Abstract
Purpose:
To review the use of molecular diagnostic techniques in the management of ocular infectious disease
Design:
Retrospective review.
Methods:
Combination of literature review and personal recollections.
Results:
While the broad term molecular diagnostics may encompass techniques to identify pathogens via protein or metabolomic signatures, this review concentrates on detection of pathogen nucleic acid as an indicator of infection. The introduction of the polymerase chain reaction (PCR) in 1985 opened a new era in analysis of nucleic acids. This technique was soon applied to the detection of potential pathogen DNA and RNA, including viruses, bacteria, and parasites in infectious eye disease. Advances in PCR have allowed class-specific diagnostics (i.e. pan-bacterial and pan-fungal), quantitation of pathogen DNA, and multiplexed testing. The Human Genome Project in the early 2000’s greatly accelerated development of DNA sequencers, ushering in the era of ‘Next Generation Sequencing’, and permitting pathogen-agnostic methods for detection of potential infectious agents. Most recently, new technologies such as nanopore sequencing have brought down both cost and equipment requirements for whole genome sequencing; when coupled with real-time sequence analysis methods, these methods offer the promise of true, real-time, point-of-service ocular infectious disease diagnostics.
Conclusions:
Molecular methods for pathogen detection have greatly advanced the diagnosis of ocular infectious disease. Further methodologic advances will directly impact the management of these conditions.
Table of contents statement
For the 78th Edward L. Jackson, MD lecture, the author reviews the history and utility of nucleic acid molecular biologic techniques for the diagnosis and characterization of ocular infectious disease.
Introduction
I am deeply grateful to the selection committee of the American Journal of Ophthalmology and to the American Academy of Ophthalmology for the singular honor of presenting the 78th Edward L. Jackson, MD lecture at the American Academy of Ophthalmology annual meeting in 2021. When I review the roster of past Jackson lecturers, I am humbled by their singular and aggregate contributions to our specialty. My own contributions have been quite modest by comparison; but I am grateful to have the opportunity to share the story of progress in the field of ocular infectious disease diagnostics with the AAO membership and AJO readership. Four other Jackson lecturers – Michael Hogan, MD (toxoplasmosis, 19571), John M. McClean, MD (ocular mycosis, 19632), and my friends and uveitis specialist colleagues Gary Holland, MD (toxoplasmosis, 20033, 4), and Douglas Jabs, MD (cytomegalovirus, 20105), have chosen topics in ocular infectious diseases as subjects for their Jackson lectures. I am glad to have the opportunity to point the spotlight on ocular infectious diseases once again, particularly in a year in which a new infectious agent – SARS-CoV2, causing COVID-19 disease – has so dominated our news and our lives; and a year in which terms like ‘PCR test’ have entered the general lexicon.
Dr. Edward L. Jackson was a luminary in the early days of American ophthalmology. He served as one of the first Presidents of the American Academy of Ophthalmology and Otolaryngology in 1904, as the second editor of the modern version of the American Journal of Ophthalmology, and as the founder of the American Board of Ophthalmology among many other accomplishments in a career marked by service to the profession. His life and accomplishments have been well-reviewed by several previous Jackson lecturers.6–9 Dr. Jackson passed away 79 years ago, in October of 1942, at the age of 86. If Dr. Jackson’s ghost returned in 2021, what aspects of ophthalmic practice would seem unfamiliar to him? Many of the daily aspects of modern care would surely raise the ghost’s eyebrows, including the electronic medical record and telehealth. I am sure he would be astounded at the work required for documentation and billing, and at the number of employees whose sole job is to work with insurers to garner payment for services. In terms of ophthalmic surgery, femtosecond small incision cataract surgery with foldable multifocal lenses would seem something out ‘Brave New World’. Lamellar keratoplasty, vitrectomy, and microincisional glaucoma devices would be remarkable to Dr. Jackson’s ghost. In ophthalmic diagnostics, the direct ophthalmoscope (invented by von Helmholtz in the 1880s) and the slit lamp (invented by von Gullestrand in 1911) would seem familiar, and indeed have changed little in the past 70 years; likewise, trial lens sets and the phoropter are in essentially unchanged condition, and no doubt Dr. Jackson’s ghost would take great satisfaction at seeing his own contribution, the Jackson Cross, still in widespread use for measurement of astigmatism. However, the binocular indirect ophthalmoscopy post-dated Dr. Jackson’s passing by a decade, as did standardized visual field testing. Fluorescein angiography was established in Dr. Jackson’s lifetime, but our other, widely used imaging modalities such as optical coherence tomography, OCT angiography, ophthalmic ultrasound, and magnetic resonance imaging were not even conceivable in Dr. Jackson’s day.
However, if Dr. Jackson’s ghost were to watch an ophthalmologist in 2021 manage a corneal ulcer, he would feel immediately familiar with the process. In his 1899 textbook, “A Manual of the Diagnosis and Treatment of Diseases of the Eye”, Dr. Jackson discussed suppurative corneal ulcer, stating, “The causes…may be the pneumococcus… or the diplobacillus, or the diphtheria bacillus, or one of these with the pus cocci…The exact organisms of infection can only be determined by careful microscopic and culture examinations of the scrapings obtained from the surface of the ulcer.” The culturing of the ulcer with a spatula or sterile scalpel blade, the streaking of blood agar and chocolate agar plates, the use of anaerobic broths such as ‘Brain-heart infusion’ or Sabauraud’s media (both introduced in the 1890’s), and performance of Gram stain (1884) were all well established in Jackson’s lifetime. Indeed, the use of blood agar plates has been essentially unchanged since these were first developed by Robert Koch’s fellow, Walter Hesse, in 1888. (Of note, it was Walter’s wife Fannie who made the key observation that agar – a seaweed extract used in making of fruit preserves – would be ideal for culture plates). The differential diagnosis for bacterial keratitis – with bacteria such as Staphylococcus, Streptococcus, and Pseudomonas, was well known in Jackson’s time (as were herpetic and zoster keratitis, and syphilitic interstitial keratitis, which are described in Dr. Jackson’s text). The use of topical antibiotics for ocular surface disease likewise has a long history overlapping Dr. Jackson’s life. Dr. Jackson commends a 10% solution of cassareep (cassava oil), “both to relieve pain and favorably influence the course of the disease”. Carl Credé, MD, first used silver nitrate as a prophylaxis for ophthalmia neonatorum in 1880; however, Jackson notes that “(f)or ulcers that are spreading at all rapidly, the infiltration extending beyond them into neighboring tissues, caustics, like silver nitrate, or strong solutions of mercuric clorid (sic) have not sufficient penetrating power to be efficient.” The first use of topical antibiotics on the eye is attributed to Katiofsky, who used sodium sulfacetamide for treatment of eye infections including trachoma in 1939,10 several years before Dr. Jackson’s death. Other antibiotics, still in use today, emerged shortly after Dr. Jackson’s passing. Streptomycin, the first aminoglycoside antibiotic, was discovered in 1943, and tobramycin – still widely used in these infections – was discovered 54 years ago in 1967. Vancomycin has similarly long provenance, having been discovered in 1952.
Unfortunately, while time has stood relatively still for ocular infectious disease diagnosis and treatment, is not because we have reached anything close to a state of perfection. Nearly half of corneal ulcers do not yield a pathogen on culture or gram stain, and 30-45% of endophthalmitis cases similarly yield no organism to culture despite being clearly of infectious origin. This has forced use of broad spectrum antibiotics such as vancomycin and moxifloxacin, which in turn has contributed to the development of resistant antibiotic strains. In one recent study, 25% of staphylococcal isolates from healthy conjunctiva were fluoroquinolone-resistant.11 The continuous use of these 19th and 20th century diagnostic methods and treatments in the 21st century has been due to a stalled progress.
