Abstract
Prior measurements at bench-scale revealed that waterless urinal cartridges containing oily sealant fluids are capable of partitioning pharmaceuticals from urine and therefore reducing their concentration in wastewater. We sought to measure pharmaceutical removal from in-use waterless urinals. We developed a method to quantify pharmaceuticals in the sealant phase which resulted in 79 ±30% and 71 ±30% recovery of eight pharmaceuticals from two sealant fluids, respectively. The method was applied to sealant samples collected over three weeks from in-use waterless urinals on a university campus. Six of eight pharmaceuticals were present in the sealant samples from 1.4 μg/L to 241 μg/L. Loads of the six pharmaceuticals detected in the sealants were removed from the receiving wastewater from 0.02 μg/day to 3.4 μg/day across the sampling period. The concentration of the pharmaceuticals were similar over time, indicating rapid saturation and washout of the sealant. We also observed relatively rapid loss of sealant at maintenance intervals consistent with the manufacturer’s instructions. These findings indicate that while waterless urinals do remove some pharmaceuticals from the wastewater stream, meaningful changes to wastewater concentrations will only result if the sealant fluid and/or the urinal cartridge are significantly modified.
Keywords: Partitioning; wastewater; emerging contaminants; urine diversion; TrOCs; source separation, PPCPs
Graphical Abstract

2. Introduction
Pharmaceuticals have received global attention because of their persistence in aquatic environments and deleterious effects on aquatic life (Brausch and Rand, 2011; Gogoi et al., 2018; Yang et al., 2017). Pharmaceuticals are poorly removed by conventional wastewater treatment plants (Roback et al., 2018; Sharma et al., 2020; Zupanc et al., 2013) resulting in their occurrence in ground water, lakes, foodstuff, and even in drinking water at concentrations from low ng/L to μg/L (Boyd et al., 2003; Ebele et al., 2017; Gogoi et al., 2018; Kolpin et al., 2002; McEachran et al., 2016; Poustie et al., 2020; Sharma et al., 2020; Yang et al., 2017).
To mitigate the impact of pharmaceutical release, there is a need to develop new techniques to remove pharmaceuticals from wastewater. Activated carbon, advanced oxidation, and urine separation are some promising techniques to remove pharmaceuticals present in wastewater but have their respective drawbacks. For example, activated carbon removes hydrophobic pharmaceuticals well but they must compete with other wastewater organic matter, reducing the adsorption capacity and increasing the regeneration frequency. Advanced oxidation is energy intensive and can produce disinfection byproducts such as bromate (Eggen et al., 2014; Richardson and Postigo, 2012; Stamm et al., 2015). Urine separation from wastewater diverts pharmaceuticals but requires separate treatment.
Another approach to reducing the environmental loading of pharmaceuticals is to remove them from the source before dilution with greywater. Human urine is the primary source of pharmaceuticals in wastewater (Winker et al., 2008) and one study reported that 64 ±27% of active ingredients were excreted through urine, and 35 ±36% through feces when the excretion pathways of 212 pharmaceuticals were examined (Lienert et al., 2007). The greater concentrations of pharmaceuticals in urine over that in wastewater indicates that separation from urine may be less challenging than more dilute wastewater streams.
We have previously shown at bench-scale that waterless urinal sealants are capable of partitioning some pharmaceuticals from synthetic urine (Thapa and Hanigan, 2020). In this work, our overall goal was to quantify the fraction of pharmaceuticals partitioned by in-use waterless urinals. We previously quantified pharmaceutical removal based on losses from the urine phase but such an approach is not feasible for an in-use toilet, as the urine is lost to the sewer. Thus, our objectives were to i) develop extraction and quantification methodology, ii) collect samples from two in-use waterless urinals over a three-week period and apply the developed methods to quantify pharmaceuticals removed from human urine.
3. Material and Methods
3.1. Reagents
The complete list of pharmaceuticals selected for extraction and quantification by high performance liquid chromatography (HPLC-UV) and liquid chromatography mass spectrometry (LC-MS/MS) are provided in Table 1. Twelve pharmaceuticals with the range of their hydrophobicity (log Kow from −0.79 to 4.19) and pKa from −1.16 to 15.96 were selected to represent pharmaceuticals typical of municipal wastewater. From the list of twelve, six pharmaceuticals (dilantin, ethinyl estradiol, gemfibrozil, ibuprofen, primidone, and sulfamethoxazole) were initially selected and analyzed via HPLC-UV to rapidly optimize the extraction method. These compounds were selected for HPLC analysis because the range of their Kow are representative of the set of compounds to be analyzed by LC-MS/MS (Table 1) and because in-house HPLC methods were already in place, facilitating their rapid quantification. Eight pharmaceuticals which represents the selected range of log Kow from −0.79 to 4.19 (caffeine, carbamazepine, diphenhydramine, fluoxetine, meprobamate, primidone, sulfamethoxazole, and trimethoprim) were then selected to be analyzed via LC-MS/MS to confirm HPLC-UV results and further optimize the extraction method.
