Abstract
Decades of research studying the behavioral effects of damage to structures in medial temporal lobe of rhesus monkeys have documented that such damage, particularly damage to the amygdala, causes animals to become hyporesponsive to threat and hyper-social. This phenotype, a subset of the behaviors known as “Klüver-Bucy Syndrome”, is one of the most well-known phenomena in behavioral neuroscience. Carrying on the tradition of evaluating hyposensitivity to threat in monkeys with temporal lobe lesions, we evaluated the responses of rhesus monkeys with bilateral ibotenic acid lesions of the amygdala or hippocampus and procedure-matched control animals to the presentation of an unfamiliar human intruder and threatening objects of varying complexity. All animals behaved as expected — calibrating their responses to the ostensible threat value of the stimuli such that they were most responsive to the most potent stimuli and least responsive to the least potent stimuli. Contrary to an earlier report (Mason et al., 2006), lesion status did not impact the pattern of responses across multiple dependent measures (overt behaviors, position in cage, etc.). The only lesion induced difference consistent with hyposensitivity to threat was that monkeys with amygdala lesions retrieved food rewards placed near reptile-like objects more rapidly than did control animals. These findings call into question the assumption that amygdala damage causes robust, stereotyped changes to affective behavior. They also highlight the importance of replication in neuroscientific studies using nonhuman primates.
Keywords: amygdala lesion, hippocampus lesion, rhesus monkey, affect, threat
For more than a century, the functions of the temporal lobe and its constituent structures have been probed through increasingly selective neurobiological manipulations in a variety of animal models, including nonhuman primates. The earliest findings related to affective processing following resections of the entire temporal lobe in monkeys dates back to Brown and Schafer’s report in the late 1800s (Brown & Shafer, 1888). Some 50 years later, Klüver and Bucy (1939) again characterized the behavior of monkeys following temporal lobectomy, including dramatically different responses to ostensibly threatening stimuli, excessive manipulation of objects, particularly with the mouth, and alterations to social behavior in the presence of each humans and conspecifics. So impactful were their findings that the phenotype of behavioral changes they observed have since come to be known collectively as “Klüver-Bucy Syndrome.” Since the publication of Klüver and Bucy’s seminal work, the impact of temporal lobe structures on behavior have been explored in monkeys with more selective lesions of the medial temporal lobe (Aggleton & Passingham, 1981; Weiskrantz, 1956), temporal neocortex (Horel et al., 1975), hippocampus (Chudasama et al., 2009; Machado et al., 2009; Zola-Morgan et al., 1989, 1991), amygdala (Aggleton & Passingham, 1981; Chudasama et al., 2009; Izquierdo & Murray, 2004; Kalin et al., 2001; Machado et al., 2009; Machado & Bachevalier, 2008; Meunier et al., 1999; Meunier & Bachevalier, 2002; Stefanacci et al., 2003; Zola-Morgan et al., 1991), specific nuclei of the amygdaloid complex (Aggleton & Passingham, 1981; Kalin et al., 2004), and regions outside of the temporal lobe like the orbitofrontal cortex (e.g., Izquierdo et al., 2005). The results of these studies, alongside studies carried out in rodents (e.g., LaBar & LeDoux, 1996), have led researchers to conclude that some of the features of Klüver-Bucy Syndrome—namely, blunted reactivity and inappropriate social behavior—can be tied to specific functions of the amygdala. In accordance with this, the amygdala has been widely implicated in “fear” despite the fact that it is active during the perception and experience of many different emotions (for a meta-analysis see: Lindquist et al., 2012), as well as demonstrated domain general roles in salience processing (e.g., Cunningham & Brosch, 2012) and reward (e.g., Baxter & Murray, 2002).
Monkeys with lesions of the amygdala and hippocampus have been tested on a variety of tasks to quantify aspects of affective reactivity and social behavior, including dyadic social interactions (e.g., Emery et al., 2001), affective learning via classical conditioning (e.g., Antoniadis et al., 2007; Pribram et al., 1979), discrimination learning (e.g., Izquierdo & Murray, 2007; Machado & Bachevalier, 2007; Zola-Morgan et al., 1989), and tests of responsivity to foods (e.g., Aggleton & Passingham, 1981), threatening objects (e.g., Chudasama et al., 2009), non-threatening objects (e.g., Mason et al., 2006), live animals (e.g., Kalin et al., 2001), and humans (e.g., Meunier et al., 1999) in order to determine the specific contributions of these structures to socioaffective behavior. The hyposensitivity to threat first described by Klüver and Bucy has often been investigated by exposing lesioned subjects to such stimuli as animal-like objects (e.g., children’s toys and figurines), live animals (e.g., snakes), items that have been conditioned to have negative associations over the animal’s lifespan (e.g., catcher’s nets, handling gloves), or direct eye contact with an experimenter (as in the Human Intruder Test). Monkeys are presented with these stimuli and their behaviors are recorded, including: the frequency of affective behaviors (facial and whole body), movements within the cage, manual or oral exploration of the stimulus (where possible), and, when the stimuli are presented alongside a food reward, the frequency and latency with which they retrieve this reward.
Despite consistent use of two of the behavioral testing paradigms employed in the characterization of the behaviors of amygdalectomized monkeys (Human Intruder Test and Object Responsiveness Test) and the general account of the impact of amygdala damage (Amaral, 2006), there is actually a fair degree of variation in outcomes across experiments. Some groups have reported striking changes to affective behavior following lesions of the amygdala such that amygdala lesioned monkeys exhibit dramatic reductions in aggressive and defensive behaviors alongside dramatically increased tendencies to manipulate stimuli (e.g., Aggleton & Passingham, 1981). But, other groups have reported subtle, time-restricted effects or effects on only a small set of behaviors (e.g., effect of lesions only on defensive and approach behavior but not aggressive or submissive behaviors in the presence of a rubber snake in Meunier et al., 1999; no effect of lesion beyond the first three days of the human intruder test in Mason et al., 2006; lesion group differences only in tension behaviors and not in gaze direction, position in cage, exploratory behaviors, affiliative behaviors, or facial expressions during the human intruder test in Machado & Bachevalier, 2008). Still others have reported no lesion-related effects at all in certain testing paradigms (e.g., human intruder test in Kalin et al., 2001 and Izquierdo et al., 2005). Studies of social behavior in amygdalectomized rhesus monkeys have shown similar inconsistencies in effects (see discussion in Emery et al., 2001). One of the challenges to understanding how robust and reproducible such effects are is that nonhuman primate studies are rarely replicated both for ethical and practical reasons (Bliss-Moreau et al., submitted). In the context of the small samples sizes of nonhuman primate studies, this problem is particularly salient due to the fact that these studies may be inherently underpowered and null results have traditionally been less likely to be published. Justifying carrying out similar experiments has historically required demonstrating that they expand on what is already known, rather replicate what has already been done.
The current study therefore is unique insofar as it is a replication of a published report (Mason et al., 2006) that evaluated affective reactivity following focal damage to temporal lobe structures. The present experiment expands on that previous work that only evaluated the impact of amygdala damage on reactivity to threat-related stimuli, by including a hippocampus lesioned group as well and carrying out testing both prior to and after surgery. The trial structure and stimuli within the behavioral experiments were, however, replicated exactly. We anticipated that analysis of these data, in light of the findings reported by Mason et al. (2006) and those of other groups who have carried out very similar experiments (e.g., Izquierdo & Murray, 2004; Kalin et al., 2001), would allow us to provide stronger evidence related to both the magnitude and generalizability of the effects of such lesions. We first tested the hypothesis that amygdala damage blunts responding to threat by exposing neurologically intact control subjects, amygdala lesioned subjects, and hippocampus lesioned subjects to the Human Intruder Test in order to assess their reactivity to varying potency of threat in the form of a novel human. Second, subjects were tested with novel objects resembling animals and their proclivities to manipulate these objects and to take a food reward from near them was recorded as an index of their affective reactivity to the objects (assuming that quick food retrievals indicate less reactivity than slow food retrievals and that the willingness to manipulate objects means they were not threatened by them). Finally, we report on the evaluation of the effects of the lesions, years after surgery with repeated exposures to animal-like objects in order to investigate whether or not effects observed shortly after surgery persisted over time and to explicitly test whether or not animals habituated to stimuli over the course of the experiment.
Methods
All experimental procedures were developed in consultation with the staff at the California National Primate Research Center (CNPRC). All protocols were approved by the University of California, Davis Institutional Animal Care and Use Committee.
Subjects and Living Arrangements
Eighteen adult male, experimentally naïve rhesus monkeys (Macaca mulatta) served as subjects. All animals were born at the CNPRC and raised in semi-naturalistic outdoor social groups ranging from 70 to 120 monkeys. Animals were 6-9 years of age when assigned to the current project. Once assigned, the animals were moved to temperature controlled indoor rooms with automatically regulated lighting (12 hour light/dark cycle with lights on at 6:00 am and off at 6:00 pm) and housed individually in standard home cages (61 cm width x 66 cm depth x 81 cm height). They were fed twice daily (7:00 am and 2:00 pm) on monkey chow (Ralston Purina, St. Louis, MO) supplemented with fresh fruit and vegetables and ad libitum water. Approximately one year after relocation, animals were randomly assigned to receive bilateral ibotenic acid lesions of the amygdala (amygdala group, n = 6), of the hippocampus (hippocampus group, n = 6), or underwent a sham surgical procedure (operated control group, n = 6). In addition to the current experiments, these animals served as subjects in two previously published studies (Antoniadis et al., 2007; Banta Lavenex et al., 2006). The only other testing experiences that these animals had prior to the current experiments were tests of dyadic social interactions (data not published). The experiments described by Antoniadis et al., (2007) and Banta Lavenex et al., (2006) occurred after the conclusion of the present experiments.
Surgical Procedures
Lesion surgeries occurred approximately 14 months after relocation indoors. The surgical procedures summarized here are described in greater detail in previous publications (Antoniadis et al., 2007; Banta Lavenex et al., 2006). Individualized stereotaxic atlases were generated using magnetic resonance imaging. Animals were anesthetized with ketamine hydrochloride (15 mg/kg, i.m.) and medetomidine (25-50 μg/kg, i.m.) and images were obtained using a 1.5 T Gyroscan magnet (GE Healthcare, Little Chalfont, Buckinghamshire, UK). Images were exported to Photoshop (version 5; Adobe Systems, San Jose, CA) and then Canvas (version 5; Deneba System, Miami, FL), to superimpose a calibrated grid that was used to calculate the coordinates of injections. At the time of surgery, anesthesia was induced with ketamine hydrochloride (10 mg/kg, i.m.) and maintained with isoflurane (1.2-2%) prior to placement in the stereotaxic apparatus (Crist Instruments Co., Inc., Damascus, MD). Anesthesia was maintained throughout surgery with isoflurane and fentanyl (7-10 μg/kg/min, i.v.). Under sterile conditions, the skull was exposed by way of a midline incision on the scalp and craniotomies were made over the amygdala or hippocampus. Electrophysiological activity was used to verify the locations of these structures by lowering a tungsten microelectrode connected to an amplifier and speaker to the MRI determined spatial target and listening to neuronal activity as it descended to confirm the location of neurons versus white matter. Adjustments to the coordinates were made as needed. Ibotenic acid (10 mg/ml in 0.1M PBS; Biosearch Technologies, Novato, CA) was simultaneously infused bilaterally into the amygdala or hippocampus using 10 μl Hamilton syringes. Following ibotenic acid injections, the dura was sutured, the craniotomies were filled with GelFoam (Amersham Biosciences, Peapack, NJ), and the fascia and skin were sutured in three layers. Operated controls were exposed to the same pre-surgical preparation, midline incision, and skull exposure. They were anesthetized for the average duration of the lesion surgeries. All animals were monitored by veterinary staff following surgery; post-op antibiotics (50 mg/kg Clarofan, three times daily for 5 days) and analgesics (0.15 mg/kg oxymorphone, three times daily for 2 days) were administered.
