Abstract
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). COVID-19 has become a worldwide pandemic, and there is a pressing need for the rapid development of novel therapeutic strategies. SARS-CoV-2 viral entry is mediated by interaction between the receptor binding domain (RBD) of the SARS-CoV-2 Spike protein and host cellular receptor, human angiotensin converting enzyme 2 (ACE2). The lack of a high throughput screening (HTS) platform for candidate drug screening means that no targeted COVID-19 treatments have been developed to date. To overcome this limitation, we developed a novel, rapid, simple, and HTS binding assay platform to screen potential inhibitors of the RBD-ACE2 complex. Three “neutralizing” mouse monoclonal antibodies capable of blocking the RBD-ACE2 interaction were identified using our binding assay and pseudovirus neutralization assay followed by further validation with the Focus Reduction Neutralization Test (FRNT), which analyzes the neutralization capacity of samples in the presence of live SARS-CoV-2. Furthermore, the consistency of our binding assay and FRNT results (R2 = 0.68) was demonstrated by patients' serum, of which were COVID-19 positive (n = 34) and COVID-19 negative (n = 76). Several small molecules selected for their potential to inhibit the Spike-ACE2 complex in silico were also confirmed with the binding assay. In addition, we have evaluated vaccine efficacy using binding assay platform and validated through pseudovirus neutralization assay. The correlation between binding assay & psuedovirus assay of the post vaccinated serum showed well correlated (R2 = 0.09) Moreover, our binding assay platform successfully validated different Spike RBD mutants. These results indicate that our binding assay can be used as a platform for in vitro screening of small molecules and monoclonal antibodies, and high-throughput assessment of antibody levels after vaccination. When conducting drug screening, computer virtual screening lacks actual basis, construction of pseudoviruses is relatively complicated, and even FRNT requires a P3 laboratory. There are few methods to determine the competitiveness of the target drug and SRBD or ACE2. Our binding assay can fill this gap and accelerate the process and efficiency of COVID-19 drug screening.
Keywords: COVID-19, SARS-CoV-2, RBD, ACE2, FRNT, Spike-mutant, Inhibitor screening, Neutralization antibody, Vaccine, Binding assay
1. Introduction
COVID-19 has made a catastrophic impact worldwide, with nearly 141 million confirmed cases and 3.01 million deaths as of April 2021 (Zhu et al., 2020). A novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is closely related to SARS-CoV, was detected in patients with COVID-19. SARS-CoV-2 is believed to be the causative agent of the atypical pneumonia observed in patients with COVID-19. Coronaviruses of the β-genus that are transmitted in humans include three highly pathogenic coronaviruses, SARS-CoV, MERS-CoV, and SARS-CoV-2, and four coronaviruses with low pathogenicity, HCoV-OC43, HCoV-HKU1, HCoV-NL63, and HCoV-229E (Walls et al., 2020).
For coronavirus to infect cells, the Spike (S) glycoprotein needs to form a homotrimer on the coronavirus surface. The S protein is composed of two subunits with different functions. The S1 subunit is responsible for binding to host cell receptors including ACE2, NRP1, and AXL, and the S2 subunit is responsible for viral fusion with the host cell membrane (Duan et al., 2020). SARS-CoV-2 cellular entry is mainly mediated by the angiotensin-converting enzyme 2 (ACE2) cellular receptor (Mittal et al., 2020). SARS-CoV-2 and SARS-CoV cellular entry both occur through binding to ACE2 on the host cell membrane.
Very recent findings indicate that, in addition to the ACE2 receptor, SARS-CoV-2 can enter cells through two other membrane receptors, Neuropilin 1 (NRP1) and tyrosine-protein kinase receptor UFO (AXL). AXL receptor specifically interacts with the N-terminal domain of the Spike S1 subunit. In addition, cofactors including transmembrane protease serine 2 (TMPRSS2) (Hoffmann et al., 2020) and NRP1 (Cantuti-Castelvetri et al., 2020) can promote S1 and ACE2 binding, thus contributing to viral infection. However, NRP1 alone is insufficient to enhance virus entry into the host and requires assistance from ACE2 and TMPRSS2.
There are several major SARS-CoV-2 variants circulating in the world. SARS-CoV-2 B.1.1.7 is the main strain in the UK and has greater infectiousness compared to its parental strain (Xie et al., 2021; Ali et al., 2021). SARS-CoV-2 B.1.1.7 contains D614G and N501Y mutations, the latter of which is within the S1 receptor binding domain (RBD). The SARS-CoV-2 B.1.351 variant first emerged in South Africa and rapidly became a more contagious major strain in the local area. In addition to the D614G mutation, the SARS-CoV-2 B.1.351 variant has three S1RBD mutations (K417N, E484K, and N501Y) (Zhou et al., 2021). Similar to the South African strain, the Brazilian P1 strain also has three S1RBD mutations (K417T, E484K, and N501Y) (Khan et al., 2021). Mutated viruses may lead to increased infectiousness and lethality. The emergence of multiple SARS-CoV-2 variants may limit the usefulness of previous research efforts, mainly based on the wildtype, Wuhan SARS-CoV-2 strain, and could affect vaccine and drug efficacy.
COVID-19 can be controlled by designing neutralizing antibodies (Nabs) or small molecule drugs based on the process of viral binding to cell receptors. Other methods to block viruses from entering cells include preventing virus replication, preventing virus release, and activating natural killer (NK) cells in the human body to kill virus-infected cells. A variety of monoclonal antibodies, polyclonal antibodies and small molecule drugs are undergoing clinical trials in different phases, and these drugs also show different neutralizing effects. Due to the continuous emergence of new virus mutants, more drugs need to be screened for use (Kalhor et al., 2020; Berber and Doluca, 2021; Levi-Schaffer and de Marco, 2021). Moreover, most of the drugs currently in clinical trials as suitable SARS-CoV-2 inhibitors were identified by virtual, computer-based screening of existing drug libraries (Akhtar, 2020; Durdagi, 2020; Usha et al., 2021). Existing drugs for the treatment of COVID-19 mainly rely on alleviating the inflammatory response and regulating the immune response. No drug capable of directly targeting SARS-CoV-2 cellular invasion has been identified or developed.
To date, there is no effective, in vitro, and high-throughput method for SARS-CoV-2 inhibitor screening. Herein, we describe the novel S-ACE2 binding assay method for rapid, simple, sensitive, and high-throughput screening of small molecules, peptides, and antibodies. This binding assay can be utilized to characterize the binding affinity of the S-ACE2 complex in the presence of potential inhibitors within a short time frame of only 4 h. This S-ACE2 binding assay can be used to screen inhibitor activity, inform vaccine development, and test potential COVID-19 therapeutic drugs.
2. Materials and methods
2.1. Materials
African Green monkey kidney-derived Vero C1008 cells and Human alveolar basal epithelial A549 cells were purchased from ATCC (Manassas, VA, USA). Cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin in 5% CO2 at 37 °C and maintained according to the manufacturer's protocols.