The development of PCR as a diagnostic test for infectious disease
It is important to note here that the ‘gold standard’ for linking infection to disease remains ‘Koch’s postulates’, which state that ascribing disease causation to a micro-organism requires isolating and culturing the suspected organism, infecting a naïve host with the purified pathogen, inducing the anticipated disease, and again recovering the micro-organism or virus from the infected host.12 When Koch promulgated these postulates at the turn of the 20th century, however, it was not recognized that many organisms cannot be readily cultured; nor that immunity may prevent recapitulation of disease is specific hosts. It has thus become clinical standard-of-care to utilize pathogen detection as a proxy test for microbial disease. Whether the detected pathogen is causative of disease is a deeper question that must be evaluated in each case based on clinical circumstances.
Bacterial stains such as Gram stain and bacterial cultures are direct diagnostic tests; the organism is either physically identified from a biopsy sample or grown from a sample to prove its presence. The use of indirect tests for infectious organisms was known in Jackson’s era. The Wasserman test for syphilis (a hemagglutination based on anti-cardiolipin antibodies, published in 1906) and the Sabin-Feldman dye test for toxoplasmosis (published in 1948) are examples of indirect tests whereby presence of an antibody generated in response to a specific infection is taken as evidence for infection. Viruses, first identified by Peyton Rous in 1911, can only be directly visualized by electron microscopy (which, remarkably was first done in Jackson’s lifetime, in 1940), but this technique has never been practical for clinical use. Rather, viral infections have been diagnosed using indirect tests such as cytology and particularly serologic tests, as well as plaque-based infection assays on purified cells when necessary.
The idea that all pathogens are nucleic acid-based lifeforms (i.e. DNA or RNA) arose slowly over the early- and mid- 20th century. Indeed, the Avery-McLeod-McCarty definitive demonstration that DNA is hereditary material13 was based on bacterial transformation with DNA, and the Hershey-Chase “blender experiment” of 195214, which cemented the role of DNA rather than protein in hereditary transmission, was based on bacteriophage, a form of bacterial virus. The phage researchers of the 1940’s and 1950’s, including Delbruck, Luria, and Benzer, established the molecular biology of heredity, and Kornberg’s elucidation of DNA replication as well as Jacob and Monod’s discovery of dynamic gene regulation were all performed in E. coli bacteria. The first ‘organisms’ to have their RNA and DNA genomes fully sequenced were MS2 bacteriophage by Fiers and colleagues15 in 1976, and PhiX174 bacteriophage by Sanger and colleagues16, in 1978. (The first bacterium to be fully sequenced, H. influenzae, was not sequenced until 1995 by Craig Venter and associates17). It was clear by the 1980’s that each pathogen would have a unique genetic sequence. What was missing was a technique to allow for the rapid and specific detection of nucleic acids.
The polymerase chain reaction (PCR) had its intellectual roots in the work of Nobel Prize winner Gobind Khorana. Khorana, who in later years would turn his research interests to rhodopsin and mechanisms of vision, was best known for solving large portions of the genetic code for translating nucleic acid sequences to protein sequences. In 1971, he and colleagues proposed a means for ‘amplifying’ DNA in vitro using a DNA polymerase;18 in essence, this was an early description of PCR. The definitive description of modern-day PCR was published by Kary Mullis and colleagues, first in 198519 and then in more current form in 1988.20 (Mullis also won the Nobel prize for this work in 1993). I remember distinctly the buzz that PCR generated in the molecular biology research community. I was a graduate student at Stanford when the 1988 paper came out – the next week I remember trying to do a PCR experiment, using three heat blocks of different temperatures, and manually transferring samples between temperatures, while adding DNA polymerase enzyme each round. (Suffice to say, my first efforts to replicate this work were not successful; I subsequently used PCR almost daily in my thesis research).
In its essence, PCR is DNA replication stripped of all inessentials (Figure 1). To perform this technique, one needs a DNA template (for instance, DNA purified from a biopsy sample), a DNA polymerase, deoxynucleotide triphosphate ‘building blocks’ for synthesizing DNA, and – most importantly – two small oligonucleotide primers oligonucleotide primers that have sequences flanking the regions of the gene of interest, typically within a relatively short distance (< 1000 base pairs) of each other. This latter requirement gives PCR its specificity; under correct salt and temperature conditions, the oligonucleotide primers will bind only to their cognate sequence and will thus only amplify the specific gene they are directed toward. PCR is performed by cycling the temperature, from an annealing temperature (typically between 50 and 60 degrees C) for the oligonucleotides to bind, to an extension temperature (typically 72 degrees, during which the DNA is synthesized), and then a melting temperature (usually above 95 degrees C) where parent and daughter strand are dissociated by heat. The technique as we know it today was facilitated by the purification of heat-stable DNA polymerase from a thermophilic bacterial species (the Thermus aquaticus bacteria living in the geothermal boiling hot springs at Yellowstone National Park). As the DNA polymerase from this species can withstand the 95 degree C denaturation step, this allows the PCR process to occur in a single tube within an automated thermal cycler, without having to add additional reagents during the thermal cycling.
Figure 1.

Schematic of the polymerase chain reaction. Starting with a patient sample (i.e., aqueous or vitreous biopsy, conjunctival swab, or blood), DNA is partially purified. Oligonucleotide primers (red = upstream, blue = downstream) are synthesized and allowed to anneal to the target DNA. As the temperature is increased, the DNA polymerase in the reaction becomes active, allowing template-directed DNA replication of the target sequence. On further elevation of the reaction temperature, the original and newly synthesized DNA strands denature and separate. When the temperature is again lowered, a set of primers can anneal to both the original target (black) and newly synthesized (red) DNA strands, and the process is repeated. PCR will thus give a theoretical yield of 2 N molecules for each starting molecule, amplified through N rounds of annealing, elongation, and denaturation. From 81 with permission.
In addition to being highly specific, PCR is also highly sensitive. Because each ‘daughter’ copy of DNA produced by one of the two primers can become the template for another round of PCR from the other primer, for each N rounds of amplification, 2N copies of the initial DNA are produced. This is the basis of the ‘chain reaction’ part of the PCR name. DNA is produced exponentially; after 35 rounds of PCR (which is often used in clinical testing), a single molecule of DNA in a complex mixture may be transformed into nearly 35 billion copies, or nanogram to microgram quantities that can easily be macroscopically visualized on gel electrophoresis, for example.
Application to known eye infections
It did not take long for this technique to be applied to detection of pathogen DNA in different fields of medicine, and the first two demonstrations of PCR to detect pathogens in the eye (one showing HIV and HHV-6 in AIDS-associated retinitis21, and another showing human papillomavirus type 16 in conjunctival neoplastic lesions22) were both published in 1989, just a year after the initial description of modern PCR. Within another year, PCR was being used to detect herpes family viruses in keratitis23 and retinitis.24
It was about this time, in the early to mid-1990’s, that I began to do research in this area. I had done my neuroscience PhD as part of the NIH Medical Sciences Training Program at Stanford in the late 1980’s and early 1990’s, and had done my thesis work in the laboratories of Drs. Jack Barchas in the Department of Psychiatry and Mark Krasnow in the Department of Biochemistry. Part of my thesis work had been to develop a non-PCR technique for amplifying messenger RNA25 (which turned out to have its own utility for gene expression profiling), and so I was familiar with the nucleic acid amplification field. I used PCR extensively in my thesis research to generate hybridization probes from a large collection of cDNA clones of genes expressed in Drosophila melanogaster heads to test these for circadian rhythmicity.26 By 1995 I was a PGY2 ophthalmology resident at Barnes Hospital/Washington University in St. Louis, when my chairman (and later uveitis fellowship mentor) Hank Kaplan, MD asked if I would be interested in co-authoring a book chapter in an upcoming uveitis text on PCR diagnostics. After we finished this chapter, Dr. Kaplan asked what it would take to set up a PCR laboratory in our department. I remember spending an afternoon at the 1996 AAO meeting in Chicago in a local library putting together a proposal for a diagnostics lab at Wash U, which Dr. Kaplan ultimately approved, and allowed me to lead while I was still a resident. The lab, on the 12th floor of the McMillan building at Barnes Hospital, was just large enough for two people to work in without tripping over each other and was outfitted frugally with hand-me-down centrifuges and equipment (some tracing back to my esteemed mentor and long-serving Wash U chair Bernard Becker, MD). This lab served us very well through my assistant professorship at Wash U.
