Abstract
Introduction:
Demonstrating human papillomavirus (HPV) vaccine impact is critical for informing guidelines to increase vaccination coverage and decrease HPV-related outcomes, particularly in states with suboptimal vaccination coverage, such as Tennessee. This study examines HPV vaccine impact among Tennessee Medicaid (TennCare) enrollees by assessing trends in high-grade cervical lesion incidence among all women aged 18–39 years and the subset of women who were screened for cervical cancer.
Methods:
Using a validated claims-based model to identify incident cervical intraepithelial neoplasia Grades 2 or 3 or adenocarcinoma in situ (CIN2+) events, annual age group–specific incidence rates from TennCare billing data, 2008–2018, were calculated. Significant trends, annual percentage changes, and average annual percentage changes (AAPCs) were determined by Joinpoint. Analyses were conducted in 2020.
Results:
From 2008 to 2018, CIN2+ incidence significantly declined in women aged 18–20 years (AAPC= −31.9, 95% CI= −38.6, −24.6), 21–24 years (AAPC= −12.9, 95% CI= −22.3, −2.4), and 25–29 years (AAPC= −6.4, 95% CI= −8.1, −4.6). Among screened women, CIN2+ incidence significantly declined for ages 18–20 years (AAPC= −20.3, 95% CI= −25.3, −15.0), 21–24 years (AAPC= −10.2, 95% CI= −12.6, −7.8), and 25–29 years (AAPC= −2.6, 95% CI= −3.9, −1.2). Trends from 2008 to 2018 were stable for older age groups (30–34 and 35–39 years).
Conclusions:
Results from this ecologic study show reduced CIN2+ incidence among ages most likely to have benefited from the HPV vaccine. Declines among young, screened women suggest causes other than the reduction in screening. Evidence of HPV vaccine impact in populations with low vaccination coverage, such as Tennessee, is promising.
INTRODUCTION
Cervical cancer and precancer (high-grade cervical lesions) are preventable outcomes and associated with considerable costs, including premature death, direct medical expenses, and loss of productivity.1–3 Roughly 90% of cervical cancer and 76% of high-grade cervical lesions are attributable to human papillomavirus (HPV) types 16/18/31/33/44/53/58, which are all covered by the nonavalent HPV vaccine.4–6 Despite the Advisory Committee on Immunization Practices’ recommendations for routine adolescent HPV vaccination,7 HPV vaccination lags behind other adolescent vaccines in the U.S.8 In 2019, HPV vaccination among U.S. adolescents aged 13–17 years was 72% for initiation (≥1 dose) and 54% for up to date (all recommended doses), with large variation across states, ranging from 49% to 92% for initiation and 31% to 79% for up-to-date coverage.8
Tennessee consistently ranks in the lowest quartile for HPV vaccination, with initiation coverage of 62% and up-to-date coverage of 43%, among adolescents aged 13–17 years in 2019.8,9 Understanding the HPV vaccine’s impact is critical for informing guidelines to increase vaccination coverage and decrease cervical cancer and precancer incidence, particularly for states with suboptimal vaccination coverage, such as Tennessee.
Timing of the vaccine’s approval, changes in vaccine recommendations, and vaccine uptake are important considerations in assessing vaccine impact. Changes in cervical cancer screening and management guidelines complicate this assessment. Specifically, updated screening guidelines in 2012 by the American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology recommended against screening before age 21 years.10 For women aged <25 years, management guidelines include reserving colposcopies with a biopsy for high-grade abnormalities or low-grade abnormalities persisting for ≥2 years.11 These updates contribute to changes in disease detection, an important consideration when interpreting trends.
Although the median latency between initial HPV exposure and development of cervical cancer is 7–12 years,12 cervical precancers, including cervical intraepithelial neoplasia (CIN) Grades 2 and 3 and adenocarcinoma in situ (collectively, CIN2+), may be detected within a few years of infection.13,14 Thus, CIN2+ has been used to monitor HPV vaccine impact as an intermediate outcome for cancer.15–19 Studies have shown notable decreases in CIN2+ incidence among ages most likely to have benefited from the HPV vaccine15–19; however, few of these studies have focused on populations with suboptimal vaccination coverage.
Only 1 study, which used data from the New Mexico HPV Pap registry, has assessed the HPV vaccine’s impact on reducing CIN2+ incidence in a population with low vaccination coverage.19 Similar ecologic studies documenting vaccine impact on CIN2+ have been conducted by the HPV Vaccine Impact Monitoring Project (HPV-IMPACT), a surveillance program that began in 2008 and is funded by the Centers for Disease Control and Prevention in 5 catchment areas across the U.S.20 However, these studies are limited to areas with adequate population-based cervical biopsy data from partnering institutions within the program and do not specifically focus on assessing vaccine impact in low-vaccination populations.
A surrogate metric for capturing CIN2+ events may be insurance billing claims. Only 1 U.S. study has used claims to examine CIN2+ trends from 2007 to 2014.21 Utilizing recent claims data to assess trends has been limited by the transition from ICD-9 to ICD-10 in 2015.22 A claims-based model for capturing CIN2+ events in both ICD eras was recently validated to expand CIN2+ surveillance to states without access to population-based cervical biopsy data.23 Thus, the present study aims to examine the HPV vaccine’s impact in a state with suboptimal vaccination coverage by assessing trends in CIN2+ incidence from 2008 to 2018 among women aged 18–39 years enrolled in Tennessee Medicaid (TennCare) and the subset who were screened for cervical cancer, accounting for changes in screening patterns over time.
