Highlights
-
•
Secondary infections can complicate the course of 10% of hospitalized patients with coronavirus disease 2019.
-
•
Elderly, diabetic and severely ill patients are at greatest risk of secondary infections.
-
•
Length of hospital stay of patients with secondary infections was almost twice as long as that of patients without secondary infections.
-
•
Patients with secondary infections had higher requirements for oxygen and intensive care unit care.
-
•
The most common type of secondary infection was urinary tract infection, followed by bloodstream infection.
Keywords: Secondary infections, Bacteria, Fungi, Antimicrobials, COVID-19
Abstract
Objective
To gain better insight into the extent of secondary bacterial and fungal infections in hospitalized patients in India, and to assess how these alter the course of coronavirus disease 2019 (COVID-19) so that control measures can be suggested.
Methods
In this retrospective, multicentre study, the data of all patients who tested positive for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) on reverse transcriptase polymerase chain reaction (RT-PCR), admitted to hospital between March 2020 and July 2021, were accessed from the electronic health records of a network of 10 hospitals across five states in North India.
Results
Of 19,852 patients testing positive for SARS-CoV-2 on RT-PCR and admitted to the study hospitals during the study period, 1940 (9.8%) patients developed secondary infections (SIs). Patients with SIs were, on average, 8 years older than patients without SIs (median age 62.6 vs 54.3 years; P<0.001). The risk of SIs was significantly (P<0.001) associated with age, severity of disease at admission, diabetes, admission to the intensive care unit (ICU), and ventilator use. The most common site of infection was urine (41.7%), followed by blood (30.8%) and sputum/bronchoalveolar lavage/endotracheal fluid (24.8%); the least common was pus/wound discharge (2.6%). Gram-negative bacilli (GNB) were the most common organisms (63.2%), followed by Gram-positive cocci (GPC) (19.6%) and fungi (17.3%). Most patients with SIs were on multiple antimicrobials. The most commonly used antibiotics against GNB were beta-lactam/beta-lactamase inhibitors (76.9%), carbapenems (57.7%), cephalosporins (53.9%), and antibiotics against carbapenem-resistant Enterobacteriaceae (47.1%). Empirical use of antibiotics against GPC was seen in 58.9% of patients with SIs, and empirical use of antifungals was observed in 56.9% of patients with SIs. The average length of hospital stay for patients with SIs was almost twice as long as that of patients without SIs (median 13 vs 7 days). Overall mortality among patients with SIs (40.3%) was more than eight times higher than that among patients without SIs (4.6%). Only 1.2% of patients with SIs with mild COVID-19 at admission died, compared with 17.5% of those with moderate COVID-19 at admission and 58.5% of those with severe COVID-19 at admission (P<0.001). The mortality rate was highest in patients with bloodstream infections (49.8%), followed by those with hospital-acquired pneumonia (47.9%), urinary tract infections (29.4%), and skin and soft tissue infections (29.4%). The mortality rate in patients with diabetes with SIs was 45.2%, compared with 34.3% in those without diabetes (P<0.001).
Conclusions
SIs complicate the course of patients hospitalized with COVID-19. These patients tend to have a much longer hospital stay, a higher requirement for oxygen and ICU care, and a significantly higher mortality rate compared with those without SIs. The groups most vulnerable to SIs are patients with more severe COVID-19, elderly patients and patients with diabetes. Judicious empirical use of combination antimicrobials in these groups of vulnerable patients can save lives. It is desirable to have region- or country-specific guidelines for appropriate use of antibiotics and antifungals to prevent their overuse.
Introduction
Viral infections, particularly severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection, may predispose to secondary infections (SIs) (Bengoechea and Bamford, 2020; Manna et al., 2020). Various explanations given for this phenomenon include direct damage to the respiratory epithelium caused by the virus, the effect on innate and adaptive immunity, and SARS-CoV-2-associated perturbation of gut homeostasis (Bengoechea and Bamford, 2020; Manna et al., 2020).
SIs have been noted to be a significant contributor to increased morbidity and mortality in influenza pandemics, during seasonal influenza and in other respiratory diseases (Morens et al., 2008; Morris et al., 2017) Shafran et al. (2021). found that patients with coronavirus disease 2019 (COVID-19) had a higher rate of SIs compared with patients with influenza (12.6% vs 8.7%; P=0.006). Other studies have suggested that superinfections, especially in the later stage of illness, were encountered in 8% of patients with COVID-19, usually those with more severe disease and those who died (Huang et al., 2020; Chen et al., 2020; Zhou et al., 2020; Shafran et al., 2021).
Due to concerns regarding increased mortality in patients with bacterial superinfections during influenza pandemics, several guidelines advocate the use of empirical antibiotics for patients with severe COVID-19 (Alhazzani et al., 2020; World Health Organization, 2020). This, however, has the potential for antibiotic overuse and increasing antimicrobial resistance (Huttner et al., 2020). As the prevalence of secondary bacterial and fungal infections in patients with COVID-19 in India is not known, a better understanding is crucial to treat COVID-19 and to help ensure the responsible use of antimicrobials to minimize the negative consequences of overuse.
This study was undertaken to gain better insight into the extent of secondary bacterial and fungal infections in hospitalized patients with COVID-19 in India, and to assess how these alter the course of the disease. Evaluating the treatment strategies used in this group of patients may help in the design of appropriate guidelines for empirical use of antimicrobials in patients with COVID-19 in India.
Methods
In this retrospective, multicentre study, the data of all patients who tested positive for SARS-CoV-2 on reverse transcriptase polymerase chain reaction (RT-PCR), admitted to hospital between March 2020 and July 2021, were accessed from the electronic health records (EHRs) of a network of 10 hospitals across five states in North India. Patients were categorized as mild, moderate or severe COVID-19, in accordance with the criteria of the Government of India (Ministry of Health and Family Welfare, 2021). The data included the demographic profile of patients, presence of diabetes, various investigations [e.g. C-reactive protein (CRP), D-dimer, interleukin-6 (IL-6), ferritin, creatine phosphokinase (CPK), lactate dehydrogenase (LDH), Trop-I and lymphocyte counts, high-resolution computed tomography chest severity (CTSS) score], various treatment modalities (e.g. steroids, remdesivir, convalescent plasma), average length of hospital stay and in-hospital mortality.
Detailed data were available for SIs. Microbiological data, in the form of culture results from blood, urine, pus/wound discharge, sputum, bronchoalveolar lavage (BAL) fluid culture, and endotracheal (ET) secretion cultures, were analysed and patients were categorized into four types of SIs: bloodstream infection (BSI), urinary tract infection (UTI), skin and soft tissue infection (SSTI), and hospital-acquired pneumonia (HAP).
Patients with SIs were compared with those without SIs for the parameters listed above. Use of antibiotics, antifungals and antivirals was studied in patients who developed SIs. A substantial number of patients were on multiple antimicrobials, and many had multiple sites of infection. For statistical analysis, these were included under one predominant or primary site of infection if the same organism was isolated from different sites. Patients who had more than two sites infected with the same micro-organism were categorized as the primary site of infection, as clinically evident or supported by radiological evidence.