Lesion Evaluation
The extent of damage to the amygdala and hippocampus was evaluated through histological methods as previously reported in detail (Antoniadis et al., 2007; Banta Lavenex et al., 2006). In brief, animals were deeply anesthetized and perfused intracardially with 1% and 4% paraformaldehyde in 0.1M phosphate buffer. The brains were removed, blocked, post-fixed, cryoprotected, frozen in isopentane, and stored at −70°C until they were sectioned. Thirty μm sections were obtained on a freezing, sliding microtome and a series of one-in-eight sections were mounted stained by the Nissl method with thionin (Lavenex et al., 2009). Lesion extent for hippocampus and amygdala lesions were evaluated microscopically on an AusJena microfiche reader (Zeiss, Oberkochen, Germany). Amygdala volumes were estimated with the Cavalieri method (Gundersen and Jensen, 1987). Unlesioned monkey brains were analyzed for comparison.
Detailed and individual descriptions of the amygdala (Antoniadis et al., 2007) and hippocampus (Banta Lavenex et al., 2006) lesions have been reported previously and thus will not be described here. For amygdala lesions, consistent damage (>80%) was achieved bilaterally in all cases but two, where damage was still greater than 60%. Minimal extraneous damage to adjacent areas occurred. Qualitative descriptions of the extraneous damage are detailed by Antoniadis et al., (2007). The lesion extent in the amygdala for the amygdala-lesioned subjects is presented in Table 1; Table 1 also includes the percent atrophy of the amygdala for animals in the Mason et al. (2006) study for the sake of comparison. Hippocampal lesions were intended to selectively damage the dentate gyrus, hippocampus, subiculum, presubiculum, and parasubiculum, and spare the entorhinal, perirhinal, and parahippocampal cortices. Quantitative evaluation of the damaged areas was not completed for these lesions. Consistent and heavy damage was done to all of the targeted areas and consistent sparing was accomplished in the entorhinal cortex, perirhinal cortex, and amygdala (see Banta Lavenex et al., 2006 for detailed descriptions of the hippocampal lesions).
Table 1.
Lesion Extent
| Amygdala |
||
|---|---|---|
| Subject | Left | Right |
| Present Study | ||
| A | 98% | 97% |
| B | 93% | 89% |
| C | 83% | 93% |
| D | 92% | 81% |
| E | 88% | 68% |
| F | 64% | 73% |
| Mean | 86% | 84% |
|
| ||
| Mason et al., 2006 | ||
| A | 83% | 85% |
| B | 76% | 88% |
| C | 61% | 90% |
| D | 72% | 80% |
| E | 96% | 70% |
| F | 74% | 59% |
| Mean | 77% | 79% |
Behavioral Experiments
Three behavioral experiments — Human Intruder and two runs of Object Responsiveness — were carried out to evaluate the effects of damage to the amygdala and hippocampus on affective reactivity. Animals completed two of the experiments, Human Intruder Test and Object Responsiveness Test I, both before and after surgery in order to evaluate changes in reactivity as a result of the lesions. Object Responsiveness II was completed only after surgery in order to test the effects of damage to the amygdala and hippocampus on habituation to aversive stimuli across repeated presentations. Testing was done with all animals in the same sequence at comparable intervals relative to the time of surgery. All testing was completed when animals were not involved in any other experimental procedures. Animal testing order was counterbalanced for lesion condition across each test. Figure 1 shows the sequence of experiments and testing dates relative to relocation, surgery, and sacrifice.
Figure 1.

Sequence of relative timing of experiments. Human Intruder Test (HIT) highlighted in blue, Object Responsiveness Test I (ORT I) highlighted in green, and Object Responsiveness Test II (ORT II) highlighted in yellow.
Human Intruder Test
Testing took place across two 5-day periods (one prior to and one following surgery). Preoperative testing occurred at approximately 13 months following relocation indoors and 1-3 months prior to surgery. Postoperative testing occurred at approximately 10 months after surgery. Animals were moved out of their home rooms and tested individually in a standard primate cage (60 cm wide x 75 cm x 75 cm). Similar procedures were used to those previously published by our group and others (e.g., Bliss-Moreau & Baxter, 2018; Bliss-Moreau & Moadab, 2016; Gottlieb et al., 2013; Kalin & Shelton, 1989, 1998). Animals were exposed to unfamiliar male experimenter in four different conditions following one minute of acclimation. The four conditions each lasted 30 seconds and were presented in the following order: 1) profile far: presentation of left profile (facing 90 degrees away from the cage) at 1 m distance; 2) profile near: presentation of left profile at 0.3 m; 3) stare far: direct stare at the animal at 1 m; 4) stare near: direct stare at the animal at 0.3 m. After all animals were tested, a 30-second food test was performed for each animal. The experimenter presented one raisin to the animal every 2 seconds by holding it in front of the cage without making any eye contact. Animals were tested between 12:00 and 2:00 pm.
Behavioral data were collected by trained observers using a 1/0 sampling method and 5 second bins (as in Bliss-Moreau & Baxter, 2018; Capitanio, 1999). Position in cage (based on the location of the animal’s head, either in the front or back half of the cage) and affect-related behaviors were scored. These behaviors included: lipsmack face, grimace, threat, lunge, cage shake, yawn, and tooth grind. Behaviors that occurred within a 5 second bin were given a score of 1 and those not occurring within a bin were given a score of 0. Affect-related scores were summed within and across bins to generate an index of affective reactivity. Observers were trained to the lab standard which is greater than 85% interrater reliability and were blind to lesion condition.
Object Responsiveness Test
In the first run of object responsiveness, testing took place across two 6-day periods (one prior to and one following surgery). Preoperative testing occurred approximately 8 months after relocation indoors and 6-8 months prior to surgery. Postoperative testing occurred approximately 2-4 months following surgery. Animals were tested in an adapted lab care cage (80.01 cm wide x 83.32 cm x 101.6 cm) with a plexiglass front, i.e., “the object responsiveness cage”. The plexiglass front contained two vertical openings (25 cm height x 5 cm width) separated by 5.08 cm and centered 32.25 cm from the left and right sides of the cage. An opaque guillotine door could be raised and lowered by a rope-and-pulley system in front of the plexiglass. Contiguous with the front of the cage was a platform containing a central food well (2.54 cm length x 2.54 cm width) situated 5.18 cm from the front of the cage and a frame directly behind the food well (22.86 cm length x 15.24 cm width) for securing the bases attached to the test objects. The back and right side of the cage were opaque and left side of the cage, which also included a door for relocating the animal, consisted of stainless-steel bars. Animals were tested between 9:00am and 1:00pm.
One week prior to testing, animals underwent acclimation training to ensure that they would readily retrieve a food reward (half of a grape, marshmallow, raisin, or M&M, depending on food preference) from the object platform. Animals received 25 trials in which the guillotine door was raised, and the animal had 30 seconds to retrieve the food reward. In order to pass the pre-testing phase animals were required to retrieve the food within 30 seconds on 20 out of 25 trials during two consecutive acclimation sessions. All animals met the criterion to retrieve a single food reward over two consecutive days. Following acclimation, animals advanced to the object testing sessions. Objects were presented one at a time on the platform concurrently with a food reward. Twelve stimulus object sets were used in this task and no object was seen more than once. Each object set was designed to represent increasing levels of complexity and consisted of a simple, intermediate, and complex form similar to those described previously (Mason et al., 2006). The most complex stimuli were children’s toys resembling animals or animal-like figures (see Figure 2). The intermediate stimuli were similar to the complex stimuli excepting the removal of distinctive features, largely from the face. The simple stimuli were wooden blocks of similar size, shape, and color to the other stimuli in the set. Complexity of stimuli was varied in an effort to vary the affective value of the stimuli (simple ostensibly least threatening and complex ostensibly most threatening). One object set was presented on each test day. Each session consisted of five 30-second trials presented in the following fixed order: food only trial, simple object + food, intermediate object + food, complex object + food, and a final food only trial. No animals other than the test subject were present in the test room. Two observers sat diagonally to the right of the test cage (approximately 2 m away). This allowed the animal to view the observers while the opaque guillotine door was raised. A camera was positioned directly in front of the test cage next to the observer on the right.
Figure 2.
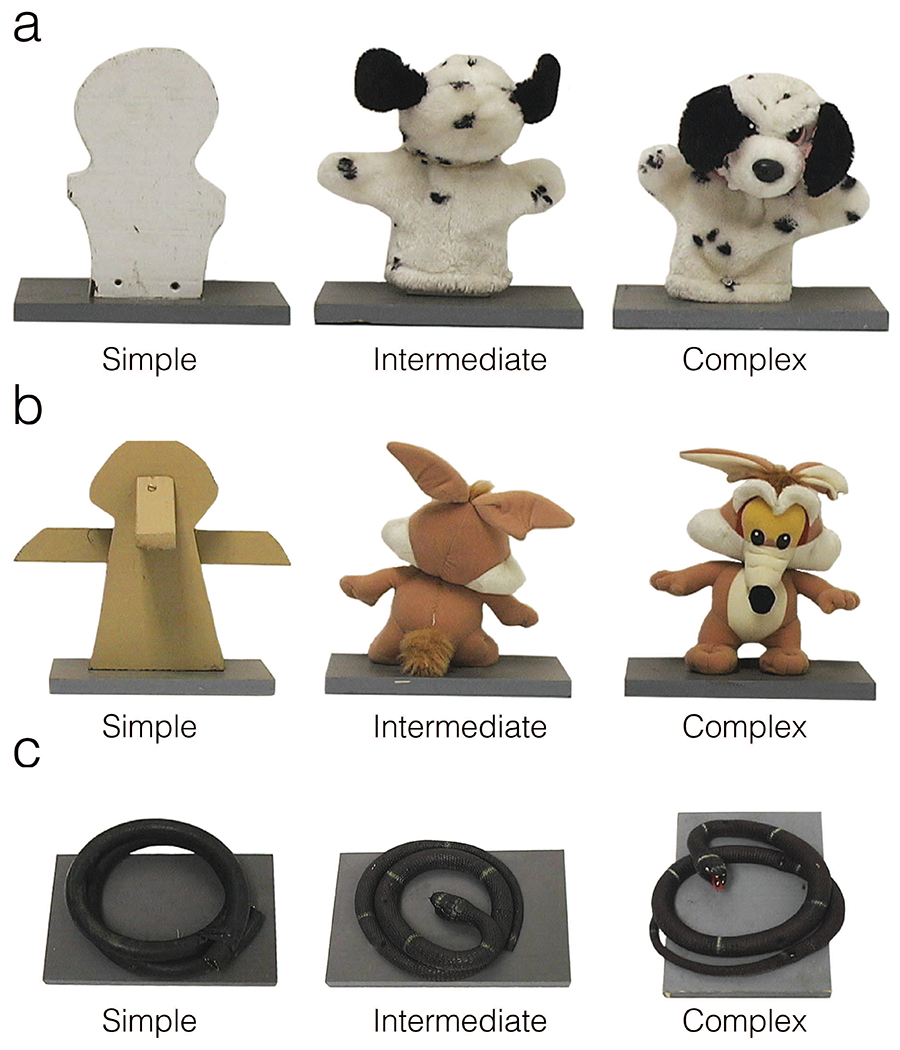
Photographs of exemplar object series (a, dog; b, coyote; c, snake), including simple (wood block or coiled hose), intermediate (backwards-facing to obscure facial features), and complex (front-facing to show facial features) objects.
