Abstract
Chlamydia pneumoniae is frequently found in atherosclerotic lesions, and high titers of specific antibodies are associated with increased risk for acute myocardial infarction. However, a causative relation has not been established yet. We performed a prospective study of 93 patients undergoing percutaneous transluminal coronary angioplasty (PTCA) to investigate whether angioplasty influences Chlamydia-specific antibody titers and whether there is an association with restenosis. Blood samples were obtained before and 1 and 6 months after angioplasty. Antibodies against chlamydial lipopolysaccharide and against purified C. pneumoniae elementary bodies were measured by enzyme-linked immunosorbent assay (ELISA). After angioplasty, the prevalence of antibodies to lipopolysaccharide rose from 20 to 26% for immunoglobulin A (IgA), from 53 to 64% for IgG, and from 2 to 7% for IgM (P = 0.021, 0.004, and 0.046, respectively). There was a rapid increase of mean antibody titers of all antibody classes within 1 month of PTCA. During the following 5 months, antibody titers decreased slightly but were still higher than baseline values. Results of the C. pneumoniae-specific ELISA were essentially the same. The rise of anti-Chlamydia antibodies was not caused by unspecific reactivation of the immune system, as levels of antibodies against cytomegalovirus did not change. Neither seropositivity nor antibody titers were related to restenosis. However, increases in mean IgA and IgM titers were restricted to patients who had suffered from myocardial infarction earlier in their lives. In conclusion, we show that PTCA induces a stimulation of the humoral immune response against C. pneumoniae. These data support the idea that plaque disruption during angioplasty might make hidden chlamydial antigens accessible to the immune system.
Chlamydia pneumoniae is an important respiratory pathogen that accounts for up to 10% of cases of community-acquired pneumonia (7, 15). It reaches very high rates of endemic infection in the general population, and seroepidemiologic research indicates that virtually everyone becomes infected at least once during his lifetime (10). Recently, C. pneumoniae has also been implicated in atherogenesis. Epidemiological studies demonstrate a consistent association between elevated C. pneumoniae antibody titers and acute myocardial infarction or chronic coronary heart disease, with odds ratios of 2 or more (5). Antigens and/or DNA of the pathogen are found in up to 60% of investigated atheromatous coronary arteries but not in unaffected vessels (3, 19, 25). Successful culture of C. pneumoniae from plaques suggests the endovascular presence of viable bacteria (8, 13, 23). Whether the organism contributes to disease progression or resides within plaque lesions as a harmless commensal is unknown. Because of its widespread presence in coronary plaques, it is straightforward to investigate whether there is an association between prior or acute C. pneumoniae infection and the development of restenosis after percutaneous transluminal coronary angioplasty (PTCA). As far as we know, only one study addressing this question has been published until now (4). Retrospective analysis of a subgroup of 148 patients from the VERAS trial (30) showed no association between C. pneumoniae serology before PTCA and restenosis. We performed a prospective study in PTCA patients to investigate whether the angioplasty procedure would have an influence on the specific humoral immune reaction against C. pneumoniae antigens and to reveal a possible association with restenosis.
MATERIALS AND METHODS
Patient population and blood sampling.
Between December 1994 and October 1995, 106 patients (68 men and 38 women; mean age, 62.9 years; range, 34 to 82) who were consecutive candidates for elective PTCA of a de novo lesion were enrolled in the study. One patient developed liver carcinoma during the follow-up period and was excluded from the study. From 12 other patients, we were not able to obtain blood samples at follow-up investigations for various reasons. Study analysis was done on the remaining 93 patients. All patients had given written informed consent to participate in this study prior to the PTCA procedure. Quantitative analysis of the lesions and of the PTCA result were performed using the Cardiovascular Measurement System (9). Blood was drawn immediately before PTCA and 1 and 6 months after PTCA. At the defined time points, patients also had clinical examinations, including bicycle exercise testing. In cases of suspected restenosis, repeat angiography and, if indicated, repeat PTCA was performed. After the 6-month observation period, each patient was classified by two experienced cardiologists who were unaware of the outcome of laboratory tests to define the clinical outcome of the study. Definitions of clinical end points were (i) recurrent ischemia, defined as either progression or recurrence of anginal complaints and/or a positive exercise test and (ii) restenosis that required repeat revascularization in the same segment as the primary stenosis.