Table 1.
Chemical characteristics of pharmaceuticals selected for extraction optimization and quantification.
| Compound | Molecular formula |
Molecular weight (Da) |
pKa a | Log Kow a |
Log Dow a at pH 6.6 |
Method Reporting limit (μg/L) |
Use |
|---|---|---|---|---|---|---|---|
| Caffeine | C8H10N4O2 | 195 | −1.16 | −0.79 | −0.79 | 0.03 | Simulant |
| Carbamazepine | C15H12N2O | 236 | 15.96 | 3.22 | 3.22 | 0.06 | Antiepileptic |
| Diphenhydramine | C12H11N | 255 | 0.78 | 3.30 | 1.33 | 0.01 | Antihistamine |
| Dilantin | C15H12N2O2 | 252 | 8.5 | 2.15 | 2.14 | * | Anticonvulsant |
| Ethinyl estradiol | C20H24O2 | 296 | 10.3 | 3.9 | 3.9 | * | Hormone |
| Fluoxetine | C17H18F3NO | 310 | 9.80 | 4.19 | 1.07 | 0.07 | Antidepressant |
| Gemfibrozil | C15H22O3 | 250 | 4.4 | 4.39 | 2.41 | * | Lipid regulator |
| Ibuprofen | C13H18O2 | 206 | 4.9 | 3.84 | 2.29 | * | Anti-inflammatory |
| Meprobamate | C9H18N2O4 | 219 | 15.63 | 0.96 | 0.96 | 0.02 | Anxiety disorder |
| Primidone | C12H14N2O2 | 218 | 11.62 | 1.49 | 1.12 | 0.60 | Antiepileptic |
| Sulfamethoxazole | C10H11N3O3S | 253 | 6.16 | 0.79 | 0.43 | 0.03 | Antibacterial |
| Trimethoprim | C14H18N4O3 | 291 | 7.2 | 1.28 | 0.53 | 0.02 | Antibacterial |
Only HPLC semi-quantitative evaluation was performed (not part of LC-MS/MS method)
Caffeine, carbamazepine, diphenhydramine, fluoxetine, meprobamate, trimethoprim, caffeine-d3, carbamazepine-d10, diphenhydramine-d3, and meprobamate-d3 were purchased from Sigma-Aldrich (St. Louis, MO). Sulfamethoxazole was purchased from either MP Biomedicals (Solon, OH) or Sigma-Aldrich (St. Louis, MO). Fluoxetine-d5 was purchased from Cayman Chemicals (Ann Arbor, MI). Primidone-d5, sulfamethoxazole-d4, and trimethoprim-d3 were purchased from Santa Cruz Biotechnology (Dallas, TX). Analytical standards were ≥98% pure. Solvents were ≥98% pure. Methanol (MeOH), acetonitrile (ACN), ultrapure water, 0.1% formic acid (v/v) in water and 0.1% formic acid (v/v) in acetonitrile, ethyl acetate (EtOAc), hexane, dimethyl sulfoxide (DMSO), methylene chloride, and n-hexane were obtained from Thermo Fisher Scientific (Pittsburgh, PA). Ammonium acetate (≥97% purity) was obtained from VWR International, LLC (Solon, OH). Dispersive solid phase extraction tubes (dSPE) were also obtained from Thermo Fisher Scientific (Pittsburgh, PA).
Two commercial waterless urinal sealants were examined: American Standard (Sealant A) (Xela Innovations, Glendale, WI), and Aqua Green Urinal Sealant (Sealant B) (Zurn Industries, Sanford, NC). The waterless urinal cartridges were from Sloan (WES-150, Franklin Park, IL). In our previous research we found these two sealants are similar in chemistry and likely to be derived from vegetable oil, and that they partition some hydrophobic pharmaceuticals from urine into the sealant phase (Thapa and Hanigan, 2020).
3.2. Extraction method development
Extraction method development and optimization focused on liquid-liquid extraction (LLE) because previously published studies focusing on extraction of pesticides from oily substances have been successful with LLE. Methods attempted were similar to those in published literature (Ferrer et al., 2005; Nguyen et al., 2010; Zhao et al., 2019). ACN, DMSO, EtOAC, hexane, MeOH, methylene chloride, and n-hexane were selected as extraction solvents on the basis of their relative polarity.