Our early work in PCR-based diagnostics was aimed at making two technical advances to the field. First, PCR as originally designed required a separate reaction for each pathogen being tested. With the very limited volumes of aqueous and vitreous that we could acquire from patient biopsies, we were often limited to testing a few likely entities on the differential diagnosis but not being able to test the entire differential. Multiplex PCR is a technique in which a nucleic acid sample from a biopsy can be tested for multiple potential pathogens simultaneously, in a single reaction. Positive results can be detected by several techniques including nested amplification or hybridization methods. Using a computer program to help find compatible primers and amplicons, we were able to design a multiplex PCR capable of detecting four common retinitis pathogens – herpes simplex, herpes zoster, cytomegalovirus, and toxoplasmosis – with high sensitivity.27 The second technical advance we tackled was to bring quantitation to these measures. In its original instantiation, PCR products were visualized on ethidium bromide-stained agarose gels, yielding a ‘yes/no’ answer to presence of pathogen DNA or RNA. Several techniques, including SYBR-green intercalating dye and specific TaqMan fluorescent probes allow quantitation of the PCR with performance, in real-time. This technique, known as either real-time PCR (RT-PCR) or quantitative PCR (qPCR) has become standard for quantifying the starting quantities of nucleic acids in PCR experiments. Such techniques can also be applied to RNA viruses, such as HIV (where calculation of viral load has become largely standard-of-care) by incorporating a reverse transcription step to convert RNA to DNA (confusingly, also sometimes referred to as RT-PCR). We were able to successfully generate the first quantitative PCR assay for detection of herpes viruses and toxoplasmosis in ocular tissues28 (see Figure 2), a technique that has become standard-of-care for diagnosis of acute posterior uveitis.
Figure 2.

Standard curves for monoplex and multiplex real-time PCR pathogen detection. Threshold cycle crossings for SYBR-labeled PCR were calculated and plotted as log-linear regressions, as both monoplex (black line) and multiplex (red line) reactions. Data demonstrate preserved sensitivity with multiplex PCR. From 28 with permission.
Bacteria and fungi are special cases for PCR. Both kingdoms have highly conserved genes for their ribosomal RNA components; specific sequences are required to correctly fold the ribosomal structural elements and are therefore found in nearly all bacteria (16S ribosomal RNA) and fungi (18S and 28S ribosomal RNA). It is thus possible to design PCR primers that will amplify all bacteria or fungi in a given sample. While the presence of bacteria or fungi can be rapidly determined, precise determination of the genus or species of the amplified material is less straightforward, and requires some other method to analyze the amplicon, such as DNA sequencing of amplicons or differential gel electrophoresis. To determine the diagnostic potential of this approach for microbial keratitis, we analyzed 108 corneal ulcers collected by my then-student Elma Kim at Aravind Eye Hospital in India (where about 2/3 of ulcers are typically fungal).29 Culture-positive rate in this population was 51%, with 25 bacterial and 31 fungal pathogens discovered. PCR produced a positive band for either bacteria or fungal in 89% of our samples. Agreement with culture was excellent with 26/29 fungal PCR-positive samples agreeing with culture results, and 12/19 bacterial PCR results agreed with culture. However, the real power of this technique was in the culture-negative samples. 17 were found to be bacterial and 29 fungal, roughly the same ratio of bacterial:fungal pathogens as found in culture-positive samples. While sequencing the PCR inserts of bacterial culture-negative, PCR-positive samples predominantly demonstrated bacteria routinely found in corneal ulcers, the novel PCR-positive fungal samples were more interesting, with sequencing of inserts identifying genera such as Sordaria and Phythium in addition the more common Aspergillis and Fusarium. All told, we found sequences of 14 of 46 PCR-positive, culture-negative samples corresponded to either novel or unusual micro-organisms. These results suggest that, for corneal ulcers at least, PCR-based diagnostics are more sensitive than traditional culture, and may reveal unexpected organisms not readily grown in vitro. In our subsequent work, we have been able to identify microsporidia30, Actinomyces31, Ebola virus32 and most interestingly, Prototheca wickerhamii33 (a non-photosynthetic alga, Figure 3) by PCR in specific cases.
Figure 3.
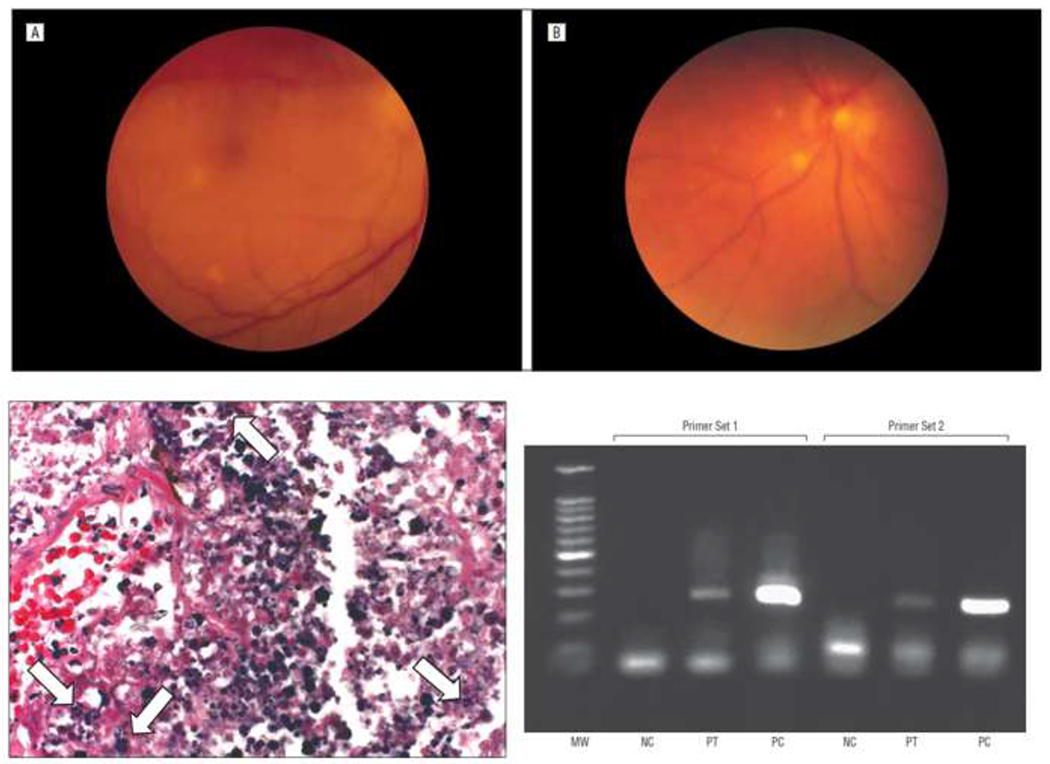
Top: Fundus appearance of 58 year old patient with bilateral choroiditis. Lower left: autopsy specimen of the left choroid demonstrating potential spores (arrows) Lower right: PCR for Prototheca wickerhamii from choroidal lesions from right eye at autopsy using two independent specific primer sets. MW = molecular weight, NC = negative control, PT = patient sample, PC = P wickerhamii positive control. From 33 with permission.
