Abstract
Background
Decisions to isolate patients at risk of having coronavirus disease 2019 (COVID-19) in the emergency department (ED) must be rapid and accurate to ensure prompt treatment and maintain patient flow whilst minimising nosocomial spread. Reverse transcription polymerase chain reaction (RT-PCR) assays are too slow to achieve this, and near-patient testing is being used increasingly to facilitate triage. The ID NOW severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) assay is an isothermal nucleic acid amplification near-patient test which targets the RNA-dependent RNA-polymerase gene.
Aim
To assess the diagnostic performance of ID NOW as a COVID-19 triage tool for medical admissions from the ED of a large acute hospital.
Methods
All adult acute medical admissions from the ED between 31st March and 31st July 2021 with valid ID NOW and RT-PCR results were included. The diagnostic accuracy of ID NOW [sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV)] was calculated against the laboratory reference standard. Discrepant results were explored further using cycle threshold values and clinical data.
Findings
Two percent (124/6050) of medical admissions were SARS-CoV-2 positive on RT-PCR. Compared with PCR, ID NOW had sensitivity and specificity of 83.1% [95% confidence interval (CI) 75.4–88.7] and 99.5% (95% CI 99.3–99.6), respectively. PPV and NPV were 76.9% (95% CI 69.0–83.2) and 99.6% (95% CI 99.5–99.8), respectively. The median time from arrival in the ED to ID NOW result was 59 min.
Conclusion
ID NOW provides a rapid and reliable adjunct for the safe triage of patients with COVID-19, and can work effectively when integrated into an ED triage algorithm.
Keywords: COVID-19, SARS-CoV-2, ID NOW, Triage, Emergency department, Near-patient testing
Background
Coronavirus disease 2019 (COVID-19), an acute respiratory viral infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), continues to place a huge burden on secondary healthcare facilities. By the end of 2021, 640,829 patients had been admitted to hospital with COVID-19 across the UK, with a 7-day rolling average of 2183 daily admissions [1].
The symptoms of COVID-19 may be non-specific, and a diagnosis cannot be made or refuted based on clinical criteria and radiology alone [2,3]. Fast and effective triage is vital to the safe functioning of a hospital, and decisions to isolate patients at risk of COVID-19 must be rapid and accurate to ensure prompt treatment and maintain patient flow whilst minimising nosocomial spread [4]. Patients admitted from the emergency department (ED) with suspected or possible COVID-19 and an outstanding SARS-CoV-2 test must be isolated from other patients pending the result.
The gold standard for diagnosis is reverse transcription polymerase chain reaction (RT-PCR) from a nasopharyngeal swab, but even the fastest available assays have a run time ≥45 min and require laboratory-based technological expertise and equipment [5,6]. Sample transport, ribonucleic acid extraction, and result reporting add to the PCR turnaround time. Laboratory-based PCR results are therefore too slow to make isolation and treatment decisions in the ED [7].
Several rapid tests have emerged to allow the prompt diagnosis and triage of patients with COVID-19 [4,8]. Accurate near-patient testing plays a crucial role in triage and admission pathways, allowing prompt initiation of specific COVID-19 treatment and optimal use of bed capacity, as well as ensuring that other conditions can be managed effectively without the risk of nosocomial spread.
The ID NOW COVID-19 assay (Abbott Diagnostics, Chicago, IL, USA) is an isothermal nucleic acid amplification test which targets the unique region of the RNA-dependent RNA polymerase gene. The instrument uses dry nasal swabs, has a small footprint, is user-friendly and does not require additional equipment, making it ideal for use as a ‘near-patient’ test for EDs. The run time is as little as 5 min for a positive result and 13 min for a negative result [9]. Results can be integrated automatically with hospital records which saves time and error by avoiding manual recording, and allows easy evaluation and audit. The reported performance of ID NOW in the literature to date has mainly come from small studies across a range of settings, with sensitivity ranging from 55% to 98% and specificity ranging from 95% to 100% when using RT-PCR as the gold standard [8,[10], [11], [12], [13], [14], [15], [16], [17], [18], [19]]. Sensitivity approaches 100% when PCR results with a cycle threshold (Ct) value >30, suggesting a lower viral load, are excluded [15,[19], [20], [21]]. This article describes the real-world diagnostic performance of ID NOW as a COVID-19 triage tool in the ED of a large acute hospital. To the authors' knowledge, this is the largest single real-world study of the diagnostic performance of ID NOW, and the first to study its routine use in consecutive medical admissions in the UK.