Measurement of chlamydial antibodies.
Serological analyses were carried out without prior knowledge of clinical data. All serum samples of a single patient were measured subsequently on the same microtiter plate.
(i) Chlamydial LPS ELISA.
Tests for antibodies (immunoglobulin G [IgG], IgA, and IgM) to chlamydial lipopolysaccharide (LPS) were done with a commercially available, recombinant enzyme-linked immunosorbent assay (ELISA) kit (MEDAC GmbH, Hamburg, Germany) on a fully automated ELISA processor (Libertas, Iason, Vienna, Austria). This ELISA includes a chemically pure structure of a recombinant LPS which contains a genus-specific epitope of the Chlamydia spp. pathogenic to humans (1). The IgG, IgA, and IgM cutoff values were calculated as prescribed by the manufacturer. Concentrations of chlamydial antibodies were expressed as an index calculated as the optical density of the sample/cutoff.
(ii) Chlamydial EB ELISA.
Specific IgG and IgA antibodies to C. pneumoniae (strain TW-183) were determined by an in-house-developed enzyme immunoassay as described for C. trachomatis (21). Briefly, C. pneumoniae was propagated in 6-well microtiter plates using optimal conditions (17). Chlamydial elementary bodies (EBs) were partially purified by differential centrifugation through a layer of 35% sodium diatrizoate and used to coat microtiter plates. Serum specimens were diluted 1:1,000 for IgG determinations and 1:500 for IgA determinations. The absorbance of control antigen consisting of partially purified mock-infected cells was subtracted from the absorbance of the C. pneumoniae antigen. A titer was calculated by using a twofold dilution series of a positive control included on each plate. Pipetting was carried out using a robotic pipettor (Canberra Packard, Tilburg, The Netherlands) and the microtiter plates were processed with an immunoassay processor (Dynatech Immunoassay System, DPC Nederland BV, Apeldoorn, The Netherlands). Measured antibody titers as well as the sensitivity of the ELISA to detect seropositive individuals show a high correlation with results obtained by the microimmunofluorescence test (11, 20).
Measurement of antibodies to CMV.
Quantitative determination of cytomegalovirus (CMV)-specific IgG antibody was performed as described previously (27). Briefly, microtiter plates were coated with protein extracts made from late-stage CMV-infected fibroblasts and with extracts from mock-infected fibroblasts as a control. Serum samples were added in serial twofold dilution beginning with 1:200. To standardize the test run, selected CMV-positive serum samples were pooled and included on each plate as standard serum. Undiluted pool serum was arbitrarily assigned to 100%. The amount of antibody present in the patient’s serum was expressed as a percentage of the antibody present in the reference serum. This procedure resulted in highly reproducible values of antibody concentrations, because dose response curves (optical density versus dilution) of unknown samples were related to the dose response curve of the standard pool. The previously determined cutoff value for CMV seropositivity is >1% for IgG.
Statistical methods.
Statistical analysis was performed on a personal computer using the Systat software package version 5.01 (Systat Inc., Evanston, Ill.). The increase in seroprevalence was statistically tested using McNemar’s symmetry chi-square test. Analyses of frequency counts were performed with the use of Fisher’s exact test for small samples. Because of lack of normal distribution, we used Wilcoxon matched pairs test to test for changes in mean antibody titers over time. All tests were two sided and P values smaller than 0.05 were considered statistically significant.
RESULTS
Distributions of age, sex, and several known risk factors for atherosclerosis in the 93 patients included in the study are shown in Table 1. In a total of 103 lesions, the residual diameter increased from a mean (standard deviation [SD]) of 0.95 (0.37) mm to 2.04 (0.59) mm. The percentage of diameter stenosis decreased from a mean (SD) of 63.2% (14%) to 26.5% (11%). The reference diameter did not change [mean (SD), 2.66 (0.66) mm to 2.77 (0.71) mm]. The prevalence of several potential risk factors for restenosis, such as diabetes, a lesion in the left anterior descending artery, small vessel diameter (<2.5 mm), hypertension, hypercholesterolemia, and male sex did not differ according to the status of IgG and IgA antibodies to LPS of the patients (data not shown).