After initial experiments which visually examined solvent miscibility, three polar organic solvents (MeOH, ACN, and DMSO) were selected to proceed with further method optimization. Each of the solvents were investigated following the extraction procedure described below and semi-quantitative results were initially obtained by HPLC-UV. EtOAc and n-hexane were selected on the basis of published literature to improve the recovery and semi-quantitative results were obtained by HPLC-UV. Follow-on experiments were conducted for MeOH, ACN, EtOAc, and n-hexane on LC-MS/MS to quantify pharmaceutical recovery.
For semi-quantitative recovery studies with HPLC-UV, 2 mL of each sealant was placed into separate 15 mL polypropylene centrifuge tubes and 200 μL of the six pharmaceuticals combined working standard solution was added to each tube to make the concentration between 103 and 148 mg/L (500 μM). The relatively high concentration of pharmaceuticals spiked was to facilitate evaluation by HPLC. The mixture was shaken vigorously for 1 min and vortexed for 5 min, and allowed to equilibrate for 24 h. For samples analyzed by LC-MS/MS, we followed the same sample preparation process except the combined working standard solution was added to make concentration of 1000 μg/L for eight pharmaceuticals. The lower concentration of working standard was used for LC-MS/MS because we expected lower concentrations (ng/L to μg/L) of pharmaceuticals in the sealant sample and LC-MS/MS provided much greater sensitivity.
The attempted extraction methods are as follows. For all extraction methods, 2 mL of each sealant was spiked with pharmaceuticals and was placed in a 15 mL polypropylene centrifuge tube. All extracted samples were stored at −20 °C until LC-MS/MS analysis. LLE-1 through LLE-4 are used to identify four separate LLE methods.
LLE-1: 2 mL MeOH was added to the solution and the solution was vortexed for 15 min. The mixture was allowed to separate at room temperature for >12 h and the upper layer of MeOH was decanted. 1 mL of the decantate was filtered with a 0.22 μm PTFE syringe filter and internal standards were added to make the sample 100 μg/L of each analyte.
LLE-2: 2 mL of ACN was added and the samples were vortexed for 15 min, followed by centrifugation for 5 min at 25 °C and 3118 relative centrifugal force (RCF). 1 mL of the supernatant was filtered with a 0.22 μm filter and internal standards were added to the samples to make the concentration 100 μg/L of each analyte.
LLE-3: 5 mL of n-hexane, 5 mL of ACN, and 0.5 g of MgSO4 was added and the mixture was vortexed for 15 min. The sample was then centrifuged for 3 min at 3118 RCF and 4 °C. The supernatant was transferred to another polypropylene tube and the process was repeated on the remaining lower layer but only with 5 mL of ACN as the solvent. The supernatants were combined and stored at −20 °C for 4 h, causing carried over oily substances to partially solidify. The cold extract was immediately centrifuged for 3 min at 3118 RCF and 0 °C and the supernatant was immediately transferred to 2 mL dispersive solid phase extraction (dSPE) centrifuge tube containing HyperSep (50 mg C18, 50 mg primary/secondary amine exchange material, 150 mg MgSO4, and 50 mg carbon (Thermo Fisher Scientific, Waltham, MA)). The dSPE tube was vortexed for 2 min followed by centrifugation for 2 min at 1344 RCF and 4 °C. 1 mL of supernatant was filtered using a 0.22 μm filter and transferred to an autosampler vial. Internal standards were spiked to the supernatant to make the concentration 100 μg/L of each analyte.
LLE-4: 5 mL of 20:80 (v/v) with EtOAc/ACN solution was added and vortexed for 15 min. The tube was then centrifuged at 4928 RCF for 5 min and the supernatant was transferred to another tube. The remaining lower layer was extracted again with 5 mL 20:80 (v/v) with EtOAc/ACN solution and centrifugation, and the supernatants were combined. 1 mL of the combined supernatants was filtered using 0.22 μm and transferred to a 2 mL auto sample vial and spiked with internals standard to make the concentration 100 μg/L of each analyte.
3.3. In-use urinal sealant sampling
Samples were taken from two waterless urinals in a male restroom on the first floor of Joe Crowley Student Union, at University of Nevada, Reno during March and April 2019. The restroom receives a large number of users from a café located approximately 200 ft away, indicating that users of the restroom are likely to have recently consumed caffeine. Data provided by the University indicates that the building has between 170,000 and 200,000 visitors during March and April, respectively.(University of Nevada Reno, 2017) Visitors in March were lower than April because the University had a 1 week closure for spring break during the building traffic measurement period (2017). Our sampling (2019) occurred after spring break and therefore, the number of visitors was likely closer to the April estimate; 200,000/mo or 50,000/wk.