Methods
Patient cohort
This study used data entered prospectively into a near-patient testing database, and extracted retrospectively from electronic patient records at Northwick Park Hospital, a large 600-bed district general hospital serving a diverse population in North-West London. Prior to the pandemic, the hospital received over 100,000 ED attendances and 50,000 medical admissions each year. Patients were included in the study if they were aged ≥16 years on arrival at the ED and required admission to a medical ward between 31st March and 31st July 2021 inclusive.
COVID-19 triage and testing procedures
Over the study period, all patients who presented to the ED were triaged at initial assessment by the attending clinician according to their risk of COVID-19 as ‘likely’, ‘uncertain’ or ‘unlikely’ (Table S1, see online supplementary material). In brief, patients with a clinical syndrome or chest radiology consistent with COVID-19, a COVID-19 contact history, or a positive PCR or lateral flow antigen test (LFT) result within 14 days prior to arrival were classified as ‘likely’. Those with fever, cough, shortness of breath, diarrhoea, abdominal pain and/or confusion who did not meet the ‘likely’ criteria were classified as ‘uncertain’, and other patients were classified as ‘unlikely’. Isolation in a side room or admission to a COVID-19 cohort area was based on clinical risk of COVID-19 and ID NOW result.
All patients were tested for SARS-CoV-2 with ID NOW, except those who had a positive community SARS-CoV-2 PCR between 14 and 90 days before arrival and were asymptomatic. A positive ID NOW result in such patients would likely represent a postinfectious period rather than a current infection with risk of onward transmission. A dry nasal swab was taken by an ED nurse and tested in the ED by trained technicians using the ID NOW platform in accordance with the manufacturer's instructions for use (IFU) [9]. If the first ID NOW result was invalid, the test was repeated a second time. Patients requiring medical admission had a separate nasopharyngeal swab sent to the laboratory in viral transport media for SARS-CoV-2 PCR testing. Laboratory SARS-CoV-2 testing was performed using the Panther Fusion SARS-CoV-2 (Hologic Inc, Santa Clara, CA, USA), Abbott RealTime SARS-CoV-2 (Abbott Park), an extraction-free SARS-CoV RT-PCR assay developed by Health Services Laboratories (London, UK) [22], Xpert Xpress SARS-CoV-2 (Cepheid, Sunnyvale, CA, USA) or SAMBA II SARS-CoV-2 (Diagnostics for the Real World, San Jose, CA, USA).
Data collection
Consecutive adult medical admissions from the ED with valid ID NOW results were included. Patients without a valid ID NOW result on admission or a valid PCR result within 48 h of admission were excluded from the analysis. ID NOW results and the time that the result became available were recorded prospectively. Time of arrival, demographic data, vital signs [including National Early Warning Score (NEWS)] on arrival at the ED, laboratory PCR results, and thoracic imaging reports were extracted retrospectively from electronic patient records and hospital information technology systems.
Reference standard and definitions
The diagnostic performance of ID NOW for detecting COVID-19 was estimated using a single RT-PCR reference standard. Patients were defined as having COVID-19 or not based on the first valid PCR result up to 48 h after admission. Given that a single PCR is not a perfect reference standard [3], discrepant results were explored further using the RT-PCR Ct value, admission symptoms, chest x-ray report, discharge diagnosis, community SARS-CoV-2 results prior to admission (PCR and LFT), and further PCR results later in admission. Where Ct values for more than one gene target were available for a single PCR result, the lowest value was taken.
The time between arrival at the ED and a valid ID NOW result becoming available was calculated. Thoracic imaging was reported and coded based upon guidelines on COVID-19 from the British Society of Thoracic Imaging at the time of reporting by radiologists [23].
Statistical analyses
Using laboratory-based RT-PCR as the reference standard, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated for ID NOW. Baseline characteristics were compared using Wilcoxon rank-sum test for continuous variables and Pearson's Chi-squared test for categorical variables. Kaplan–Meier time-to-event analysis was used to describe ID NOW result data and the log rank test was used to compare groups. All statistical tests were two-sided with an α-value of 0.05. All statistical analyses were performed using Stata Version 17.0 (StataCorp, LLC, College Station, TX, USA).
Ethics
ID NOW testing was part of routine care to support the diagnosis and triage of patients with COVID-19. The study was approved by the London North West University Hospitals NHS Trust Research and Development Committee. As the study used routinely collected clinical data, formal ethical approval and patient consent were not required. The results are reported in compliance with the STARD (Standards for the Reporting of Diagnostic Accuracy Studies) guidelines [24].