The following definitions were used in this study. Hospital-acquired infection was defined as SI occurring >48 h after hospitalization for SARS-CoV-2. Ventilator-associated pneumonia (VAP), a subset of HAP, was defined as pneumonia occurring after >48 h of ET intubation. For possible HAP/VAP diagnosis, indicators of worsening oxygenation (increase in fraction of inspired oxygen by ≥0.20 or increase in positive end-expiratory pressure by ≥3 cm H2O) over 48 h and purulent respiratory secretions and/or a positive culture for a respiratory pathogen were required (Søgaard et al., 2021).
BSI was defined as the presence of viable bacterial or fungal micro-organisms in the bloodstream (later demonstrated by the positivity of one or more blood cultures) that elicit or have elicited an inflammatory response, characterized by the alteration of clinical, laboratory and haemodynamic parameters (Viscoli, 2016).
UTI was defined as microbial infiltration of the otherwise sterile urinary tract, and is one of the most common bacterial infections worldwide. UTIs encompass infections of the urethra (urethritis), bladder (cystitis), ureters (ureteritis) and kidney (pyelonephritis) (Barber et al., 2013). Cases of isolated candiduria, defined as the presence of Candida spp. >104 colony-forming units/mL and pyuria (Gharaghani et al., 2018), were segregated from the urine-culture-positive cases.
SSTIs encompass a variety of pathological conditions that involve the skin and underlying subcutaneous tissue, fascia or muscle, ranging from simple superficial infections to severe necrotizing infections (Sartelli et al., 2018).
Statistical analysis
Data have been presented as counts and percentages for qualitative characteristics (e.g. sex, place of admission, use of oxygen), and as mean and standard deviation (SD) for quantitative characteristics (e.g. age). Length of hospital stay and laboratory parameters have been summarized in terms of median and interquartile range (IQR) because of their highly skewed distribution. The significance of the difference between cases with SIs and cases without SIs was assessed by Chi-squared test or Student's t-test. Fisher's exact test was used to compare small (<5) frequencies. The Wilcoxon–Mann–Whitney test was used for highly skewed distributions (e.g. inflammatory markers). P<0.05 was considered to indicate significance, although the number of cases was so large for some categories that P-values must be interpreted with caution. SPSS Version 21 (IBM Corp., Armonk, NY, USA) was used for calculations.
Results
Demographics
In total, 19,852 patients who tested positive for SARS-CoV-2 on RT-PCR were admitted to the study hospitals during the study period. Their records were retrieved from the EHR system. Of these, 1940 (9.8%) patients developed SIs. No significant (P=0.100) gender difference was observed, but the patients with SIs were, on average, 8 years older than those without SIs (median age 62.6 vs 54.3 years; P<0.001) (Table 1). The incidence of SIs increased with age, from 4.0% in patients aged <45 years to 18.4% in patients aged ≥75 years. More than one-fifth (22.6%) of patients in ICUs had SIs, compared with 3.0% of patients on hospital wards (P<0.001). Patients requiring oxygen supplementation were significantly (P<0.0010) more likely to have SIs compared with those not requiring oxygen supplementation (12.1% vs 4.9%), and the incidence of SIs increased with increasing need for oxygen, from oxygen via nasal prongs/face mask to non-invasive ventilation (NIV) to mechanical ventilation (MV). Details for patients receiving convalescent plasma therapy, steroids and remdesivir are given in Table 1. The median length of hospital stay was almost two-fold higher in patients with SIs compared with those without SIs (13 vs 7 days; P <0.001) (Table 1).
Table 1.
Comparison of characteristics of cases with and without secondary infections (SIs)
| Parameters | Total cases |
Cases with SIs |
Cases without SIs |
P-value | ||||
|---|---|---|---|---|---|---|---|---|
| n | % of total | n | % infected | n | % not infected | |||
| Total | 19,852 | 100.0 | 1940 | 9.8 | 17,912 | 90.2 | X | |
| Sex | Male | 13,175 | 66.4 | 1255 | 9.5 | 11,920 | 90.5 | 0.100 |
| Female | 6677 | 33.6 | 685 | 10.3 | 5992 | 89.7 | ||
| Age (years) | <45 | 5547 | 27.9 | 222 | 4.0 | 5325 | 96.0 | <0.001 |
| 45–59 | 6369 | 32.1 | 523 | 8.2 | 5846 | 91.8 | ||
| 60–74 | 6112 | 30.8 | 860 | 14.1 | 5252 | 85.9 | ||
| ≥75 | 1824 | 9.2 | 335 | 18.4 | 1489 | 81.6 | ||
| Mean age (years) (SD) | 55.0 (15.9) | 62.6 (13.9) | 54.4 (15.9) | <0.001 | ||||
| Admission | ICU | 6845 | 34.5 | 1548 | 22.6 | 5297 | 77.4 | <0.001 |
| Ward | 13,007 | 65.5 | 392 | 3.0 | 12,615 | 97.0 | ||
| Oxygen | No | 6402 | 32.2 | 312 | 4.9 | 6090 | 95.1 | <0.001 |
| Yes | 13,450 | 67.8 | 1628 | 12.1 | 11,822 | 87.9 | ||
| Type of oxygen (percentage out of those who received oxygen) | Oxygen (NP/FM/NRBM) | 9842 | 49.6 | 822 | 8.4 | 9020 | 91.6 | <0.001 |
| NIV | 1800 | 9.1 | 390 | 21.7 | 1410 | 78.3 | ||
| MV | 1808 | 9.1 | 416 | 23.0 | 1392 | 77.0 | ||
| CPT | Given | 2542 | 12.8 | 685 | 26.9 | 1857 | 73.1 | <0.001 |
| Not given | 17,310 | 87.2 | 1255 | 7.3 | 16,055 | 92.7 | ||
| Steroids (drugs) | Given | 18,344 | 92.4 | 1893 | 10.3 | 16,451 | 89.7 | <0.001 |
| Not given | 1508 | 7.6 | 47 | 3.1 | 1461 | 96.9 | ||
| Remdesivir | Given | 12,017 | 60.5 | 1273 | 10.6 | 10,744 | 89.4 | <0.001 |
| Not given | 7835 | 39.5 | 667 | 8.5 | 7168 | 91.5 | ||
| Length of hospital stay (days) | Median (IQR) | 7 (5.12–10.14) | 13 (7.87–20.05) | 7 (5.05–9.86) | <0.001 | |||
SD, standard deviation; ICU, intensive care unit; NP, nasal prongs; FM, face mask; NRBM, non-rebreather mask; NIV, non-invasive ventilation; MV, mechanical ventilation; CPT, convalescent plasma therapy; IQR, interquartile range.
Characteristics of SIs
Of the 1940 patients with SIs, 598 (30.8%) had positive blood cultures, 809 (41.7%) had positive urine cultures, 51 (2.6%) had positive cultures from pus/wound discharge, and 482 (24.8%) had positive cultures from sputum/BAL fluid/ET secretions (Table 2). Thus, urine was the most common site of infection, followed by blood.
