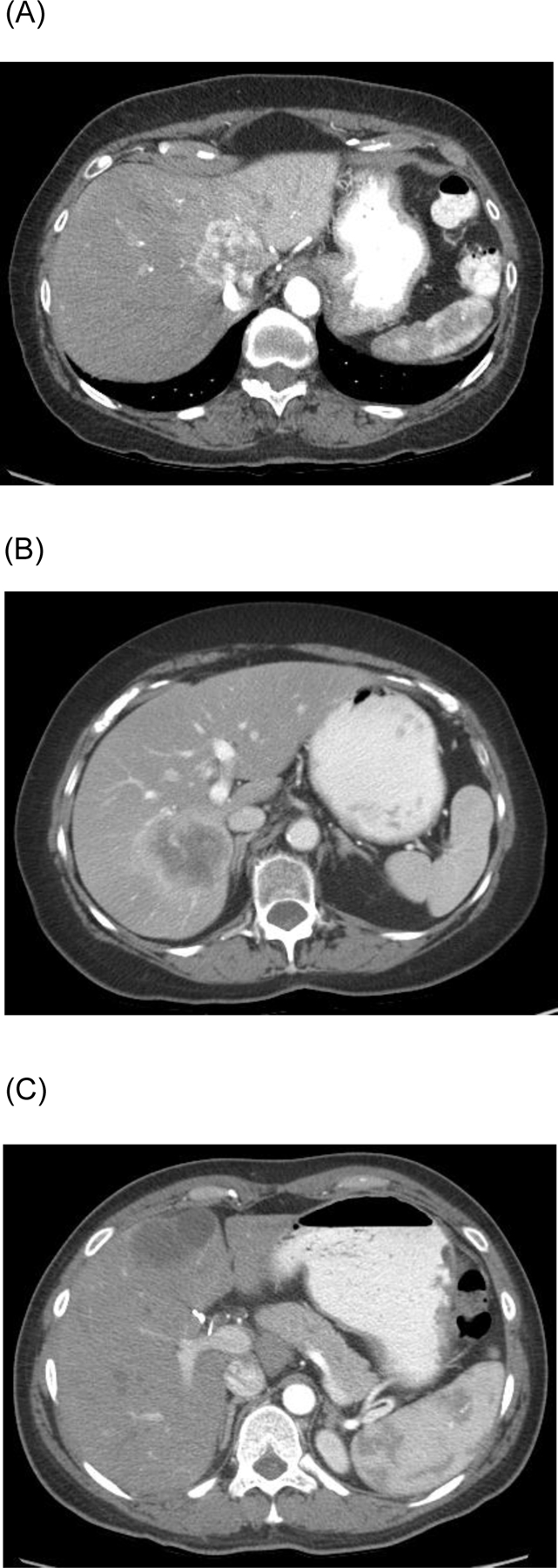
Typical computed tomography appearance of (A) hypervascular, (B) peripherally enhancing, and (C) hypovascular intrahepatic cholangiocarcinoma.
In categorizing the arterial enhancement patterns of the tumors, the radiologists took into account focal fatty changes, blood vessels, transient hepatic attenuation differences, and artifacts altering the appearance of the tumor density. For example, if the attenuation of the surrounding liver was altered by perfusion changes (e.g., related to portal vein narrowing or occlusion), the tumor was compared with an unaffected portion of the liver on the same slice. In cases of multiple lesions, the largest tumor was analyzed for classification.
The relationship between tumor density on CT and arterial enhancement pattern was evaluated in patients who underwent surgery and patients with unresectable ICC.
Survival analysis
OS was assessed in patients who underwent ICC resection and patients with unresectable ICC. OS was measured from the time of surgery to the time of death for patients who underwent ICC resection and from the time of diagnosis to the time of death for patients with unresectable ICC. For patients who underwent ICC resection, recurrence-free survival (RFS) was also assessed. RFS was measured from the time of surgery to the time of first recurrence.
Statistical analyses
Continuous variables were compared using the Mann-Whitney U test with a Bonferroni correction for multiple comparisons, while categorical variables were compared using the χ2 test. Survival curves were generated using the Kaplan-Meier method, and differences on survival between groups were evaluated using Cox proportional hazards model analyses.24,25 A Cox proportional hazards model analysis for patients initially included arterial enhancement pattern and clinicopathologic factors. A backward elimination with a threshold P value of .20 was used to select variables for the final models. HRs and 95% confidence intervals (CI) were calculated for each factor. All statistical tests were 2-sided, and P<.05 was considered statistically significant. Statistical analysis was conducted with SAS 9.4 (SAS Institute).
