Abstract
The study was aimed to examine the effects of age and dietary beta-hydroxybutyric acid (BHBA) on blood BHBA and blood health indicators in goat kids. Thirty male goats of five ages (1, 2, 3, 6, and 12 months old) were selected for blood sampling to determine the influence of age. Another 64 goat kids (half males and half females) were weaned at 1 month old and fed with starter diets with control, low, medium, and high BHBA doses (0, 3, 6, and 9 g/animal/day, respectively). Six goats per treatment were selected for blood analysis at 2 and 3 months of age. There were significant effects (p < 0.01) of ages on the blood parameters of goat kids. The 6- and 12-month-old goats showed significantly lower blood total protein, globulin, BHBA, IgA, and IgM concentrations than did young goats, while they had a higher albumin-to-globulin ratio than young goats. The blood glucose decreased (p < 0.01) and IgG increased over time (p < 0.01). In blood, growth hormone (GH) and insulin-like growth factor I (IGF-I) were lower (p < 0.01) at 1- and 3-month-old goats than 12-month-old goats. The high dietary BHBA improved (p < 0.05) the ratio of albumin to globulin of 2-month-old kids compared with control. The blood GH and IGF-I were lower (p < 0.01) in the medium BHBA dose at 2 months of age than control. These results suggested that age greatly impacted blood composition, especially around weaning, and dietary BHBA showed beneficial regulating effects on blood total protein level in young goats.
Keywords: beta-hydroxybutyric acid, age, weaning, blood chemical composition, immunoglobulins
Introduction
The blood metabolites can reflect the nutritional state of animals (1), which is associated with performance and health status. For example, globulin (GLB) and immunoglobulins (Ig) are used to indicate immune status (2). Both the blood proteins and immune status improvement are associated with the secretion of growth hormone (GH) (2), which stimulates the synthesis of insulin-like growth factor I (IGF-I) in the liver (3). As a growth-promoting hormone, IGF-I is considered to regulate tissue growth and differentiation (4). At the same time, insulin (INS) is synthesized in the pancreatic β-cells (5). The anabolic role of plasma IGF-I and INS are stimulating the uptake of amino acids and glucose (GLU) (6–8). Therefore, blood metabolites, immunoglobulins, and hormones in goats are interconnected and deserve extensive investigation.
Goats (Caprinae) start as monogastric and become small ruminants within 2 months of age (9). The blood indexes of goats can be affected by the dynamics and processes of weaning and the maturation of the rumen, liver, and immune system during the transition from pre-ruminant to ruminant (10). The volatile fatty acids (VFA), especially the butyric acid produced by the rumen fermentation of solid feed, were considered the main stimulators of rumen development (11–13). Butyrate absorbed by rumen appears as beta-hydroxybutyric acid (BHBA) and acetoacetate in portal blood (14), suggesting that BHBA may be a further stimulator of rumen development. It has been indicated that blood BHBA and ketogenesis processes could be affected by diet, stress, or age (15). Indeed, blood BHBA of calves increased with the increase of starter intake and was higher at 7 weeks than at 6 weeks (15). In addition, BHBA has been used to indicate subclinical ketosis in dairy cattle due to its positive correlation with milk BHBA (16), which suggests a relationship between BHBA and health indicators. However, the relationship between blood BHBA and gut development over time, and between dietary BHBA and blood BHBA has not been described in goats. This study hypothesized that blood BHBA and health indicators of goats could vary with age, especially around weaning, and the dietary BHBA could regulate these changes due to its role in rumen development. Therefore, the study objectives were (1) to examine the effect of age on blood BHBA and other metabolites, Ig, and hormones and (2) to evaluate the impact of BHBA-based diets on blood metabolites, Ig, and hormones in growing goats.
Materials and Methods
This study was conducted between June 10 and August 10, 2020, at Haimen goat farm, Jiangsu, China (latitude, 31°53′N; longitude 121°09′E). The experimental work was performed following the guidelines approved by the Animal Ethics Committee of the Chinese Academy of Agricultural Sciences (protocol number: AEC-CAAS-20200605; approval date: June 3, 2020).
Animals and Experimental Design
The Yangtze River Delta white goat is a unique indigenous Chinese breed that is widely used for meat and high-quality bush hair. The Yangtze River Delta white goat was included in two research experiments. Experiment 1 was designed to determine the effect of age on blood BHBA and blood health indicators. Thirty male goats were randomly selected from the farm at different ages (1, 2, 3, 6, and 12 months) for blood collection. Experiment 2 was designed to determine the effect of age, dietary BHBA, and its interaction on blood BHBA and blood health indicators. For this goal, 64 goats at 1 month old were separated from their dams and randomly assigned to a 60-day feeding trial. Kids were reared in one shed, and one male and one female were assigned to one pen (2 kids/pen; 2 m × 2 m) based on body weight (5.14 ± 0.13 kg birth weight; mean ± SEM). In this way, the goats were randomly assigned to 1 of 4 groups in 32 pens: control, 3 g/day of BHBA (low dose), 6 g/day of BHBA (medium dose), and 9 g/day of BHBA (high dose). At 2 and 3 months old, six goats were per treatment were selected to study the short- and long-term effects of dietary BHBA supplementation on blood composition. The body weight of the abovementioned selected goat kids was recorded before the blood sampling. After 3 months of age, the kids were transferred into wider pens (20 kids per pen; 4 m × 4 m).
Diets and Feeding Management
Diets for goat kids were formulated to meet growth requirements according to the National Research Council (NRC) nutrient specification as previously described (17). Goats had free access to water. Weaned goats were fed with milk replacer thrice as 2% of body weight and starter ad libitum twice a day at 0800 and 1400 h (Table 1). After 2 months, the milk replacer was removed, and kids were fed with only starter ad libitum. After 3 months of age, the kids were offered concentrate ad libitum and corn and soybean straw. For experiment 2, the goats were fed with the abovementioned control diet, or the diets were supplemented with different doses of BHBA. The chemical composition and ingredients of the milk replacer and concentrate diets are shown in Table 1.
Table 1.
