Abstract
Background
Multisystem inflammatory syndrome in children (MIS-C) is a hyperinflammatory condition associated with antecedent SARS-CoV-2 infection. In the USA, reporting of MIS-C after vaccination is required under COVID-19 vaccine emergency use authorisations. We aimed to investigate reports of individuals aged 12–20 years with MIS-C after COVID-19 vaccination reported to passive surveillance systems or through clinician outreach to the US Centers for Disease Control and Prevention (CDC).
Methods
In this surveillance activity, we investigated potential cases of MIS-C after COVID-19 vaccination reported to CDC's MIS-C national surveillance system, the Vaccine Adverse Event Reporting System (co-administered by CDC and the US Food and Drug Administration), and CDC's Clinical Immunization Safety Assessment Project. A multidisciplinary team adjudicated cases by use of the CDC MIS-C definition. Any positive SARS-CoV-2 serology test satisfied case criteria; although anti-nucleocapsid antibodies indicate previous SARS-CoV-2 infection, anti-spike protein antibodies indicate either past or recent infection or COVID-19 vaccination. We describe the demographic and clinical features of cases, stratified by laboratory evidence of SARS-CoV-2 infection. To calculate the reporting rate of MIS-C, we divided the count of all individuals meeting the MIS-C case definition, and of those without evidence of SARS-CoV-2 infection, by the number of individuals aged 12–20 years in the USA who received one or more COVID-19 vaccine doses up to Aug 31, 2021, obtained from CDC national vaccine surveillance data.
Findings
Using surveillance results from Dec 14, 2020, to Aug 31, 2021, we identified 21 individuals with MIS-C after COVID-19 vaccination. Of these 21 individuals, median age was 16 years (range 12–20); 13 (62%) were male and eight (38%) were female. All 21 were hospitalised: 12 (57%) were admitted to an intensive care unit and all were discharged home. 15 (71%) of 21 individuals had laboratory evidence of past or recent SARS-CoV-2 infection, and six (29%) did not. As of Aug 31, 2021, 21 335 331 individuals aged 12–20 years had received one or more doses of a COVID-19 vaccine, making the overall reporting rate for MIS-C after vaccination 1·0 case per million individuals receiving one or more doses in this age group. The reporting rate in only those without evidence of SARS-CoV-2 infection was 0·3 cases per million vaccinated individuals.
Interpretation
Here, we describe a small number of individuals with MIS-C who had received one or more doses of a COVID-19 vaccine before illness onset; the contribution of vaccination to these illnesses is unknown. Our findings suggest that MIS-C after COVID-19 vaccination is rare. Continued reporting of potential cases and surveillance for MIS-C illnesses after COVID-19 vaccination is warranted.
Funding
US Centers for Disease Control and Prevention.
Introduction
Multisystem inflammatory syndrome in children (MIS-C), also known as paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2, is a rare but serious complication of SARS-CoV-2 infection in children and adolescents that generally occurs 2–6 weeks after SARS-CoV-2 infection.1 MIS-C, first recognised in April, 2020, is characterised by fever, systemic inflammation, and multisystem organ involvement.1, 2, 3, 4 From May 14, 2020, to Nov 30, 2021, 5973 cases were reported to the MIS-C national surveillance system of the US Centers for Disease Control and Prevention (CDC).5 The pathogenesis of MIS-C is hypothesised to involve a dysregulated immune response to SARS-CoV-2 infection, and host genetics might alter susceptibility to developing MIS-C.6, 7, 8, 9
On Dec 11, 2020, the US Food and Drug Administration (FDA) issued an emergency use authorisation for use of the mRNA COVID-19 vaccine BNT162b2 (tozinameran; Pfizer–BioNTech) in individuals aged 16 years or older, and on May 10, 2021, expanded the authorisation to include individuals aged 12–15 years.10 The CDC and the FDA included MIS-C on a list of adverse events of special interest for COVID-19 vaccine safety monitoring after emergency use authorisation of COVID-19 vaccines, because of its known association with SARS-CoV-2 infection.11, 12 All COVID-19 vaccines currently authorised for use in the USA require reporting of this condition after COVID-19 vaccination.10 International vaccine and pharmacovigilance experts have also supported the need for close monitoring of MIS-C after COVID-19 vaccination.13, 14
Research in context.
Evidence before this study
We searched PubMed for articles published up to Jan 17, 2022, using the search terms “multisystem inflammatory syndrome in children”, “MIS-C”, “MISC”, “multisystem inflammatory syndrome in adults”, “MIS-A”, “MISA”, “paediatric inflammatory multisystem syndrome”, and “PIMS-TS” each with any COVID-19 vaccine type, without age or language restrictions. We identified reports of eight individuals aged 12–20 years described in detail in the literature who developed MIS-C after COVID-19 vaccination, excluding aggregate counts from larger analyses assessing the effect of vaccination on preventing MIS-C.
Added value of this study
We conducted integrated surveillance for MIS-C in the USA after COVID-19 vaccination using two passive surveillance systems, the US Centers for Disease Control and Prevention (CDC) MIS-C national surveillance and the Vaccine Adverse Event Reporting System (co-administered by the CDC and the US Food and Drug Administration), as well as clinician or health department outreach to the CDC, including through the Clinical Immunization Safety Assessment Project consultations. We investigated 47 reports of potential MIS-C occurring from Dec 14, 2020, to Aug 31, 2021, in individuals aged 12─20 years any time after receipt of COVID-19 vaccine, and identified 21 who met the CDC MIS-C case definition. Most had laboratory evidence of past or recent SARS-CoV-2 infection. Although no direct comparator background rate exists, our overall reporting rate of MIS-C after one or more doses of a COVID-19 vaccine—1·0 case per million individuals—is substantially lower than the previously published incidence of MIS-C among unvaccinated individuals in this age group in the USA who had SARS-CoV-2 infection (approximately 200 cases per million SARS-CoV-2 infections).
Implications of all the available evidence
During the first 9 months of the COVID-19 vaccination programme in the USA, more than 21 million individuals aged 12–20 years received one or more doses of a COVID-19 vaccine as of Aug 31, 2021. This case series describes MIS-C with onset after vaccination in 21 individuals, most of whom also had evidence of SARS-CoV-2 infection. Although our surveillance has limitations, our findings suggest that MIS-C as identified in this report after COVID-19 vaccination is rare. In evaluating individuals with a clinical presentation consistent with MIS-C after COVID-19 vaccination, it is important to consider alternative diagnoses and to determine if previous SARS-CoV-2 infection has occurred. In this regard, anti-nucleocapsid antibody testing, ideally from a serum sample obtained before administration of intravenous immunoglobulin, might be helpful. Continued surveillance for MIS-C illness after COVID-19 vaccination is warranted, especially as paediatric COVID-19 vaccination is authorised and recommended for younger children who comprise the highest proportion of MIS-C cases after SARS-Cov-2 infection.