Results
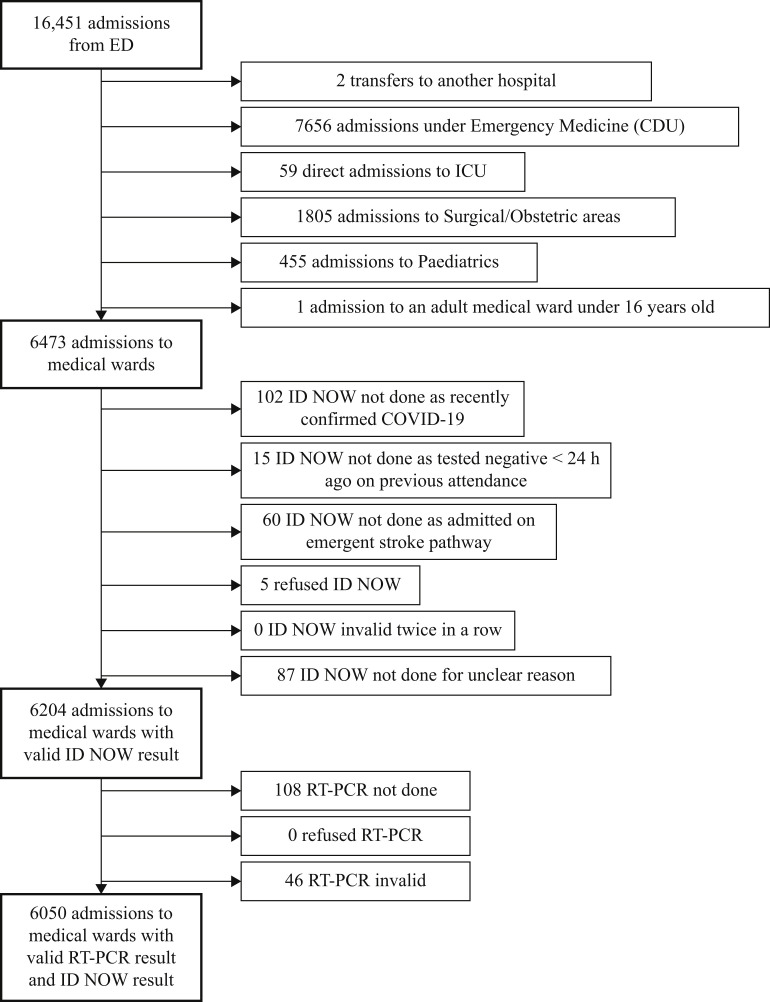
Between 31st March and 31st July 2021, valid ID NOW and RT-PCR results were available on admission for 6050 of 6473 (93.5%) patients (Figure 1 ). Of the admissions without valid paired results, 102 had had COVID-19 recently, 15 had tested negative by ID NOW during a separate attendance within 24 h prior to arrival, 60 were admitted directly to the hyperacute stroke unit, five refused ID NOW testing, and 87 had no ID NOW result for unknown reasons. None of the patients had an invalid ID NOW result that was not resolved by running the assay a second time. The median age of patients was 71 years, there were similar numbers of men and women, and the median NEWS was 2 (Table I ).
Figure 1.
Study flow-chart. RT-PCR, real-time polymerase chain reaction; ED, emergency department; COVID-19, coronavirus disease 2019; CDU, clinical decision unit; ICU, intensive care unit.
Table I.
Baseline characteristics, vital signs on presentation and ID NOW results for study patients
| Total | ID NOW negative | ID NOW positive | P-value | |||
|---|---|---|---|---|---|---|
| N | 6050 | 5916 | 134 | |||
| Age on arrival, median (IQR) | 71 (53–83) (N=6050) | 71 (53–83) (N=5916) | 62.5 (41–79) (N=134) | <0.001 | ||
| Demographics | Age on arrival >65 years | 3544 (58.6%) | 3481 (58.8%) | 63 (47.0%) | 0.006 | |
| Sex | Female | 3011 (49.8%) | 2949 (49.8%) | 62 (46.3%) | 0.41 | |
| Male | 3039 (50.2%) | 2967 (50.2%) | 72 (53.7%) | |||
| Ethnicity | Non-white | 2949 (55.4%) (N=5321) | 2875 (55.2%) (N=5205) | 74 (63.8%) (N=116) | 0.067 | |
| White | 2372 (44.6%) (N=5321) | 2330 (44.8%) (N=5205) | 42 (36.2%) (N=116) | |||
| NEWS, median (IQR) | 2 (0–4) (N=5763) | 1 (0–4) (N=5635) | 4 (1.5–7) (N=128) | <0.001 | ||
| Respiratory rate, median (IQR) | 20 (18–22) (N=5811) | 19 (18–22) (N=5683) | 22.5 (19–28) (N=128) | <0.001 | ||
| Baseline observations | Oxygen saturation <94% | 477 (8.2%) (N=5791) | 449 (7.9%) (N=5663) | 28 (21.9%) (N=128) | <0.001 | |
| Requiring supplemental oxygen | 387 (6.7%) (N=5816) | 360 (6.3%) (N=5688) | 27 (21.1%) (N=128) | <0.001 | ||
| Temperature >38.0 °C | 489 (8.4%) (N=5798) | 452 (8.0%) (N=5670) | 37 (28.9%) (N=128) | <0.001 | ||
NEWS, National Early Warning Score; IQR, interquartile range.