Ingredients and chemical composition (dry matter basis) of the concentrate diet and milk replacer.
| Items | Concentrate diet | Milk replacer |
|---|---|---|
| Ingredients, % | ||
| Corn | 50.0 | – |
| Soybean meal | 25.0 | – |
| Bran | 10.0 | – |
| Premixa | 2.50 | – |
| Salt | 0.50 | – |
| Sodium bicarbonate | 1.00 | – |
| Calcium bicarbonate | 4.00 | – |
| Corn husk | 15.0 | – |
| Chemical composition | ||
| Dry matter, % | 91.2 | 95.5 |
| Metabolizable energyb, Mcal/kg | 3.20 | 4.63 |
| Ether extract, % | 3.73 | 16.0 |
| Crude protein, % | 19.4 | 25.5 |
| Calcium, % | 0.95 | 1.02 |
| Phosphorus, % | 0.70 | 0.66 |
The premix provided the followings per kg of diet: VA, 12,000 IU; VD, 2,000 IU; VE, 30 IU; Cu, 12 mg; Fe, 64 mg; Mn, 56 mg; Zn, 60 mg; I, 1.2 mg; Se, 0.4 mg; Co, 0.4 mg; Ca, 3.2 g; P, 1.2 g; NaCl, 6.4 g.
The metabolizable energy was calculated by the equations from NRC (18).
Blood Collection and Analysis
Blood samples obtained from jugular venipuncture were collected in 10-ml Vacutainer tubes without anticoagulants. After centrifugation at 3,000 rpm for 10 min, the supernatant was transferred to 1.5-ml Eppendorf tubes and stored at −20°C until further analysis. Blood biochemical analyses were performed at the laboratory of Beijing Jinhai Keyu Biotechnology Development Co., Ltd., Beijing, China. Determinations of biochemical indices as blood total protein (TP), albumin (ALB), and GLU were performed using a KHB-1280 automatic biochemical analyzer (Kehua Biological Engineering Co., Ltd., Shanghai, China). In addition, blood IgA, IgG, and IgM were quantified colorimetrically using a KHB-1280 Automatic Biochemical Analyzer Kehua Biological Engineering Co., Ltd., Shanghai, China). The blood GLB was determined by the difference between TP and ALB concentrations. The ratio between ALB and GLB (A/G) was calculated. The blood INS, GH, IGF-I, and BHBA concentrations were determined using an ELISA commercial kit (Beijing Jinhai Keyu Biotechnology Development Co., Ltd.), following the manufacturer's instructions, and an ST-360 microplate reader (Kehua Biological Engineering Co., Ltd., Shanghai, China). No cross-reactions were observed with other soluble structural analogs. All coefficients of variation for intra- and inter-assay were <10%. The assay sensitivity was <0.1 mIU/L for INS, 0.1 ng/ml for GH, 1.0 ng/ml for IGF-I, and 0.1 mmol/L for BHBA.
Statistics
Data were tested for normality distribution (Shapiro–Wilk test) before the statistical analysis. The data showed a normal distribution (p > 0.05). The data for goats at different ages (1, 2, 3, 6, and 12 months of age) were analyzed by the one-way ANOVA of SAS (SAS Enterprise Guide 5.1, SAS Institute Inc., Cary, NC, USA). The data of goats fed with BHBA were analyzed by the two-way ANOVA of SAS that included the fixed effects of age (2 and 3 months) and dietary BHBA (control, low, medium, and high doses of 0, 3, 6, and 9 g/animal/day of BHBA, respectively) and the interaction between age and BHBA dose. Data were presented as arithmetic means and standard errors. Significant differences between groups were determined by Tukey's post-hoc test. Significance was set at p < 0.05. In addition, Pearson's correlation coefficient between the blood parameters was estimated in R studio (v. 1.3.1073), in which a probability of p < 0.05 indicated the significant differences.
Results
Experiment 1
Animal age effects on blood parameters are shown in Table 2. Low blood TP and GLB (p < 0.01) were observed at 6 and 12 months old compared with younger goats, while the TP values of 12-month-old goats were higher than those of 6-month-old goats. Meanwhile, the blood ALB did not show significant differences over time. However, the A/G ratios of both 6- and 12-month-old kids were higher (p < 0.01) than those of the younger kids. In contrast, the blood GLU concentrations at 1 month old were higher (p < 0.01) than in the following months. In addition, the blood BHBA concentrations were higher in younger goats (1–3 months; p < 0.01) than senior kids (6–12 months).
Table 2.
Blood parameters of goats from 1 to 12 months of age (n = 6 per group).
| Parameters | Age | SEM | p-value | ||||
|---|---|---|---|---|---|---|---|
| 1 months | 2 months | 3 months | 6 months | 12 months | |||
| Metabolites | |||||||
| TP, g/L | 68.9a | 70.7a | 69.0a | 58.8c | 63.6b | 1.02 | <0.01 |
| ALB, g/L | 34.7 | 33.1 | 33.0 | 33.1 | 33.8 | 0.31 | 0.37 |
| GLB, g/L | 34.3ab | 37.6a | 35.1a | 25.6c | 29.7bc | 0.96 | <0.01 |
| A/G, % | 1.01b | 0.88b | 0.92b | 1.29a | 1.17a | 0.03 | <0.01 |
| GLU, mmol/L | 5.36a | 3.85b | 3.33b | 3.73b | 3.51b | 0.15 | <0.01 |
| BHBA, mmol/L | 1.00a | 0.90a | 0.96a | 0.53b | 0.51b | 0.05 | <0.01 |
| Immunoglobulins | |||||||
| IgA, g/L | 0.92a | 0.84ab | 0.79b | 0.42c | 0.52c | 0.04 | <0.01 |
| IgG, g/L | 9.75c | 10.4c | 11.0c | 17.6b | 20.5a | 0.89 | <0.01 |
| IgM, g/L | 2.31a | 2.64a | 2.43a | 0.98b | 1.32b | 0.14 | <0.01 |
| Hormones | |||||||
| INS, mIU/L | 20.8 | 14.1 | 13.1 | 16.2 | 22.5 | 1.31 | 0.08 |
| GH, ng/ml | 3.42bc | 3.85ab | 3.09c | 3.44bc | 4.09a | 0.10 | 0.01 |
| IGF-I, ng/ml | 53.1c | 63.8abc | 54.6bc | 64.9ab | 71.9a | 1.68 | <0.01 |
TP, total protein; ALB, albumin; GLB, globulin; A/G, ratio between albumin and globulin; GLU, glucose; BHBA, beta-hydroxybutyric acid; INS, insulin; GH, growth hormone; IGF-I, insulin-like growth factor I.
Means with different superscripts in each row differ significantly (p < 0.05).