RESULTS
Patient and tumor characteristics
Of 106 patients who underwent resection of ICC during the study period, 56 patients were included in the study (Figure 2A); hereafter, this patient group is termed the surgical cohort. Of 302 patients with unresectable ICC treated during the study period, 89 patients were included in the study; hereafter, this patient group is termed the medical cohort (Figure 2B). Demographic and clinicopathologic characteristics of the surgical and medical cohorts are summarized in Table 1.
Fig. 2.

Inclusion criteria for patients with mass-forming intrahepatic cholangiocarcinoma in the (A) surgical and (B) medical cohorts. Abbreviations: CT, computed tomography; GEMCIS, gemcitabine + cisplatin.
Table 1.
Demographic and Clinicopathologic Characteristics of 56 Patients Who Underwent Resection of ICC and 89 Patients with Unresectable ICC
| Characteristic | Surgical cohort (n=56) | Medical cohort (n=89) | P value |
|---|---|---|---|
| Patient factors | |||
| Age, mean (SD), yr | 61.0 (11.0) | 60.6 (11.7) | .646 |
| Sex, n (%) | |||
| Male | 18 (32) | 45 (50) | .029 |
| Female | 38 (68) | 44 (50) | |
| Tumor clinical factors | |||
| CA19–9 level at diagnosis, mean (SD), U/mL | 629.4 (2570.9) | 5470.7 (18826.8) | .002 |
| CA19–9 >37 U/mL, n (%) | 26 (46) | 54 (61) | .093 |
| Neoadjuvant chemotherapy, n (%) | 19 (34) | - | - |
| Number of cycles, mean (SD) | 6.7 (3.4) | - | - |
| Partial response, n (%) | 8 (42) | - | - |
| Adjuvant chemotherapy, n (%) | 40 (71) | - | - |
| History of viral hepatitis, n (%)a | 5 (13) | 8 (19) | .474 |
| Tumor histopathologic factors | |||
| Poorly differentiated tumor, n (%) | 23 (41) | 36 (51) | .247 |
| Maximum tumor size, median (SD), cm | 6.1 (2.3) | 10.8 (3.8)b | <.001 |
| Multiple tumors, n (%) | 8 (14) | 56 (63)b | <.001 |
| Number of tumors, mean (SD) | 6.9 (12.9) | 11.2 (18.0)b | <.001 |
| Positive lymph nodes, n (%)c | 7 (33) | - | - |
| T category ≥3, n (%) | 12 (21) | - | - |
| R1 surgical margin, n (%) | 15 (27) | - | - |
| Cirrhosis, n (%) | 3 (5) | - | - |
| Arterial enhancement pattern | |||
| Hypervascular, n (%) | 17 (30) | 13 (15) | .023 |
| Peripherally enhancing, n (%) | 29 (52) | 43 (48) | .684 |
| Hypovascular, n (%) | 10 (18) | 33 (37) | <.001 |
| Tumor density, mean (SD), HU | 84.3 (36.4) | 63.9 (21.5) | <.001 |
| Tumor/liver density ratio, mean (SD) | 1.18 (0.5) | 0.85 (0.3) | .002 |
Abbreviations: CA19–9, carbohydrate antigen 19–9; HU, Hounsfield unit; ICC, intrahepatic cholangiocarcinoma; SD, standard deviation.
Data on viral hepatitis were unavailable for 17 patients in the surgical cohort and 46 patients in the medical cohort.
Radiologic data.
Data on lymph node status were unavailable for 35 patients who did not undergo lymphadenectomy.
The rate of hypervascular arterial enhancement pattern was significantly higher in the surgical cohort than in the medical cohort (30% vs 15%, P=.023), whereas the rate of hypovascular arterial enhancement pattern was significantly lower in the surgical cohort than in the medical cohort (18% vs 37%, P<.001). The rate of peripherally enhancing arterial enhancement pattern was similar in the 2 cohorts (surgical, 52%; medical, 48%; P=.68). In arterial phase, tumors in the surgical cohort were more radiodense than tumors in the medical cohort (mean [SD], 84.3 [36.4] Hounsfield units (HU) vs 63.9 [21.5] HU, P<.001), with a higher mean (SD) tumor/liver density ratio (1.18 [0.5] vs 0.85 [0.3], P=.002).