Surveillance for MIS-C after COVID-19 vaccination is challenging because MIS-C in general is a difficult diagnosis to make as it has no specific biomarkers and might resemble other disease processes, including acute COVID-19 infection, Kawasaki disease, and toxic shock syndrome.1, 2, 3 Additionally, with wide circulation of SARS-CoV-2 occurring concurrently with administration of millions of COVID-19 vaccine doses, some cases of MIS-C caused by SARS-CoV-2 infections acquired before full vaccination are expected to occur post-vaccination, and will appear to be temporally associated with the vaccine. Furthermore, after full vaccination, some MIS-C cases caused by SARS-CoV-2 infection might occur if protection against infection is incomplete.
Recognising these challenges, we conducted integrated surveillance for MIS-C after COVID-19 vaccination using two passive surveillance systems: CDC's MIS-C national surveillance system and the Vaccine Adverse Event Reporting System (VAERS), as well as clinician or health department outreach to CDC, including through Clinical Immunization Safety Assessment (CISA) Project consultations.5, 15, 16, 17 We investigated reports of potential MIS-C in individuals aged 12–20 years who had previously received a COVID-19 vaccine during the initial months of the US COVID-19 vaccination programme, a period when SARS-CoV-2 circulation was widespread. We aimed to describe the demographic and clinical features of MIS-C after COVID-19 vaccination, including information on past SARS-CoV-2 infection and COVID-19 vaccination.
Methods
Investigation design and case definition
We identified potential MIS-C cases occurring any time after COVID-19 vaccination in individuals aged 12–20 years at time of MIS-C illness onset through CDC's MIS-C national surveillance system, VAERS, and clinician or health department outreach to CDC and the CISA Project.5, 15, 16, 17 We investigated reports to determine if the illnesses met the CDC MIS-C case definition. This definition requires fever, hospitalisation with an illness with multisystem organ involvement, laboratory evidence of inflammation, and one of the following: a positive SARS-CoV-2 RT-PCR, viral antigen, or serology test or recent exposure to a confirmed COVID-19 case (panel ).4 This definition was published before COVID-19 vaccination authorisation. Although anti-nucleocapsid antibodies are indicative of past or recent SARS-CoV-2 infection, anti-spike protein antibodies can be induced either by SARS-CoV-2 infection or by COVID-19 vaccination (including the three vaccines authorised in the USA, BNT162b2, mRNA-1273 [elasomeran; Moderna], and Ad26.COV2.S [Janssen]).10 A positive result for anti-spike or anti-nucleocapsid antibodies can be used to satisfy the SARS-CoV-2 test criterion of the CDC case definition.
Panel. US Centers for Disease Control and Prevention case definition for multisystem inflammatory syndrome in children.
Must meet all the following clinical and laboratory criteria:
-
•
Age younger than 21 years with subjective or objective (>38·0 °C) fever for 24 h or longer
-
•
Clinically severe illness requiring hospitalisation
-
•
Multisystem (two or more) organ system involvement
-
•Cardiac: includes elevated troponin, elevated B-type natriuretic peptide or N-terminal pro hormone BNP, arrythmia, coronary artery aneurysm, cardiac dysfunction, or shock
-
•Renal: includes acute kidney injury or renal failure
-
•Respiratory: includes pneumonia, acute respiratory distress syndrome, or pleural effusion
-
•Haematological: includes elevated D-dimer, thrombophilia, or thrombocytopenia
-
•Gastrointestinal: includes elevated bilirubin, elevated liver enzymes, or diarrhoea
-
•Dermatological: includes rash or mucocutaneous lesions
-
•Neurological: includes cerebrovascular accident, aseptic meningitis encephalopathy, or headache
-
•
-
•
No alternative plausible diagnosis
-
•
Laboratory evidence of inflammation: elevated C-reactive protein, erythrocyte sedimentation rate, fibrinogen, procalcitonin, D-dimer, ferritin, lactic acid dehydrogenase, interleukin 6, or neutrophils; or reduced lymphocytes or albumin
-
•
Current or recent positive SARS-CoV-2 RT-PCR*, antigen, or serology test; or exposure to a suspected or confirmed COVID-19 case within the 4 weeks before onset of symptoms†
This activity was determined by the CDC to meet criteria for public health surveillance as defined in Title 45 of the Code of Federal Regulations, part 46.102(l)(2), and thus, no institutional review board approval or informed consent was required.
Surveillance systems
CDC's national MIS-C surveillance is a passive reporting system in which health departments voluntarily report cases of MIS-C; the collection of COVID-19 vaccination status began on May 21, 2021.5 We queried the national MIS-C surveillance system twice per week for individuals with MIS-C illness onset date occurring after their vaccination date. We also reviewed reports weekly of individuals with MIS-C onset after COVID-19 vaccination made to VAERS, a passive national surveillance system for vaccine adverse events jointly managed by CDC and FDA, which receives spontaneous reports from health-care providers, health departments, vaccine manufacturers, and the public.16, 17 We searched VAERS weekly for reports with coding or free-text mention of possible multisystem inflammation or MIS-C (appendix p 2). Additionally, clinicians at the CDC and FDA reviewing selected VAERS reports as part of COVID-19 vaccine safety referred reports of potential MIS-C to our multidisciplinary team for further review. We also received notification of potential cases when a provider contacted the MIS-C national surveillance team or requested a CISA consultation.15 We encouraged reporting to VAERS for cases first detected by MIS-C national surveillance or through outreach to the CDC. We did not specify a minimum or maximum time interval from COVID-19 vaccination to illness onset in any system searches.
