Abstract
Purpose
Dexamethasone is the only drug that has consistently reduced mortality in patients with COVID-19, especially in patients needing oxygen or invasive mechanical ventilation. However, there is a growing concern about the relation of dexamethasone with the unprecedented rates of ICU-acquired respiratory tract infections (ICU-RTI) observed in patients with severe COVID-19.
Methods
This was a multicenter, prospective cohort study; conducted in ten countries in Latin America and Europe. We included patients older than 18 with confirmed SARS-CoV-2 requiring ICU admission. A multivariate logistic regression and propensity score matching (PSM) analysis was conducted to determine the relation between dexamethasone treatment and ICU-RTI.
Results
A total of 3777 patients were included. 2065 (54.7%) were treated with dexamethasone within the first 24 h of admission. After performing the PSM, patients treated with dexamethasone showed significantly higher proportions of VAP (282/1652 [17.1%] Vs. 218/1652 [13.2%], p = 0.014). Also, dexamethasone treatment was identified as an adjusted risk factor of ICU-RTI in the multivariate logistic regression model (OR 1.64; 95%CI: 1.37–1.97; p < 0.001).
Conclusion
Patients treated with dexamethasone for severe COVID-19 had a higher risk of developing ICU-acquired respiratory tract infections after adjusting for days of invasive mechanical ventilation and ICU length of stay, suggesting a cautious use of this treatment.
Keywords: Dexamethasone, COVID-19, Critical care, Severe COVID-19, Pneumonia
Abbreviations: ICU-RTI, Intensive care unit-acquired respiratory tract infections; HIV-AIDS, Human immunodeficiency virus- Acquired Immunodeficiency Disease Syndrome; PaO2, Partial arterial oxygen concentration; PaCO2, Partial arterial carbon dioxide concentration; FiO2, Inspired Fraction of Oxygen; VAP, Ventilator-Associated Pneumonia; VAT, Ventilator-Associated Tracheobronquitis; HFNC, High Flow Nasal Cannula; HAP, Hospital-acquired pneumonia; LOS, Length of stay.
1. Background
Over the last year, more than 200 million people have been infected by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), and more than 4 million people have died worldwide due to this infection [1,2,3]. It is estimated that more than 10 billion dollars have been expended taking care of these patients only in the United States, and around 20 billion dollars were invested in developing the new vaccines currently available worldwide [4]. Moreover, about 30% of hospitalized patients develop severe infections and require organ support, including advanced ventilatory treatments and admission to the intensive care unit (ICU), increasing the mortality and costs of patient care [5,6]. During the pandemic, several medications have been tested to improve clinical outcomes in patients with SARS-CoV-2, especially in critically ill patients [7,8]. However, data evaluating the efficacy of these treatments have been contradictory. Notably, dexamethasone was the first medication to show a mortality benefit consistently in patients requiring oxygen support or invasive mechanical ventilation; thus, it has become the standard of care for severe COVID-19 patients globally [9].
On the other hand, up to 50% of patients admitted to the ICU due to severe COVID-19 develop ICU-acquired respiratory tract infections (RTI) such as ventilator-associated pneumonia (VAP), ventilator-associated tracheobronchitis (VAT), and hospital acquired-pneumonia (HAP) [10,11]. Moreover, patients that develop ICU-RTI are frequently infected by multidrug-resistant pathogens (e.g., Pseudomonas aeruginosa and carbapenem-resistant Klebsiella pneumoniae). Consequently, these patients have higher mortality rates, longer ICU length of stay, and higher patient care-associated costs [10]. The mechanisms underlying these higher rates of ICU-RTI remained widely unknown, and few studies have documented the risk factors associated with these infections. The high incidence of ICU-RTI observed in severe SARS-CoV-2 patients is contradictory because these patients are in strict isolation, theoretically decreasing hospital-acquired infections. However, there may be other factors such as a lower implementation of bundles in pneumonia prevention, longer duration of ventilation, prolonged length of stay, and uncontrolled usage of broad-spectrum antibiotics. A growing concern has also emerged that treatment with immunosuppressors, such as dexamethasone, might be associated with ICU-RTI; however, data exploring this unintended effect is lacking. Therefore, this study will attempt to bring novel data in this regard.
Corticosteroids have been associated with immunosuppression states and subsequent superinfections before the SARS-CoV-2 pandemic [12]. However, as they are the only medications that have proved to improve clinical outcomes early during the pandemic, they have been widely used, especially in patients admitted to the ICU. We hypothesize that patients treated with dexamethasone are at a higher risk of developing ICU-RTI. To test this hypothesis, we performed a propensity score matching analysis of patients admitted to the ICU in Latin America and Europe before recommending dexamethasone and after becoming the standard of care to determine whether this corticosteroid is associated with ICU-RTI.
2. Materials and methods
This was a multicenter, prospective cohort study of subjects admitted to 84 ICUs due to severe SARS-CoV-2 infection in ten countries (i.e., Spain, Ireland, Bolivia, Colombia, Chile, Ecuador, Mexico, Argentina, Uruguay, and Brazil) between March 2020 and January 2021 [13]. The patients were included in a voluntary prospective registry created by the Latin American Intensive Care Network (Red LIVEN - https://www.redliven.org/web/) and the Spanish Society of Intensive Care Medicine (SEMICYUC)(NCT04948242). The attending physicians collected data by reviewing medical records and laboratory data. All subsequent cases admitted who met the entry criteria were included in the registry. The Ethics Committee approved the study of the Clínica Universidad de La Sabana (IRB#2020AN28) and Hospital Joan XXIII (IRB#CEIM/066/2020). All data were anonymized, allowing the requirement for informed consent to be waived. Treatment decisions were not standardized between centers; therefore, they were left to the discretion of the attending physicians.
2.1. Subjects
The cohort includes patients older than 18 years hospitalized in the ICU due to severe SARS-CoV-2 infection. Confirmed SARS-CoV-2 infection was determined by reverse transcription-polymerase chain reaction (rt-PCR) in a respiratory sample at each hospital based on local protocols. Then, we used a modified World Health Organization severity criteria to identify patients with severe and critical SARS-CoV-2 illness, defined as individuals who have SpO2 < 94% on room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) <300 mmHg, a respiratory rate > 30 breaths/min, or lung infiltrates >50% or individuals who have respiratory failure, septic shock, and/or multiple organ dysfunction. All patients who fit the definition above were admitted to ICU and included in the study. The exclusion criteria were patients receiving dexamethasone outside the established timeframe (24 h of admission) and patients receiving corticosteroids other than dexamethasone. The attending physician determined the dosage and duration of dexamethasone. All patients in the control group (i.e., without dexamethasone) were enrolled before July 2021, when the results of the RECOVERY trial were available.
2.2. Data collection
The following variables were recorded during ICU admission: demographic data, comorbidities, symptoms, physiological variables collected during the first 24 h of ICU admission, systemic complications, and treatments used during the admission (Table 1 ). The indication for corticosteroid treatment was reported in the database and was confirmed by medical records.
Table 1.