TABLE 1.
Patient characteristics (n = 93 patients)
| Characteristica | Value |
|---|---|
| Sex (no. of males/no. of females) | 62/31 |
| Age (yr) | |
| Mean (SD) | 62.6 (10.0) |
| Range | 34–82 |
| Previous ischemic events (n) | |
| Previous CABG | 8 |
| Previous PTCA (other lesion) | 10 |
| Previous myocardial infarction | 35 |
| Risk factors (n) | |
| Diabetes | 7 |
| Hypertension | 21 |
| Hypercholesterolemia | 30 |
| Lesions (n) | |
| LAD | 31 |
| Cx | 36 |
| RCA | 35 |
| LM | 1 |
CABG, coronary artery bypass graft; LAD, left anterior descending coronary artery; Cx, circumflex artery; RCA, right coronary artery; LM, left main coronary artery.
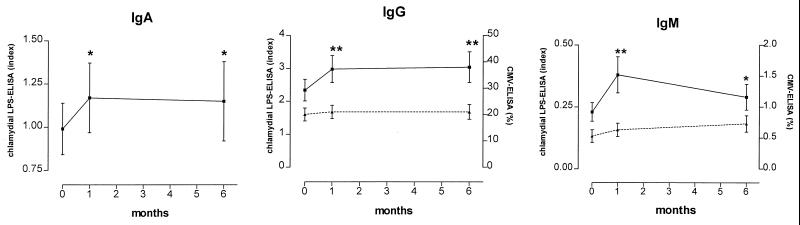
At the time of angioplasty, 19 (20%), 49 (53%), and 2 (2%) of the 93 patients had antibodies to LPS of classes IgA, IgG, and IgM, respectively. Within the follow-up period, seroprevalence rose significantly to 29, 64, and 7% for IgA, IgG, and IgM (P = 0.021, 0.004, and 0.046), respectively. There was a rapid increase of mean antibody concentrations of all classes within 1 month after PTCA (Fig. 1). During the following 5 months, antibodies decreased slightly, but were still higher than baseline values. Applying the criteria for acute Chlamydia infection proposed by Verkooyen et al. (29), four patients had acute infections during the study period. None of them experienced restenosis and only one had had myocarial infarction prior to PTCA.
FIG. 1.
Concentrations of antibodies to chlamydial LPS (IgA, IgG, and IgM; solid line) and to human CMV (IgG and IgM; dotted line) at baseline and 1 and 6 months after PTCA. Concentrations of chlamydial antibodies are expressed as an index (calculated as the optical density of the sample/cutoff), and antibodies to CMV are expressed as a percentage of antibodies present in the reference serum. Error bars indicate standard errors of the means. ∗, P < 0.05; ∗∗, P < 0.005 versus baseline levels.
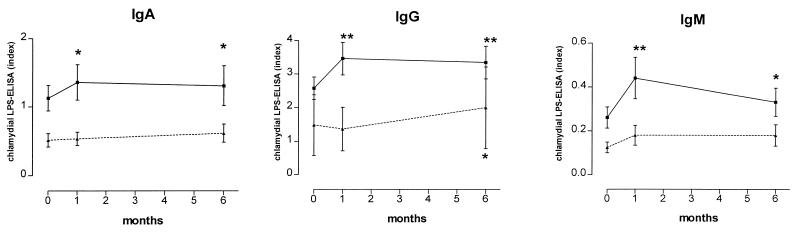
We also measured antibody titers against purified EBs of C. pneumoniae. In this EB ELISA, 72 of 93 patients (77%) tested positive for IgG. Mean titers of IgG antibodies before and 1 and 6 months after PTCA were 3,599, 4,294 (P < 0.00001), and 3,870 (P = 0.010), respectively. Mean titers of IgA antibodies at the same time points were 2,163, 2,416 (P = 0.228), and 2,018 (P = 0.054). Table 2 shows the prevalence of chlamydial IgG antibodies determined by LPS and EB ELISA. Although there was no correlation between IgG antibody concentrations in the EB and the LPS IgG ELISAs (r2 = 0.389), EB IgG titers also showed an significant increase after PTCA. Moreover, the increase in titers occurred in essentially the same patients. In the analyses described above, we analyzed mean antibody titers of all patients. We also grouped our patients into chlamydia-negative and chlamydia-positive categories, defined as having IgG antibodies in the EB ELISA before PTCA. The results shown in Fig. 2 suggest that a rise in antibody titers to LPS is not a common phenomenon but occurs almost exclusively in chlamydia-positive individuals.
