Abstract
Background
The use of COVID-19 vaccines has been prioritised to protect the most vulnerable—notably, older people. Because of fluctuations in vaccine availability, strategies such as delayed second dose and heterologous prime-boost have been used. However, the effectiveness of these strategies in frail, older people are unknown. We aimed to assess the antigenicity of mRNA-based COVID-19 vaccines in frail, older people in a real-world setting, with a rationed interval dosing of 16 weeks between the prime and boost doses.
Methods
This prospective observational cohort study was done across 12 long-term care facilities of the Montréal Centre-Sud – Integrated University Health and Social Services Centre in Montréal, Québec, Canada. Under a rationing strategy mandated by the provincial government, adults aged 65 years and older residing in long-term care facilities in Québec, Canada, with or without previously documented SARS-CoV-2 infection, were administered homologous or heterologous mRNA vaccines, with an extended 16-week interval between doses. All older residents in participating long-term care facilities who received two vaccine doses were eligible for inclusion in this study. Participants were enrolled from Dec 31, 2020, to Feb 16, 2021, and data were collected up to June 9, 2021. Clinical data and blood samples were serially collected from participants at the following timepoints: at baseline, before the first dose; 4 weeks after the first dose; 6–10 weeks after the first dose; 16 weeks after the first dose, up to 2 days before administration of the second dose; and 4 weeks after the second dose. Sera were tested for SARS-CoV-2-specific IgG antibodies (to the trimeric spike protein, the receptor-binding domain [RBD] of the spike protein, and the nucleocapsid protein) by automated chemiluminescent ELISA. Two cohorts were used in this study: a discovery cohort, for which blood samples were collected before administration of the first vaccine dose and longitudinally thereafter; and a confirmatory cohort, for which blood samples were only collected from 4 weeks after the prime dose. Analyses were done in the discovery cohort, with validation in the confirmatory cohort, when applicable.
Findings
The total study sample consisted of 185 participants. 65 participants received two doses of mRNA-1273 (Spikevax; Moderna), 36 received two doses of BNT162b2 (Comirnaty; Pfizer–BioNTech), and 84 received mRNA-1273 followed by BNT162b2. In the discovery cohort, after a significant increase in anti-RBD and anti-spike IgG concentrations 4 weeks after the prime dose (from 4·86 log binding antibody units [BAU]/mL to 8·53 log BAU/mL for anti-RBD IgG and from 5·21 log BAU/mL to 8·05 log BAU/mL for anti-spike IgG), there was a significant decline in anti-RBD and anti-spike IgG concentrations until the boost dose (7·10 log BAU/mL for anti-RBD IgG and 7·60 log BAU/mL for anti-spike IgG), followed by an increase 4 weeks later for both vaccines (9·58 log BAU/mL for anti-RBD IgG and 9·23 log BAU/mL for anti-spike IgG). SARS-CoV-2-naive individuals showed lower antibody responses than previously infected individuals at all timepoints tested up to 16 weeks after the prime dose, but achieved similar antibody responses to previously infected participants by 4 weeks after the second dose. Individuals primed with the BNT162b2 vaccine showed a larger decrease in mean anti-RBD and anti-spike IgG concentrations with a 16-week interval between doses (from 8·12 log BAU/mL to 4·25 log BAU/mL for anti-RBD IgG responses and from 8·18 log BAU/mL to 6·66 log BAU/mL for anti-spike IgG responses) than did those who received the mRNA-1273 vaccine (two doses of mRNA-1273: from 8·06 log BAU/mL to 7·49 log BAU/mL for anti-RBD IgG responses and from 6·82 log BAU/mL to 7·56 log BAU/mL for anti-spike IgG responses; mRNA-1273 followed by BNT162b2: from 8·83 log BAU/mL to 7·95 log BAU/mL for anti-RBD IgG responses and from 8·50 log BAU/mL to 7·97 log BAU/mL for anti-spike IgG responses). No differences in antibody responses 4 weeks after the second dose were noted between the two vaccines, in either homologous or heterologous combinations.
Interpretation
Interim results of this ongoing longitudinal study show that among frail, older people, previous SARS-CoV-2 infection and the type of mRNA vaccine influenced antibody responses when used with a 16-week interval between doses. In these cohorts of frail, older individuals with a similar age and comorbidity distribution, we found that serological responses were similar and clinically equivalent between the discovery and confirmatory cohorts. Homologous and heterologous use of mRNA vaccines was not associated with significant differences in antibody responses 4 weeks following the second dose, supporting their interchangeability.
Funding
Public Health Agency of Canada, Vaccine Surveillance Reference Group; and the COVID-19 Immunity Task Force.
Translation
For the French translation of the abstract see Supplementary Materials section.
Introduction
The COVID-19 pandemic has been devastating, particularly for older individuals.1, 2 Accelerated vaccine development via different platforms has been an important advancement in the fight against the COVID-19 pandemic. Because the mRNA-based vaccines mRNA-1273 (Spikevax; Moderna) and BNT162b2 (Comirnaty; Pfizer–BioNTech) showed significant serological responses after the prime dose in clinical trials,3, 4 coupled with the scarcity of their supply globally, public health measures in several countries involved rationing these vaccines by deferring boost doses in order to prioritise administering the first dose to as many high-risk individuals as possible.5 This approach resulted in delayed administration of the second dose (relative to the 3–4-week interval studied in clinical trials) and heterologous prime-boost vaccination (whereby the second dose is different from the first dose; also known as mixing). Data on the serological response associated with these approaches are scarce, particularly in frail, older people. This uncertainty is concerning because of immunosenescence in older populations, resulting in diminished immunological capacity to respond not only to infections, underlying their heightened risk for severe COVID-19, but also to vaccines, potentially contributing to the ongoing risk of SARS-CoV-2 infection.
Research in context.