Characteristics and outcomes comparing dexamethasone and no dexamethasone group before and after propensity score-matching analysis.
| Original cohort |
Match cohort* |
|||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | All n = 3777 |
Dexamethasone n = 2065 |
No Dexamethasone n = 1712 |
p-value | All n = 3304 |
Dexamethasone n = 1652 |
No Dexamethasone n = 1652 | p value |
| Demographic | ||||||||
| Age, mean (SD) | 60.3 (13.1) | 60.1 (12.9) | 60.6 (13.2) | 0.22 | 60.8 (13.0) | 60.6 (12.9) | 61.3 (13.3) | 0.12 |
| Male, n (%) | 2598 (68.8) | 1454 (70.4) | 1144 (66.8) | 0.010 | 2268 (68.7) | 1133 (68.6) | 1136 (68.8) | 0.91 |
| Country, n (%) | ||||||||
| Spain | 2456 (65.0) | 1217 (58.9) | 1239 (72.4) | <0.001 | 2358 (71.4) | 1169 (70.8) | 1189 (72.0) | <0.001 |
| Ireland | 44 (1.2) | 6 (0.3) | 38 (2.2) | 30 (0.9) | 6 (0.4) | 24 (1.5) | ||
| Colombia | 631 (16.7) | 476 (23.1) | 155 (9.1) | 351 (10.6) | 200 (12.1) | 151 (9.1) | ||
| Ecuador | 317 (8.4) | 185 (9) | 132 (7.7) | 241 (7.3) | 121 (7.3) | 120 (7.3) | ||
| Chile | 208 (5.5) | 96 (4.6) | 112 (6.5) | 197 (6.0) | 80 (4.8) | 117 (7.1) | ||
| Argentina | 80 (2.1) | 62 (3) | 18 (1.1) | 84 (2.5) | 54 (3.3) | 30 (1.8) | ||
| Uruguay | 21 (0.6) | 19 (0.9) | 2 (0.1) | 27 (0.8) | 19 (1.2) | 8 (0.5) | ||
| Brazil | 12 (0.3) | 0 (0) | 12 (0.7) | 8 (0.2) | 0 (0) | 8 (0.5) | ||
| Mexico | 7 (0.2) | 4 (0.2) | 3 (0.2) | 7 (0.2) | 3 (0.2) | 4 (0.2) | ||
| Bolivia | 1 (0) | 0 (0) | 1 (0.1) | 1 (0) | 0 (0) | 1 (0.1) | ||
| Comorbid conditions a, n (%) | ||||||||
| Congestive heart failure | 233 (6.2) | 132 (6.4) | 101 (5.9) | 0.53 | 189 (5.7) | 93 (5.6) | 97 (5.9) | 0.80 |
| Hypertension | 1654 (43.8) | 909 (44.0) | 745 (43.5) | 0.75 | 1505 (45.6) | 750 (45.4) | 758 (45.9) | 0.27 |
| Chronic pulmonary disease | 297 (7.9) | 155 (7.5) | 142 (8.3) | 0.37 | 262 (7.9) | 137 (8.3) | 119 (7.2) | 0.35 |
| Asthma | 163 (4.3) | 68 (3.3) | 95 (5.5) | 0.001 | 128 (3.9) | 59 (3.6) | 75 (4.5) | 0.24 |
| Chronic kidney disease | 232 (6.1) | 142 (6.9) | 90 (5.3) | 0.030 | 199 (6.0) | 99 (6.0) | 101 (6.1) | 0.90 |
| Neurologic disease | 50 (1.3) | 27 (1.3) | 23 (1.3) | 0.92 | 41 (1.3) | 21 (1.3) | 20 (1.2) | 0.92 |
| Hematological disease | 103 (2.7) | 52 (2.5) | 51 (3.0) | 0.38 | 94 (2.8) | 43 (2.6) | 55 (3.3) | 0.32 |
| HIV-AIDS | 16 (0.4) | 9 (0.4) | 7 (0.4) | 0.89 | 17 (0.5) | 8 (0.5) | 10 (0.6) | 0.68 |
| Obesity | 1206 (31.9) | 708 (34.3) | 498 (29.1) | 0.001 | 1075 (32.5) | 548 (33.2) | 516 (31.2) | 0.32 |
| Diabetes | 822 (21.8) | 500 (24.2) | 322 (18.8) | <0.001 | 704 (21.3) | 371 (22.5) | 313 (19.0) | 0.047 |
| Rheumatological disease | 94 (2.5) | 46 (2.2) | 48 (2.8) | 0.25 | 87 (2.6) | 43 (2.6) | 44 (2.7) | 0.89 |
| Laboratories at ICU admission | ||||||||
| FiO2%, mean (SD) | 63.4 (27.4) | 65 (27.0) | 61.4 (27.7) | <0.001 | 63 (27.5) | 63.4 (27.2) | 62.3 (28.1) | 0.23 |
| PaO2 mmHg, mean (SD) | 73.7 (24.7) | 72.9 (23.9) | 74.6 (25.5) | 0.06 | 74.3 (27.5) | 74.4 (24.5) | 74.2 (28.1) | 0.80 |
| PaO2/FiO2 Ratio, n (%) | ||||||||
| 0–50 | 124 (3.3) | 72 (3.5) | 52 (3.0) | 0.001 | 98 (3.0) | 43 (2.6) | 55 (3.3) | 0.40 |
| 50–100 | 1394 (36.9) | 816 (39.5) | 578 (33.8) | 1194 (36.1) | 612 (37.0) | 582 (35.2) | ||
| 100–150 | 925 (24.5) | 517 (25.0) | 408 (23.8) | 832 (25.2) | 442 (26.8) | 390 (23.6) | ||
| 150–200 | 535 (14.2) | 271 (13.1) | 264 (15.4) | 466 (14.1) | 215 (13.0) | 251 (15.2) | ||
| 200–250 | 308 (8.2) | 157 (7.6) | 151 (8.8) | 273 (8.3) | 142 (8.6) | 131 (7.9) | ||
| 250–300 | 210 (5.6) | 97 (4.7) | 113 (6.6) | 171 (5.2) | 86 (5.2) | 85 (5.1) | ||
| 300–350 | 122 (3.2) | 54 (2.6) | 68 (4.0) | 118 (3.6) | 39 (2.4) | 79 (4.8) | ||
| 350–400 | 71 (1.9) | 37 (1.8) | 34 (2.0) | 69 (2.1) | 34 (2.1) | 35 (2.1) | ||
| >400 | 88 (2.3) | 44 (2.1) | 44 (2.6) | 83 (2.5) | 39 (2.3) | 44 (2.7) | ||
| pH, n (%) | ||||||||
| 6.6–6.8 | 6 (0.2) | 3 (0.1) | 3 (0.2) | 0.33 | 5 (0.2) | 3 (0.2) | 2 (0.1) | 0.021 |
| 6.8–7.0 | 23 (0.6) | 12 (0.6) | 11 (0.6) | 20 (0.6) | 11 (0.7) | 9 (0.5) | ||
| 7.0–7.2 | 179 (4.7) | 99 (4.8) | 80 (4.7) | 158 (4.8) | 69 (4.2) | 89 (5.4) | ||
| 7.2–7.4 | 1461 (38.7) | 765 (37.0) | 696 (40.6) | 1242 (37.6) | 594 (36.0) | 648 (39.2) | ||
| 7.4–7.6 | 2105 (55.7) | 1184 (57.3) | 921 (53.8) | 1878 (56.8) | 975 (59.0) | 902 (54.7) | ||
| 7.6–7.8 | 1 (0.1) | 0 (0) | 1 (0.1) | 1 (0) | 0 (0) | 1 (0.1) | ||
| 7.8–8.0 | 2 (0.1) | 2 (0.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Leucocytes, 109 cells/L, mean (SD) | 10.2 (5.5) | 10.5 (5.5) | 9.8 (5.4) | <0.001 | 10.1 (5.2) | 10.1 (5.2) | 10 (5.3) | 0.85 |
| Creatinine, mg/dL, median (IQR) | 1 (1–1) | 1 (1–1) | 1 (1–1) | 0.42 | 1 (1–1) | 1.1 (1–1) | 1 (1–1) | 0.14 |
| C-Reactive Protein mg/dL, median (IQR) | 16 (8–29) | 15 (8–26) | 16 (9–28) | 0.30 | 16 (8–28) | 15 (8–28) | 17 (9–29) | 0.022 |
| Pulmonary infiltrates, n (%) | 3426 (90.7) | 1862 (90.2) | 1564 (91.4) | 0.21 | 3066 (91.2) | 1503 (91.0) | 1563 (91.4) | 0.70 |
| ICU interventions | ||||||||
| Tocilizumab, n (%) | 482 (12.8) | 187 (9.1) | 295 (17.2) | <0.001 | 349 (10.6) | 166 (10.0) | 192 (11.6) | 0.23 |
| HFNC, n (%) | 1034 (27.4) | 710 (34.4) | 324 (18.9) | <0.001 | 1085 (32.8) | 646 (39.1) | 334 (20.2) | <0.001 |
| Pronation, n (%) | 1954 (51.8) | 1181 (57.2) | 773 (45.2) | <0.001 | 1714 (51.9) | 895 (54.1) | 781 (47.3) | 0.001 |
| ICU Outcomes | ||||||||
| VAPb, n (%) | 535 (14.2) | 341 (16.5) | 194 (11.3) | <0.001 | 522 (15.8) | 282 (17.1) | 218 (13.2) | 0.014 |
| VATb, n (%) | 275 (7.3) | 185 (9.0) | 90 (5.3) | <0.001 | 195 (5.9) | 107 (6.5) | 79 (4.8) | 0.09 |
| HAP, n (%) | 51 (1.4) | 46 (2.2) | 5 (0.3) | <0.001 | 25 (0.8) | 19 (1.2) | 0 (0) | 0.07 |