Furthermore, the blood IgA concentrations were lower (p < 0.01) at 3 months than at 1 month. Then, the IgA concentrations were also lower at 6 and 12 months than younger goats. Low blood levels of IgM (p < 0.01) were observed within 6 to 12 months of age. However, the blood IgG increased (p < 0.01) over time, and that of 6-month-old kids were higher than that of younger goat. In addition, the 12-month-old kids showed the highest IgG concentrations.
In addition, blood GH was lower (p < 0.01) at 1 month than 12 months, and it was lower at 3 months than at both 2 and 12 months of age. In addition, the blood IGF-I values were lower at 1-month-old goats than 6- and 12-month-old goats (p < 0.01), and the 3-month-old goats showed lower (p < 0.01) values than the 12-month-old goats. However, the blood INS did not show significant differences over time.
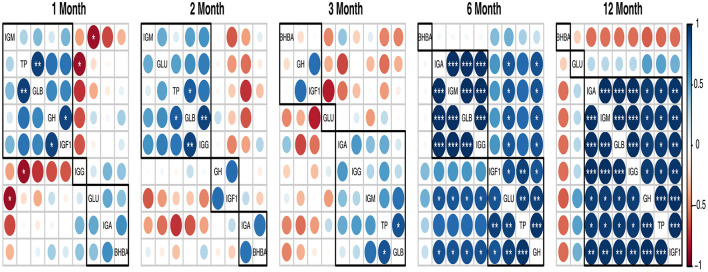
In Figure 1, the blood TP, GLB, BHBA, IgA, and IgM were positively correlated (p < 0.01), but they were negatively correlated (p < 0.01) with A/G ratio and IgG. ALB had a positive correlation (p < 0.01) with TP and GLU. In addition, the blood GLU was positively correlated with IgA (p < 0.01), TP, and BHBA (p < 0.05), but it was negatively correlated with IgG (p < 0.05). In addition, INS was positively correlated (p < 0.05) with the A/G ratio but negatively correlated (p < 0.05) with GLB and IgM. Furthermore, the GH was positively correlated with IgG (p < 0.05) and IGF-I (p < 0.01). Moreover, blood IGF-I had a positive correlation with IgG (p < 0.01) and a negative correlation with IgA, IgM (p < 0.05), and BHBA (p < 0.01).
Figure 1.
A heatmap illustrating Pearson's correlation coefficients between blood parameters in goats from 1 to 12 months of age. Significance levels: *p < 0.05, **p < 0.01, and ***p < 0.001.
Experiment 2
The effect of dietary BHBA on the blood parameters of goat kids is shown in Table 3. High dietary BHBA dose increased blood ALB (p < 0.05) compared with that of the control group. Moreover, both low and high BHBA doses showed a higher A/G ratio (p < 0.01) in comparison with that of the control group. The blood GLU was lower (p < 0.01) in the medium BHBA dose than the low and high BHBA doses.
Table 3.
Effect of dietary BHBA on blood parameters of goat kids (n = 6 per group).
| Parameters | Age | Treatments | SEM | p -value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Low | Medium | High | Mean | Age | Treatment | Interaction | |||
| Metabolites | ||||||||||
| TP, g/L | 2 months | 70.7 | 69.8 | 69.1 | 69.9 | 69.9 | 0.80 | 0.33 | 0.48 | 0.65 |
| 3 months | 69.0 | 69.4 | 68.7 | 70.1 | 69.3 | |||||
| Mean | 69.9 | 69.6 | 68.9 | 70.0 | ||||||
| ALB, g/L | 2 months | 33.1 | 34.0 | 33.8 | 34.9 | 33.9 | 0.51 | 0.30 | 0.03 | 0.84 |
| 3 months | 33.0 | 34.0 | 33.1 | 34.2 | 33.6 | |||||
| Mean | 33.0B | 34.0AB | 33.4AB | 34.5A | ||||||
| GLB, g/L | 2 months | 37.6a | 35.7b | 35.3b | 35.0b | 35.9 | 0.40 | 0.26 | 0.10 | <0.01 |
| 3 months | 35.1b | 35.3b | 35.9ab | 35.9ab | 35.6 | |||||
| Mean | 36.4 | 35.5 | 35.6 | 35.5 | ||||||
| A/G, % | 2 months | 0.88b | 0.95ab | 0.96ab | 1.00a | 0.95 | 0.02 | 0.33 | 0.01 | 0.04 |
| 3 months | 0.92ab | 0.96ab | 0.90b | 0.95ab | 0.93 | |||||
| Mean | 0.90B | 0.96A | 0.93AB | 0.98A | ||||||
| GLU, mmol/L | 2 months | 3.85 | 4.06 | 3.92 | 4.41 | 4.06A | 0.18 | <0.01 | 0.01 | 0.08 |
| 3 months | 3.33 | 3.71 | 2.78 | 3.33 | 3.29b | |||||
| Mean | 3.59AB | 3.88A | 3.35B | 3.87A | ||||||
| BHBA, mmol/L | 2 months | 0.90 | 0.92 | 0.98 | 1.00 | 0.95 | 0.06 | 0.60 | 0.89 | 0.55 |
| 3 months | 0.96 | 1.02 | 0.98 | 0.94 | 0.97 | |||||
| Mean | 0.93 | 0.96 | 0.98 | 0.97 | ||||||
| Immunoglobulins | ||||||||||
| IgA, g/L | 2 months | 0.84 | 0.79 | 0.88 | 0.88 | 0.85 | 0.04 | 0.72 | 0.34 | 0.20 |
| 3 months | 0.79 | 0.88 | 0.83 | 0.87 | 0.84 | |||||
| Mean | 0.81 | 0.83 | 0.86 | 0.88 | ||||||
| IgG, g/L | 2 months | 10.4 | 9.38 | 9.69 | 9.84 | 9.82 | 0.74 | 0.14 | 0.68 | 0.81 |
| 3 months | 11.0 | 11.0 | 9.84 | 10.7 | 10.6 | |||||
| Mean | 10.7 | 10.1 | 9.76 | 10.3 | ||||||
| IgM, g/L | 2 months | 2.64 | 2.61 | 2.77 | 2.41 | 2.61 | 0.19 | 0.53 | 0.63 | 0.88 |
| 3 months | 2.43 | 2.56 | 2.61 | 2.48 | 2.52 | |||||
| Mean | 2.53 | 2.58 | 2.69 | 2.44 | ||||||
| Hormones | ||||||||||
| INS, mIU/L | 2 months | 14.1 | 17.8 | 16.9 | 18.0 | 16.7 | 2.05 | 0.09 | 0.42 | 0.90 |
| 3 months | 13.1 | 15.6 | 12.9 | 14.9 | 14.1 | |||||
| Mean | 13.6 | 16.8 | 14.9 | 16.45 | ||||||
| GH, ng/ml | 2 months | 3.85a | 3.79ab | 3.19bc | 3.66abc | 3.62A | 0.14 | <0.01 | 0.38 | 0.01 |
| 3 months | 3.09c | 3.08c | 3.30abc | 3.21bc | 3.17B | |||||
| Mean | 3.47 | 3.44 | 3.24 | 3.43 | ||||||
| IGF-I, ng/ml | 2 months | 63.8ab | 68.7a | 56.9bcd | 62.2abc | 62.9A | 1.96 | <0.01 | 0.22 | 0.01 |
| 3 months | 54.6cd | 49.8d | 54.4cd | 55.6bcd | 53.6b | |||||
| Mean | 59.2 | 59.2 | 55.7 | 58.9 | ||||||
TP, total protein; ALB, albumin; GLB, globulin; A/G, ratio between albumin and globulin; GLU, glucose; BHBA, beta-hydroxybutyric acid; INS, insulin; GH, growth hormone; IGF-I, insulin-like growth factor I.