METHODS
Study Population
TennCare claims data were used to identify women aged 18–39 years with ≥1 year of consecutive enrollment, 2008–2018. The annual subpopulation of women screened for cervical cancer were identified by having ≥1 billing code for: HPV screening examination, Pap smear/test, or HPV DNA test (Appendix Table 1 provides specific codes). Ages 18–39 years were chosen to compare with studies examining HPV vaccine impact on CIN2+. Further, women in this age range (particularly 20–39 years) have the highest CIN2+ rates compared with older women,6 and meaningful vaccine impact in older women is not expected during the study period owing to the timing of vaccine introduction and age ineligibility. This study was considered public health surveillance and exempt by IRBs at Vanderbilt University and the Tennessee Department of Health. This research activity was reviewed and approved by the Tennessee Department of Finance and Administration Division of TennCare.
Measures
This study defined CIN2+ as CIN2, CIN3, or adenocarcinoma in situ. A validated claims-based model23 was used to identify CIN2+ events from 2008 to 2018. The validated model was built by least absolute shrinkage and selection operator logistic regression and used a linear combination of diagnosis, screening, and treatment codes to calculate prediction scores among women with cervical diagnostic procedures (Appendix Table 1 provides β coefficients and prediction score equation). Predicted probabilities were then generated using the following equation: . Finally, CIN2+ events were defined as a woman’s first event with a predicted probability ≥0.5.
For model-identified CIN2+ events, the corresponding diagnostic procedure date was a proxy for diagnosis date because not all events had a specific ICD diagnosis code for CIN2+. Events were only counted among women with ≥1 year of consecutive TennCare enrollment from their diagnostic procedure date. For the subpopulation of screened women, model-identified CIN2+ events were only counted if the screening date was within 1 year prior to the diagnostic procedure date. Incident events were defined as those among women who were event free for ≥1 year prior to their diagnostic procedure. Additional information regarding model building and validation has been described previously.23
Statistical Analysis
Assuming the occurrence of events, additions, and losses was homogenously distributed, annual (2008–2018) person-time was estimated by counting the annual number of women enrolled in TennCare on July 1 with ≥1 year of consecutive enrollment, stratified by age group (18–20, 21–24, 25–29, and 30–39 years). To estimate annual person-time for the subpopulation of screened women, women enrolled in TennCare on July 1 of each year with ≥1 year of consecutive enrollment who were screened for cervical cancer within 1 year prior to the current year (person-time estimation for 2008 included women screened between July 1, 2007 and July 1, 2008) were counted, stratified by age group.
Annual incidence rates were calculated by dividing the number of women meeting the incident CIN2+ case definition by the total person-time for each year and age group and multiplying by 100,000 to express incidence per 100,000 person-years. Joinpoint Desktop Software, version 4.5.0.124 was used to identify significant trends, determined by the best fitting log-linear model with the fewest inflection years. Annual percentage changes (APCs, β-coefficients for each trend) and average APCs (AAPCs, weighted averages of APCs before and after the detected inflection year) were estimated using permutation tests with Poisson variance. With a threshold 2-sided α of 0.05, 95% CIs that excluded 0 were considered statistically significant. All analyses were conducted in 2020.
RESULTS
A total of 549,671 TennCare-enrolled women aged 18–39 years contributed 2.3 million person-years of data over 11 years (2008–2018). Of those with known demographics, 34.1% were White, 65.2% were aged 25–39 years, and 72.9% lived in a metropolitan statistical area (Table 1). Among all TennCare-enrolled women aged 18–39 years, annual screening for cervical cancer decreased from 2008 to 2018 (40.9% to 25.4%), with largest declines among ages 18–20 years (46.0% to 12.4%) and 21–24 years (49.2% to 30.1%) (Appendix Table 2). Among screened women, the distribution of ages 18–20 and 21–24 years decreased from 2008 to 2018 (20.4% to 7.3% for 18–20 years, and 24.8% to 18.3% for 21–24 years) (Table 1).