Discovery of novel pathogens and linkage of pathogens to idiopathic disease
In our studies of corneal ulcers with PCR, we found at least three sequences which were “uncultured” 16S or 18S sequences. The finding of novel variants by 16S and 18S metagenomics is not unusual, and indeed has been the basis for many high-profile expeditions to catalogue unseen organisms in environments such as the ocean.34,35 Some of the earliest success in PCR have been in its use to identify new or novel bacteria or other pathogens. Several high-profile examples have occurred in disease. Relman et al.36, for instance, used 16S PCR to identify Tropheryma whippelii, the bacteria responsible for Whipple disease, which is a rare cause of panuveitis.37 Similarly, the pathogen responsible for cat scratch disease and some cases of Leber stellate neuroretinitis, now known as Bartonella hensellae, was identified in infected material by 16S PCR.38,39 Viruses have also been identified by purely molecular means. Kaposi’s sarcoma (KS) had been identified as a potentially virally-mediated complication of immunosuppression from AIDS. Chang et al.40 used a PCR-based differential expression experiment to identify a novel herpes virus, HHV-8, associated with KS. Subsequent studies suggested that treatment with valganciclovir or other antivirals could decrease the risk of KS41 as well as CMV retinitis.
PCR can also be used to link particular pathogens to known conditions. Two stand out in the past several years. Fuchs heterochromic iridocyclitis was first described by Ernst Fuchs in 1906, as a unilateral condition frequently featuring iris heterochromia, chronic indolent uveitis, ocular hypertension, and premature cataract. While numerous infectious organisms had been suspected in this unilateral condition42–44, it was Quentin and Reiber45 who associated the presence of Rubella virus with the condition, through a combination of quantitative measurement of intraocular antibody, and reverse-transcription PCR for the virus itself. Birnbaum and colleagues subsequently demonstrated that the incidence of this condition is declining as would be expected given near-universal vaccination for this virus.46 Glaucomatocyclitic crisis, or Posner-Schlossman Syndrome, is another unilateral condition featuring episodic anterior uveitis and severe ocular hypertension. This condition was linked to cytomegalovirus through PCR of aqueous humor paracentesis.47–49 This was the first condition in immunocompetent patients linked to CMV, and has led to recognition of a spectrum of CMV-associated anterior uveitis and ocular hypertension.50
The transition to deep DNA sequencing
PCR is a remarkably powerful technique, but it suffers from some deficiencies. First, the technique requires knowledge of the DNA sequences being sought. One must have a formed differential diagnosis, and test for those organisms specifically. In many cases, the kingdom of the organism causing infection may not even be known – is the disease viral, bacterial, fungal, or parasitic, for instance? In these cases, PCR may be of limited value. PCR is also prone to false-positive results from contamination. Its sensitivity may turn to a two-edged sword as small amounts of amplified material from previous reactions or reagent contamination can lead to false-positive results.51–53 Finally, PCR provides only a modicum of information about the organism detected; for example, very rarely will strain or subtype be characterized in a simple PCR.
The NIH Human Genome Project was an audacious program begun in the 1990’s to fully sequence the human genome. I remember first hearing about this project at a Stanford Department of Biochemistry retreat in 1991. At that time, cloning of specific genes was in its heyday – a major portion of a typical PhD thesis involved cloning one or two genes encoding important proteins in a model system, and sequencing them. Cloning was a laborious process of transferring phage plaques or bacterial colonies to nitrocellulose filters and ‘probing’ them with radioactive cDNA or RNA probes; sequencing was also very primitive, involving use of radioactive sulfur dideoxynucleotide triphosphates in a Sanger sequencing reaction, and then separating the products on long acrylamide gels run under several-thousand volt potentials. The equipment for doing these experiments was generally homemade, and more than one graduate student in our program suffered near electrocution while sequencing. (I spent most of the year 1991 sequencing about 10,000 base pairs of two circadianly expressed Drosophila genes26, 54 but thankfully survived the experience unharmed). As noted above, the first full bacterial genome sequence was not completed until 1995. So when Professor Ron Davis asked the gathered students and postdoctoral fellows what were we all going to do with our time when worm, fly, mouse, and human genomes were fully sequenced, we all laughed. But of course Professor Davis was correct, and by 2001 the draft 3 billion base pair human genome was published.55, 56
One side benefit of this project was that the cost of DNA sequencing fell precipitously beginning in the early 1990’s. In particular, the development of highly parallel DNA sequencers such as 454 pyrosequencing and the Illumina polony sequencing method (reviewed in 57) resulted in a ‘Moore’s law squared’ improvement in the cost of sequencing, such that the cost of a whole human genome sequence in 2001 – $100,000,000 – had fallen to about $1000 by 2020. I was fortunate that Washington University, where I remained on faculty, was one of the major sequencing centers for the genome project (leading the C. elegans component), and had early access to some of these new sequencing technologies. Under the guidance of my NIH K08 mentor Jeff Milbrandt and senior colleague Jeff Gordon, we began to apply these techniques to ocular samples in hopes of finding new pathogens. At the time, the costs of sequencing were still too high to apply to many samples, and so we developed a technique we called Biome Representational In Silico Karyotyping,58 or BRiSK, in which we utilized an unusual Type IIS DNA restriction enzyme that cut 33 bp of DNA around its 6-bp recognition sequence. As this sequence occurs on average every 46 = 4096 bp, this enzyme effectively creates a representation of ~0.8% of the genomic DNA. Working with then-medical students Valli Muthappan and Aaron Lee, we developed a technique to directly sequence these rapidly on the Illumina platform. At about this time, in 2008, I relocated to my present position as Boyd K. Bucey Chair of the Department of Ophthalmology at University of Washington, which also had a very strong DNA sequencing community.
One of our first applications of this new technology was in understanding the ocular surface microbiome. It had been known for many years that the ocular surface is paucibacterial – that is, it has few culturable bacteria, largely due to intrinsic antimicrobial properties of the conjunctiva and of tears.59, 60 The bacteria that are present tend to be from four genera – Staphylococcus, Streptococcus, Corynebacteria, and Cutibacteria (formerly Propionibacteria). However, several papers in the early 2010’s applying 16S PCR metagenomic techniques to the ocular surface had suggested the presence of hundreds of previously undocumented species from conjunctival swabs.61–63 It is conceivable that PCR might have such greater sensitivity for detection of bacteria on the ocular surface, or that many bacteria are not readily culturable and only detectable by molecular means. However, it is also possible that 16S PCR, when applied to a paucibacterial sample, results in artefactual amplification of sequences, as has been observed in other systems.64 To answer this question our group (led by thenuveitis fellow Thuy Doan) applied BRiSK alongside 16S metagenomics to conjunctival samples of normal subjects.65 We found that while 16S yielded a large number of possible organisms (including unlikely ocular surface residents such as Bradyrhizobium), BRiSK largely identified the same four genera found in culture. Further, when we applied quantitative 16S PCR to the normal ocular surface, we discovered that our total DNA yield from the swab was on the order of 50 bacteria per swab, making the finding of hundreds of species from the ocular surface likely to be artefactual. Such artefacts have been well-described, as it is nearly impossible to rid the PCR reagents of residual bacterial DNA (the so-called ‘kitome’), and the high sensitivity of PCR amplification may reveal these as well as very low-abundance environmental DNA.
One of the more interesting findings from this study, though, came from the ability of BRiSK to identify DNA from any source. We found a substantial number of samples harbored viral sequences. In particular, about half of the samples had DNA from Torque Teno Virus (TTV), while a number also had DNA from Merkel Cell Polyoma Virus (MCPV). These are both small DNA viruses; the former, an anellovirus and the latter a polyoma virus. TTV was first discovered in the 1990’s associated with liver transplant patients, and originally thought of as ‘transfusion transmitted virus’.66 It has since been found to be nearly ubiquitous with 100% of normal subjects infected in some studies.67 Measurement of serum TTV levels can be utilized to assess the degree of immunosuppression in an individual. To date, TTV has not been definitively linked to any disease, however. MCPV was initially discovered as an oncogenic virus underlying Merkel cell carcinoma,68 a rare but potentially fatal cancer of the Merkel cells of the skin (which occurs with some frequency on the periocular skin). Thus, it appears the ocular surface has both a resident paucibacterial microbiome, but also a resident virome.
