Abstract
Vaccine-induced immune thrombotic thrombocytopenia (VITT; synonym, thrombosis with thrombocytopenia syndrome, is associated with high-titer immunoglobulin G antibodies directed against platelet factor 4 (PF4). These antibodies activate platelets via platelet FcγIIa receptors, with platelet activation greatly enhanced by PF4. Here we summarize the current concepts in the pathogenesis of VITT. We first address parallels between heparin-induced thrombocytopenia and VITT, and provide recent findings on binding of PF4 to adenovirus particles and non-assembled adenovirus proteins in the 2 adenovirus vector-based COVID-19 vaccines, ChAdOx1 nCoV-19 and Ad26.COV2.S. Further, we discuss the potential role of vaccine constituents such as glycosaminoglycans, EDTA, polysorbate 80, human cell-line proteins and nucleotides as potential binding partners of PF4. The immune response towards PF4 in VITT is likely triggered by a proinflammatory milieu. Human cell-line proteins, non-assembled virus proteins, and potentially EDTA may contribute to the proinflammatory state. The transient nature of the immune response towards PF4 in VITT makes it likely that—as in heparin-induced thrombocytopenia —marginal zone B cells are key for antibody production. Once high-titer anti-PF4 antibodies have been formed 5 to 20 days after vaccination, they activate platelets and granulocytes. Activated granulocytes undergo NETosis and the released DNA also forms complexes with PF4, which fuels the Fcγ receptor-dependent cell activation process, ultimately leading to massive thrombin generation. Finally, we summarize our initial observations indicating that VITT-like antibodies might also be present in rare patients with recurrent venous and arterial thrombotic complications, independent of vaccination.
Keywords: VITT, TTS, Platelet factor 4, Vaccination, COVID-19, ChAdOx1 nCoV-19
Introduction
Vaccination against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is an important countermeasure to fight the ongoing COVID-19 pandemic. The European Medicines Agency has approved 2 adenovirus vector-based vaccines (recombinant chimpanzee adenoviral [ChAdOx1-S] vector, COVID-19 Vaccine AstraZeneca [Vaxzevria], and recombinant human adenovirus type 26 vector, COVID-19 Vaccine Janssen), both encoding the spike glycoprotein of SARS-CoV-2. Both vaccines, ChAdOx1 nCoV-19 and Ad26.COV2.S, are produced in human cell lines, T-REx-293 cells (human embryonic kidney cell, a HEK293 derivate) and PER.C6 TetR cells (human embryonic retinal cells), respectively [1,2,3].
Beginning in March 2021, otherwise healthy individuals developed complications starting 5 to 20 days following receipt of one of these 2 vaccines (almost always after the first injection, in the case of the 2-dose regimen for ChAdOx1 nCoV-19 vaccine). Key features were cerebral venous sinus thrombosis (CVST), splanchnic vein thrombosis, or other often severe thrombotic events in combination with thrombocytopenia. This novel disorder, “vaccine-induced immune thrombotic thrombocytopenia” (VITT; synonym, thrombosis with thrombocytopenia syndrome, TTS), is associated with high-titer immunoglobulin G (IgG) class antibodies directed against the cationic platelet chemokine, platelet factor 4 (PF4). These antibodies activate platelets via platelet FcγIIa receptors, with platelet activation greatly enhanced by PF4.
PF4 as a label of pathogens
PF4 opsonizes negatively-charged surfaces of microbial pathogens, facilitating the binding of anti-PF4 antibodies [4] and subsequent phagocytosis, as shown in detail for bacteria [5]. Binding of PF4 to Gram-positive and Gram-negative bacteria [6] is thought to be charge-mediated, as heparin and other polyanions dissociate this interaction. In vivo, mice challenged with polymicrobial sepsis produce anti-PF4/heparin antibodies [4].
No adverse clinical outcomes are seen in the majority of individuals who develop an anti-PF4 response. Such antibodies are even present in 5 to 6% of healthy blood donors, although typically in low titers [7]. The anti-PF4 positivity rate is even higher in individuals with chronic periodontal disease [8]. But even in this population, the presence of anti-PF4 antibodies is not associated with an increased risk for cardiovascular disease or thrombotic complications [8]. Indirect evidence indicates that this immune response is part of the innate antibody repertoire. Anti-PF4 antibody-producing B-cells can be found in nearly all individuals [9]. When B-cells of healthy donors are stimulated ex vivo they can produce anti-PF4 antibodies. Furthermore, when PF4 knockout mice undergo polymicrobial sepsis, they produce anti-PF4 antibodies [10] (despite these mice never having previously been exposed to this antigen), and B cells of newborns stimulated ex vivo also produce anti-PF4 antibodies [10].
Our current understanding is that the anti-PF4 antibody response is likely an evolutionary conserved immune defense mechanism. PF4 binds to bacterial pathogens, and the innate anti-PF4 antibody repertoire labels these pathogens by opsonization. This helps to bridge the interval between initial infection and the time needed for the adaptive immune system to produce specific antibodies targeting these bacteria.
Conformational changes in PF4
It is important to mention that PF4 undergoes conformational changes in secondary structure after binding to bacteria. Otherwise, anti-PF4 antibodies would constantly bind to (unmodificed) PF4 on platelets and endothelial cells, inducing platelet and leukocyte activation and chronic inflammation.
We have shown in detail that PF4 undergoes conformational change when binding to certain strongly negatively-charged molecules [11,12]. The negative charge allows the fusion of the positive charge cloud of 2 or more PF4 molecules. This provides the energy to induce conformational changes within PF4. These conformational changes expose neoepitope(s) to which anti-PF4 antibodies bind [12,13]. In other words, formation of a single charge cloud around several PF4 molecules squeezes PF4 into an altered conformation.
PF4 binding to adenovirus
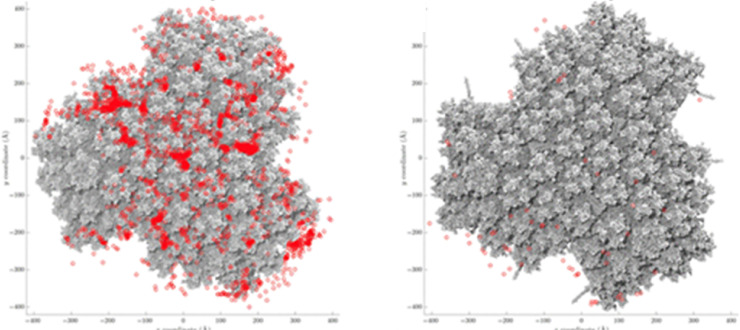
PF4 also binds to adenovirus [14,15]. Baker et al. 2021 [16] determined the structure of the ChAdOx1 viral vector and demonstrated an electrostatic interaction mechanism with PF4. They confirmed binding of PF4 to adenovirus using a technique known as plasmon resonance. Using highly purified preparations of the adenovirus-derived vaccine vectors, Ad26, Ad5, and ChAdOx1, they showed binding of PF4 to all 3 adenovirus types, although with slightly different affinities. However, whether the binding of PF4 to adenovirus results in a structural change of PF4 is currently unresolved. The major capsid protein hexon provides most of the negative charge, and interestingly, the hexon protein of ChAdOx1 is more negatively charged than the hexon proteins of the other adenoviruses tested. A major strength of this study is that the biophysical assessment and Brownian dynamics simulation identified potential binding sites of PF4 on the virus surface (Fig. 1 ). In this study, binding of PF4 to adenovirus occured with low affinity (Kd 300 nmol) [16]. This is much lower than the interaction forces of PF4 with polyanions exposing the epitope to which antibodies characteristic for heparin-induced thrombocytopenia (HIT) bind [12]. A further observation of this study is that PF4 bound with higher affinity to Ad26, although ChAdOx1 exposes more negative charges. These findings raise the possibility that—besides the virus—additional binding partners (ligands) may be required to induce immunogenicity of PF4. These findings will potentially lead to modifications of the structure of adenoviral proteins to develop adenoviral vectors with a reduced risk of inducing anti-PF4 antibodies.