Procedures
A multidisciplinary team consisting of clinical and surveillance staff from the CDC and FDA and investigators from CDC's CISA Project adjudicated cases together at least twice per month. CDC physicians reviewed medical records and presented case summaries to the team. Some potential cases were also discussed with the treating clinicians and health department officials when additional information was needed for adjudication. The investigation team sometimes provided suggestions for laboratory testing; however, clinical evaluation of alternative diagnoses was at the discretion of the treating clinicians. Individuals for whom the investigation team considered myocarditis as a plausible alternative diagnosis on the basis of clinical judgment received additional review with CISA cardiologists to differentiate between myocarditis and cardiac manifestations of MIS-C.18
Individuals with potential MIS-C after vaccination were classified into two groups: those meeting the CDC MIS-C case definition, and those not meeting the CDC MIS-C case definition. When adjudicating cases, we did not use the criterion of exposure to a COVID-19 case within 4 weeks before illness onset (panel). Individuals with MIS-C were further stratified by laboratory evidence of past or recent SARS-CoV-2 infection. Laboratory evidence of infection was defined as any positive SARS-CoV-2 nucleic acid amplification test (NAAT), including RT-PCR, or viral antigen test before or during MIS-C illness evaluation, or a positive anti-nucleocapsid antibody test during MIS-C illness evaluation. Individuals were classified as having no laboratory evidence of SARS-CoV-2 infection if they satisfied all of the following: no known history of a positive SARS-CoV-2 test before MIS-C illness onset; a negative SARS-CoV-2 NAAT or viral antigen test during MIS-C illness evaluation; and a negative anti-nucleocapsid antibody test during MIS-C illness evaluation. Individuals who met these criteria and tested positive for anti-spike antibodies were also considered to have no laboratory evidence of SARS-CoV-2 infection because anti-spike antibodies were presumed to be vaccine induced. All potential cases were also assessed by use of the case definition of the Brighton Collaboration for MIS-C cases; this definition differs from that of the CDC in that it uses a tiered approach to diagnostic certainty and uniquely includes COVID-19 vaccination status as a criterion.13
We collected demographic and clinical features of individuals with MIS-C from records provided by reporting providers and hospitals. We defined clinical phenotypes using CDC MIS-C organ system involvement criteria (panel). We stratified temporal elements such as time from previous infection and time from most recent COVID-19 vaccine dose to illness onset by laboratory evidence of SARS-CoV-2 infection and number of vaccine doses. We described the same characteristics for individuals with illness not meeting the case definition because of the absence of a positive SARS-CoV-2 test (eg, negative NAAT, negative anti-nucleocapsid antibody test, and no anti-spike antibody test obtained). These individuals would presumably have met the case definition had an anti-spike antibody test been obtained because of the presence of vaccine-induced anti-spike antibodies. We summarised illness features and possible alternative diagnoses for individuals not meeting the MIS-C case definition because of the presence of a plausible alternative diagnosis.
Statistical analysis
This is a descriptive investigation; to protect privacy, we present individuals by age group, and present demographic and clinical details in aggregate. As described elsewhere in detail,19 the calculation of confidence intervals is generally not advised for VAERS data, and thus was not done for our reporting rates.
We obtained the number of individuals aged 12–20 years who had received one or more doses up to Aug 31, 2021, from CDC national vaccine surveillance data.20 We divided the count of all individuals meeting the MIS-C case definition, and of those without evidence of SARS-CoV-2 infection, by the number of individuals aged 12–20 years who received one or more vaccine doses to calculate the reporting rate of MIS-C for this investigation. We used a Research Electronic Data Capture (REDCap 12.0.8) database for data collection and did analyses using Microsoft Excel 365 and R, version 4.0.2.
Role of the funding source
The funder led data collection, data analysis, data interpretation, writing, and submission of the manuscript.
Results
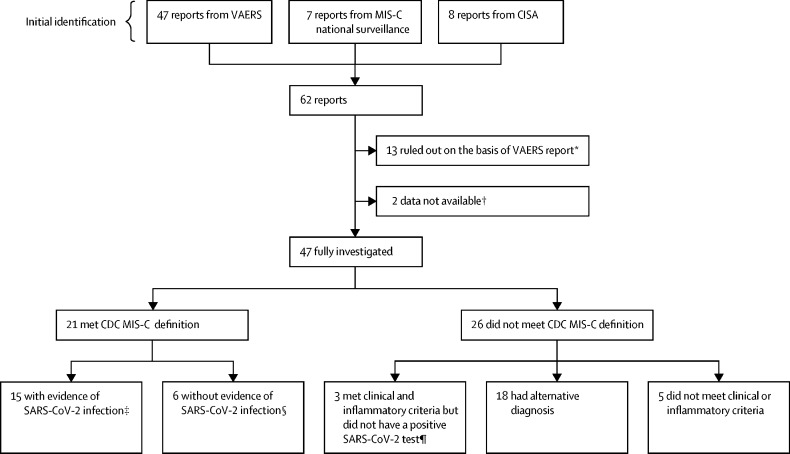
From Dec 14, 2020, to Aug 31, 2021, after removing duplicates, we identified 62 reports of individuals with potential MIS-C who had received a COVID-19 vaccine (figure ). We excluded 13 reports on the basis of information in the VAERS report alone, records were unavailable to fully investigate two reports, and 47 reports were fully investigated. Of these 47 reports, 21 (45%) described illness meeting the CDC MIS-C case definition, and 26 (55%) did not. All 21 individuals with MIS-C (median age 16 years, IQR 13–18, range 12–20) had evaluations for alternate diagnoses, but no alternative diagnoses were deemed as plausible as MIS-C. No individuals had a previous history of MIS-C. Of the 26 individuals with illness not meeting the MIS-C case definition, three (12%) met MIS-C clinical and inflammatory criteria and did not have an alternative diagnosis, but they did not meet the case definition because of absence of a positive SARS-CoV-2 test (figure, appendix pp 3–4). An additional 18 (69%) individuals did not meet case definition because they had an alternative diagnosis that was more likely, and five (19%) did not meet other clinical or inflammatory criteria of the case definition (appendix p 5).
Figure.
Investigation of potential MIS-C in individuals who had received a COVID-19 vaccine
CDC=US Centers for Disease Control and Prevention. CISA=Clinical Immunization Safety Assessment. MIS-C=multisystem inflammatory syndrome in children. NAAT=nucleic acid amplification test. VAERS=Vaccine Adverse Event Reporting System. *If the individuals were the incorrect age or if MIS-C could be clearly ruled out on the basis of the VAERS report. †Two individuals were reported to MIS-C national surveillance but not to VAERS, and medical records were not obtained; both had reported MIS-C after one dose of COVID-19 vaccine, and both were positive for SARS-CoV-2 NAAT and IgG, but no further details were available; both clinically improved and were discharged home. ‡Defined as an illness meeting the CDC MIS-C clinical and inflammatory criteria with a positive NAAT or viral antigen test during or before MIS-C illness evaluation, or a positive anti-nucleocapsid antibody test during MIS-C illness evaluation. §Defined as an illness meeting the CDC MIS-C clinical and inflammatory criteria with negative NAAT and anti-nucleocapsid antibody tests and a positive anti-spike antibody test during MIS-C illness evaluation, with no known history of a positive SARS-CoV-2 test before MIS-C illness onset. ¶Three individuals with an illness after vaccination meeting the CDC MIS-C clinical and inflammatory criteria, a negative anti-nucleocapsid antibody test and negative NAAT test during MIS-C evaluation, and anti-spike antibody test not obtained.