Table 2.
Comparison of characteristics of cases with secondary infections (SIs) at different sites (n=1940)
| Parameters | BSI |
UTI |
SSTI |
HAP |
P-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |||
| Total – % of total COVID-19 cases | 598 | 3.0 | 809 | 4.1 | 51 | 0.3 | 482 | 2.4 | ||
| Total – % of cases with SIs | 598 | 30.8 | 809 | 41.7 | 51 | 2.6 | 482 | 24.8 | ||
| Sex | Male | 395 | 31.5 | 474 | 37.8 | 31 | 2.5 | 355 | 28.3 | <0.001 |
| Female | 203 | 29.6 | 335 | 48.9 | 20 | 2.9 | 127 | 18.5 | ||
| Age (years) | Mean (SD) | 62.6 (13.8) | 62.9 (14.1) | 61.3 (15.0) | 62.4 (13.5) | 0.315 | ||||
| Diabetes | Yes | 350 | 32.8 | 463 | 43.4 | 14 | 1.3 | 239 | 22.4 | <0.001 |
| No | 248 | 28.4 | 346 | 39.6 | 37 | 4.2 | 243 | 27.8 | ||
BSI, bloodstream infection; UTI, urinary tract infection; SSTI, skin and soft tissue infection; HAP, hospital-acquired pneumonia; COVID-19, coronavirus disease 2019; SD, standard deviation.
Of 482 cases of HAP, 50 (10.4%) were receiving MV and 181 (37.6%) were receiving NIV. Of 598 cases of BSI, 341 (57.0%) had a central line inserted. Of 809 cases of UTI, 197 (24.4%) had a Foley catheter inserted.
The mean age of patients with SIs at different sites was not significantly different (P=0.315). Of all infections, HAP was significantly less common in females compared with males (18.5% vs 28.3%), and UTIs were significantly more common in females compared with males (48.9% vs. 37.8%; P<0.0010). There were more cases of BSI and UTI among in patients with diabetes, and fewer cases of SSTI and HAP.
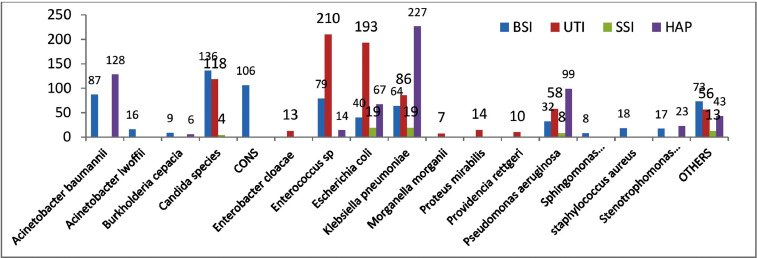
A significant number of patients were infected by more than one organism (261/1940, 13.4%), and 662 patients (34.1%) had positive cultures from more than one site. Overall, there were 685 positive cultures in blood (80.4% bacterial and 19.6% fungal), 893 positive cultures in urine (72.4% bacterial and 27.6% fungal), 607 positive cultures from any of the respiratory secretions (99.7% bacterial and two samples positive for Candida auris), and 63 positive cultures from pus/wound discharge (93.7% bacterial and 6.3% fungal). Species details are given in Figure 1. Overall, there were 2248 positive isolates (bacterial and fungal) from samples of 1940 patients with SIs. Of these, 1420 (63.2%) were Gram-negative bacilli (GNB), 440 (19.6%) were Gram-positive cocci (GPC) and 388 (17.3%) were fungi (Candida spp.). Candida spp. in blood and urine are shown in Table 3. Candida albicans and Candida tropicalis were more common in urine, and C. auris was more common in blood.
Figure 1.
Microbiological flora causing secondary infections. BSI, bloodstream infection; UTI, urinary tract infection; SSI, skin and soft tissue infection; HAP, hospital-acquired pneumonia.
Table 3.
Candida species in bloodstream infections (BSIs), urinary tract infections (UTIs) with BSIs, and Candiduria alone
| Candida spp. | BSI |
UTI + BSI |
Candiduria alone |
|||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Candida albicans | 23 | 16.9 | 21 | 17.8 | 50 | 39.1 |
| Candida auris | 35 | 25.7 | 33 | 28.0 | 0 | 0 |
| Candida catenulate | 1 | 0.7 | 0 | 0 | 0 | 0 |
| Candida ciferrii | 1 | 0.7 | 0 | 0 | 0 | 0 |
| Candida famata | 3 | 2.2 | 0 | 0 | 0 | 0 |
| Candida duobushaemulonii | 0 | 0 | 0 | 0 | 5 | 3.9 |
| Candida glabrata | 15 | 11.0 | 15 | 12.7 | 2 | 1.6 |
| Candida guilliermondii | 1 | 0.7 | 1 | 0.8 | 0 | 0 |
| Candida kefyr | 1 | 0.7 | 0 | 0 | 0 | 0 |
| Candida krusei | 2 | 1.5 | 1 | 0.8 | 0 | 0 |
| Candida parapsilosis | 13 | 9.6 | 7 | 5.9 | 0 | 0 |
| Candida tropicalis | 41 | 30.1 | 40 | 33.9 | 71 | 55.5 |
| Total | 136 | 100.0 | 118 | 100.0 | 128 | 100.0 |
Antimicrobial resistance pattern
Antibiotic sensitivity testing was conducted among the samples tested for SIs. Carbapenem resistance was observed in 68% of Acinetobacter baumanii, 48% of Klebsiella pneumoniae, 39% of Escherichia coli and 43% of Pseudomonas aeruginosa isolates. Fluconazole resistance was studied in cultures positive for fungal infection, and was found in 54% of C. auris, 10% of C. albicans and 19% of non-albicans Candida isolates.
Antimicrobial treatment
The use of various antimicrobial agents (antibiotics, antifungals and antivirals) as initial empirical therapy in those patients who developed SIs was also studied. Almost all of these patients were receiving multiple antibiotics and/or antifungals. In terms of use of antimicrobials for COVID-19, the most commonly used medications were remdesivir (74.8%), favipiravir (21.2%), doxycycline (50.2%), ivermectin (43.5%) and azithromycin (29.3%). For empirical treatment of SIs, the most commonly used antibiotics were those directed against GNB. The most commonly used antibiotics against GNB were beta-lactam/beta-lactamase inhibitors (76.9%), carbapenems (57.7%), cephalosporins (53.9%), and antibiotics against carbapenem-resistant Enterobacteriaceae (47.1%). Empirical use of antibiotics against GPC was seen in 58.9% of patients with SIs. Interestingly, empirical use of antifungals was observed in 56.9% of patients with SIs (Table 4).
Table 4.