Relationship between enhancement patterns and tumor density
In the surgical cohort, mean (SD) tumor density in the hypervascular, peripherally enhancing, and hypovascular groups was 119.3 (45.2) HU, 72.1 (15.9) HU, and 59.9 (14.4) HU, respectively. Differences between the hypervascular and peripherally enhancing groups and between the hypervascular and hypovascular groups were significant (both P<.001). Mean tumor/liver density ratio in the hypervascular, peripherally enhancing, and hypovascular groups was 1.7 (0.6), 0.9 (0.2), and 0.7 (0.2), respectively. Differences between the hypervascular and peripherally enhancing groups and between the hypervascular and hypovascular groups were significant (both P<.001). The rates of lymph node metastasis were significantly different between the three groups: 5.9% in the hypervascular group, 6.9% in the peripherally enhancing group, and 40.0% in the hypervascular group, P=.015.
In the medical cohort, mean tumor density in the hypervascular, peripherally enhancing, and hypovascular groups was 93.6 (17.5) HU, 66.6 (16.2) HU, and 48.7 (14.3) HU, respectively. Differences between the hypervascular and peripherally enhancing groups, between the hypervascular and hypovascular groups, and between the peripherally enhancingand hypovascular groups were significant (all P<.001). Mean (SD) tumor/liver density ratio in the hypervascular, peripherally enhancing, and hypovascular groups was 1.3 (0.2), 0.9 (0.3), and 0.6 (0.2), respectively. Differences between the hypervascular and peripherally enhancing groups, between the hypervascular and hypovascular groups, and between the peripherally enhancing and hypovascular groups were significant (all P<.001). The rates of extrahepatic disease did not differ significantly between the groups (P = .071).
OS analysis and risk factors for OS in the surgical cohort
In the surgical cohort, 34 (61%) patients died. The 5-year OS rate was significantly better in the hypervascular group than in the hypovascular group (68% vs 22%, P=.038), but the 5-year OS rate did not differ significantly between the hypervascular group and the peripherally enhancing group (68% vs 43%, P=.157) or between the peripherally enhancing group and the hypovascular group (43% vs 22%, P=.297) (Figure 3A). Multivariable Cox proportional hazards model analysis showed that age, male sex, positive lymph nodes and hypovascular arterial enhancement pattern were significantly associated with worse OS (Table 2).