Table 1.
Characteristics of TennCare-Enrolled Women Aged 18‒39 Years With at Least 1-year of Consecutive Enrollment From 2008‒2018 and Among Those Screened for Cervical Cancer
| Characteristic | Year | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall % | 2008 % | 2009 % | 2010 % | 2011 % | 2012 % | 2013 % | 2014 % | 2015 % | 2016 % | 2017 % | 2018 % | |
| All women | ||||||||||||
| Total PY, n | 2,332,477 | 164,695 | 166,528 | 179,207 | 185,209 | 185,571 | 184,990 | 204,357 | 263,169 | 300,837 | 249,254 | 248,660 |
| Age group, years | ||||||||||||
| 18–20 | 16.7 | 18.1 | 19.1 | 19.1 | 18.4 | 17.0 | 16.3 | 16.2 | 15.9 | 15.3 | 15.5 | 14.9 |
| 21–24 | 18.2 | 20.7 | 19.3 | 19.7 | 18.7 | 18.1 | 17.8 | 18.0 | 18.9 | 19.1 | 15.5 | 15.5 |
| 25–29 | 24.1 | 23.6 | 23.6 | 23.4 | 24.0 | 24.5 | 24.7 | 24.7 | 24.3 | 24.1 | 23.4 | 24.1 |
| 30–34 | 22.2 | 19.5 | 20.2 | 20.6 | 21.7 | 22.7 | 23.2 | 22.9 | 22.3 | 22.3 | 23.8 | 23.8 |
| 35–39 | 18.9 | 18.1 | 17.9 | 17.2 | 17.1 | 17.7 | 18.1 | 18.3 | 18.6 | 19.1 | 21.8 | 21.7 |
| Race | ||||||||||||
| White | 34.1 | 41.1 | 39.8 | 39.4 | 37.8 | 36.0 | 33.7 | 31.2 | 29.6 | 28.9 | 31.2 | 33.9 |
| Black | 17.5 | 23.7 | 22.9 | 21.7 | 20.6 | 19.7 | 18.0 | 15.8 | 14.5 | 13.9 | 14.0 | 14.9 |
| Hispanic | 0.7 | 0.7 | 0.7 | 0.8 | 0.8 | 0.8 | 0.7 | 0.7 | 0.6 | 0.6 | 0.7 | 0.8 |
| Other/Unknown | 47.7 | 34.4 | 36.6 | 38.2 | 40.8 | 43.5 | 47.5 | 52.3 | 55.2 | 56.6 | 54.2 | 50.4 |
| Urbanicitya | ||||||||||||
| MSA | 72.9 | 72.8 | 72.9 | 72.9 | 73.2 | 73.4 | 73.2 | 73.0 | 72.9 | 72.9 | 72.4 | 72.1 |
| Non-MSA | 27.1 | 27.1 | 26.9 | 27.0 | 26.7 | 26.5 | 26.7 | 27.0 | 27.1 | 27.1 | 27.5 | 27.9 |
| Women screened for cervical cancer | ||||||||||||
| Total PY, n | 759,269 | 67,322 | 70,633 | 74,885 | 72,018 | 67,947 | 62,683 | 62,908 | 72,928 | 79,327 | 65,500 | 63,118 |
| Age group, years | ||||||||||||
| 18–20 | 13.8 | 20.4 | 20.5 | 20.1 | 17.6 | 14.3 | 12.5 | 10.9 | 9.9 | 8.8 | 8.5 | 7.3 |
| 21–24 | 22.2 | 24.8 | 23.1 | 23.7 | 23.1 | 22.7 | 22.2 | 21.9 | 22.6 | 22.5 | 18.8 | 18.3 |
| 25–29 | 26.9 | 25.1 | 25.1 | 24.6 | 25.9 | 27.2 | 27.8 | 28.2 | 28.1 | 28.1 | 27.9 | 29.0 |
| 30–34 | 21.4 | 16.6 | 18.0 | 18.6 | 20.3 | 21.8 | 22.7 | 23.1 | 22.6 | 23.2 | 24.6 | 25.1 |
| 35–39 | 15.6 | 13.1 | 13.3 | 13.0 | 13.0 | 14.0 | 14.8 | 16.0 | 16.8 | 17.4 | 20.2 | 20.3 |
| Race | ||||||||||||
| White | 31.3 | 37.1 | 35.8 | 35.5 | 33.4 | 31.3 | 29.0 | 27.5 | 27.5 | 27.2 | 28.7 | 30.9 |
| Black | 19.9 | 23.8 | 23.2 | 22.2 | 21.8 | 21.1 | 20.0 | 18.5 | 17.0 | 16.9 | 16.8 | 16.9 |
| Hispanic | 0.7 | 0.6 | 0.6 | 0.7 | 0.7 | 0.7 | 0.6 | 0.6 | 0.7 | 0.6 | 0.7 | 0.8 |
| Other/Unknown | 48.2 | 38.5 | 38.5 | 41.6 | 44.1 | 47.0 | 50.4 | 53.4 | 54.8 | 55.3 | 53.8 | 51.4 |
| Urbanicitya | ||||||||||||
| MSA | 74.2 | 73.4 | 73.4 | 73.6 | 74.0 | 74.7 | 75.0 | 74.9 | 74.5 | 74.3 | 74.2 | 73.7 |
| Non-MSA | 25.8 | 26.5 | 26.6 | 26.4 | 26.0 | 25.3 | 25.0 | 25.1 | 25.5 | 25.6 | 25.8 | 26.3 |
Urbanicity was categorized by county of residence using MSA definitions and boundaries set by the U.S. Census Bureau, which classifies MSAs as counties associated with at least one urbanized area that has a population of at least 50,000 persons.
MSA, metropolitan statistical area; TennCare, Tennessee Medicaid; TN, Tennessee; PY, person-years.
Among all TennCare-enrolled women, from 2008 to 2018, CIN2+ incidence was highest for ages 21–24 years (646.4/100,000 person-years) and 25–29 years (666.1/100,000 person-years) (Table 2, Figure 1). Across the 11-year study period, steepest declines in CIN2+ incidence were in the youngest age group, 18–20 years, from 720.1/100,000 person-years in 2008 to 24.2/100,000 person-years in 2018 (AAPC= −31.9, 95% CI= −38.6, −24.6) (Tables 2 and 3, Figure 1). Significant declines in CIN2+ incidence were observed among women aged 21–24 years, from 1,193.6/100,000 person-years in 2008 to 293.7/100,000 person-years in 2018 (AAPC= −12.9, 95% CI= −22.3, −2.4). Women aged 25–29 years experienced a smaller, yet significant decline in incidence, from 845.7/100,000 person-years in 2008 to 526.6/100,000 person-years in 2018 (AAPC= −6.4, 95% CI= −8.1, −4.6). Among older ages (30–34 years and 35–39 years), trends in CIN2+ incidence from 2008 to 2018 were not significant.
Table 2.