Evidence before this study
The BNT162b2 (Comirnaty; Pfizer–BioNTech) and mRNA-1273 (Spikevax; Moderna) mRNA vaccines were authorised for emergency use against SARS-CoV-2 in Québec, Canada, on Dec 14, 2020. We searched the PubMed database and the preprint servers medRxiv, bioRxiv, and SSRN for articles published in English and in French up to Sept 23, 2021, using the search terms “coronavirus”, “SARS-CoV-2”, “COVID”, “COVID-19”, “Pfizer”, “BNT162b2”, “Moderna”, “mRNA-1273”, “vaccine”, “mRNA”, “heterologous”, “homologous”, “mix and(&) match”, “elderly”, “aged”, “senior”, “institutionalized”, “frail”, “long term care”, and “extended interval”, “prolonged interval”, “delayed dosing”, “prime”, “first dose”, “boost”, “second dose” but NOT exclusively “ChAdOx1”, “Astra Zeneca”, “Oxford”, “adenovirus”, or “adenoviral vector”. The search identified three articles and one editorial commentary at the time that characterised the immune responses to various COVID-19 vaccines in people aged 65 years and older following their first dose, including the impact of previous SARS-CoV-2 infection on these responses. We found no studies comparing heterologous versus homologous use of the mRNA-based vaccines for prime-boost immunisation in frail, older people, although numerous studies are emerging on the heterologous use of either of the two mRNA-based vaccines in combination with the ChAdOx1 nCoV-19 (AZD1222; Oxford–AstraZeneca) vaccine. We also found no studies at the time evaluating the impact of a 16-week interval between mRNA-based vaccine doses on antibody responses in this setting.
Added value of this study
To our knowledge, this study is one of the first longitudinal prospective cohort studies assessing the antigenicity and interchangeability of mRNA-based vaccines in frail, older people in a real-world setting with a rationed interval dosing of 16 weeks between the prime and boost doses. By comparing antibody responses to the spike protein, receptor-binding domain (RBD), and nucleocapsid antigens, our study found that frail, older individuals with previously documented SARS-CoV-2 infection mounted more robust humoral responses following immunisation than did those who were infection-naive, reiterating previous findings by Paul Moss and colleagues. We also showed that administration of the second dose at the end of the 16-week interval results in similar antibody concentrations in both infection-naive and previously infected individuals 1 month later. Furthermore, we found differences in the kinetics of the antibody responses based on the mRNA vaccine used for the first dose. Specifically, we found that anti-spike and anti-RBD IgG concentrations decreased significantly faster at 16 weeks following the BNT162b2 vaccine as the prime dose, relative to the mRNA-1273 vaccine, regardless of previous infection status. Use of either mRNA vaccine as the boost dose resulted in similar titres 1 month later.
Implications of all the available evidence
This interim report demonstrates the antigenicity of a real-world vaccination approach (ie, an extended dosing interval of 16 weeks) in a cohort of frail, older people. These findings have practical implications for vaccination strategies in the face of ongoing vaccine supply issues, particularly in delineating a maximum interval of 16 weeks between the prime and boost doses with mRNA-based vaccines, especially in frail, older people who are infection-naive or who received BNT162b2 as the prime dose. We also provide real-world serological evidence for the heterologous use, and thus interchangeability, of the current mRNA-based vaccines in this demographic. Longer follow-ups will assess the durability of antibody responses beyond 5 months and the effectiveness of these strategies in the face of emerging variants, which might suggest the need for additional doses.
In Québec, Canada, the vaccination strategy mandated by the provincial government resulted in an extended interval of 16 weeks between first and second vaccine doses for older people (aged ≥65 years) residing in long-term care. Although this policy widely expanded first-dose protection to this high-risk population, resulting in decreased hospital admissions and deaths due to COVID-19,6 the antigenicity of this strategy beyond the first dose in frail, older people is unknown. Specifically, due to the urgent need to vaccinate older people in long-term care facilities, all residents wishing to be vaccinated received the vaccine, regardless of their previous SARS-CoV-2 infection status. At present, it is unclear how previous infection affects the magnitude and kinetics of the vaccine response in this population. Additionally, because of the variability in vaccine supply, the boost doses administered were either the same (homologous) or different (heterologous) from the prime doses, although data on the interchangeability of mRNA vaccines were not widely available at the time of the study. The UNCoVER (UNderstanding Co-V2 Vaccination in Elderly Residents) study aimed to evaluate serological responses to a real-world COVID-19 vaccination strategy in older people residing in long-term care facilities. Here, we report the interim results of this prospective observational cohort study.
Methods
Study procedure and participants
In Québec, Canada, the COVID-19 vaccination campaign was launched by the provincial Ministry of Health on Dec 14, 2020, with people aged 65 years and older residing in long-term care facilities prioritised to receive available mRNA-1273 or BNT162b2 vaccine doses. This study was done across 12 long-term care facilities of the Montréal Centre-Sud – Integrated University Health and Social Services Centre (CIUSSS du Centre-Sud-de-l'Île-de-Montréal), Montréal, QC, Canada, and was approved by its research ethics board (Comité d'éthique de la recherche Vieillissement-Neuroimagerie, protocol number 20-21-36 MP; appendix 2 pp 1–4). The long-term care facilities included in this study (termed residential and long-term care centres, known as CHSLDs [centre d'hébergement et de soins de longue durée] in Quebec) focus primarily on the care of older people requiring more than 3 h of care daily. Because of the limited vaccine supply, the province-wide prioritisation strategy consisted of administering the first dose to as many long-term care residents as possible, which necessitated delayed administration of the second dose by up to 16 weeks (±2 days) after the first dose, rather than the 3-week or 4-week interval used in the clinical trials. At the time of the second dose, the mRNA vaccine used could be either homologous or heterologous to the first one, based on availability.