In the second test of object responsiveness, testing took place across three 6-day phases. The same testing conditions were used as in the first test. One week intervened the first and second phases and four weeks intervened the second and third phases. Six novel stimulus series of the same structure as those previously described were presented to the animals across the five testing days in each phase. Four series contained objects resembling mammalian animals and two series contained objects resembling reptilian animals. The same presentation order was used in each of the three phases: day 1: bear series; day 2: pig series; day 3: lion series; day 4: alligator series; day 5: snake series; day 6: doll series. The trial number and structure was the same each day: food only trial, simple object + food, intermediate object + food, complex object + food, and a final food only trial.
Behavioral data were collected with The Observer software (Noldus, Sterling, VA, USA) by two observers who were blind to lesion status (lab standard ≥ 85% inter-rater reliability). The following behavioral measures were collected live per trial: 1) latency to retrieve the food reward and first explore the object (full trial length was used on trials where the subject did not engage in these behaviors); 2) frequency of species-typical behaviors, spatial location in the cage, food retrieval, and manual exploration of the object, and 3) duration of spatial location in the cage and manual exploration of the object. The behavioral ethogram used is found in Table 2. As in the Human Intruder Test, an index of affect reactivity was generated by summing the frequencies of affect-related behaviors: lipsmack face, grimace, threat, lunge, cage shake, yawn, and tooth grind. Previous experiments have shown differences in the responses of amygdala lesioned animals to objects intended to resemble reptiles versus animals intended to resemble mammals (Bliss- Moreau et al., 2011). As such, for this experiment we analyzed the responses obtained during mammalian object (bear, pig, lion, and doll) and reptilian object (snake and alligator) trials separately. As the same object series were presented in the same sequence across all three phases of this experiment, phase was used as a repeated measure to observe the effect of habituation on reactivity to these two different classes of stimuli.
Table 2.
Behavioral Ethogram
| Behavior | Description |
|---|---|
| Affective behaviors | |
| Lipsmack | Rapid lip movement usually with pursed lips and accompanied by a smacking sound |
| Grimace | Exaggerated grin with teeth showing |
| Threat | At least two of the following: open mouth stare, head-bob, ear slaps, bark vocalizations, or lunges |
| Lunge | Rapid body movement toward the front of the cage |
| Cage shake | Grasping of cage parts and shaking |
| Yawn | Open mouth, exposing teeth |
| Tooth grind | Repetitive, audible rubbing of upper and lower teeth |
| Tactile explore | Contact is made with the presented object with the hands, fingers, or feet |
| Take food | Animal retrieves the food from the food well |
| Coo | Clear, soft, sound, moderate in pitch and intensity |
| Bark | Low-pitched, loud, guttural sound often accompanied by some threat |
| Grunt | Deep, muffled, low intensity vocalization; sometimes given with lipsmacks |
| Positions | |
| Front vs. Back | Scored by location of the animal’s head in the front half or the back half of the cage |
Statistical Analysis
Statistical analyses were carried out in R version 4.0.4 (R Core Team, 2020) using the following packages (lme4, (Bates et al., 2015); ImerTest, (Kuznetsova et al., 2017); lsmeans, (Lenth, 2016), rstatix, (Kassambara, 2020), sjstats, (Ludecke, 2020)). Data were checked for normality using the Shapiro-Wilk normality test and visual inspection of QQ plots. Equal variances were verified using Mauchly’s test of sphericity. Repeated-measures analysis of variance (ANOVA) were used to analyze the data. Tukey posthoc tests were used to evaluate pair-wise comparisons of lesions following ANOVAs. For the sake of completeness, because lesion experiments are unlikely to be repeated, follow-up ANOVAs with fewer factors were carried out in cases where the omnibus ANOVA or marginal means with 95% confidence intervals suggested that there might be an effect. For data that were not normally distributed, the Kruskal-Wallis test was used. In these cases, pairwise comparisons were made with the Wilcoxon test using the Benjamini & Hochberg correction. In some cases, frequencies of behavior were extremely low and so statistical tests were not carried out.
Results
Human Intruder Test
Affective reactivity.
A repeated measures ANOVA with lesion group as a between subjects factor and condition, testing day (1-5), and phase (pre- vs. post-lesion) as within subjects factors revealed no main effect of lesion group (F(2, 15) = 0.37, p = 0.70, η2 = 0.001) nor any significant interactions between lesion group and other factors (group x condition: F(6, 45) = 0.40, p = 0.88, η2 = 0.003; group x day: F(8, 60) = 0.43, p = 0.90, η2 = 0.004; group x phase: F(2, 15) = 0.26, p = 0.77, η2 = 0.001). As expected, there were significant main effects of condition (F(3, 45) = 33.65, p < 0.001, η2 = 0.124) and day (F(4, 60) = 8.42, p < 0.001, η2 = 0.041), such that subjects were least reactive in the profile near (M = 0.49, SE = 0.09) and profile far (M = 0.49, SE = 0.09) conditions and most reactive in the stare near (M = 4.26, SE = 0.28) condition. Reactivity decreased across test days (day 1: M = 2.61, SE = 0.27; day 3: M = 1.94, SE = 0.26; day 5: M = 1.51, SE = 0.22). There was a significant three-way interaction between group, condition, and phase (F(24, 570) = 2.31, p = 0.03, η2 = 0.027). To investigate this interaction, we ran follow-up ANOVAs on each group with measures collapsed across days. The condition by phase interaction was significant for the control group (F(3,15) = 12.04, p < 0.001, η2 = 0.306), but was not significant for the amygdala lesioned (F(3, 15) = 1.44, p = 0.27, η2 = 0.065) or hippocampus lesioned (F(3, 15) = 3.03, p = 0.06, η2 = 0.165) groups. See Figure 3.
Figure 3.
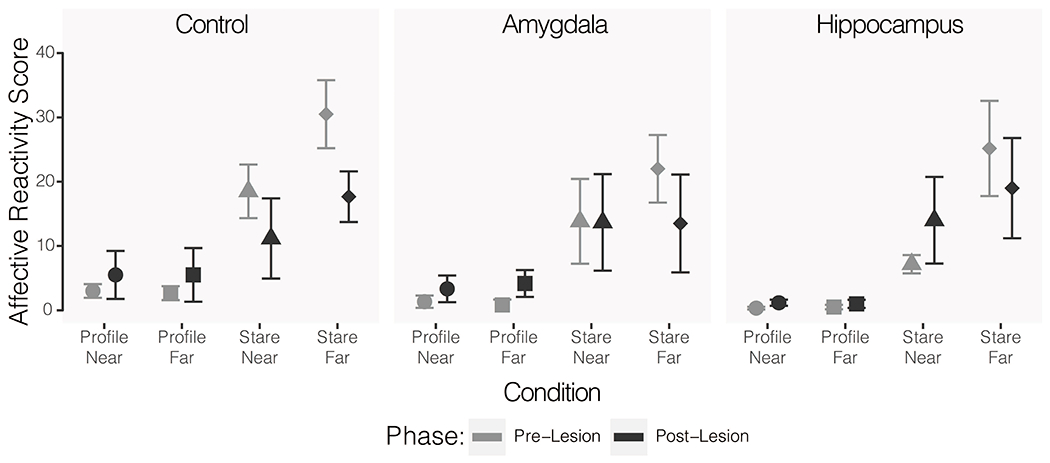
Mean reactivity scores for across all Human Intruder Test conditions for each of the three groups. Lighter grey shapes indicate the pre-operative scores and darker grey shapes indicate the post-operative scores. Circles show reactivity in the profile-near condition, squares show profile-far, triangles show stare-near, and diamonds show stare-far. Error bars indicate SEM.
Retrieval of food reward.
There were no significant differences across lesion groups in the number of raisins retrieved from the intruder (F(2, 30) = 0.97, p = 0.39, η2 = 0.011). There was a significant effect of day (F(4, 120) = 9.56, p < 0.001, η2 = 0.216) such that all subjects retrieved increasing numbers of raisins across testing days during both pre- and post-operative testing (controls: day 1 – M = 10.67, SE = 0.43, day 5 – M = 11.91, SE = 0.31; amygdala lesioned: day 1 – M = 10.17, SE = 0.51, day 5 – M = 12.25, SE = 0.22; hippocampus lesioned: day 1– M = 9.75, SE = 0.88, day 5 – M = 10.83, SE = 0.95). There was no significant effect of phase (F(1, 30) = 0.17, p = 0.69, η2 = 0.001).
Position in cage.
There was not a significant main effect of lesion group (F(2,15) = 0.37, p = 0.70, η2 = 0.001), or day (F(4, 60) = 0.338, p = 0.851, η2 = 0.002) on the animals’ positions in the cage. There was a main effect of condition (F(3, 45) = 33.65, p < 0.001, η2 = 0.046) and phase (F(1, 15) = 9.79, p = 0.007, η2 = 0.015). There was a significant three-way interaction between group, day, and phase (F(8, 465) = 2.04, p = 0.04, η2 = 0.025). Follow up ANOVAs were conducted for each group individually to determine the source of the interaction. The day x phase interaction failed to reach conventional levels of significance for control group (F(4, 200) = 2.26, p = 0.06, η2 = 0.041), but was significant for the hippocampus lesioned group (F(4, 200) = 2.88, p = 0.02, η2 = 0.051). In the pre-lesion testing phase controls and hippocampus lesioned animals showed a reduction in time spent at the front of the cage during later testing days as compared to the first day (controls: day 1 – M = 5.17, SE = 0.30, days 5-6 M = 4.17, SE = 0.25; hippocampus lesioned: day 1 – M = 4.50, SE = 0.50, days 5-6 M = 3.99, SE = 0.27). In the post-lesion testing phase the mean duration at the front of the cage was similar across all days of testing for controls and showed some increase for hippocampus lesioned subjects (day 1 – M = 4.67, SE = 0.39, days 5-6 M = 5.53, SE = 0.13). The interaction was not significant for the amygdala lesioned group (F(4, 200) = 0.95, p = 0.43, η2 = 0.017), who exhibited similar mean durations across days during both pre- and post-lesion testing.