Fig. 1.
PF4 binds to adenovirus.
(A) The ChAdOx1 nCoV-19 vaccine preparation binds PF4. Brownian dynamics simulations show frequent interactions (red spots) between the PF4 tetramer and the ChAdOx1 surface (grey). (B) this interaction is inhibited in the presence of the polyanion fondaparinux. Taken from [16].
Besides hexon proteins on the viral surface, Michalik et al. 2022 identified free hexon proteins in both vaccines [17] implicated in causing VITT. In addition, super-resolution microscopy of the PF4 complexes with the ChAdOx1 vaccine support the notion that complex formation of PF4 and vaccine constituents requires more than virus/PF4 interaction.
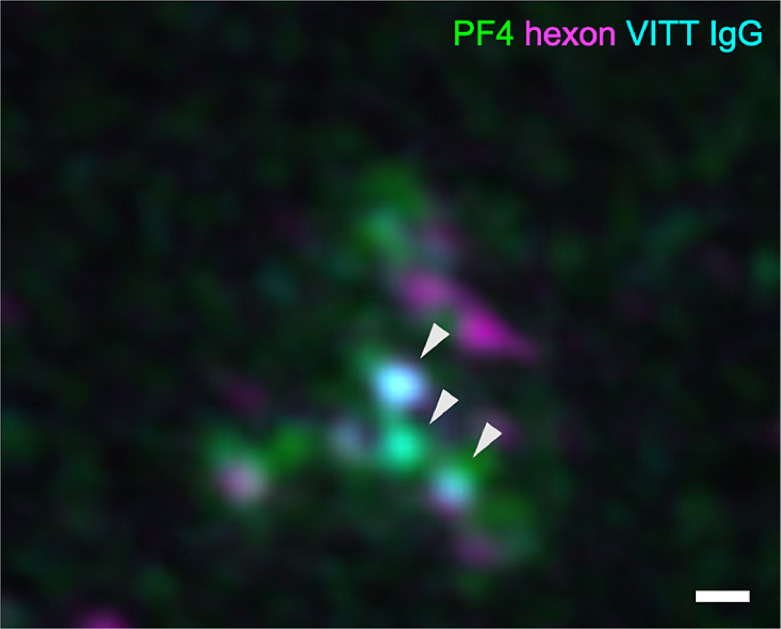
As shown in Fig. 2 , PF4 (green) and ChAdOx1 hexon protein (magenta) form multimolecular complexes to which the affinity-purified anti-PF4 antibodies from VITT patients (cyan) bind. However, some parts of the complexes of PF4 did not stain for the hexon protein, also indicating that potentially additional vaccine constituents are essential for complex formation.
Fig. 2.
Super-resolution imaging of PF4 vaccine complexes with VITT anti-PF4 antibodies.
3D structured illumination microscopy (3D-SIM) shows binding of ChAdOx1 nCoV-19 components (hexon polypeptide in magenta) to complexes of VITT IgG (cyan) bound to PF4 (green). Arrowheads indicate localization of VITT IgG on PF4-hexon complexes. The scale bar indicates 200 nm.
As in HIT the epitope(s) to which anti-PF4 antibodies bind require(s) strong negative charges; thus, characterization of the binding epitope(s) of VITT antibodies is of great interest to understand the interactions on a molecular level.
The binding site of VITT antibodies on PF4
Huynh et al. 2021 [18] used alanine scanning mutagenesis to systematically modify single amino acids in the PF4 molecule. The resulting constructs allowed them to identify amino acids critical for anti-PF4 antibody binding. They showed that anti-PF4 antibodies identified in VITT patients bind to a different epitope than anti-PF4 antibodies found in HIT patients. The anti-PF4 antibodies in VITT recognize amino acids that are also relevant for heparin binding to PF4. This has major implications for the treatment of VITT. As heparin often inhibits the binding of VITT antibodies, it could act as an antidote by blocking antibody/antigen binding.
An additional tool to characterize binding sites are monoclonal antibodies, which compete with the patient-derived anti-PF4 antibodies for the same binding site. Vayne et al. 2022 [19] characterized the antibody 1E12, the first VITT-like monoclonal antibody. 1E12 is a chimeric anti-PF4 antibody with a human Fc fragment that fully mimics the effects of human VITT antibodies. In summary, the binding epitopes of VITT antibodies largely overlap with the binding site of heparin on PF4 and with the binding site of the monoclonal antibody, 1E12. Therefore, the antibody 1E12 will become an important tool for further experiments to better understand the pathogenesis of VITT in animal models, and to distinguish between antibody binding profiles of different anti-PF4 disorders, such as HIT and VITT.
VITT antibodies are not cross-reactive anti-spike protein antibodies
VITT antibodies activate platelets through their FcγIIa receptors. Antibodies activating platelets through FcγIIa receptors have also been identified in COVID-19 patients. These findings raised concerns that vaccination-induced antibodies against the SARS-CoV-2 spike protein may cross-react with PF4, especially as in silico prediction tools and 3D modeling showed similarities of the heparin-binding site of PF4 (to which VITT antibodies bind) with an epitope on the spike protein. To exclude this possibility, we purified anti-PF4 antibodies from patients with VITT and tested them against recombinant SARS-CoV-2 spike protein. In parallel, using a cohort of 222 PCR-confirmed COVID-19 patients, we assessed for a potential association between platelet-activating anti-PF4 antibodies with adverse outcomes such as thrombocytopenia and thrombotic complications in COVID-19 patients. None of the affinity-purified anti-PF4 antibodies from 14 VITT patients cross-reacted with the SARS-CoV-2 spike protein, and the presence of anti-PF4 antibodies in COVID-19 patients was not associated with greater frequency of adverse outcomes, especially thrombocytopenia and thrombosis. Others have confirmed the lack of correlation between anti-PF4 and anti-SARS-CoV-2 antibodies [20]. In addition, compelling negative data are provided by VITT patients, as they do not develop thrombocytopenia or thrombosis when vaccinated with an mRNA vaccine that induces the spike protein while their platelet-activating anti-PF4 antibodies were still circulating [21]. In addition, at least one VITT patient acquired a SARS-CoV-2 infection after VITT and in this patient, no increase in anti-PF4 antibody titers was observed [22].
The search for other non-hexon binding partners of PF4
As stated above, beyond the hexon polypeptide, PF4 likely requires additional vaccine constituents to form multimolecular complexes and to undergo a conformational change that triggers generation of high-affinity anti-PF4 antibodies.
Glycosaminoglycans: As HIT is induced by a complex of PF4 and glycosaminoglycans (GAG), sulfated GAG [23] from the T-REx-293 HEK cells are important candidate binding partners of PF4. It is technically challenging to detect low concentrations of GAGs in a complex matrix such as a vaccine, which contains many proteins. Alban et al. 2021 [24] overcame this technical challenge by using 30 mL of the vaccine as starting volume and by digesting the proteins with proteinase K. Then, they removed the digested peptides and small-molecule excipients, including the disaccharide sucrose by dialysis (1Kd exclusion), and subsequently concentrated the processed vaccine 60-fold by lyophilization. By this procedure, they excluded that more than 0.5 µg/mL of GAG with a molecular weight > 1 kDa are present in the vaccine. This is sufficiently sensitive to detect GAG in concentrations relevant for forming immunogenic complexes with PF4, for which typically 2-3 µg/mL GAG are required [13].