Use of various antimicrobial agents in patients with secondary infections (SIs)
| Antimicrobial agent | Subagents | n of cases | % of total SI cases |
|---|---|---|---|
| Antibiotics for GNB CRE | Polymyxin B (589, 64.4%), colistin (166, 18.2%), fosfomycin (83, 9.1%), minocycline (43, 4.7%), tigicycline (33, 3.6%) | 914 | 47.1 |
| Carbapenems for GNB | Meropenem (949, 84.7%), doripenem (42, 3.8%), imipenem (20, 1.8%), ertapenem (109, 9.7%) | 1120 | 57.7 |
| BL/BLI for GNB | Piperacillin-tazobactum (846, 56.7%), ceftriaxone-sulbactum (373, 25%), ticarcillin-clavulanate (139, 9.3%), others (133, 8.9%) | 1491 | 76.9 |
| Cephalosporins for GNB | Ceftriaxone (468, 44.8%), cefepime (326, 31.2%), cefuroxime (170, 16.3%), others (81, 7.8%) | 1045 | 53.9 |
| Antibiotics for GPC | Amoxicillin clavulanate (83, 7.4%), vancomycin (38, 3.4%), linezolid (344, 30.0%), teicoplanin (651, 56.9%), levonadifloxacin (22, 1.9%) daptomycin (4, 0.3%) | 1144 | 58.9 |
| Antibiotics for anaerobes | Clindamycin (138, 45.1%), metronidazole (168, 54.9%) | 306 | 15.8 |
| Aminoglycosides for GNB | Amikacin (108, 75%), gentamycin (22, 15.3%), tobramycin (10, 6.9%), netilmycin (2, 1.4%), streptomycin (2, 1.4%) | 144 | 7.4 |
| Quinolones for GNB | Ciprofloxacin (14, 31.1%), ofloxacin (10, 22.2%), moxifloxacin (6, 13.3%), levofloxacin (2, 4.4%), prulifloxacin (1, 2.2%) | 45 | 2.3 |
| Other antibiotics for GNB | Trimethoprim-sulfamethoxazole (50, 55.6%), nitrofurantoin (38, 42.2%), chloramphenicol (2, 2.2%) | 90 | 4.6 |
| Other antimicrobials for presumed activity against COVID-19 (many on multiple drugs) | Azithromycin (569, 29.3%), doxycyline (973, 50.2%), ivermectin (843, 43.5%) | 1940 | 100.0 |
| Antifungals | Azoles, echinocandins, amphotericin BAzoles: fluconazole (410, 61.4%), itraconazole (4, 0.6%), voriconazole (228, 34.1%), posaconazole (25, 3.7%), isavuconazole (1, 0.1%)Echinocandins: caspofungin (58, 29.1%), anadulafungin (46, 23.1%), micafungin (95, 47.7%)Amphotericin B (238, 100%) | 1105668 | 56.934.4 |
| 199 | 10.3 | ||
| 238 | 12.3 | ||
| Antivirals | Remdesivir (1273, 74.8%), favipiravir (360, 21.2%), oseltamivir (40, 2.3%), lopinavir-ritonavir (11, 0.6%), aciclovir (20, 1.1%) | 1704 | 87.8 |
GNB, gram-negative bacilli; CRE, carbapenem-resistant Enterobacteriaceae; BL-BLI, beta-lactam/beta-lactamase inhibitors; GPC, Gram-positive cocci; COVID-19, coronavirus disease 2019.
Note: Most patients with SIs were on multiple antibiotics and/or antifungals.
Mortality and its correlates
As shown in Table 5, mortality (40.3%) was more than eight times higher in patients with SIs compared with those without SIs (40.3% vs 4.6%). The proportion of patients with SIs increased with the severity of COVID-19 and mortality. Only 166 (2.6%) patients with mild COVID-19 had SIs, compared with 628 (9.5%) patients with moderate COVID-19 and 1146 (16.7%) patients with severe COVID-19. Only 1.2% of patients with SIs with mild COVID-19 at admission died, compared with 17.5% of those with moderate COVID-19 at admission and 58.5% of those with severe COVID-19 at admission.
Table 5.
Correlation of mortality with secondary infections (SIs) and severity of coronavirus disease 2019
| Disease severity | Total cases |
With SIs |
Without SIs |
P-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases |
Survived | Died |
Cases |
Survived | Died |
||||||||
| n | % | n | % | n | % | n | % | n | % | ||||
| Mild | 6402 | 32.2 | 166 | 2.6 | 164 | 2 | 1.2 | 6236 | 97.4 | 6211 | 25 | 0.4 | 0.154 |
| Moderate | 6598 | 33.2 | 628 | 9.5 | 518 | 110 | 17.5 | 5970 | 90.5 | 5808 | 162 | 2.7 | <0.001 |
| Severe | 6852 | 34.5 | 1146 | 16.7 | 476 | 670 | 58.5 | 5706 | 83.3 | 5066 | 640 | 11.2 | <0.001 |
| Total | 19,852 | 100.0 | 1940 | 9.8 | 1158 | 782 | 40.3 | 17912 | 90.2 | 17,085 | 827 | 4.6 | <0.001 |
The mortality rate was highest (49.8%) in patients with BSIs, followed by HAP (47.9%), UTIs (29.4%) and SSTIs (29.4%) (Table 6).
Table 6.
Cases with different sites of infection and mortality
| Site of infection | Total with infection |
P-value | |||
|---|---|---|---|---|---|
| n of cases | % of cases | n of deaths | % mortality | ||
| BSI | 598 | 30.8 | 298 | 49.8 | <0.001 |
| UTI | 809 | 41.7 | 238 | 29.4 | |
| SSTI | 51 | 2.6 | 15 | 29.4 | |
| HAP | 482 | 24.8 | 231 | 47.9 | |
| Total | 1940 | 100 | 782 | 40.3 | |
| With one organism | |||||
| Site of infection | n of cases | % of cases | n of deaths | % mortality | |
| BSI | 523 | 31.1 | 245 | 46.8 | <0.001 |
| UTI | 734 | 43.7 | 212 | 28.9 | |
| SSTI | 39 | 2.3 | 9 | 23.1 | |
| HAP | 383 | 22.8 | 169 | 44.1 | |
| Total | 1679 | 100 | 635 | 37.8 | |
| With multiple organisms | |||||
| Site of infection | n of cases | % of cases | n of deaths | % mortality | |
| BSI | 75 | 28.7 | 53 | 70.7 | <0.001 |
| UTI | 75 | 28.7 | 26 | 34.7 | |
| SSTI | 12 | 4.6 | 6 | 50 | |
| HAP | 99 | 37.9 | 62 | 62.6 | |
| Total | 261 | 100 | 147 | 56.3 | |
| Number of sites | |||||
| n of sites | n of cases | % of cases | n of deaths | % mortality | |
| 1 | 1278 | 65.9 | 368 | 28.8 | <0.001 |
| 2 | 428 | 22.1 | 266 | 62.1 | |
| 3 | 231 | 11.9 | 147 | 63.6 | |
| 4 | 3 | 0.2 | 1 | 33.3 | |
| Total | 1940 | 100 | 782 | 40.3 | |
BSI, bloodstream infection; UTI, urinary tract infection; SSTI, skin and soft tissue infection; HAP, hospital-acquired pneumonia.