100 mL of two sealants (sealant A and B) were transferred each into a waterless urinal cartridge installed inside a urinal. The sealant was replaced every week during the sampling period. The sealant samples were collected 2 to 3 times per week for three weeks. 5 mL of sealant sample was collected each time from the urinals in a 10 mL washed and pre-combusted clear glass vial. Samples were collected using a 3 mL high-density polyethylene (HDPE) syringe with the needle inserted into the hole in the cartridge. The samples were immediately wrapped with aluminum foil and placed in a freezer at −20°C until extracted.
3.4. Analytical methods
Samples collected from the semi-quantitative extraction optimization method were analyzed with an Agilent 1260 Infinity HPLC. Separations were performed on a ZORBAX Eclipse plus C18 column from Agilent Technologies (4.6 mm I.D, 150 mm length, and 5 μm). Pharmaceuticals were analyzed method similar to our previous work Thapa and Hanigan (2020). Detailed methodology is provided in SI.
LC-MS/MS experiments were conducted with a Thermo Scientific Ultimate 3000 UPHLC with a Zorbax C-18 column (2.1×50 mm) with a packing size of 1.8 μm. Mass spectrometry was performed on Thermo Scientific TSQ Vantage. Pharmaceuticals were analyzed using method similar to Sharma et al. (2020) Details of the method and optimized LC-ESI-MS/MS parameters are provided in SI. Metabolites were not quantified for the sealant samples as they have varying levels of environmental and human activity were not the focus of this research. A ten-level calibration curve from 1 μg/L to 1000 μg/L resulted in linear fits with R2 ≥0.99. For each set of samples, solvent blanks and check standards were analyzed every 10 samples. Method reporting limits (MRLs) are provided in Table 1.
4. Results and Discussion
4.1. Recovery of spiked pharmaceuticals from sealants
We attempted to use various nonpolar and polar solvents (ACN, DMSO, EtOAC, hexane, MeOH, methylene chloride, and n-hexane) to liquid/liquid extract PPCPs from sealant fluids similar to prior research focused on pesticide extraction from oily substances (Ferrer et al., 2005; Nguyen et al., 2010; Zhao et al., 2019). The initial experiments focused on narrowing a broad range of solvents with varying polarity by conducting simple LLE extractions. Follow-on experiments were designed to test variations of LLE (e.g., multiple extractions, additives). Details of the individual methods are provided in Sec 3.2. Hexane and methylene chloride formed homogenous mixtures when mixed with sealant (i.e., miscible) and were not investigated further. More polar solvents (i.e., MeOH, ACN, and DMSO) were immiscible and hence selected for the further study.
In the initial set of experiments, HPLC was used to semi-quantitatively measure recovery of dilantin, ethinyl estradiol, gemfibrozil, ibuprofen, primidone, and sulfamethoxazole from the three remaining solvents (ACN, MeOH, DMSO) via comparisons of peak areas. The samples were analyzed by HPLC because of highly stable UV response factors over time, allowing for infrequent calibration and rapid sample analysis. Five out of six compounds were present when MeOH and ACN were the solvent, while only three were present with DMSO. Based on peak area, MeOH and ACN extractions were similar for most compounds.
We proceeded to optimize the extractions with ACN and MeOH at concentrations more representative of human urine and measurement via LC-MS/MS. LLE-1 resulted in average analyte recovery of 96 ±19% and 90 ±29% for sealants A and B, respectively (Figure 1, tabulated values are provided in Table SI-3). Recovery was >60% for seven out of eight pharmaceuticals in both sealants, excepting sulfamethoxazole for sealant B (41%).
Figure 1.
Recovery of 1000 μg/L of eight pharmaceuticals by three liquid-liquid extraction (LLE) methods. LLE-3 is not shown due to poor recovery. The error bars represent the standard deviation of triplicates where extractions were conducted. Quantitation was performed by LC-MS/MS with isotope dilution.
LLE-2 resulted in recoveries from 33% to 125%, with an average of 79 ±30% and 71 ±30% for sealant A and B, respectively (Figure 1). The majority of the analytes achieved high recovery (>80%) except caffeine (58% and 62%), sulfamethoxazole (33% and 41%), and trimethoprim (50% and 53%), respectively for sealant A and B. Poor recovery was also observed for primidone (63%) and diphenhydramine (33%) from sealant B. The recoveries were similar for sealant A and sealant B.