As of Aug 31, 2021, 21 335 331 individuals aged 12–20 years had received one or more doses of a COVID-19 vaccine in the USA: 18 030 614 received BNT162b2, 2 603 078 received mRNA-1273, 697 281 received Ad26.COV2.S, and 4358 did not have a manufacturer recorded.20
Of the 21 individuals with MIS-C, 15 (71%) had evidence of SARS-CoV-2 infection (Table 1, Table 2 ). Among these 15 individuals, ten (67%) had a positive NAAT or viral antigen test and five (33%) had a positive anti-nucleocapsid antibody test and negative NAAT during MIS-C illness, with no known positive NAAT or viral antigen test before MIS-C (table 2). Of the ten individuals with a positive NAAT or antigen test, four had a positive test during MIS-C illness evaluation, one (case 11) both during and 111 days before MIS-C illness, and five before MIS-C illness alone. Of the five individuals who had a positive NAAT or viral antigen test before MIS-C illness alone, four had a positive anti-nucleocapsid antibody test during MIS-C illness (cases 4, 8, 9, and 13) and one had a negative test (case 15; table 3 ).
Table 1.
Demographic characteristics and comorbidities for 21 individuals with MIS-C who had received a COVID-19 vaccine
| Total (n=21) | Laboratory evidence of SARS-CoV-2 infection (n=15) | No laboratory evidence of SARS-CoV-2 infection (n=6) | |
|---|---|---|---|
| Age at time of MIS-C onset, years | |||
| 12–15 | 10 (48%) | 7 (47%) | 3 (50%) |
| 16–17 | 5 (24%) | 5 (33%) | 0 (0%) |
| 18–20 | 6 (29%) | 3 (20%) | 3 (50%) |
| Sex | |||
| Female | 8 (38%) | 5 (33%) | 3 (50%) |
| Male | 13 (62%) | 10 (67%) | 3 (50%) |
| Race or ethnicity | |||
| White, non-Hispanic | 8 (38%) | 4 (27%) | 4 (67%) |
| Hispanic | 5 (24%) | 4 (27%) | 1 (17%) |
| Black, non-Hispanic | 3 (14%) | 3 (20%) | 0 (0%) |
| Asian or Pacific Islander, non-Hispanic | 3 (14%) | 2 (13%) | 1 (17%) |
| Other race, non-Hispanic* | 2 (10%) | 2 (13%) | 0 (0%) |
| Comorbidities | |||
| Asthma | 7 (33%) | 6 (40%) | 1 (17%) |
| Obesity | 3 (14%) | 2 (13%) | 1 (17%) |
| Seizure disorder | 1 (5%) | 0 (0%) | 1 (17%) |
Data are n (%). MIS-C=multisystem inflammatory syndrome in children.
This category includes one person with multiple races and one with unknown race.
Table 2.
SARS-CoV-2 laboratory testing in 21 individuals with MIS-C who had received a COVID-19 vaccine
| Total | Onset after first dose of COVID-19 vaccine | Onset after second dose of COVID-19 vaccine | ||
|---|---|---|---|---|
| MIS-C | 21 | 11 (52%) | 10 (48%) | |
| With evidence of SARS-CoV-2 infection | 15 | 10 (67%) | 5 (33%) | |
| Positive NAAT (past or recent)* | 10 | 5 (50%) | 5 (50%) | |
| Negative NAAT and positive anti-nucleocapsid antibody test | 5 | 4 (80%) | 1 (20%) | |
| Without evidence of SARS-CoV-2 infection† | 6 | 1 (17%) | 5 (83%) | |
Data are n or n (%). MIS-C=multisystem inflammatory syndrome in children. NAAT=nucleic acid amplification test.
Includes five individuals with positive SARS-CoV-2 NAAT before MIS-C illness, four with positive SARS-CoV-2 NAAT during MIS-C illness, and one with a positive SARS-CoV-2 NAAT before and during MIS-C illness.
Negative NAAT, negative anti-nucleocapsid antibody test, and positive anti-spike antibody test during MIS-C illness evaluation; these individuals did not have any reported positive NAAT results before MIS-C illness.
Table 3.
SARS-CoV-2 testing and temporal features of 21 individuals with MIS-C who had received a COVID-19 vaccine
| Laboratory evidence of SARS-CoV-2 infection* |
SARS-CoV-2 test results during evaluation of MIS-C illness |
Number of days from previous positive NAAT or antigen test to MIS-C illness† | Number of COVID-19 vaccine doses received before MIS-C onset | Number of days from first vaccine dose to MIS-C onset‡ | Number of days from second vaccine dose to MIS-C onset‡ | |||
|---|---|---|---|---|---|---|---|---|
| NAAT | Anti-spike antibody§ | Anti-nucleocapsid antibody | ||||||
| 1 | Yes | Positive | ND | Positive | NA | 1 | 7 | NA |
| 2 | Yes | Negative | ND | Positive | NA | 1 | 1 | NA |
| 3 | Yes | Positive | ND | Positive | NA | 1 | 10 | NA |
| 4 | Yes | Negative | ND | Positive | 39 (6 weeks) | 1 | 1 | NA |
| 5 | Yes | Negative | ND | Positive | NA | 1 | 1 | NA |
| 6 | Yes | Negative | ND | Positive | NA | 1 | 30 | NA |
| 7 | Yes | Negative | ND | Positive | NA | 1 | 1 | NA |
| 8 | Yes | Negative | ND | Positive | 42 (6 weeks) | 1 | 19 | NA |
| 9 | Yes | Negative | ND | Positive | 105 (15 weeks) | 1 | 8 | NA |
| 10 | Yes | Negative | ND | Positive | NA | 1 | 20 | NA |
| 11 | Yes | Positive | ND | ND | 111 (16 weeks) | 2 | 24 | 3 |
| 12 | Yes | Positive | Positive | ND | NA | 2 | 25 | 4 |
| 13 | Yes | Negative | Positive | Positive | 191 (27 weeks) | 2 | 39 | 5 |
| 14 | Yes | Positive | ND | ND | NA | 2 | 42 | 21 |
| 15 | Yes | Negative | Positive | Negative | 238 (34 weeks) | 2 | 69 | 48 |
| 16 | No | Negative | Positive | Negative | NA | 1 | 5 | NA |
| 17 | No | Negative | Positive | Negative | NA | 2 | 42 | 21 |
| 18 | No | Negative | Positive | Negative | NA | 2 | 35 | 14 |
| 19 | No | Negative | Positive | Negative | NA | 2 | 104 | 84 |
| 20 | No | Negative | Positive | Negative | NA | 2 | 26 | 5 |
| 21 | No | Negative¶ | Positive | Negative | NA | 2 | 21 | 0 |
MIS-C=multisystem inflammatory syndrome in children. NA=not applicable (time interval not available because date of preceding SARS-CoV-2 infection was unknown). NAAT=nucleic acid amplification test. ND=not done.