EDTA: EDTA is negatively charged. By NMR we found EDTA in the PF4 preparation used for the anti-PF4 antibody enzyme-immunoassay (EIA). We therefore tested whether the anti-PF4 antibodies in VITT patient sera potentially bind to PF4 in the presence of EDTA. However, neither increasing concentrations of EDTA, nor competitive binding of EDTA using ferric chloride (very high affinity to EDTA and thereby inhibiting competitively EDTA binding to PF4) had any effects on VITT antibody binding to PF4.
Nucleic acids: Nucleic acids such as DNA, RNA, and aptamers interact with PF4 and induce conformational changes to which anti-PF4 antibodies bind. In addition, PF4/aptamer complexes induced anti-PF4 antibodies in a mouse model [25]. The vaccine of course contains virions with adenoviral DNA. Whether it also contains DNA from the cell line in which the virus is propagated is unresolved, but unlikely as the vaccine is treated with DNAses during production.
Polysorbate 80: It has been proposed by Choi [26] that polysorbate 80, which is an excipient of both adenovirus vector vaccines but not present in RNA-based SARS-CoV-2 vaccines, could play an important role in VITT pathogenesis. Polysorbate 80 is a commonly used non-ionic surfactant and drug stabilizer that is known to efficiently home to brain endothelial cells and cross the blood-brain barrier when complexed with nanoparticles [27]. However, polysorbate 80 is also used as a solubilizing agent in intravenous formulations of several drugs, e.g., the antiarrhythmic drug amiodarone, or some influenza vaccines. To date, no signs of an increased risk for delayed thrombotic complications have been observed by pharmacovigilance systems worldwide with these drugs; thus polysorbate 80 seems an unlikely candidate.
Proteins: Both vaccines contain proteins derived from the cell line in which the adenovirus vector is propagated [28,17]. The total protein concentration of the ChAdOx1 nCoV-19 vaccine is approximately 3.4-times higher than that of the Ad26.COV2.S vaccine (mean: 102 ng/µL vs 29.8 ng/µL). In addition, the protein pattern of ChAdOx1 nCoV-19 is more complex than the protein pattern of Ad26.COV2.S, and mass spectrometric analysis identified a much higher proportion (44.5 %-59.2 % vs only 0.26 %-0.96 %) and number (NChAdOx1 nCoV-19 = 1571 ± 31 vs NAd26.COV2.S = 59±14; 2-sided t-test P-value = 8.709 × 10−6) of host cell-derived human proteins. However, none of the top 10 most abundant human proteins in ChAdOx1 nCoV-19 was detected in Ad26.COV2.S; Table . Adenoviral proteins comprised 40.8% to 55.5% (ChAdOx1 nCoV-19) and 99.04% to 99.74% (Ad26.COV2.S) of total ion intensity (parameter to measure protein content by proteomics). Thus, one vaccine dose (500 µL) of ChAdOx1 nCoV-19 contains 19.1 to 33.8 µg host cell-derived human proteins, whereas the Ad26.COV2.S vaccine contains only 0.04 to 0.19 µg host cell-derived human proteins.
Table.
Proteins identified in ChAdOx1 nCoV-19 and Ad26.COV2.S (more details and proteome data of both vaccines are given in [17]).
 |
In ChAdOx1 nCoV-19, 23.3 to 26.3 µg chimpanzee adenovirus proteins were found, and in Ad26.COV2.S, 10.2 to 19.2 µg adenoviral proteins were found per vaccination dose, which exceeds the amount of viral proteins in assembled virions. Approximately 5 × 1010 virions per vaccine dose weigh about 12.5 µg, and thus both vaccines contain unassembled virus proteins, mostly hexon proteins. In agreement with this notion, viral proteins were still observed in the supernatant of ChAdOx1 nCoV-19 after ultracentrifugation [17].
In summary, PF4 binds to adenovirus vectors, which is mainly mediated by hexon proteins. Also, the free hexon proteins in both vaccines are involved in PF4 complex formation (see Fig. 2, super resolution microscopy). But it is unclear whether additional binding partners are involved in complex formation. In the meantime, it has been shown that GAG are likely not involved, as their concentration in ChAdOx1 is rather low (if any GAG are present at all). A role for polysorbate 80 is unlikely. The highly complex protein patterns of the vaccines, especially of ChAdOx1, make it very difficult to exclude that one of the more than 2000 proteins identified in the ChAdOx1 vaccine may facilitate interaction with PF4. An argument against a major role of proteins in the vaccine in complex formation with PF4, however, is the little overlap in the proteins of both vaccines (Table).
VITT seems to be 3 times more frequent after the ChAdOx1 vaccine compared the Ad26.COV2.S vaccine [29,30]. As described below, the human cell line proteins and the free virus proteins may contribute indirectly to the immune response in VITT by inducing an inflammatory reaction, which is likely more pronounced when more cell line proteins and unassembled virus proteins are in the vaccine preparation.
The dilemma of epitope spreading in strong (auto-)antibody responses
The usual straightforward approach to identify the binding partner of PF4, which is causing the conformational change inducing the immune reaction, is to incubate PF4 in the presence and absence of different potential binding partners and measure whether anti-PF4 antibodies bind to putative complexes. This approach, however, cannot be used in VITT because the antibodies became autoantibodies that cluster PF4 by themselves [18] and do not require any cofactor which might initially have been required to trigger the immune response.
Broadened reactivity of antibodies in a boosted immune response is a hallmark of certain disorders besides VITT. For example, autoimmune heparin-induced thrombocytopenia (aHIT) features heparin-dependent reactivity that extends to include heparin-independent reactivity [31,32]. Similarly, post-transfusion purpura [33] reflects a strong alloimmune response that progresses to include autoreactive properties [33].
Reexposure to COVID-19 vaccines
In adverse immune reactions against drugs, recurrence of symptoms during re-exposure of the suspected compound is often considered proof of causality. In this regard, the observation that 34 VITT patients of the German cohort and 40 VITT patients of the UK cohort received their second vaccination shot with mRNA vaccines without signs of thrombosis or decreasing platelet counts is relevant [34,35,36]. This observation is strong in vivo evidence that the mRNA vaccines do not contain or induce cofactor(s) required for anti-PF4 antibody-mediated prothrombotic activation of platelets; especially as in the German cohort, several patients still had circulating platelet-activating anti-PF4 antibodies in their plasma at the time of the second shot mRNA vaccination. However, Lacy et al. 2021 [36] recently described 5 patients (1 with confirmed and 4 with possible VITT) who received a second vaccination dose with ChAdOx1 nCoV-19. These patients showed no relapse of VITT or any adverse reaction. Whether platelet-activating antibodies were still detectable at the time of second vaccination with ChAdOx1 was not analyzed. However, it is also known from HIT that a brief re-exposure to heparin is well tolerated in most patients when platelet-activating anti-PF4 HIT antibodies are no longer present at the time of re-exposure [37]. Therefore, safe re-exposure with the vaccines does not exclude that the vaccine contains the factor(s) complexing with PF4 that initially triggered VITT.