To improve the MS signal and decrease the detection limit, we attempted another extraction method. We mixed the sealant with n-hexane and ACN extraction twice on the same sample (LLE-3) and further cleaned up the extract with QuEChERS dSPE (Rybak et al., 2015; Zhao et al., 2019). Little to no recovery was observed for all analytes. QuEChERS SPE materials are designed to clean up pesticide samples but did not appear to be selective enough to allow for general pharmaceutical analysis.
The final strategy to improve the recovery of some compounds poorly recovered by LLE-2 was to extract the sample two times with 5 ml of 20:80 (v/v) EtOAc/ACN, similar to Zhao et al. (2019) (LLE-4). The supernatants were combined after extraction. Analyte recovery did not improve compared to LLE-2 with recoveries of 24 ±26% and 23 ±23% for sealants A and B, respectively. Recoveries were <65% for both the sealants A and B.
Together, recovery by LLE-2 was typically >70% and resulted in coefficient of variation of (38% and 43% for sealant A and B, respectively). LLE-1 has better recovery with best coefficient of variation (20% and 32% for sealant A and B, respectively), but was subject to recovery >100% for four of eight analytes and relatively high variability between replicates for some analytes. LLE-3 and LLE-4 resulted in poor recovery. Because of the nature of the samples which were collected from in-use urinals and therefore cannot be reproduced, reproducibility and minimizing matrix effects/false positives was prioritized over raw recovery, and all further extractions were conducted with LLE-2. Based on previous measurements of detection and reporting limits with this instrument (Table 1) and recovery measured here, reporting limits for the analytes ranged from 10 ng/L (diphenhydramine) to 600 ng/L (primidone) in the sealant fluids.
4.2. Quantification of pharmaceuticals in in-use waterless urinals samples
The developed method (LLE-2) was applied to quantify eight pharmaceuticals in sealant samples collected from two waterless urinals on the first floor of a student union building on a university campus. The quantified pharmaceuticals were selected to represent a broad range of hydrophobicity. The number of building visitors during the sampling campaign was ~50,000/wk (University of Nevada Reno, 2017). We collected three sets of sealant samples each week for three weeks, except week two, in which only two samples were collected due to a lack of building access during a sampling event.
During initial monitoring of two waterless urinals we noted that after 15 days, there was no sealant remaining in the urinal cartridges due to high use and loss to the sewer. To ensure the presence of sealant during the sampling campaign and to avoid cross contamination between cartridges, a new urinal cartridge filled with sealant was placed weekly.
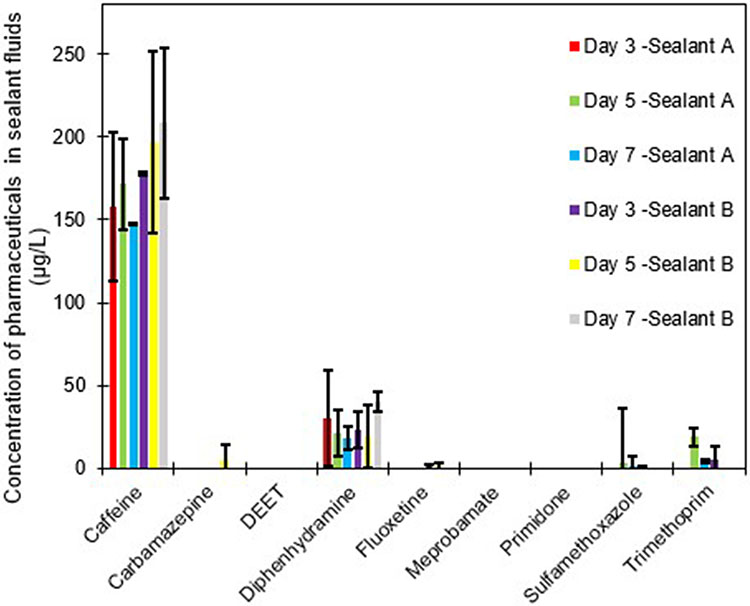
Six of eight compounds were present above the MRLs (Figure 2, tabulated values are provided in Table SI-4), up to 241 μg/L (all reported concentrations are unadjusted for method recovery). Caffeine was the most abundant compound and was present in every sample with concentrations from 111 μg/ L to 241 μg/ L, averaging 160 ± 30 μg/L and 193 ± 37 μg/L for sealant A and B, respectively (Figure 2). Caffeine is widely consumed and ubiquitously present in wastewater but is relatively hydrophilic at typical urine pH (log Dow at pH 6.6 = −0.79) (Thomas and Foster, 2005). We have previously shown at bench-scale that more hydrophobic compounds (log Dow > ~2) are more susceptible to partitioning to the sealant fluid (Thapa and Hanigan, 2020). Thus, we did not expect caffeine to be the most abundant in the sealant phase, but this unexpected finding may be due to rather high use of the urinals, proximity to a café, and natural variability in urine pH, which is not well reflected in our bench-scale experiments. Diphenhydramine, an antihistamine, was also present in all samples of both sealants with concentrations ranging from 8 μg/ L to 64 μg/ L. The concentration between sealants was similar, 24 ± 18 μg/ L and 26 ± 14 μg/ L in sealant A and B, respectively (Figure 2). The highest concentration of diphenhydramine (64 μg/ L) was observed in sealant A (first sample of week 1).