Object Responsiveness Test I
Exploration of the object.
Manual contact with the object was not necessary to retrieve the food reward but did occur in all groups. The frequency and duration of manual object manipulation was analyzed across groups with phase (pre- vs. post-lesion) and object complexity (simple, intermediate, or complex) as repeated measures in an ANOVA. There was not a significant lesion group difference in either frequency (F(2, 15) = 0.59, p = 0.57, η2 = 0.014) or duration (F(2, 15) = 1.27, p = 0.31, η2 = 0.025) of this exploration. There was a significant main effect of phase for both frequency (F(1, 15) = 7.96, p = 0.013, η2 = 0.094) and duration (F(1, 15) = 6.69, p = 0.02, η2 = 0.065). All groups explored objects less frequently and for shorter durations when tested post-surgery as compared to their pre-surgery testing. While there was no significant effect of object complexity on the frequency of manual exploration (F(2, 30) = 0.93, p = 0.41, η2 = 0.022), there was a significant effect of complexity on the duration of exploration (F(2, 60) = 6.04, p = 0.004, η2 = 0.118). Across all three groups of subjects and both phases of testing, subjects spent more time manipulating the complex (M = 1.22 s, SE = 0.29 s) and intermediate (M = 1.27 s, SE = 0.32 s) objects than the simple objects (M = 0.65 s, SE = 0.16 s). There was not a significant effect of lesion group on the latency to manually explore the objects (F(2, 15) = 0.23, p = 0.79, η2 = 0.006, Figure 4C). As in the frequency and duration data, there was a significant effect of phase on the latency to explore (F(1, 15) = 9.67, p = 0.007, η2 = 0.122). All groups were slower to manually explore the objects across all complexity levels after surgery as compared to their pre-surgery baselines. See Figure 4.
Figure 4.
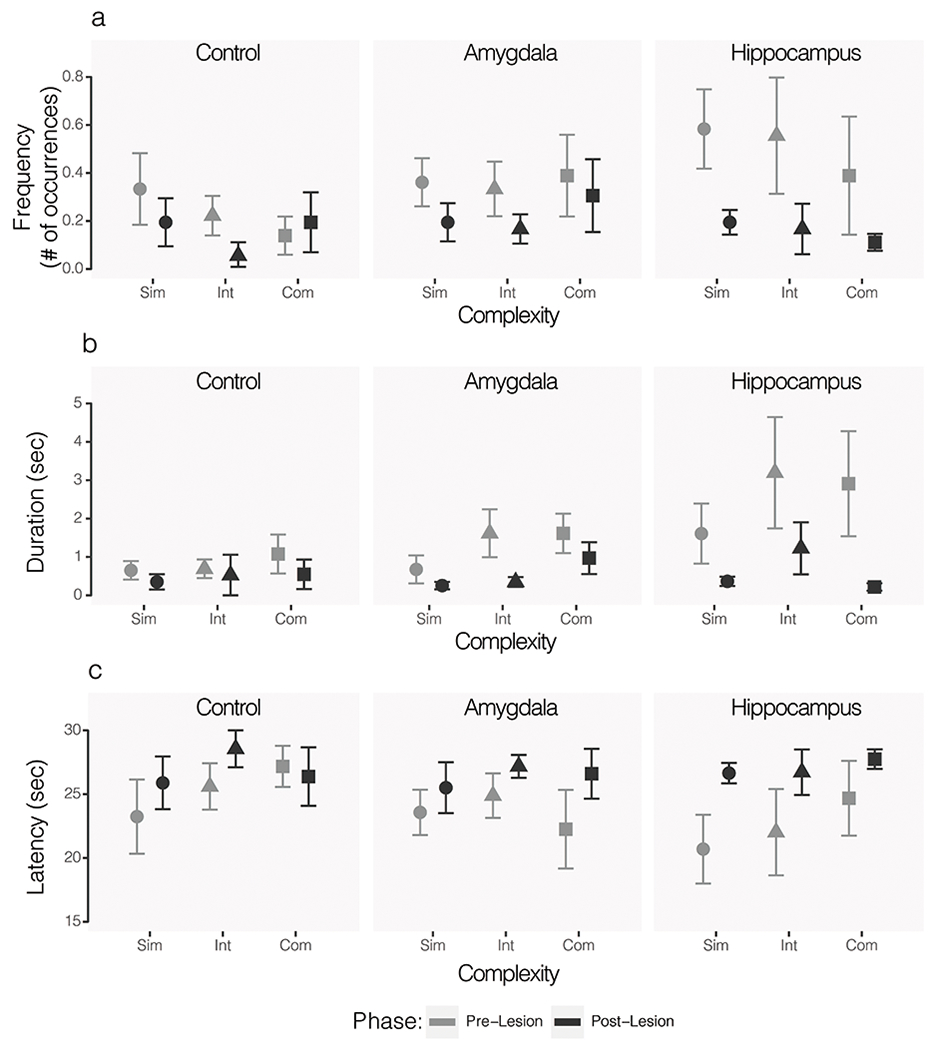
Mean frequency (A), duration (B), and latency (C) of object exploration by animals in the control (left), amygdala lesioned (middle), and hippocampus lesioned (right) groups. Lighter grey shapes indicate pre-operative baseline and darker grey shapes indicate post-operative values. Behavior in the presence of simple (circle), intermediate (triangle), and complex (square) objects are shown for each group. Error bars show SEM.
Retrieval of food reward.
There was not a significant lesion group difference in food retrieval frequency (F(2, 15) = 0.77, p = 0.48, η2 = 0.018) or latency (F(2, 15) = 0.90, p = 0.43, η2 = 0.017). There was a significant effect of complexity on the frequency of food retrieval (F(2, 30) = 5.51, p = 0.009, η2 = 0.129). Foods were retrieved more frequently on simple object trials (M = 93%, SE = 2%) than intermediate (M = 85%, SE = 2%) or complex (M = 84%, SE = 3%) object trials. A very similar effect of complexity on latency to retrieve the food reward was also observed (F(2, 30) = 13.46, p < 0.001, η2 = 0.258) – foods were retrieved fastest on simple object trials and slowest on complex object trials. There was not a significant effect of phase on food retrieval measures (frequency: F(1, 15) = 1.45, p = 0.25, η2 = 0.017; latency: F(1, 15) = 0.23, p = 0.64, η2 = 0.002) in contrast with the effects seen in the propensity of subjects to explore the objects.
Position in cage.
While the effect of group on position in cage during Object Responsiveness Test I did not reach conventional levels of significance, it is the only analysis that suggested that there might be lesion group differences relative to objects (F(2, 15) = 3.40, p = 0.06, η2 = 0.084). Post hoc tests indicated a potential group difference between the operated control and hippocampus subjects (p = 0.06), but no difference between the control and amygdala lesioned subjects (p = 0.81) or amygdala and hippocampus lesioned subjects (p = 0.18) groups. Consistent with the data on object exploration, there was a significant main effect of phase on time spent at the front of the cage (F(1, 15) = 13.63, p = 0.002, η2 = 0.168). The duration of time spent at the front of the cage during the postoperative testing phase was significantly lower than the duration of time spent at the front of cage during the preoperative testing phase. There was an interaction between complexity and phase, but it did not reach conventional levels of significance (F(2, 30) = 2.94, p = 0.07, η2 = 0.073). Follow up ANOVAs revealed a significant effect of phase on the time spent at front of cage in the presence of simple objects (F(1, 34) = 7.72, p = 0.008, η2 = 0.185) and an effect for intermediate objects that did not reach conventional levels of significance (F(1, 34) = 3.38, p = 0.07, η2 = 0.090) with groups combined. The effect of phase on complex objects was not significant (F(1, 34) = 2.74, p = 0.11, η2 = 0.074). See Figure 5.
Figure 5.
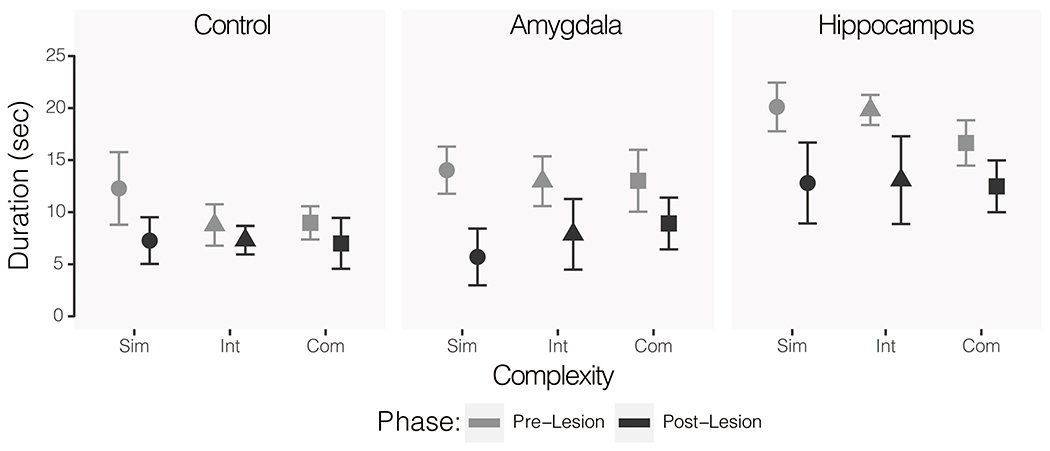
Mean duration of time spent at the front of the cage for control (left), amygdala lesioned (middle), and hippocampus lesioned (right) animals. Lighter grey shapes indicate pre-operative baseline and darker grey shapes indicate post-operative values. Behavior in the presence of simple (circle), intermediate (triangle), and complex (square) objects are shown for each group. Error bars show SEM.
Affective reactivity.
Affect-related behaviors were too infrequent to perform formal statistical analyses on the reactivity indices generated from the frequencies of these behaviors. All animals were essentially at floor when the mean number of reactivity behaviors across test conditions was evaluated. During preoperative testing, reactivity indices were generally higher during complex object trials (controls: M = 0.42, SE = 0.22; amygdala lesioned: M = 0.08, SE = 0.05; hippocampus lesioned: M = 0.50; SE = 0.18) as compared to intermediate (controls: M = 0.17, SE = 0.12; amygdala lesioned: M = 0.00, SE = 0.00; hippocampus lesioned: M = 0.31; SE = 0.12) or simple (controls: M = 0.00, SE = 0.00; amygdala lesioned: M = 0.00, SE = 0.00; hippocampus lesioned: M = 0.19; SE = 0.11) object trials. Postoperative scores indicate an overall reduction in reactivity from pre- to post- surgery, with only small differences between complex (controls: M = 0.06, SE = 0.04; amygdala lesioned: M = 0.08, SE = 0.05; hippocampus lesioned: M = 0.11; SE = 0.07), intermediate (controls: M = 0.00, SE = 0.00; amygdala lesioned: M = 0.06, SE = 0.06; hippocampus lesioned: M = 0.08; SE = 0.06), and simple (controls: M = 0.00, SE = 0.00; amygdala lesioned: M = 0.14, SE = 0.08; hippocampus lesioned: M = 0.00; SE = 0.00) object trials. Given the very low rates of behavior, we elected not to carry out formalized statistical analyses because observed differences would not be psychologically or behaviorally meaningful.