| Invasive Mechanical Ventilation, n (%) | 2622 (69.4) | 1506 (72.9) | 1116 (65.2) | <0.001 | 2308 (69.9) | 1153 (69.8) | 1157 (70.0) | 0.91 |
| Days of Invasive Mechanical Ventilation, median (IQR) | 8 (0–16) | 8 (0–17) | 7 (0–15) | <0.001 | 8 (0–17) | 8 (0–17) | 8 (0–17) | 0.98 |
| Non-Invasive Mechanical Ventilation, n (%) | 438 (11.6) | 287 (13.9) | 151 (8.8) | <0.001 | 403 (12.2) | 235 (14.2) | 133 (8.1) | <0.001 |
| Hospital LOS, median (IQR) | 20 (12–33) | 19 (12−32) | 20 (11–34) | 0.34 | 20 (12–34) | 20 (13−33) | 21 (11–35) | 0.88 |
| ICU LOS, median (IQR) | 12 (6–21) | 12 (6–21) | 12 (5–21) | 0.010 | 12 (6–22) | 12 (6–22) | 12 (5–22) | 0.43 |
| Death, n (%) | 1136 (30.1) | 642 (31.1) | 494 (28.9) | 0.13 | 1009 (30.5) | 501 (30.3) | 512 (31.0) | 0.74 |
HIV-AIDS: Human immunodeficiency virus- Acquired Immunodeficiency Disease Syndrome; PaO2: Partial arterial oxygen concentration; PaCO2: Partial arterial carbon dioxide concentration; FiO2: Inspired Fraction of Oxygen; VAP: Ventilator-Associated Pneumonia; VAT: Ventilator-Associated Tracheobronquitis; HFNC: High Flow Nasal Cannula; HAP: Hospital-acquired pneumonia; LOS: Length of stay; IQR: Interquartile range.*Nearest Neighbor Matching were used; aPatients may present more than one comorbidity; bSeven patients presented VAP and VAT together.
2.3. Study definitions
ICU-RTI was defined as a clinical syndrome of ventilator-associated pneumonia (VAP), ventilator-associated tracheobronchitis (VAT), or hospital-acquired pneumonia (HAP) as defined by the American Thoracic Society and the Infectious Diseases Society of America (ATS/IDSA) guidelines [14]. VAP was defined as pneumonia that arises more than 48 h after endotracheal intubation; VAT as fever with no other recognizable cause, with new or increased sputum production, positive endotracheal aspirates (ETA) culture (>106 CFU/mL) yielding a new bacterium and no radiographic evidence of nosocomial pneumonia. HAP was defined as pneumonia that occurs 48 h or more after admission to the ICU in patients without invasive mechanical ventilation, which was not incubating at the time of admission [14]. Additionally, only patients with the isolation of respiratory pathogens in a respiratory sample (e.g., tracheal aspirate, bronchoalveolar lavage, sputum, or pleural fluid) after the first 48 h of ICU admission were considered to have ICU-RTI. Finally, two groups were defined: 1. the dexamethasone group (who received dexamethasone within the first 24 h of hospital admission and no other corticosteroid during ICU stay) and 2. the no dexamethasone group (who received no corticosteroid treatment at all).
2.4. Outcomes
Our primary outcome was to determine the association of dexamethasone with ICU-RTI. Our secondary outcomes were to describe the characteristic of patients treated with dexamethasone and explore the effect of ICU-RTI on clinical outcomes.
2.5. Statistical analysis
Discrete variables were expressed as percentages. Continuous variables with normal distribution were expressed as means (standard deviation); variables with no normal distribution were expressed as median (interquartile ranges). Categorical variables are presented in counts (percentages) and were evaluated through the Chi-square test. For continuous variables with normal distribution, the t Student test was performed, and for variables with no normal distribution Wilcoxon-Mann-Whitney test was used.
Two multivariate logistic regression models were developed to evaluate dexamethasone's relation with ICU-RTI in patients with severe SARS-CoV-2 infection and the factors associated with dexamethasone administration (dependent variables). The explanatory variables included demographic, comorbid conditions, physiological variables collected during the first 24 h of ICU admission, days of invasive mechanical ventilation (IMV), ICU, and hospital length of stay. Variables with a p < 0.25 in the initial bivariate analysis were included in the logistic regression model. The best model was evaluated in terms of AUC, and the goodness of fit for the model was assessed with the Hosmer-Lemeshow test.
Moreover, a propensity score matching (PSM) analysis was developed to reduce selection bias from different baseline characteristics and disease severity between individuals treated or not with dexamethasone during the first 24 h of admission (sex, age, congestive heart failure, hypertension, chronic pulmonary disease, asthma, chronic kidney disease, neurologic disease, hematological disease, HIV-AIDS, obesity, diabetes, rheumatological disease, IMV, days of IMV, ICU LOS, hospital LOS, leucocytes, creatinine, C-Reactive Protein, PaO2, FiO2, tocilizumab, income country). The balance of the variables between the treated and untreated groups was evaluated through standardized means. After that, a propensity score was estimated through a logistic regression model. The matching was performed using nearest neighbor matching (NNM). The balance pre and post-matching were compared using the standardized mean differences and the Rubin index to ensure a good balance of the treated and not-treated groups. After matching the subjects, a calculation of average treatment effect (ATE) and average treatment effects among treated subjects (ATET) were made with their corresponding 95% confidence intervals (95% CI). Finally, to evaluate possible confounding factors in the development of ICU-RIT associated with tocilizumab administration among subjects who received dexamethasone, a stratified analysis was performed using the Mantel-Haenszel method. Statistical significance was set at 0.05. All statistical analysis was carried out in the R studio 1.3.1056, STATA 14, and IBM SPSS 28 for MAC.