Annual Age-Group-Specific CIN2+ Incidencea Among TennCare-Enrolled Women and Those Screened for Cervical Cancer, 2008‒2018
| Year | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age group, years | Overall IR | 2008 IR | 2009 IR | 2010 IR | 2011 IR | 2012 IR | 2013 IR | 2014 IR | 2015 IR | 2016 IR | 2017 IR | 2018 IR |
| All women | ||||||||||||
| 18–20 | 244.6 | 720.1 | 693.1 | 557.7 | 317.3 | 294.6 | 146.3 | 93.6 | 59.7 | 13.0 | 20.7 | 24.2 |
| 21–24 | 646.4 | 1193.6 | 1020.3 | 1118.2 | 939.3 | 835.0 | 721.1 | 444.1 | 396.3 | 302.4 | 310.4 | 293.7 |
| 25–29 | 666.1 | 845.7 | 913.9 | 880.7 | 754.8 | 791.9 | 752.1 | 569.5 | 540.3 | 556.7 | 493.6 | 526.6 |
| 30–34 | 463.1 | 487.9 | 598.7 | 611.9 | 539.6 | 518.3 | 494.2 | 432.1 | 411.1 | 361.9 | 411.3 | 409.1 |
| 35–39 | 313.9 | 334.9 | 402.9 | 380.2 | 378.1 | 332.0 | 308.4 | 270.0 | 273.8 | 273.5 | 287.5 | 306.1 |
| Women screened for cervical cancer | ||||||||||||
| 18–20 | 877.1 | 1,485.5 | 1,495.9 | 1,268.5 | 787.7 | 924.5 | 562.4 | 406.6 | 333.9 | 71.5 | 144.0 | 173.5 |
| 21–24 | 1,521.0 | 2,230.1 | 1,909.1 | 2,173.9 | 1,855.1 | 1,726.3 | 1,625.3 | 1,126.9 | 1,110.2 | 879.4 | 812.0 | 864.6 |
| 25–29 | 1,716.4 | 1,868.4 | 1,955.1 | 1,930.4 | 1,718.6 | 1,833.2 | 1,877.7 | 1,496.1 | 1,572.1 | 1,649.0 | 1,448.9 | 1,580.5 |
| 30–34 | 1,374.8 | 1,318.6 | 1,493.1 | 1,501.1 | 1,405.0 | 1,406.1 | 1,398.0 | 1,309.5 | 1,412.5 | 1,201.7 | 1,373.7 | 1,353.2 |
| 35–39 | 1,076.1 | 1,067.9 | 1,196.3 | 1,137.4 | 1,138.7 | 1,071.7 | 956.6 | 985.5 | 1,026.2 | 1,055.2 | 1,033.9 | 1,175.9 |
Incidence rates are expressed per 100,000 person-years.
CIN, cervical intraepithelial lesion; IR, incidence rate; TennCare, Tennessee Medicaid.
Figure 1.
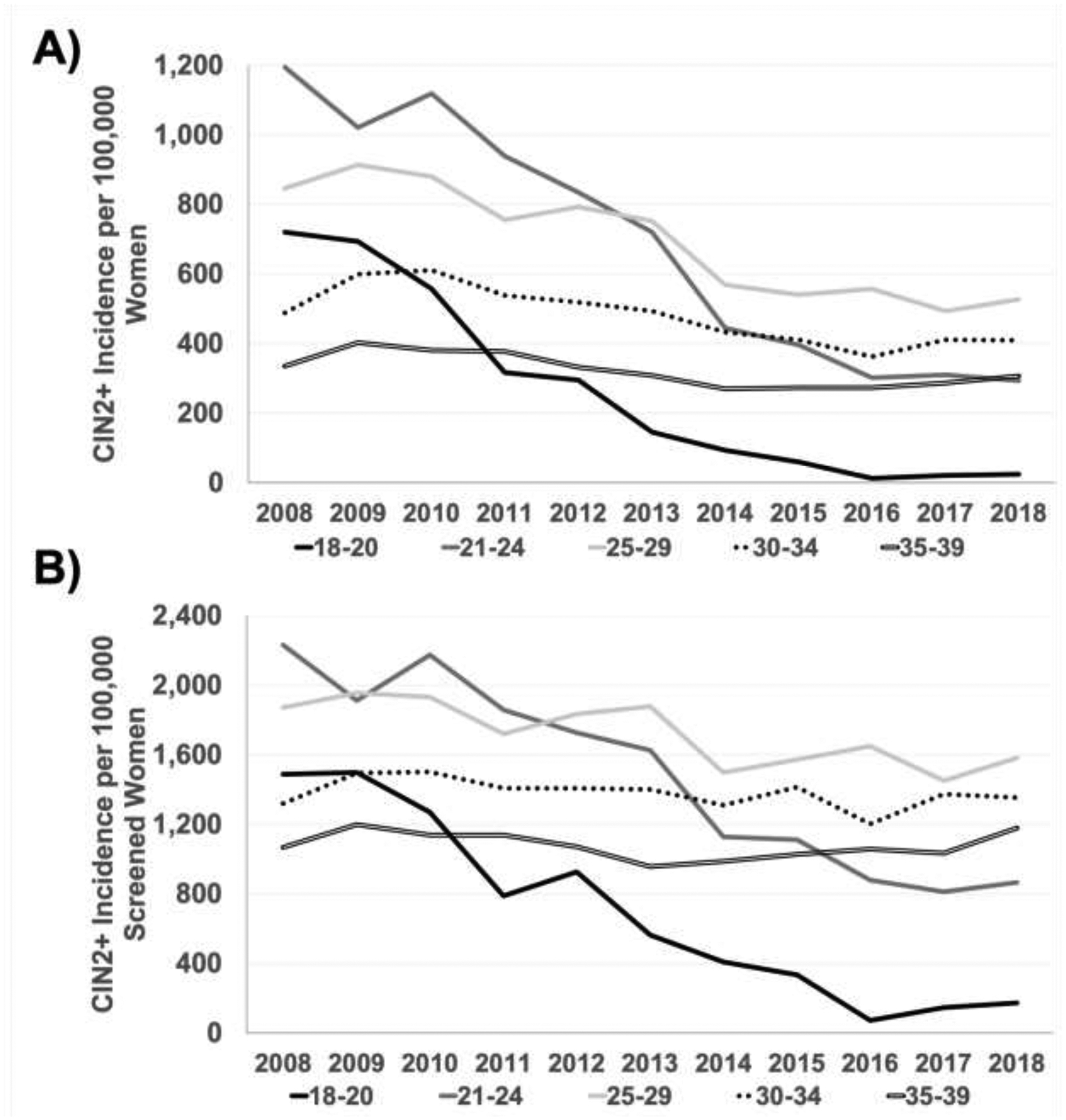
Age-group-specifica annual CIN2+ incidence among (A) TennCare-enrolled women and (B) TennCare-enrolled women screened for cervical cancer, 2008‒2018.