We next turned our attention to an unmet clinical need: the determination of organisms underlying endophthalmitis. Bacterial endophthalmitis remains one of the most challenging complications of ocular surgery and procedures. While strict attention to aseptic technique and use of prophylactic antibiotics has reduced the rate of endophthalmitis following cataract surgery to between 0.05% and 0.3%.69–71 However, the development of anti-VEGF drugs has resulted in millions more intraocular procedures annually, and a substantial increase in the total number of endophthalmitis cases in the US.72 Remarkably, even though the timecourse of endophthalmitis makes it clear that there is an infectious etiology, in 30% to 45% of cases, no bacterium is cultured. We elected to apply our deep sequencing techniques to endophthalmitis. Working with UW colleagues Cecilia and Aaron Lee, and my friend and former faculty colleague from Wash U, Sunir Garg (now at Wills Eye Hospital in Philadelphia) along with my longtime lab staff Lakshmi Akileswaran, PhD and lab manager Angela Sandt, we first applied 16S PCR to a series of endophthalmitis cases.73 We found that culture-positive cases had detectable bacteria by quantitative 16S PCR, but that culture-negative cases generally did not (Figure 4; note that the cases with less than 2 x 103 16S copies/ml – corresponding to 1 genome per microliter or less – corresponded to the culture-negative cases). Upon applying BRiSK to these samples we found the presence of TTV in the intraocular biopsies in 15/21 samples including 7/7 of the culture negative/PCR negative samples. We followed this up with a much larger study of 50 consecutive endophthalmitis samples in which ‘shotgun’ metagenomics was applied and clinical outcomes were also tracked.74 In this larger population, 48% were culture-positive. Metagenomic sequencing results generally agreed with culture results. A majority of culture-negative samples were also PCR-negative, suggesting that most culture-negative endophthalmitis is truly pathogen-negative (excepting the possibility of RNA viruses). In this study, however, 7/26 of the culture-negative samples were positive for S. epidermidis on deep sequencing, and near-full length bacterial genomic sequences could be reconstructed demonstrating presence of the potential pathogen. Remarkably, however, the combination of PCR/culture positivity and presence or absence of TTV determined outcome. With respect to overall outcomes, we found that culture-negative cases and cases positive for S. epidermidis generally had good outcomes, while all other pathogens led to poor outcomes. We also found that of cases that were PCR-negative and TTV-negative, none required secondary vitrectomy for treatment. Conversely, of those samples cases that were both culture-positive and TTV-positive, all required secondary vitrectomy. Positivity for either culture or TTV gave intermediate risk. Whether TTV is directly pathogenic in endophthalmitis, or represents other risk factors for poor outcome remains to be determined. Overall, these results suggest that knowledge of the intraocular pathogens in bacterial endophthalmitis provides critical data for prognosis and likely treatment. One would hope for refinement of treatment algorithms once real-time point-of-service diagnostics are available (see below).
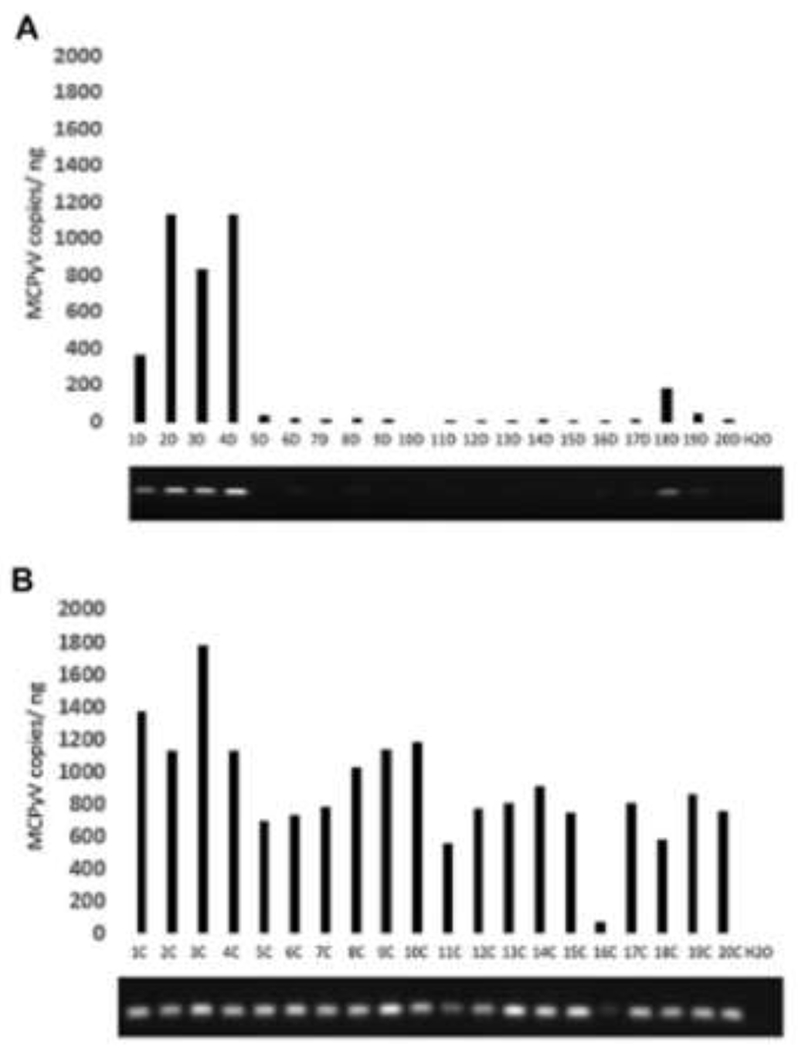
Figure 4.

Figure 1. Results of real-time polymerase chain reaction (RT-PCR) analysis of universal bacterial 16S ribosomal primers, primers for torque teno virus (TTV), and b-actin in (A) endophthalmitis samples with negative control and quantitative results for 16S and (B) normal uninflamed vitreous humor samples with positive control. Bar graph (A, top) shows bacterial real time PCR quantitation 16S copies per milliliter of fluid. NC = negative control; PC = positive control. Samples with less than ~3 x 103 copies/ml were also culture-negative. From 73 with permission.
Interestingly, we found another ocular condition that strongly affected the ocular surface virome. We sought to determine whether enucleation would change the ocular surface flora. Working with then-medical student Michal Gutowski and oculoplastics fellow Nora Siegel as well as my colleague Chris Chambers, we performed BRiSK and metagenomic sequencing on 20 samples from patients with one eye enucleated.75 While there appeared to be no difference in detected bacteria between enucleated and non-enucleated conjunctiva, we found a marked increase in MCPV on the enucleated surface (Figure 5). The clinical significance of this remains unclear; but this work does demonstrate that the normal ocular structures help regulate the ocular surface community including resident viruses.
Figure 5.
Quantitative and qualitative PCR for Merkel cell polyoma virus (MCPyV) in control (A) and anophthalmic (B) conjunctiva. MCPyV = Merkel Cell Polyoma Virus. From 75 with permission.
Applications to epidemic keratoconjunctivitis
One of the most interesting applications we have found for molecular diagnostics has been in the characterization of adenoviruses causing conjunctivitis. These ~35 kilobase non-enveloped DNA viruses fall into five major species and over 100 types. They are capable of causing many human diseases including severe respiratory disease and gastroenteritis. Adenoviruses are also the most common causes of conjunctivitis, particularly epidemic keratoconjunctivitis (EKC). Adenoviruses have traditionally been speciated and typed by serologic testing. Five major species, A-E, are in circulation in the world, with over 60 individual types within these species. The D-species adenoviruses, specifically D8, D19 (recently reclassified D64), D37 have been thought to underlie most EKC.