How could uneventful re-exposure with the ChAdOx1 vaccine be explained in VITT patients? By analogy with HIT, the anti-PF4 response in VITT likely also requires an inflammatory co-signal. In HIT, this was shown in a prospective trial comparing patients with minor and major trauma who received the same dose and duration of heparin; however, the anti-PF4 response was nearly exclusively restricted to patients undergoing major trauma surgery [38]. Therefore, if the proinflammatory signal is less pronounced with second vaccination, it is plausible that a resulting anti-PF4 response would be induced much less often. In this regard, it is highly interesting that by clinical observation the acute reactions of the immune system during the first 24 to 48 hours after the second vaccination shot with the ChAdOx1 vaccine seem to be much less pronounced compared to the acute reactions after the first vaccination shot.
Induction of the anti-PF4 immune response requires a proinflammatory milieu
In analogy to HIT, we have suggested that VITT proceeds via a 2-step mechanism. Initially, shortly after vaccination, PF4 antigens are formed (likely by a minor conformational change in PF4) along with a proinflammatory danger signal (a trigger for B-cell activation). Subsequently, between days 5 and 14, high-titer anti-PF4 antibodies appear that activate platelets [28] via FcγIIa receptors.
The proinflammatory signal clinically manifests as typical reactions towards vaccination within the first 24 to 48 hours, which include fever, chills, and headache. These acute reactions are likely induced by proteins in the vaccine, especially the non-assembled hexon proteins (see above), which can bind to innate immune cell receptors [39], inducing inflammation.
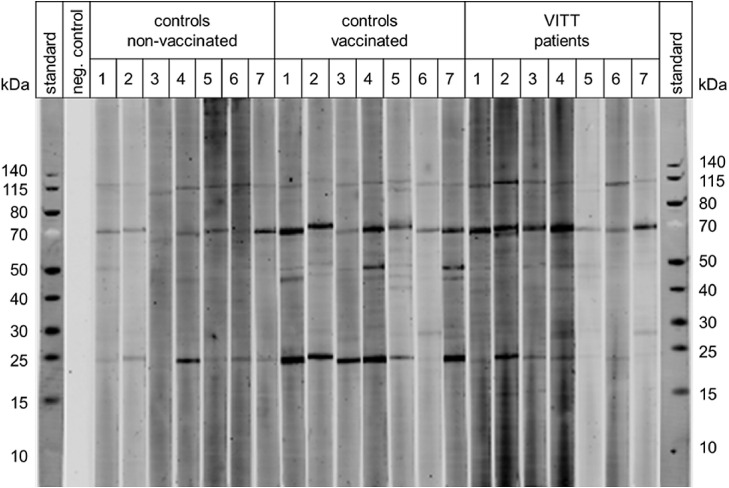
In addition, proteins originating from the human kidney-derived production cell line (T-REx HEK-293) can disseminate into the blood, where they are recognized by preformed (natural) IgG antibodies [40]. It is known that many normal individuals have such antibodies that recognize intracellular proteins, and that function to remove degraded cell components from the circulation. Binding of these natural antibodies to the host cell line proteins in the vaccine presumably results in the formation of immune complexes. Fig. 3 shows immunoblots of IgG antibodies binding to proteins in the ChAdOx1 vaccine. All individuals tested have these antibodies in their serum with stronger reactivities in vaccinated individuals compared to non-vaccinated individuals. This indirectly indicates that the immune system was boosted by vaccination to produce these antibodies.
Fig. 3.
Binding of human antibodies to ChAdOx1 nCoV-19 vaccine components.
Binding of IgG antibodies to the vaccine was determined with a LI-COR fluorescence detection system after Western blotting using sera from non-vaccinated and vaccinated healthy individuals (each n = 7) and VITT patients (n = 7) at a serum dilution of 1:40. PageRuler Prestained Protein Ladder was used (Invitrogen/Thermo Fisher) as a molecular mass standard and for the negative control lane no serum was used to assess unspecific binding of the secondary antibody. The binding of antibodies to vaccine components was determined by immunoblotting using a LI-COR Odyssey CLx imaging system (LI-COR, Lincoln, NE, USA) for quantitation of signal intensities according to the manufacturers protocol.
Together, free virus proteins, human cell line proteins and potentially also some adenovirus entering the lymphatic system and/or the bloodstream together contribute to clinical symptoms within 8 to 24 hours following vaccination. These symptoms are reminiscent of systemic inflammation (fever, chills, large joint arthralgia, occasionally skin lesions, probably reflecting a similar process as in serum sickness or serum sickness-like illness) [41]. Such symptoms have also been observed as acute vasculitis-like reaction when a column used for immunoadsorption leaked protein A with bound antibodies [42]. This inflammatory response could provide an important co-signal that stimulates antibody production by preformed B-cells capable of producing anti-PF4 antibodies, as is known to occur in the pathogenesis of classic HIT [25,9].
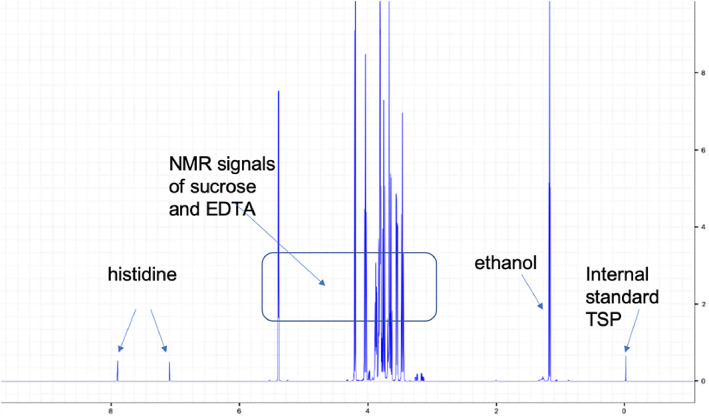
For the ChAdOx1 vaccine, this inflammatory co-signal is enhanced by EDTA in the vaccine. Fig. 4 shows the detection of EDTA in the ChAdOx1 vaccine by NMR. EDTA in the vaccine increases capillary leakage at the inoculation site, likely by endothelial (VE)-cadherin disassembly [43]. This facilitates entry of proteins found in the vaccine into the bloodstream. Induction of increased capillary leakage has been shown for the ChAdOx1 vaccine by 2 animal models, a mouse model with intradermal injection of the vaccine [28] and a zebrafish model with intramuscular injection of the vaccine [17].
Fig. 4.
1H-NMR spectrum of ChAdOx1 nCoV-19 vaccine.
Analysis of ChAdOx1 nCoV-19 vaccine confirmed the solution composition and showed the NMR signals of sucrose, ethanol, and histidine. EDTA was detected (approx. 0.1 mM). TRIS was not found in the vaccine. X-axis: NMR chemical shift signals in ppm relative to internal standard TSP; y-axis: relative signal intensity.
The anti-PF4 immune response in VITT is a secondary but transient immune response
In all VITT patients of the Greifswald cohort with available data, the onset of clinical symptoms of VITT occurred in the typical time window between days 5 and 20 after vaccination, with very high anti-PF4/heparin IgG EIA optical density (OD) values. High-titer anti-PF4 antibodies occurring as early as 5 days after vaccination cannot be explained by a primary immune response. As in HIT [44], the immune response in VITT therefore shows features characteristic of a secondary immune response. Whether prior exposure to pathogens that trigger a primary anti-PF4 immune response (as in HIT) [4], is fundamental to explaining the VITT immune response, is currently unresolved. However, after a strong secondary immune response, antibodies typically persist for months and years, especially if induced as a secondary response to vaccination. This is clearly not the case in the majority of patients with VITT [22]. The temporal decline in anti-PF4 IgG antibody levels after development of VITT is similar to the antibody dynamics in HIT [45]. However, the time to seroreversion seems to take somewhat longer than in HIT, likely because initial antibody levels are unusually high during acute VITT [46]. The rapid decline of platelet-activating antibody levels indicates that the immune response in VITT does not follow the typical “classic” antibody response pattern.