aAge groups are expressed in years.
CIN, cervical intraepithelial neoplasia; TennCare, Tennessee Medicaid.
Table 3.
Age-Group-Specific Trends in CIN2+ Incidence Among TennCare-Enrolled Women and Those Screened for Cervical Cancer, 2008‒2018
| Age group, years | Average annual percent changea | Annual percent changeb | |||
|---|---|---|---|---|---|
| Time period | AAPC (95% CI) | Inflection year | Time period | APC (95% CI) | |
| All women | |||||
| 18–20 | 2008–2018 | −31.9 (−38.6, −24.6) | 2010 | 2008–2010 | −12.0 (−40.2, 29.6) |
| 18–20 | 2010–2018 | −36.2 (−43.8, −27.5) | |||
| 21–24 | 2008–2018 | −12.9 (−22.3, −2.4) | 2012 | 2008–2012 | −7.4 (−18.5, 5.3) |
| 21–24 | 2016 | 2012–2016 | −23.6 (-41.3, −0.6) | ||
| 21–24 | 2016–2018 | 0.2 (−51.1, 105.4) | |||
| 25–29 | 2008–2018 | −6.4 (−8.1, −4.6) | – | – | – |
| 30–34 | 2008–2018 | −2.0 (−4.4, 0.6) | 2010 | 2008–2010 | 9.8 (−4.9, 26.7) |
| 30–34 | 2016 | 2010–2016 | −7.9 (−10.6, −5.3) | ||
| 30–34 | 2016–2018 | 5.9 (−6.3, 19.6) | |||
| 35–39 | 2008–2018 | −1.6 (−3.7, 0.6) | 2010 | 2008–2010 | 8.0 (−3.9, 21.4) |
| 35–39 | 2014 | 2010–2014 | −9.8 (−14.9, −4.4) | ||
| 35–39 | 2014–2018 | 2.6 (−0.6, 5.6) | |||
| Women screened for cervical cancer | |||||
| 18–20 | 2008–2018 | −20.3 (−25.3, −15.0) | – | – | – |
| 21–24 | 2008–2018 | −10.2 (−12.6, −7.8) | – | – | – |
| 25–29 | 2008–2018 | −2.6 (−3.9, −1.2) | – | – | – |
| 30–34 | 2008–2018 | −1.0 (−2.2, 0.3) | – | – | – |
| 35–39 | 2008–2018 | −0.4 (−1.9, 1.1) | – | – | – |
Notes: Boldface indicates statistical significance (p<0.05).
Average annual percent changes are weighted averages of the annual percent changes of all time periods or the average annual percent changes across the entire 11-year study period.
Annual percent changes were determined by the β-coefficient of the best fit log-linear model using a permutation test and Poisson variance; if no inflection year was detected, then only the AAPC is reported because the AAPC is equal to the APC.
AAPC, average annual percent change; APC, annual percent change; CIN, cervical intraepithelial lesion; TennCare, Tennessee Medicaid.
The APCs for trend segments were determined after identifying none, 1, or 2 Joinpoint-detected inflections (Table 3, Figure 1). Among women aged 18–20 years, declines in CIN2+ incidence were not significant until 2010 (APC [2010–2018]= −36.2, 95% CI= −43.8, −27.5). Among women aged 21–24 years, CIN2+ incidence significantly declined from 2012 to 2016 (APC= −23.6, 95% CI= −41.3, −0.6), but was stable from 2016 to 2018. Among women aged 30–34 years, CIN2+ incidence initially non-significantly increased from 2008 to 2010, then significantly declined from 2010 to 2016 (APC= −7.9, 95% CI= −10.6, −5.3), followed by a stable trend from 2016 to 2018. Similarly, among women aged 35–39 years, CIN2+ incidence non-significantly increased from 2008 to 2010, then significantly decreased (APC= −9.8, 95% CI= −14.9, −4.4) from 2010 to 2014, followed by a stable trend from 2014 to 2018.
The CIN2+ incidence from 2008 to 2018 among screened women was highest for ages 21–24 years (1,521.0/100,000 person-years) and 25–29 years (1,716.4/100,000 person-years) (Table 2, Figure 1). CIN2+ trends across the 11-year study period (2008–2018) among screened women mirrored that of all women—steepest declines were in the youngest age group, from 1,485.5/100,000 person-years to 173.5/100,000 person-years (AAPC= −20.3, 95% CI= −25.3, −15.0) (Tables 2 and 3, Figure 1). Significant declines were observed for ages 21–24 years, from 2,230.1/100,000 person-years in 2008 to 864.6/100,000 person-years in 2018 (APC= −10.2, 95% CI= −12.6, −7.8), and 25–29 years, from 1,868.4/100,000 person-years in 2008 to 1,580.5/100,000 person-years in 2018 (APC= −2.6, 95% CI= −3.9, −1.2). CIN2+ incidence among older age groups (30–34 years and 36–39 years) was not significant. Among screened women, no significant trend shifts (joinpoints) were detected for any age group.