Recombinant SARS-CoV-2 Spike glycoprotein RBD (Cat# 230–30,193) and recombinant C-terminal His-tagged human Angiotensin-converting Enzyme 2 (ACE2) (Cat# 230–30,165) were from RayBiotech Inc. (Peachtree Corners, GA, USA).
Mouse anti-SARS-CoV-2-S1RBD (1H9-G1-A1) (Cat# 130–10-807), mouse anti-SARS-CoV-2 S1RBD (4A9-C2-G3-F10) (Cat# 130–10,844), biotinylated mouse anti-SARS-CoV-2 S1RBD (1F9-F2-C1-G9) (Cat# 130–10,845), mouse anti-SARS-CoV-2 S1RBD (1F10-D4-B1) (Cat# 130–10,808), mouse anti-SARS-COV-2 N-Protein (4C10-F1-C2) (Cat# 130–10,817), and mouse anti-SARS-COV-2 N-Protein (1A10-H10-H6) (Cat# 130–10,822) antibodies were from RayBiotech Inc.
Small molecule inhibitor GW280264X (CAS: 866924–39-2; Cat# AOB3632) was purchased from Aobious Inc. (Gloucester, MA, USA), K22 (CAS:2141978–86-9; Cat# 4295–0370) was purchased from ChemDiv (San Diego, CA, USA), oxocarbazate (CAS: 1014405–03-8; Cat#: 564753) was from MedKoo (Morrisville, NC, USA), zafirlukast (CAS: 107753–78-6; Cat# T6736) and camostat mesylate (CAS: 59721–29-8; Cat# T2391) were supplied by TargetMol (Boston, MA, USA), and SSAA09E (Cas# 883944–52-3; Cat# B2723–1) was supplied by Amsbio (Cambridge, MA, USA).
2.2. Serum isolation
COVID-19-tested patient samples were collected with informed consent according to our Institutional/CLIA lab protocol (Sterling IRB: 8291-BZhang). Blood was collected in heparinized tubes and was centrifuged at 4 °C and 1000 g for 10 min. The supernatant was transferred to a clean 15- or 50-ml centrifuge tube. The SARS-CoV-2 virus was inactivated by the addition of Triton X-100 (4% final concentration) at room temperature for 5 min and was further heat inactivated at 56 °C for 1 h. For cross-reactivity studies with non-SARS viruses (Fig. 1E), serum samples were purchased from BEI Resources (Manassas, VA, USA).
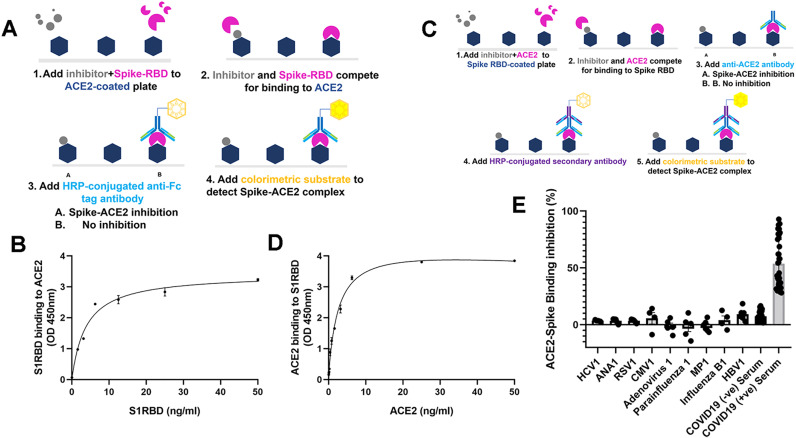
Fig. 1.
The dose responsiveness and specificity of S1RBD-ACE2 and ACE2-S1RBD binding assay. (A) Schematic diagram of the S1RBD-ACE2 binding assay. (B) Detection of ACE2- S1RBD binding interaction where ACE2 protein was coated on a microplate and S1RBD concentration was varied. (C) Schematic diagram of the ACE2-S1RBD binding assay. (D) Detection of S1RBD-ACE2 binding interaction where S1RBD protein was coated on a microplate and ACE2 concentration was varied. (E) Selectivity of binding assay for non-SARS viruses in patient sera. Data points represent individual patients seropositive for the virus indicated. Binding inhibition is the percentage of signal (OD450) in the presence of seropositive sera relative to no sera. In B and D, the average of three independent experiments is shown. Error bars indicate standard error of the mean (SEM).
2.3. Validation of the S1RBD-ACE2 binding assay ELISA-like platform
Methods for cross-reactivity study using multiple viruses were as follows. 96-well high binding strip plates (Greiner Bio-One, NC, USA) were coated overnight at 4 °C with 100 μL/well of either recombinant ACE2 (Cat# 230–30,165-100) at 2 μg/mL in phosphate buffer (pH 9.6) or recombinantly-expressed human S1RBD (Cat# 230–30,162) at 2 μg/mL in phosphate buffer (pH 9.6). After being washed 3 times with PBS with 0.05% Tween 20 (PBS-T), plates were blocked with 100 μL/well of BSA blocking buffer (Rockland Inc.) After another wash, either ACE2 protein or S1RBD protein, depending on the coated antigen, was added at 100 μL/well in a range of concentrations from 100 to 0 ng/mL to the coated plate and incubated at room temperature (RT) for 2.5 h. Plates were washed with PBS-T, and 100 μL/well of a 1:1,00 dilution of HRP-conjugated IgG antibody was added and incubated for 1 h at RT. Plates were washed with PBS-T and 100 μL/well of tetramethylbenzidine (TMB) was added for 30 min at RT for color change; the reaction was stopped with the addition of 50 μL/well 0.2 M H2SO4. Absorbance was measured at optical density 450 nm (OD450) immediately using a plate reader (Fig. 1A and C). The percentage of S1RBD binding inhibition in the presence of antibodies, small molecules, or sera was determined as [1 – (OD450 of with inhibitor / OD450 without inhibitor)] x 100.
2.4. Binding assay for screening of neutralizing antibodies (Nabs), small molecules, and serum samples
All materials were equilibrated to room temperature (18–25 °C) before use. 96-well high binding strip plates (Greiner Bio-One) were coated overnight at 4 °C with 100 μL/well of recombinant angiotensin converting enzyme 2 (ACE2) at 2 μg/mL in phosphate buffer (pH 9.6). After being washed 3 times with PBS with 0.05% Tween 20 (PBS-T), plates were blocked with 100 μL/well of blocking buffer After another wash, monoclonal antibodies (mAbs) were added at 2 μg/mL to 100 μL of recombinant SARS-CoV-2 Spike glycoprotein RBD (S1RBD) solution and mixed. This mAb-S1RBD mixture was added to each well of the 96-well ACE2 coated plate and incubated at room temperature (RT) for 2.5 h. Plates were washed with PBS-T, and 100 μl/well of a 1:1000 dilution of HRP-conjugated IgG antibody was added and incubated for 1 h at RT. Plates were washed with PBS-T and 100 μL/well of TMB was added for 30 min at RT for color change; the reaction was stopped with the addition of 50 μL/well 0.2 M H2SO4. Absorbance was measured at OD450 immediately using a plate reader. All subsequent binding assays followed the same protocol described above for coating of ACE-2. Indicated small molecules were added in 4-fold dilutions to 100 μL of recombinant S1RBD solution and mixed. This small molecule-S1RBD mixture was added to each well of the 96-well ACE2 coated plate and incubated at 37 °C for 1 h. Indicated patient serum samples were added at a dilution of 1:50 in 100 μL of recombinant SARS-CoV-2 Spike glycoprotein RBD (S1RBD) solution and mixed. This serum-S1RBD mixture was added to each well of the 96-well ACE2 coated plate. Addition of HRP solution, subsequent development, and reading were as described in the above protocol.