3. Results
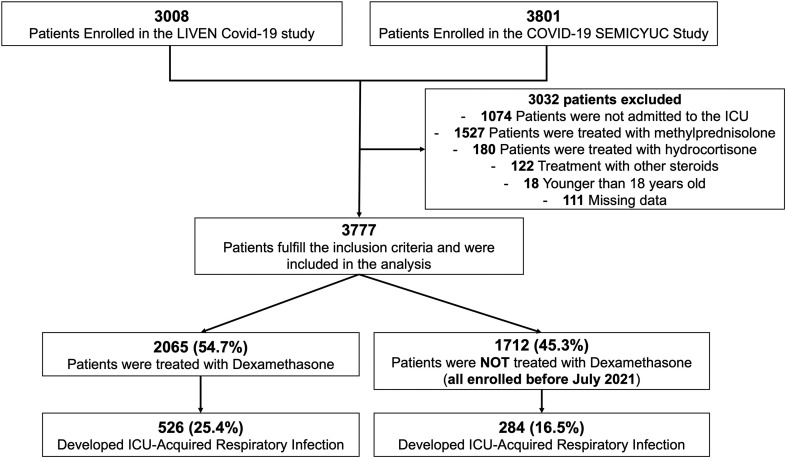
A total of 3777 patients were included in this study (Fig. 1 ). The mean (SD) age was 60.3 years (13.1), and 68.8% (2598/3777) were male. Additionally, the most frequent comorbidities were hypertension 43.8% (1654/3777) and obesity 31.9% (1206/3777) (Table 1 ). Inspired fraction of oxygen and partial oxygen pressure mean (SD) at admission were 63.4 (27.4) and 73.7 (24.7), respectively. Of all patients in the analysis, 12.8% (482/3777) received tocilizumab, 69.4% (2622/3777) required invasive mechanical ventilation, and 30.1% (1136/3777) of patients died. The median (IQR) number of days under IMV, ICU- LOS, and Hospital-LOS were 8 (0–16), 12 (6–21), and 20 (12−33), respectively. VAP was observed in 14.2% (535/3777) of the subjects included in the study.
Fig. 1.
Flow chart of patients included in the analysis.
3.1. ICU-RTI analysis
The mean (SD) age of patients that developed ICU-RTI was 61.6 (12.4) years. In that group, the most frequent comorbidities were hypertension 44.7% (382/854) and obesity 29.7% (254/854). When the ICU-RTI group were compared with those who did not develop ICU-RTI, patients were more frequently male (72.2% [617/854] vs 67.8% [1981/2923]; p = 0.01), with diabetes (24.8% [212/854] vs 20.9% [610/2923]; p = 0.01) and with hematological diseases (4.1% [35/854] vs 2.3% [68/2923]; p = 0.01). Patients who developed ICU-RTI had more days under IMV (median [IQR]: 17 [10−31] vs 5 [0−12]; p < 0.001), had longer ICU-LOS (median [IQR]: 21 [12–36] vs 10 [[6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18]]; p < 0.001) and hospital-LOS (median [IQR]: 28 [16–50] vs 18 [11−30]; p < 0.001). Notably, mortality was also significantly higher in those with ICU-RTI (39.8% [340/854] vs 27.2% [796/2923]; p < 0.001) (Supplement Table S1).
3.2. Comparison between subjects with and without dexamethasone treatment
A total of 2065/3777 (54.7%) patients were treated with dexamethasone within the first 24 h of hospital admission. Demographic characteristics between the dexamethasone and no dexamethasone group are presented in Table 1. The usage of invasive mechanical ventilation was different between groups (72.9% [1506/2065] vs. 65.2% [1116/1712]; p < 0.001). Also, the median number of days under IMV were different among patients who were treated with dexamethasone and those that did not (median [IQR]: 8 [0–17] vs. 7 [0–15]; p < 0.001), as well as ICU-LOS (median [IQR]: 12 [[7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22]] vs. 12 [[6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22]]; p = 0.01). Patients treated with dexamethasone more frequently developed VAP (16.5% [341/2065] vs 11.3% [194/1712]; p < 0.001), VAT (9.0% [185/2065] vs 5.3% [90/1712]; p < 0.001) and HAP (2.2% [46/2065] vs 0.3% [5/1712]; p < 0.001).
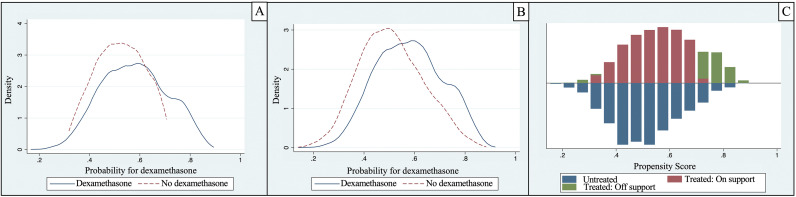
After the PSM, 3304 patients were matched 1:1 using PSM's closest neighbor (1652 in the dexamethasone group and 1652 in the non-dexamethasone group). The study groups were well balanced in terms of different baseline characteristics and disease severity; the region of common support was wide enough to assure the stability of estimates obtained by the matching method (Table 1 , Fig. 2 ). After matching, the Rubin index decreased from 55.7 to 18. Those who received dexamethasone therapy developed more frequently VAP (282/1652 [17.1%] vs. 218/1652 [13.2%], p = 0.014). The differences continued to show statistical significance when evaluated using the ATE and ATET by NNM (Table 3); according to ATE and ATET results [95% CI], there is a difference of 6% [3%–8%] of superinfection between subjects receiving dexamethasone compared to those who did not receive it (p < 0.001).
Fig. 2.
Distribution before (A) and after (B) Propensity Score Matching between patients with dexamethasone and patients without dexamethasone administration. C, region of common support between patients with and without dexamethasone administration.
Table 3.
Effect of dexamethasone administration on causing ICU-RTI.
| Method | ATE | 95%CI | p-value | ATET | 95%CI | p-value |
|---|---|---|---|---|---|---|
| Nearest Neighbor matching | 0.06 | 0.03–0.08 | <0.001 | 0.06 | 0.03–0.09 | <0.001 |
A sensitivity analysis by performing a stratified analysis to assess the risk of dexamethasone and ICU-RTI in patients treated with another immunosuppressor treatment was developed. Adjusted for tocilizumab administration; dexamethasone remained significantly associated with superinfection regardless of whether tocilizumab was received (unadjusted RR:1.63, 95%CI:1.39–1.80; p < 0.001 vs. Mantel-Haenszel RR:1.59, 95%CI:1.39–1.80; p < 0.001).