Fig. 3.
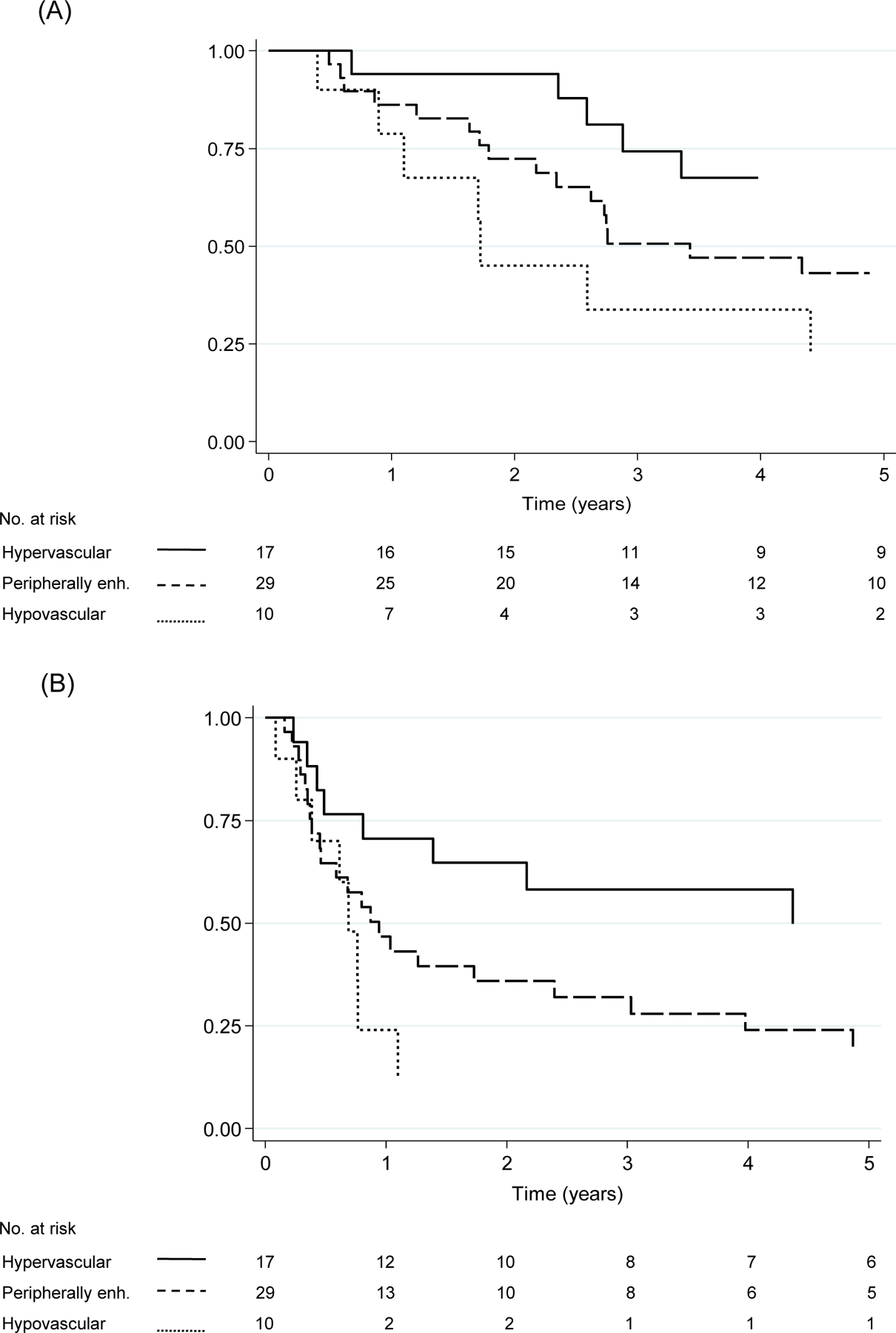
Overall survival (A) and recurrence-free survival (B) by arterial enhancement pattern in the surgical cohort. Abbreviations: OS, overall survival; peripherally enh., peripherally enhancing; RFS, recurrence-free survival. (A) OS rate was longer in the hypervascular group than in the hypovascular group (P=.038), whereas OS rates did not differ significantly between the hypervascular group and the peripherally enhancing group (P=.157) or between the peripherally enhancing group and the hypovascular group (P=.297) (B) RFS rate was longer in the hypervascular group than in the hypovascular group (P=.020), whereas RFS rates did not differ significantly between the hypervascular group and the peripherally enhancing group (P=.052) or between the peripherally enhancing group and the hypovascular group (P=.351).
Table 2.
Multivariable Cox Proportional Hazards Model Analysis for Overall Survival in 56 Patients Who Underwent Resection of ICC*
| Variable | No. of patients | No. of events | HR | 95% CI | P value |
|---|---|---|---|---|---|
| Age | - | - | 1.06 | 1.02–1.11 | 0.003 |
| Sex | |||||
| Male | 18 | 14 | 3.95 | 1.76–8.84 | < 0.001 |
| Female | 38 | 20 | Reference | ||
| Positive lymph nodes | |||||
| Yes | 7 | 6 | 4.45 | 1.67–11.87 | 0.003 |
| No | 49 | 28 | Reference | ||
| Arterial enhancement pattern | |||||
| Hypovascular | 10 | 7 | 5.33 | 1.52–19.90 | 0.009 |
| Peripherally enhancing | 29 | 20 | 1.65 | 0.67–4.09 | 0.280 |
| Hypervascular | 17 | 7 | Reference |
Abbreviations: ICC, intrahepatic cholangiocarcinoma; HR, hazard ratio.
A Cox proportional hazards model analysis initially included age (continuous variable), sex, prehepatectomy CA19–9 level (> 37 vs ≤ 37 ng/mL), prehepatectomy chemotherapy, lymph node metastasis, number of ICC (multiple vs single), largest ICC diameter (> 3 vs ≤ 3 cm), and arterial enhancement patter. A backward elimination with a threshold P value of 0.20 was used to select variables for the final models.