Different superscripts in each row of mean (doses) and column of mean (age) differ significantly (p < 0.05).
Different superscripts in the interaction of BHBA dose and age indicate significant differences (p < 0.05). The control group was fed without dietary supplementation; and the low-, medium-, and high-dose groups were fed with 2, 6, and 9 g/animal/day of BHBA, respectively.
Blood GLU, GH, and IGF-I decreased (p < 0.01) over time. Blood GH and IGF-I showed a significant interaction between diet and age (p < 0.01), and the control group had higher GH than the medium BHBA dose group at 2 months. In addition, the low dose of BHBA showed elevated blood IGF-I than the medium BHBA dose at 2 months. However, the blood concentrations of TP, GLB, BHBA, INS, and Ig were not affected by dietary BHBA and age.
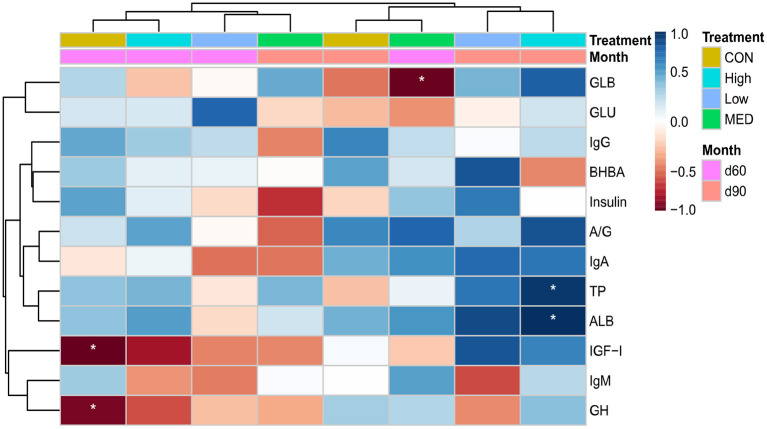
In the control group at 2 months, body weight had a negative relationship (p < 0.05) with GH and IGF-I (Figure 2). In addition, the body weight was negatively correlated (p < 0.05) with blood GLB in medium BHBA dose during 2 months of age. However, the blood TP and ALB were positively (p < 0.05) correlated with body weight in the high BHBA dose group at 3 months of age.
Figure 2.
A heatmap illustrating Pearson's correlation between body weight and blood parameters of goat kids supplemented with different concentrations of BHBA. Significance levels: *p < 0.05. The control group was fed without dietary supplementation; and the low-, medium-, and high-dose groups were fed with 2, 6, and 9 g/animal/day of BHBA, respectively. BHBA, beta-hydroxybutyric acid.
Discussion
Experiment 1
Blood proteins are indicators for neonates' immunity and growth (19). The optimal blood protein levels could reduce the mortality rates in young animals (20). In this study, the blood TP and GLB concentrations were higher in 1–3-month-old than 6-month-old goats, but ALB was not affected by age. The weaning stress of goat kids could influence the immune status (21) by increasing blood GLB concentration (22). However, these parameters showed a subsequent increase at 12 months than at 6 months of age. This should be related to the age growth and physiological maturity of kids. Indeed, the blood TP, ALB, and GLB concentration of kids increased with age (23). The enhanced protein intake due to physiological maturity caused extensive degradation of dietary protein and metabolism of absorbed amino acids, which eventually led to the possible increase of blood protein level (24).
After 1 month of age, blood GLU in goats decreased over time. Blood GLU concentration decreased (p < 0.001) with the increasing age of kids (25). Interestingly, blood GLU concentrations in Saharan goats were high 1 week after parturition and then significantly decreased at 8, 11, and 12 weeks of lactation due to the negative energy balance (26). Goat kids had significantly high blood GLU in the non-ruminant phase (10), in which colostrum and milk keep high contents of body GLU (27), while the decrease of milk and the developed rumen caused a reduction in blood GLU (28, 29). Rumen development caused low GLU and high starter intake over time (30). The developed rumen replaces GLU with rumen fermentation products as the main energy source (31, 32). Both GLU and butyrate at birth have the same oxidation rate, while butyrate metabolism to ketones increases six-fold at weaning (33). Early weaning could enhance growth by increasing VFA (34). Despite the beneficial effects of BHBA for rumen development, the higher blood ketones bodies (including BHBA) indicate energy deficiency and are considered an indicator of subclinical ketosis (35). In this study, the blood BHBA was lower in goats at 6 and 12 months than younger goats, indicating the health status of goat kids and the better nutrition state. In this study, blood BHBA had a positive relationship with blood GLU, which is inconsistent with the results of Zarrin (36), who demonstrated that the infusion of BHBA increased the blood INS and decreased the blood GLU in Holstein dairy cows. Further research is still needed to clarify the relation between these metabolites.
IgG is the most preponderant blood antibody compared with IgA and IgM (37). In this study, IgG contents increased over time, which is consistent with the results of Piccione (38), who found a low level of serum IgG in newborn kids compared with the mothers. In addition, the IgG showed a positive correlation with the days of life of newborns (38). The rising concentration of IgG can be linked to the maturation of the immune system caused by antigenic stimulation (39). However, blood IgM and IgA concentrations were high in younger goats and decreased over time, suggesting that both IgA and IgM were the first antibodies to prevent weaning stress. The IgA also has local activity in the gut against diarrhea. The increase in blood IgG and the decrease in blood IgA and IgM over time in goat kids suggest the imbalance of the internal environment and increased immune stimulation (40).