Includes previous laboratory evidence of SARS-CoV-2 infection (ie, history of previous positive SARS-CoV-2 NAAT or antigen test) or laboratory evidence of SARS-CoV-2 infection during MIS-C illness evaluation (ie, positive SARS-CoV-2 NAAT, antigen test, or anti-nucleocapsid antibody test); anti-spike antibody assay results are not included in this variable.
When the month of previous COVID-19 infection was known but not the day, the 15th of the month was used to calculate the time interval from previous infection to MIS-C illness onset; Individual 1 had household exposure to SARS-CoV-2 approximately 5 weeks and 9 weeks before MIS-C onset; Individual 2 had household exposure approximately 26 weeks before MIS-C onset; Individual 10 had MIS-C onset after the first vaccine dose but was not hospitalised until after the second dose.
For individuals who received two vaccine doses, median time from first dose to MIS-C illness onset was 39 days (IQR 25–42, range 24–69) for those with laboratory evidence of SARS-CoV-2 infection and 35 days (26–42, 21–104) for those without.
Tests reported as not done were tests reported as such in the medical notes or not present as per our review of available medical records.
Individual 21 had a SARS-CoV-2 antigen test done during MIS-C illness instead of NAAT.
Of 15 individuals with evidence of SARS-CoV-2 infection, seven (47%) were aged 12–15 years, five (33%) aged 16–17 years, and three (20%) aged 18–20 years (table 1); ten (67%) were male and five (33%) were female; and four (27%) were Hispanic, four (27%) were White non-Hispanic, and three (20%) were Black non-Hispanic. The following organ systems were most commonly involved during MIS-C illness: 14 (93%) gastrointestinal, 13 (87%) haematological, and 13 (87%) cardiac (appendix p 7). Upon assessment with the Brighton case definition, 12 (80%) individuals were considered definitive or probable MIS-C cases and three (20%) would not be considered cases (appendix p 7).
All 15 individuals with laboratory evidence of SARS-CoV-2 infection had received BNT162b2 (the only COVID-19 vaccine authorised in the USA for use in individuals younger than 18 years during our surveillance), with ten (66%) receiving one dose and five (33%) receiving two doses before MIS-C illness onset (table 2). Median time from most recent vaccine dose to MIS-C onset was 8 days (IQR 1–8, range 1–30) for those who had received only one dose, and 5 days (4–21, 3–48) for those who had received two doses (table 3, appendix p 9).
During their MIS-C hospitalisation, 13 (87%) individuals were treated with intravenous immunoglobulin, 12 (80%) with systemic steroids, and five (33%) with an immune modulator (table 4 ). Eight (53%) were admitted to an intensive care unit. Median length of hospital stay was 7 days (IQR 4–9, range 2–20). All 15 individuals clinically improved and were discharged home.
Table 4.
Treatment and outcomes for 21 individuals with MIS-C who had received a COVID-19 vaccine
| Total (n=21) | Laboratory evidence of SARS-CoV-2 infection (n=15) | No laboratory evidence of SARS-CoV-2 infection (n=6) | ||
|---|---|---|---|---|
| Inpatient MIS-C treatment | ||||
| Intravenous immunoglobulin | 17 (81%) | 13 (87%) | 4 (67%) | |
| Systemic steroids | 16 (76%) | 12 (80%) | 4 (67%) | |
| Immune modulators | 5 (23%) | 5 (33%)* | 0 (0%) | |
| Remdesivir | 1 (5%) | 1 (7%)† | 0 (0%) | |
| None | 3 (14%) | 1 (7%) | 2 (33%) | |
| Admitted to intensive care unit | 12 (57%) | 8 (53%) | 4 (67%) | |
| Vasopressors | 8 (38%) | 6 (40%) | 2 (33%) | |
| Invasive mechanical ventilation | 3 (14%) | 2 (13%) | 1 (17%) | |
| Length of hospitalisation, days | 6 (4–9, 2–21) | 7 (4–9, 2–20) | 6 (5–7, 3–7) | |
| Discharged home | 21 (100%) | 15 (100%) | 6 (100%) | |
| Discharge MIS-C medications | ||||
| Systemic steroids | 15 (71%) | 11 (73%) | 4 (67%) | |
| Aspirin | 14 (67%) | 10 (67%) | 4 (67%) | |
| Enoxaparin | 2 (10%) | 2 (13%) | 0 (0%) | |
| Angiotensin-converting enzyme inhibitors | 2 (10%) | 2 (13%) | 0 (0%) | |
| Other‡ | 2 (10%) | 2 (13%) | 0 (0%) | |
| None | 4 (19%) | 2 (13%) | 2 (33%) | |
Data are n (%) or median (IQR, range). MIS-C=multisystem inflammatory syndrome in children. NAAT=nucleic acid amplification test.
Three individuals treated with anakinra, and two individuals treated with infliximab.
In addition, one individual (not counted here) with a positive SARS-CoV-2 NAAT during MIS-C illness received an unspecified antiviral medication.
Anakinra, furosemide, and metoprolol.
Of 21 individuals with MIS-C, six (29%) had positive anti-spike antibody test alone (Table 1, Table 2) and were classified as not having laboratory evidence of SARS-CoV-2 infection (figure). None of these individuals had a history of a positive SARS-CoV-2 test before MIS-C illness and all had negative SARS-CoV-2 NAAT and anti-nucleocapsid antibody test during evaluation of MIS-C illness (table 3). Of these six individuals, three (50%) were aged 12–15 years and three (50%) aged 18–20 years, three (50%) were male and three (50%) were female, and four (67%) were White non-Hispanic (table 1). These individuals presented with varied organ system involvement: six (100%) cardiac (including two with shock), and five (83%) haematological (appendix p 8). Applying the Brighton case definition, all were classified as definitive or probable cases (appendix p 8).