Given the many similarities between VITT and HIT, findings obtained by a mouse model of HIT might be of special relevance also for VITT. In this mouse model, the anti-PF4 immune response has been attributed to marginal zone B cells [9], a B-cell subpopulation known to provide a first-line of defense by rapidly producing IgM and class-switched IgG antibodies in response to infections by blood-borne viruses and encapsulated bacteria. The clinical observations on the timing of VITT suggest that marginal zone B-cells are also likely candidates for producing anti-PF4 antibodies in VITT. In addition, Nicolai et al [47]. have demonstrated in a mouse model that ChAdOx1 binds to platelets after intravenous injection, and the virus-covered platelets interact with B-lymphocytes in the marginal zone of the spleen. This also implies that platelets could represent antigen presenting cells in VITT. Beyond VITT, co-presentation of platelet antigens together with virus antigens bears a strongly enhanced risk for induction of anti-platelet autoantibodies. In fact, we have found that approximately 30% of VITT patients also produce glycoprotein-specific anti-platelet antibodies, which are detectable in their serum (unpublished observations).
However, in a subgroup of VITT patients, persisting platelet-activating anti-PF4 antibodies [21] beyond 6 months have been observed [48]. Whether this persisting immune response involves B cells other than marginal zone B-cells is unresolved.
Multimolecular complexes containing PF4 also activate the complement system[49,50]. Complement bound to PF4 aggregates subsequently allows binding of the complexes to B-cells via their complement receptor [49]. So far, it remains unexplored whether the complement system might has also a role in the immune response towards PF4 in VITT.
VITT antibodies activate platelets and leukocytes via FcγIIa receptors
Once B cells are triggered to produce anti-PF4 antibodies, it presumably takes between 5 and 10 days until anti-PF4 antibodies are formed (although it may taken several more days or weeks before clinical manifestations lead to diagnosis of VITT). As a result, antibodies of different biological relevance are produced. In more than 5 % of vaccinated individuals, low-titer anti-PF4 antibodies are found, which do not activate platelets, and which are not associated with thrombocytopenia or thrombotic complications [51]. In contrast, in all VITT patients investigated in our laboratory, platelet-activating anti-PF4 antibodies were present [52]. These antibodies induce platelet activation mediated by the platelet FcγIIa receptors. This can be blocked in vitro by high concentrations of IVIG and an Fc receptor-specific monoclonal antibody (MoAb IV.3). Even more convincingly, administration of high-dose IVIG inhibits platelet activation by these antibodies (ex vivo experiments) [53,46,54].
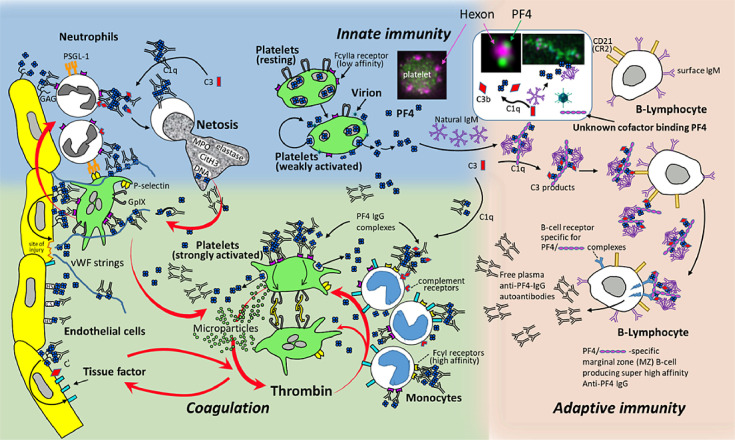
Activated platelets expose phosphatidylserine, which is the catalytic surface for thrombin generation [55]. At the same time, platelets release PF4. PF4 binds to chondroitin sulfates on the surface of granulocytes and granulocytes become activated when subsequently the anti-PF4 antibodies bind and cross-link their FcγIIa receptors. This results in the release of nuclear DNA in a process known as NETosis [56, 28]. To these DNA neutrophil extracellular traps (NETs), PF4 first binds, and then anti-PF4 antibodies.This allows for the formation of large immune complexes, causing a self-enhancing cycle of cell activation [57], further fueling the VITT prothrombotic response (Fig. 5 ). The in vitro experiments indicating this mechanism have been elegantly confirmed in vivo in a mouse model [58]. In addition, markers of NETosis are strongly elevated in VITT patients and NETs are found in the thrombi extracted from cerebral sinus veins of VITT patients [28].
Fig. 5.
Summary of pathogenesis of VITT.
The initial mechanisms underlying B-cell activation are still hypothetical and are derived from the known mechanisms in HIT. For a more detailed description please see the “summary section” of the manuscript. Figure is a modified version of a figure on pathogenesis of VITT in [68].
Anti-PF4 antibodies are a cause of unusual thromboses beyond VITT
Elucidating the pathogenesis of VITT has demonstrated that anti-PF4 antibodies can cause unusual thrombosis. Several recent observations indicate that these antibodies are not specific for COVID-19 vaccination or for adenoviral vector vaccines at all. A recently described case of VITT after vaccination against the human papillomavirus (Gardasil) is especially interesting. The patient developed the typical clinical picture of VITT with anti-PF4/heparin IgG antibodies and PF4-dependent, platelet-activating antibodies [59].
Another study [60] reported a patient with a persisting PF4-reactive monoclonal IgG paraprotein that directly activated platelets via their FcγIIa receptors in association with chronic thrombocytopenia and recurrent thrombosis. The patient had a chronic hypercoagulability state with a strong correlation between the degree of thrombocytopenia and elevated D-dimer levels. Also, a previous case of spontaneous HIT syndrome associated with IgG-κ paraprotein has been reported [61]. Based on these anecdotes, the spectrum of anti-PF4 mediated hypercoagulability states, with Fc receptor-mediated platelet activation, can be expanded beyond heparin or vaccine exposure to include association with monoclonal proteins. Potentially, anti-PF4 antibodies are also the underlying cause in other patients with recurrent venous and arterial thromboses. Better understanding of the pathogenesis and improving laboratory methods to detect these antibodies might allow the identification of other patients who suffer from (recurrent) venous and arterial thrombotic complications.