RFS analysis and risk factors for RFS in the surgical cohort
In the surgical cohort, 38 (68%) patients experienced recurrence. The 5-year RFS rate was significantly better in the hypervascular group than in the hypovascular group (50% vs 12%, P=.020), whereas it did not differ significantly between the hypervascular group and the peripherally enhancing group (50% vs 20%, P=.052) or between the peripherally enhancing group and the hypovascular group (20% vs 12%, P=.351) (Figure 3B). Multivariable Cox proportional hazards model analysis showed that positive lymph nodes and hypovascular arterial enhancement pattern were significantly associated with worse RFS (Supplementary Table 1).
OS analysis and risk factors for OS in the medical cohort
In the medical cohort, 85 (96%) patients died. The 5-year OS rate was significantly better in the hypervascular group than in the hypovascular group (15% vs 0%, P=.030), whereas it did not differ significantly between the hypervascular group and the peripherally enhancing group (15% vs 0%, P=.096) or between the peripherally enhancing group and the hypovascular group (0% vs 0%, P=.396) (Figure 4). A Cox proportional hazards model analysis showed that only hypovascular arterial enhancement pattern was significantly associated with worse OS: the hypovascular group vs. the hypervascular group (P=.042) and the peripherally enhancing group vs. the hypervascular group (P=.121) (Table 3).
Fig. 4.
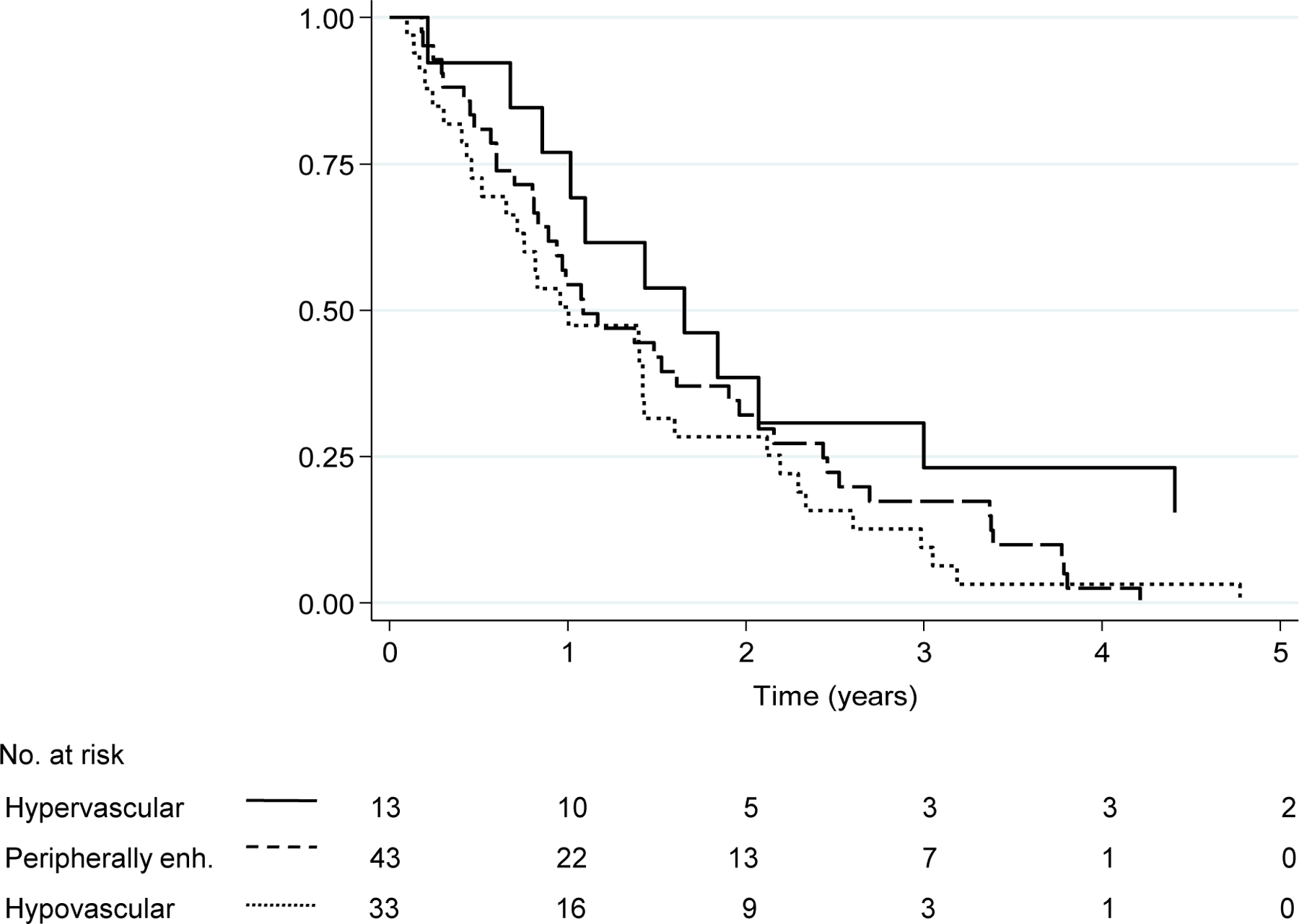
Overall survival by arterial enhancement pattern in the medical cohort. Abbreviations: OS, overall survival. OS rate was longer in the hypervascular group than in the hypovascular group (P=.030), whereas OS rates did not differ significantly between the hypervascular group and the peripherally enhancing group (P=.096) or between the peripherally enhancing group and the hypovascular group (P=.396).