Figure 2.
Concentration of pharmaceuticals detected in the sealant samples for sealant A and B. Bars represent the mean of three sampling events conducted across three weeks in March and April 2019. Error bars represent one standard deviation.
Sulfamethoxazole and trimethoprim, two commonly used antibiotics, were present with less frequency, being present in 6 of 16 samples, at concentrations of trimethoprim ranging from 8.5 μg/L to 57.0 μg/L and for sulfamethoxazole from 1.3 μg/L to 8.9 μg/ L (samples in which both compounds were detected). Trimethoprim and sulfamethoxazole are administered concurrently in many cases, at a 1:5 mass ratio (Kemnic and Coleman, 2019; Masters et al., 2003). Based on method recovery (51% for trimethoprim and 37% for sulfamethoxazole) and published metabolism/excretion (50% and 30% excreted in urine as the parent drug for trimethoprim and sulfamethoxazole, respectively (Kaplan et al., 1973)) the expected mass ratio in the sealant is ~1:2 for trimethoprim:sulfamethoxazole (Figure 1). However, the ratios of trimethoprim: sulfamethoxazole ranged from 4:1 to 15:1 in the sealant samples. Notably, the log Dow of sulfamethoxazole and trimethoprim are 0.43 and 0.53, respectively, and we have previously shown a strong dependence on partitioning to the urinal sealants, particularly below log Dow = 1 (Thapa and Hanigan, 2020). The inversion of trimethoprim to sulfamethoxazole ratios highlights the strong partitioning dependence on hydrophobicity.
Carbamazepine and fluoxetine were present in a limited number of samples. Meprobamate, and primidone were not detected in any samples. The low occurrence or absence of these compounds is likely due to their low use in the student population (primary users of the urinals) or poor partitioning to the sealant fluid.
We did not generally observe accumulation of detected pharmaceuticals over the weeks in which sampling was conducted. Because caffeine is rather hydrophilic, it is possible that either high use of the urinal caused some caffeine to be lost in the urine of users who did not have caffeine in their urine (i.e., low concentration in subsequent users’ urine causes partitioning of the caffeine from the sealant to the urine), or that the sealant was saturated within 2-3 days of the use.
4.3. Impact of waterless urinal pharmaceutical diversion
We detected six out of eight compounds in the sealant samples collected from the urinals and calculated the total mass removal over the sampling period. Caffeine was removed by both the sealants across three weeks of sampling ranged from 2.1 μg/day to 3.4 μg/day. Similarly, the loads of diphenhydramine removed by the sealants ranged from 0.1 μg/day to 0.6 μg/day. Removal of other compounds was lower, from 0.02 μg/day to 0.2 μg/day for trimethoprim, sulfamethoxazole, carbamazepine, and fluoxetine. Other compounds were not removed to a detectable extent. Given that there were likely several urinations daily containing pharmaceuticals in the ng/L to μg/L range, we find that the removal of the studied pharmaceuticals by current waterless urinal sealants and cartridge designs is insufficient to significantly reduce wastewater concentrations for most pharmaceuticals. However, we believe the limited removal described provides the foundation and motivation to adapt future iterations of waterless urinals with the specific intent of removing pharmaceuticals rather than simply obstructing sewer gas migration. Notably, the sealants were likely saturated with at least two compounds within the first two days after placement, indicating the potential of this technology for pharmaceutical removal from urine if challenges identified in this work can be overcome. Also, we can collect urine from waterless urinals and provide additional on-site treatments like granulated activated carbon, advance oxidation and changing pH of the urine (Ikehata et al., 2006; Köpping et al., 2020).
Loss of sealant during urination was also identified as a barrier to implementation and is likely a result of urine flow sweeping sealant from the cartridge. The lost sealant likely contains some of the partitioned pharmaceuticals and therefore this is a challenge that must be overcome in any design intended to reduce pharmaceutical loading to the environment. To overcome this, the replacement frequency of the sealant may be shortened significantly to remove pharmaceuticals before they are lost to the sewer, or, the cartridge redesigned to better retain the sealant. This study provides insight into the challenges to be overcome by both manufacturers and scientists.