TABLE 2.
Comparison of the occurrence of anti-Chlamydia IgG antibodies among 93 PTCA patients as determined by the LPS ELISA and the EB ELISA
| LPS ELISA result | EB ELISA result
|
Total (n) | |
|---|---|---|---|
| Negative (n) | Positive (n) | ||
| Negative | 17 | 24 | 41 |
| Positive | 4 | 48 | 52 |
| Total | 21 | 72 | 93 |
FIG. 2.
Concentrations of antibodies (IgA, IgG, and IgM) to chlamydial LPS at baseline and 1 and 6 months after PTCA in patients with positive anti-C. pneumoniae antibody titers at the time of PTCA (determined by EB ELISA; solid line) and seronegative individuals (dotted line). Concentrations of chlamydial antibodies are expressed as an index (optical density of the sample/cutoff). Error bars indicate standard errors of the means. ∗, P < 0.05; ∗∗, P < 0.005 versus baseline levels.
The 93 patients included in the study were followed for a period of 6 months to correlate C. pneumoniae serology and occurrence of restenosis. With 75 patients, an exercise test was performed which was positive in 13 patients. Because of severe complaints, four patients were immediately admitted to coronary angiography without performing an exercise test. Positive exercise test results were correlated with angiographic restenosis (P = 0.004) but not with C. pneumoniae serostatus. According to the definitions described in Materials and Methods, 27 patients (29%) were suspect for restenosis after clinical examination. Twenty four of the patients underwent coronary angiography and in 12 (13%), a restenosis in the same segment as the primary stenosis could be confirmed. The baseline seroprevalence of LPS IgG antibodies was 42% in subjects who experienced restenosis and 54% in those who did not. Mean titer indices of IgG antibodies in patients with restenosis were 1.8, 2.7, and 2.5 at baseline, 1 month, and 6 months, respectively. Antibody indices in patients without restenosis at these time points were 2.6, 3.0, and 3.1, respectively. The differences between the two groups were not statistically significant. Seropositivity and antibody titers of the other Ig classes were also not related to clinically diagnosed or angiographically verified restenosis (data not shown).
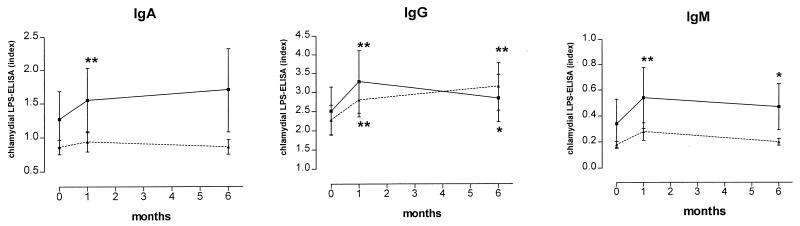
Several studies have shown that high titers of antibodies to C. pneumoniae are frequently found in patients suffering from acute myocardial infarction. Investigating this issue, we found a close correlation between the rise of chlamydial antibodies after PTCA and previous myocardial infarction (Fig. 3). Significant increases in mean IgA and IgM antibodies occurred only in patients who had suffered from myocardial infarction earlier in their lives. To elucidate if the rise in Chlamydia-specific antibody titers was due to an unspecific stimulation of the humoral immune system by the angioplasty procedure, we also measured antibodies to human CMV in these samples. However, mean antibodies to CMV did not change during the observation period (Fig. 1).
FIG. 3.