All older residents in participating long-term care facilities were eligible for inclusion in this study. Residents deemed unfit by the health-care staff (at the long-term care facilities) to provide blood samples for immunological analyses were excluded from the study. Participating long-term care residents or their legally authorised representatives provided verbal informed consent before enrolment. Clinical data were sourced from the individual's medical chart stored at the participating long-term care facility and were collected at baseline and updated at the timepoints mentioned below. Clinical data collection included information on the participant's medical history (ie, comorbidities), past SARS-CoV-2 diagnosis based on RT-PCR test results done in a diagnostic microbiology laboratory, and COVID-19 vaccine administration. Frailty status was assessed with the Clinical Frailty Scale completed by the nursing staff.7 Blood samples were collected at the following timepoints (±2 days), subject to availability of long-term care facility staff and scheduling: within 1 day of the first dose administration, before the administration of the vaccine (t1); at approximately 4 weeks after the first dose, which coincides with the timing of the putative second dose, as done in the clinical trials (t2); at 6–10 weeks after the first dose (t3); up to 2 days before the administration of the second dose, 16 weeks after the prime dose (t4); and at 4 weeks after the second dose (t5). These time measurements constitute this interim report.
Due to logistical constraints under emergency pandemic measures, this observational study established two cohorts. In the first cohort, blood samples were collected before administration of the first vaccine dose (t1) and at each timepoint thereafter. In the second cohort, blood samples were only collected from the t2 timepoint onwards. Because of this difference in sampling, we did our analyses in the first cohort (the discovery cohort), with validation in the second cohort (the confirmatory cohort), when applicable.
Thus, this interim analysis was done from Dec 31, 2020, to June 9, 2021. Longitudinal data collection at later timepoints is in progress.
Samples
Blood was collected in acid citrate dextrose from participants and processed within 6 h of collection. Plasma was stored at −80°C until ready for batch testing.
Assessment of SARS-CoV-2 antibody responses
Briefly, longitudinal serological responses to vaccines were measured by automated chemiluminescent ELISA to detect IgG antibodies to the SARS-CoV-2 trimeric spike protein, nucleocapsid, and the receptor-binding domain (RBD) of the spike protein to discriminate between vaccine-induced antibody response and convalescence from natural SARS-CoV-2 infection. Scaled luminescence values were measured and converted to binding antibody units (BAU) per mL to provide a titre (appendix 2 pp 5–6).
Statistical analysis
Participants' demographic and clinical characteristics were reported as proportions for categorical data and as means (ranges) for continuous data. All residents aged 65 years and older from the participating long-term care facilities who received two COVID-19 vaccine doses were eligible for analysis. Participants were not excluded on the basis of missing data and there was no imputation for missing data. The antibody response profiles (for spike protein, RBD, and nucleocapsid) were displayed visually. Logarithmic transformation8 was used to normalise antibody responses in BAU/mL (titres). Linear mixed-effects models with random intercepts were used to evaluate change in antibody concentrations over time, accounting for within-individual variability, and to test the interaction between change in antibody concentrations over time and vaccine type, homology, previous SARS-CoV-2 infection status, age, or the absence or presence of medical comorbidities by type of comorbidity. To determine whether previous infection status affected the antigenicity of these vaccine strategies, we separately analysed the impact of vaccine homology in individuals without and those with a history of SARS-CoV-2 infection. To determine whether antigenicity varies with age, we categorised the age of participants in the discovery cohort into decades and assessed their serological responses. The types of comorbidities examined were cognitive impairment, cardiovascular disease, chronic lung disease, history of diabetes, and history of cancer. The timepoints for blood sample collection were treated as a categorical variable in the model. Our sample size had 80% statistical power to detect a medium effect size of 0·5 (approximately 22% difference in RBD and spike protein, and approximately 60% difference in nucleocapsid) based on a two-sided significance level of 0·05. The power analysis was a post-hoc analysis for a test against a null difference of log antibody concentrations. No correction for multiple comparisons was applied. Although there is no consensus threshold of protective antibody titres, a difference of 20% (log BAU/mL) in antibody concentrations would require a sample size larger than what was feasible in this observational study. None of the differences observed by age or sex exceeded 20%. Analyses were done in SAS (version 9.4).
Role of the funding source
Members of the Executive Scientific Committee of the COVID-19 Immunity Task Force, the funding source, contributed to study design and revision of the Article for publication. The funding source had no role in data collection, data analyses, data interpretation, or writing of the report.
Results
Between Dec 31, 2020, and Feb 16, 2021, 228 (71%) of 321 eligible residents contacted by the research team agreed to participate in the study (appendix 2 p 7) and were enrolled. 14 participants were excluded because they did not receive the second vaccine dose, due to death, refusal of the second vaccine, or withdrawal from the study. An additional 29 participants were excluded because they were younger than 65 years. Thus, the total study sample consisted of 185 participants. Among these participants, the median age was 83 years (IQR 76–90), 128 (69%) were female, 166 (90%) were White, 86 (46%) had been previously diagnosed with SARS-CoV-2 infection (confirmed by RT-PCR in the respective diagnostic laboratory), and 181 (98%) had at least one coexisting condition. The mean score on the Clinical Frailty Scale7 of the included participants was 6·57 (SD 1·03; median 7·00 [6·00–7·00], range 1–8). The first vaccine dose was mRNA-1273 (Spikevax) for 149 (81%) participants and BNT162b2 (Comirnaty) for 36 (19%) participants. The second dose was mRNA-1273 (Spikevax) for 65 (35%) participants and BNT162b2 (Comirnaty) for 120 (65%) participants. 65 participants received two doses of mRNA-1273, 36 received two doses of BNT162b2, and 84 received mRNA-1273 followed by BNT162b2. All recruited participants were available for follow-up at t5. Comparison of cohorts showed that the discovery cohort (n=78) and confirmatory cohort (n=107) were comparable in age, sex, race, comorbidity, frailty, and time (in days) between the first and second vaccine doses, although differences were observed in the proportion of participants with previous SARS-CoV-2 infection and vaccine homology; the discovery cohort had a higher proportion of participants with a previous SARS-CoV-2 infection and a higher proportion who received a heterologous prime-boost compared to the confirmatory cohort (table ). The median time between the first and second vaccine dose for all participants was 111 days (IQR 111–112; range 81–169). The median time between the first and second vaccine dose was 111 days (IQR 111–112; range 95–169) for the discovery cohort and 112 days (IQR 111–112; range 81–113) for the confirmatory cohort. The median time between the first and second vaccine dose for those who received two doses of mRNA-1273 was 112 days (IQR 112–112; range 111–169); for those who received two doses of BNT162b2 it was 96 days (IQR 95–112; range 81–113); and for those who received mRNA-1273 followed by BNT162b2 it was 111 days (IQR 111–111; range 111–137).