DISCUSSION
In a state with suboptimal HPV vaccination coverage, such as Tennessee (62% initiation and 43% up-to-date vaccination in 2019 among adolescents aged 13–17 years),8,9 reductions in CIN2+ incidence coinciding with the HPV vaccine’s introduction were observed among TennCare-enrolled women. Most notable declines were in young women aged 18–20 years, an age group most likely to have benefited from the vaccine’s approval in 2006 and the Advisory Committee on Immunization Practices’ recommendations for routine HPV vaccination among adolescents aged 11–12 years.25 Declines were also observed among women aged 21–24 and 25–29 years, whereas stable trends were detected among older women in age groups that were less likely to have benefited from the HPV vaccine (30–34 and 35–39 years). After restricting to women screened for cervical cancer, declines were still observed in young women (aged 18–20, 21–24, and 25–29 years), suggesting these declines were not simply due to detecting less CIN2+ incidence from decreased screening. Though management guidelines may have reduced CIN2+ detection in women aged 18–24 years, decreases in screened women aged 25–29 years for whom management guidelines did not change suggest that decreases in observed disease are from decreased incidence rather than decreased detection.
Cervical cancer screening and management patterns have changed over time, contributing to changes in the detection of CIN2+. In the study, decreases in screening proportions for all age groups were observed. Declines were largest in women aged <21 years, which were expected given updates in cervical screening guidelines. Prior to 2009, cervical cancer screening was recommended for women after sexual debut, regardless of age; however, in 2009, the American College of Obstetricians and Gynecologists recommended screening to begin at age 21 years.26 Because HPV infections are common and typically clear spontaneously within 24 months of infection,27,28 guidelines were updated again in 2012 to recommend against screening for women aged <21 years to protect young women from unnecessary invasive procedures that could put them at risk for cervical damage.10 Additionally, since 2012, management guidelines for women aged <25 years changed in that colposcopy with biopsy was recommended only for high-grade abnormalities or low-grade abnormalities persisting for ≥2 years, leading to decreased diagnoses of CIN2+ in young women.11 For women aged ≥21 years, cervical cancer screening guidelines also changed in 2012, including recommendations for less frequent screening, such as every 3 or 5 years depending on age, medical history, and screening modality.10 For women aged ≥30 years, the preferred screening method was co-testing with a Pap and HPV DNA test (a more sensitive test), instead of Pap alone,10 which may have contributed to more disease detection in older women. Thus, changes in screening and management patterns should be considered when interpreting trends in CIN2+ incidence.
To account for screening and management changes over time, age group–specific CIN2+ incidence among the subpopulation of women screened for cervical cancer was examined. Among screened women aged 18–20, 21–24, and 25–29 years, declining trends in CIN2+ incidence were statistically significant, yet less pronounced, compared with declines among all women; this is likely because trends among all women are due to both declines in screening and HPV vaccine impact, whereas trends among screened women removes the confounding effect of declines in screening.
Declines in CIN2+ incidence were expected among younger women, particularly because of increasing HPV vaccination trends in Tennessee. In 2019, the HPV vaccine initiation coverage among female Tennessee residents aged 13–17 years was 70%, versus just 30% in 2008.8 Conversely, stable trends in CIN2+ incidence were expected among older women because of vaccine ineligibility at the time of the vaccine’s first approval in 2006,25 and because of low HPV vaccination coverage among even age-eligible adults (aged <26 years).29 In 2019, the Advisory Committee on Immunization Practices recommended that patients aged 27–45 years who are at risk for a new HPV infection may consider getting the vaccine through shared decision making with their provider.30 Therefore, continued monitoring of the impact of vaccination on HPV-related health outcomes is warranted, particularly in older age groups.
The results corroborate findings of other ecologic studies demonstrating HPV vaccine impact on reducing cervical precancers in population-based surveillance sites across the U.S., which also reported significant declines in CIN2+ incidence among young women and no significant declines among older women.6,16–19,31 Compared with populations with cervical biopsy data, similar declining CIN2+ incidence was observed in this study using a validated claims-based model. HPV-IMPACT monitoring project’s most recent analysis6 reported average annual decreases in CIN2+ incidence of 38% and 15% among women aged 18–19 and 20–24 years, respectively, from 2008 to 2016, compared with average annual decreases of 32% and 13% among women aged 18–20 and 21–24 years, respectively, from 2008 to 2018 in this study.
Data from the HPV-IMPACT monitoring project also showed decreases in CIN2+ incidence among women aged 25–29 years from 2008 to 2016; however, declines were not significant.6 With the inclusion of more recent data through 2018, the present study observed significant declines in CIN2+ incidence among women aged 25–29 years. As younger cohorts begin to age and vaccination rates continue to rise, increased evidence of the HPV vaccine’s impact may continue to show, as demonstrated by the findings from this study.
This is the first U.S. claims-based study to examine CIN2+ incident trends across ICD-9 and ICD-10 eras using a validated model and aggregated at the state-level. Prior claims-based studies were unable to assess trends past 201521 because the transition from ICD-9 to ICD-10 occurred on October 1, 2015 and the discriminative ability to identify CIN2+ events across eras was unknown. Since then, a validated claims-based CIN2+ model for both ICD eras was validated, reporting no significant differences in performance by era; therefore, more recent CIN2+ data were included in this analysis to expand prior research and CIN2+ surveillance. Moreover, utilizing claims-based data is an efficient method to monitor HPV vaccine impact through capturing frequency of clinical events such as CIN2+. This study is one of the first to examine CIN2+ incident trends in a U.S. population outside of the catchment areas with population-based cervical biopsy data from the New Mexico HPV Pap registry and the HPV-IMPACT monitoring project, where the most recent CIN2+ trends have only been analyzed through 2016.