Object Responsiveness Test II
Exploration of the object.
As in Object Responsiveness I, contact or exploration of the object was not necessary to retrieve the food reward. Subjects very rarely manipulated the objects in this experiment. Contact was made with the objects in only 35 instances across 972 object trials. Formal statistical analyses were not completed due to the rarity of engagement in this behavior. Of the 35 instances of object manipulation, 30 occurred during mammalian object trials and 5 occurred during reptilian object trials. Across mammalian object trials, there were 20 instances of object manipulation by control subjects, 3 instances by amygdala lesioned animals, and 7 by hippocampus lesioned animals. Of the 5 instances of exploration during reptilian object trials, 3 of these were by control subjects and 1 instance occurred for each the amygdala lesioned and hippocampus lesioned groups.
Retrieval of food reward.
There were no group differences in frequency or latency to retrieve the food reward during simple (frequency: H(2) = 2.11, p = 0.35, ε2 = 0.040; latency: H(2) = 0.87, p = 0.65, ε2 = 0.016) or intermediate (frequency: H(2) = 2.85, p = 0.24, ε2 = 0.054; latency: H(2) = 4.19, p = 0.12, ε2 = 0.079) mammalian object trials. There was no significant group difference in latency to retrieve the food during complex object trials H(2) = 4.18, p = 0.12, ε2 = 0.079), but there was a significant effect of group on frequency of food retrieval during these trials H(2) = 7.20, p = 0.03, ε2 = 0.136). The hippocampus lesioned group differed significantly from the control group on this measure (p = 0.04), but did not differ significantly from the amygdala lesioned group (p = 0.28). The control and amygdala lesioned groups also did not differ significantly (p = 0.16). As expected, animals retrieved the food reward less frequently and with longer latency for the more complex stimuli. Frequency and latency of food retrieval for mammalian object trials are displayed in Figure 6.
Figure 6.
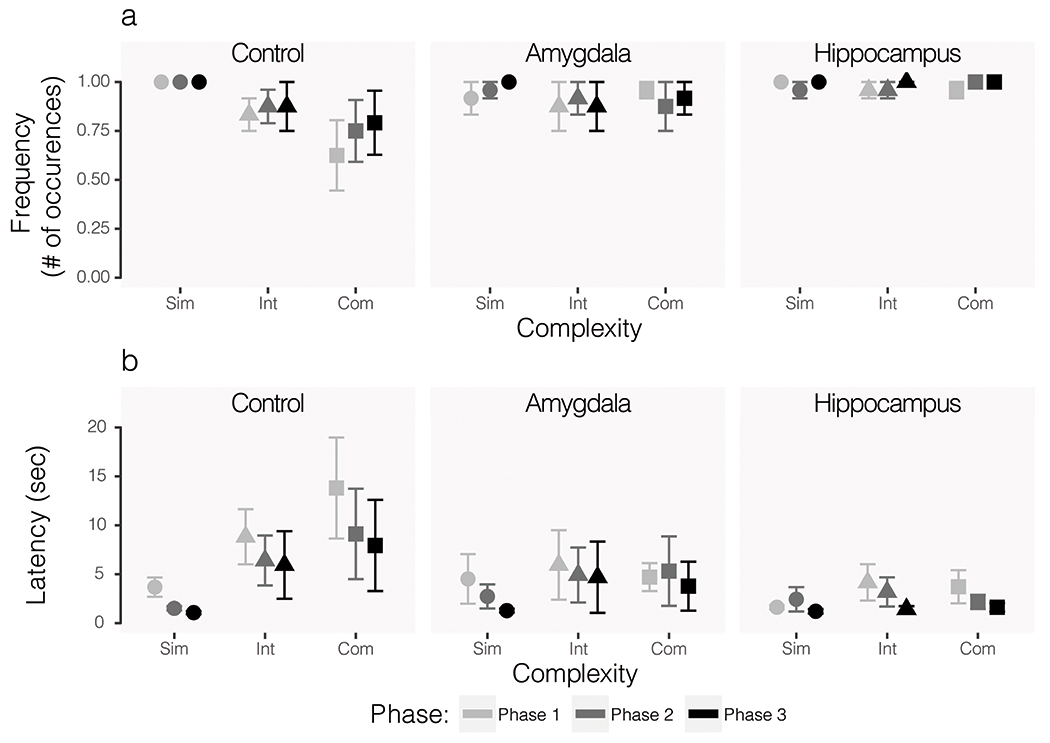
Food retrieval frequency (A) and latency (B) during mammal-like object trials only. All phases represent post-operative data and exposure to the same series of objects. The first iteration of testing (Phase 1) is shown in the lightest grey, the second (Phase 2) is shown in the second lightest grey, and the third (Phase 3) is shown in the darkest color. Circular data points show average responses across simple object trials, triangles show intermediate, and squares show complex. Error bars show SEM.
Analysis of the same behaviors during reptilian object trials revealed a different pattern of effects. There were no group differences in frequency or latency to retrieve the food reward during simple reptilian object trials (frequency: H(2) = 3.03, p = 0.22, ε2 = 0.057; latency: H(2) = 1.08, p = 0.58, ε2 = 0.020). However, the effect of lesion group was significant for intermediate (frequency: H(2) = 10.64, ε2 = 0.201, p = 0.005; latency: H(2) = 8.72, p = 0.01, ε2 = 0.164) and complex (frequency: H(2) = 7.78, p = 0.02, ε2 = 0.147; latency: H(2) = 11.85, p = 0.003, ε2 = 0.224) reptilian objects. In all cases of significant group effects, pairwise comparisons revealed significant differences between the control and amygdala lesioned groups for these measures (all p < 0.03). Controls and hippocampus lesioned subjects differed significantly only in frequency of retrieval during intermediate reptilian object trials (p = 0.02). The amygdala and hippocampus lesioned groups never differed significantly from each other on these measures (all p > 0.05). As was true for mammalian object trials, control subjects showed the expected trends in behavior with less frequent food retrieval and longer latencies as object complexity increased. Frequency of retrieval increased across phases and latency to retrieve decreased. Food retrieval behaviors during reptilian object trials are shown in Figure 7.
Figure 7.
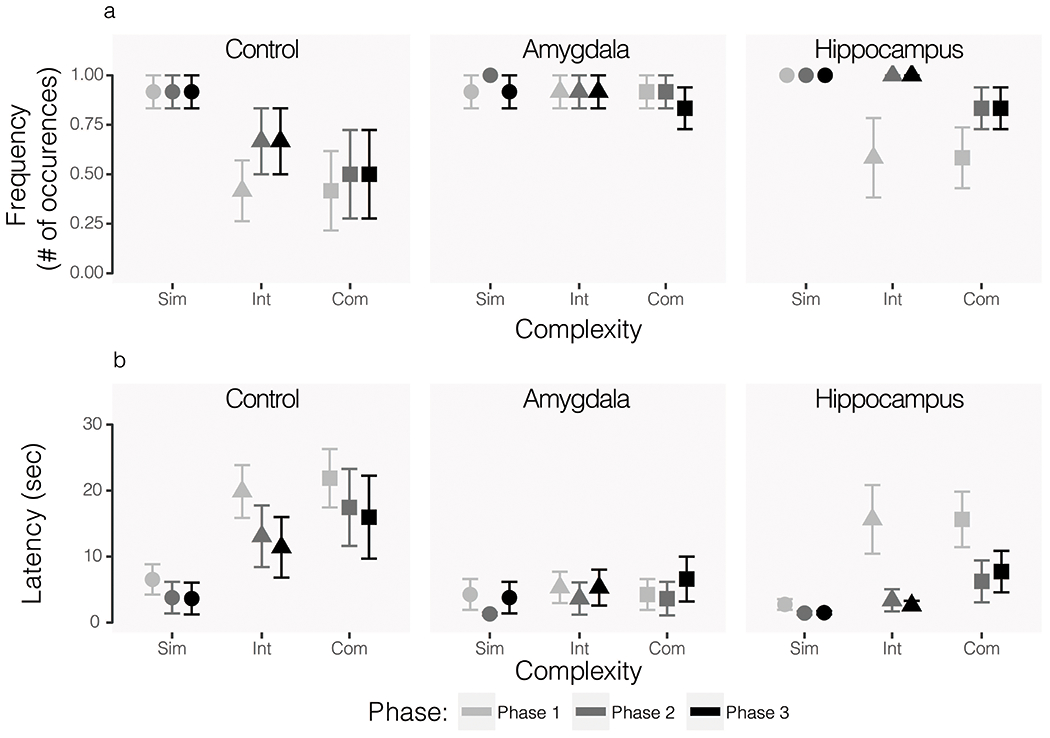
Food retrieval frequency (A) and latency (B) during reptilian-like object trials only. All phases represent post-operative data and exposure to the same series of objects. The first iteration of testing (Phase 1) is shown in the lightest grey, the second (Phase 2) is shown in the second lightest grey, and the third (Phase 3) is shown in the darkest color. Circular data points show average responses across simple object trials, triangles show intermediate, and squares show complex. Error bars show SEM.
Position in cage.
There was no significant effect of group on the duration of time spent at the front of the cage during mammalian object trials (F(2,14) = 2.29, p = 0.14, η2 = 0.039). The effect of phase was also not significant (F(2, 28) = 0.54, p = 0.59, η2 = 0.009). There was a significant effect of complexity (F(2, 28) = 7.17, p = 0.003, η2 = 0.121). On average, subjects spent less time at the front of the cage as the objects became more complex (simple: M = 8.10, SE = 0.89; intermediate: M = 6.46, SE = 0.60; complex: M = 5.52, SE = 0.51). During reptilian object trials, there were also no main effects of lesion group (F(2, 14) = 0.40, p = 0.68, η2 = 0.006) or phase (F(2, 28) = 0.33, p = 0.72, η2 = 0.005). As with mammalian object trials, there was a significant effect of complexity (F(2, 28) = 4.45, p = 0.02, η2 = 0.069). There was a significant three-way interaction between group, complexity, and phase (F(8, 56) = 2.29, p = 0.03, η2 = 0.143). Follow up ANOVAs for each group revealed that there was a significant interaction of complexity and phase for the control group (F(4, 20) = 3.38, p = 0.03, η2 = 0.306), but not for the amygdala lesioned (F(4, 30) = 1.73, p 0.17, η2 = 0.058) or hippocampus lesioned (F(4, 16) = 0.95, p = 0.46, η2 = 0.110) groups. The mean durations of time spent at the front of the cage by group, object complexity, and phase for reptilian object trials are shown in Figure 8.