Concentrations of antibodies (IgA, IgG, and IgM) to chlamydial LPS at baseline and 1 and 6 months after PTCA in patients who had suffered from myocardial infarction prior to PTCA (n = 35; solid line) and in patients who had not (n = 58; dotted line); concentrations of chlamydial antibodies are expressed as an index (optical density of the sample/cutoff). Error bars indicate standard errors of the means. ∗, P < 0.05; ∗∗, P < 0.005 versus baseline levels.
DISCUSSION
In this study, we showed that PTCA induces a significant rise in mean anti-Chlamydia antibodies of IgA, IgG, and IgM classes. This increase was specific for C. pneumoniae and occurred primarily in patients with prior myocardial infarction. An association with restenosis was not observed. A rise in antibody titer is generally considered evidence of an acute chlamydial infection and/or reinfection. A twofold or more rise in titer has been proposed as a diagnostic criterion (29). In the present study, most changes in antibody titer were smaller. Only four patients showed serological evidence of acute infection during the surveillance period and thus the prevalence of acute chlamydial infection was not higher than in the general population (10). We believe rather that the observed rise in antibody level reflects an immunologic reaction induced by angioplasty. Balloon angioplasty causes serious damage to the target artery, including plaque disruption, fissuring, and endothelial denudation (18). As a consequence of this injury and of the upcoming healing process, antigens from Chlamydia microorganisms already present within the plaque may gain access to cells of the immune system and in this way trigger a rise in antibody levels.
The conclusion of this paper that angioplasty induces an immunologic reaction to C. pneumoniae by releasing or exposing chlamydial antigens requires that these antigens be present in the plaque. Indeed, C. pneumoniae is constantly found in atherosclerotic lesions. However, the reported rates of positive tissue findings vary considerably, mainly depending on the particular detection technique used. By comparing several of these techniques, we have recently shown that some of the known C. pneumoniae antigens are always present in advanced atherosclerotic lesions, but nearly never DNA or RNA (16). Therefore, we can assume the presence of C. pneumoniae antigens in the lesions of most if not all patients.
Acute chlamydial infections within the vessel walls may constitute a risk factor for a vascular event. The previously reported detection of high antibody titers and of Chlamydia-specific immune complexes in the months before and after an acute myocardial infarction and our findings are in agreement with this hypothesis (12, 24). It is interesting that the rise in mean antibody levels of IgA and IgM classes was restricted almost completely to patients who had had a myocardial infarction earlier in their lives. IgA and IgM classes are known to react quickly to acute reinfections (28). The data suggest that those patients who already suffered from an acute cardiac event may have a higher density of chlamydial antigen and/or a higher immunological activity within the plaques. Consequently, patients with unstable angina should have higher antibody titers than those with stable angina. A recent publication reported such an association between high chlamydial antibody levels and instability (26), and a study further exploring this issue is in progress in our laboratory. For detection of antichlamydial antibodies, we used initially a commercially available recombinant LPS ELISA (1). This LPS ELISA is not able to differentiate between antibodies against various Chlamydia species, as the LPS used as antigen is also part of C. trachomatis and C. psittaci. However, it is not very probable that these species contributed substantially to seropositivity in our study. Infections with C. trachomatis are rare in the age group of our patients, who furthermore do not