SARS-CoV-2 spike proteins and platelets
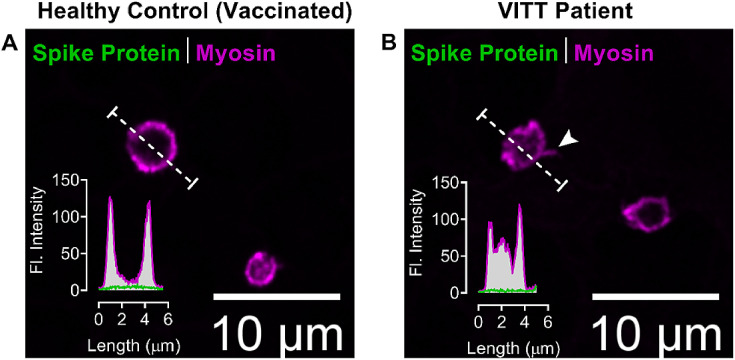
Platelets are known to interact and sequester viruses such as dengue [62], HIV [63], and influenza [64]. Recently Koupenova et al.[65] demonstrated that platelets can also interact with, attach to, and sequester SARS-CoV-2 in vitro and SARS-CoV-2 derived proteins in vivo in COVID-19 patients. Although there is convincing evidence showing systemic entry of SARS-CoV-2 leads to platelet-driven immunothrombosis, vascular coagulopathy, thromboembolism and thrombocytopenia, the exact interacting ligand of SARS-CoV-2 on platelets largely remains unknown [66]. It has been suggested that in VITT patients vaccinated with adenoviral vector-based COVID-19 vaccines, alternative splice events can lead to C-terminal truncated soluble spike protein variants that may enter the bloodstream and potentially initiate adverse reactions [67]. To address this theoretical mechanism, we performed immunofluorescent microscopy of platelets in the blood smears of VITT patients and from vaccinated healthy individuals to identify potential spike protein associated with platelets (Fig. 6 ). In these preliminary experiments, we found signs of strongly activated platelets in VITT patients as indicated by reorganization of the platelet cytoskeleton, but we were unable to detect the presence of SARS-CoV-2 spike protein.
Fig. 6.
Confocal immunofluorescence microscopy images of platelets from blood smears
(A) COVID-19 vaccinated healthy individual and from (B) VITT patient labeled for spike protein (green) and platelet myosin (magenta). The inset graphs show the fluorescence intensities (in grayscale) of both spike protein and myosin from line profiles (dotted line). The arrowhead (B) points at filopodia-like projection, a typically activated platelet morphology in acute VITT patients.
Summary
Fig. 5 summarizes the the current understanding of the pathogenesis of VITT. The scheme includes recent laboratory data but also considers data obtained in HIT. PF4 interacts with constituents in the vaccine, especially non-assembled hexon proteins, and also binds to the adenovirus vector. This results in complex formation with PF4. It is known from HIT that multimolecular complexes of PF4 bind natural IgM, leading to deposition of complement. These complexes bind to the complement receptor of B cells, with B cells expressing the B cell receptors specific for PF4 becoming activated. Please note that this part of the scheme remains hypothetical.
Once high-titer anti-PF4 antibodies are formed, they bind PF4, forming immune complexes which activate platelets, monocytes and (through activated platelets) also granulocytes. It is highly likely that the antibodies also bind to PF4 bound to heparin sulfate on endothelial cells. This causes activation of endothelial cells, which release von Willebrand factor strings. These von Willebrand factor strings again bind PF4 and subsequently anti-PF4 antibodies. This results in further activation of platelets and via activated platelets to activation of granulocytes. Granulocytes undergo NETosis which release DNA. PF4 binds to DNA, forming larger complexes to which anti-PF4 antibodies bind, further enhancing activation. Anticoagulation to block thrombin and high-dose IVIG to block Fcγ receptor-mediated cell activation are the mainstays of treatment.
Conflicts of interest
Dr Schönborn is the recipient of a young investigator grant of the medical faculty of the Universitätsmedizin Greifswald. Dr. Greinacher reports grants and non-financial support from Aspen, Boehringer Ingelheim, MSD, Bristol Myers Squibb (BMS), Paringenix, Bayer Healthcare, Gore Inc., Rovi, Sagent, Biomarin/Prosensa, personal fees from Aspen, Boehringer Ingelheim, MSD, Macopharma, BMS, Chromatec, Instrumentation Laboratory, nonfinancial support from Boehringer Ingelheim, Portola, Ergomed, GTH e.V. outside the submitted work. Dr. Thiele reports personal fees from Bristol Myers Squibb, Bayer, Daichii Sankyo, Pfizer, Novo Nordisk, Chugai Pharma, and Novartis, all of which are outside of the submitted manuscript. None of the other authors has to declare a conflict of interest.
Footnotes
This work has been supported by Deutsche Forschungsgemeinschaft (Grant/Award Number: 374031971-TRR 240) to A.G., by Bundesministerium für Bildung und Forschung (Grant 01GM1518B, STOP FSGS to N.E.) and by the Ministerium für Wirtschaft, Arbeit und Gesundheit Mecklenburg-Vorpommern (project COVIDPROTECT to U.V.). Linda Schönborn is supported by the Domagk Program of the Universitätsmedizin Greifswald.
Part of the results reported have been obtained in a study conducted by Universitätsmedizin Greifswald under service contract No. EMA/2021/17/TDA. The views expressed are the personal views of the authors and may not be understood or quoted as being made on behalf of or reflecting the position of the European Medicines Agency or one of its Committees or Working Parties.”
References
- 1.European Medicines Agency (EMA) Vaxzevria (previously covid-19 vaccine astra zeneca): Epar - product information. 2021 [Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/vaxzevria-previously-covid-19-vaccine-astrazeneca accessed February 28, 2022.
- 2.European Medicines Agency & Healthcare products Regulatory Agency- Public assessment report authorisation for temporary supply - covid-19 vaccine astrazeneca, solution for injection in multidose container covid-19 vaccine (chadox1-s [recombinant]). 2021 [Available from: https://www.ema.europa.eu/en/documents/assessment-report/vaxzevria-previously-covid-19-vaccine-astrazeneca-epar-public-assessment-report_en.pdf accessed February 28, 2022.
- 3.European Medicines Agency (EMA) COVID-19 Vaccine Janssen (COVID-19 vaccine (Ad26.COV2-S [recombinant])) 2021 [Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/covid-19-vaccine-janssen accessed February 28, 2022.