Table.
Characteristics of participants at baseline
| Discovery cohort (n=78) | Confirmatory cohort (n=107) | All participants (n=185) | p values | |
|---|---|---|---|---|
| Sex (%) | ||||
| Male | 18 (23%) | 39 (36%) | 57 (31%) | 0·052 |
| Female | 60 (77%) | 68 (64%) | 128 (69%) | .. |
| Age, years | ||||
| Median | 85 (79–91) | 83 (75–89) | 83 (76–90) | 0·13 |
| Range | 65–101 | 65–103 | 65–103 | .. |
| Age distribution, years (%) | ||||
| 65–79 | 8 (10%) | 14 (13%) | 22 (12%) | 0·23 |
| 70–79 | 13 (17%) | 30 (28%) | 43 (23%) | .. |
| 80–89 | 36 (46%) | 38 (36%) | 74 (40%) | .. |
| ≥90 | 21 (27%) | 25 (23%) | 46 (25%) | .. |
| Race or ethnic group (%)* | ||||
| White | 68 (87%) | 98 (92%) | 166 (90%) | 0·077 |
| Black | 4 (5%) | 7 (7%) | 11 (6%) | .. |
| Asian | 4 (5%) | .. | 4 (2%) | .. |
| Middle Eastern | 2 (3%) | .. | 2 (1%) | .. |
| Latin American | .. | 1 (<1%) | 1 (<1%) | .. |
| Indigenous | .. | 1 (<1%) | 1 (<1%) | .. |
| Clinical Frailty Scale† | ||||
| Mean | 6·6 (1·07) | 6·5 (1·14) | 6·5 (1·03) | .. |
| Range | 2–8 | 1–8 | 1–8 | .. |
| Previous SARS-CoV-2 infection (%) | ||||
| Yes | 49 (63%) | 37 (35%) | 86 (46%) | <0·0001 |
| No | 29 (37%) | 70 (65%) | 99 (54%) | .. |
| Previous coexisting disease (%)* | ||||
| Cognitive impairment | 68 (87%) | 77 (72%) | 145 (78%) | 0·013 |
| Cardiovascular disease | 57 (73%) | 88 (82%) | 145 (78%) | 0·14 |
| History of cancer | 10 (13%) | 14 (13%) | 24 (13%) | 0·96 |
| Diabetes | 19 (24%) | 32 (30%) | 51 (28%) | 0·40 |
| Chronic lung disease | 23 (29%) | 49 (46%) | 72 (39%) | 0·03 |
| Participant with at least one of the above comorbidities | 76 (97%) | 105 (98%) | 181 (98%) | 1·00 |
| Vaccine‡ | ||||
| mRNA-1273 (Moderna) | 18 (23%) | 47 (44%) | 65 (35%) | 0·0018 |
| BNT162b2 (Pfizer–BioNTech) | 13 (17%) | 23 (21%) | 36 (19%) | .. |
| mRNA-1273 followed by BNT162b2 | 47 (60%) | 37 (35%) | 84 (45%) | .. |
Data are n (%), unless otherwise indicated. In the discovery cohort (the first cohort), blood samples were collected before administration of the first vaccine dose and longitudinally thereafter. In the confirmatory cohort (the second cohort), blood samples were only collected from the t2 timepoint (4 weeks after the first dose) onwards.
Race or ethnic group and previous coexisting disease were recorded in the participants' medical health records.
Clinical Frailty Scale between 1 (very fit) and 9 (terminally ill).
Homologous vaccination consisted of both doses being either mRNA-1273 or BNT162b2, while heterologous vaccination consisted of mRNA-1273 followed by BNT162b2.
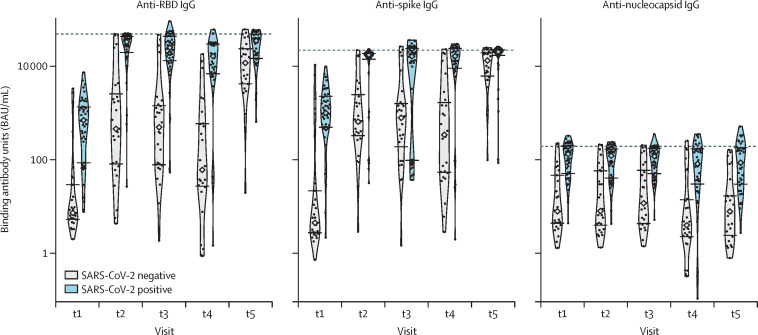
In the discovery group, 49 (63%) of 78 participants had previous, microbiologically confirmed, natural SARS-CoV-2 infection. These statuses were reflected in the mean anti-nucleocapsid IgG concentrations at baseline (t1): 2·55 (SD 1·50) log BAU/mL for the uninfected and 4·56 (0·90) log BAU/mL for the infected; p<0·0001; figure 1 ). Those who were previously infected maintained anti-nucleocapsid reactivity over the subsequent timepoints and at higher levels than those who were previously uninfected. For the anti-spike IgG response, contrasting means were noted at t1 based on the status of previous infection: 2·73 (SD 2·66) log BAU/mL for those who were uninfected and 6·68 (1·65) log BAU/mL for those who were infected (p<0·0001). A significant increase in anti-spike IgG concentrations was seen 4 weeks after the prime dose regardless of infection status (from 4·86 [SD 1·04] log BAU/mL to 8·53 [1·14] log BAU/mL for anti-RBD IgG and from 5·21 [1·22] log BAU/mL to 8·05 [0·95] log BAU/mL for anti-spike IgG). Comparable levels were detected at t2, 4 weeks after the first dose (6·61 [SD 2·00] log BAU/mL vs 8·95 [1·80] log BAU/mL, p<0·0001) and at t3, 6–10 weeks after the first dose (6·44 [2·20] log BAU/mL vs 8·14 [2·56] log BAU/mL; p=0·0047). Notably, at t4 (16 weeks after first dose), the mean anti-spike IgG response in previously uninfected participants declined to lower levels than in those who had recovered from natural SARS-CoV-2 infection (5·84 [SD 2·49] log BAU/mL vs 8·82 [2·25] log BAU/mL; p<0·0001). This difference resolved by t5, 4 weeks after the second dose (9·01 [SD 1·25] log BAU/mL vs 9·38 [1·31] log BAU/mL; p=0·25). Anti-RBD IgG concentrations at baseline (t1) were significantly higher in previously infected individuals: 2·85 (SD 2·01) log