3.3. Factors associated with dexamethasone administration in the logistic regression model
Factors independently associated with the administration of dexamethasone were low-middle income countries (OR:1.72; 95%CI:1.55–1.90; p < 0.001), obesity (OR:1.36; 95%CI:1.17–1.58; p < 0.001), diabetes (OR:1.32; 95%CI:1.11–1.57; p = 0.001), sex (OR:1.17; 95%CI: 1.01–1.36; p = 0.033), higher leukocytes count (OR:1.01; 95%CI: 1.00–1.03; p = 0.003), and higher inspired fraction of oxygen at admission (OR:1.004; 95%CI:1.002–1.007; p < 0.001) (Table 2 ).
Table 2.
Factors associated with dexamethasone treatment and characteristics associated with the risk of ICU-RTI in the multivariate logistic regression models.
| Variable | Odds Ratio | 95% CI | p-value |
|---|---|---|---|
| Dexamethasone treatment | |||
| Sex | 1.17 | 1.01–1.36 | 0.033 |
| Chronic pulmonary disease | 0.74 | 0.57–0.95 | 0.020 |
| Asthma | 0.70 | 0.50–0.98 | 0.040 |
| Obesity | 1.36 | 1.17–1.58 | <0.001 |
| Diabetes | 1.32 | 1.11–1.57 | 0.001 |
| Days of IMV | 1.01 | 1.00–1.02 | 0.010 |
| Leucocytes, 109 cells/L | 1.01 | 1.00–1.03 | 0.003 |
| C-Reactive Protein, mg/dL | 0.996 | 0.994–0.997 | <0.001 |
| Higher FiO2 support, % | 1.004 | 1.002–1.007 | <0.001 |
| Tocilizumab | 0.61 | 0.49–0.75 | <0.001 |
| Low-middle income country | 1.72 | 1.55–1.90 | <0.001 |
| Risk of ICU-RTI | |||
| IMV | 27.46 | 13.45–56.03 | <0.001 |
| Dexamethasone | 1.64 | 1.37–1.97 | <0.001 |
| pH < 7.4 | 1.35 | 1.13–1.61 | 0.001 |
| PaO2/FiO2 Ratio | 0.996 | 0.995–0.997 | <0.001 |
| Days of IMV | 1.04 | 1.03–1.06 | <0.001 |
| ICU LOS, days | 1.02 | 1.01–1.04 | 0.001 |
| Hospital LOS, days | 0.98 | 0.98–0.99 | <0.001 |
| Middle-income country | 1.97 | 1.39–2.81 | <0.001 |
| Low-middle income country | 4.55 | 3.66–5.66 | <0.001 |
3.4. Factors associated with the risk of ICU-RTI in the logistic regression model
The risk of developing ICU-RTI in patients treated with dexamethasone was significantly higher (OR 1.64; 95%CI: 1.37–1.97; p < 0.001), independent of the use of IMV (OR 27.46; 95%CI: 13.45–56.03; p < 0.001), longer days under IMV (OR 1.04; 95%CI: 1.03–1.06; p < 0.001) and longer ICU-LOS (OR 1.02; 95%CI: 1.01–1.04; p = 0.001). Middle income countries and low-middle income countries also were related with the risk of superinfection (OR 1.97; 95%CI: 1.39–2.81; p < 0.001) and (OR 4.55; 95%CI: 3.66–5.66; p < 0.001), respectively (Table 2).
4. Discussion
In this large cohort of patients admitted to the ICU in Latin America and Europe, after a comprehensive statistical analysis (including PSM), we found that treatment with dexamethasone in patients with severe infection by SARS-CoV2 was associated with a higher risk of developing ICU-RTI independently of disease severity at admission and days on IMV. Also, we found that patients that developed ICU-RTI had higher mortality rates. Moreover, we found that lower PaO2/FiO2 Ratio on admission, more days on IMV, and longer ICU-LOS were also independently associated with higher mortality rates.
The effect of corticosteroids in patients with severe pneumonia had already been studied before the pandemic as a possible adjuvant treatment in cases of severe inflammatory states [12,15]. Thus, steroids were used early in the pandemic as a strategy to modulate the inflammatory reaction observed in COVID-19, especially in severely ill patients [[16], [17], [18]]. As a result of this potential benefit, systemic steroids were included in the platform trials launched to identify treatments to improve clinical outcomes in COVID-19 patients, such as the REMAP-CAP the RECOVERY trial, among others [9,19,20]. Dexamethasone was found to reduce the 28-day mortality in hospitalized patients with COVID-19 who received respiratory support in the RECOVERY trial [9]. Also, a metanalysis carried out by The World Health Organization found a mortality reduction in patients treated with systemic corticosteroids [19]. Therefore, dexamethasone has become the standard of care in patients admitted to the hospital due to COVID-19. Notably, some experts have postulated that there are still unresolved issues regarding using systemic corticosteroids in COVID-19 patients, particularly secondary infections. Superinfections in COVID-19 patients are more frequent than in other lower respiratory tract infections, such as influenza pneumonia [10]. Our study found that dexamethasone was associated with a higher frequency of ICU-RTI, even though patients without dexamethasone had similar comorbidities. Martinez-Martinez M et al., in a small unicentric analysis with 336 patients, observed that corticosteroids (OR:3.26; 95%CI:1.78–5.97) and tocilizumab (OR:1.85; 95%CI:1.02–3.38) were independently associated with the development of VAP. These findings are in alignment with our results. To the best of our knowledge, this is the first multicenter and multinational study with a considerable number of critical patients and a robust statistical approach to describe the association between ICU-RTI and dexamethasone administration in patients with severe COVID-19.
The association of SARS-Cov2 infection and ICU-RTI has been described in up to 50.5% of patients admitted to the ICU [10,21]. It is well known that pathogens involved are mostly multidrug-resistant pathogens (e.g., P. aeruginosa, carbapenem-resistant K. pneumoniae, Enterobacter spp., among many others) [[22], [23], [24], [25]]. Although multiple factors have been shown to contribute to the mortality associated with these infections, it is mainly attributable to the failure or delays of adequate antibiotic therapy; importantly, we did not assess this aspect in this study. Patients in these scenarios have a higher risk of ICU mortality, ranging from 20 to 50% and even up to 76% [14,22,23]. Mortality in patients with COVID-19 and VAP is not widely described yet; however, Luyt et al. described patients with severe infection due to SARS-Cov2 with VAP and showed a mortality rate of 34%, comparable to patients with influenza pneumonia and VAP (40%) [21]. These ranges coincide with our study results since 39.8% of patients with ICU-RTI died during their ICU stay. This should be of great concern to the health community, as secondary infections such as VAP, VAT, and HAP are generally preventable. All possible risk factors associated with them must be identified and avoided where possible.
Our study has several strengths and limitations that should be acknowledged. First, although this was a multicenter collaboration allowing a more significant sample and excellent generalizability, treatments between the different ICUs and, therefore, dexamethasone administration were not standardized. However, international guidelines were homogeneous at the beginning of the pandemic. All societies recommended 8 mg of dexamethasone for ten days, which was the scheme used per protocol in all participating centers. Secondly, statistical adjustments through PSM matching analysis could have limitations like unmeasured severity scores at ICU admission (e.g., APACHE or SOFA), or there might have been differences in antibiotic use in terms of agents used, the timing of initiation dose and administration; all of which may have been confounding variables. However, other severity variables were considered, such as laboratory parameters on admission, physiological variables on admission, FiO2 on admission, IMV and days of IMV, ICU-LOS, and Hospital-LOS. Notably, the PSM analysis is a methodology that permits close simulation to randomization, balancing the sample and reducing observational bias. Mortality in patients who developed ICU-RTI was not adjusted for covariables; however, this study aimed to approximate the survival in patients who had been superinfected and not determine the factors associated with increased mortality. Further studies are needed to confirm this potential unintended effect.