There were 241 (82.6%) deaths among the 341 patients with BSI who had a central line inserted; in comparison, the mortality rate was 17.4% among patients with BSI without a central line.
The mortality rate in patients with only one organism identified was 37.8%, compared with 56.3% in patients with more than one organism identified; this was significantly associated with the site of infection in both cases (P<0.001). The proportionate pattern of mortality in cases with single and multiple organisms identified was similar for all sites of infection, although the differences were significant (P<0.001) due to the large sample size in this study (Table 6). Similarly, mortality in patients with one site of infection was 28.8%, compared with 62.5% in patients with multiple sites of infection (P<0.001).
More than half (1066, 54.9%) of the 1940 patients with SIs had diabetes. The mortality rate in patients with diabetes was 45.2%, compared with 34.3% in those without diabetes (Table 7); this difference was significant (P<0.001).
Table 7.
Association of mortality with diabetes
| Diabetes | n of cases with SIs | % of cases | n of deaths | % mortality | P-value |
|---|---|---|---|---|---|
| No | 874 | 45.1 | 300 | 34.3 | <0.001 |
| Yes | 1066 | 54.9 | 482 | 45.2 | |
| Total | 1940 | 100 | 782 | 40.3 |
SI, secondary infection.
Various inflammatory markers commonly used for monitoring patients admitted to hospital with COVID-19 were higher in patients with SIs compared with those without SIs. The median values (Table 8) for CRP, D-dimer, ferritin, IL-6, LDH and CPK were higher in patients with SIs, and the median absolute lymphocyte count was lower in patients with SIs. This difference was even greater when comparing the values for patients who died with those for patients who survived, across both groups. However, the difference in the median levels of inflammatory markers in patients who died was not very different between patients with and without SIs, and the median values of some markers (i.e. CRP, IL-6, LDH and CPK) were actually higher in patients without SIs who died. The median CTSS score for the overall group of patients with SIs was 15 (IQR 10-19), compared with 10 (IQR 7–14) for those without SIs. Again, the CTSS score was similar (median 17) for patients with SIs who died and patients without SIs who died (Table 8).
Table 8.
Correlation with inflammatory markers
| Laboratory parameters | Total cases |
Cases with SIs |
Cases without SIs |
||||||
|---|---|---|---|---|---|---|---|---|---|
| All | Survived | Died | All | Survived | Died | All | Survived | Died | |
| CRP – n | 13,670 | 12,633 | 1037 | 1493 | 902 | 591 | 12,177 | 11,731 | 446 |
| Median (IQR) | 9.8 (2.3–37.9) | 8.9 (2.1–33.0) | 34.8 (10.8–120.5) | 17.3 (5.5–81.4) | 13.4 (3.8–52.9) | 30.7 (8.9–112.3) | 9.3 (2.1–35.4) | 8.6 (1.9–32.5) | 35.5 (12.7–123.8) |
| D-dimer – n | 13,490 | 12,434 | 1056 | 1514 | 896 | 618 | 11,976 | 11,538 | 438 |
| Median (IQR) | 232.4 (145.7–431.0) | 220.8 (140.0–381.1) | 660.5 (318.7–2210.3) | 507.5 (260.2–1225.8) | 399.7 (222.0–903.6) | 793.0 (356.1–2406.8) | 222.0 (141.0–387.1) | 215.4 (137.4–363.0) | 591.0 (306.3–2121.1) |
| Ferritin – n | 11,895 | 10,940 | 955 | 1350 | 800 | 550 | 10545 | 10140 | 405 |
| Median (IQR) | 236.6 (102.1–493.4) | 217.8 (94.7–455.5) | 526.8 (270.3–1069.9) | 419.6 (180.4–841.6) | 316.6 (140.7–653.8) | 578.6 (283.3–1068.8) | 224.0 (97.1–465.0) | 212.4 (93.1–443.4) | 504.0 (258.1–1071.1) |
| IL-6 – n | 11,237 | 10,218 | 1019 | 1408 | 810 | 598 | 9829 | 9408 | 421 |
| Median (IQR) | 21.6 (7.7–56.8) | 19.3 (7.0–49.0) | 82.6 (31.8–215.4) | 50.1 (18.8–127.6) | 35.4 (12.9–86.7) | 73.2 (31.9–172.5) | 20.0 (7.2–51.5) | 18.7 (6.8–46.2) | 86.5 (33.8–232.0) |
| LDH – n | 9773 | 8945 | 828 | 1092 | 629 | 463 | 8681 | 8316 | 365 |
| Median (IQR) | 301.0 (234.0–402.0) | 291.0 (230.0–381.0) | 517.0 (375.8–741.0) | 410.0 (300.8–573.8) | 345.0 (261.0–469.0) | 517.0 (389.0–720.0) | 295.0 (231.0–390.6) | 288.0 (229.0–377.0) | 519.0 (361.5–767.0) |
| CPK – n | 6131 | 5577 | 554 | 648 | 334 | 314 | 5483 | 5243 | 240 |
| Median (IQR) | 100.0 (59.0–200.0) | 97.0 (58.0–187.4) | 162.0 (69.0–370.5) | 112.5 (55.0–246.0) | 102.5 (50.0–200.3) | 133.0 (58.7–316.0) | 99.0 (59.0–196.0) | 97.0 (58.0–188.0) | 183.0 (77.0–444.0) |
| Trop-1 – n | 5989 | 5185 | 804 | 998 | 531 | 467 | 4991 | 4654 | 337 |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.2) | 0.0 (0.0–0.1) | 0.0 (0.0–0.0) | 0.1 (0.0–0.2) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.3) |
| ALC – n | 16,620 | 15,129 | 1491 | 1883 | 1105 | 778 | 14,737 | 14024 | 713 |
| Median (IQR) | 1.2 (0.8–1.7) | 1.2 (0.8–1.7) | 0.8 (0.5–1.3) | 0.9 (0.6–1.5) | 1.1 (0.7–1.6) | 0.8 (0.5–1.3) | 1.2 (0.8–1.7) | 1.2 (0.8–1.7) | 0.8 (0.5–1.2) |
| CTSS – n | 9030 | 8536 | 494 | 847 | 564 | 283 | 8183 | 7972 | 211 |
| Median (IQR) | 11.0 (7.0–15.0) | 10.0 (6.0–14.0) | 17.0 (13.0–21.0) | 15.0 (10.0–19.0) | 14.0 (9.0–18.0) | 17.0 (14.0–21.0) | 10.0 (7.0–14.0) | 10.0 (6.0–14.0) | 17.0 (13.0–21.0) |
SI, secondary infection; CRP, C-reactive protein; IQR, interquartile range; IL-6, interleukin-6; LDH, lactate dehydrogenase; CPK, creatine phosphokinase; ALC, absolute lymphocyte count; CTSS, high-resolution computed tomography chest severity score.