All six individuals had received BNT162b2; one (11%) received only one dose 5 days before MIS-C onset, and five (83%) received two doses before MIS-C illness onset (table 2). Median time from vaccination with the second dose to MIS-C onset was 14 days (IQR 5–21, range 0–84) for those who received two doses (table 3, appendix p 10). Four (67%) individuals were treated for MIS-C with intravenous immunoglobulin and four (67%) with systemic steroids (table 4). Median length of stay was 6 days (IQR 5–7, range 3–7). Four (67%) individuals were admitted to an intensive care unit, and all six clinically improved and were discharged home.
The reporting rate for all reported MIS-C cases in individuals who received vaccine was 21 in 21 335 331, or 1·0 case per million individuals aged 12–20 years who had received one or more doses of any COVID-19 vaccine. The reporting rate for MIS-C cases without laboratory evidence of SARS-CoV-2 infection was six in 21 335 331, or 0·3 cases per million individuals aged 12–20 years who had received one or more doses.
Discussion
As part of the USA's comprehensive efforts to monitor COVID-19 vaccine safety after authorisation, we investigated reported potential MIS-C cases among individuals in the USA aged 12–20 years who had received at least one dose of COVID-19 vaccine during a period of widespread SARS-CoV-2 circulation. In this case series, we described 21 individuals with illness meeting the CDC's MIS-C case definition. All received BNT162b2, consistent with the age eligibility of COVID-19 vaccines during the investigation period. Most cases had laboratory evidence of SARS-CoV-2 infection, although previous positive NAAT in three individuals occurred outside the typical timeframe for MIS-C (105 days, 191 days, and 238 days before illness onset; table 3), and one other had household exposure outside the typical period. In four others, no known exposure was available to inform the timing of infection that resulted in their anti-nucleocapsid antibody positivity. Overall, the reporting rate for MIS-C was 1·0 case per million vaccinated individuals aged 12–20 years, when including all cases meeting the case definition, regardless of timing of any previous SARS-CoV-2 infection. Although no direct comparator background rate exists, the reporting rate of illness meeting the MIS-C definition in individuals who had received a COVID-19 vaccine is substantially lower than the previously published incidence of MIS-C among unvaccinated individuals who had SARS-CoV-2 infection. Using a denominator of SARS-CoV-2 infections among unvaccinated individuals, a previous study estimated an adjusted incidence of MIS-C from April to June, 2020, of 224 per million SARS-CoV-2 infections (95% CI 160–312) in children aged 11–15 years and 164 per million (110–243) in those aged 16–20 years.21
The reporting rate for cases of MIS-C that occurred after receipt of COVID-19 vaccine and without evidence of SARS-CoV-2 infection was 0·3 cases per million vaccinated individuals aged 12–20 years. It has been hypothesised that a dysregulated immune response associated with SARS-CoV-2 infection might also be associated with exposure to a COVID-19 vaccine.8, 13, 22 As with the individuals in our investigation who had evidence of previous infection outside of the usual period for development of MIS-C, the contribution of vaccination, if any, to the illnesses in individuals without evidence of infection is unknown and cannot be determined with our surveillance data. It is possible that some of these six individuals had other unrecognised inflammatory conditions. Because the pre-pandemic background incidence of illnesses with unidentified diagnosis that would meet the clinical criteria of the MIS-C case definition is unknown, we cannot estimate how often such illnesses would be expected to occur temporally associated with vaccine by chance alone. Additionally, given the limitations of laboratory assays and detection sensitivities of each test, some of these six individuals might have been infected with SARS-CoV-2 in the recent past, and vaccination might be coincidental to the subsequent MIS-C illness. Children often have unrecognised SARS-CoV-2 infection associated with mild or absent symptoms.3 Individuals with mild or asymptomatic illness might be less likely to generate anti-nucleocapsid antibodies, and anti-nucleocapsid antibodies from a previous infection wane over time, particularly in those with mild infection.6, 23, 24 These limitations could lead to misclassification of SARS-CoV-2 infection status. In addition to the six individuals without evidence of SARS-CoV-2 infection, we identified three others who met clinical and inflammatory criteria and did not have evidence of SARS-CoV-2 infection but did not meet MIS-C case definition because an anti-spike antibody test was not obtained (although presumably would have been positive from vaccination).
Multisystem inflammatory syndrome in adults was not reported in clinical trials of COVID-19 vaccines used in the USA, and MIS-C was not observed in the 46 000 individuals aged 16 years or older who participated in safety clinical trials for BNT162b2.10, 25 Globally, MIS-C in individuals who had received a COVID-19 vaccine has been described in detail in the literature for eight individuals younger than 21 years,22, 26, 27, 28, 29, 30, 31 excluding aggregate counts from larger analyses assessing the effect of vaccination on preventing MIS-C.32, 33 From the USA, two reports included three cases that are also in our surveillance results,22, 26 and one report described a 14-year-old child with evidence of previous SARS-CoV-2 infection (positive anti-nucleocapsid antibody test) with MIS-C onset 2 months after a second dose with BNT162b2.31 From outside the USA, we found reports of two individuals without evidence of previous or recent SARS-CoV-2 infection and who tested negative for anti-nucleocapsid antibodies: from Denmark, a 17-year-old with MIS-C onset 5 days after dose two of BNT162b2;28 and from Turkey, a 12-year-old with onset 27 days after dose one of BNT162b2.27 Two other reports described one person each for whom SARS-CoV-2 infection status was unclear; NAAT or antigen tests were negative but anti-nucleocapsid antibody testing was not done or not described.29, 30
This investigation highlights the challenges of diagnosing MIS-C and importance of a thorough clinical evaluation. Although the CDC's MIS-C case definition can be met with any type of SARS-CoV-2 antibody test (anti-spike, anti-nucleocapsid, or undifferentiated), testing for anti-nucleocapsid antibodies in individuals with suspected MIS-C after COVID-19 vaccination, ideally from a serum sample obtained before administration of intravenous immunoglobulin, might be helpful in identifying those with antibodies induced by SARS-CoV-2 infection. However, as noted previously, antibody titres can wane over time. Conversely, as cumulatively higher rates of SARS-CoV-2 infection result in higher anti-nucleocapsid seroprevalence among children, without a test available to indicate how recently SARS-CoV-2 infection occurred, detection of anti-nucleocapsid antibodies might be coincidental and might not distinguish MIS-C from other clinically similar syndromes (eg, toxic shock syndrome). Therefore, a thorough clinical evaluation to elucidate alternative diagnoses is important, as many conditions can mimic MIS-C (appendix pp 5–6).