To sum up, the benefit of corticosteroids in severe COVID-19 patients is widely recognized, and most treatment guidelines recommend their use. However, some complications are derived by their use as the increased risk of superinfection reported in our study; thus, cautious use of this treatment is warranted, especially in patients with no severe infection. Further studies are needed to determine strategies to reduce the rate of ICU-RTI in patients with severe COVID-19.
Author contributions
LFR, AR, and IML were responsible for data acquisition, study design analysis, interpretation, preparing the first draft of the manuscript, and study data integrity. LFR, AR, AB, DPT, YVF, EGG, and IML were responsible for statistical analysis and interpretation. LFR, AR, AB, DPT, YVF, EGG, GM, GOT, GH, ES, AMD, MJ, MVA, ED, MB, JSV, RF, AAM, LS, AE, ALV, RJG, IS, and IMG acquired the data and contributed intellectually to this work. All authors had an opportunity to review the manuscript and approve its final submitted version.
Funding
This work was supported by Universidad de La Sabana (LFR) and the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC).
Declaration of Competing Interest
The authors do not have a Conflict of interest for this publication.
Acknowledgments
LIVEN-COVID-19 Investigators: Fabio Varon, Luis Antonio Gorordo, Ricardo Buitrago, Marcela Poveda, Lina Maria Saucedo, Edwin Silva, Elisa Estenssoro, Guillermo Ortiz, Nicolas Nin, Alfonso Jose Arango, Alvaro Aguilar, Andrea Lizeth Ayala, Andrea Viviana Bayona, Andrea Lizeth Ayala, Angelica Rodriguez, Carol Viviana Aponte, Carolina Forero-Carreño, Conny Stefanny Muñoz, Cristian Augusto Estrada, Cristopher Romero, Danilo Trujillo, Diego Holguin, Jesus Chavez-Villegas, Faure Rodriguez, Francisco Franco, Hernan Sánchez, Janett Vanessa Moncayo, Jennifer A. Pinedo, Jesica Valeria Bravo, Jose David Cruz, Jose Miguel Angel, Jovany Castro-Lara, Karen Andrea Mantilla, Lorena Garcia, Lorena Pabón, Luis Arturo Lopez, Luis Fernando Mamani, Marisa Lucrecia Yupa, Valeria Catalina Quevedo,
COVID-19 SEMICYUC Investigators:
Andalucía: UCI Hospital Universitario Virgen de Valme (Sevilla): Ana Loza; UCI Hospital Quirón (Huelva): Diego Matallana Zapata; UCI Hospital Universitario Puerto Real (Cádiz): Isabel Díaz Torres, Sonia Ibañez Cuadros, María Recuerda Nuñez, Maria Luz Carmona Pérez, Jorge Gómez Ramos, Alba Villares Casas; UCI Hospital Universitario Virgen de la Macarena (Sevilla): María Luisa Cantón, José Javier González Contreras, Helena Pérez Chomón, Nerissa Alvarez Chicote, Alberto Sousa González; UCI Hospital Universitario Reina Sofía (Córdoba): María De Alba Aparicio, Juan Carlos Pozo Laderas; UCI Hospital Universitario de Jerez (Jerez de la Frontera): Angel Estella, Sara moreno Cano. UCI Hospital Infanta Elena (Huelva): Diego Matallana Zapata.
Aragón: UCI Hospital Nuestra Señora de Gracia (Zaragoza): Ruth Jorge García; UCI Hospital Clínico Universitario Lozano Blesa (Zaragoza): Laura Sánchez Montori, Sandra Herrero García, Paula Abanses Moreno, Carlos Mayordomo García. UCI Hospital General San Jorge(Huesca): Tomás Mallor Bonet, Paula Omedas Bonafonte, Enric Franquesa Gonzalez, Nestor Bueno Vidales, Paula Ocabo Buil, Carlos Serón Arbeloa; UCI Hospital Universitario Miguel Servet(Zaragoza): Isabel Sancho, Pablo Guerrero Ibañez, Pablo Gutierrez, UCI Hospital Obispo Polanco (Teruel): María Concepción Valdovinos, Raquel Canto.UCI Hospital Nuestra Señora de Gracia (Zaragoza): Ruth Jorge García; UCI Hospital Clínico Universitario Lozano Blesa (Zaragoza): Laura Sánchez Montori, Sandra Herrero García, Paula Abanses Moreno, Carlos Mayordomo García. UCI Hospital General San Jorge(Huesca): Tomás Mallor Bonet, Paula Omedas Bonafonte, Enric Franquesa Gonzalez, Nestor Bueno Vidales, Paula Ocabo Buil, Carlos Serón Arbeloa; UCI Hospital Universitario Miguel Servet(Zaragoza): Isabel Sancho, Pablo Guerrero Ibañez, Pablo Gutierrez, UCI Hospital Obispo Polanco (Teruel): María Concepción Valdovinos, Raquel Canto.
Asturias: UCI Hospital Universitario San Agustín (Avilés): Ana Luz Balán Mariño, María José Gutiérrez Fernández, Marta Martín Cuadrado, Belén García Arias; UCI Hospital Universitario Central de Asturias (Oviedo): Lorena Forcelledo Espina, Lucía Viña Soria, Lorena Martín Iglesias, Lucía López Amor, Elisabet Fernández Rey, Emilio García Prieto. UCI Hospital Cabueñes (Gijón): Débora Fernández Ruíz, Carla Martínez González.
Baleares: UCI hospital Universitario Son Llatzer (Palma de Mallorca): Lorenzo Socias, Marcio Borges-Sá, María Aranda Pérez, Antonia Socias. UCI Hospital Quirón Salud Palmaplanas (Palma de Mallorca): José Ma Bonell Goytisolo, Inmaculada Alcalde Mayayo, Carlos Corradini, Isabel Ceniceros, Edwin Rodríguez; UCI Hospital Universitario Son Espases (Palma de Mallorca): Jose Ignacio Ayestarán Rota, Mariana Andrea Novo Novo, Joaquim Colomina Climent, Albert Figueras Castilla, Tomàs Leal Rullan, Maria Magdalena Garcias Sastre; UCI Hospital Comarcal d'Inca(Inca): Rossana Pérez Senoff; UCI Hospital Mateu Orfila (Mao): Ramón Fernández-Cid Bouza.
Canarias: UCI Complejo Hospitalario Universitario Insular—Materno Infantil (Las Palmas de G.C): Juan Carlos Martín González, Carmen Pérez Ortiz, José Luciano Cabrera Santana, Juan José Cáceres Agra, Domingo González Romero, Ana Casamitjana Ortega; UCI Hospital General de la Palma (Tenerife): Luis Alberto Ramos Gómez, Carolina Montelongo Ojeda; UCI Hospital Universitario Dr. Negrín (Las Palmas de G.C): Jordi Solé-Violán.