Discussion
Patients with COVID-19 are at higher risk of secondary bacterial and fungal infections, and these are associated with increased morbidity and mortality (Morens et al., 2008; Morris et al., 2017; Bengoechea and Bamford, 2020; Manna et al., 2020). Several factors are known to contribute to the higher risk of SIs in these patients. The damage to the respiratory epithelium caused by the virus, as well as the effects on innate and adaptive immunity, and antagonizing interferon responses that enhance bacterial adherence, colonization, growth and invasion into healthy sites in the respiratory tract are important mechanisms (Bengoechea and Bamford, 2020; Manna et al., 2020). Downregulation and differential regulation of immune genes are mechanisms that may create a conducive environment for the occurrence of SIs, favouring bacterial attachment to host structural cells and a pro-inflammatory environment conducive to suppression of antibacterial host defences. In addition, Bengoechea and Bamford (2020) suggested SARS-CoV-2-associated perturbation of gut homeostasis as a mechanism that may potentially affect the disease outcomes in patients with severe COVID-19, including predisposing to secondary lung infections.
Overall incidence of SIs
Overall, 9.8% of all hospitalized patients with COVID-19 in the hospital network were diagnosed with SIs. A retrospective study by Vijay et al. (2021) on 17,534 patients with COVID-19 admitted to 10 hospitals in the Indian Council of Medical Research Antimicrobial Resistance Surveillance Network in India reported SIs in only 3.6% of cases. A meta-analysis of 24 studies, including 3338 patients with COVID-19, by Langford et al. (2020) reported overall bacterial infection in 6.9% of patients and in 8.1% of critically ill patients Shafran et al. (2021). studied 1384 cases (642 cases of COVID-19 and 742 cases of influenza) for blood and sputum culture results, clinical parameters and outcomes, and compared these parameters between the cases with COVID-19 and the cases with influenza. A higher rate of bacterial infection was found in patients with COVID-19 compared with those with influenza (12.6% vs 8.7%). A review of secondary pulmonary infections in patients with COVID-19 pneumonia by Chong et al. (2021) from the USA reported that the incidence of secondary pulmonary infections was 16% for bacterial infections and 6.3% for fungal infections. Secondary pulmonary infections were predominantly found in critically ill hospitalized cases. Thus, the incidence of SIs in patients with COVID-19 could be in the range of 5–15%, and increased with the severity of disease.
Correlates of SIs
Several factors that increased the risk of developing SIs in patients with COVID-19 were identified. On average, patients with SIs were 8 years older than patients without SIs (median age 62.6 vs 54.3 years; P<0.001). As age increased, the risk of developing SIs also increased. Only 4% of patients aged <45 years had SIs compared with 18.4% of patients aged >75 years. In the previous Indian study by Vijay et al. (2021), the mean age of patients with COVID-19 diagnosed with SIs was 53.3 (SD 9.36) years. They found that patients with SIs were older than those without SIs, with a clear upward gradient for the incidence of SIs with increasing age.
In the present study, 54.9% of patients with SIs had diabetes, whereas the overall prevalence of diabetes in this study was 43.8% (Budhiraja et al., 2021). This difference was significant (P<0.001). A US study by Adelman et al. (2021) of 774 patients with COVID-19 found hypertension in 75.5% of cases and diabetes in 45.7% of cases. A case–control study conducted in Pakistan by Nasir et al. (2021) found that diabetes and hypertension were the most common comorbidities in patients with SIs. In the present study, there were more cases of BSI and UTI among patients with diabetes, and fewer cases of SSTI and HAP.
Clear correlation was found between the severity of COVID-19 at the time of admission and the risk of developing SIs. Only 2.6% of patients with mild COVID-19 at admission developed SIs, compared with 9.5% and 16.7% of patients with moderate and severe COVID-19 at admission, respectively. This may be due, in part, to the need for hospitalization and ICU stay among patients with moderate and severe COVID-19 Nasir et al. (2021). reported that patients who were critically ill at admission were 4.42 times more likely to develop SIs Chong et al. (2021). also found secondary pulmonary infections predominantly in critically ill hospitalized cases. A clear relationship was found between higher incidence of SIs and the severity of COVID-19 at admission.
In the present study, 22.6% of patients admitted to ICUs developed SIs, compared with 3% of patients admitted to hospital wards (P<0.001) Vijay et al. (2021). reported that among the cases with confirmed SIs, 71.7% were in ICUs and 28.3% were on hospital wards at the time of SI diagnosis. ICU admission seems to have a definite association with SIs.
It may be difficult to draw a cause–effect relationship between the need for oxygen and the risk of developing SIs, but this study found that SIs developed in 12.1% of patients receiving oxygen supplementation compared with 4.9% of those not receiving oxygen supplementation (P<0.001). Similarly, the risk of developing SIs increased with increasing need for oxygen and ventilator support. The risk of developing SIs for patients receiving oxygen by nasal prongs/face mask was 8.4%, compared with 21.7% and 23% for patients receiving oxygen by NIV and MV, respectively. This could also reflect more severe disease in the patients with COVID-19 who developed serious SIs and hence needed greater oxygen support.
Features of SIs
The most common site of SI in this study was urine (41.7%), followed by blood (41.7%) and pneumonia (24.8%). SSTI was the least common infection (2.6%). This differs from the results reported by Vijay et al. (2021), who found that blood and respiratory secretions were the most common sites of SI.
Almost 13.4% of patients with SIs were infected by more than one micro-organism, and 34.1% had multiple sites of infection Shafran et al. (2021). reported the presence of more than one co-infection in only 4.5% of patients with COVID-19.
Adelman et al. (2021) reported that 30.7% of cases required MV; of these, 27.3% had positive respiratory cultures, with Staphylococcus aureus (34.5%) being the most common bacteria followed by P. aeruginosa (19.0%) and Klebsiella spp. (16.7%). Out of 774 cases, blood sample cultures were positive in 76% of cases and 4.7% (36) had BSIs; the majority of the patients had been admitted to an ICU (66.7%, 24/36 cases) Shafran et al. (2021). reported that blood sample cultures were positive in 85% of cases, compared with 14.2% of respiratory sample cultures Vijay et al. (2021)., from India, found that GNB (78.03%) were the main pathogens isolated, and the most common isolates were K. pneumoniae (29.3%), A. baumanii (21.07%), P. aeruginosa (9.6%) and E. coli (8.2%). Candida spp. were isolated from 6% of admitted cases, of which 1.2% were C. auris. K. pneumoniae was the most common isolate in blood (29.7%) and respiratory specimens (35%), and the most common isolates in urine were E. coli (27.17%), K. pneumoniae (18.4%) and Candida spp. (18.4%). Such varying findings indicate that the spectrum of SIs may be population specific.
Treatment
The number of patients who developed SIs was examined in relation to various treatment modalities used for the treatment of patients with COVID-19. This may not necessarily mean that these drugs increased the risk of SIs, but may simply reflect high use of these medicines, particularly in more seriously ill patients. Among the patients who received steroids, 10.3% had SIs, compared with 3.1% of patients who did not receive steroids. More than one-quarter (26.9%) of patients who received convalescent plasma developed SIs, compared with 7.3% of those who did not receive convalescent plasma. In the patients who received remdesivir, 10.6% developed SIs, compared with 8.5% of those who did not receive remdesivir Nasir et al. (2021). noted that the use of systemic steroids was significantly higher among cases with SIs than in those without SIs (92% vs 62%).