Our data are subject to additional limitations. The national MIS-C and VAERS surveillance platforms are both passive reporting systems and probably not all cases are reported, particularly since receipt of vaccine is not part of the MIS-C case definition; therefore, our calculated reporting rates are probably underestimated. Vaccinated individuals being evaluated who have negative SARS-CoV-2 NAAT and anti-nucleocapsid serology tests (and do not have an anti-spike antibody test done) might not be reported because they do not satisfy the CDC case definition and might not be suspected of having MIS-C. We identified three such individuals with our surveillance procedures and acknowledge that such cases would be incompletely captured. For practicality, we used a limited number of specific terms for our search strategy in the VAERS component of our surveillance. However, our VAERS text search strategy does not depend on reports listing a final diagnosis of MIS-C. Time from vaccine receipt to MIS-C onset might be underestimated for the subset that had shorter intervals because fever and headache are both common short-term reactogenicity events after COVID-19 vaccination and symptoms of MIS-C. Clinical and laboratory evaluation was not standardised across cases, and although cases were adjudicated by an interdisciplinary team, no definitive diagnostic test exists to confirm MIS-C.
In conclusion, using surveillance results from the first 9 months of the COVID-19 vaccination programme in the USA, a period when SARS-CoV-2 was widely circulating, we identified a small number of individuals aged 12─20 years with MIS-C after COVID-19 vaccination; most had laboratory evidence of past or recent SARS-CoV-2 infection. The surveillance has limitations, but our findings suggest that MIS-C without evidence of SARS-CoV-2 infection is rare after COVID-19 vaccination (reporting rate lower than 1 per million vaccinated individuals aged 12–20 years). In evaluating individuals with a MIS-C clinical presentation after COVID-19 vaccination, it is important to consider alternative diagnoses, and anti-nucleocapsid antibody testing might be helpful. Continued surveillance for MIS-C illness after COVID-19 vaccination is warranted, especially as paediatric COVID-19 vaccination is authorised for younger children, who comprise the highest proportion of MIS-C cases after SARS-CoV-2 infection. US providers are encouraged to report potential MIS-C cases after COVID-19 vaccination to VAERS.
Data sharing
Patient data from this public health investigation are not available to be shared publicly. Limited, deidentified VAERS data are publicly available at https://vaers.hhs.gov/data.html.
Acknowledgments
Acknowledgments
We thank Michael Melgar, Katherine Lindsey, Allison D Miller, Michael Wu, Laura D Zambrano, Frank Destefano, Tom Shimabukuro, Theresa Harrington, Narayan Nair, Elaine Miller, Adam Schiller, Brian Kit, Jeffrey Dendy, Allison Lale, and The Clinical Immunization Safety Assessment Project. We thank all local, state, and territorial health departments that contributed MIS-C reports to this investigation. We thank all health-care providers who made reports to VAERS and who were involved in the care of the patients described in this investigation. The CDC provided financial support for the CDC authors' salaries and project materials. This work was also supported by the CDC CISA Project contracts 200–2012–50430–0005 to Vanderbilt University Medical Center and 20,012–53661–0008 to Cincinnati Children's Hospital Medical Center. Other authors received salary support from their institutions. CDC funded and led data collection, data analysis, data interpretation, writing, and submission of the manuscript. ARY and APC were not precluded from accessing data in the investigation and they accept responsibility to submit for publication. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC or the FDA. Mention of a product or company name is for identification purposes only and does not constitute endorsement by the CDC or the FDA.
MIS-C Investigation Authorship Group
Oidda Museru, Leigh M Howard, Monica Parise, John J Openshaw, Chloe LeMarchand, Lauren E Finn, Moon Kim, Kiran V Raman, Kenneth K Komatsu, Bryce L Spiker, Cole P Burkholder, Sean M Lang, Jonathan H Soslow.
Contributors
ARY, MMC, AWT, KRB, and APC conceived of and designed the investigation. ARY, MMC, AT, and APC wrote the initial draft. ARY, AWT, and MMC produced figures and tables, had direct data access, and verified the underlying data reported in this manuscript. ARY, MMC, AWT, JMW, AYG, DWM, SK, PM, OM, MP, JRS, MBS, JJO, CL, LEF, MK, KVR, KKK, BLS, and CPB helped to procure and clean the data. ARY, MMC, AWT, KRB, JMW, AYG, DWM, SK, EDB, APC, MEO, EPS, KME, CBC, MAS, LMH, DT, SML, and JHS adjudicated cases. All authors edited the manuscript, provided feedback on the investigation, and approved the final manuscript.
Declaration of interests
Affiliations
CDC COVID-19 Response Team, Centers for Disease Control and Prevention, Atlanta, GA, USA (O Museru MSN, M Parise MD); Division of Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, TN, USA (L M Howard MD); California Department of Public Health, Sacramento, CA, USA (J J Openshaw MD, C LeMarchand MD); Los Angeles County Department of Public Health, Los Angeles, CA, USA (L E Finn MPH, M Kim MD); Arizona Department of Health Services, Phoenix, AZ, USA (K V Raman MD, K K Komatsu MPH); Michigan Department of Health and Human Services, Lansing, MI, USA (B L Spiker MPH, C P Burkholder MPH); Division of Cardiology, Department of Pediatrics, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, USA (S M Lang MD); Heart Institute, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, TN, USA (J H Soslow MD).
Footnotes
For this investigation, this criterion could be satisfied by any type of nucleic acid amplification test.
The exposure criterion was not used in this investigation.