Wilcoxon rank-sum test for continuous variables, Pearson's Chi-squared test for categorical variables. P-values are shown for the comparison between ID NOW positive and negative results, by variable.
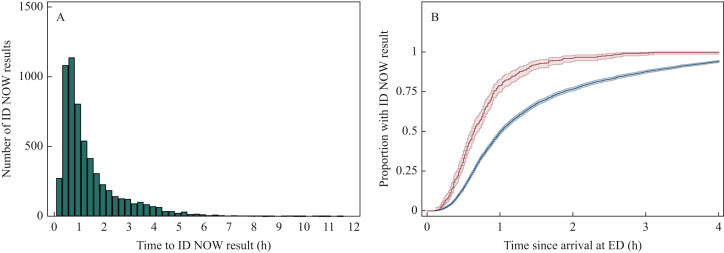
Overall, the median time between arrival at the ED and ID NOW result was 59 min [interquartile range (IQR) 37–107] (Figure 2 A). The median time to result for admissions requiring oxygen on arrival was shorter (39 min) (Figure 2B). Positive ID NOW results were available slightly more quickly (median 53 min, IQR 33–76) than negative results (59 min, IQR 37–108) (P<0.001).
Figure 2.
Time from arrival at the emergency department (ED) to ID NOW result. Time between arrival at the ED and a valid ID NOW result becoming available was calculated for each admission. These data are presented as a histogram (A) showing the distribution of time to ID NOW result for all admissions (N=6050). Kaplan–Meier time-to-event curves showing the proportion of admissions with a valid ID NOW result within the first 4 h after arrival at the ED are shown (B). 95% confidence intervals are shown. Admissions requiring supplemental oxygen on arrival (red) are compared with admissions not requiring supplemental oxygen on arrival (blue) (B) (N=5816, 3.9% missing data).
Overall, 124 of 6050 (2.0%) patients tested positive for SARS-CoV-2 by RT-PCR. The sensitivity and specificity of ID NOW were 83.1% [95% confidence interval (CI) 75.4–88.7] and 99.5% (95% CI 99.3–99.6), respectively. PPV and NPV were 76.9% (95% CI 69.0–83.2) and 99.6% (95% CI 99.5–99.8), respectively (Table II ). There were 31 patients with false-positive ID NOW results and 21 patients with false-negative ID NOW results.
Table II.
Measures of diagnostic performance of ID NOW, compared with the reverse transcription polymerase chain reaction (RT-PCR) reference standard
| A – 2x2 table | ||||
|---|---|---|---|---|
| RT-PCR result | ||||
| Positive | Negative | Total | ||
| Positive | 103 | 31 | 134 | |
| ID NOW result | Negative | 21 | 5895 | 5916 |
| Total | 124 | 5926 | 6050 | |
| B – Estimated diagnostic performance | |
|---|---|
| Measurement | Diagnostic performance |
| Sensitivity | 83.1% (103/124); CI 75.4–88.7 |
| Specificity | 99.5% (5895/5926); CI 99.3–99.6 |
| Positive predictive value | 76.9% (103/134); CI 69.0–83.2 |
| Negative predictive value | 99.6% (5895/5916); CI 99.5–99.8 |
CI, confidence interval.
False-positive results
Overall, 31 of 6050 admissions had a positive ID NOW result and a negative PCR result on admission. Five of 31 false-positive results (with two of five relating to the same patient) had a positive PCR result between 14 and 90 days prior to arrival (range 17–71 days). Nine of 31 false-positive results had a positive COVID-19 result (PCR or LFT) within 14 days of admission, or a subsequent positive PCR result within 5 days after admission. All nine were regarded as cases of COVID-19 with a false-negative PCR by the treating clinicians. Whilst six of these nine patients presented with a typical clinical syndrome and radiological features of COVID-19 pneumonitis, the remaining three patients presented with non-COVID-19 illnesses and were regarded as incidental asymptomatic infections by the treating clinicians given their additional recent positive community tests. Overall, 14 of 31 false-positive results were thought to be due to an inadequate reference standard, and these patients were considered to have had concurrent or recent COVID-19.