Most of the patients with SIs in the present study received multiple antimicrobials. The most commonly used antibiotics were against GNB. Beta-lactam/beta-lactamase inhibitor combination therapy was found to be the most common treatment (76.9%), followed by carbapenems (57.7%) and cephalosporins (53.9%). Antibiotics directed against GNB-CRE organisms, such as polymyxin B, colistin, fosfomycin, minocycline and tigecycline, were used in 47.1% of patients. This matched with the microbiological flora, as GNB were found to be the cause of SIs in 63.2% of cases. The high prevalence of CRE in GNB (68% in A. baumanii, 48% in K. pneumoniae, 39% in E. coli and 43% in P. aeruginosa) justified the empirical use of antibiotics against CRE. The use of antibiotics against GPC [Staphylococcus spp., coagulase-negative staphylococci (CoNS), Enterococcus spp.] in this study was high (58.9%), whereas the actual microbiological culture data revealed that these organisms could only be identified in 19.6% of samples. Similarly, antifungals were used in 56.9% of cases in this study, whereas fungi (Candida spp.) were only isolated in 17.3% of cases. A high degree of azole resistance in various Candida spp. (54% fluconazole resistance in C. auris, 19% resistance in non-albicans Candida and 10% in C. albicans), and a high level of isolation of C. auris (25.7% of Candida spp. isolates in BSIs and 13.4% in UTIs) and C. tropicalis (30.1% in BSIs and 45.1% in UTIs) justifies the empirical use of echinocandins (10.3%) and amphotericin B (12.3%). However, there is scope for significant improvement in terms of rationalizing the use of antimicrobials, especially empirical coverage against GPC and fungi. Each country needs to develop empiric antibiotic guidelines for hospitalized patients with COVID-19 to optimize the therapy and reduce potential harm caused by future development of antimicrobial resistance.
The drug resistance profiles of isolated pathogens were studied by Vijay et al. (2021), and 47.1% of patients were found to be infected with multi-drug resistant organisms . Three-quarters (74.2%) of GNB isolates were resistant to carbapenems alone Vijay et al. (2021). reported the use of third-generation cephalosporins (16.6%), beta-lactam/beta-lactamase inhibitor combination therapy (57.3%) and carbapenems (43.7%) in the management of patients with COVID-19 with SIs. Vancomycin or teicoplanin was prescribed to 24.9% of patients Vijay et al. (2021). also reported that the empirical cover for Gram-positive pathogens may not be warranted as the SIs were predominantly caused by GNB (78.3%) in their cohort. They also found that 10% of patients received antifungals without any evidence of fungal infection Shafran et al. (2021). found that culture reports in cases with either influenza or COVID-19 showed that P. aeruginosa and S. aureus were the most common causes of SIs. GNB represented 75% of cases in both groups. Interestingly, Enterococcus spp. infection was found to be more prevalent in patients with COVID-19 than in patients with influenza (8.6% vs 0%), and late infection with GPC was also more common in patients with COVID-19 Langford et al. (2020). analysed the use of antibiotics in patients with COVID-19 and found that >70% of cases received antibiotics, with the majority being broad-spectrum antibiotics such as third-generation cephalosporins and fluoroquinolones Adelman et al. (2021). reported that the most common organisms isolated were GNB (28.6%), S. aureus (16.7%), Candida spp. (16.7%) and CoNS (11.9%). Nearly 50% of infections were central-line-associated BSIs Nasir et al. (2021). found that among the SIs, GNB (85%) were more common than GPC. The most common isolate from blood was multi-drug-resistant Acinetobacter spp., followed by E. coli, Enterococcus spp. and K. pneumoniae. Among cases with secondary bacterial HAP, the most common isolate was multi-drug-resistant Acinetobacter spp., followed by multi-drug-resistant P. aeruginosa. Chong et al. reported the use of antibiotics in 60–100% of cases in the studies they reviewed, and the most common bacterial micro-organism was P. aeruginosa (21%), followed by Klebsiella spp. (17.2%), S. aureus (13.5%), E. coli (10%), and Stenotrophomonas maltophilia (3%). Aspergillus fumigatus was the most common fungus in patients with COVID-19. Most studies showed that multi-drug-resistant GNB were the most common organisms causing SIs in patients with COVID-19.
Outcome
The average length of hospital stay in the patients with SIs in this study was twice as long as that for those without SIs (13 vs 7 days; P<0.001). Several risk factors were identified which increased mortality more than eight-fold in patients with SIs compared with those without SIs (40.3% vs 4.6%) Vijay et al. (2021). noted that among cases of COVID-19 with SIs, the mortality rate was higher among critically ill patients (68%) compared with patients on hospital wards (27.6%) Nasir et al. (2021). found that cases with COVID-19 with SIs had a greater proportion of deaths compared with the control patients (42% vs 18%). SIs are a significant factor for mortality, and many are treatable. Thus, at least some of these deaths can be avoided.
Among the patients who developed SIs, correlation was found between the severity of COVID-19 at the time of admission and mortality. The mortality rates in patients with mild, moderate and severe COVID-19 at admission were 1.2%, 17.5% and 58.5%, respectively. The mortality rate would be expected to increase with the severity of COVID-19, but SIs may have contributed to the steep rise in the gradient. The mortality rate in patients with SIs who had diabetes was 45.2%, compared with 34.3% in those without diabetes (P<0.001). The mortality rate was highest in patients with BSIs (49.8%), followed by patients with HAP (47.9%), UTIs (29.4%) and SSTIs (29.4%) Adelman et al. (2021). found a significantly higher overall mortality rate in patients with COVID-19 who developed BSIs compared with those without BSIs (50% vs 13.8%). However, they did not find any significant difference in mortality rates between intubated cases with bacterial respiratory pathogens and those without bacterial respiratory pathogens.
The mortality rate in the study group of patients with SIs who only had one micro-organism identified was 37.8%, compared with 56.3% in patients who had more than one micro-organism identified (P<0.001) Shafran et al. (2021). reported an overall mortality rate (in patients with COVID-19 and influenza combined) of 13.2% in cases without infection, while the mortality rates were 33% and 61% in patients with one infection and patients with two infections, respectively. However, in patients with COVID-19, the mortality rate was 48.1% in cases with one infection and 75.9% in cases with more than one infection. In the present study, patients with SIs at a single site had a mortality rate of 28.8%, compared with 62.5% in patients with multiple sites of infection (P<0.001). These findings suggest that SIs are a significant contributing factor for disease severity among patients with COVID-19, leading to a higher mortality rate.