This study observed a positive correlation between blood proteins (TP and GLB) and immunoglobulins (IgA and IgM). Similarly, dietary protein promotes the absorption of immunoglobulins and IGF-I in calves fed with colostrum (41). In addition, a ewe protein diet in late pregnancy increased the absorption efficiency of IgG (42). However, the uptake of these large immunomolecules decreases during the first days of life due to gut closure (43). Consequently, the increase of TP, GLB, IgA, and IgM in this study could be due to weaning stress more than the improvement of absorption efficiency.
A significant reduction was observed in GH and IGF-I at 1- and 3-month-old goats than 12-month-old goats. At birth and after weaning, newborn animals are probably exposed to various morphological, metabolic, and physiological changes that can affect blood metabolites and enzymes (44). Thus, weaning decreased blood IGF-I (22, 45) due to reduced circulating IGF-I during poor nutrition (46). In lambs fed with milk, Sun (47) showed that milk was rich in IGF-I and had higher blood IGF-I than lambs fed with milk plus starter diets. Subsequently, INS, GH, and IGF-I increased over time, possibly due to improved nutrition and rumen development. Energy and protein intake are considered the main factors affecting plasma IGF-I concentration (48, 49). In this regard, Shen et al. (50) indicated that young goats fed with high-energy diets had higher plasma IGF-I concentrations and expression of IGF-I receptors in the rumen epithelium than goats fed with low-energy diets. In addition, Hua et al. (51) reported that exogenous bovine GH increased (p < 0.01) the plasma levels of IGF-I in the fed with rather than fasted sheep; in addition, fasting decreased the levels of IGF-I.
Experiment 2
Blood proteins are used as indicators of gut metabolic function and health status (52, 53). In this study, the group with the high dose of BHBA had an elevated blood ALB and A/G ratio as compared with those of the control group. Furthermore, the blood TP and ALB were positively correlated with body weight in the high BHBA dose. Such beneficial effects could be related to stimulated ruminant digestive system particularly for digestion of protein and fat (54). BHBA is linked with the high intake of solid feed, microbial communities, and rumen development of fermentation and absorption processes (15, 55). Moreover, the solid feed intake can increase VFA concentrations, alter the rumen microbiome (56), and improve rumen epithelium metabolic function (57). The development of rumen epithelium depends on VFA absorption, transportation, and metabolism (58). Accordingly, the supply of BHBA in this study can promote rumen functioning, and this was reflected by the improvement of blood proteins and animal health status. On the other hand, diets linked with low rumen VFA, such as milk replacer, showed slower development of the rumen and the small intestine (59). The lower blood ALB found in the control group could be related to the interruption of suckling and the incomplete digestive system to degrade solid diets (53, 60).
However, health risks have been reported in livestock due to elevated blood ketones (35). The feed intake of the medium BHBA group decreased while the high BHBA group did not show a similar response due to the adaptation behavior (61). Only medium BHBA dose decreased blood GLU (overall) and IGF-I (only at 2 months) compared with the low BHBA dose. Herrick (62) showed that infusing butyrate increases blood butyrate and BHBA concentrations and decreases GLU. In this study, blood BHBA showed a numerical increase in kids receiving BHBA than the control group at 2 months of age. Several studies showed a positive relationship between blood BHBA and the starter feed intake (55, 63). However, other studies reported the importance of the age factor than the starter feed on BHBA production in rumen epithelium (64, 65), which could be the most likely cause of nonsignificant effect of dietary BHBA on blood ketones after weaning.
The GH and IGF-I were negatively correlated with body weight in the control group at 2 months. The abrupt alteration in the diet caused by weaning may be associated with a period of decreased or stagnant development (66, 67). However, the control group showed high GH levels only at 2 months, which could be due to dehydration due to the abrupt diet change. In another study, frequent diarrhea, mortality, and body weight loss are the possible signs of stress within 2 weeks after weaning (68). On the other hand, the high GH could be due to under-nutrition status after weaning (45).
Conclusions
According to blood parameters, this longitudinal study showed that the blood concentrations of TP, GLB, IgA, IgM, and BHBA were higher in young kids before and after weaning (1–3 months) than in older goats (6 and 12 months). However, the A/G ratio, GH, and IGF-I were lower in young animals than adult animals. The dietary BHBA supplementation did not affect blood BHBA, but at high BHBA dosing, it elicited a higher blood ALB and A/G ratio than the control group. Future research is still needed to investigate the usefulness of BHBA in improving rumen development growth performance and health status in young ruminants.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was approved according to the Animal Ethics Committee of the Chinese Academy of Agricultural Sciences (Protocol number: AEC-CAAS-20200605).
Author Contributions
MA contributed to the methodology, software, writing—original draft, and writing—review and editing. EV-B-P contributed to the validation and writing—review and editing. YZ and YF contributed to the methodology. NZ contributed to the investigation, project administration, resources, supervision, validation, visualization, and writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by grants from the National Natural Science Foundation of China (31872385) and the National Key R&D Program Projects (2018YFD0501902).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank the goat farm for their cooperation in animal handling. We also appreciate the assistance in sampling collection from Animal Husbandry Institute of Jiangsu Academy of Agricultural Sciences.