Seventeen of the 31 patients with false-positive results had no radiological or clinical evidence of current COVID-19 and did not have a discharge diagnosis of COVID-19.
False-negative results
Overall, 21 of 6050 admissions had a negative ID NOW result and a positive PCR result on admission (Table S2, see online supplementary material). On initial assessment, five of these 21 admissions were identified as ‘likely COVID-19’; therefore, based on the triage algorithm, these false-negative ID NOW results would not have led to de-isolation to non-COVID-19 areas. Fifteen of these 21 patients were de-isolated based on their false-negative result. The records of clinical triage were missing for one patient.
The duration of symptoms was similar between false-negative cases (median 5.5 days, IQR 1.5–14, 23.8% missing) and true-positive cases (median 6 days, IQR 3–8, 13.6% missing) (P=0.986). Seven of 21 (33.3%) false-negative cases had radiographic features typical for COVID-19.
A Ct value was recorded for 14 of 21 (66.7%) false-negative results and 61 of 103 (59.2%) true-positive results. Compared with true-positive cases, false-negative cases had higher Ct values, suggesting a lower viral load (median 36.7 vs 26.4; P=0.001). The sensitivity of ID NOW was higher with lower Ct values (Table III ). The sensitivity for PCR-positive admissions with Ct values <35 was 91.8% (56/61, 95% CI 81.5–96.6). Two false-negative cases had very low Ct values (18.8, 14.7). Both patients were young males who presented with non-COVID-19-related issues and may represent poor sampling with the dry nasal swab.
Table III.
Sensitivity of Abbott ID NOW using reverse transcription polymerase chain reaction (RT-PCR) as a reference standard, by cycle threshold (Ct) value
| Ct value | Positive on Abbott ID NOW/positive on RT-PCR | Sensitivity (%) |
|---|---|---|
| ≤25 (low) | 25/27 | 92.6 |
| 25–30 (medium–low) | 18/18 | 100 |
| 30–35 (medium–high) | 13/16 | 81.3 |
| ≥35 (high) | 5/14 | 35.7 |
Discussion
This article presents a large real-world evaluation of the Abbott ID NOW rapid test within a front-door COVID-19 triage algorithm for adult medical admissions. ID NOW was found to have sensitivity and specificity of 83.1% and 99.5%, respectively, compared with RT-PCR. This meets the acceptable performance characteristics for sensitivity (>80%, 95% CI 70–100%) and desirable characteristics for specificity (>99%, 95% CI 97–100%) according to the UK Government target product profile for SARS-CoV-2 rapid testing [25]. These estimates of diagnostic accuracy were derived from real-world data from a busy ED in a large district general hospital as part of routine care. The median time from arrival at the ED to ID NOW result was <60 min. Results were therefore available quickly enough to inform bed allocation before patients needed to be moved to a ward, likely improving infection control and reducing nosocomial spread of SARS-CoV-2. Clinicians prioritised rapid testing for the most unwell patients, particularly those requiring supplemental oxygen on arrival. Rapid and specific diagnosis of COVID-19 may reduce delays to life-saving treatments in such patients.
These results are in keeping with previously published data which have shown sensitivity of 70.3–98.0% and specificity of 95.3–100% compared with RT-PCR [8,10,13,17,18,20,21,26]. A Cochrane review published in March 2021 included 12 studies with overall prevalence of 34.2% (634/1853), and reported sensitivity of 78.6% (95% CI 73.7–82.8%) and specificity of 99.8% (95% CI 98.7–99.9%), although most studies did not use dry nasal swabs as per the manufacturer's IFU. When limited to those studies that were adherent with the manufacturer's IFU, sensitivity was 73.0% (95% CI 66.8–78.5) and specificity was 99.7% (95% CI 98.7–99.9%), with prevalence of 27.3% (222/812) [27]. This may be because studies following the manufacturer's IFU were more likely to be real-world studies which are less controlled, with limitations such as sample quality. LFTs are an alternative approach to isothermal nucleic acid amplification tests for SARS-CoV-2 rapid testing in the ED [28]. In a large meta-analysis not confined to use in the ED, LFTs had pooled sensitivity of 76.3% (95% CI 73.1–79.2%) when the manufacturer's IFU were followed [29]. Along with improved sensitivity, ID NOW has a faster turnaround time than LFTs, and results can be integrated automatically into electronic records.
There have been few trials in lower prevalence clinical settings. This study found that ID NOW performed well at 2.0% prevalence. Tu et al. evaluated 974 patients in an outpatient clinic setting, and found prevalence of 2.4%, positive percentage agreement of 91.3% and negative percentage agreement (NPA) of 100%, with only two false-negative results with Ct values of 36.5 and 38.1 [17]. Another large cohort taken from asymptomatic pre-operative outpatient screening found four cases among 1100 screened (prevalence 0.36%), with an excellent NPA of 99.7% [30].