The NovaBay NVC-422 study76 was a large, multi-national randomized controlled clinical trial testing the antiseptic and antiviral compound auricloscene for treatment of EKC-type conjunctivitis. The study enrolled 500 subjects with typical conjunctivitis from India, Brazil, Sri Lanka, and the United States. While the trial did not show a benefit to auricloscene, the collected samples – conjunctival swabs taken at presentation and days 3, 6, 11, and 18 – provided a wealth of potential data on this very common ocular infectious condition. My late friend and colleague David Stroman, PhD, was the chief scientific officer at NovaBay, having previously headed infectious disease drug development at Alcon. He contacted me after the study to ask if we could help resolve an odd issue with their data. Even though all enrolled subjects had signs and symptoms of EKC, about 20% appeared to have no adenovirus by hexon-based PCR testing. We obtained these samples and ran BRiSK to look for adenovirus or other viral causes. BRiSK also failed to find virus. Given that the average PCR-positive sample had viral loads in the 106/ml range by qPCR, the negative results suggest some other pathogen was at play. As BRiSK is not able to ‘see’ RNA-based viruses, these would be the leading candidates for these cases (for instance, Coxsackie virus and enterovirus, both known to cause hemorrhagic conjunctivitis, are both RNA viruses).
However, more surprising was the distribution of types determined by PCR for the positive cases. While cases in Asia and South America were predominantly AdV D8, in the US this type was found in only a small minority of cases. Indeed, AdV E4 was the most common single type associated with conjunctivitis in the US. The signs and symptoms of conjunctivitis from this virus were identical to those seen with AdV D groups, although the virus appeared to clear more quickly than for D8. Importantly, the development of subepithelial infiltrates – the hallmark of EKC – was seen with comparable frequency in the AdV E4 cases as for the AdV D-affected individuals. Subsequent analysis of whole genome reconstructions of AdV E4 and D8 from affected cases has suggested that both AdV D8 and E4 have significant subclades, and, at least for the case of D8, that risk of development of SEI is linked to subclade and specific viral variants (Nakamichi et al., submitted). This finding – that seemingly identical viral types are actually composed of multiple genetic substrains, with differential pathogenicity – is made possible only through deep sequencing of pathogen DNA. This same technology has been highly prominent this year in characterizing SARS-CoV2 and identifying molecular substrains with differential pathogenicity, such as the highly infectious ‘delta variant’ that became predominant world-wide in the summer of 2021. This technique is ripe for application to many other ocular infectious diseases, including herpetic keratitis and retinitis, as well as ocular toxoplasmosis, to understand the pathogenesis of these potentially blinding conditions.
The future of molecular diagnostics for ophthalmic disease
The infectious disease diagnostic world is moving away from the 19th century of culture and stain and toward a future where primary diagnosis will be via molecular diagnostics. The ideal system for routine molecular diagnosis of ocular infectious disease would be available at point-of-care, be pathogen-agnostic, rapid, and inexpensive. While rapid point-of-care molecular diagnostics are available for specific infections (such as SHERLOCK CRISPR-based testing for SARS-CoV277 or the rapid antibody tests78 for adenoviral conjunctivitis) these are highly specific for individual pathogens.
Recent technologic developments have brought this goal into sight. As noted previously, the cost of massively parallel DNA sequencing has dropped by orders of magnitude over the past two decades; whole exome human genomic sequencing can now be obtained for less than the retail price of a bottle of brand-name moxifloxacin eye drops. One of the most impressive improvements in recent years has been the development of nanopore sequencing.79 In this method, DNA is sequenced by enzymatically drawing individual strands through an engineered membrane with nanometer-sized pores, and measuring the electrical potential change associated with each base as it goes through the membrane. This method may be greatly miniaturized, allowing devices the size of a USB dongle to sequence hundreds of millions of base-pairs of DNA daily. Such small and inexpensive instrumentation makes point-of-service testing a possibility. Further, base reads can be performed in real-time and analyzed asynchronously in cloud-based computers using rapid metagenomics matching technologies,80 which will allow for real-time matching of sequence to pathogen. When combined with techniques for enriching non-human DNA in biopsy samples, this methodology should be able to provide point-of-care results for determination of infectious causes of ocular inflammation within 1-2 hours from anywhere in the world.
I am grateful to the Jackson Memorial Lecture selection committee for the opportunity to present this work and hope this brief summary of molecular diagnostics in ocular infectious disease has been at least entertaining if not educational. I would like to thank the many institutions and individuals who have helped support this research over the years, including the National Institutes of Health/National Eye Institute, Research to Prevent Blindness, and generous gifts from the Mark J. Daily, MD Research Fund, Christopher and Alida Latham, Graham and Brenda Siddall, and Angie Karalis Johnson. I am deeply indebted to my wife Suzanne Dintzis, MD, PhD, my best friend and greatest supporter, and to our children Rachel and Maxwell, who have both followed our paths into science.
As Arthur Clarke wrote, any sufficiently advanced technology is indistinguishable from magic. We are approaching this level of technology in the domain of molecular diagnostics of ocular infectious disease. I think if Dr. Jackson returned to the AAO annual meeting in 2021, he would be impressed with the progress in many areas of ophthalmic care. But I believe he would be most impressed with the continual drive in our field to do better for our patients.
Acknowledgments
The author has no financial disclosures.
Supported by National Eye Institute grants R21EY033174 and P30EY001730, an unrestricted award from Research to Prevent Blindness, and the Mark J. Daily, MD Research Fund
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
None
References
- 1.Hogan MJ. Ocular toxoplasmosis. Am J Ophthalmol 1958;46:467–94. [DOI] [PubMed] [Google Scholar]
- 2.McLean JM. Oculomycosis. Am J Ophthalmol 1963;56:537–49. [PubMed] [Google Scholar]
- 3.Holland GN. Ocular toxoplasmosis: a global reassessment. Part I: epidemiology and course of disease. Am J Ophthalmol 2003;136:973–88. [DOI] [PubMed] [Google Scholar]
- 4.Holland GN. Ocular toxoplasmosis: a global reassessment. Part II: disease manifestations and management. Am J Ophthalmol 2004;137:1–17. [PubMed] [Google Scholar]
- 5.Jabs DA. Cytomegalovirus retinitis and the acquired immunodeficiency syndrome--bench to bedside: LXVII Edward Jackson Memorial Lecture. Am J Ophthalmol 2011;151:198–216 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liesegang TJ, Hoskins HD, Jensen AD. The significance of the Edward Jackson lecture. Am J Ophthalmol 2005;139:530–2. [DOI] [PubMed] [Google Scholar]