BAU/mL in uninfected participants versus 6·04 (1·70) log BAU/mL in previously infected participants (p<0·0001). Regardless of infection status in the discovery cohort, there was a significant decline in anti-RBD and anti-spike IgG concentrations until the boost dose (7·10 log [SD 1·43] BAU/mL for anti-RBD IgG and 7·60 [1·20] log BAU/mL for anti-spike IgG), followed by an increase 4 weeks later (9·58 [0·62] log BAU/mL for anti-RBD IgG and 9·23 [0·56] log BAU/mL for anti-spike IgG). Those who were previously infected maintained significantly higher mean anti-RBD IgG activity over the following 16 weeks (t2 to t4), compared to those who were previously uninfected, in whom concentrations declined at t4. However, by 4 weeks after boost immunisation (t5), anti-RBD IgG concentrations were comparable between the two groups. The dynamics of the anti-nucleocapsid, anti-spike, and anti-RBD IgG concentrations, starting at 4 weeks following the first dose, were confirmed in the confirmatory cohort (appendix 2 p 8), including the decline in anti-spike and anti-RBD responses at t4.
Figure 1.
Antibody responses based on previous SARS-CoV-2 infection
Antibody responses to the receptor-binding domain (RBD) of the spike protein, the spike protein, and the nucleocapsid protein, based on previous SARS-CoV-2 infection. Medians are indicated by diamonds, the IQR values are indicated by horizontal segments, and saturation thresholds are indicated by dashed lines. t1=before first dose. t2=approximately 4 weeks after first dose. t3=6–10 weeks after first dose. t4=up to 2 days before administration of second dose (16 weeks after prime dose). t5=4 weeks after second dose.
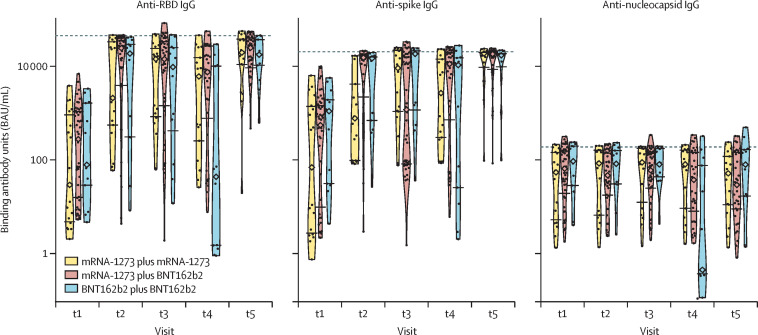
Homologous vaccination consisted of both doses being either mRNA-1273 or BNT162b2, while heterologous vaccination consisted of mRNA-1273 followed by BNT162b2. There were no participants who received BNT162b2 followed by mRNA-1273 in the current study. In the discovery cohort, 18 (23%) of 78 participants received two doses of mRNA-1273, 13 (17%) received two doses of BNT162b2, and 47 (60%) received mRNA-1273 followed by BNT162b2. At t2, the mean anti-RBD IgG concentration increased from 4·12 (SD 2·74) log BAU/mL to 8·06 (2·50) log BAU/mL in the group that received two doses of mRNA-1273, from 4·97 (2·39) log BAU/mL to 8·12 (3·00) log BAU/mL in the group that received two doses of BNT162b2, and from 5·11 (2·23) log BAU/mL to 8·83 (2·57) log BAU/mL in the group that received mRNA-1273 followed by BNT162b2 (figure 2 ). The mean anti-S IgG concentration increased from 4·29 (SD 3·29) log BAU/mL to 6·82 (2·02) log BAU/mL in the group that received two doses of mRNA-1273, from 5·84 (2·41) log BAU/mL to 8·18 (2·15) log BAU/mL in the group that received two doses of BNT162b2, and from 5·39 (2·71) log BAU/mL to 8·50 (2·13) log BAU/mL in the group that received mRNA-1273 followed by BNT162b2. The mean anti-RBD IgG reactivity over the ensuing 6–10 weeks (t3) was 8·30 (SD 2·22) log BAU/mL in the group that received two doses of mRNA-1273, 7·83 (2·95) log BAU/mL in the group that received two doses of BNT162b2, and 8·53 log BAU/mL (2·60) in the group that received mRNA-1273 followed by BNT162b2. At the time of the second dose (t4; ie, 16 weeks after the first dose), those immunised with two doses of BNT162b2 had significantly lower anti-RBD IgG concentrations than those who received two doses of mRNA-1273 or mRNA-1273 followed by BNT162b2 (4·25 [SD 4·09] log BAU/mL vs 7·49 [2·60] log BAU/mL vs 7·95 [2·72] log BAU/mL; p=0·0003). This difference was also seen in anti-spike IgG responses (6·66 [SD 3·71] log BAU/mL with two doses of BNT162b2 vs 7·56 [2·15] log BAU/mL with two doses of mRNA-1273 vs 7·97 [2·62] log BAU/mL with mRNA-1273 followed by BNT162b2; p=0·012). Mean concentrations of anti-nucleocapsid IgG antibodies did not differ significantly between homologous and heterologous vaccination across timepoints. At 4 weeks after boost immunisation (t5), anti-RBD IgG responses in those receiving BNT162b2 were similar to responses in those who had received mRNA-1273. Similar IgG responses were observed for the confirmatory cohort, and for the discovery and confirmatory cohorts combined (appendix 2 p 9). There was thus no difference in serological response between heterologous and homologous mRNA vaccination 4 weeks after the boost dose.