Of the 5926 negative PCR results, 31 patients tested positive on ID NOW. Such false-positive results may arise because of detection of residual viral RNA following recent infection in individuals who are post-infectious (likely explanation in five of 31 cases), or because a single SARS-CoV-2 PCR is an imperfect reference standard and may itself miss individuals with current infection (likely in nine of 31 cases) [3]. On further evaluation of nine ID NOW false-positive cases, Stokes et al. found that five cases were PCR positive on repeat testing of the same sample using different assays [15]. The remaining false-positive cases may represent asymptomatic COVID-19 with a false-negative PCR [31,32] or contamination. In the present study, technicians performing ID NOW conducted regular work-surface decontamination and environmental swabs as quality control, likely limiting false-positive results. Such measures must be implemented to ensure the applicability of these results to other settings, especially in the context of busy EDs.
Sensitivity of ID NOW was related to the Ct value reported by the reference RT-PCR assay. PCR-positive admissions with lower Ct values, suggesting higher viral load and greater infectiousness, were more likely to be detected by ID NOW (sensitivity for cases with Ct value <30: 95.6%; Ct value ≥30: 60.0%). This real-world observation is corroborated by the UK Technologies Validation Group report and other data [17,[19], [20], [21],[33], [34], [35]], and explained by the fact that isothermal amplification has a higher limit of viral detection than RT-PCR [36]. Two false-negative cases with low Ct values were observed, which may have occurred due to inadequate sampling.
The strengths of this study are its pragmatic design under routine clinical settings, large sample size, and the fact that it was possible to account for 93.5% of medical admissions, reducing the risk of bias. There are, however, some limitations. A single SARS-CoV-2 RT-PCR is an imperfect reference standard and does not account for PCR-negative patients with COVID-19. Specificity of ID NOW may therefore be underestimated. This was mitigated through detailed investigation of discrepant results. Several PCR platforms were used and Ct values across different assays are inconsistent [37], limiting generalisability of the estimates of ID NOW sensitivity by Ct value. This is the nature of a real-life study; a combination of platforms is required to serve the needs of the hospital.
Moreover, 39.5% of the PCR-positive results did not have a Ct value, which limits the interpretation of false-negative results. This was unavoidable as some laboratory assays did not produce a Ct value. There are also technical limitations to ID NOW itself. Unlike most RT-PCR assays, it has a single gene target which leaves it vulnerable to false-negative results if there is target failure in a future variant. This could be corrected relatively easily once identified by altering its primers. ID NOW also lacks a human control target such as RNase P, so cannot identify inadequate sampling.
In conclusion, near-patient rapid SARS-CoV-2 testing is an essential tool to ensure prompt treatment of patients with COVID-19, maintain patient flow, and minimise nosocomial transmission in acute hospitals. ID NOW provides a rapid and reliable adjunct to the safe triage of patients with COVID-19, and can work effectively when integrated into an ED triage algorithm. Reduced sensitivity compared with RT-PCR can be mitigated by applying clinical criteria within such an algorithm to ensure that negative results in patients with high pre-test probabilities are interpreted with caution.
Acknowledgements
The authors wish to thank all the clinical staff at Northwick Park Hospital who cared for the patients involved in this study, particularly the near-patient testing team.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhin.2022.02.010.