Table 3.
Univariable and Multivariable Cox Proportional Hazards Model Analysis for Overall Survival in 89 Patients with Unresectable ICC
| Factor | No. of patients | No. of events | HR | 95% CI | P value |
|---|---|---|---|---|---|
| Age | – | – | 0.99 | 0.97–1.01 | 0.194 |
| CA 19–9 level | |||||
| > 37 mU/L | 63 | 61 | 1.39 | 0.85–2.26 | 0.193 |
| ≤ 37 mU/L | 25 | 23 | Reference | ||
| Arterial enhancement pattern | |||||
| Hypovascular | 33 | 32 | 2.08 | 1.03–4.21 | 0.042 |
| Peripherally enhancing | 43 | 41 | 1.73 | 0.87–3.47 | 0.121 |
| Hypervascular | 13 | 12 | Reference |
Abbreviations: ICC, intrahepatic cholangiocarcinoma; HR, hazard ratio.
A Cox proportional hazards model analysis initially included age (continuous variable), sex, pretreatment CA19–9 level (> 37 vs ≤ 37 ng/mL), number of ICC (multiple vs single), largest ICC diameter (> 3 vs ≤ 3 cm), and arterial enhancement pattern. A backward elimination with a threshold P value of 0.20 was used to select variables for the final models.
DISCUSSION
This study shows that among patients with ICC who underwent resection and patients with unresectable ICC, hypervascular arterial enhancement pattern on CT was associated with improved survival. Previous studies assessed the prognostic relevance of arterial enhancement patterns in patients with ICC who underwent resection, but no study had investigated the prognostic relevance of arterial enhancement patterns in patients with unresectable ICC who were treated solely with chemotherapy. Min et al20. recently reported similar survival patterns according to magnetic resonance imaging (MRI) enhancement patterns in 134 patients who underwent resection of ICC. Min et al defined hypervascular tumors as those with a hyper-enhanced portion involving 70% or more of the tumor surface and peripherally enhancing tumors as tumors with 10% to 70% enhancement of the tumor surface; in contrast, in the study reported here, we used greater than 50% and 10% to 50% of the tumor exhibiting enhancement to define hypervascular and peripherally enhancing tumors, respectively, as previously described by Fujita et al19. and Kim et al21. Fujita et al19. also demonstrated worse RFS (but not OS) among patients with hypovascular ICC than among patients with peripherally enhancing and hypervascular tumors in a cohort of 47 patients who underwent resection of ICC.
Interestingly, we found that both mean tumor density and tumor/liver density ratio were significantly higher in the surgical cohort than in the medical cohort. Furthermore, hypervascular tumors were more frequently identified in the surgical cohort (30% vs 15%). These data suggest that hypervascularity may be related to more favorable tumor biology, less aggressive presentation, and higher potential for resection. Turkoglu et al18. defined ICC as hypervascular when the tumor/liver density ratio was more than 1. In our series, hypervascular tumors had a mean (SD) tumor/liver density ratio of 1.7 (0.6) in the surgical cohort and 1.3 (0.2) in the medical cohort, suggesting that further validation is required to determine an appropriate value to determine hypervascularity. The findings of our study may suggest correlation between arterial enhancement patterns with pathologic characteristics specific to each patient and could represent tumor biology aggressiveness. As such, the information on the arterial enhancement pattern may be used in treatment decision making including indications of surgery, use of neoadjuvant or adjuvant chemotherapy, and intra-arterial therapy for patents with resectable ICC.