Limitations
Individual-level vaccination data were not included to be able to capture both direct effects (from vaccination) and indirect effects (less transmission from vaccinated people resulting in “herd effects”) of the vaccine, which would only be observable through an ecologic perspective. Additionally, cervical cancer rates were not examined because ICD-9 and ICD-10 claims codes for this have not been validated. Because this was an ecologic analysis, concluding that CIN2+ incident trends were directly due to vaccination is not definitive; however, the timing of the declines and correlative results from different age groups imply the HPV vaccine’s impact on the population. This study only included women enrolled in the Tennessee Medicaid program; thus, results may not be generalizable to populations with different sociodemographic characteristics or populations in other regions. Because of eligibility for the Vaccines for Children program among low-income individuals, adolescents insured by Medicaid have historically had higher vaccination coverage compared with the general public32; thus, evidence of HPV vaccine impact on reducing CIN2+ incidence may be more prominent among the Medicaid population compared with the general population. Further, cervical cancer screening frequency and screening type may differ in TennCare compared with the general population, contributing to differences in disease detection rates. Finally, other factors may impact CIN2+ trends other than screening or direct vaccination, including behavioral factors (condom use and sexual activity trends), herd immunity, HPV type acquisition, and clearance rates.33–35
CONCLUSIONS
Both HPV vaccination and cervical cancer screening are approaches to decrease incidence of cervical precancers. The lack of vaccine impact in older women and declines in appropriate cervical cancer screening in older women reinforces the need for screening women who are at highest risk for cervical cancer. Finally, HPV vaccination coverage remains low in certain states. However, the results provide evidence of HPV vaccine impact in a population with low vaccination coverage, such as Tennessee, which is promising.
Supplementary Material
ACKNOWLEDGMENTS
The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of NIH or the Centers for Disease Control and Prevention. The authors are indebted to the Human Papillomavirus Vaccine Impact Monitoring Project and the Tennessee Division of TennCare of the Department of Finance and Administration, who provided data. Jaimie Z. Shing reports grants from NIH (5TL1TR002244, R01CA20740). Marie R. Griffin reports a grant from the Emerging Infections Cooperative Agreement from the Centers for Disease Control and Prevention (5U01C10003). Rachel S. Chang has no financial disclosures. Alicia Beeghly-Fadiel reports pilot grants from NIH (U54CA163072, U54MD010722). Staci L. Sudenga reports a grant from the National Cancer Institute (K07CA225404). James C. Slaughter has no financial disclosures. Manideepthi Pemmaraju reports a grant from the Emerging Infections Cooperative Agreement from the Centers for Disease Control and Prevention (5U01C10003). Edward F. Mitchel reports a grant from the Emerging Infections Cooperative Agreement from the Centers for Disease Control and Prevention (5U01C10003). Pamela C. Hull reports a grant from NIH (R01CA20740).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT Author Statement
Jaimie Shing: Conceptualization, Methodology, Formal analysis, Writing-Original draft preparation, Visualization. Marie Griffin: Validation, Writing-Review & Editing, Supervision, Project administration, Funding acquisition. Rachel Chang: Writing-Review & Editing. Alicia Beeghly-Fadiel: Writing-Review & Editing. Staci Sudenga: Writing-Review & Editing. James Slaughter: Validation. Manideepthi Pemmaraju: Project administration. Edward Mitchel: Data Curation. Pamela Hull: Supervision, Funding acquisition.
REFERENCES
- 1.Nwankwo C, Corman SL, Shah R, Kwon Y. HSR19–102: Direct and indirect economic burden of cervical cancer (CxCa) in the United States in 2015: a mixed-methods analysis. J Natl Compr Canc Netw. 2019;17(3.5):HSR19–102. 10.6004/jnccn.2018.7182. [DOI] [Google Scholar]
- 2.Östensson E, Silfverschiöld M, Greiff L, et al. The economic burden of human papillomavirus-related precancers and cancers in Sweden. PLoS One. 2017;12(6):e0179520. 10.1371/journal.pone.0179520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah R, Nwankwo C, Kwon Y, Corman SL. Economic and humanistic burden of cervical cancer in the United States: results from a nationally representative survey. J Womens Health (Larchmt). 2020;29(6):799–805. 10.1089/jwh.2019.7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrosky E, Bocchini JA, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2015;64(11):300–304. [PMC free article] [PubMed] [Google Scholar]
- 5.de Sanjosé S, Serrano B, Tous S, et al. Burden of human papillomavirus (HPV)-related cancers attributable to HPVs 6/11/16/18/31/33/45/52 and 58. JNCI Cancer Spectr. 2018;2(4):pky045. 10.1093/jncics/pky045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McClung NM, Gargano JW, Park IU, et al. Estimated number of cases of high-grade cervical lesions diagnosed among women — United States, 2008 and 2016. MMWR Morb Mortal Wkly Rep. 2019;68(15):337–343. 10.15585/mmwr.mm6815a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, Markowtiz LE. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68(32):698–702. 10.15585/mmwr.mm6832a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elam-Evans LD, Yankey D, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(33):1109–1116. 10.15585/mmwr.mm6933a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68(33):718–723. 10.15585/mmwr.mm6833a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172. 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perkins RB, Guido RS, Castle PE, et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24(2):102–131. 10.1097/LGT.0000000000000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ylitalo N, Josefsson A, Melbye M, et al. A prospective study showing long-term infection with human papillomavirus 16 before the development of cervical carcinoma in situ. Cancer Res. 2000;60(21):6027–6032. [PubMed] [Google Scholar]