Figure 8.
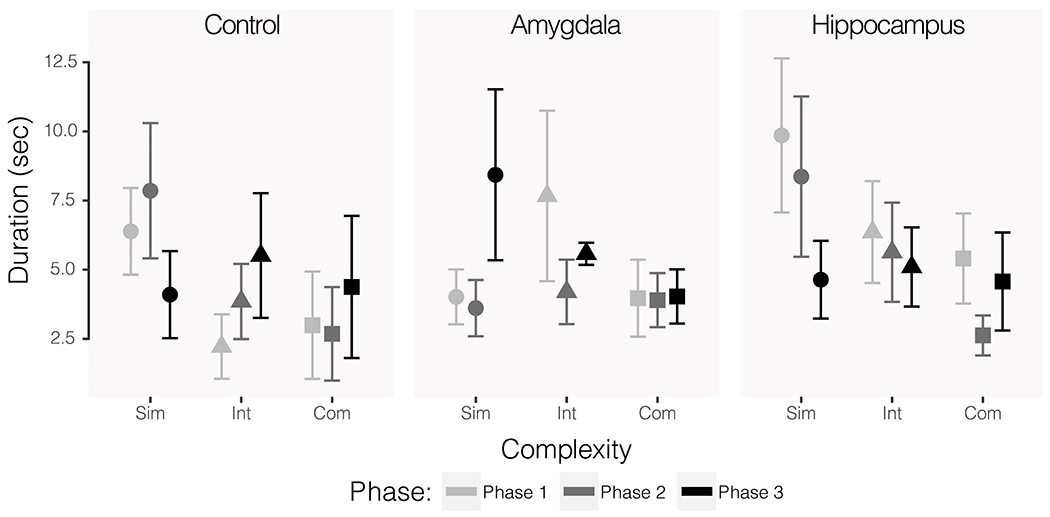
Duration of time spent at the front of the cage during reptilian-like object trials only. All phases represent post-operative data and exposure to the same series of objects. The first iteration of testing (Phase 1) is shown in the lightest grey, the second (Phase 2) is shown in the second lightest grey, and the third (Phase 3) is shown in the darkest color. Circular data points show average responses across simple object trials, triangles show intermediate, and squares show complex. Error bars show SEM.
Affectivity reactivity.
Affect-related behaviors were too infrequent to perform meaningful statistical analyses. The mean reactivity indices were generally higher for complex mammalian objects (controls: M = 0.53, SE = 0.13; amygdala lesioned: M = 0.11, SE = 0.06; hippocampus lesioned: M = 0.18, SE = 0.07) than intermediate (controls: M = 0.17, SE = 0.05; amygdala lesioned: M = 0.10, SE = 0.08; hippocampus lesioned: M = 0.04, SE = 0.02) or simple (controls: M = 0.07, SE = 0.05; amygdala lesioned: M = 0.08, SE = 0.05; hippocampus lesioned: M = 0.06, SE = 0.03) mammalian objects. Affect-related behaviors were more frequent during reptilian object trials but still very infrequent. The mean reactivity indices were again higher for the more complex objects (controls: M = 0.94, SE = 0.29; amygdala lesioned: M = 0.06, SE = 0.04; hippocampus lesioned: M = 0.53, SE = 0.20) as compared to intermediate (controls: M = 0.94, SE = 0.26; amygdala lesioned: M = 0.03, SE = 0.03; hippocampus lesioned: M = 0.39, SE = 0.14) or simple (controls: M = 0.17, SE = 0.08; amygdala lesioned: M = 0.00, SE = 0.00; hippocampus lesioned: M = 0.00, SE = 0.00) ones. Here, amygdala lesioned animals showed a reduction in affective reactivity as compared to the other two groups. When collapsed across levels of complexity group differences are particularly apparent (controls: M = 0.69, SE = 0.13; amygdala lesioned: M = 0.03, SE = 0.02; hippocampus lesioned: M = 0.31, SE = 0.08).
Discussion
The present study builds upon a number of experiments and previous reports which have used similar tasks to understand how damage to the amygdala and other temporal lobe structures impacts responsivity to threat. By directly replicating the experiments of one of these studies (Mason et al., 2006), the present study speaks to the replicability—or lack thereof—of these effects. We characterized the responses to different types of threats (objects with varying complexity and an unfamiliar human) in adult male rhesus monkeys before and after either sham surgeries or surgeries in which the amygdala or hippocampus were bilaterally removed. Since the original phenotype of temporal lobe damage was described by Klüver and Bucy (1939), an important, or even critical, role for the amygdala and hippocampus in the generation of affective responses to threat has been perpetuated in the literature by many contemporary scholars. For example, “in primates, the amygdala has an important role in mediating initial responses to fearful stimuli” (Kalin et al., 2001 p 2073). From other amygdala lesion studies, authors have concluded “these findings point to a distinction between reward processing and emotional reactions, with the amygdala having a crucial role in the latter and only a conditional role in the former” (Murray, 2007 p 494) and amygdala lesions “prevented the normal expression of defensive, adaptive responses elicited by fear-provoking stimuli (i.e., fake snake and spider), confirming an important role for the amygdala and the hippocampus in the normal expression of the fear response” (Chudasama et al. 2009 p 2336). Similarly, the amygdala and hippocampus are thought to be “essential for the normal regulation of anxiety in response to different magnitudes of danger” (Machado et al., 2008 p 938). And finally, “findings… confirm previous reports that radiofrequency or ibotenic acid lesions of the amygdala produce changes in emotional responsivity and fear” (Stefanacci et al., 2003 p 1041). In contrast with this view and the Mason et al. (2006) report, we found very few behavioral differences between operated controls and subjects with lesions of the amygdala or hippocampus. Amygdala and hippocampus lesioned subjects did not differ significantly from controls in their responses to an unfamiliar human in the Human Intruder Test. Likewise, group differences were not evident in the presence of novel objects in the first run of object responsiveness testing. In the second run of object responsiveness, group differences were evident between the control and amygdala lesioned groups only during trials where subjects faced objects intended to resemble reptiles. Even in this case, differences between the amgydala-lesioned and control animals were minimal. Amygdala lesioned subjects did not manipulate these objects any more frequently than controls manipulated objects, as has been shown previously with selective damage to the amygdala, nor did they exhibit differing frequencies of affect-related behaviors in their presence. That is, we did not replicate the findings of Mason et al. (2006).
Amygdala or hippocampus damage does not alter reactivity during the Human Intruder Test
One of the unique strengths of the current experiment is that it partially replicates previous work from our group allowing us to speak directly to replicability of effects across different samples. When monkeys from our previous study (Mason et al., 2006) were tested on the Human Intruder Test, monkeys with amygdala lesions spent significantly more time at the front of the cage across conditions (near vs. far, profile vs. stare) than did control monkeys. The present study did not replicate this effect—monkeys with amygdala lesions and control animals did not differ significantly in the time that they spent at the front of the cage. Mason et al. (2006) also found that while control monkeys calibrated their responses appropriately to the different conditions (i.e., controls responded significantly differently to near vs. far and profile vs. stare), monkeys with amygdala lesions did not show significant differences across the escalating threat conditions. We did not replicate these effects either. Monkeys with amygdala lesions in our experiment did appropriately calibrate their responses across conditions, as did control animals. One finding that was consistent across our study and the original (Mason et al., 2006) is that there were not significant differences in the frequencies of affective behaviors generated by control or amygdala lesioned monkeys during the Human Intruder Test. That said, the frequencies of affective behaviors across groups were very low. It is important to note that while our findings are not consistent with those of Mason et al. (2006) and other reports (e.g., Aggleton & Passingham, 1981; Zola-Morgan et al., 1991; Meunier & Bachevalier, 2002; Machado & Bachevalier, 2008), they are consistent with findings presented by Kalin et al. (2001) and Izquierdo et al. (2005), where amygdala lesions failed to impact responses to a human intruder. The present study provides evidence that monkeys with amygdala lesions generate the same responses to an unfamiliar and threatening human as procedure-matched control monkeys, in contrast with the results of our previous study.
Amygdala, but not hippocampus, damage results in subtle alterations only to responses to objects resembling reptiles in the Object Responsiveness Tests
The test of Object Responsiveness used by Mason et al. (“Response to Animal-Like Objects”) is directly comparable to our Object Responsiveness Test I. In their study, Mason et al. showed that monkeys with amygdala lesions did not change their responses across levels of complexity, while control monkeys exhibited increasing latencies to retrieve a food reward from beside the objects as complexity increased. We did not replicate this effect. In our study, both monkeys with amygdala lesions and controls scaled their responses relative to the level of complexity of the objects (i.e., they retrieved the food reward with a lower latency in the presence of simple objects and greater latency in the presence of complex objects). Additionally, Mason et al. found significant group differences in the amount of time monkeys spent manipulating the objects such that amygdala lesioned animals touched objects for longer durations than control animals. We did not see a difference between groups on this measure. Interestingly, the group differences that Mason et al. showed were due to the fact that monkeys with amygdala lesions touched the objects more as complexity increased, while control monkeys touched the more complex objects less. The control monkeys in our study did not exhibit this same downward trend in duration of contact, which resulted in there being no significant difference between the contact durations for the lesioned and control groups. Our Object Responsiveness Test I findings are consistent with some of those previously reported, despite inconsistencies with the Mason et al. (2006) study. For example, Machado et al. (2009) report that monkeys with amygdala lesions did not respond differently to a variety of threatening objects.
The only effect of amygdala lesions that we saw across these experiments was in the food retrieval behaviors in the presence of objects intended to resemble reptiles (a snake or an alligator). These results are in agreement with previous reports where amygdala lesions resulted in faster food retrieval in the presence of fake snakes (e.g., Kalin et al., 2001; Izquierdo et al., 2005). While amygdala lesioned subjects did not differ significantly from controls or hippocampus lesioned subjects in their food retrieval in the presence of mammalian-like objects, they did retrieve food rewards faster and more frequently in the presence of reptile-like objects. Such effects are typically interpreted as amygdala lesioned animals being less threatened by the objects, and therefore retrieving food faster. It has been hypothesized that snakes have been a significant agent in the evolution of the primate brain, with degree of snake co-existence predicting the expansion and refinement of particular pathways in the brain (Isbell, 2006). If it is the case that the amygdala functions as a protection device, as has been proposed previously (Amaral, 2002; Whalen, 1998), it follows that disruption of this circuitry might perturb responses to those stimuli which the brain has been evolutionarily hard coded to treat as threatening and potentially dangerous. Whether or not hastened food retrieval, without corresponding changes in other dependent variables, represents a true perturbation of the affect system remains up for debate.