Cataluña: UCI Hospital Universitari de Tarragona Joan XXIII (Tarragona): Alejandro Rodríguez, María Bodí, Gerard Moreno, Sandra Trefler, Laura Claverias, Raquel Carbonell, Erika Esteve, Montserrat Olona, Xavier Teixidó. UCI Hospital Uni- versitari Arnau de Vilanova (Lleida): Monserrat Vallverdú Vidal, Begoña Balsera Garrido. UCI Hospital Universitari Vall d'Hebron (Barcelona): Elisabeth Papiol Gallofré, Raquel Albertos Martell, Rosa Alcaráz Peñarrocha, Xavier Nuvials Casals, Ricard Ferrer Roca; UCI Hospital Verge de la Cinta (Tortosa): Eric Adrián Mayor Vázquez, Ferrán Roche Campo, Pablo Concha Martínez, Diego Franch Llasat;UCI Hospital del Mar (Barcelona): Joan Ramón Masclanz, Judith Marín- Corral, Purificación Pérez, Rosana Muñoz, Clara Vila; UCI Hospital Mutua de Terrasa (Terrasa): Francisco Javier González de Molina, Elisabeth Navas Moya, Josep Trenado; UCI Hospital Sant Joan (Reus): Imma Vallverdú, Eric Castañé; UCI Hospital Parc Tauli (Sabadell): Emili Díaz Santos, Gemma Goma, Edgar Moglia.
Cantabria: UCI Hospital Universitario Marqués de Valdecillas(Santander): Borja Suberviola.
Castilla La Mancha: UCI Hospital Universitario de Guadalajara (Guadalajara): Antonio Albaya Moreno, Carlos Marian Crespo. UCI Hospital Nuestra Señora del Prado (Toledo): Carmen Carolina Sena Pérez, Francisca Arbol Linde.
Castilla y León: UCI Hospital Virgen de la Concha (Zamora): Diana Monge Donaire, Vega Losada Martínez, Nuria Rodrigo Castroviejo, Gerardo Ferrigno, Reyes Beltrán, Carolina Sanmartino, Concepción Tarancón Maján, Alfredo Marcos Gutiérrez; UCI Complejo Asistencial de Segovia(Segovia): Virginia Hidalgo Valverde, Caridad Martín López; UCI Hospital universitario de Burgos (Burgos): Oihane Badallo, María del Valle Ortiz, Rebeca Vara Arlanzón, David Iglesias Posadilla; UCI Hospital Clínica de Salamanca (Salamanca): María Teresa Recio, Juan Carlos Ballesteros; UCI Complejo Asistencial Universitario de Palencia (Palencia).
Ceuta: UCI Hospital Universitario de Ceuta: Enrique Laza Laza.
Extremadura: UCI Hospital San Pedro de Alcántara (Cáceres): Elena Gallego Curto, Ma Car- men Sánchez García; UCI Hospital de Mérida(Mérida): Miguel Díaz-Tavora, Rosa Mancha.
Galicia: UCI Hospital Montecelo (Pontevedra): Ana Ortega Montes, Isabel Gallego Barbachano, Eva Sanmartín Mantiñán. UCI CHUAC A Coruña (A Coruña): María Lourdes Cordero, Raquel María Rodríguez García, Jorge Gámez Zapata, María Gestal Vázquez. UCI Centro Hospital Universitario de Ferrol (Ferrol): María José Castro Orjales, María Isabel Álvarez Diéguez. UCI Hospitalario Clínico Universitario de Santiago (Santiago de Compostela): Carmen Rivero Velasco, Beatriz Lence Massa; REA CHUAC A Coruña (A Coruña): María Gestal Vázquez. UCI Hospital Lucus Augusti (Lugo): Ignacio Martínez Varela.
Huelva: UCI Hospital Infanta Elena (Huelva): Diego Matallana Zapata.
Madrid: UCI IFEMA (Madrid): Alberto Hernández Tejedor; UCI Hospital Príncipe de Asturias (Madrid): Esther Ma López Ramos, Laura Alcázar Sánchez Elvira, Rocío Molina Montero, Ma Consuelo Pintado Delgado, María Trascasa Muñoz de la Peña, Yaiza Betania Ortiz de Zárate Ansotegui, Alejandra Acha Aranda, Juan Higuera Lucas; UCI Hospital de la Princesa (Madrid): Juan Antonio Sanchez Giralt, Marta Chicot Llano, Nuria Arevalillo Fernández, Marta Sánchez Galindo, Ricardo Andino Ruiz, Alfonso Canabal Berlanga; UCI Hospital Clinico San Carlos (Madrid): Miguel Sánchez, Mercedes Nieto; UCI Hospital HLA la Moncloa(Madrid): Eduardo Arias Sarmiento, Adoración Bueno Blázquez, Rosa María de la Casa, Fátima Martín, Samuel González López.
Murcia: UCI Hospital Morales Meseguer (Murcia): Elena Martínez Quintana, Bernardo Gil Rueda, Áurea Higon Cañigral, Laura López Gómez, Pablo Safwat Bayoumi Delis, Augusto Montenegro Muore, Ángel Andrés Agamez Luengas; UCI Hospital Clínico Universitario Virgen de la Arrixaca (Murcia): Enriqueta Andreu Soler, Ana Beatriz Pérez Pérez, José Higinio de Gea García, Rubén Jara Rubio, Silvia Sánchez Cámara, Alba Moreno Flores, José Moya Sánchez, Daniel Fran- cisco Pérez Martínez,-Ma Desamparados del Rey Carrión; UCI Hospital Reina Sofía (Murcia): María José Rico Lledó, Juana María Serrano Navarro, Juan Fran- cisco Martín Ruíz, Julián Triviño Hidalgo, África López Ferrer, Isabel Cremades Navalón; UCI Hospital Santa Lucía (Cartagena): Josefa Murcia Payá, JM Allegre Gallego; UCI Hospital Rafael Méndez (Lorca): María del Carmen Lorente; UCI Hospital Universitario Mar Menor (San Javier):Marta Gonsalvez.
Navarra: UCI Hospital Reina Sofía (Tudela): Ruth González Natera, Raquel Garrido López de Murillo, Tania Ojuel Gros,Raquel Flecha Viguera, Isabel López González; UCI Hospital García Orcoyen(Estella-Lizarra): Adriana García Herrera.
País Vasco: UCI Hospital Universitario de Donostia (Donostia): Loreto Vidaur Tello, Maialen Aseguinolaza, Itziar Eguibar.
Valencia: UCI Hospital Universitario de La Ribera (Alzira): Asunción Marqués Parra, Sergio García Marti, Alberto Lorenzo Aguilar, Laura Bellver Bosch, Victor Gascón Sanchez, Sonia De la Guía Ortega. UCI Hospital Dr. Peset (Valencia): Martín Parejo Montell, Alberto Belenguer Muncharaz, Hector Hernández Garces, Victor Ramírez Montero, Mónica Crespo Gómez, Verónica Martí Algarra; UCI Hospital Universitari i Politècnic La Fe (Valencia): Susana Sancho Chinesta, Joaquin Arguedas Cervera, Faustino Álvarez Cebrian, Begoña Balerdi Pérez, Rosa Jannone Fores, Javier Botella de Maglia; UCI Hospital Clínico Universitario de Valencia (Valencia): Nieves Carbonell Monleón, Jose Ferreres Franco, Ainhoa Serrano Lazaro, Mar Juan Díaz, María Luisa Blasco Cortés; UCI Hospital Virgen de los Lirios de Alcoy (Alicante): Laura Fayos, Julia Giménez, Gaspar Soriano, Ricardo Navarro. UCI Hospital Arnau de Vilanova (Valencia): Sonia Mas, Elena Bisbal, Laura Albert, Johncard Romero, Juan Fernández Cabreara; UCI Hospital Comarcal de Vinarós (Vinarós): Andrea Ortíz.