References
- 1.Tothova CS, Nagy O, Seidel H, Konvicna J, Farkasova Z, Kovac G. Acute phase proteins and variables of protein metabolism in dairy cows during the pre- and postpartal period. Acta Vet Brno. (2008) 77:51–7. 10.2754/avb200877010051 [DOI] [Google Scholar]
- 2.Obese FY, Ali ZS, Naazie A, Ayizanga RA. Effect of age, breed and sex on haematological and blood biochemical parameters in helmeted guinea fowl (Numida meleagris). Comp Clin Path. (2018) 27:901–9. 10.1007/s00580-018-2680-y [DOI] [Google Scholar]
- 3.Jones J, Clemmons D. Insulin-like growth-factors and their binding-proteins - biological actions. Endocr Rev. (1995) 16:3–34. 10.1210/edrv-16-1-3 [DOI] [PubMed] [Google Scholar]
- 4.Oldham J, Martyn J, Hua K, MacDonald N, Hodgkinson S, Bass J. Nutritional regulation of IGF-II, but not IGF-I, is age dependent in sheep. J Endocrinol. (1999) 163:395–402. 10.1677/joe.0.1630395 [DOI] [PubMed] [Google Scholar]
- 5.Pessin JE, Saltiel AR. Signaling pathways in insulin action: molecular targets of insulin resistance. J Clin Invest. (2000) 106:165–9. 10.1172/JCI10582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magistrelli D, Valli A, Rosi F. Insulin and IGF-1 in goat milk: influence of the diet. Ital J Anim Sci. (2005) 4:386–8. 10.4081/ijas.2005.2s.386 [DOI] [Google Scholar]
- 7.Noguchi T. Protein nutrition and insulin-like growth factor system. Br J Nutr. (2000) 84(Suppl. 2):S241–S4. 10.1079/096582197388617 [DOI] [PubMed] [Google Scholar]
- 8.Gale SM, Castracane VD, Mantzoros CS. Energy homeostasis, obesity and eating disorders: recent advances in endocrinology. J Nutr. (2004) 134:295–8. 10.1093/jn/134.2.295 [DOI] [PubMed] [Google Scholar]
- 9.Agrawal A, Karim S, Kumar R, Sahoo A, John P. Sheep and goat production: basic differences, impact on climate and molecular tools for rumen microbiome study. Int J Curr Microbiol App Sci. (2014) 3:684–706. Available online at: https://www.ijcmas.com/vol-3-1/A.R.Agrawal,%20et%20al.pdf. [Google Scholar]
- 10.Redlberger S, Fischer S, Kohler H, Diller R, Reinhold P. Age-dependent physiological dynamics in acid-base balance, electrolytes, and blood metabolites in growing goats. Vet J. (2017) 229:45–52. 10.1016/j.tvjl.2017.10.017 [DOI] [PubMed] [Google Scholar]
- 11.Yan L, Zhang B, Shen ZM. Dietary modulation of the expression of genes involved in short-chain fatty acid absorption in the rumen epithelium is related to short-chain fatty acid concentration and pH in the rumen of goats. J Dairy Sci. (2014) 97:5668–75. 10.3168/jds.2013-7807 [DOI] [PubMed] [Google Scholar]
- 12.Metzler-Zebeli BU, Hollmann M, Sabitzer S, Podstatzky-Lichtenstein L, Klein D, Zebeli Q. Epithelial response to high-grain diets involves alteration in nutrient transporters and Na+/K+-ATPase mRNA expression in rumen and colon of goats. J Anim Sci. (2013) 91:4256–66. 10.2527/jas.2012-5570 [DOI] [PubMed] [Google Scholar]
- 13.Kuzinski J, Rontgen M. The mRNA and protein expression of ruminal MCT1 is increased by feeding a mixed hay/concentrate diet compared with hay ad libitum diet (Short Communication). Arch Anim Breed. (2011) 54:280–6. 10.5194/aab-54-280-2011 [DOI] [Google Scholar]
- 14.Beck U, Emmanuel B, Giesecke D. The ketogenic effect of glucose in rumen epithelium of ovine (Ovis aries) and bovine (Bos taurus) origin. Comp Biochem Physiol B. (1984) 77:517–21. 10.1016/0305-0491(84)90268-2 [DOI] [PubMed] [Google Scholar]
- 15.Suarez-Mena F, Hu W, Dennis T, Hill T, Schlotterbeck R. β-Hydroxybutyrate (BHB) and glucose concentrations in the blood of dairy calves as influenced by age, vaccination stress, weaning, and starter intake including evaluation of BHB and glucose markers of starter intake. J Dairy Sci. (2017) 100:2614–24. 10.3168/jds.2016-12181 [DOI] [PubMed] [Google Scholar]
- 16.JeŽek J, Cincović MR, Nemec M, Belić B, Djoković R, Klinkon M, et al. Beta-hydroxybutyrate in milk as screening test for subclinical ketosis in dairy cows. Polish J Vet Sci. (2017) 20:507–12. 10.1515/pjvs-2017-0061 [DOI] [PubMed] [Google Scholar]
- 17.NRC . Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids. Washington, DC: National Academy Press; (2007). [Google Scholar]
- 18.NRC . Nutrient Requirements of Dairy Cattle. Washington, DC: National Academy of Science; (2001). [Google Scholar]
- 19.Chen J, Chang C, Peh H, Chen S. Serum protein levels and neonatal growth rate of Nubian goat kids in Taiwan area. Small Ruminant Res. (1999) 32:153–60. 10.1016/S0921-4488(98)00166-7 [DOI] [Google Scholar]
- 20.Kaneko JJ, Harvey JW, Bruss ML. Clinical Biochemistry of Domestic Animals. San Diego, CA: Academic Press; (1997). 10.1016/B978-012396305-5/50032-4 [DOI] [Google Scholar]
- 21.Li H, Diao Q-y, Zhang N-f, Tu Y, Wang J-f. Effect of different protein levels on nutrient digestion metabolism and serum biochemical indexes in calves. Sci Agric Sin. (2008) 7:375–80. 10.1016/S1671-2927(08)60079-6 [DOI] [Google Scholar]
- 22.Chai J, Diao Q, Wang H, Tu Y, Tao X, Zhang N. Effects of weaning age on growth, nutrient digestibility and metabolism, and serum parameters in Hu lambs. Anim Nutr. (2015) 1:344–8. 10.1016/j.aninu.2015.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abbas S, Rashid MA, Yousaf MS, Ashraf S, Rabbani I, Zaneb H, et al. Effect of maternal yeast feeding on dam performance and serum health biomarkers of Beetal goat kids. S Afr J Anim Sci. (2020) 50:281–90. 10.4314/sajas.v50i2.11 [DOI] [Google Scholar]
- 24.Quigley J, III, Bernard J. Effects of nutrient source and time of feeding on changes in blood metabolites in young calves. J Anim Sci. (1992) 70:1543–9. 10.2527/1992.7051543x [DOI] [PubMed] [Google Scholar]
