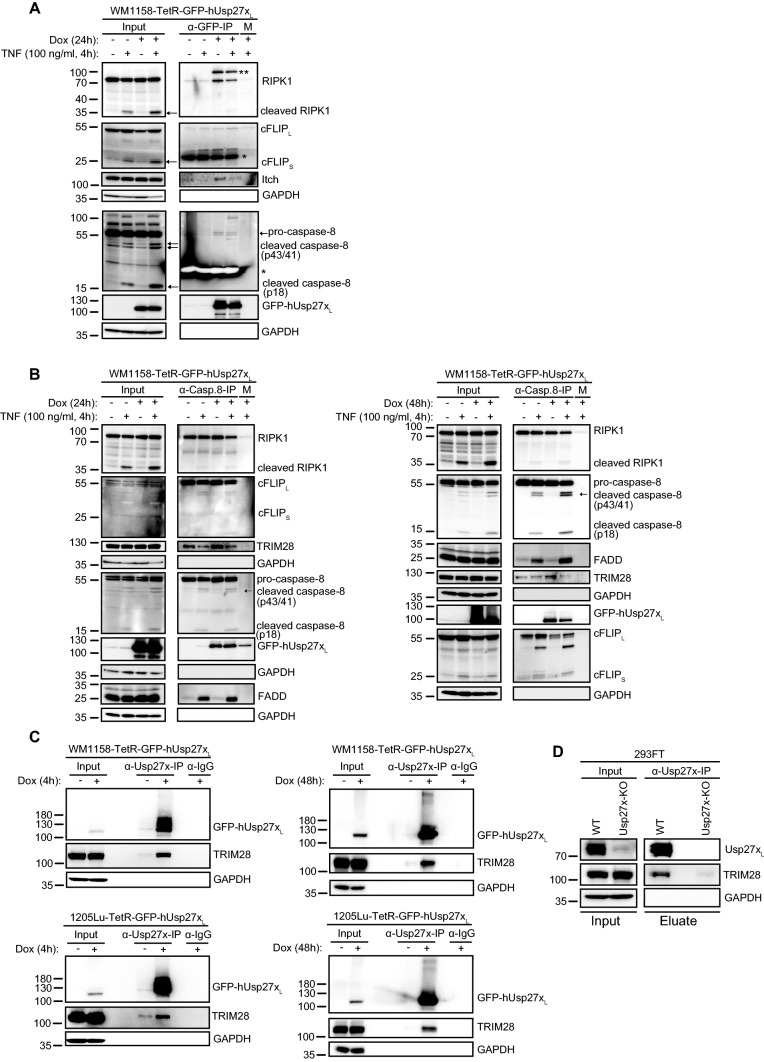
Fig. 5.
Usp27x interacts with components of the TNFR1/TLR3 death inducing signalling complex and TRIM28. A hUsp27xL is in a complex with RIPK1 and caspase-8 in WM1158 cells. Co-immunoprecipitation of GFP-hUsp27xL doxycycline inducible WM1158 cells. Cells were treated as indicated and then lysed in DISC-lysis-buffer. An antibody against GFP was used to pull down GFP-tagged human Usp27x and interaction partners were determined by Western blot (*: unspecific signal/ light chain of IP-antibody; **, unknown cross-reactive band; M: matrix control; n = 3). B Caspase-8 pulldown co-precipitates human Usp27xL, RIPK1, cFLIP, FADD and TRIM28. Co-immunoprecipitation of caspase-8 from GFP-hUsp27xL doxycycline inducible WM1158 cells (24 h dox left, 48 h dox right panel). Cells were treated as indicated and then lysed in DISC-lysis-buffer. An antibody against caspase-8 was used to pull down caspase-8 and interaction partners were determined by Western blot (n = 3). C Confirmation of Usp27xL interaction with TRIM28 in WM1158 and 1205Lu human melanoma cells. TetR-GFP-hUsp27xL WM1158 (top) or TetR-GFP-hUsp27xL 1205Lu (bottom) cells were treated as indicated (4 h dox left, 48 h dox right) and then lysed in DISC-lysis-buffer. Co-IP was performed using custom-made human Usp27x antibody. TRIM28 interaction was determined by Western blot (both n = 2). IgG: antibody control. D Usp27xL interacts with TRIM28 at endogenous levels in 293FT cells. Endogenous Usp27xL was immunoprecipitated as described for C from 293FT wild-type cells (WT) or Usp27x deficient knock out cells (Usp27x-deficient clone 2/10, see Fig. 1B, n = 2)