Indeed, high-quality CT imaging can also be used to predict lymph node metastasis in ICC with arterial and portal enhancement calculations to predict patients with a poor prognosis. A recent study by Zhu et al26. Validated hyperenhancement as an additional factor to consider during review of cross-sectional imaging for patients with ICC. Additionally, arterial enhancement pattern of ICC may be used to predict lymph node metastases because the hypovascular group showed the high rate of lymp node metastases.
There are limitations to this study. First, the analysis was retrospective and therefore subject to inherent selection bias. However, this is the first study to include patients who underwent resection and patients who had unresectable ICC and received chemotherapy, and hypervascular tumors were associated with better survival in both treatment groups. Second, more than 80 patients were excluded because baseline CT images were unavailable. As such, to use our findings in clinical practice, it is important to have CT images with a triphasic or biphasic liver protocol and to establish the scoring criteria of hypervascularity for standardization Last, MRI was not evaluated in this study because we did not routinely examine in our institute.
In conclusion, hypervascularity during the arterial CT phase is a positive prognostic biomarker in patients with ICC, both those with resectable disease and those with unresectable disease. This may be explained by less fibrotic stroma in hypervascular tumors. Hyperenhancement may provide additional information about tumor biology at the time of diagnosis, and this information may be used in treatment decision making including indications of surgery, use of neoadjuvant or adjuvant chemotherapy, and intra-arterial therapy.
Supplementary Material
Highlights.
#Vascularity may reflect tumor biology in intrahepatic cholangiocarcinoma
#Vascularity classified into 3 grades in contrast-enhanced CT images
#Better prognosis of hypervascularity in both resectable and unresectable tumors
ACKNOWLEDGMENTS
The authors thank Dr. Ramiro Manuel Fernandez Placencia for reviewing the data used in the study, Ms. Ruth Haynes for administrative support in the preparation of this manuscript and Ms. Stephanie Deming, Senior Scientific Editor at MD Anderson Cancer Center, for copyediting the manuscript.
FUNDING SUPPORT
Supported by the National Cancer Institute under award number P30CA016672, which supports the MD Anderson Cancer Center Clinical Trials Support Resource.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST DISCLOSURES
Nothing to disclose.
REFERENCES
- 1.Khan SA, Tavolaro S, Brandi G, et al. Cholangiocarcinoma: epidemiology and risk factors. Liver Int 2019;39Suppl 1:19–31. [DOI] [PubMed] [Google Scholar]
- 2.Yamashita S, Koay EJ, Passot G, et al. Local therapy reduces the risk of liver failure and improves survival in patients with intrahepatic cholangiocarcinoma: a comprehensive analysis of 362 consecutive patients. Cancer 2017;123(8):1354–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banales JM, Cardinale V, Carpino G, et al. Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol 2016;13(5):261–280. [DOI] [PubMed] [Google Scholar]
- 4.Uhlig J, Sellers CM, Cha C, et al. Intrahepatic cholangiocarcinoma: socioeconomic discrepancies, contemporary treatment approaches and survival trends from the National Center Database. Ann Surg Oncol 2019;26:1993–2000. [DOI] [PubMed] [Google Scholar]
- 5.Khan SA, Davidson BR, Goldin R, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut 2002;51Suppl 6:Vi1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jarnagin WR, Shoup M. Surgical management of cholangiocarcinoma. Semin Liver Dis 2004;24(2):189–199. [DOI] [PubMed] [Google Scholar]
- 7.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273–1281. [DOI] [PubMed] [Google Scholar]
- 8.Valle JW, Furuse J, Jitlal M, et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomized trials. Ann Oncol 2014;25:391–398. [DOI] [PubMed] [Google Scholar]