Conflict of interest statement
None declared.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.UK Government . UK Government; London: 2022. Healthcare in United Kingdom.https://coronavirus.data.gov.uk/details/healthcare Available at: [last accessed January 2022] [Google Scholar]
- 2.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta-Wright A., Macleod C.K., Barrett J., Filson S.A., Corrah T., Parris V., et al. False-negative RT-PCR for COVID-19 and a diagnostic risk score: a retrospective cohort study among patients admitted to hospital. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-047110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collier D.A., Assennato S.M., Warne B., Sithole N., Sharrocks K., Ritchie A., et al. Point of care nucleic acid testing for SARS-CoV-2 in hospitalized patients: a clinical validation trial and implementation study. Cell Rep Med. 2020;1:100062. doi: 10.1016/j.xcrm.2020.100062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wen D., Yang S., Li G., Xuan Q., Guo W., Wu W. Sample-to-answer and routine real-time RT-PCR. J Mol Diagn. 2021;23:665–670. doi: 10.1016/j.jmoldx.2021.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Assennato S.M., Ritchie A.V., Nadala C., Goel N., Tie C., Nadala L.M., et al. Performance evaluation of the SAMBA II SARS-CoV-2 test for point-of-care detection of SARS-CoV-2. J Clin Microbiol. 2020;59 doi: 10.1128/JCM.01262-20. e01262-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reynard C., Allen J.A., Shinkins B., Prestwich G., Goves J., Davies K., et al. COVID-19 rapid diagnostics: practice review. Emerg Med J. 2022;39:70–76. doi: 10.1136/emermed-2021-211814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahmoud S.A., Ganesan S., Ibrahim E., Thakre B., Teddy J.G., Raheja P., et al. Evaluation of six different rapid methods for nucleic acid detection of SARS-CoV-2 virus. J Med Virol. 2021;93:5538–5543. doi: 10.1002/jmv.27090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abbott Diagnostics Scarborough, Inc. ID NOW COVID-19 . Abbott Diagnostics; Scarborough, ME: 2020. [Google Scholar]
- 10.Thwe P.M., Maiyo E., Ren P. Abbott ID NOW COVID-19 assay performance: a year in review. Diagn Microbiol Infect Dis. 2021;101:115536. doi: 10.1016/j.diagmicrobio.2021.115536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore N.M., Li H., Schejbal D., Lindsley J., Hayden M.K. Comparison of two commercial molecular tests and a laboratory-developed modification of the CDC 2019-nCoV reverse transcriptase PCR assay for the detection of SARS-CoV-2. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.00938-20. e00938-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lephart P.R., Bachman M.A., LeBar W., McClellan S., Barron K., Schroeder L., et al. Comparative study of four SARS-CoV-2 nucleic acid amplification test (NAAT) platforms demonstrates that ID NOW performance is impaired substantially by patient and specimen type. Diagn Microbiol Infect Dis. 2021;99:115200. doi: 10.1016/j.diagmicrobio.2020.115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham M., Muhi S., Hoang T., Ballard S.A., McAuley J., Kwong J.C., et al. Multi-site point of care assessment of Abbott ID NOW rapid molecular test for SARS-CoV-2 in a low-prevalence setting. Pathology. 2021;53:912–914. doi: 10.1016/j.pathol.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burnes L.E., Clark S.T., Sheldrake E., Faheem A., Poon B.P., Christie-Holmes N., et al. One swab, two tests: validation of dual SARS-CoV-2 testing on the Abbott ID NOWTM. J Clin Virol. 2021;141:104896. doi: 10.1016/j.jcv.2021.104896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stokes W., Berenger B.M., Singh T., Adeghe I., Schneider A., Portnoy D., et al. Acceptable performance of the Abbott ID NOW among symptomatic individuals with confirmed COVID-19. J Med Microbiol. 2021;70 doi: 10.1099/jmm.0.001372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghofrani M., Casas M.T., Pelz R.K., Kroll C., Blum N., Foster S.D. Performance characteristics of the ID NOW COVID-19 assay: a regional health care system experience. Pathology. 2020 doi: 10.1101/2020.06.03.20116327. [DOI] [Google Scholar]
- 17.Tu Y.-P., Iqbal J., O’Leary T. Sensitivity of ID NOW and RT-PCR for detection of SARS-CoV-2 in an ambulatory population. ELife. 2021;10 doi: 10.7554/eLife.65726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tworek J.A., Khan F., Sekedat M.D., Scheidel C., Malani A.N. The utility of rapid nucleic acid amplification testing to triage symptomatic patients and to screen asymptomatic preprocedure patients for SARS-CoV-2. Open Forum Infect Dis. 2021;8:ofaa607. doi: 10.1093/ofid/ofaa607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smithgall M.C., Scherberkova I., Whittier S., Green D.A. Comparison of Cepheid Xpert Xpress and Abbott ID NOW to Roche Cobas for the rapid detection of SARS-CoV-2. J Clin Virol. 2020;128:104428. doi: 10.1016/j.jcv.2020.104428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen Van J.