2.5. Pseudovirus assay for screening and validation of neutralizing antibodies (Nabs), small molecules, and serum samples
G*ΔG-luciferase VSV (purchased from Kerafast: EH1020-PM) and VSV-SARS-CoV-2 pseudotyped viral particles were generated as previously described (Whitt, 2010; Nie et al., 2020; Xiong et al., 2020). One day before transfection, BHK21 cells were cultured in a 100 mm cell culture dish at the concentration of 3 × 106 cell and incubated overnight at 37 °C in 5% CO2 to reach 70% to 90% confluence. Using the Lipofectamine 3000 Transfection Reagent (Invitrogen) according to the manufacturer's instructions, BHK21 cells were transfected with 16 μg pCAGGS-G-kan plasmid. The transfected cells were subsequently infected with G*ΔG-luciferase VSV at a multiplicity of infection (MOI) of 0.1. These cells were incubated at 37 °C in 5% CO2 for 1.5 h followed by the addition of fresh complete DMEM. At 24 h post infection, culture supernatants containing G*ΔG -luciferase VSV were harvested, and aliquots were stored at −80 °C until use. To generate SARS-CoV-2 pseudovirus, BHK21 cells were transfected with 16 μg pCAGGS-SARS-COV-2 spike plasmid. The transfected cells were subsequently infected with G*ΔG-luciferase VSV at a MOI of 3–5.
Vero and A549 cells, suspended at a density of 2 × 105 cells/mL, were prepared (10 mL each) in 15 mL sterile conical polypropylene tubes using pre-warmed DMEM complete growth medium. Vero and A549 cell suspensions were seeded at 100 μL/well into Greiner white 96-well tissue culture plates with a clear base and were incubated for 24 h at 37 °C in a humidified incubator with 5% CO2. Cells were then infected with rVSV pseudoviruses. In a sterile U-shaped 96-well cell culture plate, 60 μL of pre-warmed DMEM was added as a negative control. Supernatant containing the generated rVSV pseudotype viruses was thawed on ice. Serum samples were heat inactivated at 56 °C for 30 min in a heat block. Serum samples were diluted in serum-free DMEM to 1:50 and filtered using a Millex GV Filter Unit and a plastic syringe. DMEM containing 20 μg/mL antibodies (75 μL total volume) was added to the wells using 5-fold serial dilutions to decrease antibody concentration. For serum samples, 2-fold serial dilution was used. During each dilution step, the mixture was mixed well by pipetting 10 times. Supernatant (60 μL) containing a 1:10 dilution of rVSV-ΔG-pCAGGS/Spike-luciferase pseudoviruses was added to all wells except for the negative control.
The plate was incubated at 37 °C for 1 h. Medium (90 μL) was removed from the 96-well plate seeded with Vero or A549 cells on Day 1. Then, 100 μL was transferred from each well of the U shaped 96-well cell culture plate to the corresponding wells of 96-well plate containing Vero or A549 cells. Then, the plate was incubated at 37 °C for 1.5 h in a humidified incubator with 5% CO2. Pre-warmed DMEM complete growth medium (100 μL) was added to each well and the plate was incubated at 37 °C for 24 h in a humidified incubator with 5% CO2.
Luciferase assay buffer was equilibrated to room temperature without exposure to light and the reagent was prepared. In each well, cells were rinsed with 180 μL of pre-warmed DMEM. Then 150 μL of DMEM was removed from each well and 50 μL of prepared luciferase reagent was added. The plate was incubated for 3 min on an orbital shaker at 300–600 rpm to lyse cells and equilibrate samples. Luminescence was measure in a luminometer with an integration time of 0.5–1 s.
Pseudoviruses harboring the SARS-CoV-2 Spike viral surface glycosylation protein were incubated with 2 μg/ml three different antibodies at 37 °C for 1 h and inoculated into A549 cells. At 24 h post infection, pseudotype entry was assessed by measuring luciferase activity in cell lysates, and the relative luminescence ratio compared to the “zero” concentration data is shown.
2.6. Focus reduction neutralization test (FRNT)
SARS-CoV-2 is a Biosafety Level 3 (BSL-3) pathogen. All blood samples were handled and processed according to Institutional Biosafety Guidelines. The FRNT procedure was performed in accordance with all applicable safety procedures. Refer to the literature for detailed operation steps (Vanderheiden et al., 2020).
Serially diluted patient serum and SARS-CoV-2 (100–200 focus-forming units) were combined in DMEM +1% FBS, then incubated at 37 °C for 1 h. The antibody-virus mixture was aliquoted onto a monolayer of VeroE6 cells, gently shaken to evenly distribute the mixture, and incubated at 37 °C for 1 h. Then, the antibody-virus inoculum was removed and prewarmed DMEM supplemented with 1% FBS, HEPES buffer, 2 mM l-glutamine, 1 mM sodium pyruvate, 1 x non-essential amino acids, and 1 x antibiotics (penicillin, streptomycin, and amphotericin B) was mixed with an equal volume of methylcellulose (DMEM, 1% antibiotic, 2% FBS, 2% methylcellulose) and was overlaid onto the infected VeroE6 cell layer. Plates were incubated at 37 °C for 24 h. After 24 h, plates were gently washed three times with 1 x phosphate buffered saline (PBS) and fixed with 200 μL of 2% paraformaldehyde for 30 min. Following fixation, plates were washed twice with 1 x PBS and the fixed Vero cell monolayer was incubated with 100 μL of permeabilization buffer (0.1% BSA-Saponin in PBS) for 20 min. Cells were incubated with a biotin conjugated anti-SARS-CoV-2 spike protein primary antibody at room temperature for 1–2 h. Cells were then incubated with avidin-HRP conjugated secondary antibody at room temperature for 1 h. Foci were visualized using True Blue HRP substrate and images were obtained using an ELISPOT reader. Each plate contained three positive neutralization control wells, three negative control wells containing healthy control serum mixed with SARS-CoV-2, and three mock-infected wells.
2.7. Quantification and statistical analysis
For statistical analyses, the GraphPad Prism 7 software package and origin 8.0 were used. Receiver operating characteristic (ROC) curves were generated for each clinical validation sample cohort. Optical densities (OD) for each sample were entered into Microsoft Excel for Office 365 v16, and a custom software package was used to iteratively compute the false positive rate (1-specificity) and true positive rate (sensitivity) at every OD cutoff level for each cohort. The false-positive rates (x) and true positive rates (y) were then rendered as scatter plots to generate ROC curves. Linear regression analyses were used to assess correlation.