Figure 2.
Antibody responses based on homologous versus heterologous use of mRNA vaccines
Antibody responses to the receptor-binding domain (RBD) of the spike protein, the spike protein, and the nucleocapsid protein, based on homologous versus heterologous use of mRNA vaccines. Homologous vaccination consisted of both doses being either mRNA-1273 or BNT162b2, whereas heterologous vaccination consisted of mRNA-1273 followed by BNT162b2. Medians are indicated by diamonds, the IQR values are indicated by horizontal segments, and saturation thresholds are indicated by dashed lines. t1=before first dose. t2=approximately 4 weeks after first dose. t3=6–10 weeks after first dose. t4=up to 2 days before administration of second dose (16 weeks after prime dose). t5=4 weeks after second dose.
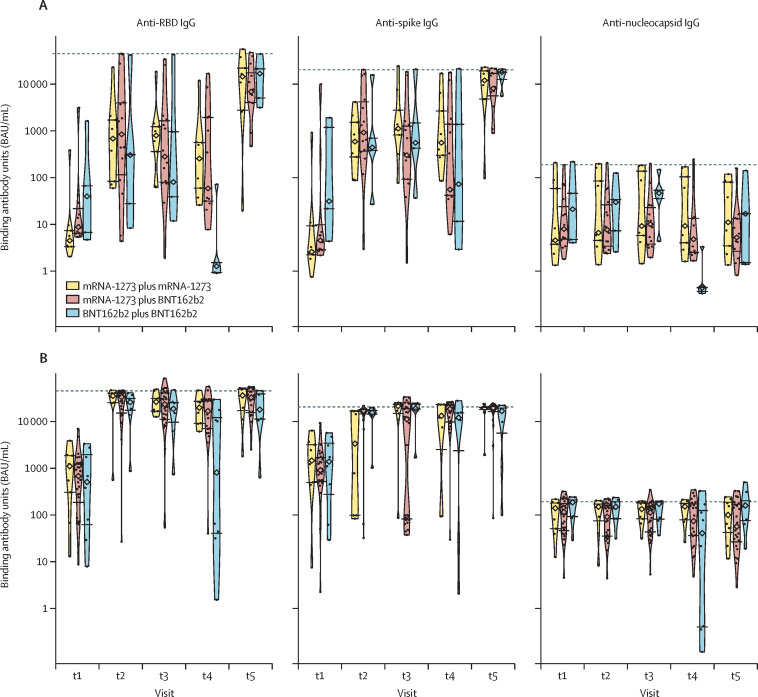
As expected, the anti-nucleocapsid IgG concentrations were higher in those with a history of SARS-CoV-2 infection for all timepoints. Compared with previously uninfected participants, anti-RBD and anti-spike IgG concentrations were consistently higher until t5 in those who had recovered from SARS-CoV-2 infection (figure 3 ), at which point concentrations were comparable. Among participants who received two doses of BNT162b2, both uninfected and previously infected individuals had a decrease in anti-RBD IgG antibodies from t2 to t4. By contrast, among those who received two doses of mRNA-1273, individuals with previous infection showed no significant change in antibody responses from t2 to t4, whereas uninfected individuals had a decrease in anti-RBD IgG antibodies, albeit a smaller decrease than their counterparts who received BNT162b2. At t5, there was an increase in antibody concentrations in all groups; no differences related to vaccine type, vaccine homology, and previous SARS-CoV-2 infection were observed.
Figure 3.
Antibody responses to homologous versus heterologous use of mRNA vaccines based on previous infection status
Antibody responses to the receptor-binding domain (RBD) of the spike protein, the spike protein, and the nucleocapsid protein, with homologous versus heterologous use of mRNA vaccines, based on the absence (A) or presence (B) of previous SARS-CoV-2 infection. Homologous vaccination consisted of both doses being either mRNA-1273 or BNT162b2, whereas heterologous vaccination consisted of mRNA-1273 followed by BNT162b2. Medians are indicated by diamonds, the IQR values are indicated by horizontal segments, and saturation thresholds are indicated by dashed lines. t1=before first dose. t2=approximately 4 weeks after first dose. t3=6–10 weeks after first dose. t4=up to 2 days before administration of second dose (16 weeks after prime dose). t5=4 weeks after second dose.
The mean anti-nucleocapsid, anti-spike, and anti-RBD IgG concentrations (in log BAU/mL) were similar, at all timepoints, across the age categories (appendix 2 p 6). We did not observe decreased antibody responses with increasing age. These findings were confirmed in the confirmatory cohort. Similarly, no differences in responses were observed in both cohorts when stratifying participants by sex or by type of comorbidity (appendix 2 pp 10–13). Similar antibody responses were observed between age groups and between men and women when stratifying participants on the basis of previous infection status or by vaccine homology (appendix 2 pp 14–17).
Discussion
Our findings show that, in frail, older individuals, the use of mRNA-based vaccines against COVID-19 can be used with a maximum extended interval of up to 16 weeks between doses. Robust antibody responses were elicited with either mRNA-1273 or BNT162b2 as the first dose, particularly in individuals with previously documented SARS-CoV-2 infection, although BNT162b2 was distinctly associated with a decline in antibody concentrations from 4 weeks after the first dose until the second dose. Under this dosing strategy, we found that similar antibody concentrations can be achieved with either homologous or heterologous use of these vaccines within 1 month after the second dose. These data can be used to shape vaccination policies globally, especially in light of vaccine supply shortages.