- 7.Albert DM. Edward Jackson in 1896: a man and his specialty at a crossroads. LIII Edward Jackson Memorial Lecture: Part 1. Am J Ophthalmol 1996;122:469–75. [DOI] [PubMed] [Google Scholar]
- 8.Lichter PR. Honoring the history of the Edward Jackson Memorial Lecture. The L Edward Jackson Memorial Lecture. Part 1. Am J Ophthalmol 1994;117:699–705. [DOI] [PubMed] [Google Scholar]
- 9.Newell FW. Edward Jackson, MD--a historical perspective of his contributions to refraction and to ophthalmology. Ophthalmology 1988;95:555–8. [DOI] [PubMed] [Google Scholar]
- 10.Katiofsky W Erfahrungen uber die Wirksamkeit der Sulranilamide bei gonorrheischen Augenerkrankungen. Klin Monatshl Augenh 1939;103:214. [Google Scholar]
- 11.Lin YH, Kang YC, Hou CH, et al. Antibiotic susceptibility profiles of ocular and nasal flora in patients undergoing cataract surgery in Taiwan: an observational and cross-sectional study. BMJ Open 2017;7:e017352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Gelder RN. Koch’s postulates and the polymerase chain reaction. Ocul Immunol Inflamm 2002;10:235–8. [DOI] [PubMed] [Google Scholar]
- 13.Avery OT, Macleod CM, McCarty M. Studies on the chemical nature of the substance inducing transformation of pneumococcal types : induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type iii. J Exp Med 1944;79:137–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hershey AD, Chase M. Independent functions of viral protein and nucleic acid in growth of bacteriophage. J Gen Physiol 1952;36:39–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiers W, Contreras R, Duerinck F, et al. Complete nucleotide sequence of bacteriophage MS2 RNA: primary and secondary structure of the replicase gene. Nature 1976;260:500–7. [DOI] [PubMed] [Google Scholar]
- 16.Sanger F, Coulson AR, Friedmann T, et al. The nucleotide sequence of bacteriophage phiX174. J Mol Biol 1978;125:225–46. [DOI] [PubMed] [Google Scholar]
- 17.Fleischmann RD, Adams MD, White O, et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science 1995;269:496–512. [DOI] [PubMed] [Google Scholar]
- 18.Kleppe K, Ohtsuka E, Kleppe R, Molineux I, Khorana HG. Studies on polynucleotides. XCVI. Repair replications of short synthetic DNA’s as catalyzed by DNA polymerases. J Mol Biol 1971;56:341–61. [DOI] [PubMed] [Google Scholar]
- 19.Saiki RK, Scharf S, Faloona F, et al. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science 1985;230:1350–4. [DOI] [PubMed] [Google Scholar]
- 20.Saiki RK, Gelfand DH, Stoffel S, et al. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 1988;239:487–91. [DOI] [PubMed] [Google Scholar]
- 21.Qavi HB, Green MT, SeGall GK, Font RL. Demonstration of HIV-1 and HHV-6 in AIDS-associated retinitis. Curr Eye Res 1989;8:379–87. [DOI] [PubMed] [Google Scholar]
- 22.McDonnell JM, McDonnell PJ, Stout WC, Martin WJ. Human papillomavirus DNA in a recurrent squamous carcinoma of the eyelid. Arch Ophthalmol 1989;107:1631–4. [DOI] [PubMed] [Google Scholar]
- 23.Crouse CA, Pflugfelder SC, Pereira I, Cleary T, Rabinowitz S, Atherton SS. Detection of herpes viral genomes in normal and diseased corneal epithelium. Curr Eye Res 1990;9:569–81. [DOI] [PubMed] [Google Scholar]
- 24.Hodge WG, Boivin JF, Shapiro SH, et al. Laboratory-based risk factors for cytomegalovirus retinitis. Can J Ophthalmol 2004;39:733–45. [DOI] [PubMed] [Google Scholar]
- 25.Van Gelder RN, von Zastrow ME, Yool A, Dement WC, Barchas JD, Eberwine JH. Amplified RNA synthesized from limited quantities of heterogeneous cDNA. Proc Natl Acad Sci U S A 1990;87:1663–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Gelder RN, Bae H, Palazzolo MJ, Krasnow MA. Extent and character of circadian gene expression in Drosophila melanogaster: identification of twenty oscillating mRNAs in the fly head. Curr Biol 1995;5:1424–36. [DOI] [PubMed] [Google Scholar]
- 27.Dabil H, Boley ML, Schmitz TM, Van Gelder RN. Validation of a diagnostic multiplex polymerase chain reaction assay for infectious posterior uveitis. Arch Ophthalmol 2001;119:1315–22. [DOI] [PubMed] [Google Scholar]
- 28.Dworkin LL, Gibler TM, Van Gelder RN. Real-time quantitative polymerase chain reaction diagnosis of infectious posterior uveitis. Arch Ophthalmol 2002;120:1534–9. [DOI] [PubMed] [Google Scholar]
- 29.Kim E, Chidambaram JD, Srinivasan M, et al. Prospective comparison of microbial culture and polymerase chain reaction in the diagnosis of corneal ulcer. Am J Ophthalmol 2008;146:714–23, 723, e1. [DOI] [PubMed] [Google Scholar]
- 30.Conners MS, Gibler TS, Van Gelder RN. Diagnosis of microsporidia keratitis by polymerase chain reaction. Arch Ophthalmol 2004;122:283–4. [DOI] [PubMed] [Google Scholar]
- 31.Milman T, Mirani N, Gibler T, Van Gelder RN, Langer PD. Actinomyces israelii endogenous endophthalmitis. Br J Ophthalmol 2008;92:427–8. [DOI] [PubMed] [Google Scholar]
- 32.Wells JR, Crozier I, Kraft CS, et al. Approach to Cataract Surgery in an Ebola Virus Disease Survivor with Prior Ocular Viral Persistence. Emerg Infect Dis 2020;26:1553–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hariprasad SM, Prasad A, Smith M, et al. Bilateral choroiditis from Prototheca wickerhamii algaemia. Arch Ophthalmol 2005;123:1138–41. [DOI] [PubMed] [Google Scholar]
- 34.Venter J, Remington K, Heidelberg J, et al. Environmental genome shotgun sequencing of the Sargasso Sea. Science 2004;304:66–74. [DOI] [PubMed] [Google Scholar]
- 35.Williamson S, Rusch D, Yooseph S, et al. The Sorcerer II Global Ocean Sampling Expedition: metagenomic characterization of viruses within aquatic microbial samples. PLoS One 2008;3:e1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Relman DA, Schmidt TM, MacDermott RP, Falkow S. Identification of the uncultured bacillus of Whipple’s disease. N Engl J Med 1992;327:293–301. [DOI] [PubMed] [Google Scholar]
- 37.Rickman LS, Freeman WR, Green WR, et al. Brief report: uveitis caused by Tropheryma whippelii (Whipple’s bacillus). N Engl J Med 1995;332:363–6. [DOI] [PubMed] [Google Scholar]
- 38.Matar GM, Koehler JE, Malcolm G, et al. Identification of Bartonella species directly in clinical specimens by PCR-restriction fragment length polymorphism analysis of a 16S rRNA gene fragment. J Clin Microbiol 1999;37:4045–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sander A, Posselt M, Bohm N, Ruess M, Altwegg M. Detection of Bartonella henselae DNA by two different PCR assays and determination of the genotypes of strains involved in histologically defined cat scratch disease. Journal of Clinical Microbiology 1999;37:993–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 1994;266:1865–9. [DOI] [PubMed] [Google Scholar]
- 41.Krown SE, Dittmer DP, Cesarman E. Pilot study of oral valganciclovir therapy in patients with classic Kaposi sarcoma. J Infect Dis 2011;203:1082–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toledo de Abreu M, Belfort R Jr., Hirata PS. Fuchs’ heterochromic cyclitis and ocular toxoplasmosis. Am J Ophthalmol 1982;93:739–44. [DOI] [PubMed] [Google Scholar]
- 43.Teyssot N, Cassoux N, Lehoang P, Bodaghi B. Fuchs heterochromic cyclitis and ocular toxocariasis. Am J Ophthalmol 2005;139:915–6. [DOI] [PubMed] [Google Scholar]
- 44.Barequet IS, Li Q, Wang Y, O’Brien TP, Hooks JJ, Stark WJ. Herpes simplex virus DNA identification from aqueous fluid in Fuchs heterochromic iridocyclitis. Am J Ophthalmol 2000;129:672–3. [DOI] [PubMed] [Google Scholar]