-C., Gerlier C., Pilmis B., Mizrahi A., Péan de Ponfilly G., Khaterchi A., et al. Prospective evaluation of ID NOW COVID-19 assay used as point-of-care test in an emergency department. J Clin Virol. 2021;145:105021. doi: 10.1016/j.jcv.2021.105021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sepulveda J.L., Abdulbaki R., Sands Z., Codoy M., Mendoza S., Isaacson N., et al. Performance of the Abbott ID NOW rapid SARS-CoV-2 amplification assay in relation to nasopharyngeal viral RNA loads. J Clin Virol. 2021;140:104843. doi: 10.1016/j.jcv.2021.104843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grant P.R., Turner M.A., Shin G.Y., Nastouli E., Levett L.J. Extraction-free COVID-19 (SARS-CoV-2) diagnosis by RT-PCR to increase capacity for national testing programmes during a pandemic. Molec Biol. 2020 doi: 10.1101/2020.04.06.028316. [DOI] [Google Scholar]
- 23.British Society of Thoracic Imaging . London: BSTI; 2020. COVID-19 CXR report proforma. Available at: https://www.bsti.org.uk/media/resources/files/BSTI_COVID_CXR_Proforma_v.3-1.pdf. [Google Scholar]
- 24.Cohen J.F., Korevaar D.A., Altman D.G., Bruns D.E., Gatsonis C.A., Hooft L., et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medicines & Healthcare Products Regulatory Agency . UK Government; London: 2021. Target product profile: point of care SARS-CoV-2 detection tests.https://www.gov.uk/government/publications/how-tests-and-testing-kits-for-coronavirus-covid-19-work/target-product-profile-point-of-care-sars-cov-2-detection-tests Available at: [last accessed December 2021]. [Google Scholar]
- 26.Harrington A., Cox B., Snowdon J., Bakst J., Ley E., Grajales P., et al. Comparison of Abbott ID NOW and Abbott m2000 methods for the detection of SARS-CoV-2 from nasopharyngeal and nasal swabs from symptomatic patients. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.00798-20. e00798–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinnes J., Deeks J.J., Berhane S., Taylor M., Adriano A., Davenport C., et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2020;8:CD013705. doi: 10.1002/14651858.CD013705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houston H., Gupta-Wright A., Toke-Bjolgerud E., Biggin-Lamming J., John L. Diagnostic accuracy and utility of SARS-CoV-2 antigen lateral flow assays in medical admissions with possible COVID-19. J Hosp Infect. 2021;110:203–205. doi: 10.1016/j.jhin.2021.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brümmer L.E., Katzenschlager S., Gaeddert M., Erdmann C., Schmitz S., Bota M., et al. Accuracy of novel antigen rapid diagnostics for SARS-CoV-2: a living systematic review and meta-analysis. PLoS Med. 2021;18 doi: 10.1371/journal.pmed.1003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tauh T., Lee S.M., Meyler P., Mozel M., McLennan M., Hoang L.M.N. Diagnostic performance and clinical application of preoperative COVID-19 bedside testing with ID NOWTM. Can J Anesth Can Anesth. 2021;68:1569–1571. doi: 10.1007/s12630-021-02035-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ra S.H., Lim J.S., Kim G., Kim M.J., Jung J., Kim S.-H. Upper respiratory viral load in asymptomatic individuals and mildly symptomatic patients with SARS-CoV-2 infection. Thorax. 2021;76:61–63. doi: 10.1136/thoraxjnl-2020-215042. [DOI] [PubMed] [Google Scholar]
- 32.Lee S., Kim T., Lee E., Lee C., Kim H., Rhee H., et al. Clinical course and molecular viral shedding among asymptomatic and symptomatic patients with SARS-CoV-2 infection in a community treatment center in the Republic of Korea. JAMA Intern Med. 2020;180:1447. doi: 10.1001/jamainternmed.2020.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Department of Health and Social Care. Technical validation of Abbott ID NOW. London: Department of Health and Social Care; 2021. Available at: https://webarchive.nationalarchives.gov.uk/ukgwa/20220107005902mp_/https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/951021/technical-validation-report_Abbot_ID_Now.pdf [last accessed March 2022].
- 34.Mitchell S.L., St George K. Evaluation of the COVID19 ID NOW EUA assay. J Clin Virol. 2020;128:104429. doi: 10.1016/j.jcv.2020.104429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farfour E., Asso-Bonnet M., Vasse M., SARS-CoV-2 Foch Hospital Study Group The ID NOW COVID-19, a high-speed high-performance assay. Eur J Clin Microbiol Infect Dis. 2021;40:2041–2045. doi: 10.1007/s10096-021-04243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fellner M.D., Bonaventura R., Basiletti J., Avaro M., Benedetti E., Campos A., et al. Evaluation of RT-qPCR and loop-mediated isothermal amplification (LAMP) assays for the detection of SARS-CoV-2 in Argentina. Genes. 2021;12:659. doi: 10.3390/genes12050659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.IDSA and AMP joint statement on the use of SARS-CoV-2 PCR cycle threshold (Ct) values for clinical decision-making. Arlington, VA. Infectious Disease Society of America and Association for Molecular Pathology. 2021. Available at: https://www.idsociety.org/globalassets/idsa/public-health/covid-19/idsa-amp-statement.pdf [last accessed April 2022] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.