belong to a high-risk population for C. trachomatis. In recent reports on the serological data of patients with coronary artery disease, IgG seroprevalence for C. trachomatis was less than 1%, and no cases of C. psittaci infection were identified (14, 29). Because of the relatively short half-life of anti-LPS antibodies and the quick increase in IgM and IgA reactivity after infection, this LPS ELISA may currently be the most sensitive serological method for the detection of acute chlamydial infection (28). In contrast, to determine the prevalence of C. pneumoniae infection, i.e., establishing the proportion of subjects with C. pneumoniae infection in the past, the microimmunofluorescence or the EB ELISA seems to be more appropriate, as the antibodies to protein antigens last much longer. We have previously shown that the EB ELISA, which we used in this study, is more specific for C. pneumoniae than the LPS ELISA and that it recognizes chlamydial antibodies as a risk factor for atherosclerosis (20). It correlates well with the microimmunofluorescence method of Wang and Grayston (10). This EB ELISA showed a much higher rate of seropositive patients (72 of 93; 77%) and there was no correlation to anti-LPS antibody titers. Both findings may be explained by the different kinetics of antibodies to LPS and to chlamydial proteins and are in good agreement with the literature (1, 28). However, there was a good concordance in classifying patients, as only 4 of 93 patients were LPS IgG positive but EB negative. Moreover, increases in antibody titers occurred essentially in the same patients. It could be possible that the rise of chlamydial antibodies after PTCA is caused by an unspecific activation by the immune system. However, as the increase in antibody concentrations occurred only in seropositive patients and the antibodies to CMV did not change, we feel certain that the reported rise of antibody levels really reflects a specific interaction between chlamydial antigen and immune system. In this study, occurrence of restenosis was associated with neither seroprevalency nor antibody titer. This is in agreement with a previously published study (4). A certain limitation of the present study may be the fact that not all angioplasty patients underwent routine follow-up cardiac catheterization. Clinical assessment of cardiac events is known to have some inaccuracy in predicting restenosis. Prior studies have indicated that 15 to 20% of asymptomatic patients have angiographic evidence of restenosis and that about 30% of patients with symptoms have no angiographic evidence of restenosis at the time of follow-up (2). This discrepancy was explained by an abnormal vasomotion in the PTCA-treated artery (22) or by the fact that moderate angiographic stenosis (40 to 70%) may not cause ischemic symptoms (6). Therefore, the lack of association between chlamydial antibodies and restenosis should be interpreted cautiously.
In conclusion, our results show a statistically significant rise in C. pneumoniae-specific antibodies induced by PTCA. Our findings prove neither an acute chlamydial reinfection nor a pathogenic relevance of this increase in regard to restenosis. However, since the increase was specific for chlamydial antibodies, it suggests that already-present microbial antigens released from within the plaque may induce a secondary or memory immune response. These results are consistent with the idea that C. pneumoniae infection plays a role in the immunopathogenesis of atherosclerosis.
ACKNOWLEDGMENTS
This work was supported by grants from the Austrian Science Foundation (grant J 01105-Med) and the Dutch Heart Foundation (grant D95-019).
We would like to thank N. Sitke and E. Winter for excellent technical assistance.
REFERENCES
- 1.Brade L, Brunnemann H, Ernst M, Fu Y, Holst O, Kosma P, Naher H, Persson K, Brade H. Occurrence of antibodies against chlamydial lipopolysaccharide in human sera as measured by ELISA using an artificial glycoconjugate antigen. FEMS Immunol Med Microbiol. 1994;8:27–41. doi: 10.1111/j.1574-695X.1994.tb00422.x. [DOI] [PubMed] [Google Scholar]