- 45.Quentin CD, Reiber H. Fuchs heterochromic cyclitis: rubella virus antibodies and genome in aqueous humor. Am J Ophthalmol 2004;138:46–54. [DOI] [PubMed] [Google Scholar]
- 46.Birnbaum AD, Tessler HH, Schultz KL, et al. Epidemiologic relationship between Fuchs heterochromic iridocyclitis and the United States rubella vaccination program. Am J Ophthalmol 2007;144:424–428. [DOI] [PubMed] [Google Scholar]
- 47.Chee SP, Bacsal K, Jap A, Se-Thoe SY, Cheng CL, Tan BH. Clinical features of cytomegalovirus anterior uveitis in immunocompetent patients. Am J Ophthalmol 2008. [DOI] [PubMed] [Google Scholar]
- 48.Chee SP, Bacsal K, Jap A, Se-Thoe SY, Cheng CL, Tan BH. Clinical features of cytomegalovirus anterior uveitis in immunocompetent patients. Am J Ophthalmol 2008;145:834–40. [DOI] [PubMed] [Google Scholar]
- 49.Chee SP, Jap A. Presumed Fuchs heterochromic iridocyclitis and Posner-Schlossman syndrome: comparison of cytomegalovirus-positive and negative eyes. Am J Ophthalmol 2008;146:883–9 e1. [DOI] [PubMed] [Google Scholar]
- 50.Sakai JI, Usui Y, Suzuki J, Kezuka T, Goto H. Clinical features of anterior uveitis caused by three different herpes viruses. Int Ophthalmol 2019;39:2785–2795. [DOI] [PubMed] [Google Scholar]
- 51.Corless CE, Guiver M, Borrow R, Edwards-Jones V, Kaczmarski EB, Fox AJ. Contamination and sensitivity issues with a real-time universal 16S rRNA PCR. J Clin Microbiol 2000;38:1747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grahn N, Olofsson M, Ellnebo-Svedlund K, Monstein HJ, Jonasson J. Identification of mixed bacterial DNA contamination in broad-range PCR amplification of 16S rDNA V1 and V3 variable regions by pyrosequencing of cloned amplicons. FEMS Microbiol Lett 2003;219:87–91. [DOI] [PubMed] [Google Scholar]
- 53.Salter SJ, Cox MJ, Turek EM, et al. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol 2014; 12:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Gelder RN, Krasnow MA. A novel circadianly expressed Drosophila melanogaster gene dependent on the period gene for its rhythmic expression. EMBO J 1996;15:1625–31. [PMC free article] [PubMed] [Google Scholar]
- 55.Venter JC, Adams MD, Myers EW, et al. The sequence of the human genome. Science 2001;291:1304–51. [DOI] [PubMed] [Google Scholar]
- 56.Lander ES, Linton LM, Birren B, et al. Initial sequencing and analysis of the human genome. Nature 2001;409:860–921. [DOI] [PubMed] [Google Scholar]
- 57.Su Z, Ning B, Fang H, et al. Next-generation sequencing and its applications in molecular diagnostics. Expert Rev Mol Diagn 2011;11:333–43. [DOI] [PubMed] [Google Scholar]
- 58.Muthappan V, Lee AY, Lamprecht TL, et al. Biome representational in silico karyotyping. Genome Res 2011;21:626–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ung L, Bispo PJM, Doan T, et al. Clinical metagenomics for infectious corneal ulcers: Rags to riches? The ocular surface 2020;18:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zegans ME, Van Gelder RN. Considerations in understanding the ocular surface microbiome. Am J Ophthalmol 2014;158:420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schabereiter-Gurtner C, Maca S, Rolleke S, et al. 16S rDNA-based identification of bacteria from conjunctival swabs by PCR and DGGE fingerprinting. Invest Ophthalmol Vis Sci 2001;42:1164–71. [PubMed] [Google Scholar]
- 62.Graham JE, Moore JE, Jiru X, et al. Ocular pathogen or commensal: a PCR-based study of surface bacterial flora in normal and dry eyes. Invest Ophthalmol Vis Sci 2007;48:5616–23. [DOI] [PubMed] [Google Scholar]
- 63.Dong Q, Brulc JM, Iovieno A, et al. Diversity of bacteria at healthy human conjunctiva. Invest Ophthalmol Vis Sci 2011;52:5408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leiby JS, McCormick K, Sherrill-Mix S, et al. Lack of detection of a human placenta microbiome in samples from preterm and term deliveries. Microbiome 2018;6:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Doan T, Akileswaran L, Andersen D, et al. Paucibacterial Microbiome and Resident DNA Virome of the Healthy Conjunctiva. Invest Ophthalmol Vis Sci 2016;57:5116–5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bruno R, Sacchi P, Debiaggi M, et al. Prevalence and histologic features of transfusion transmitted virus and hepatitis C virus coinfection in a group of HIV patients. Dig Liver Dis 2000;32:617–20. [DOI] [PubMed] [Google Scholar]
- 67.Zehender G, Manzin A, De Maddalena C, et al. Molecular epidemiology of TT virus in Italy and phylogenesis of viral isolates from subjects at different risk for parenteral exposure. J Med Virol 2001;63:76–84. [PubMed] [Google Scholar]
- 68.Feng H, Shuda M, Chang Y, Moore P. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008;319:1096–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Han DP, Wisniewski SR, Kelsey SF, Doft BH, Barza M, Pavan PR. Microbiologic yields and complication rates of vitreous needle aspiration versus mechanized vitreous biopsy in the Endophthalmitis Vitrectomy Study. Retina 1999;19:98–102. [DOI] [PubMed] [Google Scholar]
- 70.Lloyd JC, Braga-Mele R. Incidence of postoperative endophthalmitis in a high-volume cataract surgicentre in Canada. Can J Ophthalmol 2009;44:288–92. [DOI] [PubMed] [Google Scholar]
- 71.Nentwich MM, Ta CN, Kreutzer TC, et al. Incidence of postoperative endophthalmitis from 1990 to 2009 using povidone-iodine but no intracameral antibiotics at a single academic institution. J Cataract Refract Surg 2015;41:58–66. [DOI] [PubMed] [Google Scholar]
- 72.Malmin A, Syre H, Ushakova A, Utheim TP, Forsaa VA. Twenty years of endophthalmitis: Incidence, aetiology and clinical outcome. Acta Ophthalmol 2021;99:e62–e69. [DOI] [PubMed] [Google Scholar]
- 73.Lee AY, Akileswaran L, Tibbetts MD, Garg SJ, Van Gelder RN. Identification of torque teno virus in culture-negative endophthalmitis by representational deep DNA sequencing. Ophthalmology 2015;122:524–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee CS, Hong B, Kasi SK, et al. Prognostic utility of whole-genome sequencing and polymerase chain reaction tests of ocular fluids in postprocedural endophthalmitis. Am J Ophthalmol 2020;217:325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Siegal N, Gutowski M, Akileswaran L, et al. Elevated levels of Merkel cell polyoma virus in the anophthalmic conjunctiva. Sci Rep 2021;11:15366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee CS, Lee AY, Akileswaran L, et al. Determinants of Outcomes of Adenoviral Keratoconjunctivitis. Ophthalmology 2018;125:1344–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Joung J, Ladha A, Saito M, et al. Detection of SARS-CoV-2 with SHERLOCK One-Pot Testing. N Engl J Med 2020;383:1492–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sambursky R, Tauber S, Schirra F, Kozich K, Davidson R, Cohen EJ. The RPS adeno detector for diagnosing adenoviral conjunctivitis. Ophthalmology 2006;113:1758–64. [DOI] [PubMed] [Google Scholar]
- 79.Deamer D, Akerson M, Branton D. Three decades of nanopore sequencing. Nature Biotechnol. 2016; 34:518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee AY, Lee CS, Van Gelder RN. Scalable metagenomics alignment research tool (SMART): a scalable, rapid, and complete search heuristic for the classification of metagenomic sequences from complex sequence populations. BMC Bioinformatics 2016;17:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Van Gelder RN. Applications of the polymerase chain reaction to diagnosis of ophthalmic disease. Surv Ophthalmol 2001;46:248–58. [DOI] [PubMed] [Google Scholar]