3. Results
3.1. The production of S1RBD-ACE2 binding assay and its confirmation
Knowing that SARS-CoV-2, specifically the S1RBD, has a high affinity for ACE2 found on human cells, we tested our in vitro binding assay for specificity toward this viral interaction following our specific protocol (Fig. 1A and C). For this analysis we compared the S1RBD-ACE2 interaction in the presence of other common viruses including hepatitis C virus (HCV), ANA, respiratory syncytial virus (RSV), cytomegalovirus (CMV), adenovirus (ADV), parainfluenza, MP virus, and influenza B virus (IBV). We observed that, among the viruses tested, four had a slight inhibitory effect on S-ACE2 binding, while the other five viruses had no inhibitory effect (Fig. 1E). Our binding assay cross-reactivity test results were below 10% which is within the acceptable USA-FDA guideline range. To confirm that SARS-CoV-2 binding is S1RBD and ACE2 specific, we compared the binding of ACE2 to SARS-CoV-2 RBD as well as other proteins found on the SARS-CoV-2 virus (S1, S2, nucleocapsid). Binding of ACE2 to S1RBD proportionally increased with increasing ACE2 concentration (Fig. 1B and D) whereas no signal was detected at similar concentrations for other proteins found on the SARS-CoV-2 virus (data not shown), demonstrating that the observed binding is S1RBD and ACE2 specific.
3.2. Pseudovirus system construction and its confirmation
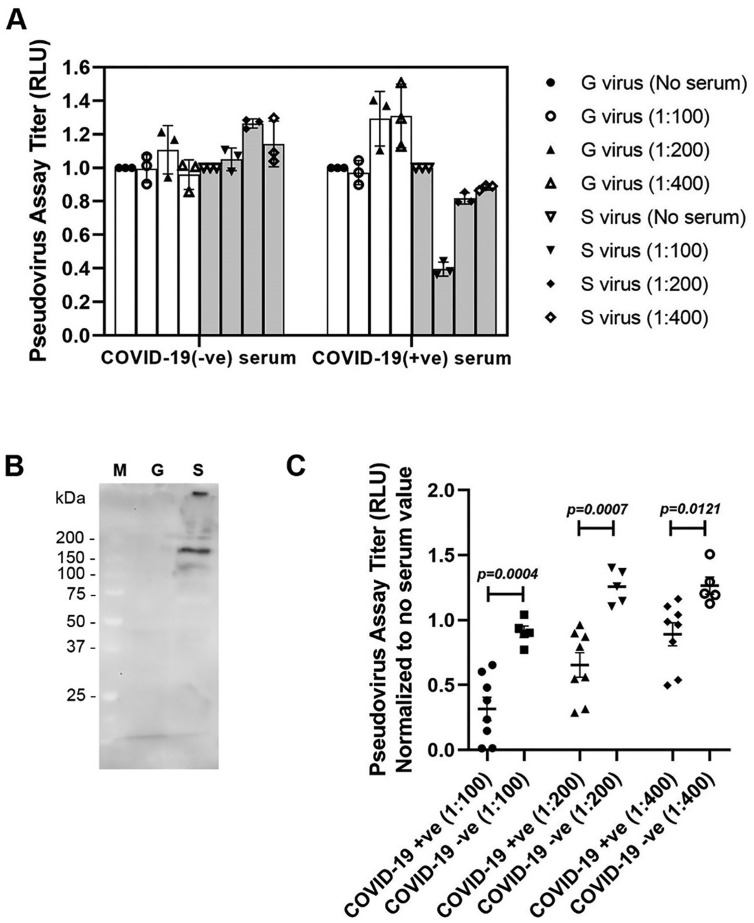
Currently, pseudovirus inhibition assays are one of the most translationally relevant assays to evaluate neutralizing antibodies to SARS-CoV-2, potential drug inhibitors, as well as vaccine candidates. Thus, to confirm the results observed in our S1RBD binding assay we ran subsequent studies utilizing the pseudovirus assay. To ensure our pseudovirus assays' functionality, we compared pseudovirus assay titers in the presence of sera from COVID-19 infected (+ve) individuals along with COVID-19 negative (−ve) samples at varying concentrations (Fig. 2A). We compared the two sample groups in using two different scenarios: a pseudovirus containing the SARS-CoV-2-S gene and a control pseudovirus containing the VSV-G gene which does not contain the S protein (Fig. 2B). The pseudoviruses used in our assay carries both GFP and luciferase reporter genes and the activity of pseudovirus-infected cells can be evaluated by observing fluorescence and detecting of luciferase activity. Next, in order to verify the reliability of the pseudovirus assay system, we used more sera from patients with COVID-19 (+ve) and normal healthy sera (COVID-19 (−ve) for verification purpose. We used only S virus for the validation purpose as we have noticed G virus has no effect (Fig. 2A). We have found that in COVID-19 (−ve) normal patients' sera there was no inhibition, however, COVID-19 (+ve) sera showed significant (p = 0.0004) inhibition (pseudovirus neutralization titer ratio went down compared to no serum) even at different S virus dilutions (100–400 fold, Fig. 2C). This results further validated that the pseudovirus system we constructed can be used for HTS.
Fig. 2.
Validation of pseudovirus system. At 24 h post infection, pseudotype viral entry into A549 was determined by measuring relative luciferase units (RLU) in cell lysates and calculating the ratio of RLU relative to a control group with no serum. (A) Pseudotypes rVSV/spike (S) virus and rVSV/G (G) virus (no S control) were treated with normal or COVID-19-positive patient sera at different dilutions (100–400 fold). (B) Western blot analysis of cell lysates infected with pseudotype rVSV/spike (S) virus and rVSV/G (G) virus. M = protein marker. Blot was probed with mouse anti-SARS-CoV-2 S1RBD monoclonal antibody (clone 1F10-D4-B1; 1:1000 dilution). Three independent experiments were performed for and pseudotype at each dilution; error bars indicate standard deviation (SD). (C) The pseudovirus assay was validated using 13 serum samples: five from normal healthy donors (COVID-19 (−ve), and eight from patients with COVID-19 (+ve). Different titers (100, 200, 400-fold) of S pseudovirus were tested. Data were normalized with no serum value.