- 25.Paez Lama S, Grilli D, Egea V, Fucili M, Allegretti L, Guevara JC. Rumen development and blood metabolites of Criollo kids under two different rearing systems. Livest Sci. (2014) 167:171–7. 10.1016/j.livsci.2014.06.018 [DOI] [Google Scholar]
- 26.Henna K, Boudjellaba S, Khammar F, Amirat Z, Chesneau D, Charallah S. Endocrine, energy, and lipid status during parturition and early lactation in indigenous goats native to the Algerian Sahara. Vet World. (2021) 14:2419–26. 10.14202/vetworld.2021.2419-2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Girard J, Ferre P, Pegorier J, Duee P. Adaptations of glucose fatty acid metabolism during perinatal period suckling-weaning transition. Physiol Rev. (1992) 72:507–62. 10.1152/physrev.1992.72.2.507 [DOI] [PubMed] [Google Scholar]
- 28.Quigley J, III, Smith Z, Heitmann R. Changes in plasma volatile fatty acids in response to weaning and feed intake in young calves. J Dairy Sci. (1991) 74:258–63. 10.3168/jds.S0022-0302(91)78168-X [DOI] [PubMed] [Google Scholar]
- 29.Chai JM, Ma T, Wang HC, Qi ML, Tu Y, Diao QY, et al. Effect of early weaning age on growth performance, nutrient digestibility, and serum parameters of lambs. Anim Prod Sci. (2017) 57:110–5. 10.1071/AN1507929767014 [DOI] [Google Scholar]
- 30.Tao H, Guo F, Tu Y, Si B-W, Xing Y-C, Huang D-J, et al. Effect of weaning age on growth performance, feed efficiency, nutrient digestibility and blood-biochemical parameters in Droughtmaster crossbred beef calves. Asian-Australas J Anim Sci. (2018) 31:864–72. 10.5713/ajas.17.0539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan MA, Lee HJ, Lee WS, Kim HS, Ki KS, Hur TY, et al. Structural growth, rumen development, and metabolic and immune responses of Holstein male calves fed milk through step-down and conventional methods. J Dairy Sci. (2007) 90:3376–87. 10.3168/jds.2007-0104 [DOI] [PubMed] [Google Scholar]
- 32.Baldwin RL, McLeod KR, Klotz JL, Heitmann RN. Rumen development, intestinal growth and hepatic metabolism in the pre- and postweaning ruminant. J Dairy Sci. (2004) 87:E55–E65. 10.3168/jds.S0022-0302(04)70061-2 [DOI] [Google Scholar]
- 33.Baldwin RLt, Jesse BW. Developmental changes in glucose and butyrate metabolism by isolated sheep ruminal cells. J Nutr. (1992) 122:1149–53. 10.1093/jn/122.5.1149 [DOI] [PubMed] [Google Scholar]
- 34.Wu S, Cui Z, Chen X, Wang P, Yao J. Changed caecal microbiota and fermentation contribute to the beneficial effects of early weaning with alfalfa hay, starter feed, and milk replacer on the growth and organ development of yak calves. Animals. (2019) 9:921. 10.3390/ani9110921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puppel K, Kuczynska B. Metabolic profiles of cow's blood; a review. J Sci Food Agric. (2016) 96:4321–8. 10.1002/jsfa.7779 [DOI] [PubMed] [Google Scholar]
- 36.Zarrin M, Grossen-Rösti L, Bruckmaier RM, Gross JJ. Elevation of blood β-hydroxybutyrate concentration affects glucose metabolism in dairy cows before and after parturition. J Dairy Sci. (2017) 100:2323–33. 10.3168/jds.2016-11714 [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez C, Castro N, Capote J, Morales-delaNuez A, Moreno-Indias I, Sanchez-Macias D, et al. Effect of colostrum immunoglobulin concentration on immunity in Majorera goat kids. J Dairy Sci. (2009) 92:1696–701. 10.3168/jds.2008-1586 [DOI] [PubMed] [Google Scholar]
- 38.Piccione G, Sciano S, Messina V, Casella S, Zumbo A. Changes in serum total proteins, protein fractions and albumin-globulin ratio during neonatal period in goat kids and their mothers after parturition. Ann Anim Sci. (2011) 11:251–60. [Google Scholar]
- 39.Stogdale L. Correlation of changes in blood chemistry with pathological changes in the animal's body: II Electrolytes, kidney function tests, serum enzymes, and liver function tests. J S Afr Vet Assoc. (1981) 52:155–64. [PubMed] [Google Scholar]
- 40.Zhang H, Li J, Cao C, Zhang B, Yang W, Shi B, et al. Pyrroloquinoline quinone inhibits the production of inflammatory cytokines via the SIRT1/NF-κB signal pathway in weaned piglet jejunum. Food Funct. (2020) 11:2137–53. 10.1039/C9FO02609F [DOI] [PubMed] [Google Scholar]
- 41.Burton JH, Hosein AA, McMillan I, Grieve DG, Wilkie BN. Immunoglobulin absorption in calves as influenced by dietary protein intakes of their dams. Can J Anim Sci. (1984) 64:185–6. 10.4141/cjas84-216 [DOI] [Google Scholar]
- 42.O'Doherty JV, Crosby TF. The effect of diet in late pregnancy on colostrum production and immunoglobulin absorption in sheep. Animal Sci. (1997) 64:87–96. 10.1017/S135772980001558730886898 [DOI] [Google Scholar]
- 43.Arthington JD, Cattell MB, Quigley JD, McCoy GC, Hurley WL. Passive immunoglobin transfer in newborn calves fed colostrum or spray-dried serum protein alone or as a supplement to colostrum of varying quality. J Dairy Sci. (2000) 83:2834–8. 10.3168/jds.S0022-0302(00)75183-6 [DOI] [PubMed] [Google Scholar]
- 44.Yu K, Canalias F, Sola-Oriol D, Arroyo L, Pato R, Saco Y, et al. Age-related serum biochemical reference intervals established for unweaned calves and piglets in the post-weaning period. Front Vet Sci. (2019) 6:123. 10.3389/fvets.2019.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carroll JA, Veum TL, Matteri RL. Endocrine responses to weaning and changes in post-weaning diet in the young pig. Domest Anim Endocrinol. (1998) 15:183–94. 10.1016/S0739-7240(98)00006-X [DOI] [PubMed] [Google Scholar]
- 46.Hoffman ML, Rokosa MA, Zinn SA, Hoagland TA, Govoni KE. Poor maternal nutrition during gestation in sheep reduces circulating concentrations of insulin-like growth factor-I and insulin-like growth factor binding protein-3 in offspring. Domest Anim Endocrinol. (2014) 49:39–48. 10.1016/j.domaniend.2014.05.002 [DOI] [PubMed] [Google Scholar]