- 4.Krauel K, Potschke C, Weber C, et al. Platelet factor 4 binds to bacteria-inducing antibodies cross-reacting with the major antigen in heparin-induced thrombocytopenia. Blood. 2011;117:1370–1378. doi: 10.1182/blood-2010-08-301424. [DOI] [PubMed] [Google Scholar]
- 5.Palankar R, Kohler TP, Krauel K, et al. Platelets kill bacteria by bridging innate and adaptive immunity via platelet factor 4 and FcgammaRIIA. J Thromb Haemost. 2018;16:1187–1197. doi: 10.1111/jth.13955. [DOI] [PubMed] [Google Scholar]
- 6.Krauel K, Weber C, Brandt S, et al. Platelet factor 4 binding to lipid A of Gram-negative bacteria exposes PF4/heparin-like epitopes. Blood. 2012;120:3345–3352. doi: 10.1182/blood-2012-06-434985. [DOI] [PubMed] [Google Scholar]
- 7.Hursting MJ, Pai PJ, McCracken JE, et al. Platelet factor 4/heparin antibodies in blood bank donors. Am j clin pathol. 2010;134:774–780. doi: 10.1309/AJCPG0MNR5NGKNFX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greinacher A, Holtfreter B, Krauel K, et al. Association of natural anti-platelet factor 4/heparin antibodies with periodontal disease. Blood. 2011;118:1395–1401. doi: 10.1182/blood-2011-03-342857. [DOI] [PubMed] [Google Scholar]
- 9.Zheng Y, Wang AW, Yu M, et al. B-cell tolerance regulates production of antibodies causing heparin-induced thrombocytopenia. Blood. 2014;123:931–934. doi: 10.1182/blood-2013-11-540781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krauel K, Schulze A, Jouni R, et al. Further insights into the anti-PF4/heparin IgM immune response. Thromb Haemost. 2016;115:752–761. doi: 10.1160/TH15-08-0654. [DOI] [PubMed] [Google Scholar]
- 11.Delcea M, Greinacher A. Biophysical tools to assess the interaction of PF4 with polyanions. Thromb Haemost. 2016;116:783–791. doi: 10.1160/TH16-04-0258. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen TH, Greinacher A, Delcea M. Quantitative description of thermodynamic and kinetic properties of the platelet factor 4/heparin bonds. Nanoscale. 2015;7:10130–10139. doi: 10.1039/c5nr02132d. [DOI] [PubMed] [Google Scholar]
- 13.Kreimann M, Brandt S, Krauel K, et al. Binding of anti-platelet factor 4/heparin antibodies depends on the thermodynamics of conformational changes in platelet factor 4. Blood. 2014;124:2442–2449. doi: 10.1182/blood-2014-03-559518. [DOI] [PubMed] [Google Scholar]
- 14.Othman M, Baker AT, Gupalo E, et al. To clot or not to clot? Ad is the question-Insights on mechanisms related to vaccine-induced thrombotic thrombocytopenia. J Thromb Haemost. 2021;19:2845–2856. doi: 10.1111/jth.15485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Othman M, Labelle A, Mazzetti I, Elbatarny HS, Lillicrap D. Adenovirus-induced thrombocytopenia: the role of von Willebrand factor and P-selectin in mediating accelerated platelet clearance. Blood. 2007;109:2832–2839. doi: 10.1182/blood-2006-06-032524. [DOI] [PubMed] [Google Scholar]
- 16.Baker AT, Boyd RJ, Sarkar D, et al. ChAdOx1 interacts with CAR and PF4 with implications for thrombosis with thrombocytopenia syndrome. Sci Adv. 2021;7:eabl8213. doi: 10.1126/sciadv.abl8213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michalik S, Siegerist F, Palankar R, et al. Comparative analysis of ChAdOx1 nCoV-19 and Ad26.COV2.S SARS-CoV-2 vector vaccines. Haematologica. 2022 Jan 20 doi: 10.3324/haematol.2021.280154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huynh A, Kelton JG, Arnold DM, Daka M, Nazy I. Antibody epitopes in vaccine-induced immune thrombotic thrombocytopaenia. Nature. 2021;596:565–569. doi: 10.1038/s41586-021-03744-4. [DOI] [PubMed] [Google Scholar]
- 19.Vayne C, Palankar R, Billy S, et al. The Deglycosylated Form of 1E12, a Monoclonal Anti-PF4 IgG, Strongly Inhibits Antibody-Triggered Cellular Activation in Vaccine-Induced Thrombotic Thrombocytopenia, and Is a Potential New Treatment for Vιττ. Blood. 2021;138:582. [Google Scholar]
- 20.Uzun G, Althaus K, Bakchoul T. No Correlation between Anti-PF4 and Anti–SARS-CoV-2 Antibodies after ChAdOx1 nCoV-19 Vaccination. New England J Med. 2021;385:1334–1336. doi: 10.1056/NEJMc2111305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schönborn L, Thiele T, Kaderali L, et al. Most anti-PF4 antibodies in vaccine-induced immune thrombotic thrombocytopenia are transient. Blood. 2022 doi: 10.1182/blood.2021014214. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schönborn LG, A. Longitudinal aspects of vaccine-induced immune thrombotic thrombocytopenia (VITT) Seminars in Haematology. 2022 doi: 10.1053/j.seminhematol.2022.03.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holen HL, Zernichow L, Fjelland KE, et al. Ephrin-B3 binds to a sulfated cell-surface receptor. Biochem J. 2011;433:215–223. doi: 10.1042/BJ20100865. [DOI] [PubMed] [Google Scholar]
- 24.Alban S, Neupane S, Girreser U, Sönnichsen FD, Greinacher A. The COVID-19 vaccine ChAdOx1-S is not contaminated with sulfated glycosaminoglycans. J Thromb Haemost. 2022 Mar;20(3):777–780. doi: 10.1111/jth.15633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaax ME, Krauel K, Marschall T, et al. Complex formation with nucleic acids and aptamers alters the antigenic properties of platelet factor 4. Blood. 2013;122:272–281. doi: 10.1182/blood-2013-01-478966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi PY. Thrombotic thrombocytopenia after ChAdOx1 nCoV-19 vaccination. The New England j med. 2021;385:e11. doi: 10.1056/NEJMc2107227. [DOI] [PubMed] [Google Scholar]
- 27.Ramge P, Unger RE, Oltrogge JB, et al. Polysorbate-80 coating enhances uptake of polybutylcyanoacrylate (PBCA)-nanoparticles by human and bovine primary brain capillary endothelial cells. Eur J Neurosci. 2000;12:1931–1940. doi: 10.1046/j.1460-9568.2000.00078.x. [DOI] [PubMed] [Google Scholar]
- 28.Greinacher A, Selleng K, Palankar R, et al. Insights in ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. Blood. 2021;138:2256–2268. doi: 10.1182/blood.2021013231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.See I, Lale A, Marquez P, et al. Case Series of Thrombosis With Thrombocytopenia Syndrome After COVID-19 Vaccination-United States, December 2020 to August 2021. Ann Internal Med. 2022 doi: 10.7326/M21-4502. epub. Jan 18:M21-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paul-Ehrlich-Institut G. Sicherheitsbericht: Verdachtsfälle von Nebenwirkungen und Impfkomplikationen nach Impfung zum Schutz vor COVID-19 seit Beginn der Impfkampagne am 27.12.2020 bis zum 30.09.2021 [cited 2021 Dec 6]. Available from: 2021 [Available from: https://www.pei.de/SharedDocs/Downloads/DE/newsroom/dossiers/sicherheitsberichte/sicherheitsbericht-27-12-20-bis-30-09-21.pdf?__blob=publicationFile&v=9.