The results that we report regarding the time spent at the front of the cage during Object Responsiveness Test II appear counterintuitive. All monkeys spent a decreasing amount of time at the front of the cage across phases. Typically, increased time at the front of the cage is interpreted as increased comfort with the testing procedure (usually as a result of habituating to the situation over repeated presentations) or interest in the objects. In this case, duration at front of cage did not correlate with object manipulation (also used as a proxy for interest in objects) as might be expected if time was a proxy for interest in objects but we also had no evidence that the animals were becoming increasingly uncomfortable with the test procedure. Our test cage has a very low perch at its rear (only a ~;6 cm off of the floor) and our anecdotal experience from other studies is that when animals “check out” of the testing because they are bored or not interested, they retreat to the perch at the rear of the cage — in this case, the decreasing time at the front of the cage would be interpreted as a lack of interest in the objects and testing procedure.
Experimental considerations
Most previous studies indexing affective reactivity in monkeys with brain lesions include the collection of only post-operative data from subjects. Here, we presented data in the Human Intruder Test and Object Responsiveness Test I from both pre-operative and post-operative testing phases. Given the great individual variance in responses to both human and object stimuli, this is an important measure to take in controlling the results of these studies. If amygdala lesions resulted in reduced responsivity to threat, then the behaviors of monkeys with amygdala lesions should appear the same as controls in the pre-lesion testing phase and dramatically different from controls in the post-lesion testing phase. However, this was not the case in these experiments — lesion groups behaved similarly at each measurement moment. And, in some cases (see Figure 4A-C, for example), the pre-operative behavior of the hippocampus group was quite different from the pre-operative of the other two groups simply due to individual differences (but the behavior of the controls and amygdala lesioned animals was similar before surgery). If pre-operative data were not collected from these animals, the results of the tests done on these data may have suggested a significant difference between the groups derived solely from natural differences between animals and not differences caused by the experimental brain lesions that they received. The objects used in pre-operative and post-operative testing were all novel, making it unlikely that the lack of effects can be explained by habituation of the monkeys to the objects. It is possible that pre-operative differences between groups may have obscured lesion effects, although by including pre- and post-operative data in our analyses and comparing animals to themselves, it should be the case that the analyses control for or take into account variation observed pre-operatively, particularly if such effects were as robust as those previously published. It is important to note that the amygdala-lesioned group and control group displayed very similar behaviors pre-operatively and it was the hippocampus-lesioned animals whose behavior differed from the other groups. We expected that amygdala lesions, and not hippocampus lesions, would have the most dramatic impact on affective responding based on the existing literature which was not the case here.
Given that the findings in these experiments do not support amygdala damage as a behavioral pattern that looks at all like the aspects of Klüver-Bucy Syndrome that have been linked to amygdala function, it is critical to note that such null effects are not a failure of the experiments to induce affective reactivity per se. All monkeys responded as expected to varying degrees (or intensities) of threat. In the Human Intruder Test, subjects were much more reactive in the stare condition as compared to the profile condition. Within each of these conditions, subjects were more reactive to the near presentation than the far presentation. In other words, monkeys appropriately modified their behavior according to the level of threat presented. In the two tests of Object Responsiveness, subjects showed varying responses based on the complexity of the objects that were presented. Interestingly, there were conceptually contradictory effects of stimulus complexity on exploration and food retrieval behavior. While subjects were slower to retrieve the food reward from beside more complex objects, suggesting a greater aversion to these objects, they also spent more time manipulating more complex objects, suggesting some level of interest in or attraction to these objects. This is consistent with the idea that food reward retrieval may not be indexing the value of objects as discussed above. Taken together, our findings demonstrate that the tasks were reasonable assays of variation in affective behavior — but neither amygdala nor hippocampus damage modified that affective behavior consistently and significantly across outcome measures.
Contextualizing the present results in the established literature
There are a number of potential explanations for the dissonance between the findings in the present study and those reported by Mason et al. (2006). The first of these is a methodological failure to completely and accurately lesion the targeted structures. That is unlikely, however, because histological verification of the lesions (reported in Antoniadis et al., 2007) detail that the lesions were nearly complete in all cases with the average destruction on each side exceeding 80%. Additionally, the amygdala lesioned subjects used in the experiments presented here were also subjected to an aversive associative learning paradigm (Antoniadis et al., 2007) in which it was clear that the putative function (see Gallagher & Holland, 1994 for a discussion of the amygdala’s role in associative learning) of the amygdala had been taken offline by the lesions. Similarly, histological evaluation of the hippocampus lesioned animals revealed complete damage (Banta Lavenex et al., 2006). These hippocampus lesioned monkeys were incapable of spatial relational learning, consistent with appropriate destruction of the hippocampal formation (Banta Lavenex et al., 2006). The combination of these behavioral and histological findings for each group give us confidence that the null effects we report in this study cannot be explained by a failure to accomplish the intended lesions of the amygdala or hippocampus or something remarkably unique about this sample that rendered them unimpacted by amygdala or hippocampus damage.
It is also unlikely that either sparing of amygdala tissue in Cases E and F or extraneous damage as a consequence of the lesion surgeries explains the failure to replicate the findings by Mason et al., (2006). Statistical analyses from the Human Intruder Test and Object Responsiveness I were repeated dropping the two cases that had the greatest sparing of amygdala tissue. The results of those analyses were identical, suggesting that those two cases were not driving the null effects. Further, minimal extraneous damage occurred in regions outside of the amygdala (including the endopiriform nucleus, ventral claustrum, and rhinal cortex) in the case of the present study (see Antoniadis et al., 2007 for detailed descriptions of extraneous damage) and in the case of the study done by Mason et al. (2006) (see Emery et al., 2001 for detailed descriptions of that extraneous damage). An additional difference between this study and the Mason et al. (2006) is the pre- and post-testing in this study, although we do not think that this methodological difference is a likely cause of the null effects. A few previous studies of amygdala lesions have used similar pre-post designs and still produced the expected effects of amygdala lesions (e.g., Kalin et al., 2001; Izquierdo et al., 2005). It is true that amygdala lesions disrupt post-operative acquisition of conditioned threat responses while they do not impact pre-operatively conditioned responses (Antoniadis et al., 2007), but we expected that affective responses that do not have to be learned should not be subject to the same effects as learned responses. Regardless, we cannot completely rule out the impact of the pre-post design on the results given that the only effects of amygdala lesions were seen in the test that was not also administered pre-operatively. It is important to note that with the exception of pre-operative testing done here, the testing experiences of the monkeys in the present study are identical to those of the monkeys tested by Mason et al. (2006).
An additional potential methodological explanation for the null effects we present here is the timing of the behavioral testing relative to the lesion surgeries. The post-operative phase for Object Responsiveness Test I did not occur until 3 months after surgery and Object Responsiveness Test II did not occur until over 2 years after lesions had been completed. Post-operative data collection for the Human Intruder Test did not occur until approximately 10 months after surgery. The behavioral testing completed by Mason et al. (2006) occurred approximately 5 months after surgery, an intermediate timepoint relative to the testing carried out here. In several reports on the effects of amygdalectomy, researchers have found diminished differences between lesion and control groups with the passage of time. Horel et al. (1975) found a significant effect of amygdala and temporal cortex lesions on what they called “emotional” behavior, but when animals were evaluated again prior to sacrifice (2-5 months after the operations were completed), this effect was no longer significant. Similarly, Stefanacci et al. (2003) tested the tendency of monkeys with amygdala lesions and controls to retrieve a food reward from beneath ostensibly threatening and neutral stimuli both 3 months and 23 months following surgery. In their study, the lesion and control groups differed significantly in approach behaviors on the initial test (at 3 months) but were not significantly different on the retest (23 months). These time effects point to the possibility that the brain is accommodating damage to the focal structures via plasticity mechanisms. For example, experimental and non-experimental lesions of the hippocampus of adult and infant monkeys cause plasticity in the amygdala (Chareyron et al., 2016). Significant cortical reorganization has been demonstrated in the secondary somatosensory cortex of adult-lesioned monkeys following lesions of the primary somatosensory cortex (Pons et al., 1988). Significant enlargement of the cingulate cortex, medial superior frontal gyrus, and medial parietal cortex has also been demonstrated in monkeys who received amygdala lesions as infants (Grayson et al., 2017). More work is needed to determine what macro- and microstructural changes may be occurring as a result of lesions of the amygdala and other structures in monkeys across the lifespan in order to determine whether this is a potential explanation for the variability that has been demonstrated here and elsewhere in the effects of such brain lesions.
While the neurobiological focus of most threat responding tasks is on the amygdala, the Object Responsiveness Test I revealed a potential effect of hippocampal lesions on the time spent at the front of the cage. In threat responsivity tasks, effects like this are traditionally interpreted as animals exhibiting less aversion to the object stimuli (i.e., they do not feel the need to retreat to the back of the cage as the objects are not sufficiently threatening), but an alternative explanation exists. Previous reports have indicated that monkeys with hippocampus lesions explore their test cage environments (including physically touching or moving through their environments) more than both operated controls and monkeys with amygdala lesions (adult lesions: Machado & Bachevalier, 2006; neonatal lesions: Bliss-Moreau et al., 2013). As such, it is possible that the increased time spent at the front of the cage is merely an indirect measure of increased total movement about the cage and tendency to explore aspects of the environment like the cage bars and other elements of the caging set up.
Concluding Remarks
In conclusion, we repeated the experiments from our group’s previous reports evaluating how lesions to temporal lobe structures impact affective reactivity and found minimal effects, and thus did not replicate the findings. Despite the largely null effects presented on reactivity to threat by monkeys with lesions of the amygdala or hippocampus, there was evidence that amygdala lesioned and control animals differentially respond to reptiles — consistent with evolutionary arguments about the threat value of snakes and the amygdala’s role as a threat detector. That said, the lack of consistent significant effects that differentiated lesion groups underscores the need for reproducibility efforts in nonhuman primate science in order to generate normative theory. Finally, our results point to a new research direction as well. The lack of significant effects may be due to a factor that has previously been largely ignored in nonhuman primate lesion studies: the role of plasticity following brain damage and its ability to help animals overcome deficits resulting from lesions. Future research will investigate such plasticity mechanisms.
Acknowledgments
This research was supported by the California National Primate Center’s base grant (current grant number OD011107) and R01-MH75702.