- 2.Califf R M, Ohman E M, Frid D J, Fortin D F, Mark D B, Hlatky M A, Herndon J E, Bengtson J R. Restenosis: the clinical issues. In: Topol E J, editor. Textbook of interventional cardiology. W. B. Philadelphia, Pa: Saunders Co.; 1990. pp. 363–394. [Google Scholar]
- 3.Campbell L A, O’Brien E R, Cappuccio A L, Kuo C C, Wang S P, Stewart D, Patton D L, Cummings P K, Grayston J T. Detection of Chlamydia pneumoniae TWAR in human coronary atherectomy tissues. J Infect Dis. 1995;172:585–588. doi: 10.1093/infdis/172.2.585. [DOI] [PubMed] [Google Scholar]
- 4.Carlsson J, Miketic S, Mueller K H, Brom J, Ross R, von Essen R, Tebbe U. Previous cytomegalovirus or Chlamydia pneumoniae infection and risk of restenosis after percutaneous transluminal coronary angioplasty. Lancet. 1997;350:1225. doi: 10.1016/S0140-6736(05)63456-3. [DOI] [PubMed] [Google Scholar]
- 5.Danesh J, Collins R, Peto R. Chronic infections and coronary heart disease: is there a link. Lancet. 1997;350:430–436. doi: 10.1016/S0140-6736(97)03079-1. [DOI] [PubMed] [Google Scholar]
- 6.Gordon P C, Friedrich S P, Piana R N, Kugelmass A D, Leidig G A, Gibson C M, Cohen D J, Carrozza J P, Kuntz R E, Baim D S. Is 40% to 70% diameter narrowing at the site of previous stenting or directional coronary atherectomy clinically significant? Am J Cardiol. 1994;74:26–32. doi: 10.1016/0002-9149(94)90486-3. [DOI] [PubMed] [Google Scholar]
- 7.Grayston J T, Campbell L A, Kuo C C, Mordhorst C H, Saikku P, Thom D H, Wang S P. A new respiratory tract pathogen: Chlamydia pneumoniae strain TWAR. J Infect Dis. 1990;161:618–625. doi: 10.1093/infdis/161.4.618. [DOI] [PubMed] [Google Scholar]
- 8.Jackson L A, Campbell L A, Kuo C C, Rodriguez D I, Lee A, Grayston J T. Isolation of Chlamydia pneumoniae from a carotid endarterectomy specimen. J Infect Dis. 1997;176:292–295. doi: 10.1086/517270. [DOI] [PubMed] [Google Scholar]
- 9.Jukema J W, Bruschke A V, van Boven A J, Reiber J H, Bal E T, Zwinderman A H, Jansen H, Boerma G J, van Rappard F M, Lie K I. Effects of lipid lowering by pravastatin on progression and regression of coronary artery disease in symptomatic men with normal to moderately elevated serum cholesterol levels. The Regression Growth Evaluation Statin Study (REGRESS) Circulation. 1995;91:2528–2540. doi: 10.1161/01.cir.91.10.2528. [DOI] [PubMed] [Google Scholar]
- 10.Kuo C C, Jackson L A, Campbell L A, Grayston J T. Chlamydia pneumoniae (TWAR) Clin Microbiol Rev. 1995;8:451–461. doi: 10.1128/cmr.8.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ladany S, Black C M, Farshy C E, Ossewaarde J M, Barnes R C. Enzyme immunoassay to determine exposure to Chlamydia pneumoniae (strain TWAR) J Clin Microbiol. 1989;27:2778–2783. doi: 10.1128/jcm.27.12.2778-2783.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leinonen M, Linnanmaki E, Mattila K, Nieminen M S, Valtonen V, Leirisalo Repo M, Saikku P. Circulating immune complexes containing chlamydial lipopolysaccharide in acute myocardial infarction. Microb Pathog. 1990;9:67–73. doi: 10.1016/0882-4010(90)90042-o. [DOI] [PubMed] [Google Scholar]
- 13.Maass M, Bartels C, Engel P M, Mamat U, Sievers H H. Endovascular presence of viable Chlamydia pneumoniae is a common phenomenon in coronary artery disease. J Am Coll Cardiol. 1998;31:827–832. doi: 10.1016/s0735-1097(98)00016-3. [DOI] [PubMed] [Google Scholar]
- 14.Maass M, Gieffers J. Prominent serological response to Chlamydia pneumoniae in cardiovascular disease. Immunol Infect Dis. 1996;6:65–70. [Google Scholar]
- 15.Marrie T J, Grayston J T, Wang S P, Kuo C C. Pneumonia associated with the TWAR strain of Chlamydia. Ann Intern Med. 1987;106:507–511. doi: 10.7326/0003-4819-106-4-507. [DOI] [PubMed] [Google Scholar]