Principado de Andorra: ICU Hospital Nostra Señyora de Meritxell (Les Esclades): Antonio Margarit Ribas, Neus Guasch.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcrc.2022.154014.
Appendix A. Supplementary data
Characteristics and outcomes comparing patients with ICU-RTI and no ICU-RTI in all studied cohorts and standardized mean difference between treatment groups of the matching cohort after the PSM.
References
- 1.Group ICC COVID-19 symptoms at hospital admission vary with age and sex: results from the ISARIC prospective multinational observational study. Infection. 2021;49(5):889–905. doi: 10.1007/s15010-021-01599-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrasa H., Rello J., Tejada S., Martin A., Balziskueta G., Vinuesa C., et al. SARS-CoV-2 in Spanish intensive care units: early experience with 15-day survival in Vitoria. Anaesth Crit care Pain Med. 2020;39:553–561. doi: 10.1016/j.accpm.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reyes LF, Murthy S, Garcia-Gallo E, Irvine M, Merson L, Martin-Loeches I, et al. Clinical characteristics, risk factors and outcomes in patients with severe COVID-19 registered in the International Severe Acute Respiratory and Emerging Infection Consortium WHO clinical characterisation protocol: a prospective, multinational, multicentre, observational study. ERJ Open Res. 2022;8(1) doi: 10.1183/23120541.00552-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carson G., Long Covid Forum G Research priorities for long Covid: refined through an international multi-stakeholder forum. BMC Med. 2021;19:84. doi: 10.1186/s12916-021-01947-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aziz S., Arabi Y.M., Alhazzani W., Evans L., Citerio G., Fischkoff K., et al. Managing ICU surge during the COVID-19 crisis: rapid guidelines. Intensive Care Med. 2020;46:1303–1325. doi: 10.1007/s00134-020-06092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grasselli G., Greco M., Zanella A., Albano G., Antonelli M., Bellani G., et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180:1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angus D.C. Optimizing the trade-off between learning and doing in a pandemic. JAMA. 2020;323:1895–1896. doi: 10.1001/jama.2020.4984. [DOI] [PubMed] [Google Scholar]
- 8.Azoulay E., de Waele J., Ferrer R., Staudinger T., Borkowska M., Povoa P., et al. International variation in the management of severe COVID-19 patients. Crit Care. 2020;24:486. doi: 10.1186/s13054-020-03194-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Group RC, Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rouze A., Martin-Loeches I., Povoa P., Makris D., Artigas A., Bouchereau M., et al. Relationship between SARS-CoV-2 infection and the incidence of ventilator-associated lower respiratory tract infections: a European multicenter cohort study. Intensive Care Med. 2021;47:188–198. doi: 10.1007/s00134-020-06323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coopersmith C.M., Antonelli M., Bauer S.R., Deutschman C.S., Evans L.E., Ferrer R., et al. The surviving sepsis campaign: research priorities for coronavirus disease 2019 in critical illness. Crit Care Med. 2021;49:598–622. doi: 10.1097/CCM.0000000000004895. [DOI] [PubMed] [Google Scholar]
- 12.Moreno G., Rodriguez A., Reyes L.F., Gomez J., Sole-Violan J., Diaz E., et al. Corticosteroid treatment in critically ill patients with severe influenza pneumonia: a propensity score matching study. Intensive Care Med. 2018;44:1470–1482. doi: 10.1007/s00134-018-5332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez A., Ruiz-Botella M., Martin-Loeches I., Jimenez Herrera M., Sole-Violan J., Gomez J., et al. Deploying unsupervised clustering analysis to derive clinical phenotypes and risk factors associated with mortality risk in 2022 critically ill patients with COVID-19 in Spain. Crit Care. 2021;(25):63. doi: 10.1186/s13054-021-03487-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalil A.C., Metersky M.L., Klompas M., Muscedere J., Sweeney D.A., Palmer L.B., et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torres A., Sibila O., Ferrer M., Polverino E., Menendez R., Mensa J., et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA. 2015;313:677–686. doi: 10.1001/jama.2015.88. [DOI] [PubMed] [Google Scholar]
- 16.Chaudhuri D., Sasaki K., Karkar A., Sharif S., Lewis K., Mammen M.J., et al. Corticosteroids in COVID-19 and non-COVID-19 ARDS: a systematic review and meta-analysis. Intensive Care Med. 2021;47:521–537. doi: 10.1007/s00134-021-06394-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Paassen J., Vos J.S., Hoekstra E.M., Neumann K.M.I., Boot P.C., Arbous S.M. Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Crit Care. 2020;24:696. doi: 10.1186/s13054-020-03400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasan S.S., Capstick T., Ahmed R., Kow C.S., Mazhar F., Merchant H.A., et al. Mortality in COVID-19 patients with acute respiratory distress syndrome and corticosteroids use: a systematic review and meta-analysis. Expert Rev Respir Med. 2020;14:1149–1163. doi: 10.1080/17476348.2020.1804365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Group WHOREAfC-TW, Sterne J.A.C., Murthy S., Diaz J.V., Slutsky A.S., Villar J., et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angus D.C., Derde L., Al-Beidh F., Annane D., Arabi Y., Beane A., et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020;324:1317–1329. doi: 10.1001/jama.2020.17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luyt C.E., Sahnoun T., Gautier M., Vidal P., Burrel S., Pineton de Chambrun M., et al. Ventilator-associated pneumonia in patients with SARS-CoV-2-associated acute respiratory distress syndrome requiring ECMO: a retrospective cohort study. Ann Intensive Care. 2020;10:158. doi: 10.1186/s13613-020-00775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalanuria A.A., Ziai W., Mirski M. Ventilator-associated pneumonia in the ICU. Crit Care. 2014;18:208. doi: 10.1186/cc13775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blot S., Koulenti D., Dimopoulos G., Martin C., Komnos A., Krueger W.A., et al. Prevalence, risk factors, and mortality for ventilator-associated pneumonia in middle-aged, old, and very old critically ill patients*. Crit Care Med. 2014;42:601–609. doi: 10.1097/01.ccm.0000435665.07446.50. [DOI] [PubMed] [Google Scholar]
- 24.Villafuerte D., Aliberti S., Soni N.J., Faverio P., Marcos P.J., Wunderink R.G., et al. Prevalence and risk factors for Enterobacteriaceae in patients hospitalized with community-acquired pneumonia. Respirology. 2020;25:543–551. doi: 10.1111/resp.13663. [DOI] [PubMed] [Google Scholar]
- 25.Gramegna A., Sotgiu G., Di Pasquale M., Radovanovic D., Terraneo S., Reyes L.F., et al. Atypical pathogens in hospitalized patients with community-acquired pneumonia: a worldwide perspective. BMC Infect Dis. 2018;18:677. doi: 10.1186/s12879-018-3565-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics and outcomes comparing patients with ICU-RTI and no ICU-RTI in all studied cohorts and standardized mean difference between treatment groups of the matching cohort after the PSM.