5. Conclusions
A robust method was developed to determine residues of pharmaceuticals in sealant samples. The optimized method was applied to quantify pharmaceuticals present in sealants collected from two waterless urinals in a single restroom on a university campus. Six of eight pharmaceutical compounds were detected in the samples from 1.3 μg/L to 241 μg/L. Caffeine and diphenhydramine were detected in all samples and were present at the highest concentrations of the analytes measured. Loads of six pharmaceuticals removed by the sealant over the three week of sampling period ranged from 0.02 μg/day to 3.4 μg/day. To be practical at full-scale, redesign of existing waterless urinal cartridges to increase the contact time and interfacial area is likely required and are opportunities for future research. Future research in this area should also note the rapid loss of the sealant to the sewer, which must be overcome in any successful cartridge redesign.
Supplementary Material
Practitioner points.
We developed a quantification method for pharmaceuticals in oily waterless urinal sealants.
Pharmaceuticals were present at relatively low concentrations in the sealant phase of two in-use waterless urinals.
We identify engineering challenges that must be overcome to meaningfully reduce pharmaceutical loads in wastewater with waterless urinals.
8. Acknowledgements
This research was supported by the National Science Foundation under Grant No. 1804255 and by AFRI grant no. 2017-69007-26309 from the USDA National Institute of Food and Agriculture. LC-MS/MS was conducted at the Nevada Proteomics Center, made possible by a grant from the National Institute of General Medical Sciences (GM103440) from the National Institutes of Health. We thank Drs. Eric Marchand, Sage Hiibel, Yu Yang, and Kerri Jean Ormerod for their insight and comments.
Footnotes
Conflicts of Interest
There are no conflicts of interest to declare.
7. Data Availability Statement
“The data that support the findings of this study are available from the corresponding author upon reasonable request."
10 References
- Boyd GR; Reemtsma H; Grimm DA; Mitra S (2003) Pharmaceuticals and personal care products (PPCPs) in surface and treated waters of Louisiana, USA and Ontario, Canada. Science of the total Environment, 311, 135–149. [DOI] [PubMed] [Google Scholar]
- Brausch JM; Rand GM (2011) A review of personal care products in the aquatic environment: environmental concentrations and toxicity. Chemosphere, 82, 1518–1532. [DOI] [PubMed] [Google Scholar]
- Chemicalize.org. (2019) Marvin was used for drawing, displaying, and prediction of chemicals and properties. Marvin v19.4.0 http://chemaxon.com. [Google Scholar]
- Ebele AJ; Abdallah MA-E; Harrad S (2017) Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerging Contaminants, 3, 1–16. [Google Scholar]
- Eggen RIL; Hollender J; Joss A; Schärer M; Stamm C (2014) Reducing the Discharge of Micropollutants in the Aquatic Environment: The Benefits of Upgrading Wastewater Treatment Plants. Environmental Science & Technology, 48, 7683–7689. [DOI] [PubMed] [Google Scholar]
- Ferrer C; Gómez MJ; García-Reyes JF; Ferrer I; Thurman EM; Fernández-Alba AR (2005) Determination of pesticide residues in olives and olive oil by matrix solid-phase dispersion followed by gas chromatography/mass spectrometry and liquid chromatography/tandem mass spectrometry. Journal of Chromatography A, 1069, 183–194. [DOI] [PubMed] [Google Scholar]
- Gogoi A; Mazumder P; Tyagi VK; Chaminda GT; An AK; Kumar M (2018) Occurrence and fate of emerging contaminants in water environment: A review. Groundwater for Sustainable Development, 6, 169–180. [Google Scholar]
- Ikehata K; Jodeiri Naghashkar N; Gamal El-Din M (2006) Degradation of aqueous pharmaceuticals by ozonation and advanced oxidation processes: a review. Ozone: Science and Engineering, 28, 353–414. [Google Scholar]
- Kaplan SA; Weinfeld RE; Abruzzo CW; McFaden K; Lewis Jack M; Weissman L (1973) Pharmacokinetic profile of trimethoprim-sulfamethoxazole in man. Journal of Infectious Diseases, 128, S547–S555. [DOI] [PubMed] [Google Scholar]
- Kemnic TR; Coleman M (2019) Trimethoprim Sulfamethoxazole, StatPearls [Internet]. StatPearls Publishing. [Google Scholar]
- Kolpin DW; Furlong ET; Meyer MT; Thurman EM; Zaugg SD; Barber LB; Buxton HT (2002) Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999-2000: a national reconnaissance. Environmental science & technology, 36, 1202–1211. [DOI] [PubMed] [Google Scholar]