- 47.Sun DM, Mao SY, Zhu WY, Liu JH. Effect of starter diet supplementation on rumen epithelial morphology and expression of genes involved in cell proliferation and metabolism in pre-weaned lambs. Animal. (2018) 12:2274–83. 10.1017/S1751731118000290 [DOI] [PubMed] [Google Scholar]
- 48.Thissen JP, Ketelslegers JM, Underwood LE. Nutritional regulation of the insulin-like growth factors. Endocr Rev. (1994) 15:80–101. 10.1210/edrv-15-1-80 [DOI] [PubMed] [Google Scholar]
- 49.Thorp CL, Wylie ARG, Steen RWJ, Shaw C, McEvoy JD. Effects of incremental changes in forage: concentrate ratio on plasma hormone and metabolite concentrations and products of rumen fermentation in fattening beef steers. Anim Sci. (2000) 71:93–109. 10.1017/S135772980005492830886898 [DOI] [Google Scholar]
- 50.Shen Z, Seyfert H-M, Löhrke B, Schneider F, Zitnan R, Chudy A, et al. An energy-rich diet causes rumen papillae proliferation associated with more IGF type 1 receptors and increased plasma IGF-1 concentrations in young goats. J Nutr. (2004) 134:11–7. 10.1093/jn/134.1.11 [DOI] [PubMed] [Google Scholar]
- 51.Hua KM, Hodgkinson SC, Bass JJ. Differential regulation of plasma levels of insulin-like growth factors-I and -II by nutrition, age and growth hormone treatment in sheep. J Endocrinol. (1995) 147:507–16. 10.1677/joe.0.1470507 [DOI] [PubMed] [Google Scholar]
- 52.Araujo APC, Venturelli BC, Santos MCB, Gardinal R, Consolo NRB, Calomeni GD, et al. Chitosan affects total nutrient digestion and ruminal fermentation in Nellore steers. Anim Feed Sci Tech. (2015) 206:114–8. 10.1016/j.anifeedsci.2015.05.016 [DOI] [Google Scholar]
- 53.Kelly D, Coutts AGP. Development of digestive and immunological function in neonates: role of early nutrition. Livest Prod Sci. (2000) 66:161–7. 10.1016/S0301-6226(00)00223-210946785 [DOI] [Google Scholar]
- 54.El-Azrak KEM, Morsy AS, Soltan YA, Hashem NM, Sallam SMA. Impact of specific essential oils blend on milk production, serum biochemical parameters and kid performance of goats. Anim Biotechnol. (2021) 15:1–9. 10.1080/10495398.2021.1898978 [DOI] [PubMed] [Google Scholar]
- 55.Rey M, Enjalbert F, Monteils V. Establishment of ruminal enzyme activities and fermentation capacity in dairy calves from birth through weaning. J Dairy Sci. (2012) 95:1500–12. 10.3168/jds.2011-4902 [DOI] [PubMed] [Google Scholar]
- 56.Drackley JK. Calf Nutrition from Birth to Breeding. Vet Clin North Am Food Anim Pract. (2008) 24:55–86. 10.1016/j.cvfa.2008.01.001 [DOI] [PubMed] [Google Scholar]
- 57.Górka P, Kowalski ZM, Zabielski R, Guilloteau P. Invited review: Use of butyrate to promote gastrointestinal tract development in calves. J Dairy Sci. (2018) 101:4785–800. 10.3168/jds.2017-14086 [DOI] [PubMed] [Google Scholar]
- 58.Li W, Gelsinger S, Edwards A, Riehle C, Koch D. Transcriptome analysis of rumen epithelium and meta-transcriptome analysis of rumen epimural microbial community in young calves with feed induced acidosis. Sci Rep. (2019) 9:4744. 10.1038/s41598-019-40375-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Górka P, Kowalski ZM, Pietrzak P, Kotunia A, Jagusiak W, Zabielski R. Is rumen development in newborn calves affected by different liquid feeds and small intestine development? J Dairy Sci. (2011) 94:3002–13. 10.3168/jds.2010-3499 [DOI] [PubMed] [Google Scholar]
- 60.McCracken BA, Gaskins HR, Ruwe-Kaiser PJ, Klasing KC, Jewell DE. Diet-dependent and diet-independent metabolic responses underlie growth stasis of pigs at weaning. J Nutr. (1995) 125:2838–45. [DOI] [PubMed] [Google Scholar]
- 61.Abdelsattar MM, Vargas-Bello-Pérez E, Zhuang Y, Fu Y, Zhang N. Impact of dietary supplementation with beta-hydroxybutyric acid on performance, nutrient digestibility, organ development and serum stress indicators in early-weaned goat kids. Anim Nutr. (2021). 10.1016/j.aninu.2021.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Herrick K, Hippen A, Kalscheur K, Schingoethe D, Ranathunga S, Anderson J, et al. Infusion of butyrate affects plasma glucose, butyrate, and β-hydroxybutyrate but not plasma insulin in lactating dairy cows. J Dairy Sci. (2018) 101:3524–36. 10.3168/jds.2017-13842 [DOI] [PubMed] [Google Scholar]
- 63.Deelen SM, Leslie KE, Steele MA, Eckert E, Brown HE, DeVries TJ. Validation of a calf-side beta-hydroxybutyrate test and its utility for estimation of starter intake in dairy calves around weaning. J Dairy Sci. (2016) 99:7624–33. 10.3168/jds.2016-11097 [DOI] [PubMed] [Google Scholar]
- 64.Bush R. Effect of age and diet on in vitro metabolism in rumen epithelium from Holstein calves. Canadian J Anim Sci. (1988) 68:1245–51. 10.4141/cjas88-139 [DOI] [Google Scholar]
- 65.Lane MA, Baldwin RLt, Jesse BW. Sheep rumen metabolic development in response to age and dietary treatments. J Anim Sci. (2000) 78:1990–6. 10.2527/2000.7871990x [DOI] [PubMed] [Google Scholar]
- 66.Budzynska M, Weary DM. Weaning distress in dairy calves: effects of alternative weaning procedures. Appl Anim Behav Sci. (2008) 112:33–9. 10.1016/j.applanim.2007.08.004 [DOI] [Google Scholar]
- 67.Jasper J, Budzynska M, Weary DM. Weaning distress in dairy calves: acute behavioural responses by limit-fed calves. Appl Anim Behav Sci. (2008) 110:136–43. 10.1016/j.applanim.2007.03.017 [DOI] [Google Scholar]
- 68.Fairbrother JM, Nadeau E, Gyles CL. Escherichia coli in postweaning diarrhea in pigs: an update on bacterial types, pathogenesis, and prevention strategies. Anim Health Res Rev. (2005) 6:17–39. 10.1079/AHR2005105 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.