Previous natural infection with SARS-CoV-2, documented by microbiological testing, was documented in 86 (46%) of 185 participants in our study; this classification was confirmed by anti-nucleocapsid antibody positivity at baseline, with concomitantly higher anti-spike and anti-RBD IgG concentrations. Here, several findings are noteworthy. First, in those with confirmed, previous SARS-CoV-2 infection, a two-dose mRNA-based vaccine strategy, with a 16-week interval between doses, resulted in elevated concentrations of all SARS-CoV-2-specific IgG antibodies, at least during the 5-month timeframe of this report. Second, among participants who were not previously infected, the anti-RBD and anti-spike IgG responses achieved were significantly lower at all timepoints before the second dose compared to participants with previous SARS-CoV-2 infection. This effect has been previously noted in people aged 65 years and older, although in smaller studies.9, 10 Third, among the infection-naive group, a notable decrease in anti-RBD and anti-spike IgG concentrations was observed at 4 months after the first dose. However, administration of the second dose at this timepoint augmented the anti-RBD and anti-spike antibodies to levels comparable to those with previous SARS-CoV-2 infection. This decline was observed in our discovery cohort and corroborated in our confirmatory cohort. Although we did not assess vaccine effectiveness against infection because of the small number of SARS-CoV-2 cases in the participating facilities during the study period, our data suggest that an extended interval of 16 weeks between doses might be the maximum period permitted to limit waning of these antibody responses, at least in individuals who have not been previously infected.
In comparing antibody responses under different vaccine mixing strategies, a consistent pattern emerges: use of BNT162b2 as the first dose results in a significantly faster decline in anti-RBD IgG responses during the 16-week interval between the two vaccine doses, both in aggregate and when stratified by previous infection status, relative to mRNA-1273. A difference in antigenicity between these mRNA vaccines is emerging;11, 12, 13 our study highlights that this effect also occurs in frail, older individuals, at least following the prime dose. Although studies on heterologous vaccine platforms have focused on the immunogenicity of adenovirus-based vaccines in combination with mRNA-based vaccines,14, 15 the interchangeability of mRNA-based vaccines and their heterologous use in frail, older people have not, to our knowledge, been evaluated in a real-world setting. Our data indicate that there might be differences in the kinetics of induced anti-RBD responses between mRNA-1273 and BNT162b2, detectable at 4 months from the first dose. Administering BNT162b2 as the second dose at this timepoint achieves a similar antibody response to homologous or heterologous use of mRNA-1273 within 1 month of administration. Due to vaccine availability, the sequence of BNT162b2 followed by mRNA-1273 was not used in this study and thus cannot be compared.
In this study, we found no age-based, sex-based, or comorbidity-based differences in IgG humoral immune responses induced by the mRNA-based SARS-CoV-2 vaccines. Residents of long-term care facilities, who are often older and frail, might have vaccine-induced immune responses that are less robust than those of younger individuals because of immunosenescence, a multifactorial process that results in declining immunity with advancing age.16 Indeed, older vaccine recipients (aged ≥60 years) have shown diminished serological responses to COVID-19 vaccination compared to younger recipients (aged <60 years).9, 10, 17, 18 Our analyses show no variation in antibody responses to mRNA-based vaccines among the different age groups within these cohorts of frail, older individuals. Similarly, sex-based differences in antibody responses to vaccines have been observed in older individuals for certain vaccines (eg, influenza, zoster, and pneumococcus).19 However, no differential responses to COVID-19 were noted in our two cohorts. Our findings suggest that, in these cohorts of frail, older individuals with a similar age and comorbidity distribution, the serological responses were similar and clinically equivalent, at least during the first 5 months from the first dose. Although larger studies in frail, older adults are required to confirm these observations, our findings could have implications for addressing immunosenescence within the age groups of this population through the use of mRNA-based vaccines.
This study has some notable limitations. First, our cohorts were small in size, reflecting the real-world observational design of the study; the long-term care facilities were distributed within different, independent regional health networks; and we had limited ability to obtain emergency research ethics board review during implementation of the provincial vaccination campaign that coincided with the December holiday period. Nonetheless, this study systematically recruited available, consenting individuals at participating sites, and had the statistical power to detect biologically meaningful differences in antibody responses that are sufficient to inform evolving health-care policies. Another limitation of this cohort is that the ethnicity of the majority of participants was White; this limitation should be considered when interpreting and applying the results to older populations of different ethnic backgrounds, as there might be race-related differences in immune responses.20 Additionally, we evaluated IgG responses. Although immune correlates of protection against COVID-19 are not currently established, the spike protein-specific antibody responses (assessed here by IgG responses to trimeric spike and RBD antigens) generally correlate with the level of neutralising antibody activity, which is thought to be an important determinant of vaccine efficacy. Although the clinical relevance of induced circulating IgA and IgM responses is less clear, we are currently evaluating these concentrations. Additionally, our study does not provide data on vaccine effectiveness against infection or disease during the study period, nor did we have a cohort to serologically compare the serial use of BNT162b2 followed by mRNA-1273. Last, cellular immune responses were not included in this interim report, given the potential impact of our findings for nascent vaccination strategies in other countries, although those immunological studies are ongoing.
In conclusion, the increased susceptibility of older people to severe COVID-19, the continued emergence of viral variants with the potential for de novo or breakthrough infection, and the ongoing global shortages in vaccine supply could require the development of rationing policies in different regions. This interim report contributes to the available evidence for strategic use of vaccines in older adults. Further analyses of this cohort, combined with additional studies by others, could help define the scope of protection afforded by previous SARS-CoV-2 infection, and by extended-interval vaccination, in protecting against new SARS-CoV-2 variants.
Data sharing
De-identified clinical data for the patients in this study might be made available to other investigators after approval by the institutional review board. Requests should be directed via email to the corresponding author.