Contributor Information
MIS-C Investigation Authorship Group:
Oidda Museru, Leigh M. Howard, Monica Parise, John J. Openshaw, Chloe LeMarchand, Lauren E. Finn, Moon Kim, Kiran V. Raman, Kenneth K. Komatsu, Bryce L. Spiker, Cole P. Burkholder, Sean M. Lang, and Jonathan H. Soslow
Supplementary Material
References
- 1.Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldstein LR, Tenforde MW, Friedman KG, et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325:1074–1087. doi: 10.1001/jama.2021.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Centers for Disease Control and Prevention Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19) 2020. https://emergency.cdc.gov/han/2020/han00432.asp
- 5.US Centers for Disease Control and Prevention Health department-reported cases of multisystem inflammatory syndrome in children (MIS-C) in the United States. 2021. https://covid.cdc.gov/covid-data-tracker/#mis-national-surveillance
- 6.Weisberg SP, Connors TJ, Zhu Y, et al. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat Immunol. 2021;22:25–31. doi: 10.1038/s41590-020-00826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rowley AH, Shulman ST, Arditi M. Immune pathogenesis of COVID-19-related multisystem inflammatory syndrome in children. J Clin Invest. 2020;130:5619–5621. doi: 10.1172/JCI143840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chou J, Platt CD, Habiballah S, et al. Mechanisms underlying genetic susceptibility to multisystem inflammatory syndrome in children (MIS-C) J Allergy Clin Immunol. 2021;148:732. doi: 10.1016/j.jaci.2021.06.024. 38.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sancho-Shimizu V, Brodin P, Cobat A, et al. SARS-CoV-2-related MIS-C: a key to the viral and genetic causes of Kawasaki disease? J Exp Med. 2021;218 doi: 10.1084/jem.20210446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US Food and Drug Administration COVID-19 vaccines. 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines
- 11.Gubernot D, Jazwa A, Niu M, et al. U.S. population-based background incidence rates of medical conditions for use in safety assessment of COVID-19 vaccines. Vaccine. 2021;39:3666–3677. doi: 10.1016/j.vaccine.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein N, Donahue J, Weintraub E. US Centers for Disease Control and Prevention; Atlanta, GA: 2021. Rapid Cycle Analysis (RCA) to monitor the safety of COVID-19 vaccines in near real-time within the Vaccine Safety Datalink. [Google Scholar]
- 13.Vogel TP, Top KA, Karatzios C, et al. Multisystem inflammatory syndrome in children and adults (MIS-C/A): case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2021;39:3037–3049. doi: 10.1016/j.vaccine.2021.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.European Medicines Agency Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) October 25–28, 2021. 2021. https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-25-28-october-2021
- 15.US Centers for Disease Control and Prevention Clinical Immunization Safety Assessment (CISA) project. 2020. https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/cisa/index.html
- 16.Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS) Vaccine. 2015;33:4398–4405. doi: 10.1016/j.vaccine.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.VAERS Team . US Centers for Disease Control and Prevention; Atlanta, GA: 2021. Vaccine Adverse Event Reporting System (VAERS) standard operating procedures for COVID-19. [Google Scholar]
- 18.Patel T, Kelleman M, West Z, et al. Comparison of MIS-C related myocarditis, classic viral myocarditis, and COVID-19 vaccine related myocarditis in children. medRxiv. 2021 doi: 10.1101/2021.10.05.21264581. published online Oct 7. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varricchio F, Iskander J, Destefano F, et al. Understanding vaccine safety information from the Vaccine Adverse Event Reporting System. Pediatr Infect Dis J. 2004;23:287–294. doi: 10.1097/00006454-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 20.US Centers for Disease Control and Prevention About COVID-19 vaccine delivered and administration data. 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/distributing/about-vaccine-data.html
- 21.Payne AB, Gilani Z, Godfred-Cato S, et al. Incidence of multisystem inflammatory syndrome in children among US persons infected with SARS-CoV-2. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.16420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salzman MB, Huang CW, O'Brien CM, Castillo RD. Multisystem inflammatory syndrome after SARS-CoV-2 infection and COVID-19 vaccination. Emerg Infect Dis. 2021;27:1944–1948. doi: 10.3201/eid2707.210594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibarrondo FJ, Fulcher JA, Goodman-Meza D, et al. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild COVID-19. N Engl J Med. 2020;383:1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersen LR, Sami S, Vuong N, et al. Lack of antibodies to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a large cohort of previously infected persons. Clin Infect Dis. 2021;73:e3066–e3073. doi: 10.1093/cid/ciaa1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belay ED, Godfred Cato S, Rao AK, et al. Multisystem inflammatory syndrome in adults after SARS-CoV-2 infection and COVID-19 vaccination. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab936. published online Nov 28. [DOI] [Google Scholar]
- 26.Poussaint TY, LaRovere KL, Newburger JW, et al. Multisystem inflammatory-like syndrome in a child following COVID-19 mRNA vaccination. Vaccines (Basel) 2022;10:43. doi: 10.3390/vaccines10010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yalcinkaya R, Oz FN, Polat M, et al. A case of multisystem inflammatory syndrome in a 12-year-old male after COVID-19 mRNA vaccine. Pediatr Infect Dis J. 2021 doi: 10.1097/INF.0000000000003432. published online Dec 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chai Q, Nygaard U, Schmidt RC, Zaremba T, Moller AM, Thorvig CM. Multisystem inflammatory syndrome in a male adolescent after his second Pfizer-BioNTech COVID-19 vaccine. Acta Paediatr. 2022;111:125–127. doi: 10.1111/apa.16141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abdelgalil AA, Saeedi FA. Multisystem inflammatory syndrome in a 12-year-old boy after mRNA-SARS-CoV-2 vaccination. Pediatr Infect Dis J. 2021 doi: 10.1097/INF.0000000000003442. published online Dec 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buchhorn R, Meyer C, Schulze-Forster K, Junker J, Heidecke H. Autoantibody release in children after corona virus mRNA vaccination: a risk factor of multisystem inflammatory syndrome? Vaccines (Basel) 2021;9 doi: 10.3390/vaccines9111353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeJong J, Sainato R, Forouhar M, Robinson D, Kunz A. Multisystem inflammatory syndrome in a previously vaccinated adolescent female with sickle cell disease. Pediatr Infect Dis J. 2021 doi: 10.1097/INF.0000000000003444. published online Dec 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levy M, Recher M, Hubert H, et al. Multisystem inflammatory syndrome in children by COVID-19 vaccination status of adolescents in France. JAMA. 2022;327:281–283. doi: 10.1001/jama.2021.23262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zambrano LDNM, Newhams MM, Olson SM, et al. Effectiveness of BNT162b2 (Pfizer-BioNTech) mRNA vaccination against multisystem inflammatory syndrome in children among persons aged 12–18 years—United States, July–December, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:52–58. doi: 10.15585/mmwr.mm7102e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Patient data from this public health investigation are not available to be shared publicly. Limited, deidentified VAERS data are publicly available at https://vaers.hhs.gov/data.html.