References
- Aggleton JP, & Passingham RE (1981). Syndrome Produced by Lesions of the Amygdala in Monkeys (Macaca mulatta). Journal of Comparative and Physiological Psychology, 95(6), 961–977. [DOI] [PubMed] [Google Scholar]
- Amaral DG (2002). The Primate Amygdala and the Neurobiology of Social Behavior: Implications for Understanding Social Anxiety. Biological Psychiatry, 51(1), 11–16. [DOI] [PubMed] [Google Scholar]
- Amaral DG (2006). The amygdala, social behavior, and danger detection. Annals of the New York Academy of Sciences, 1000(1), 337–347. 10.1196/annals.1280.015 [DOI] [PubMed] [Google Scholar]
- Antoniadis EA, Winslow JT, Davis M, & Amaral DG (2007). Role of the Primate Amygdala in Fear-Potentiated Startle: Effects of Chronic Lesions in the Rhesus Monkey. Journal of Neuroscience, 27(28), 7386–7396. 10.1523/JNEUROSCI.5643-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banta Lavenex P, Amaral DG, & Lavenex P (2006). Hippocampal Lesion Prevents Spatial Relational Learning in Adult Macaque Monkeys. Journal of Neuroscience, 26(17), 4546–4558. 10.1523/JNEUROSCI.5412-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, & Walker S (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1), 1–48. [Google Scholar]
- Baxter MG, & Murray EA (2002). The amygdala and reward. Nature Reviews Neuroscience, 3(7), 563–573. 10.1038/nrn875 [DOI] [PubMed] [Google Scholar]
- Bliss-Moreau E, Amara RR, Buffalo EA, Colman RJ, Embers ME, Morrison JH, Quillen EE, Sacha JB, & Roberts CT (2021). Improving Rigor and Reproducibility in Nonhuman Primate Research [Paper submitted for publication]. [DOI] [PMC free article] [PubMed]
- Bliss-Moreau E, Bauman MD, & Amaral DG (2011). Neonatal amygdala lesions result in globally blunted affect in adult rhesus macaques. Behavioral Neuroscience, 125(6), 848–858. 10.1037/a0025757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E, & Baxter MG (2018). Estradiol treatment in a nonhuman primate model of menopause preserves affective reactivity. Behavioral Neuroscience, 132(4), 224–229. 10.1037/bne0000253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E, & Moadab G (2016). Variation in Behavioral Reactivity Is Associated with Cooperative Restraint Training Efficiency. Journal of the American Association for Laboratory Animal Science, 55(1), 9. [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E, Moadab G, Bauman MD, & Amaral DG (2013). The Impact of Early Amygdala Damage on Juvenile Rhesus Macaque Social Behavior. Journal of Cognitive Neuroscience, 25(12), 2124–2140. 10.1162/jocn_a_00483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, & Shafer EA (1888). An investigation into the functions of the occipital and temporal lobes of the monkey’s brain. Philosophical Transactions of the Royal Society of London B, 179, 303–327. 10.1098/rstb.1888.0011 [DOI] [Google Scholar]
- Capitanio JP (1999). Personality dimensions in adult male rhesus macaques: Prediction of behaviors across time and situation. American Journal of Primatology, 47(4), 299–320. [DOI] [PubMed] [Google Scholar]
- Chareyron LJ, Amaral DG, & Lavenex P (2016). Selective lesion of the hippocampus increases the differentiation of immature neurons in the monkey amygdala. Proceedings of the National Academy of Sciences, 113(50), 14420–14425. 10.1073/pnas.1604288113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Izquierdo A, & Murray EA (2009). Distinct contributions of the amygdala and hippocampus to fear expression: Effects of amygdala and hippocampal lesions on emotion. European Journal of Neuroscience, 30(12), 2327–2337. 10.1111/j.1460-9568.2009.07012.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham WA, & Brosch T (2012). Motivational Salience: Amygdala Tuning From Traits, Needs, Values, and Goals. Current Directions in Psychological Science, 21(1), 54–59. 10.1177/0963721411430832 [DOI] [Google Scholar]
- Emery NJ, Capitanio JP, Mason WA, Machado CJ, & Mendoza SP (2001). The Effects of Bilateral Lesions of the Amygdala on Dyadic Social Interactions in Rhesus Monkeys (Macaca mulatta). Behavioral Neuroscience, 115(3), 515–544. [PubMed] [Google Scholar]
- Gallagher M, & Holland PC (1994). The amygdala complex: Multiple roles in associative learning and attention. Proceedings of the National Academy of Sciences, 91(25), 11771–11776. 10.1073/pnas.91.25.11771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb DH, Capitanio JP, & McCowan B (2013). Risk factors for stereotypic behavior and self-biting in rhesus macaques ( Macaca mulatta ): Animal’s history, current environment, and personality: Stereotypic Behavior and Self-Biting. American Journal of Primatology, 75(10), 995–1008. 10.1002/ajp.22161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson DS, Bliss-Moreau E, Bennett J, Lavenex P, & Amaral DG (2017). Neural Reorganization Due to Neonatal Amygdala Lesions in the Rhesus Monkey: Changes in Morphology and Network Structure. Cerebral Cortex, 27(6), 3240–3253. 10.1093/cercor/bhx080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horel JA, Keating EG, & Misantone LJ (1975). Partial Kluver-Bucy Syndrome Produced By Destroying Temporal Neocortex or Amygdala. Brain Research, 94, 347–359. [DOI] [PubMed] [Google Scholar]
- Isbell LA (2006). Snakes as agents of evolutionary change in primate brains. Journal of Human Evolution, 51(1), 1–35. 10.1016/j.jhev0l.2005.12.012 [DOI] [PubMed] [Google Scholar]
- Izquierdo A (2005). Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. Journal of Neuroscience, 25(37), 8534–8542. 10.1523/JNEUROSCI.1232-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, & Murray EA (2004). Combined unilateral lesions of the amygdala and orbital prefrontal cortex impair affective processing in rhesus monkeys. Journal of Neurophysiology, 91(5), 2023–2039. 10.1152/jn.00968.2003 [DOI] [PubMed] [Google Scholar]
- Izquierdo A, & Murray EA (2007). Selective Bilateral Amygdala Lesions in Rhesus Monkeys Fail to Disrupt Object Reversal Learning. Journal of Neuroscience, 27(5), 1054–1062. 10.1523/JNEUROSCI.3616-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, & Shelton SE (1989). Defensive behaviors in infant rhesus monkeys: Environmental cues and neurochemical regulation. Science, 243(4899), 1718–1721. 10.1126/science.2564702 [DOI] [PubMed] [Google Scholar]
- Kalin NH, & Shelton SE (1998). Ontogeny and stability of separation and threat-induced defensive behaviors in rhesus monkeys during the first year of life. American Journal of Primatology, 44(2), 125–135. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, & Davidson RJ (2004). The Role of the Central Nucleus of the Amygdala in Mediating Fear and Anxiety in the Primate. Journal of Neuroscience, 24(24), 5506–5515. 10.1523/JNEUROSCI.0292-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ, & Kelley AE (2001). The Primate Amygdala Mediates Acute Fear But Not the Behavioral and Physiological Components of Anxious Temperament. The Journal of Neuroscience, 21(6), 2067–2074. 10.1523/JNEUROSCI.21-06-02067.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassambara A (2020). rstatix: Pipe-Friendly Framework for Basic Statistical Tests. [Google Scholar]
- Kuznetsova A, Brockhoff PB, & Christensen RHB (2017). lmerTest Package: Tests in Linear Mixed Effects Models. Journal of Statistical Software, 82(13), 1–26. [Google Scholar]
- LaBar KS, & LeDoux JE (1996). Partial Disruption of Fear Conditioning in Rats With Unilateral Amygdala Damage: Correspondence With Unilateral Temporal Lobectomy in Humans. 110(5), 991–997. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Lavenex PB, Bennett JL, & Amaral DG (2009). Postmortem changes in the neuroanatomical characteristics of the primate brain: Hippocampal formation. The Journal of Comparative Neurology, 512(1), 27–51. 10.1002/cne.21906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth RV (2016). Least-Squares Means: The R Package lsmeans. Journal of Statistical Software, 69(1), 1–33. [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, & Barrett LF (2012). The brain basis of emotion: A meta-analytic review. Behavioral and Brain Sciences, 35(3), 121–143. 10.1017/S0140525X11000446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludecke D (2020). Statistical Functions for Regression Models (Version 0.18.0).
- Machado CJ, & Bachevalier J (2006). The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta). Behavioral Neuroscience, 120(4), 761–786. 10.1037/0735-7044.120.4.761 [DOI] [PubMed] [Google Scholar]
- Machado CJ, & Bachevalier J (2007). The effects of selective amygdala, orbital frontal cortex or hippocampal formation lesions on reward assessment in nonhuman primates: Reward assessment in nonhuman primates. European Journal of Neuroscience, 25(9), 2885–2904. 10.1111/j.1460-9568.2007.05525.x [DOI] [PubMed] [Google Scholar]
- Machado CJ, & Bachevalier J (2008). Behavioral and hormonal reactivity to threat: Effects of selective amygdala, hippocampal or orbital frontal lesions in monkeys. Psychoneuroendocrinology, 33(7), 926–941. 10.1016/j.psyneuen.2008.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Kazama AM, & Bachevalier J (2009). Impact of amygdala, orbital frontal, or hippocampal lesions on threat avoidance and emotional reactivity in nonhuman primates. Emotion, 9(2), 147–163. 10.1037/a0014539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason WA, Capitanio JP, Machado CJ, Mendoza SP, & Amaral DG (2006). Amygdalectomy and responsiveness to novelty in rhesus monkeys (Macaca mulatta): Generality and individual consistency of effects. Emotion, 6(1), 73–81. 10.1037/1528-3542.6.1.73 [DOI] [PubMed] [Google Scholar]
- Meunier M, & Bachevalier J (2002). Comparison of emotional responses in monkeys with rhinal cortex or amygdala lesions. Emotion, 2(2), 147–161. 10.1037/1528-3542.2.2.147 [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Murray EA, Málková L, & Mishkin M (1999). Effects of aspiration versus neurotoxic lesions of the amygdala on emotional responses in monkeys. European Journal of Neuroscience, 11(12), 4403–4418. 10.1046/j.1460-9568.1999.00854.x [DOI] [PubMed] [Google Scholar]
- Pons TP, Garraghty PE, & Mishkin M (1988). Lesion-induced plasticity in the second somatosensory cortex of adult macaques. Proceedings of the National Academy of Sciences, 85(14), 5279–5281. 10.1073/pnas.85.14.5279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribram KH, Reitz S, Mcneil M, & Spevack AA (1979). The effect of amygdalectomy on orienting and classical conditioning in monkeys. 14(4), 15. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Clark RE, & Zola SM (2003). Selective neurotoxic amygdala lesions in monkeys disrupt reactivity to food and object stimuli and have limited effects on memory. Behavioral Neuroscience, 117(5), 1029–1043. 10.1037/0735-7044.117.5.1029 [DOI] [PubMed] [Google Scholar]
- Weiskrantz L (1956). Behavioral changes associated with ablation of the amygdaloid complex in monkeys. Journal of Comparative and Physiological Psychology, 49(4), 381–391. 10.1037/h0088009 [DOI] [PubMed] [Google Scholar]
- Whalen PJ (1998). Fear, vigilance, and ambiguity: Initial neuroimaging studies of the human amygdala. Current Directions in Psychological Science, 7(6), 177–188. [Google Scholar]
- Zola-Morgan S, Squire LR, & Amaral DG (1989). Lesions of the amygdala that spare adjacent cortical regions do not impair memory or exacerbate the impairment following lesions of the hippocampal formation. The Journal of Neuroscience, 9(6), 1922–1936. 10.1523/JNEUROSCI.09-06-01922.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Clower RP, & Alvarez-Royo P (1991). Independence of memory functions and emotional behavior: Separate contributions of the hippocampal formation and the amygdala. Hippocampus, 1(2), 207–220. 10.1002/hipo.450010208 [DOI] [PubMed] [Google Scholar]