3.3. Screening for neutralizing antibodies (Nabs) on the S1RBD binding assay and confirmation of binding assay screening results using pseudovirus assay
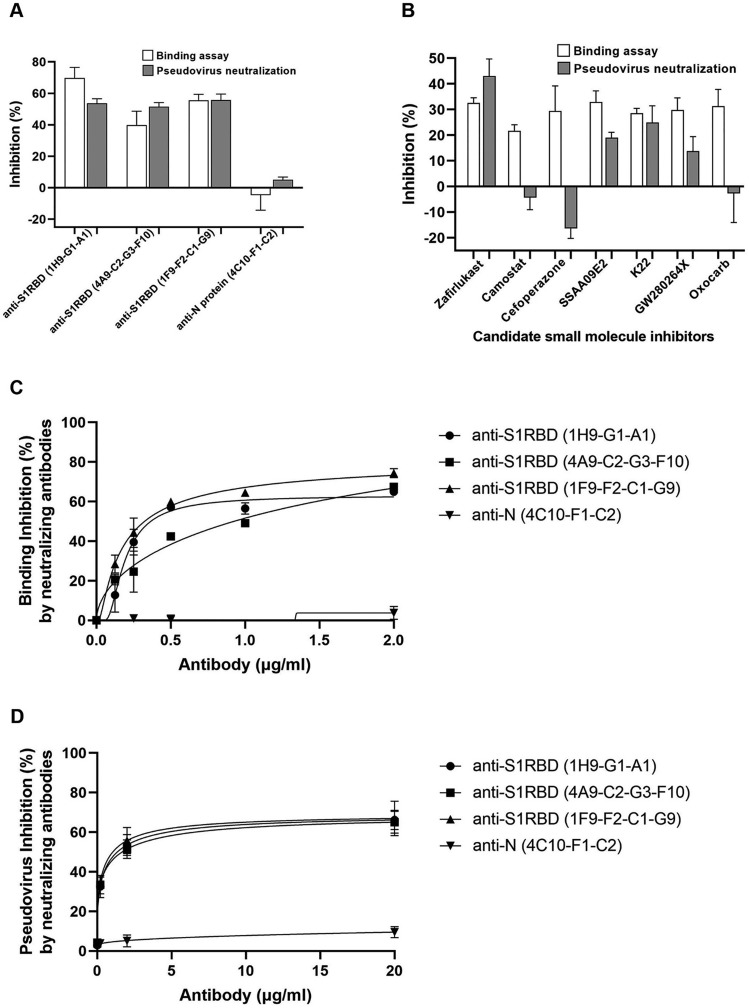
To examine whether our S1RBD binding assay could be used to screen for neutralizing antibodies (Nabs), a total of 24 S1RBD monoclonal antibodies were screened and three Nabs were identified. Three Nabs indicated as anti-SARS-CoV-2-S1RBD (clone 1H9-G1-A1), anti-SARS-CoV-2-S1RBD (clone 4A9-C2-G3-F10) (Cat# 130–10-844), and anti-SARS-COV-2-S1RBD (clone 1F9-F2-C1-G9) (Cat#130–10-845) showed up to a 90% inhibitory effect on the S1RBD-ACE2 complex, indicating their ability to bind S1RBD (Fig. 3A). Mouse anti-SARS-CoV-2 N-Protein (clone 1A10-H10-H6) (Cat# 130–10-817) binds to the SARS-CoV-2 nucleocapsid (N) protein with high affinity and therefore was used as a negative control. This negative control antibody showed an inhibition rate of about ≤10% (Fig. 3A). These results indicate that our S1RBD binding assay can screen for potential Nabs that inhibit S1RBD binding to ACE2. To confirm these results, we used the pseudovirus assay. For pseudoviruses containing the SARS-CoV-2 S gene, the addition of the S antibody led to a decrease in fluorescence signal intensity with increasing S antibody concentration. However, the addition of the N antibody resulted in no obvious change in fluorescence signal for pseudoviruses containing the SARS-CoV-2 S gene (Fig. 3A). This confirms that S Nabs screened by our S1RBD binding assay also inhibit SARS-CoV-2 pseudovirus entry into cells. The binding inhibition rates observed for the three Nabs identified are all approximately ≥50%, indicating that the binding assay can be utilized to screen Nabs in vitro.
Fig. 3.
Neutralization antibodies and drugs screening using S1RBD-ACE2 binding assay and pseudovirus harboring the SARS-CoV-2 S protein. (A) Comparison of the consistency of the results of three different neutralizing antibodies and one antibody against Nucleocapsid (N) protein as negative control using binding assay and pseudovirus assay. All antibodies were raised in mice and clone numbers are indicated for each. (B) Binding assay and pseudovirus in the presence of five small molecules (0.4 μg/ml) with known ability to interfere with the S1RBD-ACE2 interaction as well as two that target unrelated proteins (see also Table 1). (C) Titration of neutralizing antibodies in binding assay. (D) Titration of neutralizing antibodies in pseudovirus assay. Inhibition was calculated as percentage of signal (OD450 or RLU for binding assay and pseudovirus assay, respectively) in the presence of inhibitor (antibody or small molecule) relative to no inhibitor. The average of 3 independent experiments was performed with error bars indicating SEM.
3.4. Screening for small molecule inhibitors on the S1RBD binding assay and confirmation of binding assay screening results using pseudovirus assay
We next wanted to determine if our S1RBD binding assay can be used to identify small molecules that block the S1RBD-ACE2 interaction, as most pharmaceutical drug candidates are small molecules. We performed our S1RBD binding assay by using seven drugs indicated as SSAA09E2, K22, GW280264X, oxocarb, zafirlukast, cefoperazone, and camostat mesylate (Table 1 ) and DMSO as a negative control. A series of concentrations, 1800, 600, 200, 67, 22, and 0 μg/ml of each compound were assessed for their potential to strongly inhibit SARS-CoV-2. Zafirlukast showed the strongest inhibitory effects of the S1RBD-ACE2 complex formation, while cefoperazone and camostat had no obvious inhibitory effects (Fig. 3B). Clinically, zafirlukast is a leukotriene receptor antagonist. Zafirlukast has a significant inhibitory effect on the S-ACE2 complex at concentrations above 2 μg/mL, effectively blocking SARS-CoV-2 cell entry. Camostat mesylate, a potent serine protease inhibitor, is a SARS-CoV-2 inhibitor (Xiu et al., 2020). However, camostat does not have an inhibitory effect in our binding assay because it blocks SARS-CoV-2 cellular entry with a co-factor, TMPRSS2. These results indicate the binding assay can be used to identify drug candidates that block the S1RBD-ACE2 interaction. These candidates are likely to prevent viral entry in vivo. To confirm this assumption, we ran the indicated small molecules in a pseudovirus assay following the same assay parameters. With increasing concentrations of the three drugs tested, no inhibitory effect on the VSV-G virus system is observed. In the Spike virus system, increasing zafirlukast concentration led to a significant reduction in fluorescence intensity. This indicates that zafirlukast has the ability to inhibit pseudovirus entry into cells (Fig. 3B). Small molecules screened by our pseudovirus assay inhibit SARS-CoV-2 pseudovirus entry into cells at consistent levels with the S1RBD binding assay, with zafirlukast having the highest inhibitory effects.
Table 1.