Declaration of interests
DCV is funded by the Fonds de la recherche en santé du Québec clinician-scientist scholar Junior 2 Program; has received clinical trial support from Cidara Therapeutics, CSL Behring, and Janssen Pharmaceuticals; has served on advisory boards for CSL Behring, Novartis Canada, and UCB Biosciences; has received speaker honoraria from CSL Behring and Merck Canada; and has a patent application pending (Electronic Filing System ID: 40101099) and a report of invention to McGill University (Track code: D2021-0043), both unrelated to this work. J-PG is funded by a Canada Research Chair award. Production of COVID-19 reagents was financially supported by National Research Council Canada's Pandemic Response Challenge Program. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We acknowledge the ongoing collaboration of the Centre intégré universitaire de santé et de services sociaux du Centre-Sud-de-l'Île-de-Montréal for this study. We also thank the centre d'hébergement de soins de longue durée (CHSLD) residents and their family members for their participation. This project was supported by funding from the Public Health Agency of Canada through the COVID-19 Immunity Task Force and by a COVID-19 Rapid Response grant from the Canadian Institute of Health Research (VR2-172722) and by a grant supplement by the COVID-19 Immunity Task Force to M-AL.
Contributors
DCV, J-PG, and DC-S contributed to conceptualisation; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; supervision; validation; visualisation; writing the original draft; and writing, review, and editing of the manuscript. MC-M contributed to conceptualisation; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; software; supervision; visualisation; writing the original draft; and writing, review, and editing of the manuscript. SB contributed to investigation; and writing, review, and editing of the manuscript. FB, AS, and J-PB contributed to investigation; validation; and writing, review, and editing of the manuscript. AG, RD, AP, YS, YL, and LR contributed to the investigation; and writing, review, and editing of the manuscript. MJL contributed to the investigation; validation; and writing, review, and editing of the manuscript. DK contributed to the formal analysis; methodology; resources; and writing, review, and editing of the manuscript. CA, MW, and M-AL contributed to data curation; formal analysis; methodology; resources; software; and writing, review, and editing of the manuscript. MP contributed to data curation; formal analysis; methodology; resources; software; visualisation; and writing, review, and editing of the manuscript. XZ contributed to data curation; formal analysis; methodology; resources; software; visualisation; writing the original draft; and writing, review, and editing of the manuscript. BDM contributed to conceptualisation; and writing, review, and editing of the manuscript. DCV, J-PG, DC-S, and MC-M had full access to all the data in the study, accessed and verified the data, and had final responsibility for the decision to submit for publication.
Supplementary Materials
References
- 1.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 2.Pastor-Barriuso R, Pérez-Gómez B, Hernán MA, et al. Infection fatality risk for SARS-CoV-2 in community dwelling population of Spain: nationwide seroepidemiological study. BMJ. 2020;371 doi: 10.1136/bmj.m4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iversen PL, Bavari S. Extending the interval of COVID-19 vaccine regimens in individuals aged 80 years or older. Lancet Healthy Longev. 2021;2:e529–e530. doi: 10.1016/S2666-7568(21)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comité sur l'immunisation du Québec Données préliminaires sur l'efficacité vaccinale et avis complémentaire sur la stratégie de vaccination contre la COVID-19 au Québec en contexte de pénurie [Internet] Feb 12, 2021. https://inspq.qc.ca/sites/default/files/publications/3111_vaccination_covid19_2e_dose_contexte_penurie.pdf
- 7.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olivier J, Johnson WD, Marshall GD. The logarithmic transformation and the geometric mean in reporting experimental IgE results: what are they and when and why to use them? Ann Allergy Asthma Immunol. 2008;100:333–337. doi: 10.1016/S1081-1206(10)60595-9. [DOI] [PubMed] [Google Scholar]
- 9.Parry H, Bruton R, Stephens C, et al. Differential immunogenicity of BNT162b2 or ChAdOx1 vaccines after extended-interval homologous dual vaccination in older people. Immun Ageing. 2021;18:34. doi: 10.1186/s12979-021-00246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tut G, Lancaster T, Krutikov M, et al. Profile of humoral and cellular immune responses to single doses of BNT162b2 or ChAdOx1 nCoV-19 vaccines in residents and staff within residential care homes (VIVALDI): an observational study. Lancet Healthy Longev. 2021;2:e544–e553. doi: 10.1016/S2666-7568(21)00168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richards NE, Keshavarz B, Workman LJ, Nelson MR, Platts-Mills TAE, Wilson JM. Comparison of SARS-CoV-2 antibody response by age among recipients of the BNT162b2 vs the mRNA-1273 vaccine. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.24331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steensels D, Pierlet N, Penders J, Mesotten D, Heylen L. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA. 2021;326:1533–1535. doi: 10.1001/jama.2021.15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barros-Martins J, Hammerschmidt SI, Cossmann A, et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat Med. 2021;27:1525–1529. doi: 10.1038/s41591-021-01449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt T, Klemis V, Schub D, et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat Med. 2021;27:1530–1535. doi: 10.1038/s41591-021-01464-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gustafson CE, Kim C, Weyand CM, Goronzy JJ. Influence of immune aging on vaccine responses. J Allergy Clin Immunol. 2020;145:1309–1321. doi: 10.1016/j.jaci.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collier DA, Ferreira IATM, Kotagiri P, et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021;596:417–422. doi: 10.1038/s41586-021-03739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Müller L, Andrée M, Moskorz W, et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. Clin Infect Dis. 2021;73:2065–2072. doi: 10.1093/cid/ciab381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fink AL, Klein SL. The evolution of greater humoral immunity in females than males: implications for vaccine efficacy. Curr Opin Physiol. 2018;6:16–20. doi: 10.1016/j.cophys.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurupati R, Kossenkov A, Haut L, et al. Race-related differences in antibody responses to the inactivated influenza vaccine are linked to distinct pre-vaccination gene expression profiles in blood. Oncotarget. 2016;7:62898–62911. doi: 10.18632/oncotarget.11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified clinical data for the patients in this study might be made available to other investigators after approval by the institutional review board. Requests should be directed via email to the corresponding author.