- Köpping I; McArdell CS; Borowska E; Böhler MA; Udert KM (2020) Removal of pharmaceuticals from nitrified urine by adsorption on granular activated carbon. Water research X, 9, 100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienert J; Bürki T; Escher BI (2007) Reducing micropollutants with source control: substance flow analysis of 212 pharmaceuticals in faeces and urine. Water Science and Technology, 56, 87–96. [DOI] [PubMed] [Google Scholar]
- Masters PA; O'Bryan TA; Zurlo J; Miller DQ; Joshi N (2003) Trimethoprim-sulfamethoxazole revisited. Archives of Internal Medicine, 163, 402–410. [DOI] [PubMed] [Google Scholar]
- McEachran AD; Shea D; Bodnar W; Nichols EG (2016) Pharmaceutical occurrence in groundwater and surface waters in forests land-applied with municipal wastewater. Environmental toxicology and chemistry, 35, 898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TD; Lee MH; Lee GH (2010) Rapid determination of 95 pesticides in soybean oil using liquid–liquid extraction followed by centrifugation, freezing and dispersive solid phase extraction as cleanup steps and gas chromatography with mass spectrometric detection. Microchemical Journal, 95, 113–119. [Google Scholar]
- Poustie A; Yang Y; Verburg P; Pagilla KR; Hanigan D (2020) Reclaimed Wastewater as a Viable Water Source for Agricultural Irrigation: A Review of Food Crop Growth Inhibition and Promotion in the Context of Environmental Change. Science of the Total Environment, 739, 139756. [DOI] [PubMed] [Google Scholar]
- Richardson S; Postigo C (2012) Drinking Water Disinfection By-products, in: Barceló D (Ed.), Emerging Organic Contaminants and Human Health. Springer Berlin; Heidelberg, pp. 93–137. [Google Scholar]
- Roback SL; Ferrer I; Thurman EM; Ishida KP; Plumlee MH; Poustie A; Westerhoff P; Hanigan D (2018) Non-target mass spectrometry analysis of NDMA precursors in advanced treatment for potable reuse. Environmental Science: Water Research & Technology, 4, 1944–1955. [Google Scholar]
- Rybak ME; Sternberg MR; Pao C-I; Ahluwalia N; Pfeiffer CM (2015) Urine excretion of caffeine and select caffeine metabolites is common in the US population and associated with caffeine intake. The Journal of nutrition, 145, 766–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P; Poustie A; Verburg P; Pagilla K; Yang Y; Hanigan D (2020) Trace Organic Contaminants in Field-scale Cultivated Alfalfa, Soil, and Pore Water after 10 Years of Irrigation with Reclaimed Wastewater. Science of the Total Environment 744, 140698. [DOI] [PubMed] [Google Scholar]
- Stamm C; Eggen RIL; Hering JG; Hollender J; Joss A; Schärer M (2015) Micropollutant Removal from Wastewater: Facts and Decision-Making Despite Uncertainty. Environmental Science & Technology, 49, 6374–6375. [DOI] [PubMed] [Google Scholar]
- Thapa U; Hanigan D (2020) Waterless Urinals Remove Select Pharmaceuticals from Urine by Phase Partitioning. Environmental Science & Technology, 54, 6344–6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PM; Foster GD (2005) Tracking acidic pharmaceuticals, caffeine, and triclosan through the wastewater treatment process. Environmental Toxicology and Chemistry: An International Journal, 24, 25–30. [DOI] [PubMed] [Google Scholar]
- University of Nevada Reno. (2017) JCSU Traffic Counts. [Google Scholar]
- Winker M; Faika D; Gulyas H; Otterpohl R (2008) A comparison of human pharmaceutical concentrations in raw municipal wastewater and yellowwater. Science of the total environment, 399, 96–104. [DOI] [PubMed] [Google Scholar]
- Yang Y; Ok YS; Kim K-H; Kwon EE; Tsang YF (2017) Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Science of the Total Environment, 596, 303–320. [DOI] [PubMed] [Google Scholar]
- Zhao L; Szakas T; Churley M; Lucas D (2019) Multi-class multi-residue analysis of pesticides in edible oils by gas chromatography-tandem mass spectrometry using liquid-liquid extraction and enhanced matrix removal lipid cartridge cleanup. Journal of Chromatography A, 1584, 1–12. [DOI] [PubMed] [Google Scholar]
- Zupanc M; Kosjek T; Petkovšek M; Dular M; Kompare B; Širok B; Blažeka Ž; Heath E (2013) Removal of pharmaceuticals from wastewater by biological processes, hydrodynamic cavitation and UV treatment. Ultrasonics sonochemistry, 20, 1104–1112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
“The data that support the findings of this study are available from the corresponding author upon reasonable request."