Target Molecules of the Small Molecules for Spike-RBD-ACE2 Binding Assay based on Computational Screening.
| Target | Small Molecules |
|---|---|
| RBD | SSAA09E2 |
| RBD | K22 |
| ACE2 | GW280264X |
| ACE2 | Oxocarbazate |
| ACE2 | Zafirlukast |
| Penicillin-binding proteins (PBPs) | Cefoperazone |
| TMPRSS2-related proteases | Camostat |
3.5. Validation of the S1RBD binding assay in patient plasma and sera samples and confirmation using pseudovirus assay
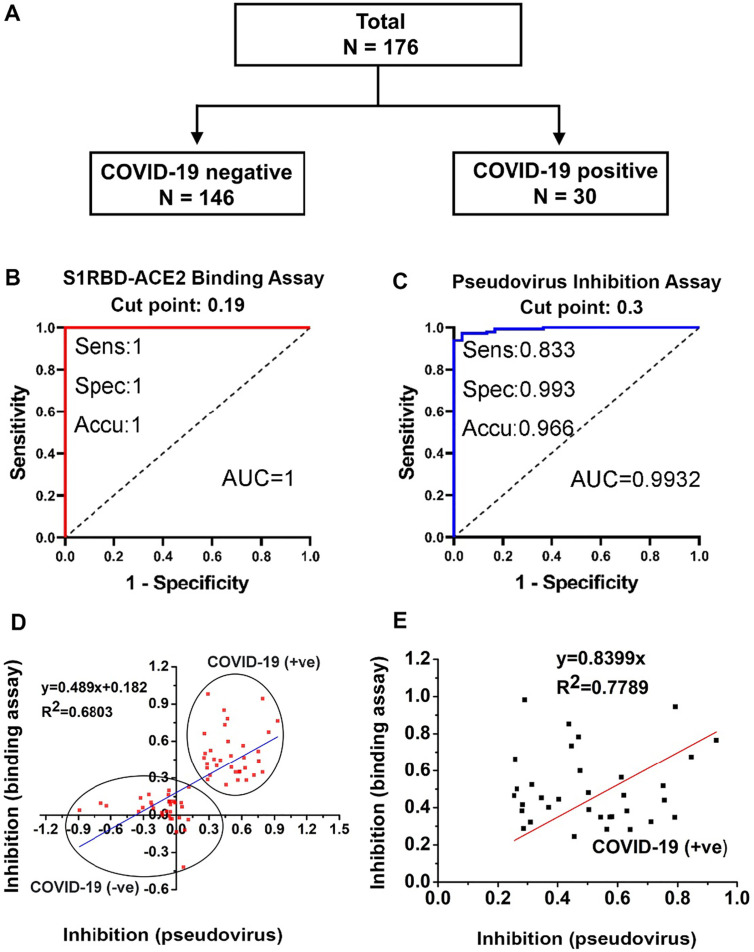
Our binding assay makes it possible to screen neutralizing antibodies and small molecule drugs for SARS-CoV-2. Nabs play an important role in virus clearance and are considered a key immune product for protection against or treatment of viral diseases. Passive antibody therapy, such as plasma fusion, is successfully used to treat infectious viral diseases, including SARS-CoV, influenza, and Ebola viruses. To further determine whether human serum containing Nabs is capable of blocking viral entry into host cells, several SARS-CoV-2 infected human serum samples and healthy human controls were tested using the S1RBD binding assay (Fig. 4A). COVID-19 negative (n = 146) and COVID-19 positive (n = 30) subjects (total n = 176) were evaluated; all samples were tested by an FDA approved RT-PCR kit for COVID-19 infection. Based on our binding inhibition assay data, we determined the cut-off level at 0.2, equivalent to 20% binding inhibition (Fig. 4B). Thus, we have estimated that if the binding inhibition of the subject's serum is >20%, it contains minimum neutralizing antibodies and the level of Nabs in the patient's serum can be assessed semi-quantitatively based on our binding inhibition rate. Sensitivity of S1RBD-ACE2 binding inhibition assay against the FDA-approved RT-PCR (positive) results is 100% (30/30, 95% CI: 88.43–100%) while the specificity of the S1RBD-ACE2 binding assay is 100% (146/146, 95% CI: 97.51–100%). The accuracy of the S1RBD-ACE2 binding assay against reference standard is 100% (176/176, 95% CI: 97.93–100%), with a Kappa value of 1 (Fig. 4B).
Fig. 4.
Evaluation of S1RBD-ACE2 binding assay and pseudovirus assay using COVID-19 patient sera. (A) Study subjects used for validation of binding assay. (B) ROC curve generated for S1RBD-ACE2 binding assay of each patient serum sample. (C) ROC curve generated for pseudovirus inhibition test in determining SARS-CoV-2 neutralization activity. (D) Correlation between binding assay and pseudovirus assay using COVID-19 (+ve) and COVID-19 (−ve) serum samples. (E) Correlation between binding assay and pseudovirus assay using COVID-19 (+ve) serum samples alone. Inhibition was calculated as the fraction of signal (OD450 or RLU for binding assay and pseudovirus assay, respectively) in the presence of serum relative to no serum.
Subsequently, pseudovirus assays were performed using the same serum samples. Biostatistical data analysis revealed a cut-off point of 0.3, indicating that if the serum showed at least 30% pseudovirus inhibition, then the serum contained minimum Nabs (Fig. 4C). These results revealed that the percentage of pseudovirus inhibition is proportionately equivalent to the level of Nabs in serum. Sensitivity of pseudovirus inhibition is 83.33% (25/30, 95% CI: 65.28–94.36%), the specificity of pseudovirus inhibition against the reference standard is 99.32% (145/146, 95% CI: 96.24–99.98%), and the accuracy of pseudovirus inhibition against the reference standard is 96.59% (170/176, 95% CI: 92.73–98.74%), with a Kappa value of 0.8727 (Fig. 4C).
Furthermore, both binding inhibition and pseudovirus entry inhibition assays were positively correlated. Comparison of the S1-ACE2 binding assay and pseudovirus assay produced a correlation coefficient R2 of 0.64 (Fig. 4D, E), indicative of a positive correlation between the assays. The results of this analysis show that the binding assay is a rapid and efficient in vitro method for detecting the presence of SARS-CoV-2 Nabs in human sera, and that the accuracy and sensitivity of detection can reach a high level. The binding assay can recognize SARS-CoV-2 Nabs in patient serum, and these results have a high degree of overlap with the results of the conventional pseudovirus system (Fig. 4E).
3.6. FRNT and the correlation between FRNT and binding and pseudovirus assays
Live virus experiments are currently the gold standard for measuring SARS neutralization. An FRNT (Suthar et al., 2020) was performed and the results were compared with those obtained from binding and pseudovirus assays. In the FRNT assay, sera from patients with COVID-19 (confirmed by FDA approved RT-PCR kit) were incubated with a SARS-CoV-2 clinical isolate and then used to infect VeroE6 cells. The neutralization ability of the sera samples is measured by a reduction in the number of virally infected foci. Sera from patients with COVID-19 (n = 34) had FRNT50 titers ranging from 1:2886 to 1:26.4, which show neutralization capacity. Sera from normal heathy control patients had an FRNT50 titer of 1:10 (Fig. 5 and Table 1). These results also show that the FRNT50 of sera from patients with COVID-19 is significantly higher than that of sera from normal healthy controls. Moreover, the FRNT assay results are consistent with those of the binding and pseudovirus assays.
Fig. 5.