Laboratory parameters
In comparison with patients without SIs, median values for CRP, D-dimer, ferritin, IL-6, LDH and CPK were higher, and the median absolute lymphocyte count was lower, in patients with SIs. This difference was greater when comparing the values for patients who died with those for patients who survived, across both groups. However, the difference in the median levels of inflammatory markers in patients who died in both groups was small, and for some markers (i.e. CRP, IL-6, LDH and CPK), the median values were actually higher in patients without SIs who died. A cytokine storm causing significant elevation of these inflammatory markers, independent of SIs, would be the most likely reason for this Nasir et al. (2021). found that median CRP (169 vs 81) and median neutrophil:lymphocyte ratio (8 vs 4) were significantly higher in patients with SIs, but no significant difference in procalcitonin (0.36 vs 0.14) was noted in patients with COVID-19 with SIs compared with those without SIs. The median CTSS for the overall group with SIs was 15 (IQR 10–19), and that for the group without SIs was 10 (IQR 7–14). Again, when comparing those who died in both groups, the CTSS score was almost similar (median 17).
Conclusions
SIs complicate the course of patients hospitalized with COVID-19. These patients tend to have a much longer hospital stay, a higher requirement for oxygen and ICU care, and a significantly higher mortality rate compared with those without SIs. The groups most vulnerable to SIs are patients with more severe COVID-19, elderly patients and patients with diabetes. Judicious empirical use of combination antimicrobials in these groups of vulnerable patients can save lives. It is desirable to have region- or country-specific guidelines for appropriate use of antibiotics and antifungals to prevent their overuse.
Acknowledgments
Acknowledgements
The authors wish to thank Taruna Sharma for her help at various stages of the study.
Author contributions
SB designed the study concept, finalized the draft, and contributed patients for the study. DJ performed collation of clinical and laboratory data. AI undertook the statistical analysis and contributed to the draft. MA wrote the initial manuscript. BT provided inputs on laboratory data and interpretation. The remaining authors are clinicians (Internal Medicine, Pulmonology, Critical Care, Laboratory Medicine and Microbiology) and contributed patient/laboratory data and/or treated patients in the present study.
Conflict of interest statement
None declared.
Funding sources
This study did not receive any financial contribution from any funding agency/source.
Ethical approval
This study was approved by the Institutional Ethics Committee, Max Super Speciality Hospital (a unit of Devki Devi Foundation) (Ref. No. BHR/RS/MSSH/DDF/SKT-2/IEC/IM/21-24, 7 September 2021). The Institutional Ethics Committe provided no objection and approved the publication of this manuscript. All patients gave consent for their anonymized data to be used for research purposes.
References
- Adelman MW, Bhamidipati DR, Hernandez-Romieu AC, Babiker A, Woodworth MH, Robichaux C, et al. Secondary bacterial pneumonias and bloodstream infections in patients hospitalized with COVID-19. Ann Am Thoracic Soc. 2021;18:1584–1587. doi: 10.1513/AnnalsATS.202009-1093RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020;46:854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AE, Norton JP, Spivak AM, Mulvey MA. Urinary tract infections: current and emerging management strategies. Clin Infect Dis. 2013;57:719–724. doi: 10.1093/cid/cit284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengoechea JA, Bamford CG. SARS-CoV-2, bacterial co-infections, and AMR: the deadly trio in COVID-19? EMBO Mol Med. 2020;12:e12560. doi: 10.15252/emmm.202012560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budhiraja S, Indrayan A, Aggarwal M, Jha V, Jain D, Tarai B, et al. Differentials in the characteristics of COVID-19 cases in Wave-1 and Wave-2 admitted to a network of hospitals in North India. medRxiv 2021.06.24.21259438. doi: 10.1101/2021.06.24.21259438. https://medrxiv.org/cgi/content/short/2021.06.24.21259438v1. [DOI]
- Chen J. Alberta Health Services; Edmonton: 2020. COVID-19 Scientific Advisory Group Rapid Response Report 2020. [Google Scholar]
- Chong WH, Saha BK, Ramani A, Chopra A. State-of-the-art review of secondary pulmonary infections in patients with COVID-19 pneumonia. Infection. 2021;49:591–605. doi: 10.1007/s15010-021-01602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharaghani M, Taghipour S, Halvaeezadeh M, Mahmoudabadi AZ. Candiduria; a review article with specific data from Iran. Turk J Urol. 2018;44:445–452. doi: 10.5152/tud.2018.54069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner BD, Catho G, Pano-Pardo JR, Pulcini C, Schouten J. COVID-19: don't neglect antimicrobial stewardship principles! Clin Microbiol Infect. 2020;26:808–810. doi: 10.1016/j.cmi.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford BJ, So M, Raybardhan S, Leung V, Westwood D, MacFadden DR, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26:1622–1629. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna S, Baindara P, Mandal SM. Molecular pathogenesis of secondary bacterial infection associated to viral infections including SARS-CoV-2. J Infect Public Health. 2020;13:1397–1404. doi: 10.1016/j.jiph.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health and Family Welfare . Government of India; New Delhi: 2021. Clinical management protocol: COVID-19.https://www.mohfw.gov.in/pdf/ClinicalManagementProtocolforCOVID19dated27062020.pdf Available at. (accessed 2 March 2022) [Google Scholar]
- Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198:962–970. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DE, Cleary DW, Clarke SC. Secondary bacterial infections associated with influenza pandemics. Front Microbiol. 2017;8:1041. doi: 10.3389/fmicb.2017.01041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasir N, Rehman F, Omair SF. Risk factors for bacterial infections in patients with moderate to severe COVID-19: a case–control study. J Med Virol. 2021;93:4564–4569. doi: 10.1002/jmv.27000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartelli M, Guirao X, Hardcastle TC. 2018 WSES/SIS-E consensus conference: recommendations for the management of skin and soft-tissue infections. World J Emerg Surg. 2018;13:58. doi: 10.1186/s13017-018-0219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafran N, Shafran I, Ben-Zvi H, Ben-Zvi H, Sofer S, Sheena L, Krause I, et al. Secondary bacterial infection in COVID-19 patients is a stronger predictor for death compared to influenza patients. Sci Rep. 2021;11:12703. doi: 10.1038/s41598-021-92220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søgaard KK, Baettig V, Osthoff M, S Marsch, Leuzinger K, Schweitzer M, et al. Community-acquired and hospital-acquired respiratory tract infection and bloodstream infection in patients hospitalized with COVID-19 pneumonia. J Intensive Care. 2021;9:10. doi: 10.1186/s40560-021-00526-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay S, Bansal N, Rao BK, Veeraraghavan B, Rodrigues C, Wattal C, et al. Secondary infections in hospitalized COVID-19 patients: Indian experience. Infect Drug Resist. 2021;14:893–1903. doi: 10.2147/IDR.S299774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscoli C. Bloodstream infections: the peak of the iceberg. Virulence. 2016;7:248–251. doi: 10.1080/21505594.2016.1152440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . WHO; Geneva: 2020. Clinical management of COVID-19: interim guidance. [Google Scholar]
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. Erratum in: Lancet 2020;395:1038. [DOI] [PMC free article] [PubMed] [Google Scholar]