- 13.Meijer CJLM, Snijders PJF, Brule A. Screening for cervical cancer: should we test for infection with high-risk HPV? CMAJ. 2000;163(5):535–538. [PMC free article] [PubMed] [Google Scholar]
- 14.Watson RA. Human papillomavirus: confronting the epidemic—a urologist’s perspective. Rev Urol. 2005;7(3):135–144. [PMC free article] [PubMed] [Google Scholar]
- 15.Markowitz LE, Gee J, Chesson H, Stokley S. Ten years of human papillomavirus vaccination in the United States. Acad Pediatr. 2018;18(2 suppl):S3–S10. 10.1016/j.acap.2017.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oakley F, Desouki MM, Pemmaraju M, et al. Trends in high-grade cervical cancer precursors in the human papillomavirus vaccine era. Am J Prev Med. 2018;55(1):19–25. 10.1016/j.amepre.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 17.Hariri S, Johnson ML, Bennett NM, et al. Population-based trends in high-grade cervical lesions in the early human papillomavirus vaccine era in the United States. Cancer. 2015;121(16):2775–2781. 10.1002/cncr.29266. [DOI] [PubMed] [Google Scholar]
- 18.Gargano JW, Park IU, Griffin MR, et al. Trends in high-grade cervical lesions and cervical cancer screening in five states, 2008‒2015. Clin Infect Dis. 2019;68(8):1282–1291. 10.1093/cid/ciy707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benard VB, Castle PE, Jenison SA, et al. Population-based incidence rates of cervical intraepithelial neoplasia in the human papillomavirus vaccine era. JAMA Oncol. 2017;3(6):833–837. 10.1001/jamaoncol.2016.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Human papillomavirus vaccine impact monitoring project (HPV-IMPACT). https://www.cdc.gov/ncird/surveillance/hpvimpact/overview.html. Published February 21, 2018. Accessed February 9, 2019.
- 21.Flagg EW, Torrone EA, Weinstock H. Ecological association of human papillomavirus vaccination with cervical dysplasia prevalence in the United States, 2007‒2014. Am J Public Health. 2016;106(12):2211–2218. 10.2105/ajph.2016.303472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cartwright DJ. ICD-9-CM to ICD-10-CM codes: What? Why? How? Adv Wound Care (New Rochelle). 2013;2(10):588–592. 10.1089/wound.2013.0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shing JZ, Griffin MR, Nguyen LD, et al. Improving cervical precancer surveillance: validity of claims-based prediction models in ICD-9 and ICD-10 eras. JNCI Cancer Spectr. 2021;5(1):pkaa112. 10.1093/jncics/pkaa112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Average Annual Percent Change (AAPC) — Joinpoint help system 4.5.0.1 https://surveillance.cancer.gov/help/joinpoint/setting-parameters/method-and-parameters-tab/average-annual-percent-change-aapc. Accessed December 4, 2017.
- 25.Centers for Disease Control and Prevention. Quadrivalent human papillomavirus vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2007;56(02):1–24.17218934 [Google Scholar]
- 26.Committee on Practice Bulletins—Gynecology. ACOG committee opinion No. 431: routine pelvic examination and cervical cytology screening. Obstet Gynecol. 2009;113(5):1190–1193. 10.1097/aog.0b013e3181a6d022. [DOI] [PubMed] [Google Scholar]
- 27.de Sanjosé S, Brotons M, Pavón MA. The natural history of human papillomavirus infection. Best Pract Res Clin Obstet Gynaecol. 2018;47:2–13. 10.1016/j.bpobgyn.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 28.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. 10.1016/s0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 29.Boersma P, Black LI. Human papillomavirus vaccination among adults aged 18‒26, 2013‒2018. NCHS Data Brief. 2020;(354):1–8. [PubMed] [Google Scholar]
- 30.Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, Markowitz LE. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68(32):698–702. 10.15585/mmwr.mm6832a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niccolai LM, Julian PJ, Meek JI, McBride V, Hadler JL, Sosa LE. Declining rates of high-grade cervical lesions in young women in Connecticut, 2008‒2011. Cancer Epidemiol Biomarkers Prev. 2013;22(8):1446–1450. 10.1158/1055-9965.epi-13-0272. [DOI] [PubMed] [Google Scholar]
- 32.Lindley MC, Smith PJ, Rodewald LE. Vaccination coverage among U.S. adolescents aged 13–17 years eligible for the Vaccines for Children Program, 2009. Public Health Rep. 2011;126(suppl 2):124–134. 10.1177/00333549111260s214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ueda P, Mercer CH, Ghaznavi C, Herbenick D. Trends in frequency of sexual activity and number of sexual partners among adults aged 18 to 44 years in the US, 2000‒2018. JAMA Netw Open. 2020;3(6):e203833. 10.1001/jamanetworkopen.2020.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Waal D, Bekkers RLM, Dick S, et al. Risk prediction of cervical abnormalities: the value of sociodemographic and lifestyle factors in addition to HPV status. Prev Med. 2020;130:105927. 10.1016/j.ypmed.2019.105927. [DOI] [PubMed] [Google Scholar]
- 35.El-Zein M, Ramanakumar AV, Naud P, et al. Determinants of acquisition and clearance of human papillomavirus infection in previously unexposed young women. Sex Transm Dis. 2019;46(10):663–669. 10.1097/olq.0000000000001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