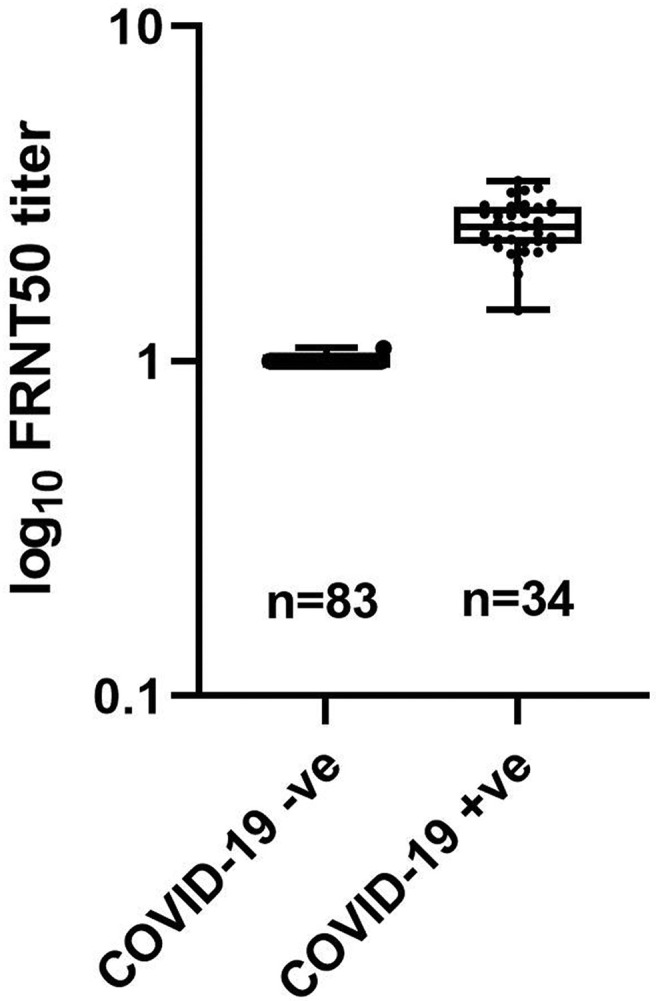
Box and whisker plot FRNT50 in COVID-19 negative (−ve) and COVID-19 positive (+ve) serum samples. Whiskers represent min to max values and all data points are shown.
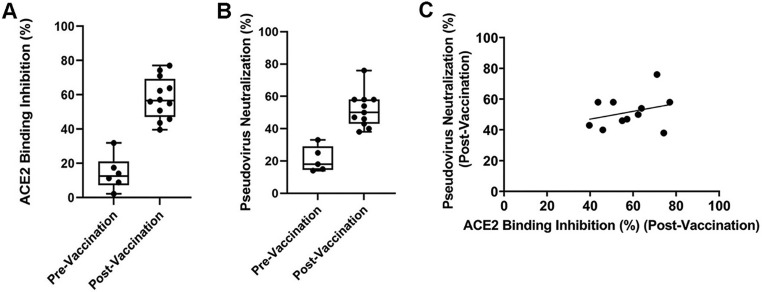
3.7. Evaluation of ACE2 binding inhibition and pseudovirus neutralization activity of vaccinated samples
We collected pre-vaccinated and 14 days post-vaccinated (either Pfizer or Moderna) blood samples from the same cohort of volunteers with their informed consent. Using the ACE2-Spike-RBD binding assay, we observed significant ACE2 binding inhibition (≥55% at 1:100 dilution) for each of the each of the post-vaccination samples, while the pre-vaccinated serum samples did not show any significant inhibition (Fig. 6A). Pseudovirus assay showed a similar trend (Fig. 6B). The correlation R2 of the post-vaccine serum between binding assay and pseudovirus assay was 0.09 (Fig. 6C).
Fig. 6.
Evaluation of vaccine efficacy using S1RBD-ACE2 binding assay and pseudovirus assay. (A) ACE2 binding inhibition (%) and (B) pseudovirus neutralization (%) in subjects pre- and post-vaccination. (C) Correlation study between binding assay and pseudovirus assay in post vaccinated sample. Whiskers represent min to max values and all data points are shown.
4. Discussion
Receptor recognition is the first step of viral infection and is a key determinant of host cell and tissue tropism. S1RBD binding with ACE2 is not the only way for SARS-CoV-2 to enter human cells, but the S1RBD-ACE2 interaction has been more thoroughly studied than have TMPRSS-, NRP- and AXL-mediated cellular entry. This is because the SARS-CoV-2 S1RBD-ACE2 interaction and cellular entry is so similar to that of SARS-CoV. Therefore, once we were able to prepare the S1RBD protein in the laboratory, we developed an assay similar to ELISA, using competitive inhibition for detection. If the analyte contains a protein or molecule that can inhibit S1RBD-ACE2 binding, such as Nabs, small molecule inhibitors, peptides, or DNA aptamers, this can be quickly screened out within 4 h.
Most of the current development of drugs for the treatment of COVID-19 is based on the following four strategies: one is neutralizing antibodies against S protein; the other is neutralizing antibodies against ACE2; the third is ACE2 analogs, which competitively bind to S, thereby blocking the binding of the virus to the receptor; the last is the antibody against the inflammatory factor storm, such as TNF-α, IL-1, IL-6, IFN-α. Based on the resolved SARS-CoV-2 crystal structures, the main COVID-19 drug screening methods mainly rely on computational modeling and in-silico models assess the binding site cavity to find a cavity similar to that of SARS-CoV-2. Then, we can find drugs suitable for binding this cavity, and perform docking fitting to verify whether the selected drug can suppress SARS-CoV-2. Therefore, the S1RBD-ACE2 binding assay can fill this gap. Using our assay, drugs can be screened in vitro and further verified using the pseudovirus assay. This approach will allow for more accurate and effective drug screening and complement the computer simulation results.
Repurposing drugs that are already on the market can greatly reduce the discovery cycle for new COVID-19 drugs. As these drugs have passed clinical trials, there is no need to conduct toxicological tests, and these drugs can more quickly enter the clinical stage.
The S1RBD-ACE2 binding assay can be used to screen small molecule inhibitors and Nabs and to evaluate the antibody changes in serum after vaccination. Currently, SARS-CoV-2 vaccines require two doses. The binding assay method can be used to test the effectiveness of each shot following administration. If follow-up testing is performed every month, this assay can also be used to determine the effective duration of the vaccine. Moreover, this binding assay also help to screen other molecules, including Nabs, that can inhibit binding of SARS-CoV-2 Spike variants.
The results of the binding assay are consistent results with those of the FRNT and pseudovirus assays. This approach simplifies the way in which COVID-19 drugs are screened, from a process requiring 3 days operating in a BSL-3 laboratory, to a process requiring only 4 h in a protein biochemical laboratory. We have developed a binding assay to screen potential COVID-19 drugs to accelerate the development of COVID-19-specific drugs. It would be beneficial to further develop this binding assay method for other receptors, such as NRP1 and AXL, to assess their interaction with the Spike protein. We are currently working to develop the Spike-NRP1 and Spike-AXL binding assay platforms and validating our assay system.