- 9.Nakanuma Y, Sasaki M, Ikeda H, et al. Pathology of peripheral intrahepatic cholangiocarcinoma with reference to tumorigenesis. Hepatol Res 2008;38(4):325–334. [DOI] [PubMed] [Google Scholar]
- 10.Xu J, Sasaki M, Harada K, et al. Intrahepatic cholangiocarcinoma arising in chronic advanced liver disease and the cholangiocarcinomatous component of hepatocellular cholangiocarcinoma share common phenotypes and cholangiocarcinogenesis. Histopathology 2011;59(6):1090–1099. [DOI] [PubMed] [Google Scholar]
- 11.Asayama Y, Yoshimitsu K, Irie H, et al. Delayed-phase dynamic CT enhancement as a prognostic factor for mass-forming intrahepatic cholangiocarcinoma. Radiology 2006;238(1):150–155. [DOI] [PubMed] [Google Scholar]
- 12.Kajiyama K, Maeda T, Takenaka K, et al. The significance of stromal desmoplasia in intrahepatic cholangiocarcinoma: a special reference of “scirrhous-type” and “nonscirrhous-type” growth. Am J SurgPathol 1999;23:892–902. [DOI] [PubMed] [Google Scholar]
- 13.Maetani Y, Itoh K, Watanabe C, et al. MR imaging of intrahepatic cholangiocarcinoma with pathologic correlation. Am J Roentgenol2001;176(6):1499–1507. [DOI] [PubMed] [Google Scholar]
- 14.Valls C, Guma A, Puig I, et al. Intrahepatic peripheral cholangiocarcinoma: CT evaluation. Abdom Imaging 2000;25(5):490–496. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto M, Ariizumi S, Otsubo T, et al. Intrahepatic cholangiocarcinoma diagnosed preoperatively as hepatocellular carcinoma. J SurgOncol 2004;87(2):80–83; discussion 3–4. [DOI] [PubMed] [Google Scholar]
- 16.Nanashima A, Sumida Y, Abo T, et al. Relationship between pattern of tumor enhancement and clinicopathologic characteristics in intrahepatic cholangiocarcinoma. J SurgOncol 2008;98(7):535–539. [DOI] [PubMed] [Google Scholar]
- 17.Ariizumi S, Kotera Y, Takahashi Y, et al. Mass-forming intrahepatic cholangiocarcinoma with marked enhancement on arterial-phase computed tomography reflects favorable surgical outcomes. J SurgOncol 2011;104(2):130–139. [DOI] [PubMed] [Google Scholar]
- 18.Turkoglu MA, Yamamoto Y, Sugiura T, et al. The favorable prognosis after operative resection of hypervascular intrahepatic cholangiocarcinoma: a clinicopathologic and immunohistochemical study. Surgery 2016;160(3):683–690. [DOI] [PubMed] [Google Scholar]
- 19.Fujita N, Asayama Y, Nishie A, et al. Mass-forming intrahepatic cholangiocarcinoma: enhancement patterns in the arterial phase of dynamic hepatic CT – Correlation with clinicopathological findings. EurRadiol 2017. Feb; 27(2):498–506. [DOI] [PubMed] [Google Scholar]
- 20.Min JH, Kim YK, Choi S, et al. Intrahepatic mass-forming cholangiocarcinoma: arterial enhancement patterns at MRI and prognosis. Radiology 2019;290:691–699. [DOI] [PubMed] [Google Scholar]
- 21.Kim SA, Lee JM, Lee KB, et al. Intrahepatic mass-forming cholangiocarcinomas: enhancement patterns at multiphasic CT, with special emphasis on arterial enhancement pattern – Correlation with clinicopathologic findings. Radiology 2011;260(1):148–157. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto Y, Turkoglu MA, Aramaki T, et al. Vascularity of intrahepatic cholangiocarcinoma on computed tomography is predictive of lymph node metastasis. Ann Surg Oncol 2016;23(Suppl 4):485–493. [DOI] [PubMed] [Google Scholar]
- 23.Loyer EM, Chin II, Du Brow RA, et al. Hepatocellular carcinoma and intrahepatic peripheral cholangiocarcinoma: enhancement patterns with quadruple phase helical CT – a comparative study. Radiology 1999;212(3):866–875. [DOI] [PubMed] [Google Scholar]
- 24.Vittinghoff E and McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol 2007;165:710–8. [DOI] [PubMed] [Google Scholar]
- 25.Chowdhury MZI and Turin TC. Variable selection strategies and its importance in clinical prediction modelling. Fam Med Community Health 2020;16;8:e000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu Y, Mao Y, Chen J, et al. Preoperative computed tomography features of intrahepatic cholangiocarcinoma for predicting lymph nodes metastasis and overall survival. J Comput Assist Tomogr 2019;43(5):729–735. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


