Introduction
Since January 2020, the ongoing COVID-19 pandemic has been a global health emergency. Vaccination is a critical measure for containing the spread of COVID-19. At the end of 2020, several vaccines became available. Multiple adverse events (AEs) have been reported with all the available COVID-19 vaccines. This report describes a case of vitiligo in a child that started 2 weeks after COVID-19 messenger RNA (mRNA) vaccine administration.
Case report
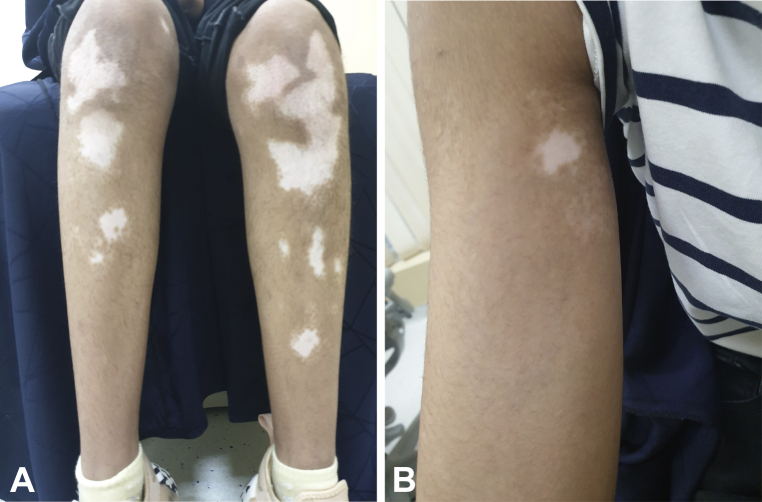
Three weeks after receiving her first dose of the BNT162b2 COVID-19 mRNA vaccine (Pfizer/BioNTech), a 13-year-old healthy girl presented to an outpatient dermatology clinic complaining of light-colored skin patches spreading across her body that started 2 weeks after vaccination. The patient had no known dermatologic diseases or medical conditions, no known allergies, and was not taking any medications. She had not used any treatment. Her father and one paternal uncle had vitiligo that had begun in adulthood; however, the patient never had vitiligo previously. Physical examination revealed a Fitzpatrick skin type IV with multiple widespread well-defined depigmented patches throughout her extremities and trunk, involving approximately 5% of her body surface area (Fig 1, A and B). Wood’s lamp examination illustrated milky-white accentuation of patches, which is consistent with clinically with vitiligo vulgaris. She was treated with a topical calcineurin inhibitor and topical steroid, as well as localized phototherapy. Her condition was stable after the second dose of the vaccine. Blood tests, including complete blood count, thyroid antibodies, antinuclear antibodies, transglutaminase, vitamin B12, and vitamin D were all within the normal limits. Follow up at 1 and 3 months showed gradual partial improvement of her condition.
Fig 1.
A, Vitiligo on the lower portion of both the legs of a 13-year-old girl. The vitiligo started to appear 2 weeks after she received her first dose of BNT162b2 COVID-19 messenger RNA vaccine. B, Vitiligo on the arm of the 13-year-old girl. The vitiligo started to appear 2 weeks after she received her first dose of BNT162b2 COVID-19 messenger RNA vaccine.
Discussion
No cutaneous AEs were reported in phase III studies of the BNT162b2 mRNA COVID-19 vaccine; however, several cutaneous AEs have been reported worldwide since January 2021, including local injection-site reactions, delayed large local reactions, urticaria, morbilliform eruption, erythromelalgia, chilblains, soft-tissue filler reactions, and pityriasis rosea.1,2 At least 4 cases of new-onset vitiligo following COVID-19 vaccination have been reported in adults,1,3, 4, 5 but to my knowledge, this is the youngest case of new-onset vitiligo following COVID-19 vaccination reported to date.
Although the precise role of the vaccine in disease pathogenesis is not fully understood, some studies have suggested that certain vaccine components with molecular resemblance to the host antigen may initiate disease in genetically predisposed individuals. Subsequently, tolerance of autoantigen is broken, and a pathogen-specific immune response is directed against host tissue, melanocytes in this case. Furthermore, vaccine antigen/adjuvant can stimulate a nonspecific innate immune response, resulting in activation of autoreactive CD8+/CD4+ T and B lymphocytes that can trigger autoimmune disease.4,6 Another possible mechanism is vaccine-induced stimulation of plasmacytoid dendritic cells to secrete type I interferon, which has an established role in host defense against SARS-CoV-2. Likewise, type I interferon and plasmacytoid dendritic cells participate in the immune response in vitiligo.7,8
Vitiligo is a common pigmentary autoimmune disease that affects approximately 0.5% to 2% of humans globally.4 Therefore, it is possible that the timing of vitiligo onset after COVID-19 vaccination was coincidental. However, the temporal relationship between onset and vaccine, as well as the emerging reports worldwide of different types of postvaccination autoimmune diseases, raise the possibility that the vaccine contributed to vitiligo pathogenesis.
In skin of color, vitiligo, though not a life-threatening disease, can have a considerable impact on a patient’s psychosocial wellbeing, as the individual may feel stigmatized and isolated.
This case report contributes to the growing medical literature regarding possible cutaneous AEs after COVID-19 vaccination. Larger-scale studies are needed to explore a possible causal relationship between COVID-19 vaccines and vitiligo. In the interim, dermatologists should be aware of possible postvaccine autoimmune cutaneous disease activation, especially in genetically susceptible patients.
However, this mild manageable adverse cutaneous reaction should not deter all eligible candidates from being vaccinated, as the risk posed by adverse cutaneous reactions is small compared with the possible fatal outcome of COVID-19.
Conflicts of interest
None disclosed.
Acknowledgments
The author would like to acknowledge the patient and her parents for giving their consent to have this case published.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Aktas H., Ertuğrul G. Vitiligo in a COVID-19-vaccinated patient with ulcerative colitis: coincidence? Clin Exp Dermatol. 2022;47(1):143–144. doi: 10.1111/ced.14842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMahon D.E., Amerson E., Rosenbach M., et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85(1):46–55. doi: 10.1016/j.jaad.2021.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaminetsky J., Rudikoff D. New-onset vitiligo following mRNA-1273 (Moderna) COVID-19 vaccination. Clin Case Rep. 2021;9(9) doi: 10.1002/ccr3.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciccarese G., Drago F., Boldrin S., Pattaro M., Parodi A. Sudden onset of vitiligo after COVID-19 vaccine. Dermatol Ther. 2022;35(1) doi: 10.1111/dth.15196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koç Yıldırım S. A new-onset vitiligo following the inactivated COVID-19 vaccine. J Cosmet Dermatol. 2022;21(2):429–430. doi: 10.1111/jocd.14677. [DOI] [PubMed] [Google Scholar]
- 6.Murali-Krishna K., Altman J.D., Suresh M., et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8(2):177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 7.Abdullah L., Awada B., Kurban M., Abbas O. Comment on 'Vitiligo in a COVID-19-vaccinated patient with ulcerative colitis: coincidence?': type I interferons as possible link between COVID-19 vaccine and vitiligo. Clin Exp Dermatol. 2022;47(2):436–437. doi: 10.1111/ced.14932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertolotti A., Boniface K., Vergier B., et al. Type I interferon signature in the initiation of the immune response in vitiligo. Pigment Cell Melanoma Res. 2014;27(3):398–407. doi: 10.1111/pcmr.12219. [DOI] [PubMed] [Google Scholar]