- 16.Meijer A, Roholl P J M, van der Vliet J A, Gielis-Proper F K, de Vries A, Ossewaarde J M. Proceedings of the Ninth International Symposium on Human Chlamydial Infection. San Francisco, Calif: International Chlamydia Symposium; 1998. Differences in detection of chlamydia pneumoniae protein, hsp60, lipopolysaccharide and DNA in abdominal aneurysms; pp. 191–194. [Google Scholar]
- 17.Meijer A, Vallinga C E, Ossewaarde J M. A microcarrier culture method for the production of large quantities of viable Chlamydia pneumoniae. Appl Microbiol Biotechnol. 1996;46:132–137. doi: 10.1007/s002530050794. [DOI] [PubMed] [Google Scholar]
- 18.Nissen S E, Gurley J C. Assessment of coronary angioplasty results by intravascular ultrasound. In: Serruys P W, Strauss B H, King S B, editors. Restenosis after intervention with new mechanical devices. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. pp. 73–96. [Google Scholar]
- 19.Ong G, Thomas B J, Mansfield A O, Davidson B R, Taylor R D. Detection and widespread distribution of Chlamydia pneumoniae in the vascular system and its possible implications. J Clin Pathol. 1996;49:102–106. doi: 10.1136/jcp.49.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ossewaarde J M, Feskens E J, de Vries A, Vallinga C E, Kromhout D. Chlamydia pneumoniae is a risk factor for coronary heart disease in symptom-free elderly men, but Helicobacter pylori and cytomegalovirus are not. Epidemiol Infect. 1998;120:93–99. doi: 10.1017/s0950268897008303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ossewaarde J M, Manten J W, Hooft H J, Hekker A C. An enzyme immunoassay to detect specific antibodies to protein and lipopolysaccharide antigens of Chlamydia trachomatis. J Immunol Methods. 1989;123:293–298. doi: 10.1016/0022-1759(89)90233-0. [DOI] [PubMed] [Google Scholar]
- 22.Quyyumi A A, Raphael M, Perrins E J, Shapiro L M, Rickards A F, Fox K M. Incidence of spasm at the site of previous successful transluminal coronary angioplasty: effect of ergometrine maleate in consecutive patients. Br Heart J. 1986;56:27–32. doi: 10.1136/hrt.56.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramirez J A. Isolation of Chlamydia pneumoniae from the coronary artery of a patient with coronary atherosclerosis. The Chlamydia pneumoniae/Atherosclerosis Study Group. Ann Intern Med. 1996;125:979–982. doi: 10.7326/0003-4819-125-12-199612150-00008. [DOI] [PubMed] [Google Scholar]
- 24.Saikku P. Chlamydia pneumoniae infection as a risk factor in acute myocardial infarction. Eur Heart J. 1993;14(Suppl. K):62–65. [PubMed] [Google Scholar]
- 25.Shor A, Kuo C C, Patton D L. Detection of Chlamydia pneumoniae in coronary arterial fatty streaks and atheromatous plaques. S Afr Med J. 1992;82:158–161. [PubMed] [Google Scholar]
- 26.Stanek G, Huber K, Glogar D, Maurer M, Kundi M. Chlamydia antibodies in patients with coronary artery disease. Hospitalis. 1997;67:18. [Google Scholar]
- 27.Van der Giessen M, Van den Berg A P, van der Bij W, Postma S, Van Son W J, The T H. Quantitative measurement of cytomegalovirus-specific IgG and IgM antibodies in relation to cytomegalovirus antigenaemia and disease activity in kidney recipients with an active cytomegalovirus infection. Clin Exp Immunol. 1990;80:56–61. doi: 10.1111/j.1365-2249.1990.tb06441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verkooyen R P. Chlamydia pneumoniae—studies on an emerging pathogen. Ph.D. thesis. Rotterdam, The Netherlands: University of Rotterdam; 1997. [Google Scholar]
- 29.Verkooyen R P, Van Lent N A, Joulandan S A M, Snijder R J, Van den Bosch J M, Van Helden H P, Verbrugh H A. Diagnosis of Chlamydia pneumoniae infection in patients with chronic obstructive pulmonary disease by microimmunofluorescence and ELISA. J Med Microbiol. 1997;46:959–964. doi: 10.1099/00222615-46-11-959. [DOI] [PubMed] [Google Scholar]
- 30.von Essen R, Ostermaier R, Grube E, Maurer W, Tebbe U, Erbel R, Roth M, Oel W, Brom J, Weidinger G. Effects of octreotide treatment on restenosis after coronary angioplasty: results of the VERAS study. Circulation. 1997;96:1482–1487. doi: 10.1161/01.cir.96.5.1482. [DOI] [PubMed] [Google Scholar]