In view of the fact that the early vaccine design and the design of antibody drugs and even small molecule inhibitors are still based on high homology between SARS-CoV-2 and SARS-CoV-1, we have chosen the competitive design of the drug against SRBD-ACE2 and built a platform. Some inhibitors need the assistance of other protease (TMPRSS2) to enter the cell. Our SRBD-ACE2 binding assay cannot fully cover all types of drug screening. Furthermore, drugs that require the help of other proteases to enter host cells, such as camostat, cannot be screened by our binding assay. This is also the limitation of our assay.
Funding
This research was funded by the following grants: RayBiotech innovative research fund, Guangzhou Innovation Leadership Team (CXLJTD-201602), Pearl River S&T Nova Program of Guangzhou (201806010035, 201806010032), Guangdong Province Key Technologies R&D Program for “Precision Medicine and Stem Cells” (2019B020231002, 2019B020227004), National Science and Technology Major Project of China (No.2018ZX10732-401-003-012), the Industry-University-Research Collaborative Innovation Special Project of Guangzhou (201802030001). Guangzhou Basic and Applied Basic Research Project (202002030242), Basic Research Plan of Guangzhou People's Livelihood Science and Technology Project (202002020021) and Guangzhou key medical discipline construction project fund, the National Key R&D Program of China (2018YFC1106300, 2017YFC1105000), the Joint Funds of the National Natural Science Foundation of China (No. U1501245), the National Natural Science Foundation of China (No.51672088).
Availability of data and materials
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author contributions
Study Design and Conceptualization: RP.H.; Methodology: S.L.; S.Z; H.T.; C.G; and T.D; Experiments: S.Z.; C.G; T.D.; S.L.; H.T.; X.Y.; T.C.; and K.M.; Data analysis: S.Z.; T.D.; C.G; S.L.; H.T.; V. J.; and RP.H.; Statistical data analysis: J.L.; S.Z.; and C.G; Writing: T.D.; S.Z.; S.L.; C.G.; and RP.H.; Review and Editing: RP.H. All authors have read and agreed to the published version of the manuscript.
Institutional review board statement
This study was approved by IRB (ID# 8291-BZhang).
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Declaration of Competing Interest
Shuhong Luo, Tuhin Das, Shuangzhe Zhang, Hao Tang, Xinyi Yao, Tarina Cho, Jingqiao Lu, Kino Maravillas, Valerie Jones, and Ruo-Pan Huang are employees of RayBiotech and have a financial stake in RayBiotech.
Acknowledgments
Authors acknowledge the Dr. Mehul Suthar's laboratory at the Emory University Medical Center, Atlanta, GA., for performing FRNT assay and Dr. Rebecca Keall for English editing.
References
- Akhtar M.J. COVID19 inhibitors: a prospective therapeutics. Bioorg. Chem. 2020;101 doi: 10.1016/j.bioorg.2020.104027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali F., Kasry A., Amin M. The new SARS-CoV-2 strain shows a stronger binding affinity to ACE2 due to N501Y mutant. Med. Drug Discov. 2021;10 doi: 10.1016/j.medidd.2021.100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berber B., Doluca O. A comprehensive drug repurposing study for COVID19 treatment: novel putative dihydroorotate dehydrogenase inhibitors show association to serotonin-dopamine receptors. Brief. Bioinform. 2021;22(2):1023–1037. doi: 10.1093/bib/bbaa379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantuti-Castelvetri L., et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370(6518) doi: 10.1126/science.abd2985. p. 856-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan L., et al. The SARS-CoV-2 spike glycoprotein biosynthesis, structure, function, and antigenicity: implications for the design of spike-based vaccine immunogens. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.576622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durdagi S. Virtual drug repurposing study against SARS-CoV-2 TMPRSS2 target. Turk. J. Biol. 2020;44(3) doi: 10.3906/biy-2005-112. p. 185-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalhor H., et al. Repurposing of the approved small molecule drugs in order to inhibit SARS-CoV-2 S protein and human ACE2 interaction through virtual screening approaches. J. Biomol. Struct. Dyn. 2020;40 (3):1299–1315. doi: 10.1080/07391102.2020.1824816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A., et al. Higher infectivity of the SARS-CoV-2 new variants is associated with K417N/T, E484K, and N501Y mutants: an insight from structural data. J. Cell. Physiol. 2021;236 (10):7045–7057. doi: 10.1002/jcp.30367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Schaffer F., de Marco A. Coronavirus disease 2019 and the revival of passive immunization: antibody therapy for inhibiting severe acute respiratory syndrome coronavirus 2 and preventing host cell infection: IUPHAR review 31. Br. J. Pharmacol. 2021;178(17):3359–3372. doi: 10.1111/bph.15359. [DOI] [PubMed] [Google Scholar]
- Mittal A., et al. COVID-19 pandemic: insights into structure, function, and hACE2 receptor recognition by SARS-CoV-2. PLoS Pathog. 2020;16(8) doi: 10.1371/journal.ppat.1008762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie J., et al. Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2. Emerg. Microbes. Infect. 2020;9(1):680–686. doi: 10.1080/22221751.2020.1743767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthar M.S., et al. Rapid generation of neutralizing antibody responses in COVID-19 patients. Cell Rep. Med. 2020;1(3) doi: 10.1101/2020.05.03.20084442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usha T., et al. Drug repurposing approaches: existing leads for novel threats and drug targets. Curr. Protein Pept. Sci. 2021;22(3):251–271. doi: 10.2174/1389203721666200921152853. [DOI] [PubMed] [Google Scholar]
- Vanderheiden A., et al. Development of a rapid focus reduction neutralization test assay for measuring SARS-CoV-2 neutralizing antibodies. Curr. Protoc. Immunol. 2020;131(1):e116. doi: 10.1002/cpim.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., et al. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2) doi: 10.1016/j.cell.2020.02.058. p. 281-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitt M.A. Generation of VSV pseudotypes using recombinant DeltaG-VSV for studies on virus entry, identification of entry inhibitors, and immune responses to vaccines. J. Virol. Methods. 2010;169(2):365–374. doi: 10.1016/j.jviromet.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., et al. bioRxiv: the Preprint Server for Biology. 2021. Neutralization of N501Y mutant SARS-CoV-2 by BNT162b2 vaccine-elicited sera. [Google Scholar]
- Xiong H.L., et al. Robust neutralization assay based on SARS-CoV-2 S-protein-bearing vesicular stomatitis virus (VSV) pseudovirus and ACE2-overexpressing BHK21 cells. Emerg. Microbes. Infect. 2020;9(1):2105–2113. doi: 10.1080/22221751.2020.1815589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiu S., et al. Inhibitors of SARS-CoV-2 entry: current and future opportunities. J. Med. Chem. 2020;63(21):12256–12274. doi: 10.1021/acs.jmedchem.0c00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D., et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell. 2021;184(9):2348–2361. doi: 10.1016/j.cell.2021.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.