- 31.Greinacher A, Selleng K, Warkentin TE. Autoimmune heparin-induced thrombocytopenia. J Thromb Haemost. 2017;15:2099–2114. doi: 10.1111/jth.13813. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen TH, Medvedev N, Delcea M, Greinacher A. Anti-platelet factor 4/polyanion antibodies mediate a new mechanism of autoimmunity. Nature commun. 2017;8:14945. doi: 10.1038/ncomms14945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hawkins J, Aster RH, Curtis BR. Post-transfusion purpura: current perspectives. J Blood Med. 2019;10:405–415. doi: 10.2147/JBM.S189176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schönborn L, Thiele T, Kaderali L, Greinacher A. Decline in Pathogenic Antibodies over Time in VITT. The New England j med. 2021;385:1815–1816. doi: 10.1056/NEJMc2112760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindhoff-Last E, Schoenborn L, Piorkowski M, et al. Heterogeneity of Vaccine-Induced Immune Thrombotic Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination and Safety of Second Vaccination with BNT162b2. Thromb Haemost. 2022;122:304–307. doi: 10.1055/a-1701-2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lacy J, Pavord S, Brown KE. VITT and Second Doses of Covid-19 Vaccine. The New England j med. 2022;386:95. doi: 10.1056/NEJMc2118507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warkentin TE, Sheppard JA. Serological investigation of patients with a previous history of heparin-induced thrombocytopenia who are reexposed to heparin. Blood. 2014;123:2485–2493. doi: 10.1182/blood-2013-10-533083. [DOI] [PubMed] [Google Scholar]
- 38.Lubenow N, Hinz P, Thomaschewski S, et al. The severity of trauma determines the immune response to PF4/heparin and the frequency of heparin-induced thrombocytopenia. Blood. 2010;115:1797–1803. doi: 10.1182/blood-2009-07-231506. [DOI] [PubMed] [Google Scholar]
- 39.Li R, Liu J, Wu S, et al. Toll-like receptor 4 signalling regulates antibody response to adenoviral vector-based vaccines by imprinting germinal centre quality. Immunology. 2018;155:251–262. doi: 10.1111/imm.12957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfueller SL, Logan D, Tran TT, Bilston RA. Naturally occurring human IgG antibodies to intracellular and cytoskeletal components of human platelets. Clin Exp Immunol. 1990;79:367–373. doi: 10.1111/j.1365-2249.1990.tb08097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chary MA, Barbuto AF, Izadmehr S, Hayes BD, Burns MM. COVID-19: therapeutics and their toxicities. J Med Toxicol. 2020;16:284–294. doi: 10.1007/s13181-020-00777-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Käbisch A, Kroll H, Wedi B, et al. Severe adverse effects of protein A immunoadsorption. Lancet. 1994;343:116. doi: 10.1016/s0140-6736(94)90843-5. [DOI] [PubMed] [Google Scholar]
- 43.Gao X, Kouklis P, Xu N, et al. Reversibility of increased microvessel permeability in response to VE-cadherin disassembly. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1218–L1225. doi: 10.1152/ajplung.2000.279.6.L1218. [DOI] [PubMed] [Google Scholar]
- 44.Greinacher A. Heparin-induced thrombocytopenia. The New England j med. 2015;373:1883–1884. doi: 10.1056/NEJMc1510993. [DOI] [PubMed] [Google Scholar]
- 45.Warkentin TE, Kelton JG. Temporal aspects of heparin-induced thrombocytopenia. The New England j med. 2001;344:1286–1292. doi: 10.1056/NEJM200104263441704. [DOI] [PubMed] [Google Scholar]
- 46.Greinacher A, Thiele T, Warkentin TE, et al. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. The New England J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nicolai L, Leunig A, Pekayvaz K, et al. Thrombocytopenia and splenic platelet directed immune responses after intravenous ChAdOx1 nCov-19 administration. BioRxiv. 2021 doi: 10.1182/blood.2021014712. preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Günther A, Brämer D, Pletz MW, et al. Complicated long term vaccine induced thrombotic immune thrombocytopenia-a case report. Vaccines (Basel) 2021;9:1344. doi: 10.3390/vaccines9111344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khandelwal S, Ravi J, Rauova L, et al. Polyreactive IgM initiates complement activation by PF4/heparin complexes through the classical pathway. Blood. 2018;132:2431–2440. doi: 10.1182/blood-2018-03-834598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arepally GM, Cines DB. Pathogenesis of heparin-induced thrombocytopenia. Transl Res. 2020;225:131–140. doi: 10.1016/j.trsl.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thiele T, Ulm L, Holtfreter S, et al. Frequency of positive anti-PF4/polyanion antibody tests after COVID-19 vaccination with ChAdOx1 nCoV-19 and BNT162b2. Blood. 2021;138:299–303. doi: 10.1182/blood.2021012217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thiele T, Weisser K, Schönborn L, et al. Laboratory confirmed vaccine-induced immune thrombotic thrombocytopenia: Retrospective analysis of reported cases after vaccination with ChAdOx-1 nCoV-19 in Germany. Lancet Reg Health Eur. 2022;12 doi: 10.1016/j.lanepe.2021.100270. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uzun G, Althaus K, Singh A, et al. The use of IV immunoglobulin in the treatment of vaccine-induced immune thrombotic thrombocytopenia. Blood. 2021;138:992–996. doi: 10.1182/blood.2021012479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bourguignon A, Arnold DM, Warkentin TE, et al. Adjunct immune globulin for vaccine-induced immune thrombotic thrombocytopenia. The New England j med. 2021;385:720–728. doi: 10.1056/NEJMoa2107051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Althaus K, Möller P, Uzun G, et al. Antibody-mediated procoagulant platelets in SARS-CoV-2-vaccination associated immune thrombotic thrombocytopenia. Haematologica. 2021;106:2170–2179. doi: 10.3324/haematol.2021.279000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holm S, Kared H, Michelsen AE, et al. Immune complexes, innate immunity, and NETosis in ChAdOx1 vaccine-induced thrombocytopenia. Eur Heart J. 2021;42:4064–4072. doi: 10.1093/eurheartj/ehab506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vayne C, et al. has identified a monoclonal antibody which competes with the binding of anti-PF4 antibodies from Vitt patients. Haematologica. 2022 in press. [Google Scholar]
- 58.Beng C, Halina L, Jose P, et al. NETosis and thrombosis in vaccine-induced immune thrombotic thrombocytopenia. Nature Portfolio. 2022 doi: 10.1038/s41467-022-32946-1. preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johansen S, Laegreid IJ, Ernstsen SL, et al. Thrombosis and thrombocytopenia after HPV vaccination. J Thromb Haemost. 2021 doi: 10.1111/jth.15604. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Greinacher A, Langer F, Schonborn L, et al. Platelet-activating anti-PF4 antibodies mimicking VITT antibodies in an unvaccinated patient with monoclonal gammopathy. Haematologica. 2021;20(2):542–543. doi: 10.3324/haematol.2021.280366. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Faille DH-NMO, A.; Amiral, J.; Huisse, M.G.; Chauveheid, M.P.; Mazighi, M.; Ajzenberg, N. . Isolation of a monoclonal IgG kappa with functional autoantibody activity against platelet factor 4/heparin from a patient with a monoclonal gammopathy of undetermined significance and clinically overt heparin thrombocytopenia Res Pract Thromb Haemost. 2017;1:1355.
- 62.Campbell RA, Schwertz H, Hottz ED, et al. Human megakaryocytes possess intrinsic antiviral immunity through regulated induction of IFITM3. Blood. 2019;133:2013–2026. doi: 10.1182/blood-2018-09-873984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Simpson SR, Singh MV, Dewhurst S, Schifitto G, Maggirwar SB. Platelets function as an acute viral reservoir during HIV-1 infection by harboring virus and T-cell complex formation. Blood advances. 2020;4:4512–4521. doi: 10.1182/bloodadvances.2020002420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koupenova M, Corkrey HA, Vitseva O, et al. The role of platelets in mediating a response to human influenza infection. Nature commun. 2019;10:1780. doi: 10.1038/s41467-019-09607-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koupenova M, Corkrey HA, Vitseva O, et al. SARS-CoV-2 Initiates Programmed Cell Death in Platelets. Circ Res. 2021;129:631–646. doi: 10.1161/CIRCRESAHA.121.319117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koupenova M. Potential role of platelets in COVID-19: Implications for thrombosis. Res Pract Thromb Haemost. 2020;4:737–740. doi: 10.1002/rth2.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eric K, Lea K, Jenny R, et al. Vaccine-Induced Covid-19 Mimicry” Syndrome:Splice reactions within the SARS-CoV-2 Spike open reading frame result in Spike protein variants that may cause thromboembolic events in patients immunized with vector-based vaccines. Research Square. 2022 preprint. [Google Scholar]
- 68.Warkentin TEG, Greinacher A. Heparin-induced thrombocytopenia. In: Murphy MR, D.; Dunbar, N.; Yazer, M., editor. Practical Transfusion Medicine. 6th ed: Whiley-Blackwell 2022. p. in press. Chichester, UK.